Introduction
Epidemiological studies have revealed that
menopausal women have a greater risk of developing dysmnesia, and
the prevalence of Alzheimer's disease in women is twice as high as
that in men (1). This sex
difference is correlated with lower levels of ovarian hormones,
particularly the potent estrogen hormone estradiol (E2) (2). The specific mechanism underlying
E2-mediated dementia is not clearly understood, although
substantial evidence indicates that an estrogen deficit leads to
glutamate toxicity, causes amyloid-β deposition, affects neural
plasticity, perturbs neurotrophic activities, evokes
neuroinflammation, and accelerates neuronal apoptosis in the brain
in menopausal women and ovariectomized (OVX) animals (3–6).
Notably, estrogen replacement therapy has been frequently used in
young women with primary ovarian insufficiency and in
postmenopausal women for preventing dementia or cognitive disorders
(7–9).
Phytoestrogens are plant-derived estrogen compounds,
which are effective substitutes for clinical estrogen usage
(10,11). The traditional Chinese medicine,
Xiaoyao San, contains Chai Hu (Bupleurum falcatum L), which
is widely used to ameliorate the postmenopausal syndrome and memory
impairment under chronic psychological stress (12–14).
The principal bioactive ingredients of Chai Hu are saikosaponins,
which comprise three major subtypes: Saikosaponin-A, saikosaponin-C
and saikosaponin-D (SSD) aglycones (15,16).
Among these subtypes, SSD (Fig. 1)
has attracted interest in medical research and clinical trials, due
to its potent bioavailability (17). For example, SSD has been reported
to significantly ameliorate postmenopausal syndrome and depressive
behaviors (18,19).
The hippocampus is pivotal for memory acquisition,
consolidation and extinction. In addition, estrogen action in the
hippocampus, particularly in the CA1 region, has important roles in
the memory process (20). The
present study aimed to determine the modulatory role of SSD in fear
memory deficit in OVX rats, as well as the underlying mechanism
relating to estrogen action in the hippocampal CA1 region. The
present findings may provide a promising therapeutic strategy for
the treatment of memory deficits, particularly in postmenopausal
women.
Materials and methods
Animals
In total, 84 adult female Sprague-Dawley rats
(weight, 180–220 g; age, 6–7 weeks) were obtained from Beijing
Vital River Laboratory Animal Technology Co., Ltd. All rats were
individually housed in cages under a 12-h light/dark cycle (lights
on at 07:00 a.m.) at a temperature of 22±2°C and a humidity of
50–65%. The rats had free access to water and rodent chow. The
experimental procedures were approved by The Institutional
Committee on The Care and Use of Animals of Nanjing University of
Chinese Medicine. Every effort was made to minimize the number of
animals used and their suffering. The experimental procedure is
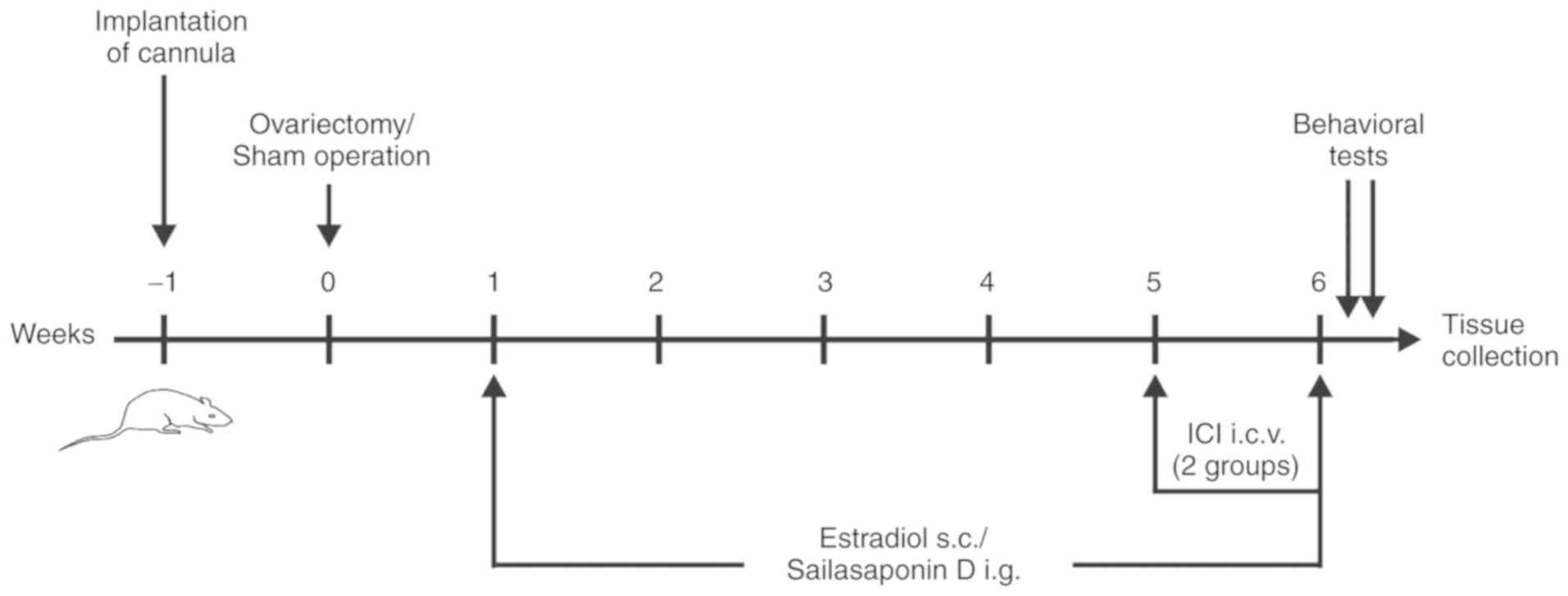
presented in Fig. 2.
Implantation of the infusion
cannula
The rats were anesthetized with sodium pentobarbital
(40 mg/kg, intraperitoneal injection; cat. no. 20021216; Sinopharm
Chemical Reagent Co., Ltd.) and were then mounted on a stereotaxic
frame (RWD Life Science Co., Ltd.) for implantation of the infusion
cannula [guide cannula: Inner diameter (ID)=0.38 mm, outer diameter
(OD)=0.56 mm; internal cannula: ID=0.36 mm, OD=0.20 mm; RWD Life
Science Co., Ltd.]. The cannula was implanted toward the right
lateral ventricle (A, −0.8; L, 1.5; H, 4.0) according to a rat
brain atlas (21). The cannula was
then anchored to the skull with dental cement and skull screws. An
osmotic mini pump was implanted subcutaneously (s.c.) on the back
and connected by a tube to the infusion cannula. After the surgery,
rats were allowed to recover for at least 7 days in their
cages.
