Introduction
Parkinson's disease (PD) is a long-term degenerative
disorder of the central nervous system. It is characterized by a
progressive loss of dopaminergic neurons in the substantia nigra.
PD affects ~6 million people worldwide and its prevalence is 1% in
the population aged 60 or over (1). To date, studies demonstrated that PD
may involve oxidative stress, mitochondrial dysfunction, lysosomal
dysfunction and neuroinflammatory changes (1,2).
Among them, oxidative stress serves a pivotal role in PD
development (3–5). Neurotoxin-based models of PD
(particularly, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MPTP)
are important in elucidating the molecular cascade of cell death in
dopaminergic neurons (1). MPTP
itself is not toxic, but its oxidized product, 1-methyl-4-phenyl
pyridine ion (MPP+), is harmful. MPP+ interferes with oxidative
phosphorylation in mitochondria by inhibiting complex I of the
electron transport chain, resulting in the depletion of ATP and
excessive formation of reactive oxygen species (ROS), ultimately
leading to cell death (6). PC12
cells originate from a rat pheochromocytoma and exhibit the
properties of neurosecretory cells and dopaminergic neurons
(7). PC12 cells are commonly used
for PD studies (8,9). In this study, PC12 cell damage
induced by MPP+ served as a cell model of PD.
At present, PD treatments are symptomatic and no
medication is available to reverse or prevent its progression. This
has led to the search for a novel pharmacological and
neuroprotective treatment. SFN, a natural phytochemical and a
second enzyme inducer, has previously drawn increasing attention
due to its beneficial effects on a number of medical conditions
(10). Studies demonstrated that
SFN has multiple anti-pathological actions including antitumor
(11,12), antioxidant (13), immune regulation (14) and anti-inflammatory effects
(15). A number of mechanisms and
signal pathways are associated with the biological effects of SFN.
Among them, Nrf2 is one of the critical signal molecules (16,17).
As an important transcription factor, Nrf2 targets genes called
antioxidant response element, which encodes several antioxidant
enzymes including (HO-1) and nicotinamide quinone oxidoreductase 1
(NQO1). These enzymes are key players in the redox balance of cells
and provide cytoprotection against prooxidant stimuli (18–22).
In the present study, whether SFN protects PC12 cells against the
toxicity of MPP+ was investigated and the possible mechanism was
explored. The present study may provide evidence that SFN could be
a potential compound to prevent PD progression.
Materials and methods
Cell culture
PC12 cells (undifferentiated) were purchased from
the Shanghai Cell Bank of Chinese Academy of Sciences and were
cultured in DMEM high glucose medium (Hyclone, GE Healthcare)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 1% penicillin-streptomycin mixture and 4 mmol/l
L-glutamine. The culture was placed in a humidified incubator with
5% CO2 and 95% air at 37°C. The cells were subcultured
every 3 days and cells at a logarithmic growth phase were used for
the experiments.
MTT colorimetric assay
The density of PC12 cells was adjusted to
5×105 cells/ml and the cells (100 µl/well) were seeded
on a 96-well microplate. Following a total of 24 h of growth in a
humidified incubator, the different compounds were added into the
cultures. A total of 5 parallel wells were used for a specific
treatment and the experiments were independently repeated three
times. For the MPP+ group, the cells were treated with serial
concentrations of MPP+ (100, 300, 500 and 700 µmol/l) and incubated
for different periods of time (12, 24 and 48 h) (23). For the SFN group, the cells were
incubated with SFN (0.5, 1.0, 2.5, 5.0 and 10 µmol/l) for 24 h. For
the SFN plus MPP+ group, the cells were pretreated with different
concentrations of SFN (0.5, 1.0, 2.5, 5.0 and 10 µmol/l) for 1 h,
then MPP+ (500 µmol/l) was added and the cultures were continued
for 24 h. Following the different treatments, cell viability was
determined by MTT colorimetric assay as described previously
(24). Briefly, 20 µl of MTT
solution (5 mg/ml) was added into each well and incubated with the
cells for 4 h at 37°C. Subsequently the medium was carefully
replaced with 150 µl DMSO and a 10 min oscillation was applied to
sufficiently dissolve the crystal. The absorbance value at 570 nm
was measured with a microplate reader. The cell survival rate was
calculated as the ratio of absorbance of the experimental groups to
that of the control group.
Flow cytometry analyses
Flow cytometry analyses were performed as described
previously (25). In short,
following the different treatments, PC12 cells were harvested and
re-suspended in a 500 µl binding buffer. The cells were labeled
with 5 µl Annexin V-FITC (Nanjing KeyGen Biotech. Co. Ltd.) and 5
µl of propidium iodide for 10 min in the dark. The apoptotic index
was determined using a flow cytometer (BD Biosciences). All data
were acquired and analyzed with Cellquest version 5.1 software (BD
Biosciences).
Western blotting
Protein expression was assessed by western blotting
according to the authors' previous study (26). In brief, the cell pellets were
lysed in a RIPA lysis buffer (cat. no. R0020; Beijing Solarbio
Science & Technology Co., Ltd.) supplemented with proteinase
and phosphatase inhibitor (Sigma-Aldrich; Merck KGaA). After
centrifugation at 16,000 × g at 4°C for 15 min, the supernatant was
harvested and the protein was quantified using the bicinchoninic
acid protein assay (Merck KGaA). Equal amounts of protein (30–40 µg
per well) were loaded on 12% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (EMD Millipore). The membrane was
blocked with a 5% nonfat milk solution for 2 h at room temperature.
Then the membrane was incubated with the primary antibodies
overnight at 4°C (Santa Cruz Biotechnology, Inc.) against Nrf2
(1:100, sc-518036), HO-1 (1:100, sc-136960) and NQO1 (1:200,
sc-376023), respectively. Following incubation with the secondary
antibody (1:1,000, goat anti-mouse IgG-HRP, sc-2005, Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature, the blot bands
were detected with ECL substrate (Clarity Max™, Bio-Rad
Laboratories, Inc.). β-actin (1:500, sc-47778, Santa Cruz
Biotechnology, Inc) was used as an internal control. The western
blot images were analyzed with ImageJ software (v1.52, National
Institutes of Health), and protein levels were expressed as the
ratio of values of the detected protein bands to that of β-actin
bands.
