|
1
|
Oh J, Yoon H and Park JH: Nanoparticle
platforms for combined photothermal and photodynamic therapy.
Biomed Eng Lett. 3:67–73. 2013. View Article : Google Scholar
|
|
2
|
Cao J, An H, Huang X, Fu G, Zhuang R, Zhu
L, Xie J and Zhang F: Monitoring of the tumor response to
nano-graphene oxide-mediated photothermal/photodynamic therapy by
diffusion-weighted and BOLD MRI. Nanoscale. 8:10152–10159. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin J, Wang S, Huang P, Wang Z, Chen S,
Niu G, Li W, He J, Cui D, Lu G, et al: Photosensitizer-loaded gold
vesicles with strong plasmonic coupling effect for imaging-guided
photothermal/photodynamic therapy. ACS Nano. 7:5320–5329. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong LQ, Chen ZG, Yu MX, Li FY, Liu C and
Huang CH: Synthesis, characterization, and in vivo targeted imaging
of amine-functionalized rare-earth up-converting nanophosphors.
Biomaterials. 30:5592–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibu ES, Hamada M, Murase N and Biju V:
Nanomaterials formulations for photothermal and photodynamic
therapy of cancer. J Photochem Photobiol C: Photochem Rev.
15:53–72. 2013. View Article : Google Scholar
|
|
6
|
Liu J, Han J, Kang Z, Golamaully R, Xu N,
Li H and Han X: In vivo near-infrared photothermal therapy and
computed tomography imaging of cancer cells using novel
tungsten-based theranostic probe. Nanoscale. 6:5770–5776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Tao H, Cheng L and Liu Z:
Near-infrared light induced in vivo photodynamic therapy of cancer
based on upconversion nanoparticles. Biomaterials. 32:6145–6154.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen J, Xu Y, Li H, Lu A and Sun S: Recent
applications of carbon nanomaterials in fluorescence biosensing and
bioimaging. Chem Commun (Camb). 51:11346–11358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu X, Ji C, Jin T and Fan X: The effects
of size and surface modification of amorphous silica particles on
biodistribution and liver metabolism in mice. Nanotechnology.
26:1751012015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo M, Shen C, Feltis BN, Martin LL,
Hughes AE, Wright PF and Turney TW: Reducing ZnO nanoparticle
cytotoxicity by surface modification. Nanoscale. 6:5791–5798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chow EK and Ho D: Cancer nanomedicine:
From drug delivery to imaging. Sci Transl Med. 5:216rv42013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jaque D, Martinez Maestro L, del Rosal B,
Haro-Gonzalez P, Benayas A, Plaza JL, Martin Rodriguez E and García
Solé J: Nanoparticles for photothermal therapies. Nanoscale.
6:9494–9530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thakor AS, Jokerst J, Zavaleta C, Massoud
TF and Gambhir SS: Gold nanoparticles: A revival in precious metal
administration to patients. Nano Lett. 11:4029–4036. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bazán-Díaz L, Mendoza-Cruz R,
Velázquez-Salazar JJ, Plascencia-Villa G, Romeu D, Reyes-Gasga J,
Herrera-Becerra R, José-Yacamán M and Guisbiers G: Gold-copper
nanostars as photo-thermal agents: Synthesis and advanced electron
microscopy characterization. Nanoscale. 7:20734–20742. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pissuwan D and Niidome T:
Polyelectrolyte-coated gold nanorods and their biomedical
applications. Nanoscale. 7:59–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Liu Y, Luehmann H, Xia X, Brown P,
Jarreau C, Welch M and Xia Y: Evaluating the pharmacokinetics and
in vivo cancer targeting capability of Au nanocages by positron
emission tomography imaging. ACS Nano. 6:5880–5888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
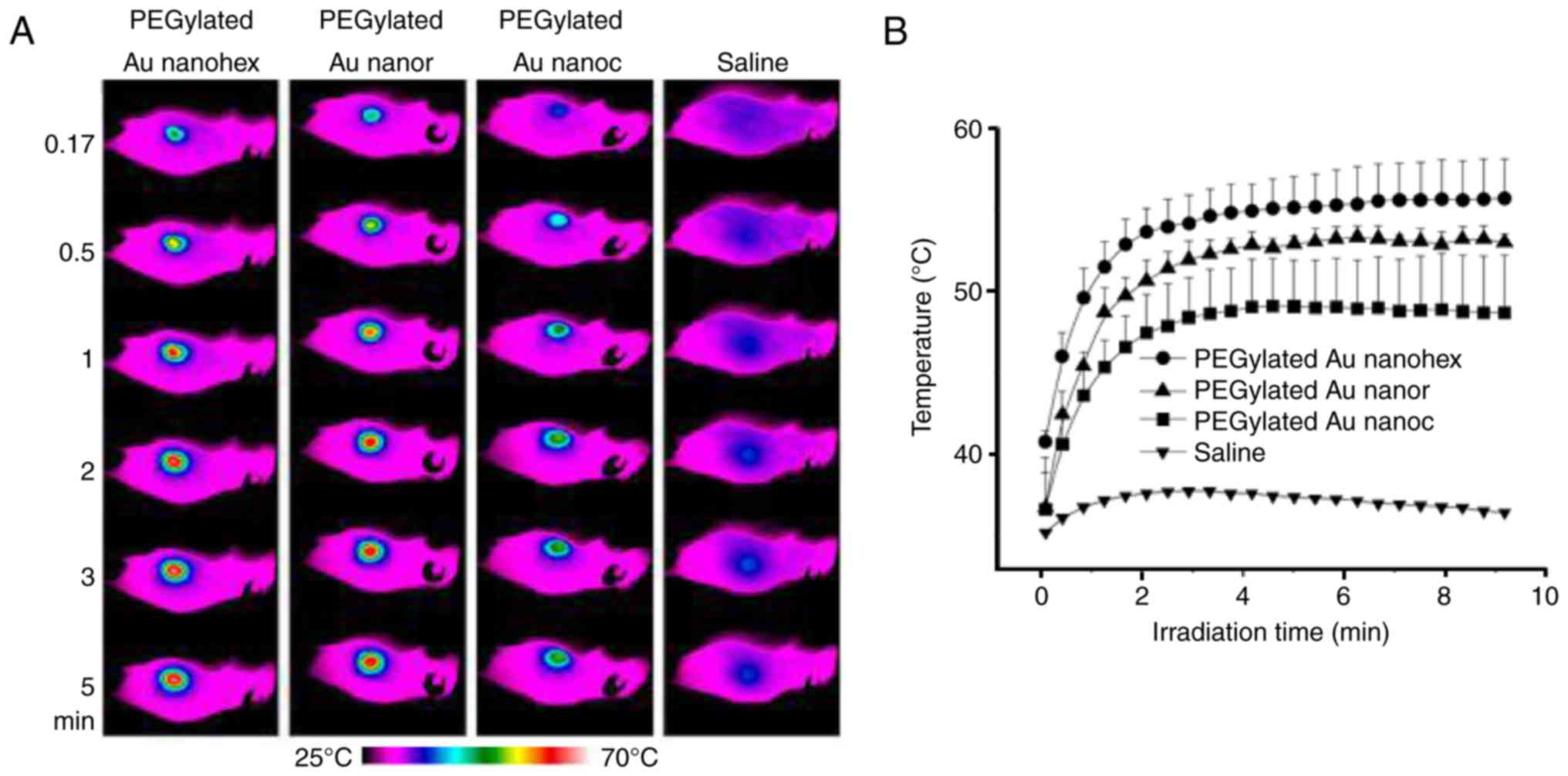
Wang Y, Black KC, Luehmann H, Li W, Zhang
Y, Cai X, Wan D, Liu SY, Li M, Kim P, et al: Comparison study of
gold nanohexapods, nanorods, and nanocages for photothermal cancer
treatment. ACS Nano. 7:2068–2077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shrestha R, Elsabahy M, Luehmann H,
Samarajeewa S, Florez-Malaver S, Lee NS, Welch MJ, Liu Y and Wooley
KL: Hierarchically assembled theranostic nanostructures for siRNA
delivery and imaging applications. J Am Chem Soc. 134:17362–17365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hasan W, Stender CL, Min HL, Nehl CL and
Lee J: Tailoring the structure of nanopyramids for optimal heat
generation. Nano Lett. 9:1555–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DY, Yu T, Cho EC, Ma Y, Park OO and
Xia Y: Synthesis of gold nano-hexapods with controllable arm
lengths and their tunable optical properties. Angew Chem Int Ed
Engl. 50:6328–6331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar A and Liang XJ: Gold nanomaterials
as prospective metal-based delivery systems for cancer treatment.