Ovariectomy
Animals were anesthetized with sodium pentobarbital,
and the bilateral ovaries were excised through flank incisions (1.5
cm inferior to the palpated rib cage; 1–2 cm from the spine). Sham
surgery was performed similarly to the ovariectomy procedure but
without ovary removal. After the surgery, rats were placed in their
cages and allowed to recover for 7 days.
Drug preparation and drug
treatment
SSD (purity ≥95%; cat. no. S9321; Sigma-Aldrich;
Merck KGaA) was first dissolved in 12% Tween-80 (cat. no. P4780;
Sigma-Aldrich; Merck KGaA) and then diluted with 0.9% saline to a
final Tween concentration of 0.5%. E2 (cat. no. E8875;
Sigma-Aldrich; Merck KGaA) was dissolved in sesame oil. The
non-selective estrogen receptor (ER) inhibitor ICI182780 (cat. no.
1047/1; Tocris Bioscience) was first dissolved in dimethyl
sulfoxide (10 mM; cat. no. d103273; Shanghai Aladdin Biochemical
Technology Co., Ltd.) and then diluted to 0.1% by volume in
artificial cerebrospinal fluid (aCSF). The composition of the aCSF
was: NaCl 124.0 mM, KCl 3.0 mM, NaHCO3 26.0 mM,
MgCl2·6H2O 1.2 mM,
NaH2PO4·2H2O 1.2 mM,
C6H12O6 10.0 mM, CaCl2
2.0 mM. All chemical reagents were purchased from Sigma-Aldrich
(Merck KGaA).
OVX rats were randomly divided into six subgroups
(n=12/subgroup): Saline subgroup, 0.9% NaCl containing 0.5%
Tween-80 was administered via intragastric gavage (i.g.) to the
rats at the same concentration as in the other groups for 5 weeks;
O-E2 subgroup, 2 µg/kg E2 (s.c.) was administered for 5 weeks;
O-SSD 1.8 subgroup, 1.8 mg/kg SSD (i.g.) was administered for 5
weeks; O-SSD 0.9 subgroup, 0.9 mg/kg SSD (i.g.) was administered
for 5 weeks; O-SSD 1.8-ICI, 1.8 mg/kg SSD was administered for 5
weeks, with 500 µg/day ICI182780 (intracerebroventricular
injection) co-administered for the final week; and O-SSD 0.9-ICI
subgroup, 0.9 mg/kg SSD was administered for 5 weeks, with
ICI182780 co-administered for the final week. The dosages of SSD
(0.9 and 1.8 mg/kg) used in the current study were based on
previous reports, on the basis of sufficient behavioral
discrimination and an absence of significant side effects (19,22,23).
Rats in the sham group (n=12) underwent no drug treatment. The
experimental procedure is shown in Fig. 2. All drugs were administered with a
comparable volume once per day at 8:00 a.m.
Contextual fear conditioning test
Fear conditioning tests were performed in a chamber
composed of a plexiglas box (27×31×36 cm) with stainless steel
grids (diameter, 5 mm; pitch, 15.7 mm) on the floor (Coulbourn
Instruments). Each rat was placed in the chamber and subjected to a
2-min adaptation period followed by a 2-min preconditioning phase
(without any stimulation) in which the freezing time was measured.
During the conditioning period, a tone (80 dB) was presented as the
conditioned stimulus for 20 sec, and then a foot shock (0.5 mA) was
delivered as an unconditioned stimulus during the last 2 sec of the
tone stimulus. After 10 tone-shock pairs with 60 sec interstimulus
intervals (all animals exhibited >12 sec freezing in a 2-min
period), the rats were returned to their cages. Context-dependent
tests were performed 1 and 24 h after the conditioning, in which
the freezing time was measured in the same contextual conditions,
but without any stimulation, for 3 min. Freezing time (%)=freezing
time/total measurement time (24).
ELISA and western blot analysis
Nine rats randomly selected from each group were
sacrificed on the day after behavioral tests under anesthesia at
8:00-9:00 a.m. The skulls were then dissected, and the bilateral
hippocampi were quickly removed and placed on ice. The hippocampal
CA1 areas were rapidly extracted and cut into ~1×1×0.5
mm3 sections according to the brain atlas (21); these sections were immediately
frozen in liquid nitrogen.
Samples from six rats in each group were analyzed by
ELISA (cat. no. KGE014; R&D Systems, Inc.), in order to
determine the content of E2 in the hippocampus, according to the
manufacturer's protocol. Absorbance was measured at 450 nm with a
microplate reader.
Samples from the remaining three rats in each group
were used for western blot analysis. Tissue proteins were extracted
with 5% RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology), and centrifuged at 13,500 × g for 15 min at 4°C,
and the protein concentration was determined using a Pierce
bicinchoninic protein assay kit (cat. no. 23223; Thermo Fisher
Scientific Inc.). Equal amounts of protein (30 µg/lane) were
separated by 7.5–12% SDS-PAGE (cat. nos. 1610171TA and 1610175TA;
Bio-Rad Laboratories, Inc.) and transferred to PVDF membranes (cat.
no. IPVH00010; EMD Millipore). The membranes were incubated with
TBS containing 0.05% Tween-20 (TBS-T; cat. no. SRE0031;
Sigma-Aldrich; Merck KGaA) and 5% non-fat dry milk for 2 h at room
temperature to block nonspecific binding and were immunoblotted at
4°C overnight with the following primary antibodies: Rabbit
anti-ERα (1:1,000, cat. no. 21244-1-AP; Proteintech Group, Inc.),
rabbit anti-ERβ, (1:1,000, cat. no. 14007-1-AP; Proteintech Group,
Inc.), rabbit anti-GAPDH (1:1,000, cat. no. AP0063; Bioworld
Technology, Inc.). After being washed by TBS-T, the membranes were
incubated with a horseradish peroxidase-conjugated secondary
antibody goat anti-rabbit immunoglobulin G (1:5,000, cat. no.
BS13278; Bioworld Technology, Inc.) at room temperature for 2 h.
Subsequently, the membranes were washed in TBS-T, and were
developed with an enhanced chemiluminescence kit (cat. no.
NEL104001EA; PerkinElmer, Inc.). Each experiment was performed
independently at least three times, and the integrated intensity
was measured using Image-Pro-Plus 6.0 (Media Cybernetics,
Inc.).
Immunohistochemistry and Nissl
staining
In each group, tissues from the remaining three rats
were processed for immunohistochemistry, according to our
previously described procedure (25). Briefly, the rats were deeply
anesthetized and transcardially perfused with 4% paraformaldehyde
(cat. no. AR1068; Wuhan Boster Biological Technology, Ltd.). The
brains were extracted, postfixed in 4% paraformaldehyde at 4°C for
24 h and embedded in paraffin; subsequently, samples were cut into
5-µm sections. The three sections containing the hippocampal CA1
region were incubated with 3% hydrogen peroxide (cat. no. AR1108;
Wuhan Boster Biological Technology, Ltd.) at room temperature for
10 min, 10% goat serum (cat. no. ab7481; Abcam) at 37°C for 30 min.