Chemicals
SFN and MPP+ were purchased from
Sigma-Aldrich and Merck KGaA.
Statistical analysis
The data are presented as the mean ± standard error
of mean and GraphPad Prism 7.0 (GraphPad Software, Inc.) was used
to perform the statistical analysis. One-way or two-way analysis of
variance was conducted to examine the differences among the
multiple groups, followed by Turkey post-hoc analysis. P<0.05
were considered to indicate a statistically significant
difference.
Results
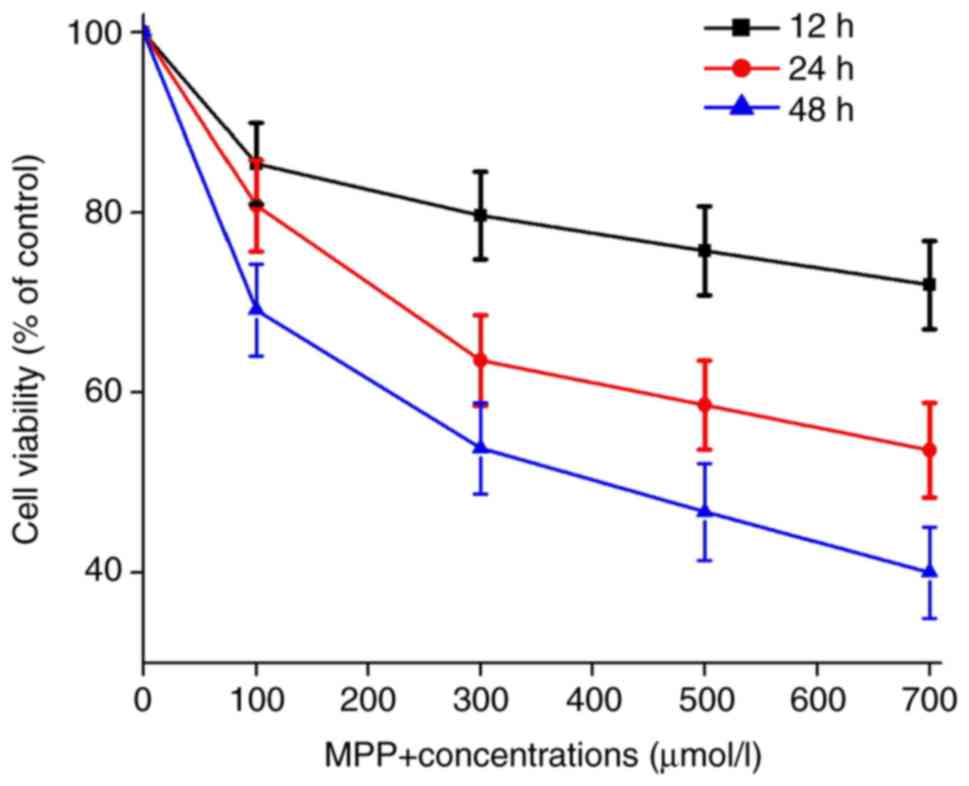
MPP+ reduces the survival rate of PC12
cells
In order to evaluate the toxic effects of MPP+ on
PC12 cells, the cells were incubated with different concentrations
of MPP+ and examined the cell viability at different time points.
It was identified that MPP+ decreased the cell viability in a dose-
and time-dependent manner (Fig.
1). The results of the present study are in line with previous
studies that MPP+ caused significant damage to PC12 cells (27,28).
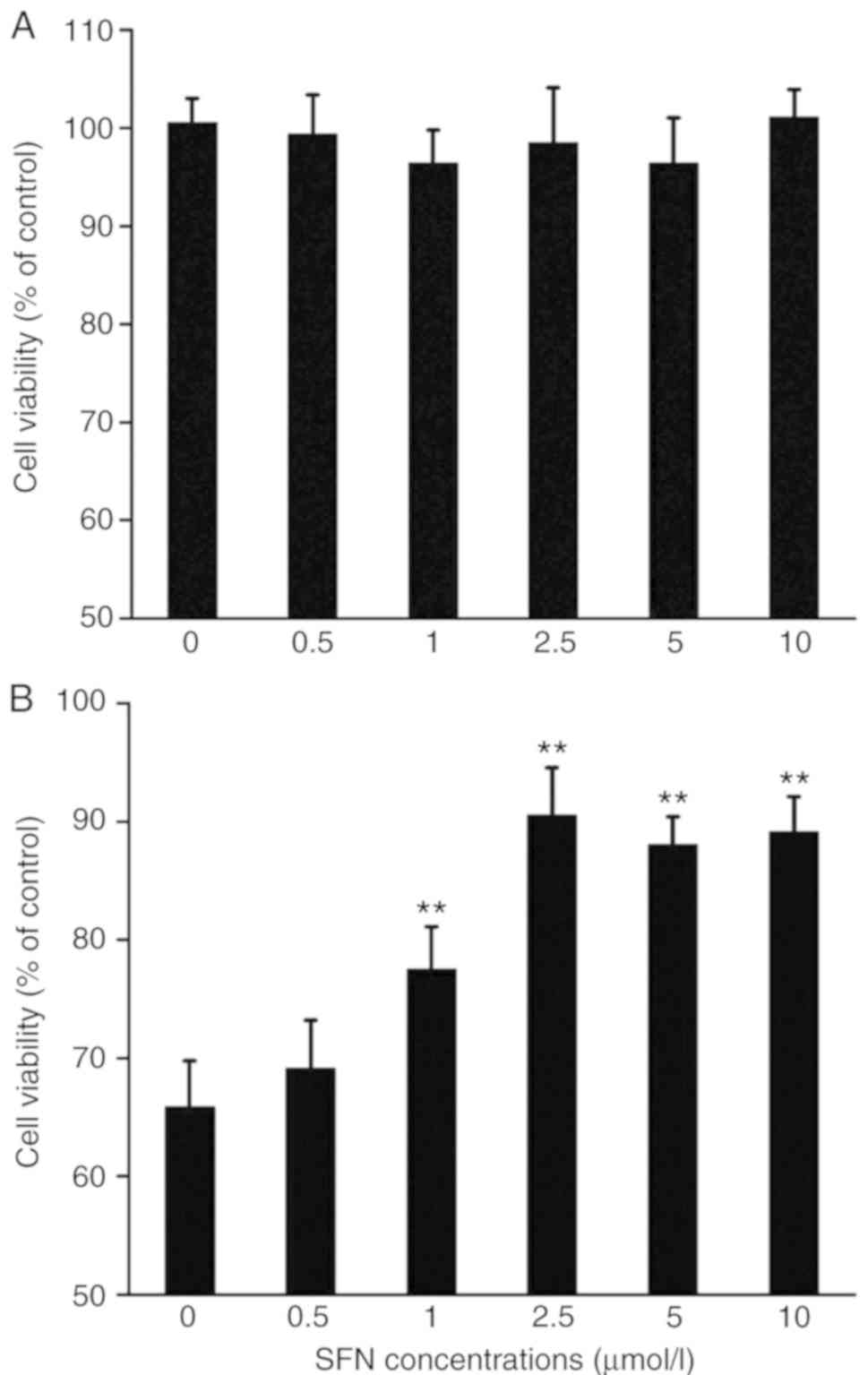
SFN protects against the cytotoxicity
induced by MPP+
Next whether SFN could protect PC12 cells against
the cytotoxicity of the MPP+ was examined. As presented in Figs. 2A and 3, SFN alone did not affect the viability
or apoptosis of PC12 cells. However, SFN (1–10 µmol/l) pretreatment
significantly attenuated the toxic effects of MPP+ on PC12 cells in
a dose-dependent fashion (P<0.01; Fig. 2B).