Kretsinger RH, Uversky VN and Permyakov EA: Encyclopedia of
Metalloproteins; Springer, New York, NY: pp. 875–887. 2013
|
|
23
|
Wu H, Yang R, Song B, Han Q, Li J, Zhang
Y, Fang Y, Tenne R and Wang C: Biocompatible inorganic
fullerene-like molybdenum disulfide nanoparticles produced by
pulsed laser ablation in water. ACS Nano. 5:1276–1281. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Wang C, Gu X, Gong H, Cheng L, Shi
X, Feng L, Sun B and Liu Z: Drug delivery with PEGylated MoS2
nano-sheets for combined photothermal and chemotherapy of cancer.
Adv Mater. 26:3433–3440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin H, Shan X, Hao L, Feng Q and Zhang Z:
Copper sulfide nanoparticle-based localized drug delivery system as
an effective cancer synergistic treatment and theranostic platform.
Acta Biomate. 54:307–320. 2017. View Article : Google Scholar
|
|
26
|
Hessel CM, Pattani VP, Rasch M, Panthani
MG, Koo B, Tunnell JW and Korgel BA: Copper selenide nanocrystals
for photothermal therapy. Nano Lett. 11:2560–2566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Z, Kong B, Yu C, Shi X, Wang M, Liu
W, Sun Y, Zhang Y, Yang H and Yang S: Tungsten oxide nanorods: an
efficient nanoplatform for tumor CT imaging and photothermal
therapy. Sci Rep. 4:36532014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
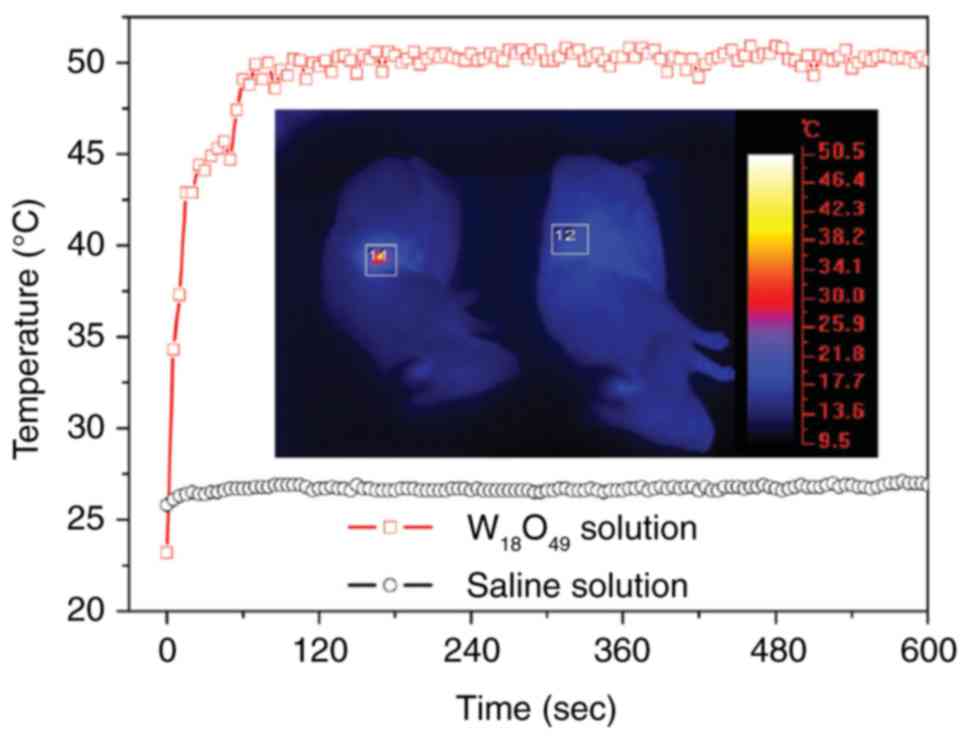
Chen Z, Wang Q, Wang H, Zhang L, Song G,
Song L, Hu J, Wang H, Liu J, Zhu M and Zhao D: Ultrathin PEGylated
W18O49 nanowires as a new 980 nm-laser-driven photothermal agent
for efficient ablation of cancer cells in vivo. Adv Mater.