Subsequently, sections were incubated with the primary antibodies
rabbit anti-ERα (1:200; cat. no. 21244-1-AP; Proteintech Group,
Inc.) or rabbit anti-ERβ (1:200, cat. no. 14007-1-AP; Proteintech
Group, Inc.) at 4°C overnight and the biotinylated secondary
antibody goat anti-rabbit IgG (1:500, cat. no. BS13278; Bioworld
Technology, Inc.) were incubated at room temperature for 30 min.
The sections were subsequently counterstained with hematoxylin
(0.5%; cat. no. H104302; Shanghai Aladdin Biochemical Technology
Co., Ltd.) at room temperature for 3 min.
The adjacent slices were selected for Nissl staining
(0.5% thionine; cat. no. 78338-22-4; Sigma-Aldrich; Merck KGaA;
37°C, 10 min), and micrographs of the hippocampal CA1 region were
captured under a light microscope (Axio Vert A1; Carl Zeiss AG).
Nissl-labeled neurons were calculated from two or three random
fields per slice, and technicians blinded to the samples manually
made the measurements. Briefly, in each Nissl-stained slide
(magnification, ×400) the number of neurons was counted in a
calibrator (125×125 µm) and the density (cells/mm2) was
subsequently calculated. The criterion for acceptance as a neuron
was clear staining of a soma and a nucleus, which were distinctly
differentiated from their backgrounds (25).
Statistical analysis
Data are expressed as the means ± SEM. The
significance of the differences between groups was evaluated by
one-way analysis of variance (ANOVA) followed by Fisher's least
significant difference post hoc test using SPSS version 16.0 (SPSS
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
SSD markedly rescues
ovariectomy-induced fear memory deficit
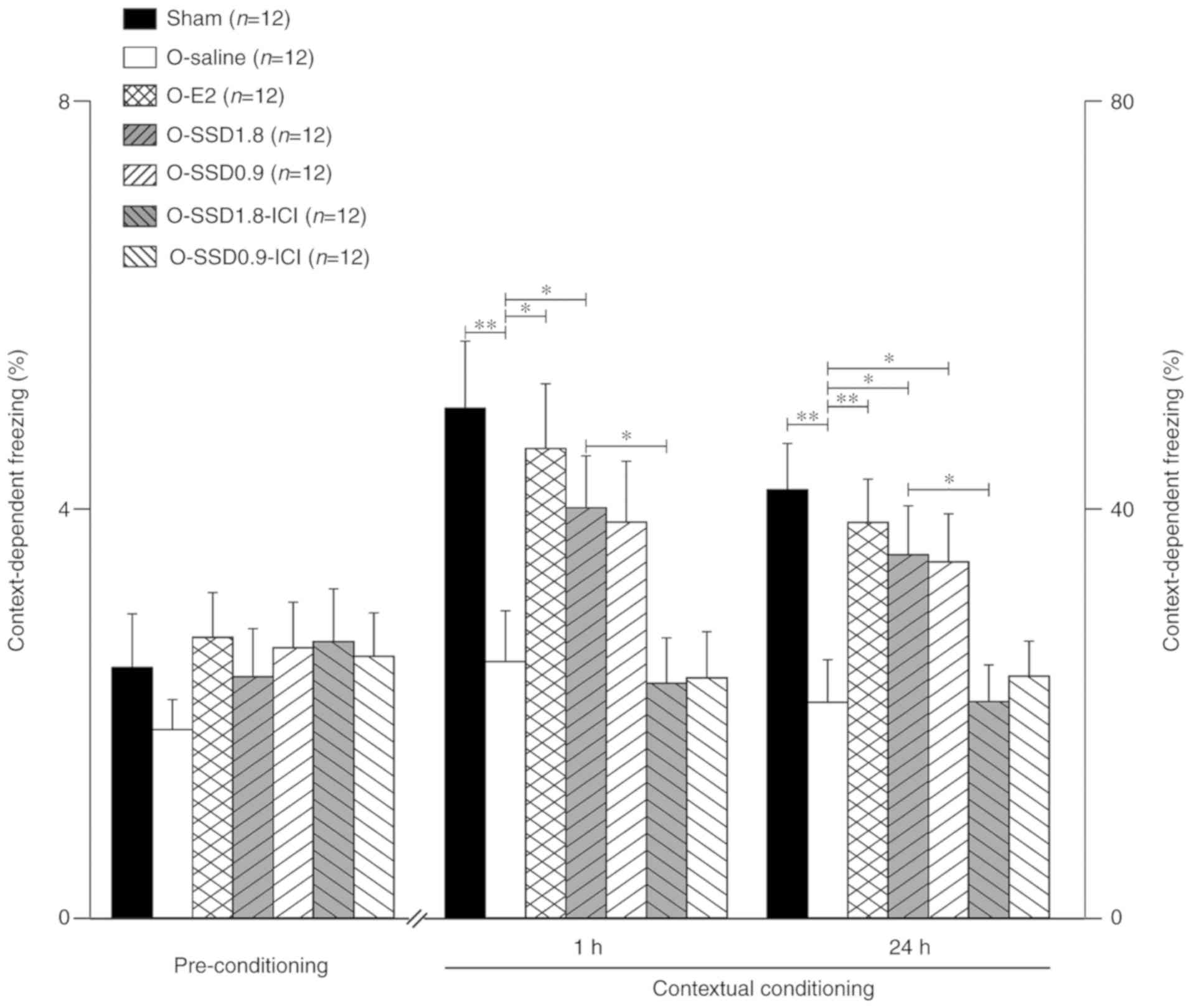
Among all groups (sham, O-saline, O-E2, O-SSD 1.8,
O-SSD 0.9, O-SSD 1.8-ICI and O-SSD 0.9-ICI), the mean freezing
fraction prior to conditioning exhibited no significant differences
(F6,77=0.47, P=0.65, one-way ANOVA; Fig. 3). Compared with the sham operation
group, ovariectomy significantly shortened the freezing time
(P<0.01, O-saline vs. sham; Fig.
3); this finding is in agreement with results from a previous
study (26). However, the freezing
time was markedly prolonged in OVX animals after E2 administration
at 1 or 24 h (P<0.05 or P<0.01, O-E2 vs. O-saline; Fig. 3), thus indicating that the memory
loss in OVX rats was due to an estrogen deficit. Notably, treatment
with SSD (SSD 1.8 and SSD 0.9) exerted a similar effect to E2
administration, which lasted for at least 24 h despite slight
attenuation (Fig. 3).