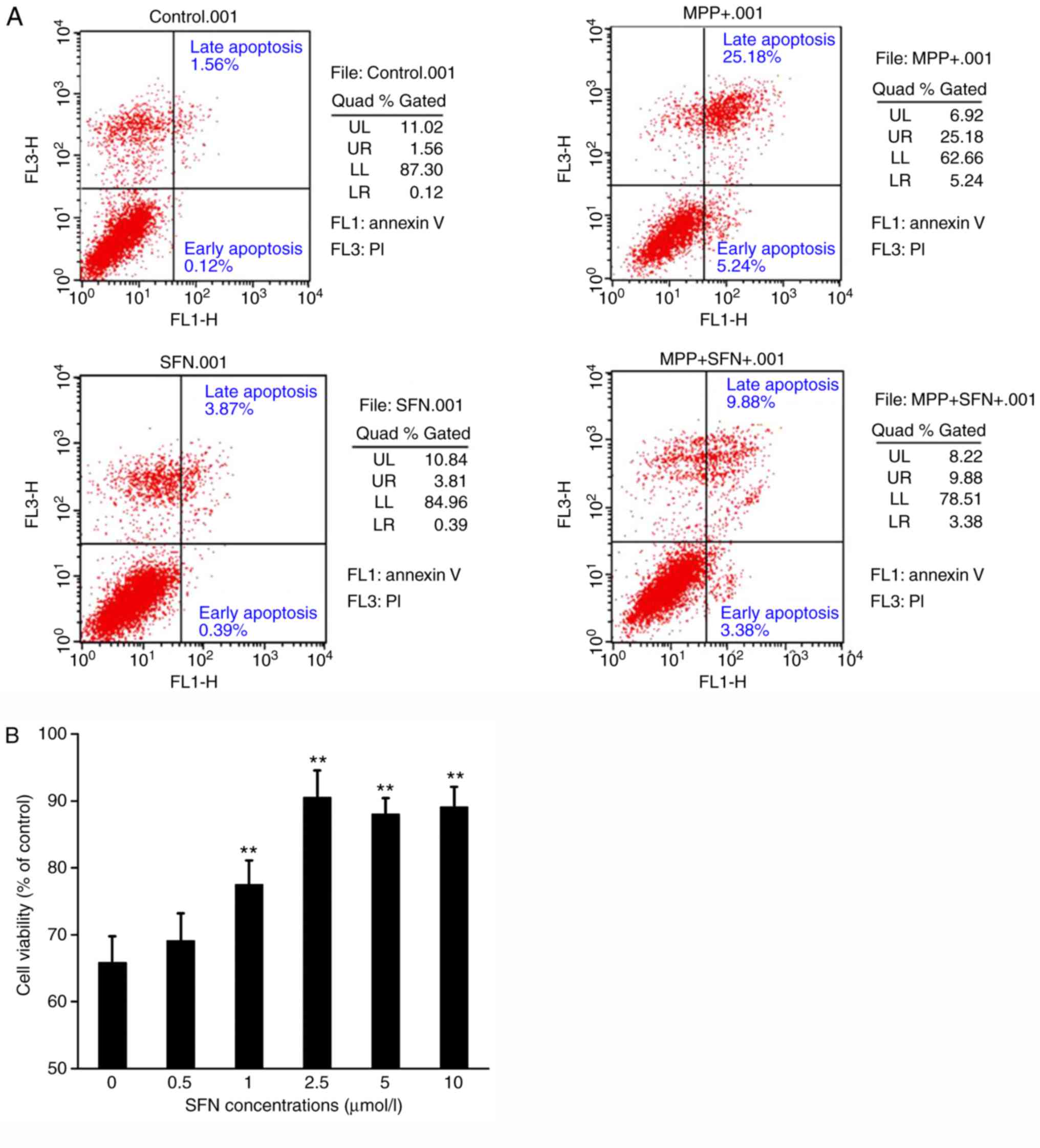 | Figure 3.Apoptosis rate of PC12 cells is
determined by flow cytometry using the annexin V-FITC/PI apoptosis
assay following different treatments. (A) Representative scatter
plots demonstrating apoptosis by Annexin V-FITC and PI staining and
flow cytometry. UL, dead cells; UR, late apoptotic cells; LL,
viable cells; LR, early apoptotic cells. (B) Summary data for
apoptosis rate of PC12 cells treated with SFN, MPP+, or SFN and
MPP+ together. **P<0.01 vs. the control group. One-way analysis
of variance was used to analyze the data. SFN, sulforaphane; MPP+,
1-methyl-4-phenyl pyridine ion; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Consistent with the MTT experiments, flow cytometry
data demonstrated that MPP+ significantly increased apoptosis of
PC12 cells. After 24 h treatment, 500 µmol/l MPP+ resulted in
30.4±0.6% apoptotic cells (Fig.