25:2095–2100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song G, Shen J, Jiang F, Hu R, Li W, An L,
Zou R, Chen Z, Qin Z and Hu J: Hydrophilic molybdenum oxide
nanomaterials with controlled morphology and strong plasmonic
absorption for photothermal ablation of cancer cells. ACS Appl
Mater Interfaces. 6:3915–3922. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
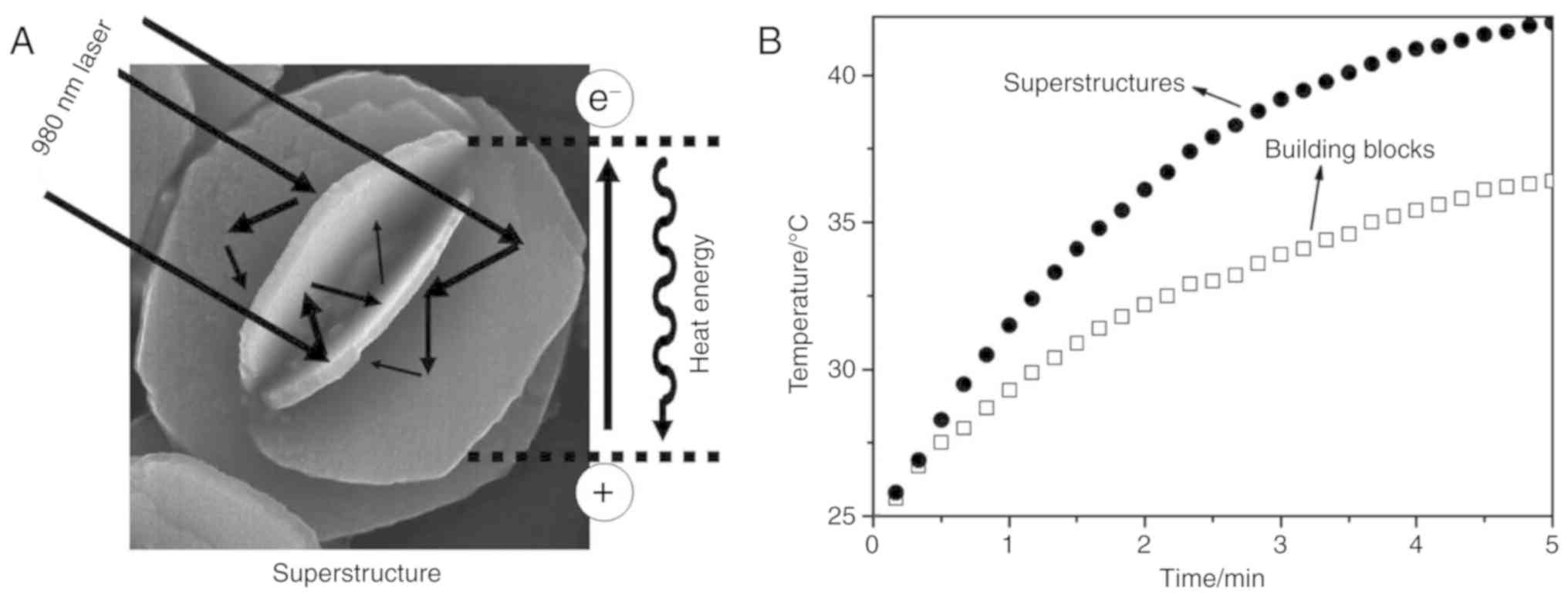
Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu
M, Yang S, Wang J, Wang J and Hu J: Hydrophilic flower-like CuS
superstructures as an efficient 980 nm laser-driven photothermal
agent for ablation of cancer cells. Adv Mater. 23:3542–3547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu
M, Yang S, Wang J, Wang J and Hu J: Hydrophilic Cu9S5 nanocrystals:
a photothermal agent with a 25.7% heat conversion efficiency for
photothermal ablation of cancer cells in vivo. ACS Nano.
