Introduction
Gastric cancer (GC) is a common malignancy in China,
with the second-highest incidence and mortality rates in the
country (1). Since GC develops
rapidly and rarely causes symptoms in the early stages, the
majority of patients present with advanced-stage GC at their first
hospital visit, making curative surgical treatment a challenge; the
5-year survival rate for the disease is~20–30%, and the median
survival time is only 11 months (2–4). The
aggressive development of GC is closely associated with the strong
proliferation capacity of the tumor cells (5); therefore, it is necessary to explore
the mechanism underlying GC cell proliferation. It has been
reported that interleukin enhancer-binding factor 3 (ILF3)
regulates transcription, translation, mRNA stability and primary
microRNA (pri-miRNA) processing; it may function as a
transcriptional activator to regulate the mRNA synthesis of target
genes (6). Abnormal expression of
ILF3 has been identified in a number of malignancies, and ILF3 has
been reported to be involved in tumor proliferation, invasion and
metastasis (7–9). Nevertheless, few studies have been
conducted to investigate the mechanism of action of ILF3 in GC. The
present study detected ILF3 protein expression in tissues from
paraffin-embedded samples. Subsequently, the ILF3 expression in GC
cells was inhibited by small interfering RNAs (siRNAs). The cell
proliferation and associated molecular mechanisms were
investigated. Meanwhile, the clinical records of participants were
obtained to evaluate the clinical significance of ILF3 detection,
based on patient prognosis. The present study may provide evidence
for further investigation of the role of ILF3 in GC development,
and also suggested that ILF3 may be a novel prognostic marker for
patients with GC.
Materials and methods
Clinical data
A total of 80 patients with GC who underwent surgery
to remove the primary lesions at Hebei Medical University Fourth
Affiliated Hospital (Shijiazhuang, China) between January 2010 and
December 2011, while not receiving any other treatment for cancer
prior to surgery (radiotherapy, chemotherapy, targeted therapy
etc.), were recruited, and paraffin-embedded samples from their
tumorous and adjacent mucosal tissues were obtained. The adjacent
tissues exhibited no trace of cancerous cell or signs of atypical
hyperplasia under microscopy. There were 57 males and 23 females
with mean age of 55.82±8.54 years, with a range of 38–78 years. The
research was approved by the Ethics Committee of Hebei Medical
University Fourth Affiliated Hospital and informed consent was
obtained from all participants.
Reagents
Rabbit-anti-human polyclonal antibodies, including
ILF3 (cat. no. HPA001897), p16 (cat. no. SAB4500072), p21 (cat. no.
SAB4500065), Cyclin D1 (cat. no. SAB4502603) and GAPDH (cat. no.
SAB2108266), were produced by Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Immunohistochemistry (IHC) kits and reagents were
purchased from ZSGB-BIO (cat. no. SP-9001; OriGene Technologies,
Inc., Beijing, China). Dulbecco's modified Eagle's medium (DMEM),
fetal bovine serum (FBS) and trypsin solution were supplied by
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). MTT was
obtained from Sigma-Aldrich (Merck KGaA). All polymerase chain
reaction (PCR) primers and siRNAs targeting ILF3 were synthesized
by Sangon Biotech Co., Ltd. (Shanghai, China).
Lipofectamine® 2000 reagent was obtained from Invitrogen
(Thermo Fisher Scientific, Inc.).
IHC assay and scoring of results
Tissue was fixed in 4% formalin for 24 h at room
temperature and embedded in paraffin. Sections (4-µm thickness)
were cut from paraffin blocks, deparaffinized and rehydrated in a
graded alcohol series and distilled water. The slides were immersed
in citrate buffer (0.01 M, pH 6.0) antigen retrieval buffer and
boiled for 3 min. Endogenous peroxidase activity was blocked with
3% H2O2 for 20 min. Sections were blocked in
normal goat serum (5%; cat. no. SP-9001; ZSGB-BIO; OriGene
Technologies, Inc.) at 37°C for 30 min and then incubated with
monoclonal antibody against ILF3 (1:100) overnight at 4°C, followed
by incubation with goat anti-rabbit polyclonal biotin-conjugated
antibody (1:100; cat. no. SP-9001; ZSGB-BIO; OriGene Technologies,
Inc.) at 37°C for 15 min. Then, the horseradish peroxidase
(HRP)-conjugated streptavidin working solution was added and
incubated at 37°C for 15 min. Subsequently, DAB substrate-chromogen
solution (1:100; ZSGB-BIO; OriGene Technologies, Inc.) was applied
to the samples to detect expression. Images were captured under a
light microscope (magnification, ×400) using a digital camera
(Olympus DP25; Olympus Corporation, Tokyo, Japan); five randomly
selected fields were analyzed per sample. The results were scored
by two pathologists using a bi-parametric scoring system based on
the quantity of cells with different staining intensities between 0
and 3 (0, <25%; 1, 25–50%; 2, 51–75%; 3, >76%) and the
intensity of staining between 0 and 3 (0, no positive staining; 1,
light yellow; 2, yellow; 3, brownish yellow). The summation of the
two scores was marked as follows: 0, -; 1–2, +; 3–4, ++; and 5–6,
+++. ILF3 staining was considered positive if the summation score
was >0.
Cell lines and cell culture
Gastric cell lines BGC823, AGS and SGC7901, and the
gastric epithelial cell line GES-1 were obtained from the American
Type Culture Collection (Manassas, VA, USA), and were preserved in
the Research Centre of Hebei Medical University Fourth Affiliated
Hospital. These cell lines were cultured in standard DMEM
supplemented with 10% FBS. The incubators were maintained at 5%
CO2 and 37°C. Among these GC cells, BGC823 and AGS are
poorly differentiated gastric adenocarcinoma cell lines, while
SGC7901 is a moderately differentiated gastric adenocarcinoma cell
line.
ILF3-siRNA transfection and
groupings
ILF3-siRNA (5′GCGGAUCCGACUACAACUACG-3′) and the
negative control (5′CGGCUGCAAUCGAUUGAUAGC-3′) were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Transfection of
ILF3 specific siRNA and the negative control were performed at a
concentration of 20 µmol/l using Lipofectamine 2000 reagent.
