Introduction
Parkinson's disease (PD), a progressive
neurodegenerative disorder, affects ~1% of the population aged ≥60
years. Clinically, PD is characterized by bradykinesia, tremor,
rigidity and postural instability. Pathologically, these
manifestations are primarily caused by loss of dopaminergic neurons
in the substantia nigra (1). While
significant progress has been made in the elucidation of the
etiology of PD, precise targeted therapies interfering with the
pathological mechanisms of neurodegeneration are not available at
present. Increasing evidence has indicated that chronic
inflammation is a key risk factor for aggravating the disease
(2). Microglia, the dominant
immune cells in the nervous system, maintain a ramified structure
in the brain under normal physiological conditions. Once activated
by inflammatory cytokines, microglia synthesize and secrete
pro-inflammatory cytokines to promote and amplify inflammatory
cascades. Gradually, the resulting inflammatory microenvironment
facilitates the death of midbrain dopaminergic neurons.
Furthermore, the number of microglia is strictly controlled, in
part by ‘activation-induced cell death’ (3). Therefore, inhibiting microglia
activation and maintaining a dynamic balance of microglial
proliferation and apoptosis has become a strategy for the treatment
of PD.
A comprehensive understanding of the molecular
mechanisms underlying the occurrence and development of PD is
important. MicroRNAs (miRNAs/miRs) have been identified as critical
factors that participate in the pathophysiological processes of
multiple diseases. The identification of miRNAs over a decade
previously has improved the understanding of the
post-transcriptional regulation of gene expression. miRNAs are
small non-coding RNAs with an average length of 22–25 nucleotides.
Compelling evidence has demonstrated that the miRNA machinery in
neurodegenerative diseases, particularly in PD, is critical for the
survival of neurons (4). Numerous
studies have indicated that miRNAs are crucially involved in
inhibiting or facilitating inflammation (5), and consequently affect the occurrence
and development of PD (6). Of
note, miR-195 was identified as a potential key biomarker for PD
(7,8). However, the precise role of miR-195
in PD and the underlying mechanisms remain to be fully elucidated.
Preliminary evidence implied that miR-195 inhibits the release of
pro-inflammatory factors via diverse signaling pathways (9,10).
Rho-associated kinase 1 (ROCK1) is a major
downstream effector of the small GTPase RhoA and serves a pivotal
role in cancer, particularly in cell motility, metastasis and
angiogenesis. Previously, the pathophysiological effects of ROCK1
regarding other types of cell behaviors and cellular physiological
processes have attracted increasing attention (11). It was suggested that ROCK1 may
function as a trigger for the inflammatory response (12,13),
suggesting the participation of ROCK1 in neuroinflammation
associated with PD. Notably, ROCK1 has been demonstrated to be a
direct target gene of miR-195 (14). Therefore, in the present study, it
was hypothesized that miR-195 inhibits microglia activation, at
least in part via inhibition of ROCK1.
Materials and methods
Cell culture
The BV2 cells used in the present study are
immortalized microglia and were purchased from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). BV2 cells were cultured in Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd., Hangzhou, China), 100
U/ml penicillin and 100 µg/ml streptomycin (Biosharp, Hefei,
China). The BV2 cells were incubated in a cell incubator with a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cell transfection
miR-195 mimics (5′-UAGCAGCACAGAAAUAUUGGC-3′), miRNA
mimics negative control (miR-NC1; 5′-UCACAACCUCCUAGAAAGAGUAGA-3′),
miR-195 inhibitor (5′-GCCAAUAUUUCUGUCUGCUA-3′) and miRNA inhibitor
negative control (miR-NC2; 5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were
purchased from Guangzhou RiboBio Co., Ltd., (Guangzhou, China).
ROCK1 small interfering (si)RNA (siROCK1; cat. no. sc-36432) and
control siRNA (cat no. sc-37007) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Cell transfection was
performed when cell confluence reached 75%.
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for cell transfection according to the
manufacturer's protocols. After 6 h, the transfection mixture was
replaced with fresh culture medium and the cells used for
subsequent experimentation. The concentration of miR-195 mimics,
miR-195 inhibitor and ROCK1 siRNA was 100 nM.
RNA extraction and reverse
transcription quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from BV2 cells using
TRIzol® reagent according to the protocol provided by
the manufacturer (Thermo Fisher Scientific, Inc.). RT-qPCR was
performed according to a previously described protocol (15). U6 and GAPDH were used as the
invariant controls and the mRNA expression of target genes was
expressed as fold changes following normalization to GAPDH or U6.
The primers used are summarized in Table I.
 | Table I.Primers used for analyses of mRNA
expression in BV2 cells. |
Table I.
Primers used for analyses of mRNA
expression in BV2 cells.
| Gene | Orientation | Primers
sequences |
|---|
| miR195 | Forward |
5′-ACGATAGCAGCACAGAAAT-3′ |
|
| Reverse |
5′-GTGCAGGGTCCGAGGT-3′ |
| iNOS | Forward |
5′-CCTTGTTCAGCTACGCCTTC-3′ |
|
| Reverse |
5′-CTGAGGGCTCTGTTGAGGTC-3′ |
| IL-6 | Forward |
5′-ATGAACTCCTTCTCCACAAGC-3′ |
|
| Reverse |
5′-CTACATTTGCCGAAGAGCCCTCAGGCTGGACTG-3′ |
| TNF-α | Forward |
5′-ATGAGCACTGAAAGCATGATC-3′ |
|
| Reverse |
5′-TCACAGGGCAATGATCCCAAAGTAGACCTGCCC-3′ |
| IL-4 | Forward |
5′-TCTCACCTCCCAACTGCTTC-3′ |
|
| Reverse |
5′-AGAGGTTCCTGTCGAGCCGTTTCA-3′ |
| IL-10 | Forward |
5′-AGGGCACCCAGTCTGAGAACA-3′ |
|
| Reverse |
5′-CGGCCTTGCTCTTGTTTTCAC-3′ |
| U6 | Forward |
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| Reverse |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| GAPDH | Forward |
5′-CTATGACCACAGTCCATGC-3′ |
|
| Reverse | 5
-CACATTGGGGGTAGGAACAC-3′ |
ELISA
Microglia cells were treated with 1 µg/ml LPS for 24
h and transfection with 100 nM miR-195 mimics for another 6 h. The
levels of inducible nitric oxide synthase (iNOS; cat. no.,
A014-1-1), interleukin (IL)-6 (cat. no. H007), tumor necrosis
factor-α (TNF-α; cat. no. H052), IL-4 (cat. no. H005) and IL-10
(cat. no. H009) in whole cell lysate of microglia were determined
using corresponding ELISA kits (Nanjing Jiancheng Bioengineering
Institute, Jiangsu, China) according to the protocols provided by
the manufacturer.
Cell proliferation assay
The cell proliferation rate was determined using a
non-radioactive proliferation assay kit (X1327K; Exalpha
Biologicals, Inc., Shirley, MA, USA) according the manufacturer's
protocol. The ELISA is able to detect the incorporation of
5-bromo-2-deoxyuridine (BrdU) into newly-synthesized DNA in
proliferated cells. BrdU solution was added into the culture system
following treatment of the cells with 1 µg/ml LPS for 24 h and
transfection with 100 nM miR-195 inhibitor and ROCK1 siRNA for 6 h.