5:9761–9771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knowles KE, Hartstein KH, Kilburn TB,
Marchioro A, Nelson HD, Whitham PJ and Gamelin DR: Luminescent
colloidal semiconductor nanocrystals containing copper: Synthesis,
photophysics, and applications. Chem Rev. 116:10820–10851. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang S, Xie Z, Wei Y, Cheng Z, Han Y and
Lin J: DNA decorated Cu9S5 nanoparticles as
NIR light responsive drug carriers for tumor chemo-phototherapy.
Dalton Trans. 47:7916–7924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stubenvoll M, Schäfer B, Mann K, Walter A
and Zittel L: Photothermal absorption measurements for improved
thermal stability of high-power laser optics. Journal 88851R.
2013.
|
|
35
|
Miokovic T, Schulze V, Löhe D and
Vöhringer O: Influence of heating rate, cooling rate and numbers of
pulses on the microstructure of AISI 4140 after
short-time-hardening. Int J Mater Prod Technol. 24:2005. View Article : Google Scholar
|
|
36
|
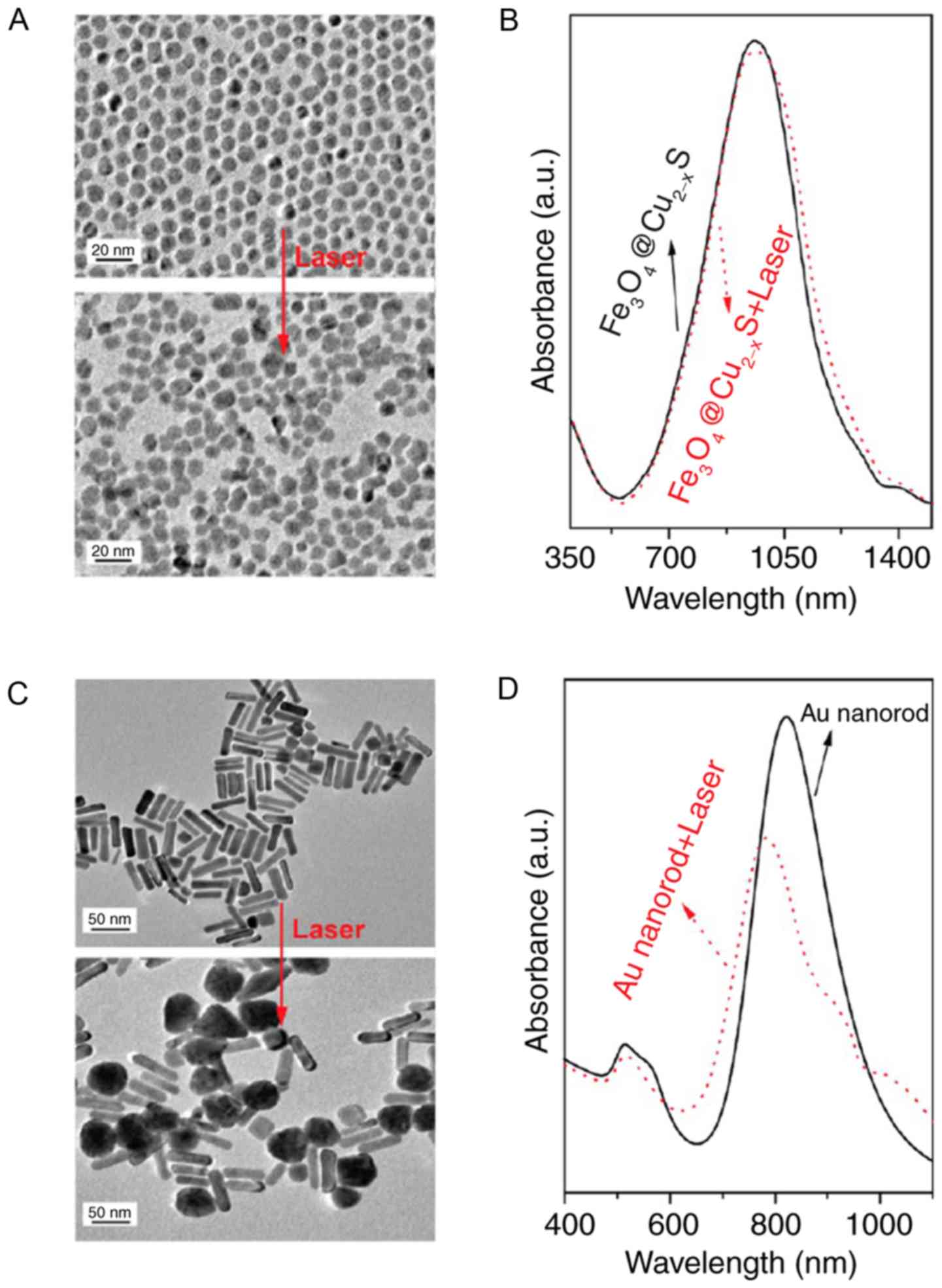
Tian Q, Hu J, Zhu Y, Zou R, Chen Z, Yang
S, Li R, Su Q, Han Y and Liu X: Sub-10 nm Fe3O4@Cu(2-x)S core-shell
nanoparticles for dual-modal imaging and photothermal therapy. J Am
Chem Soc. 135:8571–8577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murali VS, Wang R, Mikoryak CA, Pantano P
and Draper RK: The impact of subcellular location on the near
infrared-mediated thermal ablation of cells by targeted carbon