According to the manufacturer's protocol, 24 h prior to
transfection, 5×105 BGC823 and SGC7901 cells/well in 2
ml DMEM were seeded into 6-well plates and cultured at 5%
CO2 and 37°C. At 24 h later, when the cells had reached
70% confluence, transfection was performed. ILF3-siRNA and negative
control siRNA were transfected into BGC823 and SGC7901 cells. At 48
h post-transfection, the subsequent experiments were performed.
Experiments were repeated three times.
Cell activity assay (MTT assay)
Each group of cells was seeded in 96-well plates at
a density of 5×104 cells/ml. Following 4 h of
incubation, a volume of 20 µl MTT reagent (5 mg/ml) was added to
each well, and cells were incubated for a further 4 h. Then, the
culture medium was discarded, followed by the addition of 150 µl
dimethyl sulfoxide to each well with gentle agitation for 15 min at
room temperature. The optical density (OD) was obtained at 490 nm
using a microtiter plate reader. Experiments were repeated three
times.
Flow cytometry for cell cycle
analysis
Cells were centrifuged at 2,000 RPM/447.2 × g for 15
min at 37°C for collection and rinsed twice with PBS. Pre-chilled
70% ethanol was added and stored at 4°C overnight. Again, the cells
were centrifuged at 2,000 RPM/447.2 × g for 15 min at 37°C and
resuspended once in 1 ml PBS. Subsequently, 50 µg/ml propidium
iodide, 100 µg/ml RNase A and 0.2% Triton X-100 PBS were added into
100 µl of a 1×106 cells/ml suspension. After a 30-min
incubation at 4°C in the dark, the cell cycle was detected using a
flow cytometer (Epics-XL II; Beckman Coulter, Inc., Brea, CA, USA)
and analyzed using Expo 32 ADC XL4 (Beckman Coulter, Inc.).
Experiments were repeated three times.
Detection of target gene mRNA by
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Total RNA (2 µg) was reverse
transcribed into cDNA using an A5000 GoScript Reverse Transcription
System (Promega Corporation, Madison, WI, USA) under the following
conditions: 25°C annealing for 5 min, 42°C extension for 60 min and
70°C inactivation of reverse transcriptase for 15 min. PCR was
conducted using an A6001 GoTaq(R) RT-qPCR Master Mix (Promega
Corporation). qPCR in a 25 µl total volume was established and
performed over 45 cycles under the following conditions: 95°C
annealing for 5 min, 95°C denaturation for 30 sec and 72°C
extension for 30 sec. The quantification cycle (Cq) was
calculated with the amplification curve using GAPDH as an internal
standard (10). The sequences of
each primer are presented in Table
I. Experiments were repeated three times.
 | Table I.Sequences of each primer. |
Table I.
Sequences of each primer.
| Gene | Forward primer
(5–3′) | Reverse primer
(5′-3′) |
|---|
| ILF3 |
GTGTCCAATCACCAGTCCTG |
GCTGAAGAAGTGGGAGTGTAGC |
| p16 |
GAGAAACCTCGGGAAACTTAG |
GGGTGATGGCATTTACAGGT |
| p21 |
CATCCCGTGTTCTCCTTT |
ACTCTTCATTTGTCTACCGTG |
| Cyclin D1 |
ACCTGAGGAGCCCCAACAAC |
GCTTCGATCTGCTCCTGGC |
| GAPDH |
GACCCCTTCATTGACCTCAAC |
CGCTCCTGGAAGATGGTGAT |
Western blot assay for the examination
of candidate proteins
Total proteins were extracted from the cell lines
using RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) supplemented with 1X protease inhibitor
cocktail (Roche Applied Science, Mannheim, Germany). The protein
concentration was determined using a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). Protein samples (40 µg)
were loaded into each well and separated via 12% SDS-PAGE. Proteins
were transferred from the gel to a polyvinylidene fluoride
membrane, followed by blocking for 1 h in 5% skim milk in TBS at
room temperature. Subsequently, the membrane was incubated in the
primary antibodies (1:1,000) at 4°C overnight. Following three
washes with TBS with Tween-20, the membrane was incubated with an
alkaline phosphatase-conjugated goat anti-rabbit IgG secondary
antibody (1:2,000; cat. no. A3687; Sigma-Aldrich; Merck KGaA) at
room temperature for 1 h. Protein bands were detected using an
Odyssey system (LI-COR Biosciences, Lincoln, NE, USA). Expression
was normalized to GAPDH. Image J v1.48 software (National
Institutes of Health, USA) was used to quantify protein expression.
Experiments were repeated three times.
Statistical analysis
All data were presented as the mean ± standard
deviation. SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was
utilized for data analysis. A χ2 test, Spearman
correlation analysis, one-way analysis of variance followed by a
least significant difference post hoc test, and the independent
t-test were applied for experimental data examination. The
Kaplan-Meier analysis was conducted for survival analysis;
significant differences between patients positive for ILF3
expression and those a lack of expression were determined using the
log-rank test. Multivariate survival analysis was performed using a
Cox regression model (forward stepwise-likelihood ratio). P<0.05
was considered to indicate a statistically significant
difference.
