Introduction
Osteoporosis (OP) is a systemic bone metabolic
disorder characterized by reduced bone density and the degeneration
of bone microstructure, which results in increased bone brittleness
and fracture risk (1,2). As one of the most common bone
metabolic disorders, OP adversely affects the health and quality of
life of elderly individuals and postmenopausal women (3,4).
Numerous studies have sought to identify genes associated with OP
and sensitive genetic markers, and then use the information to
predict the occurrence and development of OP.
Several hormones, cell types and humoral factors
regulate the bone reconstruction process by promoting or inhibiting
the development of osteoblasts and osteoclasts during the
remodeling/modeling process (5–7).
Bone marrow-derived mesenchymal stem cells (BMSCs) are the source
osteoblasts in bone tissue, and affect the numbers and functional
activities of osteoblasts (8).
BMSCs also serve a major role in bone reconstruction and help
maintain a balance between bone formation and bone resorption
(9,10). A previous study reported that BMSCs
can regulate the occurrence and development of postmenopausal OP
(11). The most fundamental reason
for abnormal bone reconstruction in cases of postmenopausal OP is
functional defects of BMSCs, including decreased proliferative
activity and adipogenic differentiation ability, and increased
lipid differentiation ability (12,13).
Therefore, BMSCs were selected as target cells in which to
investigate the occurrence and development of OP.
MicroRNAs (miRNAs) are endogenous, non-coding small
RNAs composed of 21–23 nucleotides (14), which have been reported to be
involved in numerous complex cellular processes, including cell
proliferation, differentiation, division, apoptosis and gene
regulation (15,16). In recent years, miRNAs have been
shown to be involved in the occurrence, development and prognosis
of various diseases, and have also been used to diagnose and treat
diseases (17,18). miRNAs also regulate the
differentiation of mesenchymal stem cells and osteoblasts (19,20).
During the processes of bone differentiation and regeneration,
abnormal miRNA expression is closely associated with OP (21,22).
For example, the miR-34c-induced silencing of MC3T3-E1
reduces the inhibitory effect of vaspin on osteoblast
differentiation (23);
furthermore, miRNA (miR)-433-3p can regulate osteoblastic
differentiation by targeting Dickkopf-1 (DKK1) (24). As an important miRNA, miR-144 is
known to be involved in the occurrence and development of various
human diseases, including ovarian cancer (25), cervical cancer (26) and anaplastic thyroid carcinoma
(27). For example, miR-144 acts
as a tumor suppressor in osteosarcoma by inhibiting cell
proliferation and inducing apoptosis (28). miR-144 also aggravates intestinal
hyperpermeability and destroys the epithelial barrier (29). Reports have demonstrated that
mammalian target of rapamycin, occludin and zonula occludens 1 are
all target genes of miR-144 (29,30).
However, the effect of miR-144 on the occurrence and development of
OP and the associated molecular mechanisms have not been
reported.
The regulation of osteoblast differentiation is a
complex process involving multiple genes and pathways, including
the Notch signaling pathway, bone morphogenetic protein (BMP)/Smad
signaling pathway and Wnt signaling pathway (31–33).
The Wnt/β-catenin signaling pathway serves a vital role in
regulating osteoblastic differentiation and osteogenic matrix
formation (34,35); therefore, the use of Wnt/β-catenin
pathway antagonists represents a novel approach for treating OP. As
a family of secretory glycoproteins, secreted frizzled-related
proteins (SFRPs) are a class of extracellular antagonists of the
Wnt signaling pathway (36). A
previous study demonstrated that SFRPs can antagonize the Wnt
signaling pathway by competitively binding to the Frizzled receptor
in a cysteine-rich domain (37).
SFRP1 is one of the most important proteins in the SFRPs family and
a negative regulator of human osteoblast and osteocyte survival
(37). However, whether SFPR1 is
involved in the process by which miR-144 regulates OP remains to be
elucidated.
In the present study, the expression of miR-144 and
its target gene SFPR1 were detected in clinical serum
samples obtained from patients with postmenopausal OP and in
postmenopausal women with normal bone density. Subsequently,
primary bone mesenchymal stem cells (BMSCs) were isolated from
rats, and flow cytometry and EdU and Hoechst 33258 staining methods
were used to detect cell apoptosis. Following completion of an
osteoblast induction culture, a clone formation assay was used to
assess changes in the ability of cells to form clones, and Alizarin
red staining was used to determine the extent to which the BMSCs
had differentiated into osteoblasts. The mechanism by which miR-144
regulates OP was also investigated. The results not only provide a
basis for investigating the mechanism by which miR-144 is involved
in the occurrence and development of OP, but also suggest a novel
molecular target for preventing and treating OP.
Materials and methods
Collection of clinical samples
The collection and analysis of all clinical samples
was approved by the Ethics Committee of the Affiliated Hospital of
Guilin Medical University (Guilin, China; GLMUIA2016002). In total,
15 pairs of serum samples were collected from patients with
postmenopausal OP and postmenopausal women with a normal bone
mineral density. The samples were collected from January 2016 to
May 2018 at the Affiliated Hospital of Guilin Medical University.
The donors, including the postmenopausal women with OP and women
with normal bone mineral density had no other diseases, and ranged
in age between 54 and 64 years.
Isolation and culture of BMSCs
The gradient centrifugation method was used to
isolate MSCs from the bone marrow, which had been collected from
Sprague-Dawley (SD) rats. A total of 4 male SD rats (8 weeks old;
200±20 g) were specific pathogen-free (certificate no.
SCXK2011-0015), purchased from the Animal Center of Southern
Medical University (Guangzhou, China) and housed in an animal
experimental center. The rats were housed under standard conditions
(22±2°C; 40–60% relative humidity; 12:12-h light/dark cycle).
Throughout the rearing period, the rats were provided access to
food and water ad libitum. The protocols for all animal
experiments were approved by the Animal Ethics Committee of the
Affiliated Hospital of Guilin Medical University. The BM aspirates
were cultured in MEM-a medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.). Cells were cultured in a humidity incubator at
37°C with 5% CO2. Following culture for 24 h, the
suspended cells were removed and the adherent cells were cleaned
with phosphate-buffered saline (PBS) solution. The adherent cells
were then cultured for ~10 days with two changes of medium (PBS).
When the cells reached ~75% confluence, they were harvested for use
in experiments.
Transfection
To achieve changes in miR-144 expression, BMSCs
(2×104 cells/ml, 200 µl) were seeded in 48-well plates
and transfected with miR-144 mimic (50 nmol/l) or inhibitor (50
nmol/l; Guangzhou Ribobio Co., Ltd., Guangzhou, China) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as a transfection reagent, according to the
manufacturer's instructions. Following incubation for 6 h, cells
were cultured in MEM-a complete medium for 48 h. The miR-144 mimic
and miR-144 inhibitor were used to achieve the overexpression and
knockdown of miR-144, respectively. BMSCs treated with
Lipofectamine 2000 reagent alone (Mock) were used as control cells.
The sequences of the miR-144 mimic, miR nonsense strand negative
control (NC) and miR-144 inhibitor were as follows: Mimic, sense
5′-UACAGUAUAGAUGAUGUACU-3′; miR NC strand, sense
5′-UUCUCCGAACGUGUCACGUTT-3′; inhibitor, sense
5′-AGUACAUCAUCUAUACUGUA-3′. To achieve changes in the expression of
Sfrp1, the BMSCs were treated with Sfrp1 small interfering (si)RNA
(5 pmol/l) (three Sfrp1 siRNAs purchased from Sangon Biotech Co.,
Ltd., Shanghai, China) or a nonsense strand NC. Following
incubation for 6 h, cells were cultured in MEM-a complete medium
for 48 h. The sequences of the SFRP1 siRNAs and control siRNA were
as follows: NC of siRNA, sense 5′-UUCUCCGAACGUGUCACGUTT-3′; siRNA-1
(789–811) sense 5′-GCCACAACUUCCUCAUCAUUU-3′; siRNA-2 (561–583)
sense 5′-CGUGUGACAACGAGUUGAAUU-3′; siRNA-3 (836–858) sense
5′-CUCACAGCCAUUCACAAGUUU-3′. The sfrp1 siRNAs were used to knock
down the expression of Sfrp1. siRNA-2 (siSfrp1-2) was selected for
use in subsequent knockdown experiments following initial
characterization of the three siRNAs. Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot methods were used to detect transfection
efficiency.