Intracerebroventricular administration of ICI182780
markedly blocked the increase in freezing time in the O-SSD 1.8
group at 1 h (P<0.05 O-SSD 1.8-ICI vs. O-SSD 1.8) and 24 h
(P<0.05, O-SSD 1.8-ICI vs. O-SSD 1.8; Fig. 3), and there was also a clear
tendency toward reduced freezing in the O-SSD 0.9-ICI group
(Fig. 3). These results indicated
that ERs may be involved in SSD-mediated effects.
SSD does not affect E2 levels in the
hippocampus of OVX rats
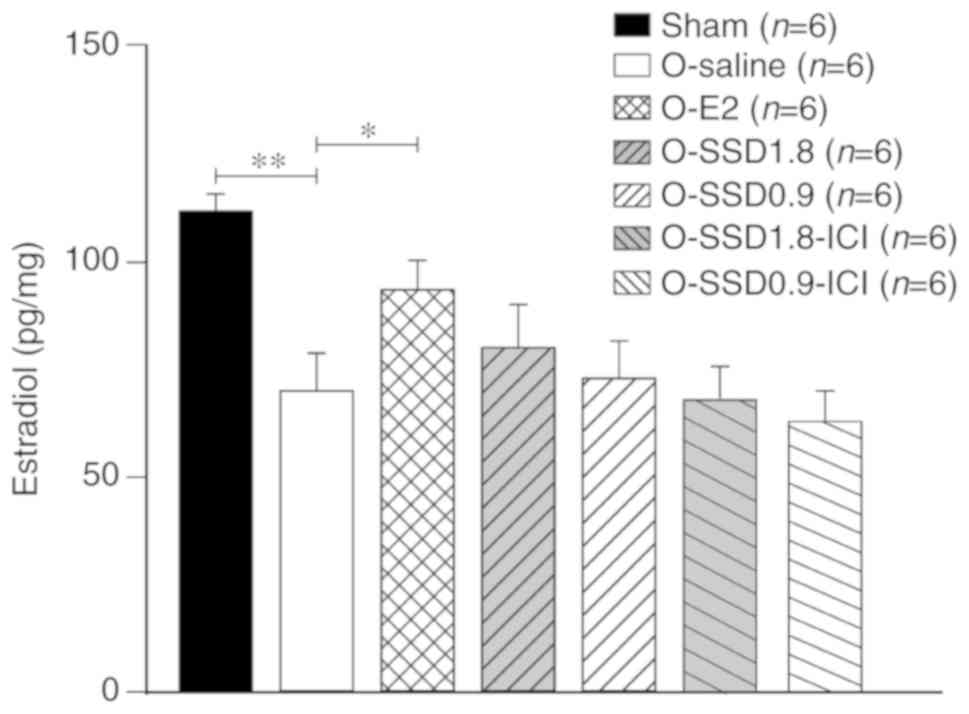
The E2 levels in the hippocampus were significantly
lower in OVX rats compared with in sham-operated animals
(P<0.01, O-saline vs. sham; Fig.
4), whereas this decrease was reversed following E2
administration (P<0.05, O-E2 vs. O-saline; Fig. 4). However, SSD treatment (O-SSD 1.8
or O-SSD 0.9) exhibited no influence on E2 levels (P=0.46 and
P=0.82, respectively, for O-SSD 1.8 or O-SSD 0.9 vs. O-saline;
Fig. 4). In addition, the ER
inhibitor ICI182780 did not influence E2 levels (P=0.22, O-SSD
1.8-ICI vs. O-SSD 1.8; P=0.59, O-SSD 0.9-ICI vs. O-SSD 0.9;
Fig. 4). These results suggested
that the SSD-mediated improvement in memory deficit in OVX rats may
not be attributed to E2 alterations in the hippocampus.
SSD enhances hippocampal expression of
ERα, but not ERβ, in OVX rats
As shown in Fig. 5,
ERα and ERβ were widely expressed in hippocampal neurons; this has
also been reported by previous studies (27–29).
ERα was present in both the extranuclear (cytoplasmic regions) and
intranuclear sites of the neurons, with prominent extranuclear
expression (Fig. 5Aa). In
addition, ERβ was mainly expressed in the extranuclear sites
(Fig. 5Bb), which is in line with
previous reports (30–32). The staining intensities for ERs
exhibited obvious differences among the groups. Western blotting
was conducted for semi-quantitative analysis of ER expression
(Fig. 5C). ERα exhibited a
significant downregulation in the hippocampus of OVX rats
(P<0.01, O-saline vs. sham; Fig.
5D), whereas E2 supplementation substantially reversed this
downregulation (P<0.01, O-E2 vs. O-saline; Fig. 5D). Notably, ERα expression in the
hippocampus was also significantly enhanced by SSD treatment in the
O-SSD 1.8 (P<0.01, O-SSD 1.8 vs. O-saline; Fig. 5D) and O-SSD 0.9 groups (P<0.05,
O-SSD 0.9 vs. O-saline; Fig. 5D)
of OVX rats. Conversely, ERβ expression exhibited no significant
differences among these groups (sham, O-saline, O-E2, O-SSD 1.8 and
O-SSD 0.9) by one-way ANOVA (F4,10=0.75, P=0.58;
Fig. 5D). These findings indicated
that SSD activated ERα expression in the hippocampus of OVX rats.
Furthermore, ERα and ERβ expression in the O-SSD groups was
markedly inhibited by ICI182780 intervention (P<0.01, O-SSD
1.8-ICI vs. O-SSD 1.8 and O-SSD 0.9-ICI vs. O-SSD 0.9; Fig. 5D), confirming that the inhibitor
ICI182780 had suppressive effects on ERα and ERβ.