Results
ILF3 protein expression in tumorous
and adjacent mucosal tissue, GC cell lines and GES-1
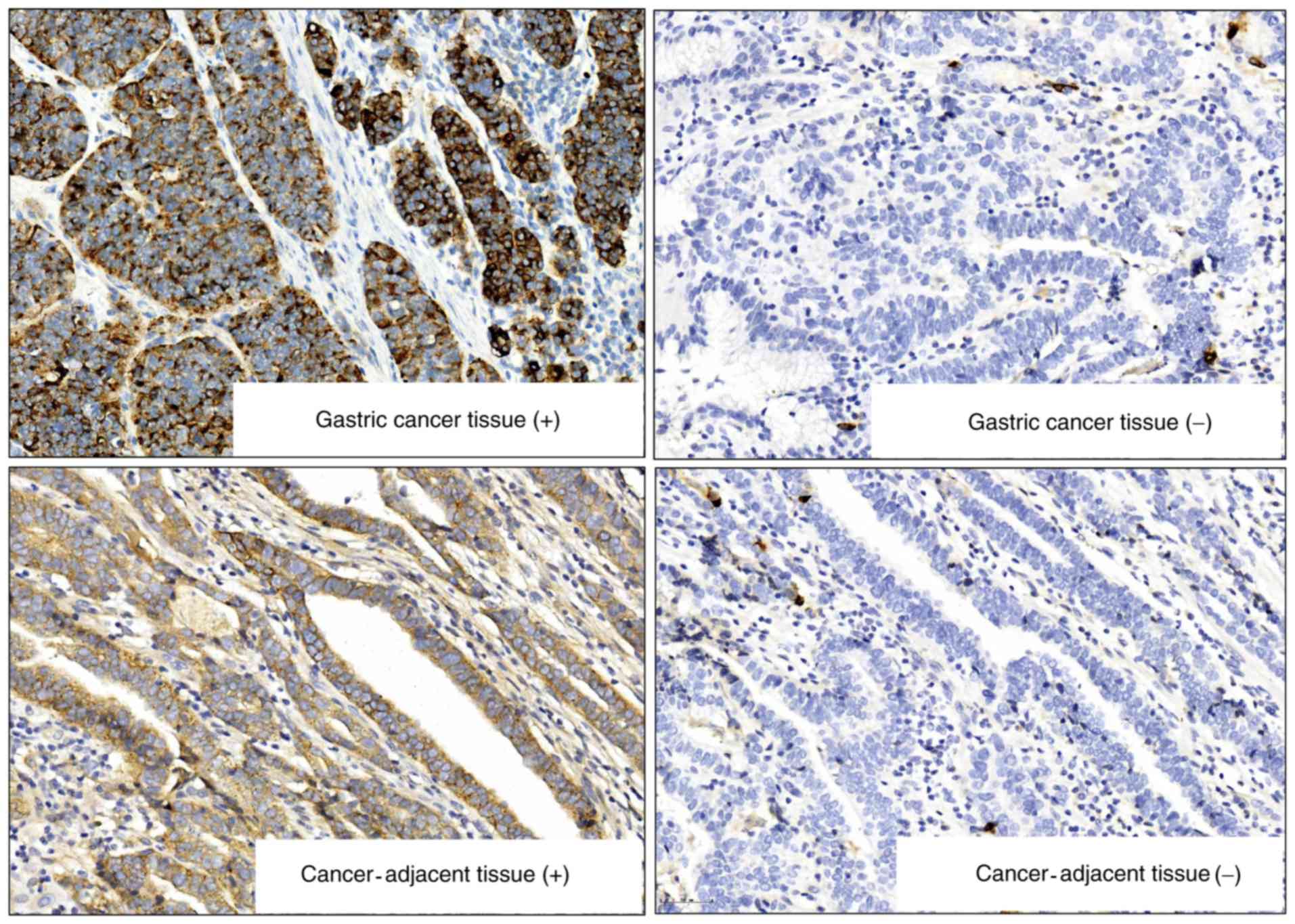
The IHC results showed 72.50 % (58/80) ILF3 positive
staining in GC tissues compared to 13.75% (14/80) in the adjacent
mucosal tissues. ILF3 expression in GC tissues was higher than that
in cancer-adjacent mucosa (χ2=48.89; P<0.001;
Fig. 1). ILF3 protein expression
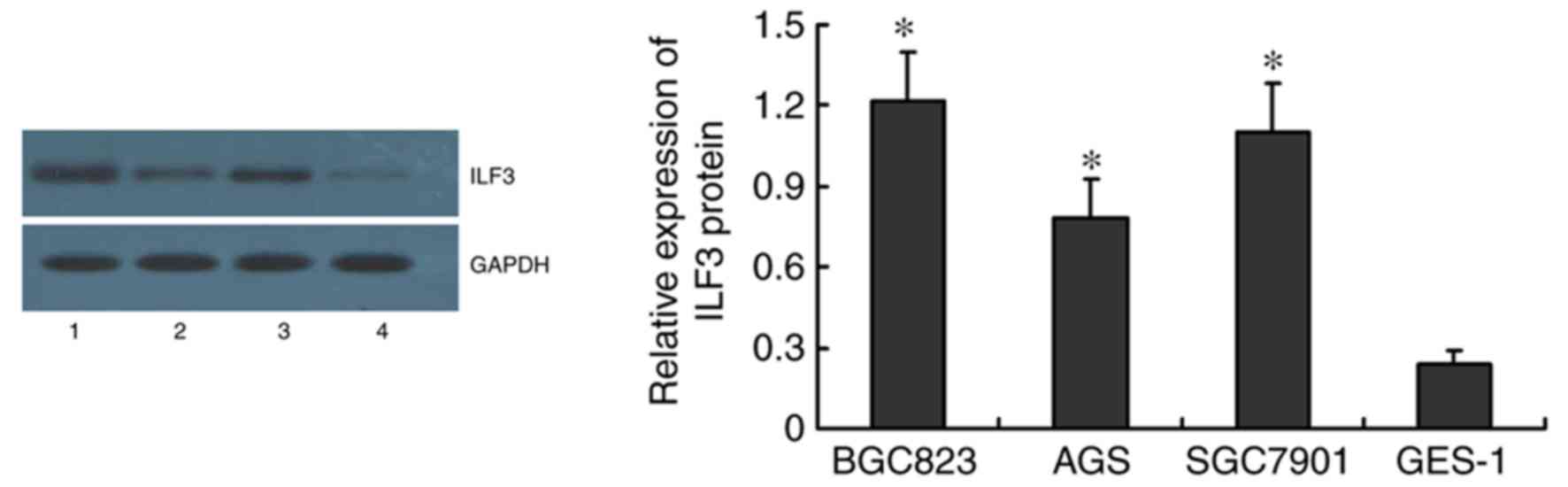
was highest in BGC823 and SGC7901 cells, as demonstrated by western
blotting (Fig. 2).