Dual luciferase reporter assay
The binding site for miR-144 in Sfrp1 mRNA was
predicted by using TargetScan release 7.2 (http://www.targetscan.org/vert_72/), and the results
indicated that Sfrp1 was a target gene for miR-144. To
confirm that Sfrp1 is a target gene for miR-144, 293T cells
(2×104 cells/ml, 200 µl; American Type Culture
Collection, Manassas, VA, USA) were seeded in 48-well plates and
transfected with 400 ng wild-type Sfrp1 3′-UTR (WT-Sfrp1) or mutant
Sfrp1 3′-UTR (MUT-Sfrp1) cloned into the psiCHECK2 plasmid (Promega
Corporation, Madison, WI, USA) together with 50 nmol/l miR-144
nonsense strand or mimic using Lipofectamine 2000. Following
incubation for 6 h, 293T cells were cultured in DMEM complete
medium for 48 h, and then luciferase activity was detected with a
dual-luciferase reporter assay (Promega Corporation). The relative
luciferase activity was calculated using firefly luciferase
activity as an internal control.
RT-qPCR analysis
The expression levels of miR-144, Sfrp1 and
Runt-related transcription factor 2 (Runx2) were detected by
RT-qPCR analysis. Total RNA was extracted from serum with a
GenElute™ Plasma/Serum RNA Purification Mini kit (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) according to the manufacturer's
instructions, and then transcribed into cDNA with the use of a
Bestar qPCR RT kit (DBI Bioscience, Ludwigshafen, Germany). RT was
conducted as follows: 37°C for 15 min and 98°C for 5 min. qPCR was
performed using a Bestar™ qPCR Master Mix (DBI Bioscience) under
the following conditions: 95°C for 2 min, followed by 40 cycles of
94°C for 20 sec, 58°C for 20 sec and 72°C for 20 sec, and finally
extension at 72°C for 4 min. The RT-qPCR analysis was performed on
an Agilent Stratagene Mx3000P Sequence Detection system (Agilent
Technologies, Inc., Santa Clara, CA, USA) using the following
primer sequences: GAPDH, forward 5′-CCTCGTCTCATAGACAAGATGGT-3′ and
reverse 5′-GGGTAGAGTCATACTGGAACATG-3′; miR-144, forward
5′-ACACTCCAGCTGGGTACAGTATAGATGATG-3′ and reverse
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGTACATC-3′; U6, forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′;
Sfrp1, forward 5′-TCATGCAGTTCTTCGGCTTC-3′ and reverse
5′-TTCAACTCGTTGTCACACGG-3′; Runx2, forward
5′-CATTCGCCTCACAAACAACC-3′ and reverse 5′-AGAAGTTTTGCTGACACGGT-3′.
GAPDH and U6 were used as the control for the relative
quantification of mRNA or miRNA, respectively. The relative levels
of gene expression were calculated using the 2−ΔΔCq
method (38).
ELISA
Blood samples from patients with postmenopausal OP
and postmenopausal women with normal bone mineral density were
collected in serum separator tubes (BD Biosciences, San Jose, CA,
USA), clotted for 15 min and then centrifuged at 3,000 × g for 5
min at 4°C. Serum were collected and stored at −80°C until
detected. The serum levels of Sfrp1 and TNF-α in patients with
postmenopausal OP and postmenopausal women with normal bone mineral
density were determined by ELISA, according to the manufacturer's
instructions. The testing kits for Sfrp1 (cat. no. CSB-E15074h) and
TNF-α (cat. no. CSB-E04740h) were purchased from Cusabio Biotech
Co., Ltd. (Wuhan, China). The absorbance was measured at 450 nm
using the SpectraMax M5 microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
5-Ethynyl-2′-deoxyuridine (EdU)
staining
Thymidine analog EdU staining was used to detect
cell proliferation. The MSCs (1×104/well) were seeded in
96-well plates (Corning Inc., Corning, NY, USA) and cultured in a
humidity incubator at 37°C with 5% CO2. In atmosphere. The MSCs wee
transfected as previously mentioned and then treated with 50 µM EdU
(cat. no. C10310-2, Guangzhou Ribobio Co., Ltd.) for 2 h, following
which, they were collected and stained with reagents in the
Apollo® 643 EdU labeling kit (cat. no. C10310-2,
Guangzhou Ribobio Co. Ltd.). The cell nuclei were stained with
Hoechst 33342 (cat. no. C1022, Beyotime Institute of Biotechnology;
Haimen, China). The stained cells were then observed and images
were captured with a fluorescence confocal microscope (Olympus
FV1000, Olympus Corporation, Tokyo, Japan).
Apoptosis assay
Flow cytometry and a Hoechst staining assay were
used to determine the effect of miR-144 on apoptosis. The BMSCs
(1×106) were seeded into 6-well plates (Corning Inc.),
and then transfected with miR-144 mock, miR-144 mimic or miR-144
inhibitor plasmids, respectively. After 48 h, the cells were
collected and stained with reagents in an Annexin V-FITC/PI
apoptosis detection kit (Sigma-Aldrich; Merck KGaA) at room
temperature for 10 min in the dark, following which, they were
immediately counted with a BD FACSCalibur flow cytometry (BD
Biosciences). CellQuest software (version 3.3; BD Biosciences) was
used to assess the resultant data. Apoptosis-induced chromatin
pycnosis in the nucleus was detected by Hoechst staining. The
transfected cells were seeded into 24-well plates and cultured for
48 h, following which, they were fixed in 4% (w/v) paraformaldehyde
at 4°C for 20 min. The cells were then stained with reagents in a
Hoechst 33258 staining kit (cat. no. C1011, Beyotime Institute of
Biotechnology) at room temperature for 30 min in the dark, and
images were captured with a laser scanning confocal microscope
(LSM700; Zeiss AG, Oberkochen, Germany).
Colony formation assay
Briefly, the BMSCs transfected with plasmids were
seeded into 6-well plates (Corning) and maintained in a medium
containing 10% FBS; the medium was refreshed every 2 days.
Following 7 days of incubation, the cells were stained with 0.1%
crystal violet solution (Sigma-Aldrich; Merck KGaA) at room
temperature for 10 min, and the numbers of colonies containing
>50 cells were counted. Colonies were observed using a
microscope imaging system (Nikon Corporation, Tokyo, Japan).
Western blot analysis
RIPA buffer (Sigma-Aldrich; Merck KGaA)
reconstituted with a proteasome inhibitor (Roche Diagnostics,
Indianapolis, IN, USA) and phosphatase inhibitors (Roche
Diagnostics) was used to lyse cells. The concentration of samples
were determined using a Pierce™ BCA Protein Assay Kit (Pierce;
Thermo Fisher Scientific, Inc.). Samples of total cellular protein
were loaded onto 5–10% SDS-polyacrylamide gels (20 µg of protein
per lane) and then separated by electrophoresis. The separated
protein bands were transferred onto PVDF membranes (EMD Millipore,
Billerica, MA, USA), then the PVDF membranes were blocked with 5%
bovine serum albumin solution (Sigma-Aldrich; Merck KGaA) for 1 h
at room temperature, and then incubated with antibodies against
Runx2 (1:1,000; cat. no. 8486, CST Biological Reagents Co., Ltd.),
cyclin-dependent kinase (CDK)4 (1:3,000; cat. no. 12790, CST
Biological Reagents Co., Ltd.), Sfrp1 (1:4,000; cat. no. 3534, CST
Biological Reagents Co., Ltd.), Wnt1 (1:1,000; cat. no. ab85060,
Abcam), β-catenin (1:5,000; cat. no. 8480, CST Biological Reagents
Co., Ltd.) or GAPDH (1:10,000; cat. no. ab8245, Abcam) overnight at
4°C. HRP-conjugated goat anti-rabbit (cat. no. BA1054) or
anti-mouse (cat. no. BA1050) antibodies (1:20,000; Boster
Biological Technology, Pleasanton, CA, USA) were used as secondary
antibodies; membranes were incubated with secondary antibodies for
2 h at room temperature. An enhanced chemiluminescence kit (GE
Healthcare, Chicago, IL, USA) was used to visualize protein
bands.