Subsequently, cells were cultured for an additional 16 h. The cell
medium was removed and the BrdU immunoreactivity was detected using
prediluted anti-BrdU Detector Antibody and 1X Peroxidase Goat
anti-Mouse IgG included in the kit. The absorbance was detected at
450 and 540 nm. The results were expressed as a fold of the vehicle
control.
Cell viability assay
Cell viability was detected using a Cell Counting
Kit-8 (CCK-8) cell viability assay (Nanjing Jiancheng
Bioengineering Institute). Cells were cultured in a 96-well plate
(1×104/well) and then treated with 1 µg/ml LPS for 24 h
and transfection with 100 nM miR-195 inhibitor and ROCK1 siRNA for
6 h. Subsequently, 10 µl CCK-8 solution was added to each well,
followed by incubation at 37°C for 1 h. The SpectraMax™ microplate
spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA, USA) was
used to detect the absorbance at 450 nm.
Cell apoptosis assay
Cell apoptosis was measured by flow cytometry (FCM)
using an Annexin V-fluorescein isothiocyanate (FITC) and propidium
iodide (PI) kit (Vazyme Biotech, Co., Ltd., Nanjing, China).
Following treatment with 1 µg/ml LPS for 24 h and transfection with
100 nM miR-195 inhibitor and ROCK1 siRNA for 6 h, BV2 cells were
detached from the culture plates with 0.25% trypsin (Beyotime
Institute of Biotechnology, Haimen, China) and washed with PBS 3
times. Following removal of the PBS, the cells were suspended in 1
ml binding buffer (Vazyme Biotech, Co., Ltd.) supplemented with 10
µl FITC and PI each. Cells were incubated in the dark for 15 min
and then measured by FCM (BD Biosciences, San Jose, CA, USA). Data
were analyzed using BD CellQuest Pro software (version 5.1; BD
Biosciences, Franklin Lakes, NJ, USA) and expressed as a fold of
the vehicle control.
Statistical analysis
All of the experimental data are expressed as the
mean ± standard deviation. The results were analyzed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The
significance of differences between groups was determined by
one-way analysis of variance followed by Dunnett's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
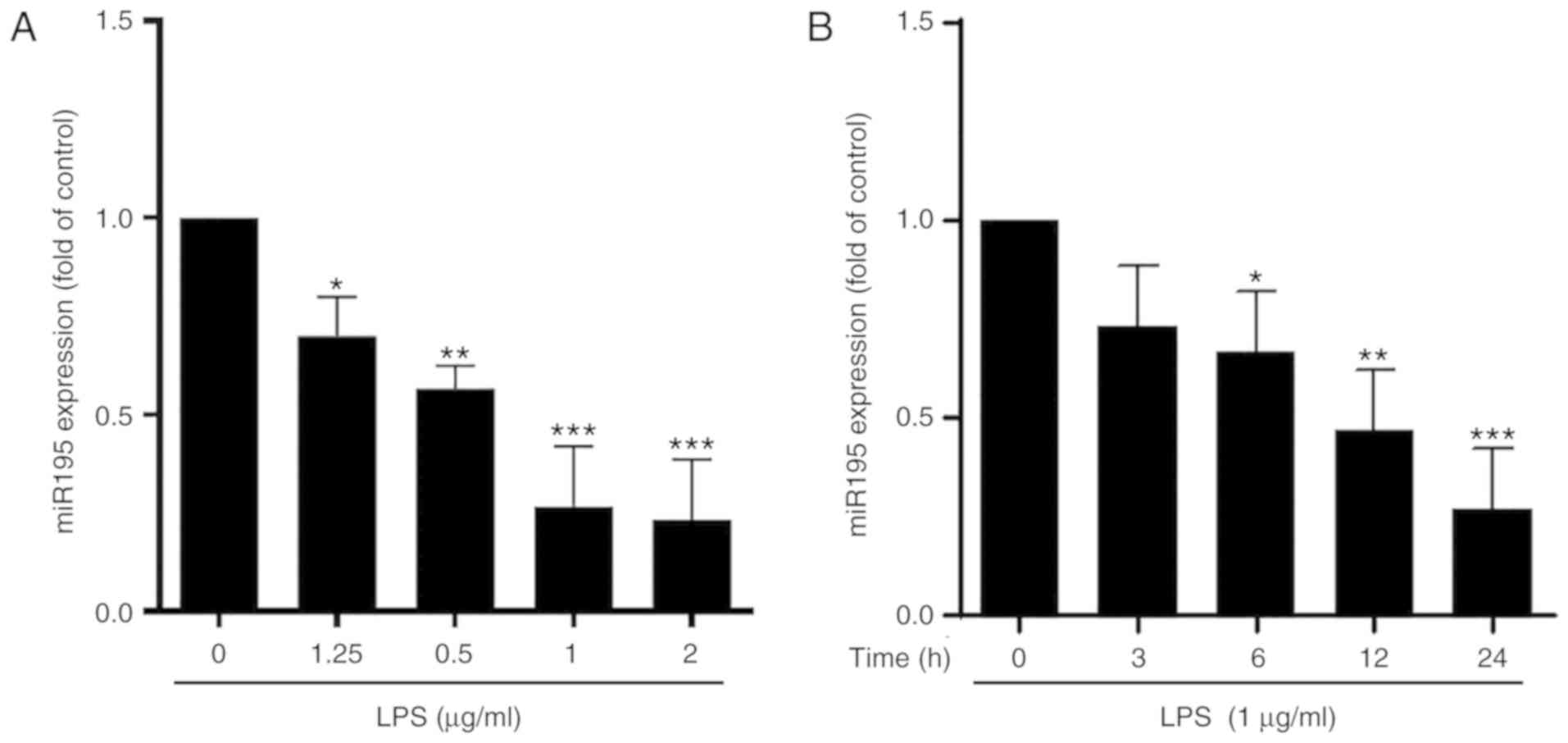
miR-195 is decreased in
lipopolysaccharide (LPS)-stimulated microglia
To preliminarily assess the association between
miR-195 expression and microglial activation, the expression of
miR-195 in ramified quiescent and activated microglia was initially
detected. LPS was used to establish an in vitro model of
microglia activation. The results indicated that, compared with the
control, LPS inhibited miR-195 expression in BV2 cells in a
concentration-dependent manner. Notably, no significant difference
in miR-195 expression between the 1 and 2 µg/ml LPS-stimulated
groups was observed (Fig. 1A). In
addition, LPS at the concentration of 1 µg/ml decreased the
expression of miR-195 in BV2 cells in a time-dependent manner
(Fig. 1B). Taken together, miR-195
expression was decreased in LPS-stimulated BV2 cells in a
concentration- and time-dependent manner.