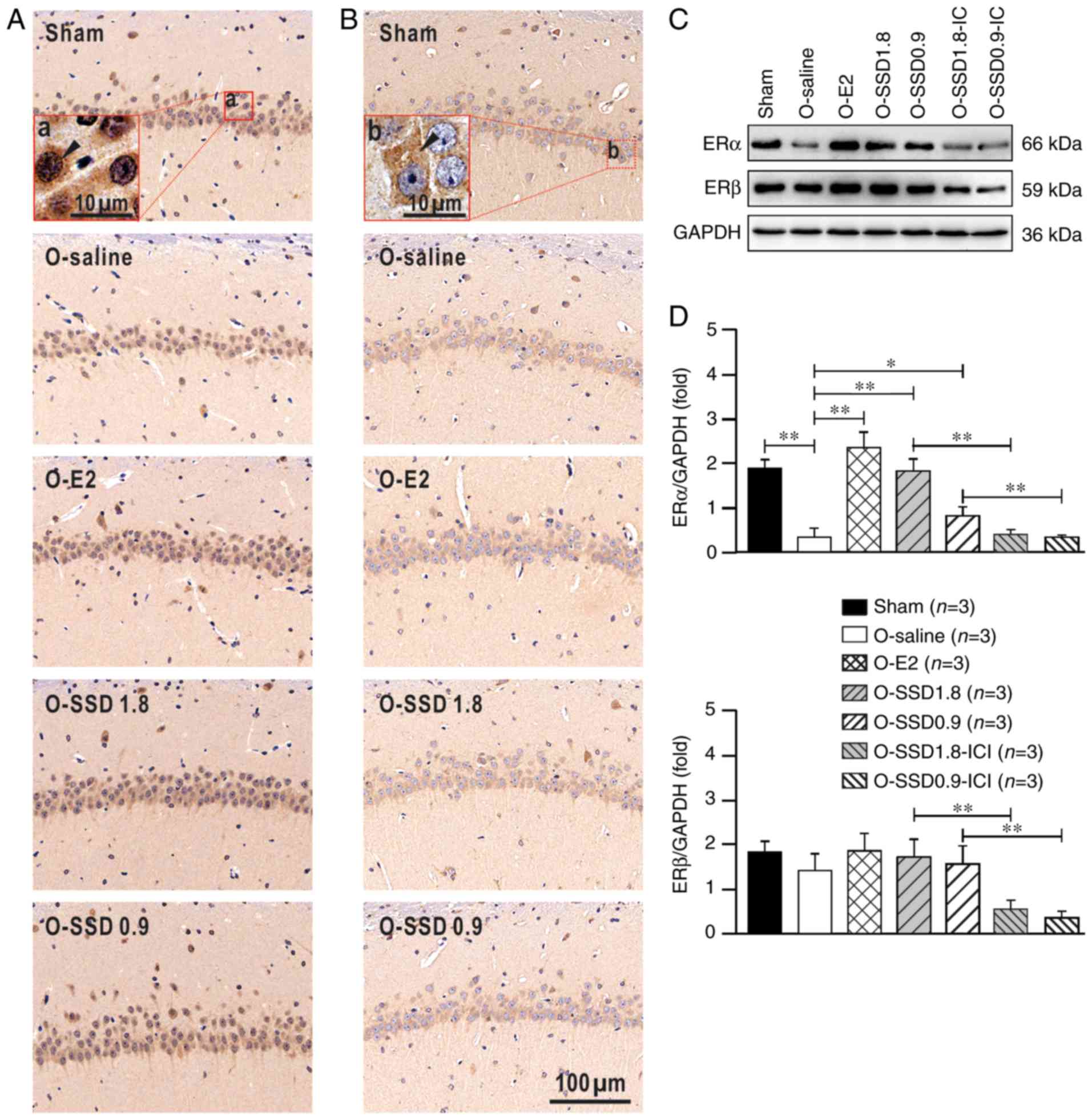 | Figure 5.ERα and ERβ expression in the
hippocampal CA1 region among groups. (A and B) Immunohistochemical
staining. Sections demonstrated that in the hippocampal CA1 region,
ERα was present in extranuclear and nuclear sites, whereas ERβ was
present in extranuclear sites. (a) The red box represents a higher
magnification, and the black triangle indicates an ERα-positive
neuron; (b) the red box represents a higher magnification, and the
black triangle indicates an ERβ positive neuron. (C and D) Western
blot analysis for semi-quantitative analysis of ERα and ERβ
expression, whose bands were 66 and 59 kDa, respectively. The
optical density of ERα or ERβ was normalized to GAPDH. Data are
presented as the means ± SEM. *P<0.05; **P<0.01, one-way
analysis of variance and Fisher's least significant difference post
hoc test. Scale bars: (A and B) 100 µm; (a and b) 10 µm. E2,
estradiol; ER, estrogen receptor; ICI, ICI182780; O,
ovariectomized; SSD, saikosaponin-D. |
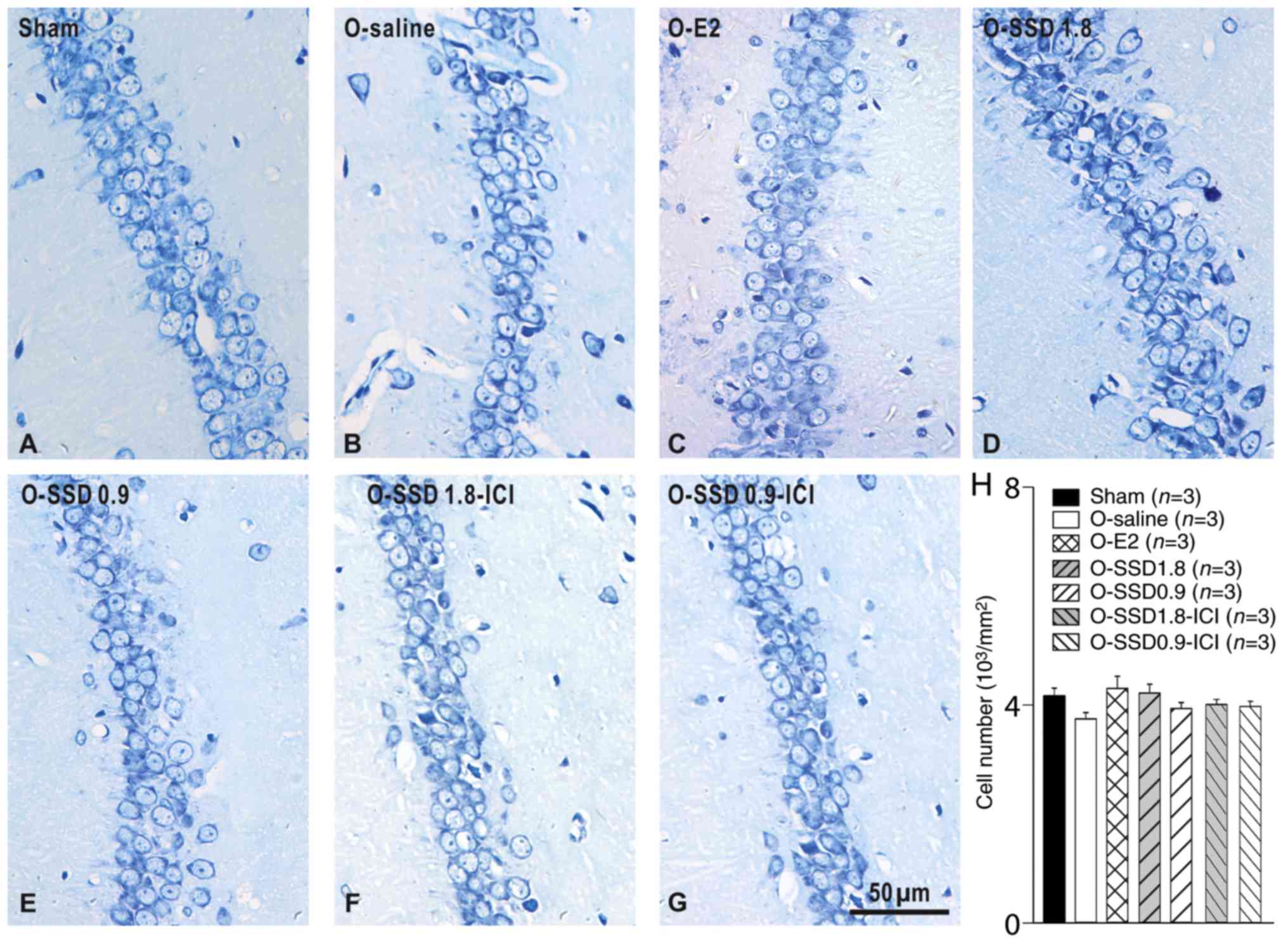
SSD does not affect the number of
neurons in the hippocampus of OVX rats
Given that neuron number conclusively influences
neural functioning in the nervous system, this study aimed to
determine whether SSD-induced behavioral improvement in OVX rats
may be associated with changes in neuron number. As shown in
Fig. 6A-H, the number of neurons
in the hippocampus exhibited no significant alterations among the
groups (sham, O-saline, O-E2, O-SSD 1.8, O-SSD 0.9, O-SSD 1.8-ICI
and O-SSD 0.9-ICI; F6,119=1.429, P=0.209; Fig. 6H), thus suggesting that
SSD-mediated improvements in memory deficits in OVX rats were not
attributed to changes in the number of neurons.