Immunofluorescence assay
The effects of miR-144 on the expression of Sfrp1
and Runx2 were investigated by immunofluorescence. Briefly, the
BMSCs were fixed and permeabilized with 4% (w/v) paraformaldehyde
at 4°C for 20 min. The cells were then incubated overnight at 4°C
with primary antibodies (anti-Sfrp1 or anti-Runx2, 1:100 dilution),
following which they were washed and stained with either
FITC-conjugated goat anti-mouse IgG (1:100; cat. no. sc-2010, Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) or
phycoerythrin-conjugated IgG (1:1,000; cat. no. sc-3738, Santa Cruz
Biotechnology, Inc.) at 4°C for 30 min in the dark. The cell nuclei
were stained with DAPI (10 µg/ml) at room temperature for 5 min in
the dark. A laser scanning confocal microscope (LSM700; Zeiss AG)
was used to capture the images.
Alizarin red staining
To induce osteoblast differentiation, the BMSCs were
cultured in complete medium for the osteogenic differentiation of
rat BMSCs (Cyagen Biosciences, Inc., Santa Clara, CA, USA),
according to the manufacturer's guidelines. After 3 weeks of
culture, calcium nodi, which are indicative of osteogenic
differentiation, were detected by Alizarin red staining. The BMSCs
were then transferred onto slides and fixed in 10% ethanol,
following which the slides were incubated in 0.1% Alizarin red
staining solution (cat. no. G8550, Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) at room temperature for 30
min and then rinsed with distilled water. Following a final wash,
images of the stained cells were captured using a microscope
imaging system (Nikon Corporation).
Alkaline phosphatase (ALP) assay
The activity of ALP, a marker of early osteoblastic
differentiation, was detected and used to demonstrate osteogenic
differentiation. ALP activity assays were performed following 14
days of osteogenic differentiation. Briefly, the cells were
collected and lysed with lysis buffer, and the total protein
concentration was analyzed with a bicinchoninic acid protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.). ALP activity was
determined using a phosphatase assay kit (Beyotime Institute of
Biotechnology), with the absorbance measured at 405 nm.
Reactive oxygen species (ROS)
assay
Flow cytometric assays were preformed to detect the
levels of ROS in BMSCs. The BMSCs (1×106) were seeded
into 6-well plates and cultured for 24 h. The BMSCs were collected
and stained with the reagents of a Reactive Oxygen Species Assay
kit (cat. no. MAK144, Sigma-Aldrich; Merck KGaA) at 37°C for 1 h.
Following staining, the cells were immediately counted with a BD
FACS Calibur flow cytometry (BD Biosciences). CellQuest Pro
software was used to analyze the data.
Statistical analysis
Each experiment was repeated at least three times,
and results are expressed as the mean ± SEM. Student's t-test and
one-way ANOVA with the Newman-Keuls post hoc test were used to
analyze the data. All statistical was analyses were performed using
GraphPad Prism Version 7.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
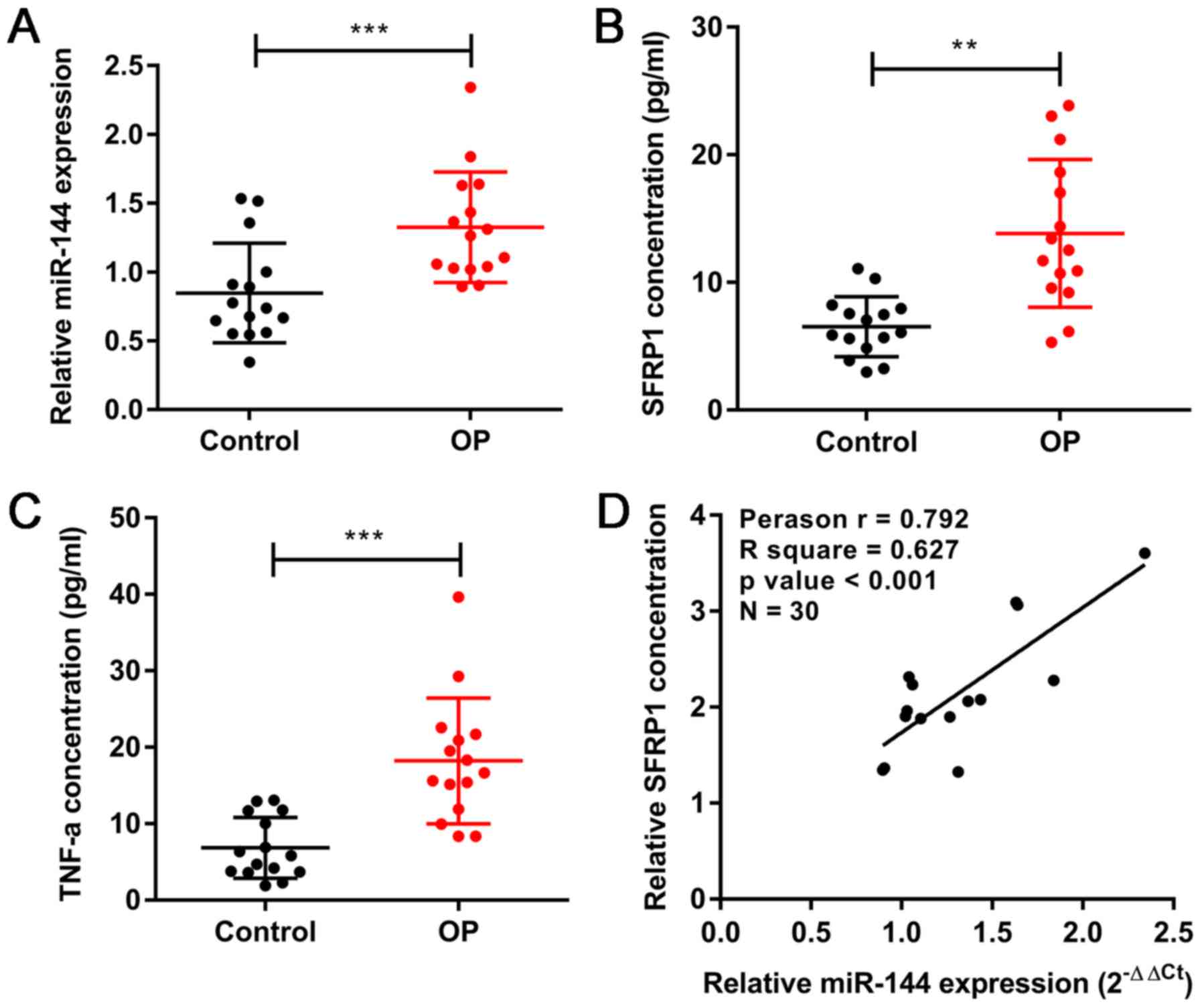
Expression of miR-144, Sfrp1 and TNF-α
in clinical samples
In order to investigate the biological roles of
miR-144, Sfrp1 and TNF-α in the development of OP, their respective
expression levels were compared clinically in patients with
postmenopausal OP (n=15) and postmenopausal women with a normal
bone density (n=15). The RT-qPCR results showed that the expression
of miR-144 was upregulated in the patients with OP compared with
its expression in the normal group (Fig. 1A). The serum levels of Sfrp1 and
TNF-α were detected by ELISA, which showed trends that were similar
to that of miR-144 (Fig. 1B and
C). In addition, correlation analysis (n=30) revealed a
significant correlation between the serum levels of miR-144 and
Sfrp1 (Fig. 1D). These results
indicated that miR-144 and Sfrp1 are involved in the development of
OP.