miR-195 suppresses LPS-induced
activation of microglia
Microglia activation is a complex process
accompanied by extensive release of inflammatory factors. Given
that the expression of miR-195 was markedly decreased in
LPS-induced activated BV2 cells, the function of miR-195 in
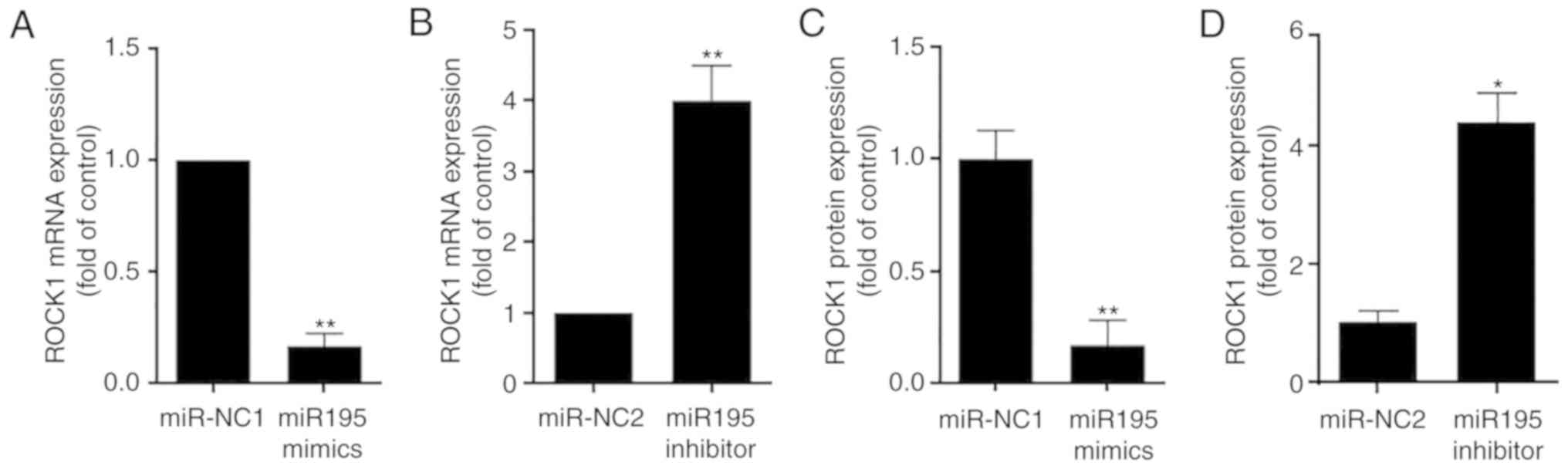
microglia activation was then investigated. It was confirmed that,
compared with the control, transfection with miR-195 mimics
markedly increased the level of miR-195 (Fig. 2A), whereas miR-195 inhibitors
decreased the level of miR-195 expression (Fig. 2B), suggesting that the gain- or
loss-of-function of miR-195 was successfully achieved in the in
vitro system. Furthermore, it was revealed that miR-195 mimics
inhibited the expression of pro-inflammatory cytokines, including
iNOS, IL-6 and TNF-α, but promoted the expression of
anti-inflammatory cytokines in LPS-treated BV2 cells, including
IL-4 and IL-10 (Fig. 2C-G).
However, miR-195 inhibitors enhanced the stimulatory effects of LPS
on the expression of these neuroinflammation-associated factors,
characterized by an increase in the expression of iNOS, IL-6 and
TNF-α, and a decrease in the expression of IL-4 and IL-10 (Fig. 2H-L). Consistent with the qPCR
results, the ELISA data indicated similar variations of iNOS, IL-6,
TNF-α, IL-4 and IL-10 in microglia following stimulation with LPS
and miR-195 mimics or inhibitor (Fig.
2M and N). Taken together, these results demonstrated that
miR-195 may have a crucial role in suppressing LPS-induced
activation of microglia.
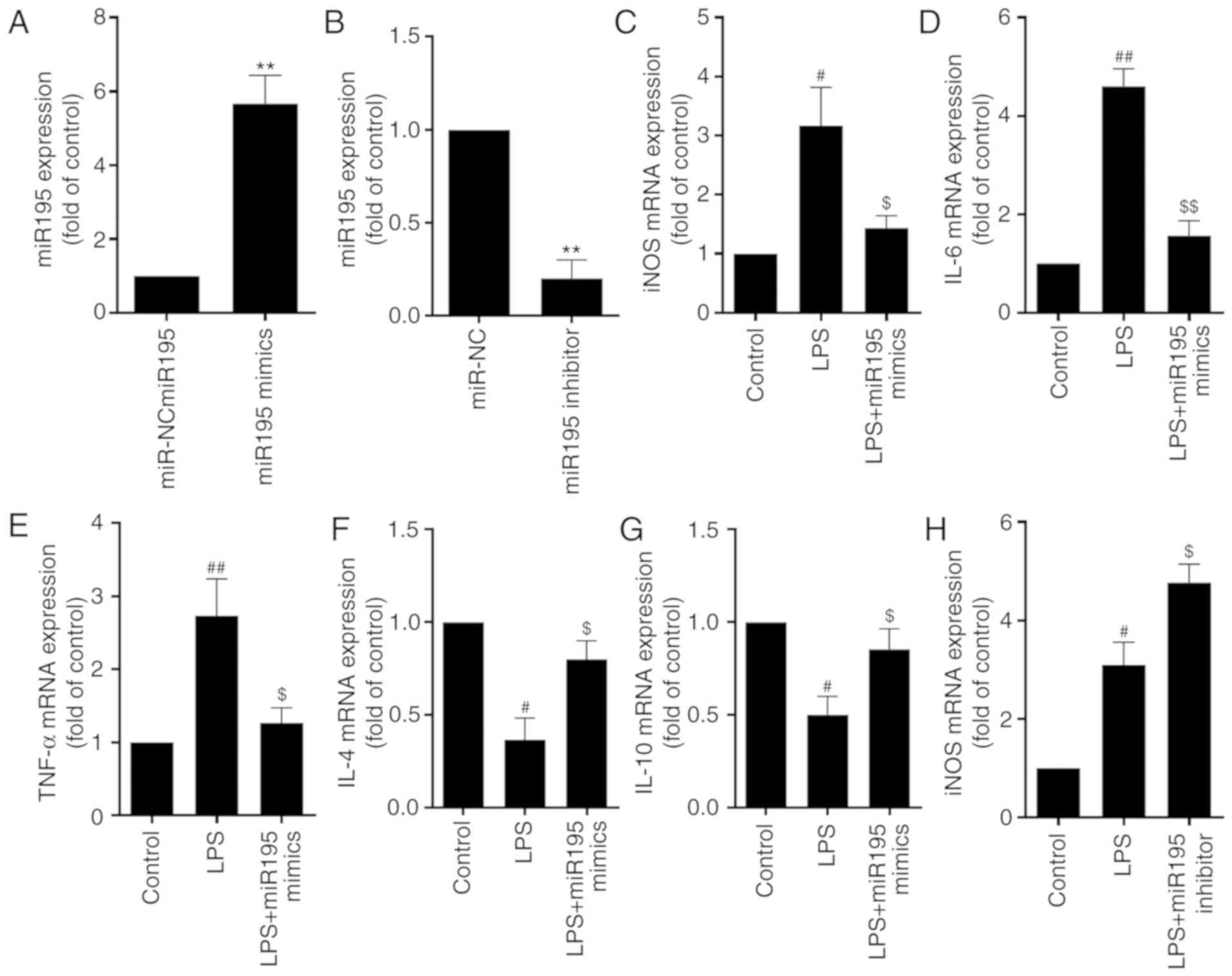 | Figure 2.miR-195 suppresses LPS-induced
activation of microglia. (A and B) BV2 cells were transfected with
(A) miR-195 mimics and miR-195 inhibitor or (B) miR-NC and miR-195
inhibitor. miR-195 expression in microglia was determined by
RT-qPCR analysis. (C-L) BV2 cells were transfected with miR195
mimics or miR-195 inhibitors and then treated with LPS (1 µg/ml)
for 24 h. The expression of (C) iNOS, (D) IL-6, (E) TNF-α, (F) IL-4
and (G) IL-10 in microglia treated with miR195 mimics was
determined by RT-qPCR analysis. Then, the expression of (H) iNOS,
(I) IL-6, (J) TNF-α, (K) IL-4 and (L) IL-10 in microglia treated
with miR195 mimics was determined by RT-qPCR analysis. GAPDH was
used as the invariant control. Analysis of iNOS, IL-6, TNF-α, IL-4
and IL-10 protein expression levels in microglia treated with (M)
miR195 mimics and (N) miR195 inhibitors was measured by ELISA.