Effect of ILF3-siRNA on ILF3
expression in BGC823 and SGC7901 cells
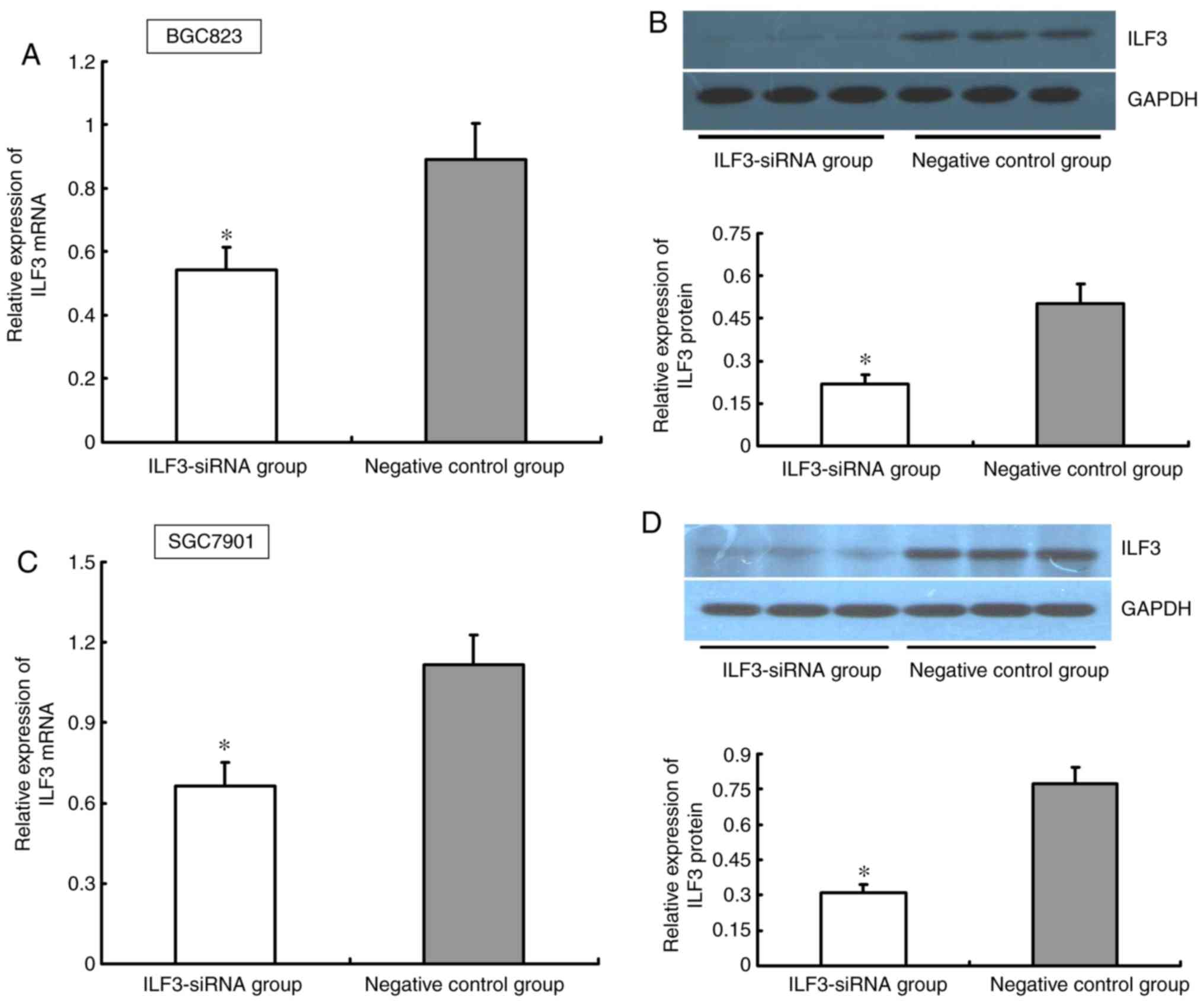
BGC823 and SGC7901 cells were transfected with
ILF3-siRNA. After 48 h of siRNA exposure, the ability of ILF3-siRNA
to inhibit ILF3 protein and mRNA expression was quantified by
western blotting and RT-qPCR, respectively. The ILF3 expression at
the mRNA and protein levels was significantly lower in the
ILF3-siRNA group compared with the negative control group in BGC823
and SGC7901 cells (P<0.05; Fig.
3).
Effect of ILF3-siRNA transfection on
BGC823 and SGC7901 cell viability
The proportion of viable BGC823 cells was
0.614±0.122 at 48 h post-ILF3-siRNA transfection relative to at 0
h, which was significantly lower than that of the cells in the
negative control group (0.975±0.128; t=−5.001; P<0.001; data not
shown) at the same time point. Similar results were confirmed in
SGC7901 cells; the proportion of viable SGC7901 cells at 48 h
post-ILF3-siRNA transfection was 0.582±0.126 in the ILF3-siRNA
group, which was significantly lower than that in the control group
(1.014± 0.178; t=−4.852; P<0.001).