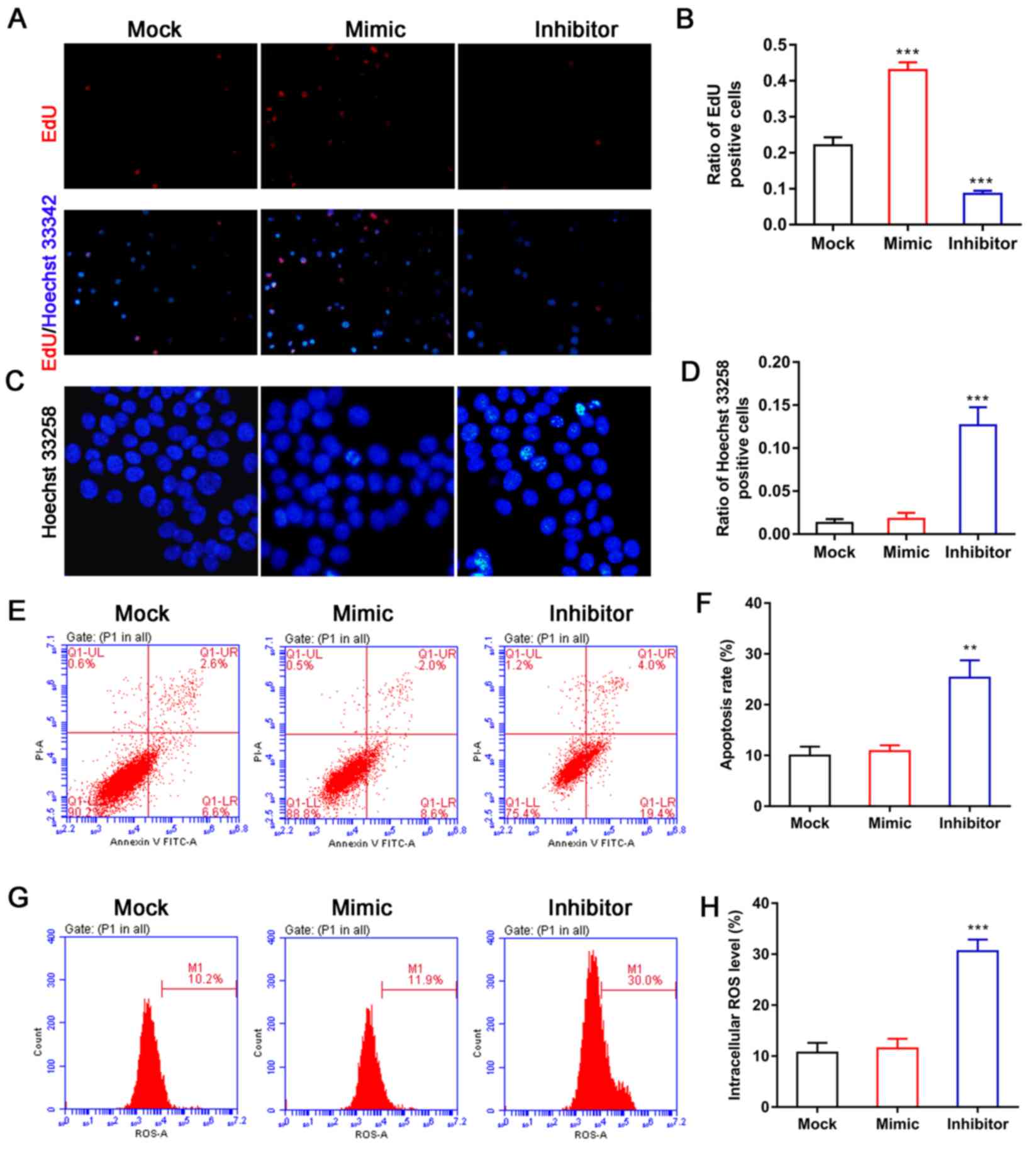
Effect of miR-144 on BMSC
proliferation and apoptosis
To examine the role of miR-144 in the function of
BMSCs, BMSCs were first isolated from rats and then transfected
with miR-144 mimic or the miR-144 inhibitor. EdU staining was used
to evaluate the effect of miR-144 on BMSC proliferation. The
results showed that overexpression of miR-144 efficiently promoted
the proliferation of BMSCs when compared with BMSCs in the mock
groups (Fig. 2A and B), whereas
the opposite effect was observed for BMSCs in the miR-144 inhibitor
group (Fig. 2A and B). In
addition, Hoechst 33258 staining was used to detect the effect of
miR-144 on the nuclear morphological features of BMSCs. As shown in
Fig. 2C and D, the number of BMSCs
with chromatin condensation was markedly increased following
treatment with the miR-144 inhibitor when compared with the BMSCs
in the mock group. However, there was no change in the BMSCs
treated with the miR-144 mimic (Fig.
2C and D). As shown in Fig. 2B and
C, the ratio of positive cells indicates the ratio of positive
to total cells. An apoptosis detection kit was then used to detect
the role of miR-144 in BMSC apoptosis. The results showed that the
miR-144 inhibitor significantly increased the numbers of apoptotic
BMSCs when compared with the BMSCs in the mock group, whereas there
was no difference between the BMSCs treated with the miR-144 mimic
and the mock group (Fig. 2E and
F). It was also found that the absence of miR-144 significantly
increased the levels of intracellular ROS in BMSCs compared with
those in BMSCs in the mock groups (Fig. 2G and H), indicating that the
absence of miR-144 may induce apoptosis in BMSCs by increasing
their concentrations of reactive oxygen species.
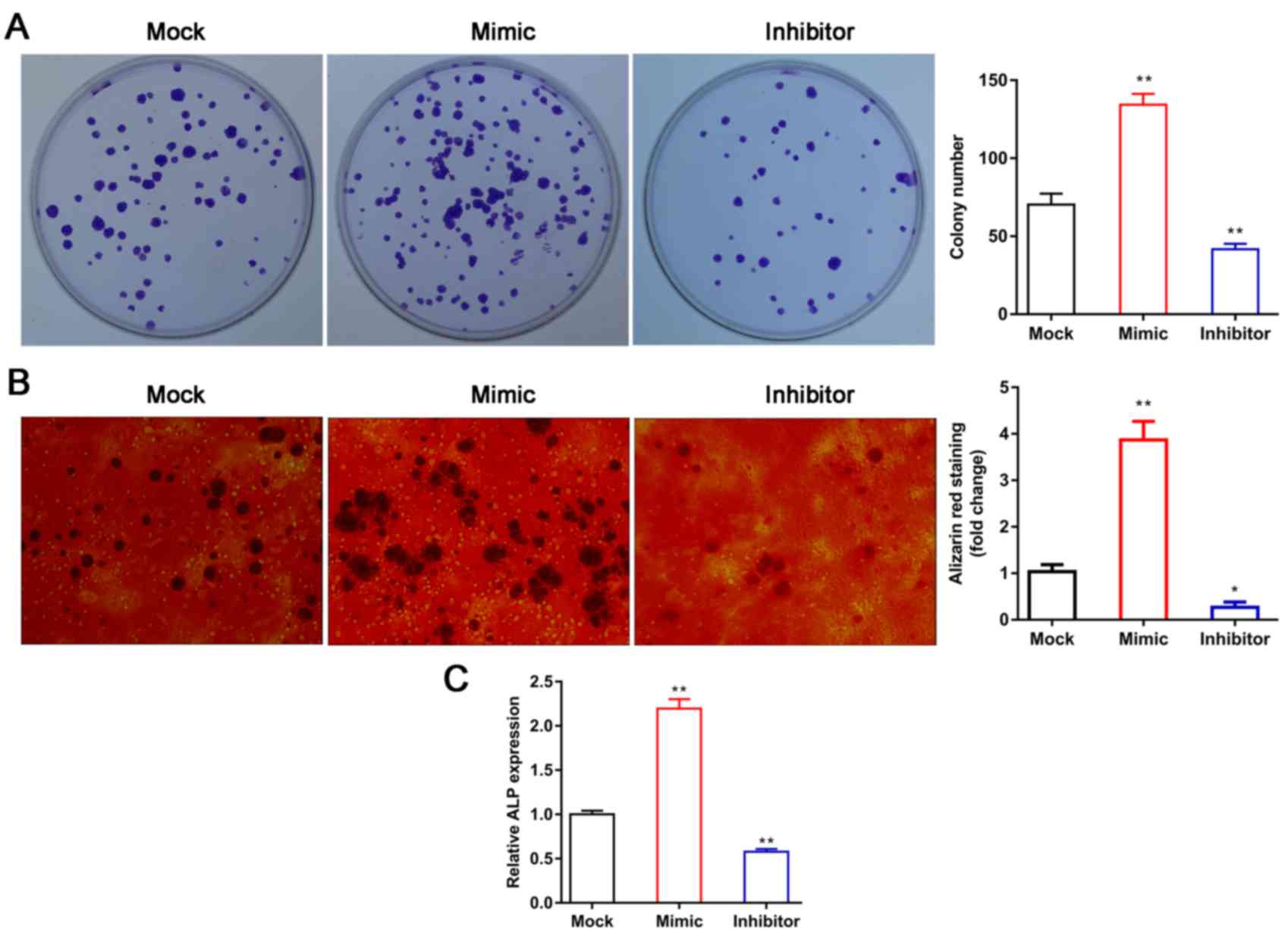
miR-144 promotes the proliferation and
differentiation of BMSCs
To further examine the effect of miR-144 on the
proliferation of BMSCs, a colony formation assay was used to
evaluate the effect of miR-144 on BMSC proliferation. The results
indicated that the overexpression of miR-144 efficiently promoted
the proliferation of BMSCs, whereas miR-144 knockdown efficiently
inhibited BMSC proliferation compared with that in the mock group
(Fig. 3A). Subsequently, Alizarin
red staining was performed to detect the numbers of calcium nodi in
the BMSCs. The results showed that the overexpression of miR-144
significantly increased the numbers of calcium nodi, whereas
miR-144 knockdown efficiently decreased the numbers of calcium nodi
in the BMSCs, compared with the numbers in the corresponding mock
groups (Fig. 3B), indicating that
miR-144 may induce the osteoblastic differentiation of BMSCs in
vitro. The ALP activity assay was used to further examine the
role of miR-144 in the osteogenic differentiation of BMSCs. The
results showed that the overexpression of miR-144 significantly
increased ALP activity, whereas miR-144 knockdown decreased ALP
activity (Fig. 3C). When taken
together, these results indicate that miR-144 can induce the
osteoblastic differentiation of BMSCs in vitro.