Discussion
Estrogen serves important roles in the central
nervous system and is involved in various processes, including
neuronal differentiation, synaptic formation and neural repair
(33–35). Accordingly, estrogen deficits lead
to substantial neural malfunction, particularly cognitive
impairment (36,37). The hippocampus is a crucial center
for cognitive processes, which is extensively modulated by
estrogen; furthermore, estrogen disorders often lead to cognitive
impairment, including fear memory deficits (38,39).
In the present study, contextual fear conditioning was used to
reveal the hippocampal action of estrogen involved in fear
memory.
E2 levels in the hippocampus are markedly decreased
in OVX rats, probably due to a local decrease in estrogenic
synthesis (40). The present
results demonstrated that decreased E2 in the hippocampus was
associated with fear memory impairment in OVX rats. Notably, this
impairment was reversed by E2 supplementation, in a manner
reminiscent of clinical estrogen replacement therapy for menopausal
women or OVX rats (34,36,41,42).
However, E2 in the hippocampus exerts distinct roles under
different conditions; for example, E2 serves an active role at low
doses, whereas higher doses exert a suppressive effect on working
memory (43,44). Notably, the phytoestrogen SSD has a
similar chemical structure and physiological function to E2, and is
frequently used as a substitute for estrogen in clinical trials. In
the present study, it was revealed that SSD prolonged freezing time
in OVX rats in a manner similar to E2. However, the E2-mediated
improvement in memory deficit was associated with elevated hormone
levels, whereas the SSD-induced improvement was independent of E2
levels in the hippocampus of OVX rats. In addition, SSD did not
exert any influence on serum E2 levels in OVX rats (Liu et
al, unpublished data), strongly indicating that the SSD-rescued
memory deficit in OVX rats does not rely on E2 levels.
Although ERs are expressed in neurons and glial
cells, the expression is highly distinct among different brain
regions (30). ERs in the
hippocampal CA1 area are mainly expressed in neurons (27–29).
In the current study, immunohistochemistry was performed to discern
the cytoplasmic and nuclear distribution of ERs, and western
blotting was used to semi-quantify protein expression. The results
revealed that ERα and ERβ were expressed in the hippocampus;
however, ERβ expression in the hippocampal CA1 region remained
unchanged in OVX rats. This outcome may be associated with elevated
serum corticosterone (Liu et al, unpublished data), since E2
deficiency stimulates the hypothalamic-pituitary-adrenal axis to
release corticosterone, which subsequently counteracts the decrease
in ERβ (45,46). In addition, SSD treatment did not
affect ERβ protein expression. Conversely, significant alterations
in ERα expression were detected in the hippocampus of OVX rats
following SSD treatment. ERα has frequently been reported to be
associated with spine structure and/or postsynaptic functions
(47,48); for example, selective activation of
ERα in OVX mice results in an increased spine density in the
hippocampus, which is inversely correlated with memory dysfunction
(49). In particular, ICI182780, a
non-selective inhibitor of ERs, markedly blocked the memory
improvement induced by SSD in OVX rats, thus corroborating a
mechanism of SSD-mediated memory restoration via ERs. Previous
studies reported that ovariectomy stimulates ERα promoter
methylation, which in turn inhibits ERα expression (47,50).
Therefore, it may be hypothesized that SSD prevents ERα promoter
methylation and induces ER mRNA expression, thus resulting in
upregulation of ERα protein expression in OVX rats. In the present
study, ovariectomy did not alter the number of neurons in the
hippocampus; this was also the case in a previous study (46). In addition, neither SSD nor E2
supplementation affected neuron number. Therefore, SSD-induced
activation of ERα in hippocampal neurons may be a potential
mechanism underlying SSD-improved fear deficits in OVX rats.
Despite the novel findings of the present study,
several limitations must be solved for further improvement.
Firstly, because the amygdala serves a pivotal role in triggering a
state of fear, and is also involved in the modulation of memory
consolidation (51), a thorough
investigation of the neural circuit for SSD-mediated promotion in
fear memory deficit is worthy of future consideration. Secondly,
although this study revealed that SSD differentially upregulates ER
expression in the hippocampus, whether SSD modulates neuronal
activities, and whether ERα and ERβ have different roles in the
hippocampus in OVX animals, remains to be determined. Thirdly, the
present study, along with various previous reports, suggested that
a suitable low dosage of SSD may benefit neurological functions
(19,52), whereas higher concentrations of SSD
may cause neurotoxicity (22,53);
although the different concentrations of SSD activate distinct
signal pathways (54), further
research is required to clarify the precise mechanisms.
In conclusion, this study demonstrated that the
phytoestrogen SSD rescued fear memory deficit in OVX rats. The
results suggested that this effect may be mediated through
activation of ERα, rather than ERβ or E2, in the hippocampus.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant nos. BK20140959 and
BK20151008); the National Natural Science Foundation of China
(grant nos. 81603578 and 81503536); the Natural Science Foundation
of Universities in Jiangsu Province (grant no. 15KJB360008); the
Key Laboratory Fund of Philosophy and Social Sciences of
Universities in Guangdong Province (Psychological Assessment and
Rehabilitation for Exceptional Children; grant no.