3). In addition, it was found that SFN significantly reduced
the apoptosis of PC12 cells induced by MPP+ (P<0.01; Fig. 3). At a concentration of 2.5 µmol/l,
SFN decreased the apoptosis rate induced by MPP+ from 30.4±0.6 to
13.3±0.2% (Fig. 3). The results
demonstrate that SFN can protect against MPP+ induced
cytotoxicity.
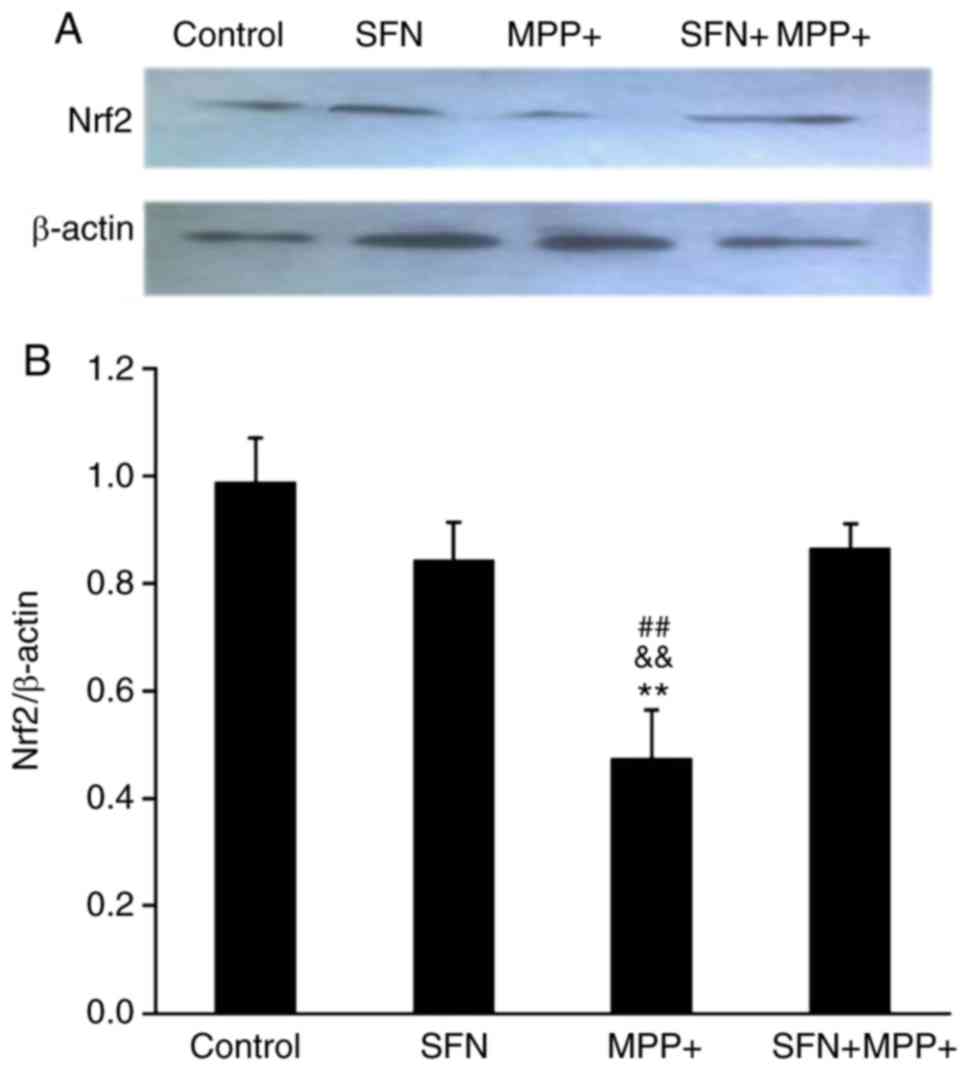
SFN upregulates the Nrf2 signaling
pathway
To investigate the signaling pathways involved in
the protective effect of SFN, the expression of Nrf2, which is
reported to be pivotal for the biological effects of SFN was
examined (29). As presented in
Fig. 4, MPP+ treatment
significantly decreased Nrf2 expression in PC12 cells by western
blotting (P<0.01). Although SFN itself did not alter the
expression of Nrf2, SFN pretreatment (2.5 µmol/l) significantly
reversed the reduced expression of Nrf2 caused by MPP+ treatment.
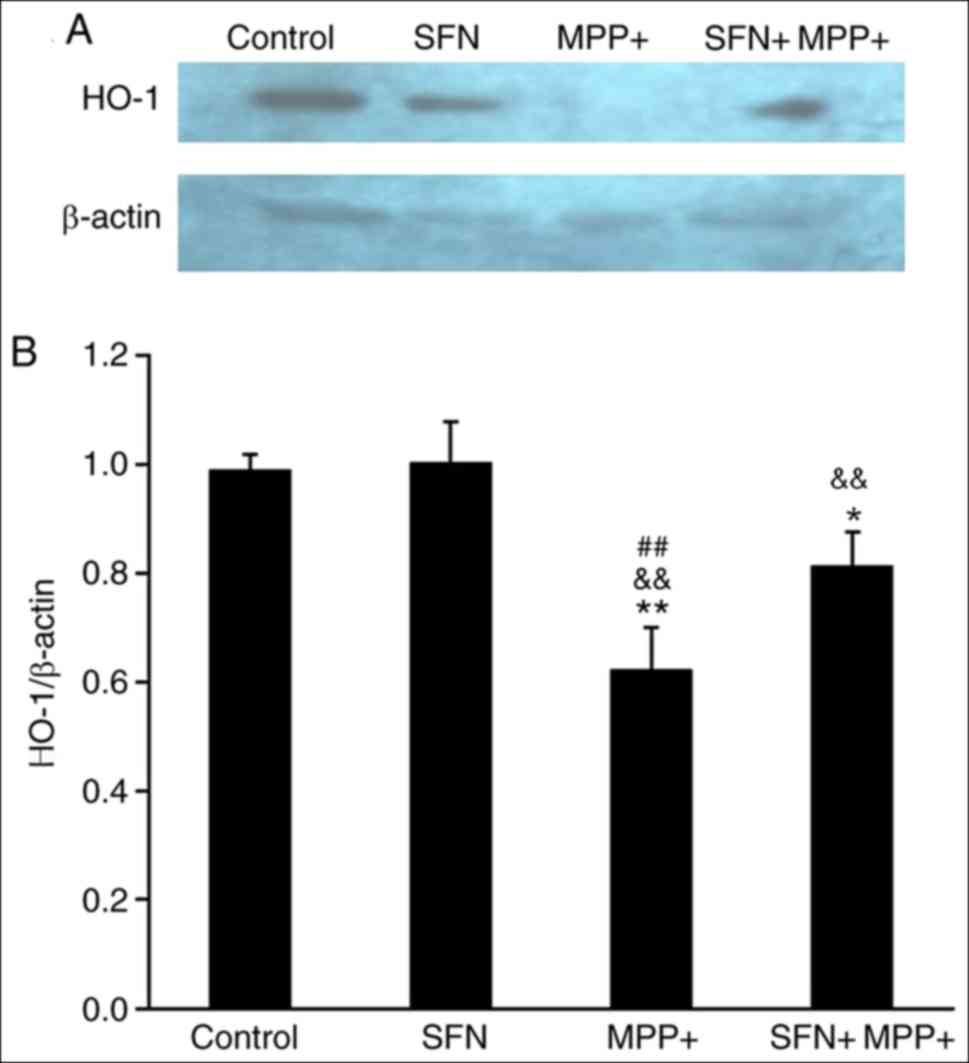
Nrf2 is an important transcription factor and can regulate the
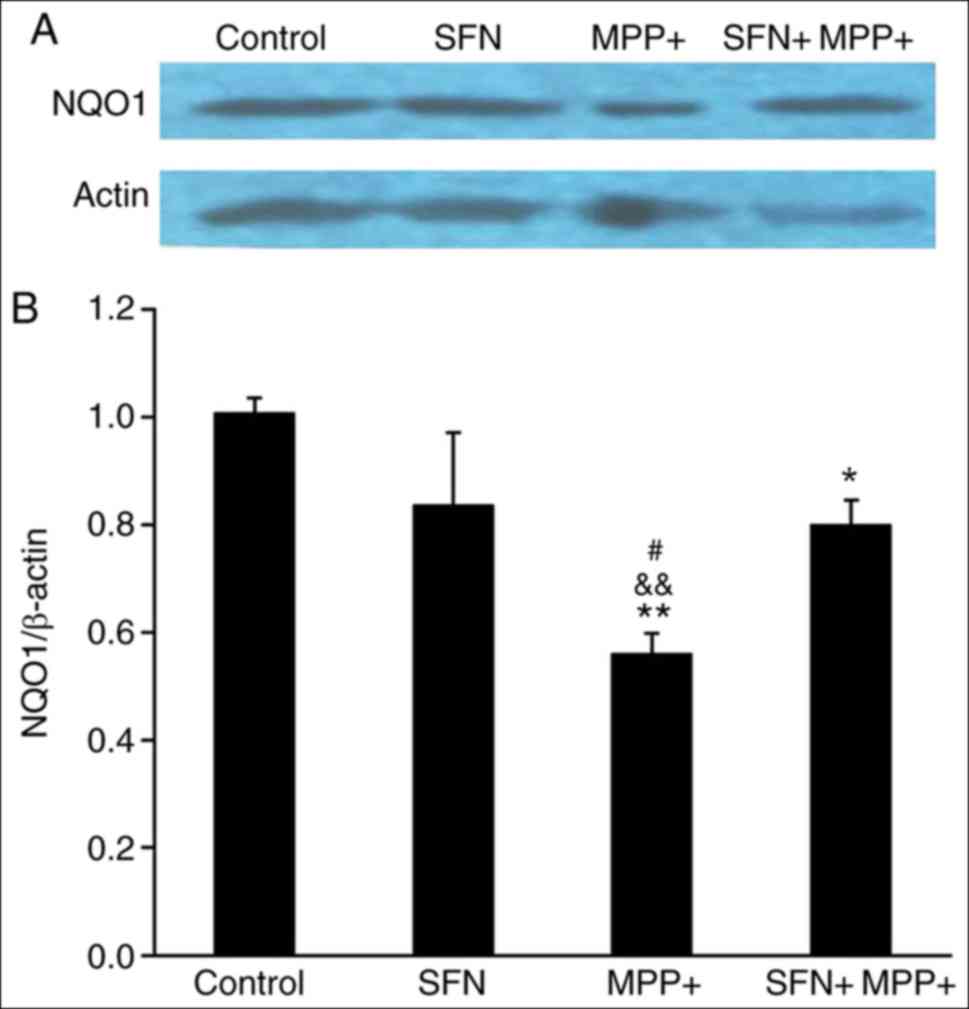
expression of several antioxidant proteins including HO-1 and NQO1
(30). Therefore the expression of
HO-1 and NQO1 was examined. Notably, the expression of HO-1 and
NQO1 proteins exhibited a parallel change (Figs. 5 and 6), namely, MPP+ significantly reduced the
expression of HO-1 and NQO1 (P<0.05), while SFN restores their
expression. It is well documented that the Nrf2 signaling pathway
serves an important role in neuron survival (30), the results of the present study
suggest that SFN may attenuate the cell toxicity by sustaining the
expression of Nrf2 and its downstream antioxidant enzymes.
Discussion
In the present study, using an MTT assay and flow
cytometry, it was demonstrated that MPP+ dose-dependently caused
damage to PC12 cells and SFN attenuated the detrimental effects of
MPP+. Furthermore, it was identified that that the protective
effects of SFN may act through restoring transcription factor Nrf2
signaling pathways.
Oxidative stress occurs due to an imbalance of free
radical production and the antioxidant response system. The central
nervous system is susceptible to oxidative stress because of its
high rate of oxygen consumption and lipid-rich content (31). It is well established that
oxidative stress is involved in a number neurodegenerative
disorders including PD and Alzheimer disease (3). It has been long recognized that MPTP,
a by-product of illegal heroin synthesis, can cause a parkinsonian
syndrome among drug abusers (1).
The nontoxic MPTP is converted to MPP+ by the enzyme of monoamine
oxidase B. MPP+ accumulates in mitochondria and binds to complex I
of the electron transport chain, leading to excessive production of
ROS and therefore cell damage (1,19,23,27,32).