Expression of Sfrp1 and Runx2 in BMSCs
is regulated by miR-144
It is generally known that miRNA binds to the 3′-UTR
of its target mRNAs and thereby regulates gene expression. The
TargetScan website (http://www.targetscan.org/) was used to predict the
binding site for miR-144, and the results indicated that
Sfrp1 was a potential target of miR-144. To investigate the
role of miR-144 in the expression of Sfrp1, BMSCs were
transfected with miR-144 mimic or inhibitor and the expression
levels of Sfrp1, miR-144 and Runx2 were detected by RT-qPCR and
western blot methods. As shown in Fig.
4, the expression levels of miR-144 in the miR-144 mimic group
and miR-144 inhibitor group were significantly upregulated and
downregulated, respectively, compared with their levels in the
corresponding mock groups (Fig.
4A). Furthermore, the mRNA and protein levels of Sfrp1 in the
BMSCs transfected with miR-144 mimic or miR-144 inhibitor were
significantly reduced and increased, respectively (Fig. 4A and B). The immunofluorescence
assay showed similar results (Fig.
4C). The present study also investigated how the mRNA and
protein levels of Runx2 were altered by miR-144, and found that
Runx2 levels in the BMSCs were significantly increased when miR-144
was overexpressed (Fig. 4A and B).
By contrast, the expression of Runx2 in BMSCs was significantly
reduced when miR-144 was knocked down (Fig. 4A and B), and the immunofluorescence
assays showed similar results (Fig.
4C). The protein levels of CDK4, Wnt1 and β-catenin were also
markedly increased by miR-144 (Fig.
4B).
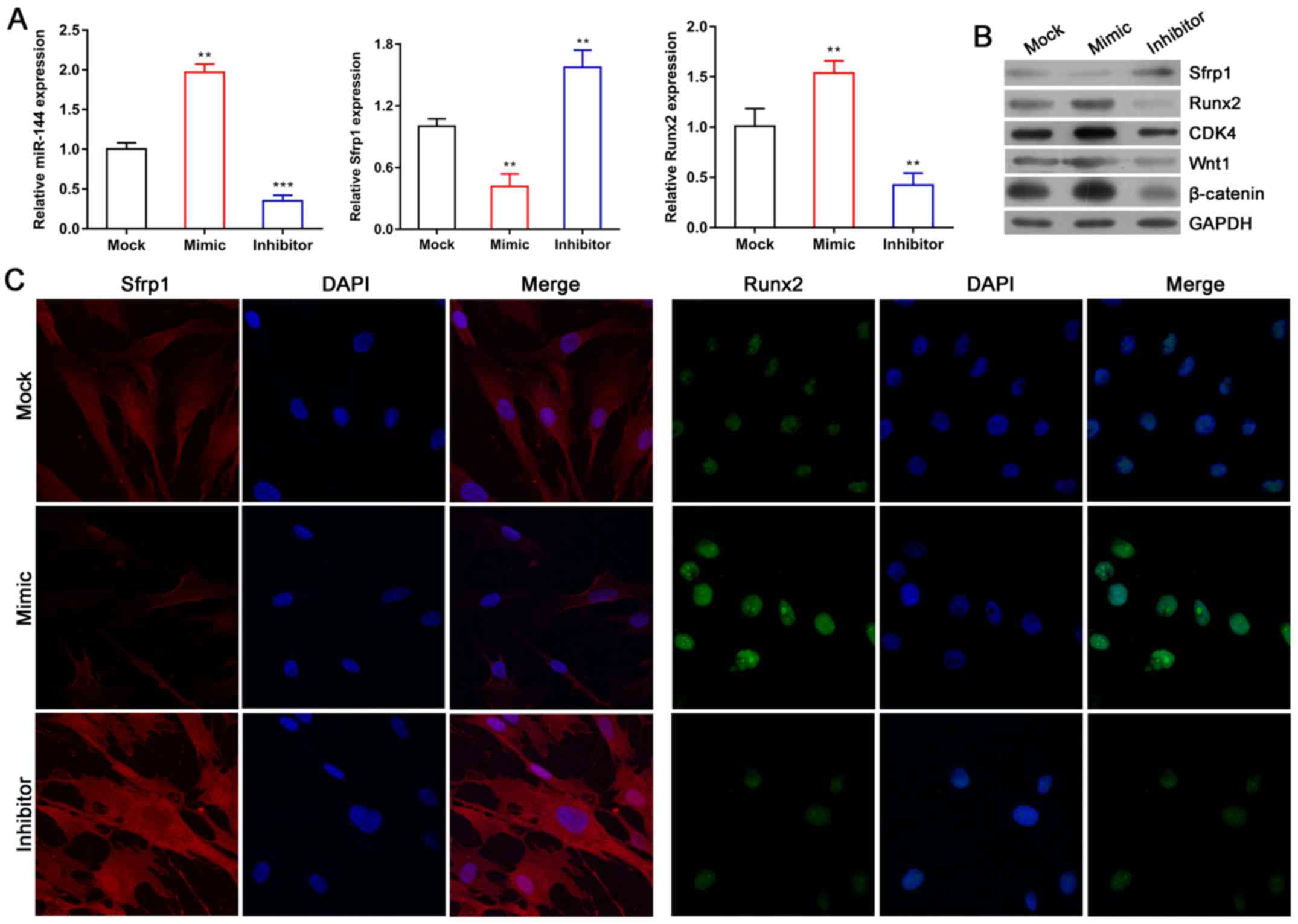 | Figure 4.Expression of Sfrp1 and Runx2 in
BMSCs is regulated by miR-144. (A) Expression levels of miR-144,
Sfrp1 and Runx2 were detected by reverse transcription-quantitative
polymerase chain reaction analysis. (B) Protein expression of
Sfrp1, Runx2, CDK4, Wnt1 and β-catenin was analyzed by western blot
analysis. (C) Distributions of Sfrp1 and Runx2 in BMSCs were
observed using an immunofluorescence assay (magnification, ×200).