2017SYSYJ01).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LL and CZ conceived and designed the study. LL, JY
and XX performed the experiments. HS, FG, JL SL and YZ analyzed the
data and revised the manuscript. LL, JY and CZ wrote the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All experimental protocols involving animal tissue
samples were approved by the Ethics Committee of Nanjing University
of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heber LE, Weuve J, Scherr PA and Evans DA:
Alzheimer disease in the United States (2010–2050) estimated using
the 2010 census. Neurology. 80:1778–1783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simpkins JW, Green PS, Gridley KE, Singh
M, de Fiebre NC and Rajakumar G: Role of estrogen replacement
therapy in memory enhancement and the prevention of neuronal loss
associated with Alzheimer's disease. Am J Med. 103:S19–S25. 1997.
View Article : Google Scholar
|
|
3
|
Correia SC, Santos RX, Cardoso S, Carvalho
C, Santos MS, Oliveira CR and Moreira PI: Effects of estrogen in
the brain: Is it a neuroprotective agent in Alzheimer's disease?
Cur Aging Sci. 3:113–126. 2010. View Article : Google Scholar
|
|
4
|
Pompili A, Arnone B and Gasbarri A:
Estrogens and memory in physiological and neuropathological
conditions. Psychoneuroendocrino. 37:1379–1396. 2012. View Article : Google Scholar
|
|
5
|
Bonomo SM, Rigamonti AE, Giunta M,
Galimberti D, Guaita A, Gagliano MG, Müller EE and Cella SG:
Menopausal transition: A possible risk factor for brain pathologic
events. Neurobiol Aging. 30:71–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karisetty BC, Maitra S, Wahul AB,
Musalamadugu A, Khandelwal N, Guntupalli S, Garikapati R,
Jhansyrani T, Kumar A and Chakravarty S: Differential effect of
chronic stress on mouse hippocampal memory and affective behavior:
Role of major ovarian hormones. Behav Brain Res. 318:36–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sullivan SD, Sarrel PM and Nelson LM:
Hormone replacement therapy in young women with primary ovarian
insufficiency and early menopause. Fertil Steril. 106:1588–1599.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osmanovic-Barilar J and Salkovic-Petrisi
M: Evaluating the role of hormone therapy in postmenopausal women
with Alzheimer's disease. Drug Aging. 33:787–808. 2016. View Article : Google Scholar
|
|
9
|
Lewis CE and Wellons MF: Menopausal
hormone therapy for primary prevention of chronic disease. JAMA.
318:21872017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kennedy DO and Scholey AB: The
psychopharmacology of European herbs with cognition-enhancing
properties. Curr Pharm Design. 12:4613–4623. 2006. View Article : Google Scholar
|
|
11
|
Atteritano M, Pernice F, Mazzaferro S,
Mantuano S, Frisina A, D'Anna R, Cannata ML, Bitto A, Squadrito F,
Frisina N and Buemi M: Effects of phytoestrogen genistein on
cytogenetic biomarkers in postmenopausal women: 1 year randomized,
placebo-controlled study. Eur J Pharmacol. 589:22–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang YH, Chen PC, Wang JD, Lee CH and Lai
JN: Prescription pattern of traditional Chinese medicine for
climacteric women in Taiwan. Climacteric. 12:541–547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee B, Shim I, Lee H and Hahm DH: Effect
of Bupleurum falcatum on the stress-induced impairment of
spatial working memory in rats. Biol Pharm Bull. 32:1392–1398.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen JX, Ji B, Lu ZL and Hu LS: Effects of
chai hu (radix burpleuri) containing formulation on plasma
beta-endorphin, epinephrine and dopamine on patients. Am J Chinese
Med. 33:737–745. 2005. View Article : Google Scholar
|
|
15
|
Morinaga O, Zhu S, Tanaka H and Shoyama Y:
Visual detection of saikosaponins by on-membrane immunoassay and
estimation of traditional Chinese medicines containing Bupleuri
radix. Biochem Bioph Res Commun. 346:687–692. 2006. View Article : Google Scholar
|
|
16
|
de Oliveira DR, Zamberlam CR, Rêgo GM,
Cavalheiro A, Cerutti JM and Cerutti SM: Effects of a
flavonoid-rich fraction on the acquisition and extinction of fear
memory: Pharmacological and molecular approaches. Front Behav
Neurosci. 9:3452016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aoyagi H, Kobayashi Y, Yamada K, Yokoyama
M, Kusakari K and Tanaka H: Efficient production of saikosaponins
in Bupleurum falcatum root fragments combined with signal
transducers. Appl Microbiol Biot. 57:482–488. 2001. View Article : Google Scholar
|
|
18
|
Lin J, Zhu J, Wang Y, Zhang N, Gober HJ,
Qiu X, Li D and Wang L: Chinese single herbs and active ingredients
for postmenopausal osteoporosis: From preclinical evidence to
action mechanism. Biosci Trends. 11:496–506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li HY, Zhao YH, Zeng MJ, Fang F, Li M, Qin
TT, Ye LY, Li HW, Qu R and Ma SP: Saikosaponin D relieves
unpredictable chronic mild stress induce d depressive-like behavior
in rats: Involvement of HPA axis and hippocampal neurogenesis.
Psychopharmacology (Berl). 234:3385–3394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McEwen B, Akama K, Alves S, Brake WG,
Bulloch K, Lee S, Li C, Yuen G and Milner TA: Tracking the estrogen
receptor in neurons: Implications for estrogen-induced synapse
formation. Proc Natl Acad Sci USA. 98:7093–7100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paxinos G and Watson C: The rat brain in
stereotaxic coordinates. Academic Press/Elsevier; Amsterdam;
Boston: 2007,
|
|
22
|
Dang SS, Wang BF, Cheng YA, Song P, Liu ZG
and Li ZF: Inhibitory effects of saikosaponin-d on CCl4-induced
hepatic fibrogenesis in rats. World J Gastroentero. 13:557–563.
2007. View Article : Google Scholar
|
|
23
|
Lu XL, He SX, Ren MD, Wang YL, Zhang YX
and Liu EQ: Chemopreventive effect of saikosaponin-d on
diethylinitrosamine-induced hepatocarcinogenesis: Involvement of
CCAAT/enhancer binding protein β and cyclooxygenase-2. Mol Med Rep.
5:637–644. 2012.PubMed/NCBI
|
|
24
|
Takuma K, Mizoguchi H, Funatsu Y, Hoshina
Y, Himeno Y, Fukuzaki E, Kitahara Y, Arai S, Ibi D, Kamei H, et al:
Combination of chronic stress and ovariectomy causes conditioned
fear memory deficits and hippocampal cholinergic neuronal loss in
mice. Neuroscience. 207:261–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Sun T, Zhou P, Zhu Q and Zhang L:
Role of muscarinic acetylcholine receptor-2 in the cerebellar
cortex in cardiovascular modulation in anaesthetized rats.