nanotubes. Nanotechnology. 27:4251022016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Madani SY, Naderi N, Dissanayake O, Tan A
and Seifalian AM: A new era of cancer treatment: Carbon nanotubes
as drug delivery tools. Int J Nanomedicine. 6:2963–2979.
2011.PubMed/NCBI
|
|
39
|
Robinson JT, Tabakman SM, Liang Y, Wang H,
Casalongue HS, Vinh D and Dai H: Ultrasmall reduced graphene oxide
with high near-infrared absorbance for photothermal therapy. J Am
Chem Soc. 133:6825–6831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang K, Wan J, Zhang S, Tian B, Zhang Y
and Liu Z: The influence of surface chemistry and size of nanoscale
graphene oxide on photothermal therapy of cancer using ultra-low
laser power. Biomaterials. 33:2206–2214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo Y, Xu H, Li Y, Wu F, Li Y, Bao Y, Yan
X, Huang Z and Xu P: Hyaluronic acid and Arg-Gly-Asp peptide
modified Graphene oxide with dual receptor-targeting function for
cancer therapy. J Biomater Appl. 32:54–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Robinson JT, Sun X and Dai H:
PEGylated nanographene oxide for delivery of water-insoluble cancer
drugs. J Am Chem Soc. 130:10876–10877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H, Lee D, Kim J, Kim TI and Kim WJ:
Photothermally triggered cytosolic drug delivery via endosome
disruption using a functionalized reduced graphene oxide. ACS Nano.