Data represent the mean ± SEM. **P<0.01 and ***P<0.001. miR,
microRNA; BMSCs, bone marrow-derived mesenchymal stem cells; Sfrp1,
secreted frizzled-related protein 1; Runx2, Runt-related
transcription factor 2; CDK4, cyclin-dependent kinase 4. |
miR-144 promotes the proliferation and
differentiation of BMSCs by downregulating the expression of
Sfrp1
The binding site for miR-144 in Sfrp1 mRNA was
predicted using the TargetScan website, and Sfrp1 was
suggested as a target gene for miR-144 in BMSCs. The luciferase
reporter assay was used to examine the direct interaction between
miR-144 and Sfrp1. The 3′-UTR sequences of the WT and MUT Srfp1
were sequenced, and the results revealed that the MUT Sfrp1 3′-UTR
gene had successfully mutated in the target region (data not
shown). The 3′-UTR sequences of the WT and MUT Srfp1 were cloned
into a psiCHECK2 plasmid in which the firefly luciferase gene is
constitutively expressed. The results showed that overexpression of
miR-144 significantly decreased the luciferase activity of the WT
Sfrp1 plasmid, compared with that of the other three groups
(Fig. 5A). By contrast, there was
no effect on the luciferase activity of the MUT Sfrp1 plasmid
compared with that in the NC group (Fig. 5A). Taken together, these results
indicate that miR-144 can bind to the 3′-UTR of Sfrp1 mRNA and
thereby regulate the expression of Sfrp1. To further examine
the effects of Sfrp1 and miR-144 on the function of BMSCs, BMSCs
were transfected with Sfrp1 siRNA. The mRNA and protein levels of
Sfrp1 were significantly reduced by Sfrp1 siRNA, particularly in
the second Sfrp1 siRNA group, compared with that in the NC group
(Fig. 5B and C). In follow-up
experiments, the second Sfrp1 siRNA was used to interfere with the
expression of Sfrp1. The colony formation assay, Alizarin
red staining and ALP activity assay were used to further examine
how Sfrp1 may influence miR-144 in promoting the proliferation and
differentiation of BMSCs. The results showed that Sfrp1 suppressed
the proliferation of BMSCs compared with that of the group of BMSCs
co-transfected with the miR-144 inhibitor and NC siRNA (Fig. 5D and F), and also decreased calcium
nodus formation (Fig. 5E and G)
and ALP activity (Fig. 5H). In
addition, the protein levels of Runx2, CDK4, Wnt1 and β-catenin
were markedly increased in the BMSCs co-transfected with the
miR-144 inhibitor and Sfrp1 siRNA compared with the those in the
group of BMSCs co-transfected with the miR-144 inhibitor and NC
siRNA (Fig. 5I). It is well known
that the Wnt/β-catenin pathway serves an important role in cell
proliferation and differentiation, and that Wnt signaling can be
downregulated by Sfrp1 (39). It
is also known that Runx2 is a key transcription factor associated
with osteoblastic differentiation. When combined with our previous
studies, these results suggest that miR-144 promotes the
proliferation and differentiation of BMSCs by upregulating the
expression of Runx2, CDK4, Wnt1 and β-catenin and downregulating
the expression of Sfrp1.
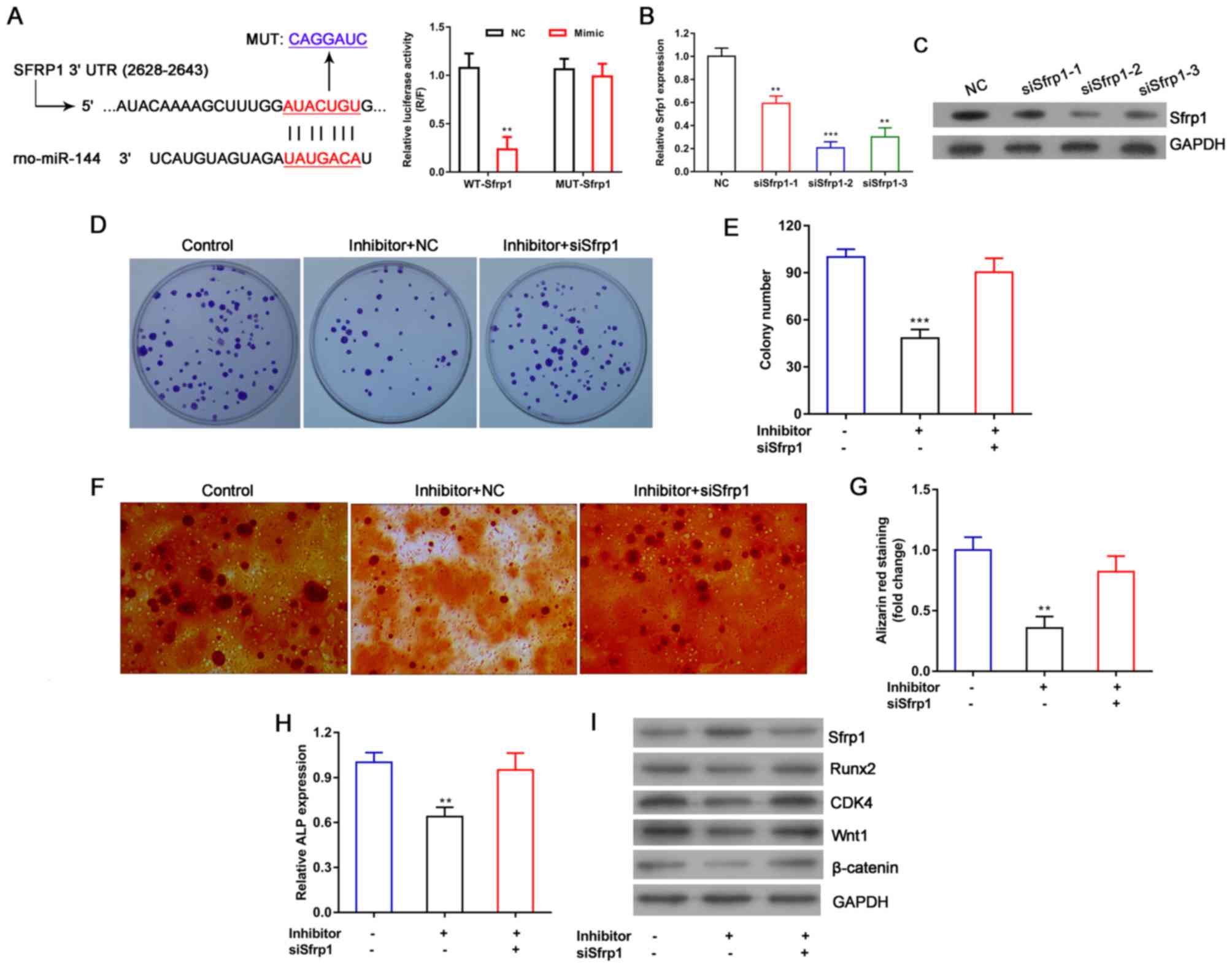 | Figure 5.miR-144 promotes the proliferation
and differentiation of BMSCs by downregulating the expression of
Sfrp1. (A) A luciferase reporter assay was used to examine the
direct interaction between miR-144 and Sfrp1. The group of BMSCs
were co-transfected with WT Sfrp1 plasmid and miR-144 mimic was
compared with the other three groups. BMSCs were transfected with
Sfrp1 siRNA, and the interference efficiency was analyzed by (B)
reverse transcription-quantitative polymerase chain reaction and
(C) western blot analysis, respectively. (D) A colony formation
assay was used to determine how Sfrp1 affected the ability of
miR-144 to promote BMSC proliferation, and (E) quantified. (F)
Alizarin red staining was performed following 3 weeks of osteogenic
differentiation to detect the calcium nodi in BMSCs (magnification,
×200), with (G) quantification of staining. (H) ALP activity assays
were performed following 14 days of osteogenic differentiation. The
group of BMSCs co-transfected with the miR-144 inhibitor and NC
siRNA was compared with other two groups. (I) Protein expression
levels of Sfrp1, Runx2, CDK4, Wnt1 and β-catenin were analyzed by
western blot analysis. GAPDH was used as an internal control. Data
represent the mean ± SEM. **P<0.01 and ***P<0.001. miR,
microRNA; BMSCs, bone marrow-derived mesenchymal stem cells; WT,
wild-type; MUT, mutant; siRNA, small interfering RNA; NC, negative
control; ALP, alkaline phosphatase; Sfrp1, secreted
frizzled-related protein 1; Runx2, Runt-related transcription
factor 2; CDK4, cyclin-dependent kinase 4. |
Discussion
OP is a common disease among older adults and
postmenopausal women (1,3). The degeneration of osteoblasts, in
terms of their function and quantity, aggravates the occurrence and
development of OP; however, BMSCs can continue to differentiate
into osteoblasts following directed induction, which has become an
important method for preventing and curing OP (10,40).