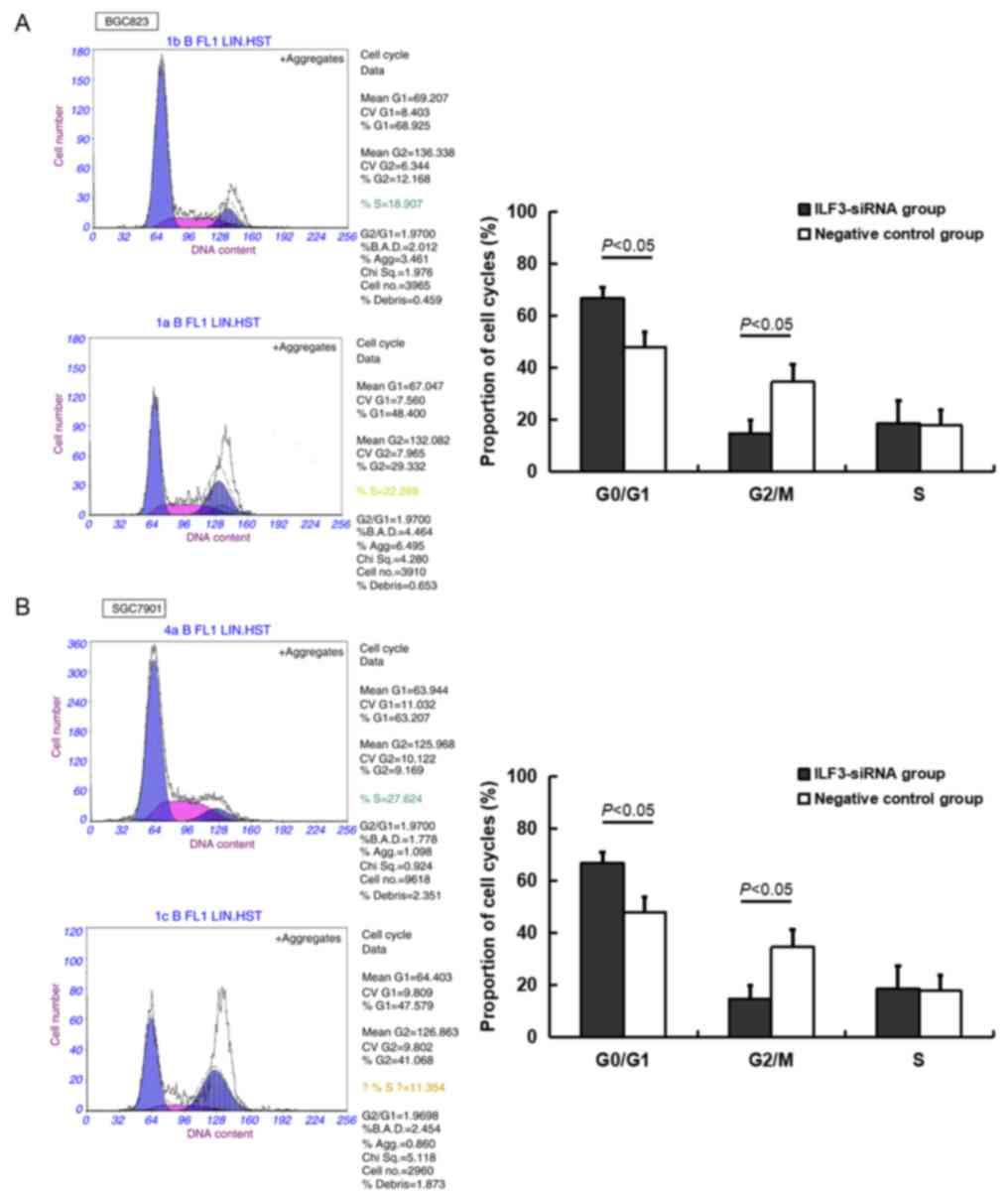
Effect of ILF3 inhibition on the
BGC823 and SGC7901 cell cycle
A higher proportion of BGC823 and SGC7901 cells in
the ILF3-siRNA transfected group were in the G0/G1 phase relative
to the negative control group, whereas the number of cells in the
G2/M phase in the ILF3-siRNA group was lower compared with the
negative control group (P<0.05; Table II and Fig. 4).
 | Table II.Effect of ILF3-siRNA transfection on
the cell cycle (n=3; cell %). |
Table II.
Effect of ILF3-siRNA transfection on
the cell cycle (n=3; cell %).
|
| BGC823 cells | SGC7901 cells |
|---|
|
|
|
|
|---|
| Cell cycle stage | ILF3-siRNA group | Negative control
group | ILF3-siRNA group | Negative control
group |
|---|
| G0/G1 |
73.73±4.17a | 58.04±9.10 |
66.75±4.09a | 47.89±5.79 |
| G2/M |
12.83±1.48a | 24.33±4.95 |
14.81±4.94a | 34.58±6.74 |
| S | 15.69±4.55 | 17.96±6.69 | 18.77±8.61 | 17.87±5.85 |
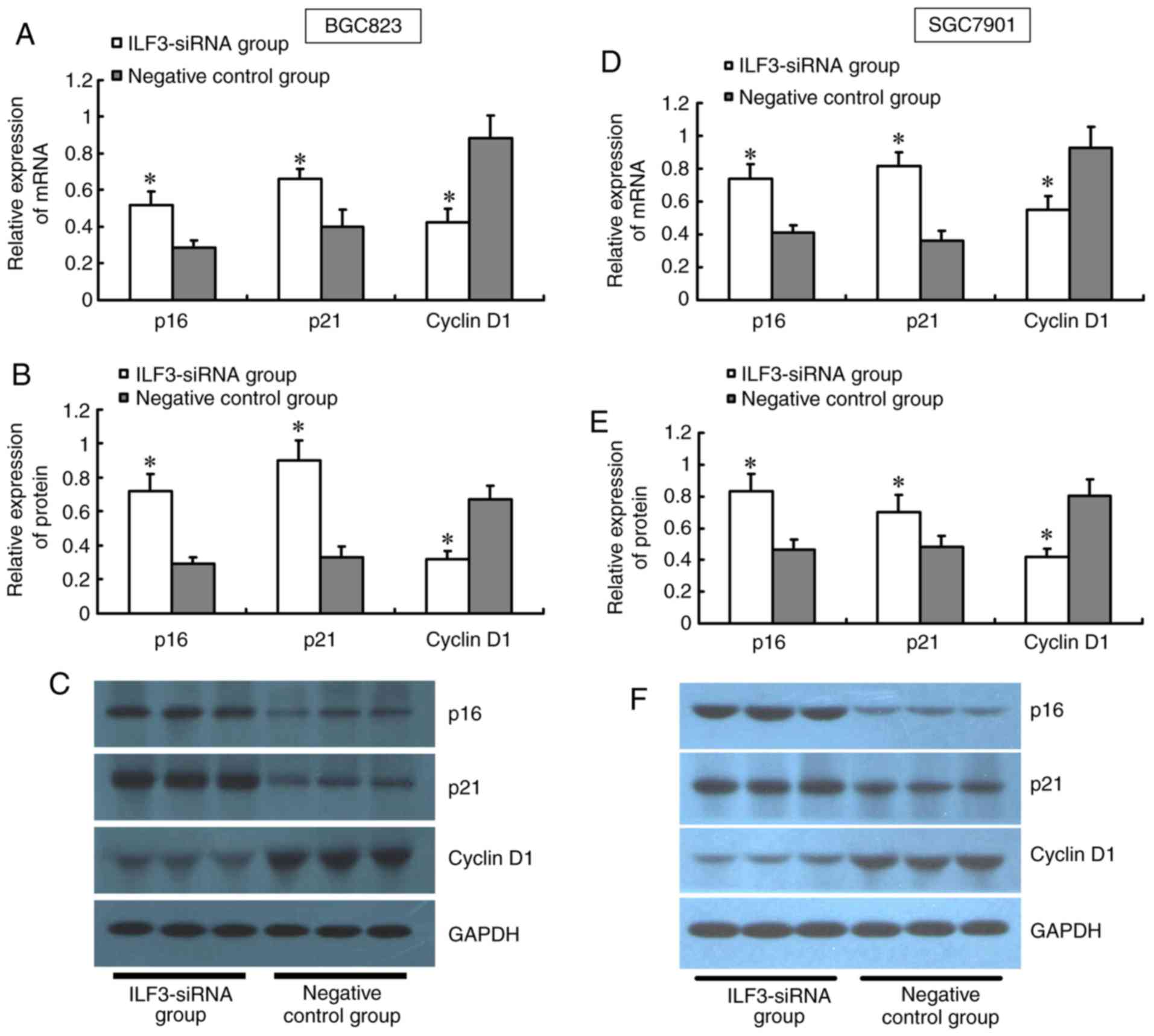
Effect of ILF3-siRNA on genes
associated with cell proliferation in BGC823 and SGC7901 cells
The results from the RT-qPCR and western blot
analyses showed a significant upregulation of p16 and p21 at the
mRNA and protein levels in the ILF3-siRNA group compared with the
control, while Cyclin D1 was significantly downregulated (Fig. 5).