7:6735–6746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu Z, Oleinick N and Hamblin MR:
Photodynamic therapy as an emerging treatment modality for cancer
and non-cancer diseases. J Anal Bioanal Tech. S1:e0012014.
View Article : Google Scholar
|
|
45
|
Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y,
Achilefu S and Gu Y: In vivo targeted deep-tissue photodynamic
therapy based on near-infrared light triggered upconversion
nanoconstruct. ACS Nano. 7:676–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ali SM and Olivo M: Mechanisms of action
of phenanthroperylenequinones in photodynamic therapy (review). Int
J Oncol. 22:1181–1191. 2003.PubMed/NCBI
|
|
47
|
Ge X, Liu J and Sun L: Controlled optical
characteristics of lanthanide doped upconversion nanoparticles for
emerging applications. Dalton Trans. 46:16729–16737. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Dong Y, Tuerxun·, Aidilibike, Liu X,
Guo J and Qin W: Growth phase diagram and upconversion luminescence
properties of NaLuF4: Yb3+/Tm3+/Gd3+ nanocrystals. RSC Adv.
7:44531–44536. 2017. View Article : Google Scholar
|
|
49
|
Wang F, Banerjee D, Liu Y, Chen X and Liu
X: Upconversion nanoparticles in biological labeling, imaging, and
therapy. Analyst. 135:1839–1854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jin S, Zhou L, Gu Z, Tian G, Yan L, Ren W,
Yin W, Liu X, Zhang X, Hu Z and Zhao Y: A new near infrared
photosensitizing nanoplatform containing blue-emitting
up-conversion nanoparticles and hypocrellin A for photodynamic
therapy of cancer cells. Nanoscale. 5:11910–11918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wei Y, Chen Q, Wu B, Zhou A and Xing D:
High-sensitivity in vivo imaging for tumors using a spectral
up-conversion nanoparticle NaYF4: Yb3+, Er3+ in cooperation with a
microtubulin inhibitor. Nanoscale. 4:3901–3909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qian HS, Guo HC, Ho PC, Mahendran R and
Zhang Y: Mesoporous-silica-coated up-conversion fluorescent
nanoparticles for photodynamic therapy. Small. 5:2285–2290. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chatterjee DK and Yong Z: Upconverting
nanoparticles as nanotransducers for photodynamic therapy in cancer
cells. Nanomedicine (Lond). 3:73–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Idris NM, Gnanasammandhan MK, Zhang J, Ho
PC, Mahendran R and Zhang Y: In vivo photodynamic therapy using
upconversion nanoparticles as remote-controlled nanotransducers.
Nat Med. 18:1580–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang L, Yan R, Huo Z, Wang L, Zeng J, Bao
J, Wang X, Peng Q and Li Y: Fluorescence resonant energy transfer
biosensor based on upconversion-luminescent nanoparticles. Angew
Chem Int Ed Engl. 44:6054–6057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiong LQ, Chen ZG, Yu MX, Li FY, Liu C and
Huang CH: Synthesis, characterization, and in vivo targeted imaging
of amine-functionalized rare-earth up-converting nanophosphors.
Biomaterials. 30:5592–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou J, Sun Y, Du X, Xiong L, Hu H and Li
F: Dual-modality in vivo imaging using rare-earth nanocrystals with
near-infrared to near-infrared (NIR-to-NIR) upconversion
luminescence and magnetic resonance properties. Biomaterials.
31:3287–3295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shen X, He F, Wu J, Xu GQ, Yao SQ and Xu
QH: Enhanced two-photon singlet oxygen generation by
photosensitizer-doped conjugated polymer nanoparticles. Langmuir.