Values are expressed as the mean ± standard deviation. **P<0.01
vs. miR-NC group; #P<0.05 and ##P<0.01
vs. control; $P<0.05 and $$P<0.01 vs.
LPS group. miR, microRNA; LPS, lipopolysaccharide; iNOS, inducible
nitric oxide synthase; IL, interleukin; TNF-α, tumor necrosis
factor-α; NC, negative control; RT-qPCR, reverse transcription
quantitative polymerase chain reaction. |
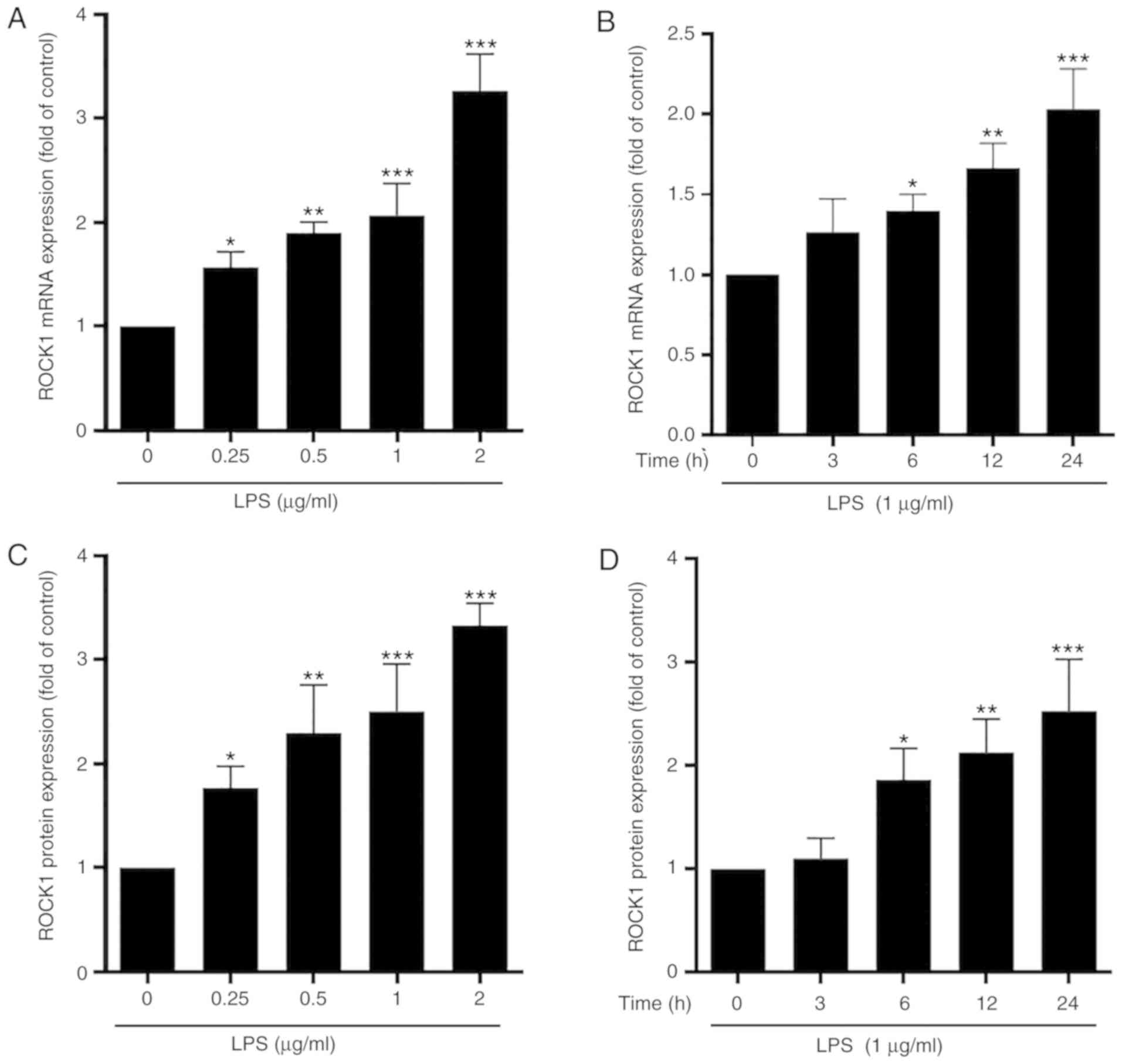
ROCK1 is a potential target of miR-195
in microglia
In view of the pivotal role of ROCK1 in modulating
inflammation, the present study investigated whether ROCK1
functions downstream of miR-195 in the inflammatory signaling
pathways of in PD. The results indicated that, compared with the
control, LPS stimulated the mRNA and protein expression of ROCK1 in
a concentration- and time-dependent manner (Fig. 3). Of note, miR-195 mimics markedly
decreased the mRNA and protein levels of ROCK1, while miR-195
inhibitors enhanced the expression of ROCK1 mRNA and protein
(Fig. 4). These results suggest
that ROCK1 expression is positively associated with microglia
activation but negatively regulated by miR-195 in microglia.
ROCK1 promotes LPS-induced microglia
activation
To systematically investigate the intricate
regulatory mechanisms, the potential role of ROCK1 in regulating
microglia activation was additionally explored. It was confirmed
that, compared with the control, siROCK1 markedly decreased the
expression of ROCK1, suggesting that ROCK1 in microglia was
efficiently knocked down (Fig.
5A). ROCK1 knockdown inhibited LPS-induced activation of BV2
cells, evidenced by decreased cellular iNOS, IL-6 and TNF-α, and
increased IL-4 and IL-10 (Fig.
5B-F). Consistent with the qPCR results, the results of the
ELISAs demonstrated similar changes in the levels of iNOS, IL-6,
TNF-α, IL-4 and IL-10 in microglia following stimulation with LPS
subsequent to transfection with siROCK1 (Fig. 5G). Taken together, these results
indicated that inhibition of ROCK1 may prevent microglia
activation.