The numbers and functions of BMSCs are key factors involved in
maintaining the normal physiological function of bones, and the
osteogenic capacity of BMSCs in bone marrow decreases when OP
occurs (8–10). Therefore, investigations on how
BMSCs differentiate into osteoblasts have become a primary focus in
the search for novel treatments for OP. A recent study showed that
miRNAs may be involved in cell proliferation and osteoblastic
differentiation (41). The present
study confirmed that the expression of miR-144 was markedly
increased in clinical samples from patients with OP. Furthermore,
miR-144 silencing inhibited the proliferation and promoted the
apoptosis of BMSCs, suggesting that miR-144 may have a key
regulatory role in the progression of OP. In addition, the numbers
of calcium nodi in BMSCs was significantly increased following
treatment with miR-144 mimic, indicating that miR-144 induced the
osteoblastic differentiation of BMSCs.
The regulation of osteoblast differentiation is a
complex process involving multiple genes and multiple pathways,
including the Notch signaling pathway, BMP/Smad signaling pathway
and Wnt signaling pathway (31–33).
The Wnt/β-catenin signaling pathway serves a vital role in
regulating the proliferation and differentiation of osteoblasts and
regeneration of bone tissue. Studies have shown that miRNAs with
abnormal expression patterns specifically regulate expression of
the Wnt signaling pathway, and are thus closely associated with the
occurrence of OP. The expression of miR-218 is regulated by the Wnt
pathway. The upregulation of miR-218 inhibits the expression of
sclerostin, DKK2 and sFRP2, to create a similar positive feedback
regulatory loop leading to osteogenic differentiation (42). Therefore, antagonists of
Wnt/β-catenin may be useful as novel agents for treating OP. In the
present study, it was found that the Wnt/β-catenin signaling
pathway was significantly activated by the overexpression of
miR-144 and sharply suppressed by miR-144 silencing, indicating
that miR-144 partially promotes osteoblast differentiation via the
Wnt/β-catenin signaling pathway. It was further shown that
Sfrp1 was a target gene of miR-144 and negatively regulated
by miR-144 in BMSCs. However, it was found that the miR-144 and
Sfrp1 were expressed at high levels in the serum of patients with
OP, which may be due to the secretion of miR-144 out of the cell.
When miR-144 is secreted out of the cell, such as in the blood,
serum detection becomes correspondingly higher. Therefore, a
positive regulation was found between miR-144 and Sfrp1 in the
serum of patients with OP. Of note, Sfrp1 is an antagonist of the
Wnt signaling pathway; it can inhibit the downward transduction of
Wnt protein, and thereby inhibit osteoblast activity and osteogenic
activity (43,44). In the present study, it was also
found that suppression of the Wnt/β-catenin signaling pathway as a
result of miR-144 knockdown was partially reversed by Sfrp1 siRNA,
suggesting that miR-144 may regulate osteoblast differentiation by
targeting Sfrp1, which is an antagonist of the Wnt signaling
pathway.
During the process by which BMSCs differentiate into
osteoblasts, numerous transcription factors become activated or
deactivated, and a series of osteoblast-related genes, including
Runx2 and Osterix, are expressed (43,45).
As an important factor affecting osteoblast differentiation and
functional activity, Runx2 is regulated by BMP, TGF-β, Wnt
and other signaling pathways (32,46).
Altering the expression of Runx2 affects the osteogenic
differentiation of BMSCs and further influences the formation of
normal osteoblasts and bone tissue. In the present study, it was
shown that the downregulation of miR-144 suppressed the expression
of Runx2; however, this effect was partially reversed
following treatment with Sfrp1 siRNA, demonstrating that miR-144
promotes the osteoblastic differentiation of BMSCs by targeting
Sfrp1. CDK4 is reported to regulate the transition from the
G1 phase to the S phase, and thus promote the growth of osteoblasts
(47). In the present study, it
was shown that miR-144 silencing significantly suppressed the
expression of CDK4, but that effect was partially reversed when
Sfrp1 was simultaneously knocked down. This indicates that miR-144
promotes the proliferation of BMSCs by enhancing the expression of
CDK4, and thereby promoting the transition from the G1 phase to the
S phase.
In conclusion, the data obtained in the present
study showed that the levels miR-144, Sfrp1 and TNF-α were
significantly increased in the serum of patients with OP, and there
was a significant positive correlation between miR-144 and Sfrp1.
In addition, functional experiments demonstrated that miR-144
promoted the proliferation of BMSCs, inhibited the apoptosis of
BMSCs and induced the osteoblastic differentiation of BMSCs,
partially by targeting Sfrp1, which is an antagonist of the Wnt
signaling pathway. These results provide a basis for investigating
the mechanism by which miR-144 is involved in the occurrence and
development of OP, and also suggest a novel molecular target for
preventing and treating OP.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health and
Family Planning Commission of Guangxi Zhuang autonomous region
(grant no. Z2016387) and the Guilin Scientific Research and
Technological Development Project (grant no. 20180106-4-5).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LT and SL conceived and supervised the study. LT, WL
and JH designed and performed experiments. XT and HZ analyzed the
data. LT and SL drafted the manuscript and made revisions. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The collection and analysis of all clinical samples
was approved by the Ethics Committee of the Affiliated Hospital of
Guilin Medical University (Guilin, China; GLMUIA2016002). The
protocols for all animal experiments were approved by the Animal
Ethics Committee of the Affiliated Hospital of Guilin Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Janiszewska M, Kulik T,
Żołnierczuk-Kieliszek D, Drop B, Firlej E and Gajewska I: General
self-efficacy level and health behaviours in women over the age of
45 years who have undergone osteoporosis treatment. Prz
Menopauzalny. 16:86–95. 2017.PubMed/NCBI
|
|
2
|
Baron R and Gori F: Targeting WNT
signaling in the treatment of osteoporosis. Curr Opin Pharmacol.
40:134–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rizou S, Chronopoulos E, Ballas M and
Lyritis GP: Clinical manifestations of osteoarthritis in
osteoporotic and osteopenic postmenopausal women. J Musculoskelet
Neuronal Interact. 18:208–214. 2018.PubMed/NCBI
|
|
4
|
Nguyen BN, Hoshino H, Togawa D and
Matsuyama Y: Cortical thickness index of the proximal femur: A
radiographic parameter for preliminary assessment of bone mineral
density and osteoporosis status in the age 50 years and over
population. Clin Orthop Surg. 10:149–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han Y, You X, Xing W, Zhang Z and Zou W:
Paracrine and endocrine actions of bone-the functions of secretory
proteins from osteoblasts. osteocytes, and osteoclasts. Bone Res.
6:162018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gómez-Cerezo N, Casarrubios L, Morales I,
Feito MJ, Vallet-Regí M, Arcos D and Portolés MT: Effects of a
mesoporous bioactive glass on osteoblasts, osteoclasts and
macrophages. J Colloid Interface Sci. 528:309–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mödinger Y, Löffler B, Huber-Lang M and
Ignatius A: Complement involvement in bone homeostasis and bone
disorders. Semin Immunol. 37:53–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang RF, Wang Q, Zhang AA, Xu JG, Zhai
LD, Yang XM and Liu XT: Low-level laser irradiation promotes the
differentiation of bone marrow stromal cells into osteoblasts
through the APN/Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci.
22:2860–2868. 2018.PubMed/NCBI
|
|
9
|
Wang R, Xu B and Xu HG: Up-Regulation of
TGF-β promotes Tendon-to-Bone healing after anterior cruciate
ligament reconstruction using Bone Marrow-Derived mesenchymal stem
cells through the TGF-β/MAPK signaling pathway in a New Zealand
White Rabbit model. Cell Physiol Biochem. 41:213–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu R, Shi G, Xu L, Gu Q, Fu Y, Zhang P,
Cheng J and Jiang H: Simvastatin improves oral implant
osseointegration via enhanced autophagy and osteogenesis of BMSCs
and inhibited osteoclast activity. J Tissue Eng Regen Med.
12:1209–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan JZ, Yang L, Meng GL, Lin YS, Wei BY,
Fan J, Hu HM, Liu YW, Chen S, Zhang JK, et al: Estrogen improves
the proliferation and differentiation of hBMSCs derived from
postmenopausal osteoporosis through notch signaling pathway. Mol
Cell Biochem. 392:85–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu J, Huang G, Na N and Chen L:
MicroRNA-214-5p/TGF-β/Smad2 signaling alters adipogenic
differentiation of bone marrow stem cells in postmenopausal
osteoporosis. Mol Med Rep. 17:6301–6310. 2018.PubMed/NCBI
|
|
13
|
Abdi J, Mutis T, Garssen J and Redegeld
FA: Toll-like receptor (TLR)-1/2 triggering of multiple myeloma
cells modulates their adhesion to bone marrow stromal cells and
enhances bortezomib-induced apoptosis. PLoS One. 9:e966082014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sárközy M, Kahán Z and Csont T: A myriad
of roles of miR-25 in health and disease. Oncotarget.