27:1739–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Y, Yang G, Wang Y, Zhao Y, Jiang H,
Han Y and Yang P: Multiple imaging and excellent anticancer
efficiency of an upconverting nanocarrier mediated by single near
infrared light. Nanoscale. 9:4759–4769. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bakker MH, Lee CC, Meijer EW, Dankers PY
and Albertazzi L: Multicomponent supramolecular polymers as a
modular platform for intracellular delivery. ACS Nano.
10:1845–1852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dong R, Zhou Y, Huang X, Zhu X, Lu Y and
Shen J: Functional supramolecular polymers for biomedical
applications. Adv Mater. 27:498–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Selvasekar CR, Birbeck N, McMillan T,
Wainwright M and Walker SJ: Photodynamic therapy and the alimentary
tract. Aliment Pharmacol Ther. 15:899–915. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Khorami HH: Preliminary study on porphyrin
derivatives as transfection reagents for mammalian cell.
Porphyrins-synthesis. Anim Cell Biotechnol. 2013.
|
|
64
|
Chen M and Scheer H: Extending the limits
of natural photosynthesis and implications for technical light
harvesting. J Porphyr Phthalocyanines. 17:1–15. 2013. View Article : Google Scholar
|
|
65
|
Stilts CE, Nelen MI, Hilmey DG, Davies SR,
Gollnick SO, Oseroff AR, Gibson SL, Hilf R and Detty MR:
Water-soluble, core-modified porphyrins as novel,
longer-wavelength-absorbing sensitizers for photodynamic therapy. J
Med Chem. 43:2403–2410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cui L, Lin Q, Jin CS, Jiang W, Huang H,
Ding L, Muhanna N, Irish JC, Wang F, Chen J and Zheng G: A
pegylation-free biomimetic porphyrin nanoplatform for personalized
cancer theranostics. ACS Nano. 9:4484–4495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rossi F, Bedogni E, Bigi F, Rimoldi T,
Cristofolini L, Pinelli S, Alinovi R, Negri M, Dhanabalan SC,
Attolini G, et al: Porphyrin conjugated SiC/SiOx nanowires for
X-ray-excited photodynamic therapy. Sci Rep. 5:76062015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Brozek-Pluska B and Kopec M: Raman
microspectroscopy of Hematoporphyrins. Imaging of the noncancerous
and the cancerous human breast tissues with photosensitizers.
Spectrochim Acta A Mol Biomol Spectrosc. 169:182–191. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huynh E and Zheng G: Porphysome
nanotechnology: A paradigm shift in lipid-based supramolecular
structures. Nano Today. 9:212–222. 2014. View Article : Google Scholar
|
|
70
|
Li H, Marotta DE, Kim S, Busch TM, Wileyto
EP and Zheng G: High payload delivery of optical imaging and
photodynamic therapy agents to tumors using
phthalocyanine-reconstituted low-density lipoprotein nanoparticles.
J Biomed Opt. 10:412032005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stefflova K, Chen J, Marotta D, Li H and
Zheng G: Photodynamic therapy agent with a built-in apoptosis
sensor for evaluating its own therapeutic outcome in situ. J Med
Chem. 49:3850–3856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng G, Chen J, Li H and Glickson JD:
Rerouting lipoprotein nanoparticles to selected alternate receptors
for the targeted delivery of cancer diagnostic and therapeutic
agents. Proc Natl Acad Sci USA. 102:17757–17762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zheng G, Chen J, Stefflova K, Jarvi M, Li
H and Wilson BC: Photodynamic molecular beacon as an activatable
photosensitizer based on protease-controlled singlet oxygen
quenching and activation. Proc Natl Acad Sci USA. 104:8989–8994.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lovell JF, Jin CS, Huynh E, Jin H, Kim C,
Rubinstein JL, Chan WC, Cao W, Wang LV and Zheng G: Porphysome
nanovesicles generated by porphyrin bilayers for use as multimodal
biophotonic contrast agents. Nat Mater. 10:324–332. 2011.
View Article : Google Scholar : PubMed/NCBI
|