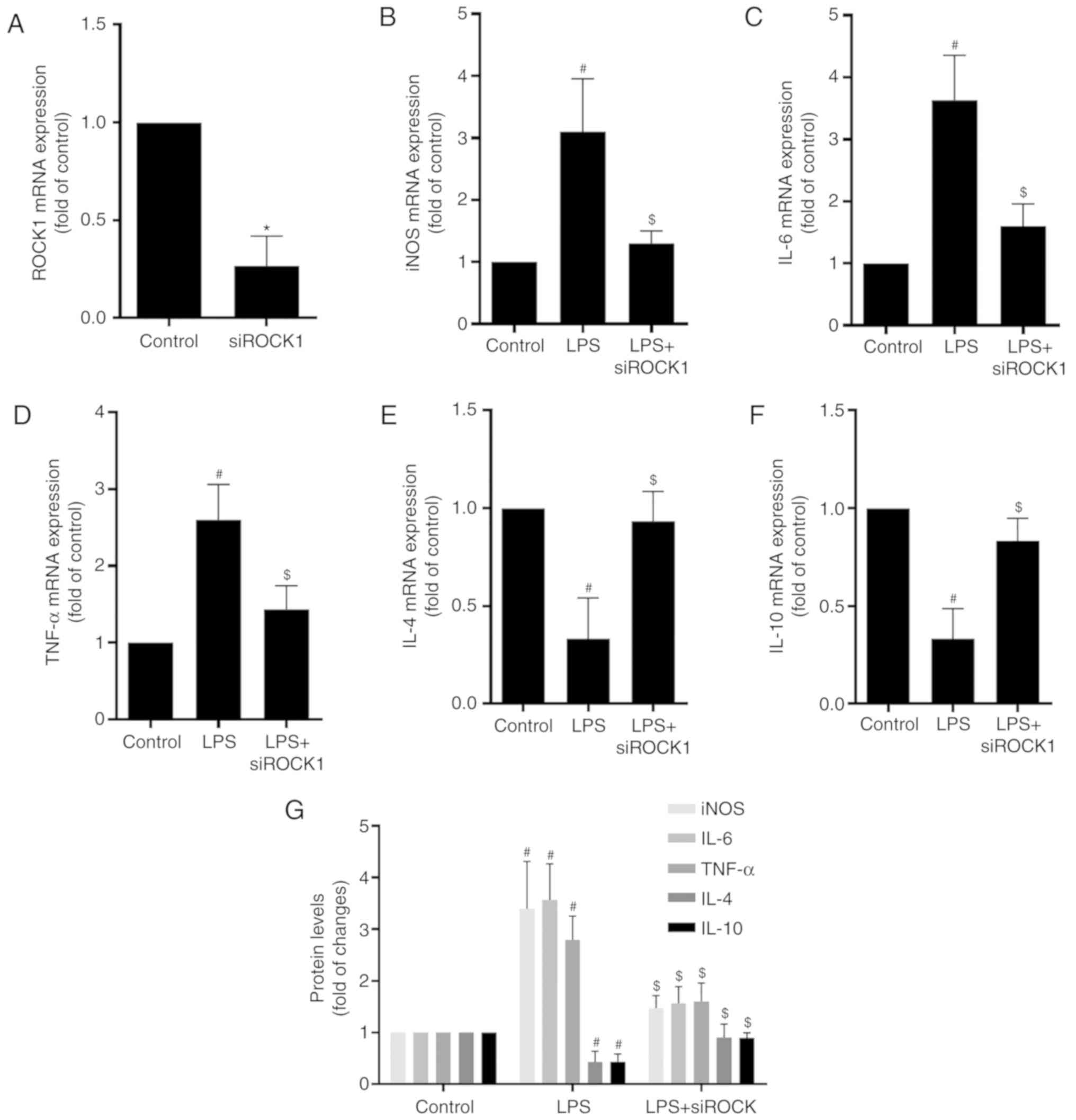 | Figure 5.ROCK1 promotes LPS-induced microglia
activation. (A) RT-qPCR analysis of ROCK1 expression in microglia.
GAPDH was used as the invariant control. BV2 cells were transfected
with siNC or siROCK1. (B-F) RT-qPCR analysis of the expression of
(B) iNOS, (C) IL-6, (D) TNF-α, (E) IL-4 and (F) IL-10 in microglia.
BV2 cells were transfected with siROCK1 and then treated with LPS
(1 µg/ml) for 24 h. (G) ELISA of iNOS, IL-6, TNF-α, IL-4 and IL-10
protein levels in microglia. Values are expressed as the mean ±
standard deviation. *P<0.05 vs. siNC group;
#P<0.05 vs. control group; $P<0.05 vs.
LPS group. LPS, lipopolysaccharide; NC, negative control; ROCK1,
Rho-associated kinase 1; siROCK1, small interfering RNA targeting
ROCK1; miR, microRNA; iNOS, inducible nitric oxide synthase; IL,
interleukin; TNF-α, tumor necrosis factor-α; RT-qPCR, reverse
transcription quantitative polymerase chain reaction. |
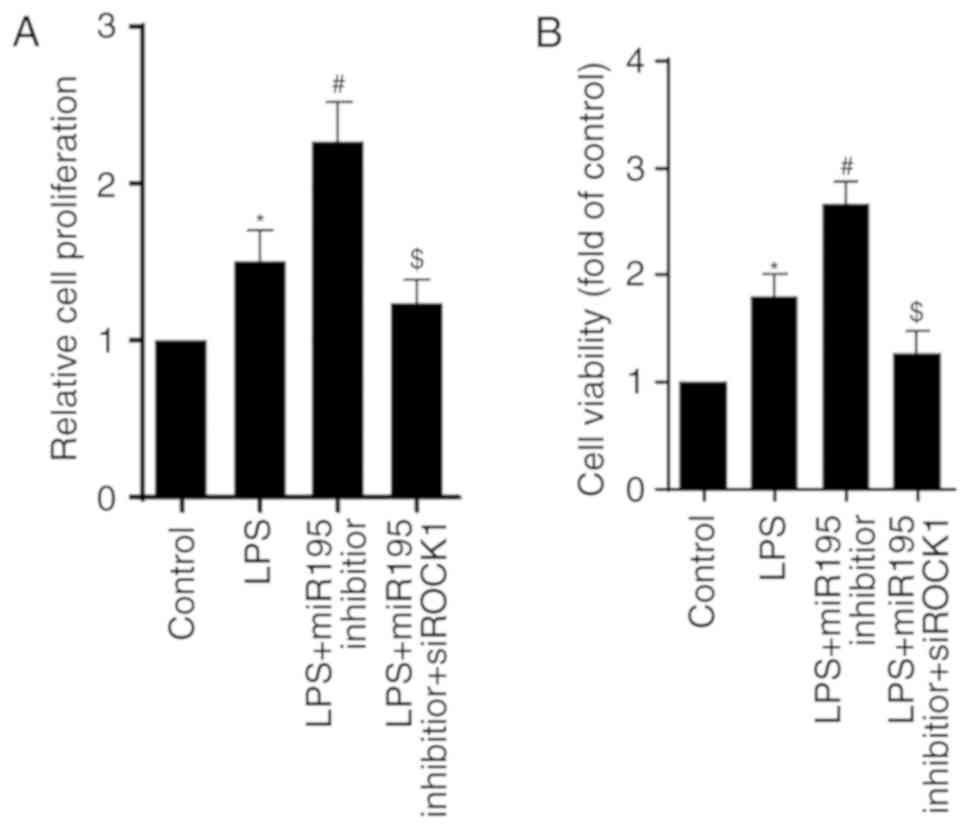
miR-195/ROCK1 pathway regulates the
proliferation and viability of BV2 cells treated with LPS
The role of the miR-195/ROCK1 pathway in regulating
cell proliferation and viability was then investigated. The results
indicated that LPS enhanced the proliferative capacity of
microglia, and this effect was additionally increased in miR-195
inhibitor-transfected microglia. Notably, ROCK1 knockdown reversed
the effects of miR-195 inhibitor (Fig.