9:21580–21612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Casciaro M, Di Salvo E, Brizzi T, Rodolico
C and Gangemi S: Involvement of miR-126 in autoimmune disorders.
Clin Mol Allergy. 16:112018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan Y, Jing J, Qiao L, Liu J, An L, Li B,
Ren D and Liu W: MiRNA-seq reveals that miR-124-3p inhibits
adipogenic differentiation of the stromal vascular fraction in
sheep via targeting C/EBPα. Domest Anim Endocrinol. 65:17–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang C, Yu M and Yao X: MicroRNA-17 and
the prognosis of human carcinomas: A systematic review and
meta-analysis. BMJ Open. 8:e0180702018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lopez-Santillan M, Larrabeiti-Etxebarria
A, Arzuaga-Mendez J, Lopez-Lopez E and Garcia-Orad A: Circulating
miRNAs as biomarkers in diffuse large B-cell lymphoma: A systematic
review. Oncotarget. 9:22850–22861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu X, Tang J, Hu X, Bao P, Pan J, Chen Z
and Xian J: MiR-27b impairs adipocyte differentiation of human
adipose tissue-derived mesenchymal stem cells by targeting LPL.
Cell Physiol Biochem. 47:545–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai F, Du P, Chang Y, Ji E, Xu Y, Wei C
and Li J: Downregulation of MiR-199b-5p inducing differentiation of
bone-marrow mesenchymal stem cells (BMSCs) toward
Cardiomyocyte-Like cells via HSF1/HSP70 pathway. Med Sci Monit.
24:2700–2710. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng Z, Xie X, Zhu Y, Liu J, Hu X, Na Q,
Zhang X, Wei G, Xu S, Liu Y, et al: miR-142-5p in bone
marrow-derived mesenchymal stem cells promotes osteoporosis
involving targeting adhesion molecule VCAM-1 and Inhibiting cell
migration. Biomed Res Int. 2018:32746412018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feichtinger X, Muschitz C, Heimel P,
Baierl A, Fahrleitner-Pammer A, Redl H, Resch H, Geiger E, Skalicky
S, Dormann R, et al: Bone-related circulating MicroRNAs miR-29b-3p,
miR-550a-3p, and miR-324-3p and their Association to bone
microstructure and histomorphometry. Sci Rep. 8:48672018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Xu F, Pei HX, Zhu X, Lin X, Song
CY, Liang QH, Liao EY and Yuan LQ: Vaspin regulates the osteogenic
differentiation of MC3T3-E1 through the PI3K-Akt/miR-34c loop. Sci
Rep. 6:255782016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang X, Lin J, Wang G and Lu J:
MicroRNA-433-3p promotes osteoblast differentiation through
targeting DKK1 expression. PLoS One. 12:e01798602017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han S, Zhu J and Zhang Y: MiR-144
potentially suppresses proliferation and migration of ovarian
cancer cells by targeting RUNX1. Med Sci Monit Basic Res. 24:40–46.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao P, Wen H, Yang B, Zhang A, Wu X and Li
Q: MiR-144 inhibits growth and metastasis of cervical cancer cells
by targeting VEGFA and VEGFC. Exp Ther Med. 15:562–568.
2018.PubMed/NCBI
|
|
27
|
Liu J, Feng L, Zhang H, Zhang J, Zhang Y,
Li S, Qin L, Yang Z and Xiong J: Effects of miR-144 on the
sensitivity of human anaplastic thyroid carcinoma cells to
cisplatin by autophagy regulation. Cancer Biol Ther. 19:484–4962.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren YF, Zhang TH, Zhong S, Zhao YT and Lv
YN: MiR-144 suppresses proliferation and induces apoptosis of
osteosarcoma cells via direct regulation of mTOR expression. Oncol
Lett. 15:1163–1169. 2018.PubMed/NCBI
|
|
29
|
Hou Q, Huang Y, Zhu S, Li P, Chen X, Hou Z
and Liu F: MiR-144 Increases Intestinal Permeability in IBS-D Rats
by Targeting OCLN and ZO1. Cell Physiol Biochem. 44:2256–2268.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiang C, Cui SP and Ke Y: MiR-144 inhibits
cell proliferation of renal cell carcinoma by targeting MTOR. J
Huazhong Univ Sci Technolog Med Sci. 36:186–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakajima K, Kho DH, Yanagawa T, Harazono
Y, Gao X, Hogan V and Raz A: Galectin-3 inhibits osteoblast
differentiation through notch signaling. Neoplasia. 16:939–949.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan P and Bonewald LF: The role of the
wnt/β-catenin signaling pathway in formation and maintenance of
bone and teeth. Int J Biochem Cell Biol. 77:23–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao F, Zhan J, Chen X, Zhang K, Lai R and
Feng Z: MiR-214 promotes periodontal ligament stem cell
osteoblastic differentiation by modulating Wnt/β-catenin signaling.
Mol Med Rep. 16:9301–9308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dejaeger M, Böhm AM, Dirckx N, Devriese J,
Nefyodova E, Cardoen R, St-Arnaud R, Tournoy J, Luyten FP and Maes
C: Integrin-Linked kinase regulates bone formation by controlling
cytoskeletal organization and modulating BMP and Wnt signaling in
osteoprogenitors. J Bone Miner Res. 32:2087–2102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Warrier S, Marimuthu R, Sekhar S,
Bhuvanalakshmi G, Arfuso F, Das AK, Bhonde R, Martins R and
Dharmarajan A: sFRP-mediated Wnt sequestration as a potential
therapeutic target for Alzheimer's disease. Int J Biochem Cell
Biol. 75:104–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amjadi-Moheb F, Hosseini SR,
Kosari-Monfared M, Ghadami E, Nooreddini H and Akhavan-Niaki H: A
specific haplotype in potential miRNAs binding sites of secreted
frizzled-related protein 1 (SFRP1) is associated with BMD variation
in osteoporosis. Gene. 677:132–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chim CS, Pang R, Fung TK, Choi CL and
Liang R: Epigenetic dysregulation of Wnt signaling pathway in
multiple myeloma. Leukemia. 21:2527–2536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen G, Ren H, Qiu T, Zhang Z, Zhao W, Yu
X, Huang J, Tang J, Liang, Yao Z, et al: Mammalian target of
rapamycin as a therapeutic target in osteoporosis. J Cell Physiol.
233:3929–3944. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Ji J, Wei W and Liu L: MiR-25
promotes proliferation, differentiation and migration of
osteoblasts by up-regulating Rac1 expression. Biomed Pharmacother.
99:622–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang
W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL,
et al: MiR-218 directs a Wnt signaling circuit to promote
differentiation of osteoblasts and osteomimicry of metastatic
cancer cells. J Biol Chem. 287:42084–42092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Holdsworth G, Greenslade K, Jose J,
Stencel Z, Kirby H, Moore A, Ke HZ and Robinson MK: Dampening of
the bone formation response following repeat dosing with sclerostin
antibody in mice is associated with up-regulation of Wnt
antagonists. Bone. 107:93–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang FS, Lin CL, Chen YJ, Wang CJ, Yang
KD, Huang YT, Sun YC and Huang HC: Secreted frizzled-related
protein 1 modulates glucocorticoid attenuation of osteogenic
activities and bone mass. Endocrinology. 146:2415–2423. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang C, Liao H and Cao Z: Role of Osterix
and MicroRNAs in bone formation and tooth development. Med Sci
Monit. 22:2934–2942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stewart S, Gomez AW, Armstrong BE, Henner
A and Stankunas K: Sequential and opposing activities of Wnt and
BMP coordinate zebrafish bone regeneration. Cell Rep. 6:482–498.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fujita M, Urano T, Horie K, Ikeda K,
Tsukui T, Fukuoka H, Tsutsumi O, Ouchi Y and Inoue S: Estrogen
activates cyclin-dependent kinases 4 and 6 through induction of
cyclin D in rat primary osteoblasts. Biochem Biophys Res Commun.
299:222–228. 2002. View Article : Google Scholar : PubMed/NCBI
|