Association of ILF3 protein expression
with clinicopathological characteristics, and its prognostic
value
Participants with positive ILF3 protein expression
exhibited deeper tumor infiltration and higher rates of lymph node
metastasis (both P<0.05), and no associations were detected
between ILF3 and other clinical pathological characteristics
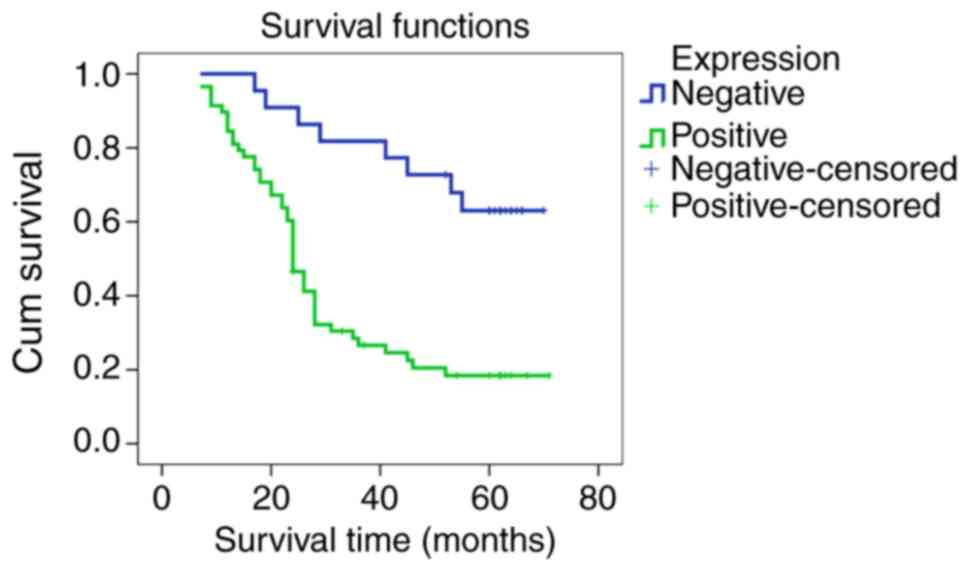
(Table III). Kaplan-Meier (K-M)
analysis for ILF3 protein indicated a lower survival rate in
participants with positive expression compared with a lack of
expression (χ2=15.683; P<0.001; Fig. 6). The results of the Cox survival
analysis demonstrated that positive expression of ILF3, TNM
staging, differentiation of cancer cells and distant metastasis
were independent risk factors for patients with GC, suggesting that
ILF3 may serve as a novel marker to predict the prognosis of
patients with GC (Table IV).
 | Table III.Association between ILF3 protein
expression and clinicopathological characteristics in gastric
cancer. |
Table III.
Association between ILF3 protein
expression and clinicopathological characteristics in gastric
cancer.
|
| ILF3 protein |
|---|
|
|
|
|---|
| Biological
characteristics (n) | Negative, n=22 | Positive, n=58 | χ2
(P-value) |
|---|
| Sex |
| Male
(55) | 13 | 42 | 1.318 (0.251) |
| Female
(25) | 9 | 16 |
|
| Age, years |
| ≥60
(25) | 5 | 20 | 1.026 (0.311) |
| <60
(55) | 17 | 38 |
|
| Diameter |
| ≥5 cm
(39) | 11 | 28 | 0.019 (0.890) |
| <5
cm (41) | 11 | 30 |
|
| Serosal
invasion |
|
Negative (31) | 15 | 16 | 11.075
(<0.001) |
|
Positive (49) | 7 | 42 |
|
| TNM staging |
| I–II
(34) | 12 | 22 | 1.802 (0.180) |
| III–IV
(46) | 10 | 36 |
|
|
Differentiation |
| Well
(55) | 12 | 43 | 2.850 (0.091) |
| Poorly
(25) | 10 | 15 |
|
| Lymphatic
metastasis |
|
Positive (52) | 10 | 42 | 5.096 (0.024) |
|
Negative (28) | 12 | 16 |
|
| Nerve/vessel
invasion |
|
Positive (45) | 12 | 33 | 0.036 (0.850) |
|
Negative (35) | 10 | 25 |
|
| Distant
metastasis |
|
Positive (7) | 3 | 4 | 0.907 (0.341) |
|
Negative (73) | 19 | 54 |
|
 | Table IV.Risk factors from Cox survival
analysis for patients with gastric cancer. |
Table IV.