6A). Consistently, the results of the cell viability assay
exhibited a similar trend (Fig.
6B). Collectively, these data suggest that the miR-195/ROCK1
pathway has a crucial role in modulating the proliferation and
viability of BV2 cells challenged with LPS.
miR-195/ROCK1 pathway regulates
apoptosis of LPS-treated BV2 cells
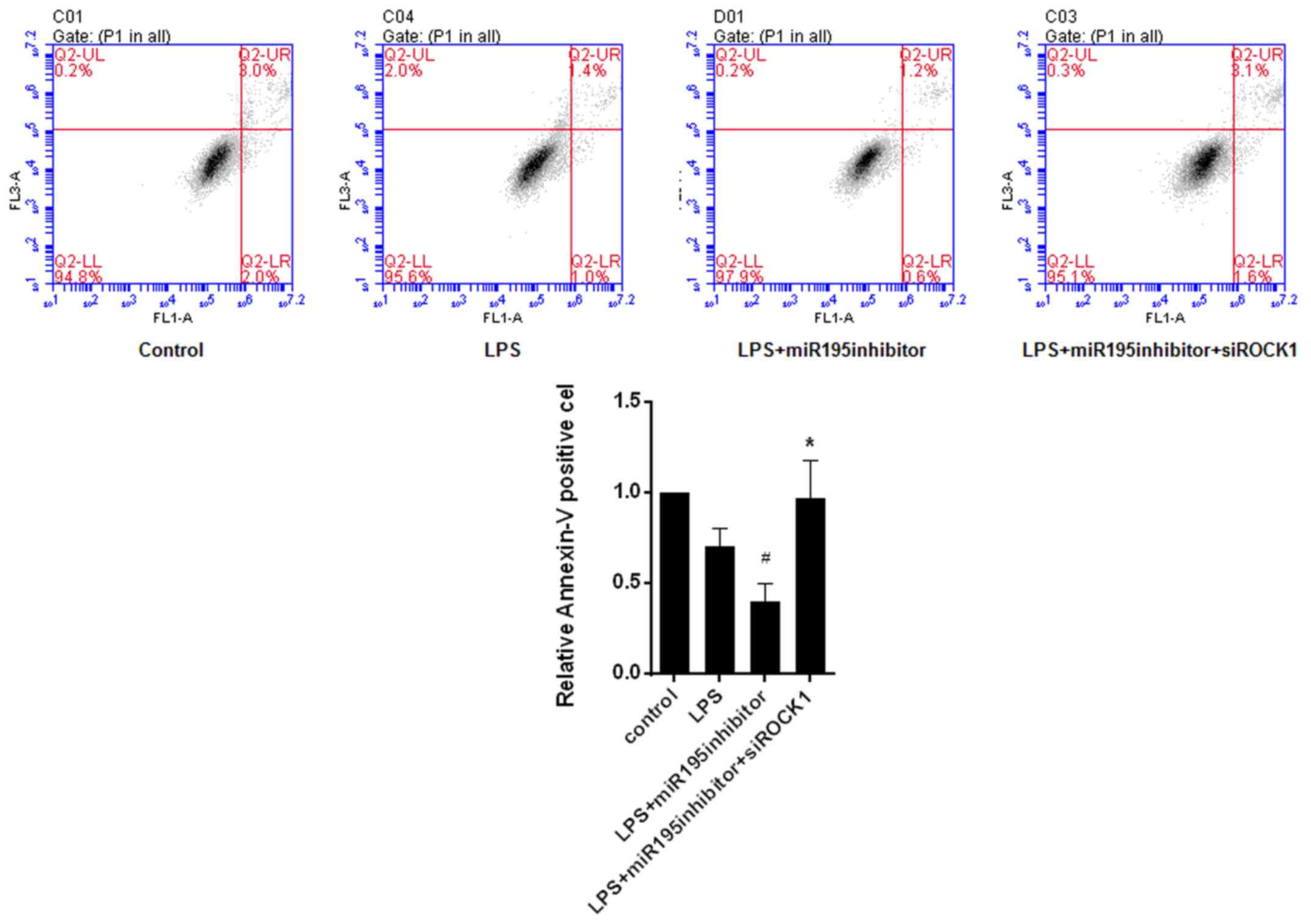
To determine whether cell apoptosis was also
affected by the miR-195/ROCK1 pathway, the Annexin V-FITC-PI
apoptosis detection kit was used and FCM analysis was performed.
The results demonstrated that miR-195 inhibitor suppressed the
apoptosis of LPS-treated BV2 cells, which, was reversed by
knockdown of ROCK1 (Fig. 7). It
was therefore indicated that the miR-195/ROCK1 pathway is involved
in regulating LPS-induced microglia apoptosis.
Discussion
PD is the second most common type of
neurodegenerative disease in older individuals. However, the
pathogenesis of PD remains to be fully elucidated. To the best of
our knowledge, the present study was the first to reveal that
miR-195 attenuates LPS-induced microglia activation and
proliferation, and promotes apoptosis by inhibiting ROCK1.
Microglia are resident immune cells with key roles
in regulating inflammation in cerebral diseases (16). When the inflammatory response is
initiated, microglia are primarily activated by LPS secreted from
adjacent inflammatory cells. Therefore, in the present study, LPS
was used to establish an in vitro model of microglia
activation. An increasing number of studies have indicated that
miR-195 is a highly sensitive blood-based diagnostic biomarker for
the early detection of PD due to its aberrant expression in the
serum of patients with PD (7,8).
Furthermore, miR-195 has been identified to participate in multiple
diseases of solid organs, particularly in cancer (17) and hepatic fibrosis (18). However, few studies have focused on
the role of miR-195 in microglia in the progression of PD. In the
present study, the expression of miR-195 was determined in
LPS-induced activated microglia. The results clearly indicated that
LPS concentration- and time-dependently decreased the expression of
miR-195, suggesting a negative association between miR-195
expression and microglia activation. This result was consistent
with those of previous studies indicating that miR-195 was
downregulated in various types of cancer, including pancreatic
(19) and prostate cancer
(17). Activated microglia cells
serve an important role in adjusting and controlling
neuroinflammation. The inflammatory response is triggered by the
extensive production and secretion of cytokines and chemokines.