In the present study, it was demonstrated that MPP+ caused PC12
cell injury and apoptosis using an MTT assay and flow cytometry.
The results agree with the previous studies (32–34).
For example, Hartley et al (32) demonstrated that MPP+ caused cell
apoptosis at low concentrations but necrosis at high
concentrations. It is worth noting that treatment with 500 µmol/l
MPP+ for 24 h caused ~42% cell death by MTT assay, while under the
same condition an apoptotic rate of ~30% was identified using flow
cytometry. It seemed that there was a 12% difference in cell death
between the two methods. Since the MTT assay can detect all kinds
of cell death which include necrosis and apoptosis, the results of
the present study suggest that the cell death induced by MPP+ was
dominantly via apoptosis, but other mechanisms including necrosis
may also contribute to cell death.
Notably, SFN was identified to dose-dependently
prevent the cell damage induced by MPP+, although SFN itself did
not affect the viability of PC12 cells. However, several studies
demonstrated that SFN alone can induce apoptosis of breast and lung
cancer cells (35–37), indicating that SFN may affect cell
viability depending upon the cell types. SFN, an organosulfur
compound derived from cruciferous vegetables, has recently gained
attention for its multiple biological functions. Studies have
demonstrated that SFN may be beneficial to a wide variety of
diseases, including neurological degenerative disease (38,39),
cancer (40–42), heart disease (43), obesity (44) and asthma (45). SFN efficacy occurs by activating
signaling molecules including Nrf2 (16,17,46–49),
uncoupling protein-1 (44) and
programmed cell death 4 (50).
Among all the SFN associated signaling pathways, Nrf2 is
well-established and is identified to be associated with the
protective effects of SFN on various diseases (16,17).
The Nrf2-antioxidant response element (ARE) pathway serves a
critical role in the regulation of the oxidative stress response in
a number of cell types (51). When
Nrf2 is activated, it enters the nucleus and binds to a specific
site, ARE, to activate an array of the phase II enzymes and
antioxidant enzymes including HO-1 and NQO1. These enzymes help to
maintain intracellular redox balance and therefore protect cells
from oxidative stress damage (18–22).
The present study identified that SFN protected PC12 cells against
the cytotoxicity of MPP+ and at the same time also restored the
expression of Nrf2, HO-1 and NQO1 that was decreased by MPP+.
Considering that these molecular signals are well-established cell
protectors (21,22), the present study hypothesized that
activation of Nrf2,-HO-1 and NQO1 may account for the protective
effects of SFN. The present study only examined HO-1 and NQO1
expression. However, the Nrf2/ARE pathway can induce the expression
of a wide variety of downstream phase II detoxifying and
antioxidant enzymes. In addition, HO-1 and NQO1, a number of other
enzymes may also be activated, including GST, superoxide dismutase
3 and glucuronosyltransferase-1a6 (52). Further studies are required to
elucidate the entire pathway for SFN protection.
Studies have demonstrated that SFN can regulate
certain neurotransmitters and their receptors (53,54).
For example, Mastrangelo et al (53) identified that SFN inhibited the
secretion of serotonin and downregulated the expression of its
receptors in human colorectal adenocarcinoma cells. While Lee et
al (54) demonstrated that SFN
increased acetylcholine production and its receptor expression in
primary cortical neurons. Progressive degeneration of dopaminergic
neurons in the substantia nigra is the main mechanism of PD.
However, reductions in dopamine content and uptake indices have
also been documented in PD (55,56).
The present study demonstrated that SFN prevented PC12 cell death
induced by MPP+. In addition, it would be also interesting to
determine whether SFN affects dopamine production or the expression
of its receptors/transporters in dopaminergic neurons.
It should be noted that one limitation of the
present study is that ROS production was not measured during the
treatments with different compounds. Although several studies have
demonstrated that ROS is a critical mediator in the MPP+-induced
cell injury, the evaluation of ROS productions in this study would
more definitively elucidate the interrelation between the Nrf2/ARE
pathway and ROS.
In conclusion, SFN, a naturally occurring compound,
protected PC12 cells from MPP+-induced damage and the protective
effects are in part due to the activation of the Nrf2-ARE signaling
pathway. The results imply that certain plant-derived supplements
including SFN can assist in modifying various biochemical and
physiological risk factors in neurodegenerative diseases like PD,
particularly environmentally induced symptoms of PD. Further
studies are needed to examine the efficacy of SFN on animal models
and ultimately in patients with neurological diseases including
PD.
Acknowledgements
The authors sincerely thank Dr Meiling Joiner,
Department of Physiology the University of Iowa, for her diligent
proofreading of the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (awarded to XPY and ZYC; grant
nos. 81760221 and 81260211), National Science & Technology
Fundational Resource Investigation Program of China (awarded to
XPY; grant no. 2018FY100900) and Youth Project of Education
Department of Jiangxi Province (awarded to BB; grant no.
151007).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BB, MQZ and XPY conceived and planned the
experiments. BB, MQZ, ZYC, XBW, ZBX and JYC performed the
experiments. BB, MQZ, ZYC, XBW, ZBX, JYC and XPY interpreted and
analyzed the data. BB and XPY wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dauer W and Przedborski S: Parkinson's
disease: Mechanisms and models. Neuron. 39:889–909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tolleson CM and Fang JY: Advances in the
mechanisms of Parkinson's disease. Discov Med. 15:61–66.
2013.PubMed/NCBI
|
|
3
|
Coyle JT and Puttfarcken P: Oxidative
stress, glutamate, and neurodegenerative disorders. Science.