Risk factors from Cox survival
analysis for patients with gastric cancer.
|
|
|
|
|
|
|
| 95.0% CI for
Exp(B) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Risk factor | B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper |
|---|
| ILF3 | 2.141 | 0.554 | 14.932 | 1 | 0.000 | 8.505 | 2.872 | 25.191 |
| Invasion | 0.764 | 0.454 | 2.826 | 1 | 0.093 | 2.146 | 0.881 | 5.230 |
| TNM stages | −1.163 | 0.475 | 5.992 | 1 | 0.014 | 0.312 | 0.123 | 0.793 |
| Lymphatic | 0.213 | 0.488 | 0.191 | 1 | 0.662 | 1.238 | 0.476 | 3.219 |
| Sex | −0.177 | 0.341 | 0.270 | 1 | 0.604 | 0.838 | 0.429 | 1.636 |
| Age | −0.054 | 0.036 | 2.291 | 1 | 0.130 | 0.947 | 0.883 | 1.016 |
|
Differentiation | 0.647 | 0.320 | 4.091 | 1 | 0.043 | 1.910 | 1.020 | 3.575 |
| Nerve vessel | −0.139 | 0.298 | 0.218 | 1 | 0.641 | 0.870 | 0.485 | 1.560 |
| Distant | 1.620 | 0.505 | 10.298 | 1 | 0.001 | 5.054 | 1.879 | 13.595 |
Discussion
GC is one of the most common cancers in China, with
high mortality due to its aggressiveness, and poor prognosis
(11–13). Although a number of genes and
signaling pathways involved in GC development have been identified
(14–17), the mechanism is still too
sophisticated to be fully understood. Identifying novel genes
closely associated with GC development is clinically necessary.
ILF3 is a transcriptional coactivator which plays a regulatory role
in the transcriptional activity of target genes (18–20).
ILF3 has been verified to be aberrantly expressed in tumorous
cells, and has been reported to be associated with tumor
proliferation and metastasis, and to act as a regulator to its
downstream genes such as Cyclins and p53 (7–9,21,22).
However, its association with GC remains unclear. In this study,
the role of ILF3 in GC was explored, and it was verified that ILF3
exhibited enhanced expression in GC tissues and cell lines, which
suggested that ILF3 may be one of the factors underlying gastric
carcinogenesis and development. ILF3 inhibition may possibly delay
the progression of GC.
In present study, we detected ILF3 in GC cell lines,
and BGC823, SGC7901 were selected to investigate the role of ILF3
in the development of GC, and the associated mechanism. After ILF3
inhibition, BGC823 and SGC7901 cells exhibited weakened cell
activity and an increased proportion of cells in the G0/G1 phase,
which indicated that ILF3 might be associated with the promotion of
gastric cancer cell proliferation. ILF3 may be a potential target
in regulating growth of GC, and it is valuable to explore the
detailed effect of ILF3 as a novel target to treat GC.
To investigate the mechanism underlying the role of
ILF3 in cell proliferation in GC, the present study further
examined the expression of candidate genes in relation to cell
growth after ILF3 suppression. p16 functions in suppressing cell
proliferation through regulation of the cell cycle (23,24).
p21 is an inhibitor of cyclin-dependent kinases (CDKs), which acts
along with the p53 gene as a regulator of the cell cycle,
participating in cancer suppression (25,26).
Cyclin D1, on the other hand, promotes cells proliferation, aiding
tumor development (27,28). In the present study, upregulation
of p16 and p21 mRNA and protein in BGC823 and SGC7901 cells were
confirmed following ILF3 suppression, while mRNA and protein of
Cyclin D1 were downregulated. This finding suggested that ILF3 is
likely to affect GC growth and progression through regulation of
such genes as p16, p21 and Cyclin D1. However, further research
into the molecular mechanism of ILF3 are needed, and the results
should be verified by more experiments in vivo.
To investigate the clinical value of ILF3 protein,
the present study analyzed the relationship between ILF3 and the
clinical and pathological characteristics of patients with GC. The
results verified that positive ILF3 expression was associated with
deeper tumor infiltration and higher rates of lymph node metastasis
in patients with GC, suggesting that ILF3 overexpression may
contribute to GC progression and metastasis. K-M prognostic
analysis demonstrated that patients with positive expression of
ILF3 protein had a lower survival rate than those with negative
expression. In addition, based on Cox survival analysis, positive
ILF3 protein expression is an independent risk factor of poor
prognosis for patients with GC. These results indicated that
positive ILF3 expression may be a novel prognostic marker for GC,
although further studies are required with more subjects, in
addition to multiple-center studies, to confirm theses preliminary
observations about the clinical value of ILF3.
The present study indicated that overexpression of
ILF3 protein in GC tissues is associated with poor prognosis in
patients with GC. Moreover, ILF3 may promote the proliferation of
GC through regulation of such genes as p16, p21 and Cyclin D1. The
limitations of this study were that the sample size was limited and
the detailed mechanism of ILF3 remains elusive. Despite these
limitations, this study verified that ILF3 plays a crucial role in
GC development. Further functional studies would be beneficial to
evaluated outcomes in GC. Also, ILF3 is likely to become a novel
therapeutic target in GC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YüL and YoL conceived, designed and supervised the
experiments. YüL, YZ, YaL, LF, NJ and QZ performed the experiments.
The data were analyzed by YüL and YoL. YüL wrote the
manuscript.
Ethics approval and consent to
participate
The research was approved by the Ethics Committee of
Hebei Medical University Fourth Affiliated Hospital and informed
consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang W, Zheng C, Fang C, Li P, Xie J, Lin
J, Zhan Y, Li W, Chen Y, Sun X, et al: Time trends of
clinicopathologic features and surgical treatment for gastric
cancer: Results from 2 high-volume institutions in southern China.
Surgery. 158:1590–1597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li ZX and Kaminishi M: A comparison of
gastric cancer between Japan and China. Gastric Cancer. 12:52–53.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bolke E, Peiper M and Budach W:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:19652008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YJ, Liu JZ, Lv P, Dang Y, Gao JY and
Wang Y: Long non-coding RNA CCAT2 promotes gastric cancer
proliferation and invasion by regulating the E-cadherin and LATS2.