Increasing evidence has suggested that iNOS is responsible for the
mass production of NO from L-arginine. Furthermore, it has been
suggested that the expression of iNOS is significantly increased in
inflammatory lesions in multiple inflammation-associated diseases
(20). The key pro-inflammatory
cytokines also include TNF-α and IL-6, while anti-inflammatory
cytokines include IL-4 and IL-10 (21). Subsequently, a gain- or
loss-of-function strategy was successfully employed in the present
study to determine the role of miR-195 in regulating
neuroinflammation during PD. The results indicated a marked
decrease in the expression of inflammatory factors and an increase
in the expression of anti-inflammatory factors in BV2 cells
transfected with miR-195 mimics, which were contrary to those in
the miR-195 inhibitor-treated group. These results collectively
validated the anti-inflammatory effects of miR-195 in PD.
Consistent with this, the anti-inflammatory effects of miR-195 have
been confirmed in various pathological conditions, including sickle
cell disease (22), hepatocellular
carcinoma (23) and activation of
macrophages in atherosclerotic plaques (10).
Extensive studies have demonstrated that inhibition
of ROCK1 ameliorates inflammation (12,13).
To identify the role of ROCK1 in microglia activation, the
expression of ROCK1 was detected in LPS-treated BV2 cells. The
results demonstrated that the expression of ROCK1 was positively
associated with microglia activation but negatively associated with
the expression of miR-195 in microglia. As ROCK1 expression was
increased in activated BV2 cells, a loss-of-function experiment was
used. As hypothesized, siRNA-mediated ROCK1 knockdown prior to LPS
challenge markedly decreased the expression of iNOS, IL-6 and
TNF-α, but induced the expression of IL-4 and IL-10 compared with
that in control-transfected cells, suggesting that the inhibition
of ROCK1 suppressed the inflammation induced by activated
microglia. Consistent with this result, a previous study
demonstrated that ROCK inhibitor Y-27632 significantly decreased
the levels of serum IL-6 and TNF-α, but increased the levels of
IL-10 in Murphy Roths Large(+/+)/lymphoproliferation
mice (24). Therefore, these
results suggested that the miR-195/ROCK1 pathway serves an
important role in regulating activated microglia-mediated
inflammatory responses.
The self-renewal of microglia, which contributes to
maintaining a stable number of microglia over a mouse or human
lifetime, is achieved based on the homeostasis between
proliferation and apoptosis (25).
To the best of our knowledge, the present study was the first to
determine the role of the miR-195/ROCK1 pathway in the
proliferation of LPS-stimulated microglia. It was indicated that
miR-195 inhibitors additionally enhanced the proliferation and
viability of LPS-treated microglia, while ROCK1 knockdown reversed
the effects of miR-195 inhibitor. These results suggested that the
miR-195/ROCK1 pathway is involved in the regulation of microglia
proliferation and viability.
As the extensive production of pro-inflammatory
cytokines may aggravate tissue damage and promote the progression
of PD, the number of activated microglia is strictly controlled by
apoptosis, which is also called ‘activation-induced cell death’.
The present results indicated that miR-195 inhibitor decreased the
level of apoptosis in LPS-treated BV2 cells, which was reversed by
siROCK1. These results suggest that the miR-195/ROCK1 pathway
mediates the induction of apoptosis of microglia activated by
LPS.
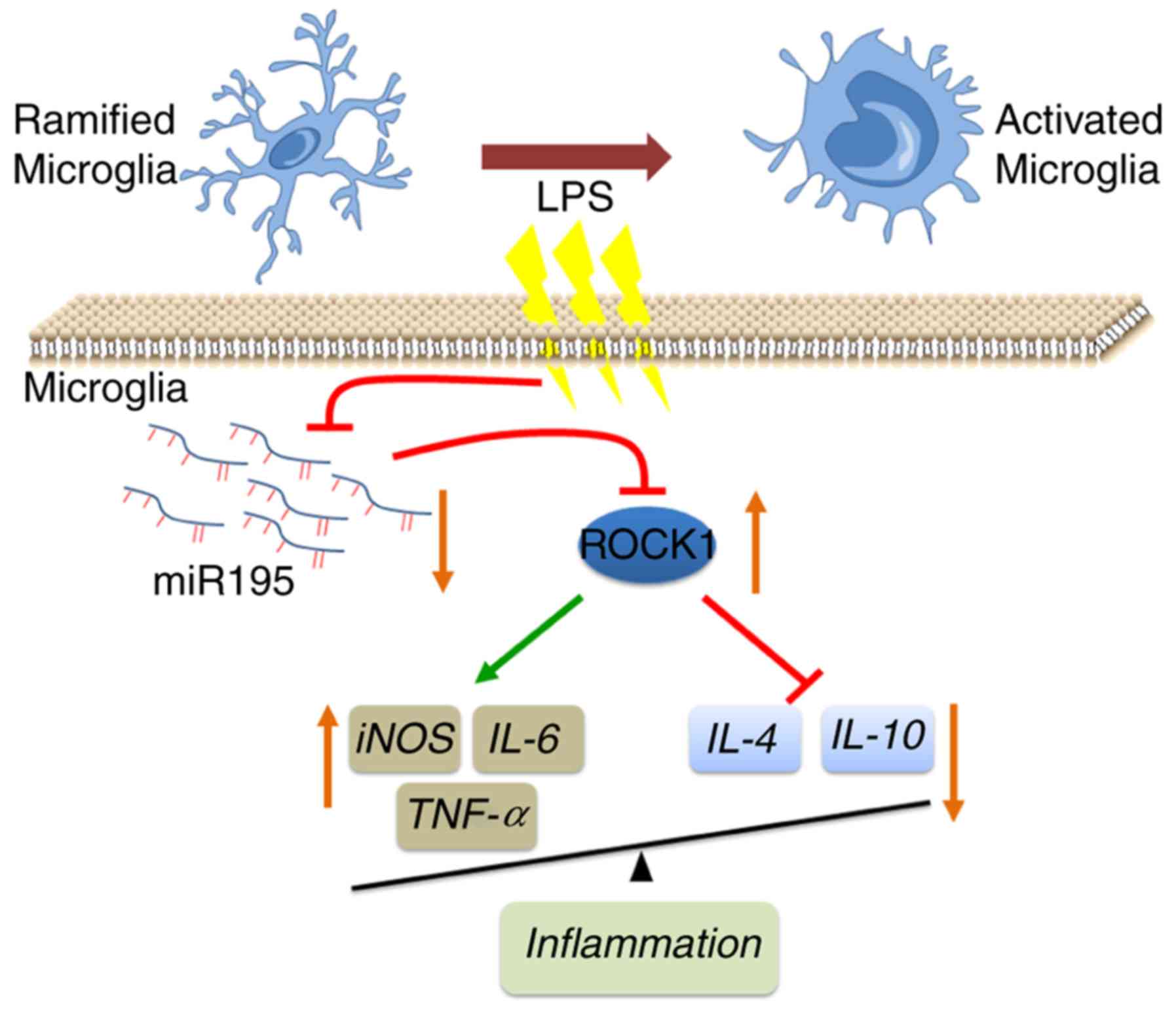
The present study demonstrated that miR-195
expression is decreased in LPS-stimulated BV2 cells. Functionally,
the decrease in miR-195 levels increased the expression of
inflammatory cytokines, promoted cell proliferation and inhibited
apoptosis in LPS-treated BV2 cells. Mechanistically, increased
ROCK1 caused by loss of miR-195 promoted the activation of
microglia and triggered neuroinflammation in the present in
vitro model of PD (Fig. 8).