262:689–695. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou C, Huang Y and Przedborski S:
Oxidative stress in Parkinson's disease: A mechanism of pathogenic
and therapeutic significance. Ann N Y Acad Sci. 1147:93–104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Zhou T, Ziegler AC, Dimitrion P and
Zuo L: Oxidative stress in neurodegenerative diseases: From
molecular mechanisms to clinical applications. Oxid Med Cell
Longev. 2017:25259672017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lerin C, Rodgers JT, Kalume DE, Kim SH,
Pandey A and Puigserver P: GCN5 acetyltransferase complex controls
glucose metabolism through transcriptional repression of
PGC-1alpha. Cell Metab. 3:429–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jha N, Kumar MJ, Boonplueang R and
Andersen JK: Glutathione decreases in dopaminergic PC12 cells
interfere with the ubiquitin protein degradation pathway: Relevance
for Parkinson's disease? J Neurochem. 80:555–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang JJ, Zhang T, Niu DB, Wang K, Li KR,
Xue B and Wang XM: Doxycycline-regulated co-expression of GDNF and
TH in PC12 cells. Neurosci Lett. 401:142–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Liu L, Ying XX, Wei WJ, Han C, Liu
Y, Han CH, Leng AJ, Ma JY and Liu J: Dried rehmannia root protects
against glutamate-induced cytotoxity to PC12 cells through energy
metabolism-related pathways. Neural Regen Res. 12:1338–1346. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soane L, Li Dai W, Fiskum G and Bambrick
LL: Sulforaphane protects immature hippocampal neurons against
death caused by exposure to hemin or to oxygen and glucose
deprivation. J Neurosci Res. 88:1355–1363. 2010.PubMed/NCBI
|
|
11
|
Xu C, Huang MT, Shen G, Yuan X, Lin W,
Khor TO, Conney AH and Kong AN: Inhibition of
7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in
C57BL/6 mice by sulforaphane is mediated by nuclear factor
E2-related factor 2. Cancer Res. 66:8293–8296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher ML, Ciavattone N, Grun D, Adhikary
G and Eckert RL: Sulforaphane reduces YAP/ΔNp63α signaling to
reduce cancer stem cell survival and tumor formation. Oncotarget.
8:73407–73418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kubo E, Chhunchha B, Singh P, Sasaki H and
Singh DP: Sulforaphane reactivates cellular antioxidant defense by
inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress.
Sci Rep. 7:141302017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thejass P and Kuttan G: Immunomodulatory
activity of sulforaphane, a naturally occurring isothiocyanate from
broccoli (Brassica oleracea). Phytomedicine. 14:538–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greaney AJ, Maier NK, Leppla SH and
Moayeri M: Sulforaphane inhibits multiple inflammasomes through an
Nrf2-independent mechanism. J Leukoc Biol. 99:189–199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dwivedi S, Rajasekar N, Hanif K, Nath C
and Shukla R: Sulforaphane ameliorates okadaic acid-induced memory
impairment in rats by activating the Nrf2/HO-1 antioxidant pathway.
Mol Neurobiol. 53:5310–5323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jang M and Cho IH: Sulforaphane
ameliorates 3-nitropropionic acid-induced striatal toxicity by
activating the keap1-Nrf2-ARE pathway and inhibiting the MAPKs and
NF-κB pathways. Mol Neurobiol. 53:2619–2635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minelli A, Conte C, Cacciatore I,
Cornacchia C and Pinnen F: Molecular mechanism underlying the
cerebral effect of Gly-Pro-Glu tripeptide bound to L-dopa in a
Parkinson's animal model. Amino Acids. 43:1359–1367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen PC, Vargas MR, Pani AK, Smeyne RJ,
Johnson DA, Kan YW and Johnson JA: Nrf2-mediated neuroprotection in
the MPTP mouse model of Parkinson's disease: Critical role for the
astrocyte. Proc Natl Acad Sci USA. 106:2933–2938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin XP, Chen ZY, Zhou J, Wu D and Bao B:
Mechanisms underlying the perifocal neuroprotective effect of the
Nrf2-ARE signaling pathway after intracranial hemorrhage. Drug Des
Devel Ther. 9:5973–5986. 2015.PubMed/NCBI
|
|
21
|
Yin XP, Wu D, Zhou J, Chen ZY, Bao B and
Xie L: Heme oxygenase 1 plays role of neuron-protection by
regulating Nrf2-ARE signaling post intracerebral hemorrhage. Int J
Clin Exp Pathol. 8:10156–10163. 2015.PubMed/NCBI
|
|
22
|
Chen G, Fang Q, Zhang J, Zhou D and Wang
Z: Role of the Nrf2-ARE pathway in early brain injury after
experimental subarachnoid hemorrhage. J Neurosci Res. 89:515–523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rostamian Delavar M, Baghi M, Safaeinejad
Z, Kiani-Esfahani A, Ghaedi K and Nasr-Esfahani MH: Differential
expression of miR-34a, miR-141, and miR-9 in MPP+-treated
differentiated PC12 cells as a model of Parkinson's disease. Gene.
662:54–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng W, Chen W, Wang P and Chu J: Asiatic
acid protects differentiated PC12 cells from
Aβ25-35-induced apoptosis and tau hyperphosphorylation
via regulating PI3K/Akt/GSK-3β signaling. Life Sci. 208:96–101.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin X, Zhang X, Wang W, Chang L, Jiang Y
and Zhang S: Perihematoma damage at different time points in
experimental intracerebral hemorrhage. J Huazhong Univ Sci
Technolog Med Sci. 26:59–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin M, Chen Z, Ouyang Y, Zhang H, Wan Z,
Wang H, Wu W and Yin X: Thrombin-induced, TNFR-dependent miR-181c
downregulation promotes MLL1 and NF-κB target gene expression in
human microglia. J Neuroinflammation. 14:1322017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kajimura Y, Aoki T, Kuramochi K, Kobayashi
S, Sugawara F, Watanabe N and Arai T: Neoechinulin A protects PC12
cells against MPP+-induced cytotoxicity. J Antibiot (Tokyo).
61:330–333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu XL, Lin YH, Wu Q, Su FJ, Ye CH, Shi L,
He BX, Huang FW, Pei Z and Yao XL: Paeonolum protects against
MPP(+)-induced neurotoxicity in zebrafish and PC12 cells. BMC
Complement Altern Med. 15:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Houghton CA, Fassett RG and Coombes JS:
Sulforaphane and other nutrigenomic Nrf2 activators: Can the
clinician's expectation be matched by the reality? Oxid Med Cell
Longev. 2016:78571862016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calkins MJ, Johnson DA, Townsend JA,
Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J and
Johnson JA: The Nrf2/ARE pathway as a potential therapeutic target
in neurodegenerative disease. Antioxid Redox Signal. 11:497–508.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilgun-Sherki Y, Rosenbaum Z, Melamed E
and Offen D: Antioxidant therapy in acute central nervous system
injury: Current state. Pharmacol Rev. 54:271–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hartley A, Stone JM, Heron C, Cooper JM
and Schapira AH: Complex I inhibitors induce dose-dependent
apoptosis in PC12 cells: Relevance to Parkinson's disease. J
Neurochem. 63:1987–1990. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Huang L, Li X, Liu C, Sun X, Wu
L, Li T, Yang H and Chen J: Potential molecular mechanisms
mediating the protective effects of tetrahydroxystilbene glucoside
on MPP+-induced PC12 cell apoptosis. Mol Cell Biochem.