Am J Cancer Res. 6:2651–2660. 2016.PubMed/NCBI
|
|
6
|
Nakadai T, Fukuda A, Shimada M, Nishimura
K and Hisatake K: The RNA binding complexes NF45-NF90 and
NF45-NF110 associate dynamically with the c-fos gene and function
as transcriptional coactivators. J Biol Chem. 290:26832–26845.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agnoletto C, Brunelli L, Melloni E,
Pastorelli R, Casciano F, Rimondi E, Rigolin GM, Cuneo A, Secchiero
P and Zauli G: The anti-leukemic activity of sodium dichloroacetate
in p53mutated/null cells is mediated by a p53-independent ILF3/p21
pathway. Oncotarget. 6:2385–2396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang W, Huang H, Ding L, Zhu P, Saiyin H,
Ji G, Zuo J, Han D, Pan Y, Ding D, et al: Regulation of cell cycle
of hepatocellular carcinoma by NF90 through modulation of cyclin E1
mRNA stability. Oncogene. 34:4460–4470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Q, Lu YY, Noh H, Hong S, Dong Z, Ding
HF, Su SB and Huang S: Interleukin enhancer-binding factor 3
promotes breast tumor progression by regulating sustained
urokinase-type plasminogen activator expression. Oncogene.
32:3933–3943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu M, Zhou Y, Jin Y, Wei XL, Wang DS, Ren
C, Bai L, Yang DJ and Xu RH: Prognostic effect of high pretreatment
neutrophil to lymphocyte ratio on survival of patients with gastric
adenocarcinoma in China. Int J Biol Markers. 30:e96–e103. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li P, He HQ, Zhu CM, Ling YH, Hu WM, Zhang
XK, Luo RZ, Yun JP, Xie D, Li YF and Cai MY: The prognostic
significance of lymphovascular invasion in patients with resectable
gastric cancer: A large retrospective study from Southern China.
BMC Cancer. 15:3702015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Q, Bi JJ, Tian YT, Feng Q, Zheng ZX
and Wang Z: Outcome after simultaneous resection of gastric primary
tumour and synchronous liver metastases: survival analysis of a
single-center experience in China. Asian Pac J Cancer Prev.
16:1665–1669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Sun Z, Han Y, Yao R, Yue L, Xu Y
and Zhang J: Rnf2 knockdown reduces cell viability and promotes
cell cycle arrest in gastric cancer cells. Oncol Lett.
13:3817–3822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei S, Li Q, Li Z, Wang L, Zhang L and Xu
Z: Correction: miR-424-5p promotes proliferation of gastric cancer
by targeting Smad3 through TGF-β signaling pathway. Oncotarget.
8:340182017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia S, Qu T, Wang X, Feng M, Yang Y, Feng
X, Ma R, Li W, Hu Y, Feng Y, et al: KIAA1199 promotes migration and
invasion by Wnt/β-catenin pathway and MMPs mediated EMT progression
and serves as a poor prognosis marker in gastric cancer. PLoS One.
12:e01750582017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Ye J, Chen Z, Wen J, Li F, Su P, Lin
Y, Hu B, Wu D, Ning L, et al: Annonaceous acetogenins mediated
up-regulation of Notch2 exerts growth inhibition in human gastric
cancer cells in vitro. Oncotarget. 8:21140–21152.
2017.PubMed/NCBI
|
|
18
|
Castella S, Bernard R, Corno M, Fradin A
and Larcher JC: Ilf3 and NF90 functions in RNA biology. Wiley
Interdiscip Rev RNA. 6:243–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Li X, Zhu W, Wang H, Mei L, Wu S,
Lin X and Han X: NF90 is a novel influenza A virus NS1-interacting
protein that antagonizes the inhibitory role of NS1 on PKR
phosphorylation. FEBS Lett. 590:2797–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jayachandran U, Grey H and Cook AG:
Nuclear factor 90 uses an ADAR2-like binding mode to recognize
specific bases in dsRNA. Nucleic Acids Res. 44:1924–1936. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wan C, Gong C, Ji L, Liu X, Wang Y, Wang
L, Shao M, Yang L, Fan S, Xiao Y, et al: NF45 overexpression is
associated with poor prognosis and enhanced cell proliferation of
pancreatic ductal adenocarcinoma. Mol Cell Biochem. 410:25–35.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masuda K, Kuwano Y, Nishida K, Rokutan K
and Imoto I: NF90 in posttranscriptional gene regulation and
microRNA biogenesis. Int J Mol Sci. 14:17111–17121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ovestad IT, Dalen I, Hansen E, Loge JL,
Dybdahl BM, Dirdal MB, Moltu P and Berland JM: Clinical value of
fully automated p16/Ki-67 dual staining in the triage of
HPV-positive women in the Norwegian Cervical Cancer Screening
Program. Cancer. 125:283–291. 2017.
|
|
24
|
Wang FL, Yang Y, Liu ZY, Qin Y and Jin T:
Correlation between methylation of the p16 promoter and cervical
cancer incidence. Eur Rev Med Pharmacol Sci. 21:2351–2356.
2017.PubMed/NCBI
|
|
25
|
Wang LL, Guo HH, Zhan Y, Feng CL, Huang S,
Han YX, Zheng WS and Jiang JD: Specific up-regulation of p21 by a
small active RNA sequence suppresses human colorectal cancer
growth. Oncotarget. 8:25055–25065. 2017.PubMed/NCBI
|
|
26
|
Li LH, Wu GY, Lu YZ, Chen XH, Liu BY,
Zheng MH and Cai JC: p21-activated protein kinase 1 induces the
invasion of gastric cancer cells through c-Jun NH2-terminal
kinase-mediated activation of matrix metalloproteinase-2. Oncol
Rep. 38:193–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng R, Liu YJ, Cui JW, Yang M, Liu XL,
Li P, Wang Z, Zhu LZ, Lu SY, Zou L, et al: Aspirin regulation of
c-myc and cyclinD1 proteins to overcome tamoxifen resistance in
estrogen receptor-positive breast cancer cells. Oncotarget.
8:30252–30302. 2017.PubMed/NCBI
|
|
28
|
Kim Y, Kim H, Park D, Lee H, Lee YS, Choe
J, Kim YM, Jeon D and Jeoung D: The pentapeptide
Gly-Thr-Gly-Lys-Thr confers sensitivity to anti-cancer drugs by
inhibition of CAGE binding to GSK3β and decreasing the expression
of cyclinD1. Oncotarget. 8:13632–13651. 2017.PubMed/NCBI
|