However, additional in-depth studies are required in the future.
The present results suggest that it may be valuable to explore the
potential applications of miR-195 mimics in the clinical treatment
of microglia-associated PD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YR and ML conceived and designed the experiments.
YR, HL, and WX performed the experiments. YR and NW analyzed the
data. YR wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BrdU
|
5-bromo-2-deoxyuridine
|
|
FCM
|
flow cytometry
|
|
FITC
|
fluorescein isothiocyanate
|
|
IL-6
|
interleukin-6
|
|
iNOS
|
inducible nitric oxide synthase
|
|
LPS
|
lipopolysaccharide
|
|
miR-195
|
microRNA-195
|
|
miR-NC1
|
microRNA mimics negative control
|
|
miR-NC2
|
microRNA inhibitor negative
control
|
|
PD
|
Parkinson's disease
|
|
PI
|
propidium iodide
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
ROCK1
|
Rho-associated kinase 1
|
References
|
1
|
Zafar S and Yaddanapudi SS: Parkinson
disease. StatPearls (Internet). Treasure Island (FL): StatPearls
Publishing; 2018
|
|
2
|
Joshi N and Singh S: Updates on immunity
and inflammation in Parkinson disease pathology. J Neurosci Res.
96:379–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sunkaria A, Wani WY, Sharma DR and Gill
KD: Dichlorvos exposure results in activation induced apoptotic
cell death in primary rat microglia. Chem Res Toxicol.
25:1762–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh SE, Park HJ, He L, Skibiel C, Junn E
and Mouradian MM: The Parkinson's disease gene product DJ-1
modulates miR-221 to promote neuronal survival against oxidative
stress. Redox Biol. 19:62–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee J, Park EJ and Kiyono H:
MicroRNA-orchestrated pathophysiologic control in gut homeostasis
and inflammation. BMB Rep. 49:263–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arshad AR, Sulaiman SA, Saperi AA, Jamal
R, Mohamed Ibrahim N and Abdul Murad NA: MicroRNAs and target genes
as biomarkers for the diagnosis of early onset of parkinson
disease. Front Mol Neurosci. 10:3522017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS
and Xue LJ: MicroRNA biomarkers of Parkinson's disease in serum
exosome-like microvesicles. Neurosci Lett. 644:94–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding H, Huang Z, Chen M, Wang C, Chen X,
Chen J and Zhang J: Identification of a panel of five serum miRNAs
as a biomarker for Parkinson's disease. Parkinsonism Relat Disord.
22:68–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma X, Yao H, Yang Y, Jin L, Wang Y, Wu L,
Yang S and Cheng K: miR-195 suppresses abdominal aortic aneurysm
through the TNF-alpha/NF-kappaB and VEGF/PI3K/Akt pathway. Int J
Mol Med. 41:2350–2358. 2018.PubMed/NCBI
|
|
10
|
Bras JP, Silva AM, Calin GA, Barbosa MA,
Santos SG and Almeida MI: miR-195 inhibits macrophages
pro-inflammatory profile and impacts the crosstalk with smooth
muscle cells. PloS One. 12:e01885302017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han C and Wang W: MicroRNA-129-5p
suppresses cell proliferation, migration and invasion via targeting
ROCK1 in osteosarcoma. Mol Med Rep. 17:4777–4784. 2018.PubMed/NCBI
|
|
12
|
Moon HG, Cao Y, Yang J, Lee JH, Choi HS
and Jin Y: Lung epithelial cell-derived extracellular vesicles
activate macrophage-mediated inflammatory responses via ROCK1
pathway. Cell Death Dis. 6:e20162015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang H, Liao M, Zhao W, Zheng X, Xu F,
Wang H and Huang J: CXCL16/ROCK1 signaling pathway exacerbates
acute kidney injury induced by ischemia-reperfusion. Biomed
Pharmacother. 98:347–356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Liu J, Wang L, Yang X and Liu X:
MicroRNA195 inhibits cell proliferation, migration and invasion in
laryngeal squamous cell carcinoma by targeting ROCK1. Mol Med Rep.
16:7154–7162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin H, Lian N, Zhang F, Chen L, Chen Q, Lu
C, Bian M, Shao J, Wu L and Zheng S: Activation of PPARgamma/P53
signaling is required for curcumin to induce hepatic stellate cell
senescence. Cell death Dis. 7:e21892016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gomez-Nicola D and Perry VH: Microglial
dynamics and role in the healthy and diseased brain: A paradigm of
functional plasticity. Neuroscientist. 21:169–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma X, Zou L, Li X, Chen Z, Lin Q and Wu X:
MicroRNA-195 regulates docetaxel resistance by targeting clusterin
in prostate cancer. Biomed Pharmacother. 99:445–450. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song LY, Ma YT, Wu CF, Wang CJ, Fang WJ
and Liu SK: MicroRNA-195 activates hepatic stellate cells in vitro
by targeting Smad7. BioMed Res Int. 2017:19456312017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou B, Sun C, Hu X, Zhan H, Zou H, Feng
Y, Qiu F, Zhang S, Wu L and Zhang B: MicroRNA-195 suppresses the
progression of pancreatic cancer by targeting DCLK1. Cell Physiol
Biochem. 44:1867–1881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tiwari A, Singh P, Jaitley P, Sharma S,
Prakash A, Mandil R, Choudhury S, Gangwar NK and Garg SK:
Eucalyptus robusta leaves methanolic extract suppresses
inflammatory mediators by specifically targeting TLR4/TLR9, MPO,
COX2, iNOS and inflammatory cytokines in experimentally-induced
endometritis in rats. J Ethnopharmacol. 213:149–158. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mavrogonatou E, Konstantinou A and Kletsas
D: Long-term exposure to TNF-alpha leads human skin fibroblasts to
a p38 MAPK- and ROS-mediated premature senescence. Biogerontology.
19:237–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gonsalves C and Kalra VK:
Endothelin-1-induced macrophage inflammatory protein-1beta
expression in monocytic cells involves hypoxia-inducible
factor-1alpha and AP-1 and is negatively regulated by microRNA-195.
J Immunol. 185:6253–6264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding J, Huang S, Wang Y, Tian Q, Zha R,
Shi H, Wang Q, Ge C, Chen T, Zhao Y, et al: Genome-wide screening
reveals that miR-195 targets the TNF-alpha/NF-kappaB pathway by
down-regulating IkappaB kinase alpha and TAB3 in hepatocellular
carcinoma. Hepatology. 58:654–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Lu Y, Chai J, Sun M, Hu X, He W,
Ge M and Xie C: Y-27632, a Rho-associated protein kinase inhibitor,
inhibits systemic lupus erythematosus. Biomed Pharmacotherapie.
88:359–366. 2017. View Article : Google Scholar
|
|
25
|
Askew K, Li K, Olmos-Alonso A,
Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA,
Riecken K, Beccari S, et al: Coupled proliferation and apoptosis
maintain the rapid turnover of microglia in the adult brain. Cell
Rep. 18:391–405. 2017. View Article : Google Scholar : PubMed/NCBI
|