436:203–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye S, Koon HK, Fan W, Xu Y, Wei W, Xu C
and Cai J: Effect of a traditional chinese herbal medicine
formulation on cell survival and apoptosis of
MPP+-treated MES 23.5 dopaminergic cells. Parkinsons
Dis. 2017:47642122017.PubMed/NCBI
|
|
35
|
Zhang Z, Li C, Shang L, Zhang Y, Zou R,
Zhan Y and Bi B: Sulforaphane induces apoptosis and inhibits
invasion in U251MG glioblastoma cells. Springerplus. 5:2352016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Żuryń A, Litwiniec A, Safiejko-Mroczka B,
Klimaszewska- Wiśniewska A, Gagat M, Krajewski A, Gackowska L and
Grzanka D: The effect of sulforaphane on the cell cycle, apoptosis
and expression of cyclin D1 and p21 in the A549 non-small cell lung
cancer cell line. Int J Oncol. 48:2521–2533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pledgie-Tracy A, Sobolewski MD and
Davidson NE: Sulforaphane induces cell type-specific apoptosis in
human breast cancer cell lines. Mol Cancer Ther. 6:1013–1021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
An YW, Jhang KA, Woo SY, Kang JL and Chong
YH: Sulforaphane exerts its anti-inflammatory effect against
amyloid-β peptide via STAT-1 dephosphorylation and activation of
Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol Aging.
38:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pearson BL, Simon JM, McCoy ES, Salazar G,
Fragola G and Zylka MJ: Identification of chemicals that mimic
transcriptional changes associated with autism, brain aging and
neurodegeneration. Nat Commun. 7:111732016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chirumbolo S and Bjørklund G: Sulforaphane
and 5-fluorouracil synergistically inducing autophagy in breast
cancer: A possible role for the Nrf2-Keap1-ARE signaling? Food Chem
Toxicol. 2018. View Article : Google Scholar
|
|
41
|
Wang F, Wang W, Li J, Zhang J, Wang X and
Wang M: Sulforaphane reverses gefitinib tolerance in human lung
cancer cells via modulation of sonic hedgehog signaling. Oncol
Lett. 15:109–114. 2018.PubMed/NCBI
|
|
42
|
Ramirez CN, Li W, Zhang C, Wu R, Su S,
Wang C, Gao L, Yin R and Kong AN: In vitro-in vivo dose response of
ursolic acid, sulforaphane, PEITC, and curcumin in cancer
prevention. AAPS J. 20:192017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Evans PC: The influence of sulforaphane on
vascular health and its relevance to nutritional approaches to
prevent cardiovascular disease. EPMA J. 2:9–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagata N, Xu L, Kohno S, Ushida Y, Aoki Y,
Umeda R, Fuke N, Zhuge F, Ni Y, Nagashimada M, et al: Glucoraphanin
ameliorates obesity and insulin resistance through adipose tissue
browning and reduction of metabolic endotoxemia in mice. Diabetes.
66:1222–1236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brown RH, Reynolds C, Brooker A, Talalay P
and Fahey JW: Sulforaphane improves the bronchoprotective response
in asthmatics through Nrf2-mediated gene pathways. Respir Res.
16:1062015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xin Y, Bai Y, Jiang X, Zhou S, Wang Y,
Wintergerst KA, Cui T, Ji H, Tan Y and Cai L: Sulforaphane prevents
angiotensin II-induced cardiomyopathy by activation of Nrf2 via
stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biol. 15:405–417.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stepniewski J, Pacholczak T, Skrzypczyk A,
Ciesla M, Szade A, Szade K, Bidanel R, Langrzyk A, Grochowski R,
Vandermeeren F, et al: Heme oxygenase-1 affects generation and
spontaneous cardiac differentiation of induced pluripotent stem
cells. IUBMB Life. 70:129–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dinkova-Kostova AT, Fahey JW, Kostov RV
and Kensler TW: KEAP1 and done? targeting the NRF2 pathway with
sulforaphane. Trends Food Sci Technol. 69:257–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Izumi Y, Kataoka H, Inose Y, Akaike A,
Koyama Y and Kume T: Neuroprotective effect of an Nrf2-ARE
activator identified from a chemical library on dopaminergic
neurons. Eur J Pharmacol. 818:470–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cho JH, Kim YW and Keum YS: Sulforaphane
suppresses LPS-induced or TPA-induced downregulation of PDCD4 in
RAW 264.7 cells. Phytother Res. 28:1606–1611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cho HY, Jedlicka AE, Reddy SP, Kensler TW,
Yamamoto M, Zhang LY and Kleeberger SR: Role of NRF2 in protection
against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol.
26:175–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mastrangelo L, Cassidy A, Mulholland F,
Wang W and Bao Y: Serotonin receptors, novel targets of
sulforaphane identified by proteomic analysis in Caco-2 cells.
Cancer Res. 68:5487–5491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee S and Kim J, Seo SG, Choi BR, Han JS,
Lee KW and Kim J: Sulforaphane alleviates scopolamine-induced
memory impairment in mice. Pharmacol Res. 85:23–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chinaglia G, Alvarez FJ, Probst A and
Palacios JM: Mesostriatal and mesolimbic dopamine uptake binding
sites are reduced in Parkinson's disease and progressive
supranuclear palsy: A quantitative autoradiographic study using
[3H]mazindol. Neuroscience. 49:317–327. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Leenders KL, Salmon EP, Tyrrell P, Perani
D, Brooks DJ, Sager H, Jones T, Marsden CD and Frackowiak RS: The
nigrostriatal dopaminergic system assessed in vivo by positron
emission tomography in healthy volunteer subjects and patients with
Parkinson's disease. Arch Neurol. 47:1290–1298. 1990. View Article : Google Scholar : PubMed/NCBI
|