Neurochem Res. 41:804–812. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mcdermott CM, Liu D, Ade C and Schrader
LA: Estradiol replacement enhances fear memory formation, impairs
extinction and reduces COMT expression levels in the hippocampus of
ovariectomized female mice. Neurobiol Learn Mem. 118:167–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shughrue PJ and Merchenthaler I: Evidence
for novel estrogen binding sites in the rat hippocampus.
Neuroscience. 99:605–612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loy R, Gerlach JL and Mcewen BS:
Autoradiographic localization of estradiol-binding neurons in the
rat hippocampal formation and entorhinal cortex. Dev Brain Res.
39:245–251. 1988. View Article : Google Scholar
|
|
29
|
Adams MM, Fink SE, Shah RA, Janssen WG,
Hayashi S, Milner TA, McEwen BS and Morrison JH: Estrogen and aging
affect the subcellular distribution of estrogen receptor-alpha in
the hippocampus of female rats. J Neurosci. 22:3608–3614. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mitterling KL, Spencer JL, Dziedzic N,
Shenoy S, McCarthy K, Waters EM, McEwen BS and Milner TA: Cellular
and subcellular localization of estrogen and progestin receptor
immunoreactivities in the mouse hippocampus. J Comp Neurol.
518:2729–2743. 2010.PubMed/NCBI
|
|
31
|
Milner TA, McEwen BS, Hayashi S, Li CJ,
Reagan LP and Alves SE: Ultrastructural evidence that hippocampal
alpha estrogen receptors are located at extranuclear sites. J Comp
Neurol. 429:355–371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Milner TA, Ayoola K, Drake CT, Herrick SP,
Tabori NE, McEwen BS, Warrier S and Alves SE: Ultrastructural
localization of estrogen receptor β immunoreactivity in the rat
hippocampal formation. J Comp Neurol. 491:81–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heberden C: Sex steroids and neurogenesis.
Biochem Pharmacol. 141:56–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hara Y, Waters EM, Mcewen BS and Morrison
JH: Estrogenzeffects on cognitive and synaptic health over the
lifecourse. Physiol Rev. 95:785–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vierk R, Bayer J, Freitag S, Muhia M,
Kutsche K, Wolbers T, Kneussel M, Sommer T and Rune GM:
Structure-function-behavior relationship in estrogen-induced
synaptic plasticity. Horm Behav. 74:139–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herrera AY, Hodis HN, Mack WJ and Mather
M: Estradiol therapy after menopause mitigates effects of stress on
cortisol and working memory. J Clin Endocrinol Metab.
102:4457–4466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Foy MR, Baudry M, Diaz BR and Thompson RF:
Estrogen and hippocampal plasticity in rodent models. J Alzheimers
Dis. 15:5892008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barha CK, Dalton GL and Galea LA: Low
doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas
higher doses of estrone and 17alpha- and 17beta-estradiol impair,
contextual fear conditioning in adult female rats.
Neuropsychopharmacol. 35:547–559. 2010. View Article : Google Scholar
|
|
39
|
Hoffman AN, Armstrong CE, Hanna JJ and
Conrad CD: Chronic stress, cyclic 17β-estradiol, and daily handling
influences on fear conditioning in the female rat. Neurobiol Learn
Mem. 94:422–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barker JM and Galea LAM: Sex and regional
differences in estradiol content in the prefrontal cortex, amygdala
and hippocampus of adult male and female rats. Gen Comp Endocr.
164:77–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rodgers SP, Bohacek J and Daniel JM:
Transient estradiol exposure during middle age in ovariectomized
rats exerts lasting effects on cognitive function and the
hippocampus. Endocrinology. 151:1194–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bean LA, Kumar A, Rani A, Guidi M, Rosario
AM, Cruz PE, Golde TE and Foster TC: Re-opening the critical window
for estrogen therapy. J Neurosci. 35:16077–16093. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Holmes MM, Wide JK and Galea LA: Low
levels of estradiol facilitate, whereas high levels of estradiol
impair, working memory performance on the radial arm maze. Behav
Neurosci. 116:928–934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wide JK, Hanratty KJ and Galea LA: High
level estradiol impairs and low level estradiol facilitates
non-spatial working memory. Behav Brain Res. 155:45–53. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei N, Yu Y, Schmidt T, Stanford C and
Hong L: Effects of glucocorticoid receptor antagonist, RU486, on
the proliferative and differentiation capabilities of bone marrow
mesenchymal stromal cells in ovariectomized rats. J Orthop Res.
31:760–767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van de Stolpe A, Slycke AJ, Reinders MO,
Zomer AW, Goodenough S, Behl C, Seasholtz AF and van der Saag PT:
Estrogen receptor (ER)-mediated transcriptional regulation of the
human corticotropin-releasing hormone-binding protein promoter:
Differential effects of ERalpha and ERbeta. Mol Endocrinol.
18:2908–2923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qu N, Zhou XY, Han L, Wang L, Xu JX, Zhang
T, Chu J, Chen Q, Wang JZ, Zhang Q and Tian Q: Combination of PPT
with LiCl treatment prevented bilateral ovariectomy-induced
hippocampal-dependent cognition deficit in rats. Mol Neurobiol.
53:894–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gujski M, Pinkas J, Wierzbińska-Stępniak
A, Owoc A and Bojar I: Does genetic testing for ERα gene
polymorphisms provide new possibilities of treatment for cognitive
function disorders in postmenopausal women? Arch Med Sci.
13:1224–1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Witty CF, Foster TC, Semple-Rowland SL and
Daniel JM: Increasing hippocampal estrogen receptor alpha levels
via viral vectors increases MAP kinase activation and enhances
memory in aging rats in the absence of ovarian estrogens. PLOS One.
7:e513852012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ianov L, Kumar A and Foster TC: Epigenetic
regulation of estrogen receptor α contributes to age-related
differences in transcription across the hippocampal regions CA1 and
CA3. Neurobiol Aging. 49:79–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Izquierdo I, Furini CR and Myskiw JC: Fear
memory. Physiol Rev. 96:695–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun X, Li X, Pan R, Xu Y, Wang Q and Song
M: Total Saikosaponins of Bupleurum yinchowense reduces depressive,
anxiety-like behavior and increases synaptic proteins expression in
chronic corticosterine-treated mice. BMC Complement Altern Med.
18:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lixing X, Zhouye J, Liting G, Ruyi Z, Rong
Q and Shiping M: Saikosaponin-d-mediated downregulation of
neurogenesis results in cognitive dysfunction by inhibiting
Akt/Foxg-1 pathwayin mice. Toxicol Lett. 284:79–85. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang P, Ren J, Tang J, Zhang D, Li B and
Li Y: Estrogen-like activities of saikosaponin-d in vitro: A pilot
study. Eur J Pharmacol. 626:159–165. 2010. View Article : Google Scholar : PubMed/NCBI
|