Introduction
Although mortality rates of coronary heart disease
worldwide have declined over the past four decades, the disease
remains responsible for approximately one-third of all deaths of
individuals over the age of 35 (1). Upon acute myocardial ischemia (MI)
due to coronary occlusion, restoring myocardial perfusion through
either percutaneous coronary intervention or thrombolytic therapy
is the most effective treatment. However, reperfusion causes
ischemia/reperfusion (I/R) injury, which aggravates myocardial
damage (2,3). Recent research suggests great
interest in alleviating reperfusion injury following myocardial
ischemia (4,5).
Glucagon-like peptide-1 (GLP-1) is a
30-amino-acid-long gut hormone secreted from intestinal endocrine
L-cells (6,7). GLP-1 exerts physiological functions
through binding to its receptor (GLP-1R), which is a class B
heterotrimeric G-protein-coupled receptor (8). GLP-1R is expressed in a number of
tissues, including pancreatic islets, heart, lung, kidney, stomach,
intestine, pituitary, skin and nervous tissue. Previous studies
have demonstrated that GLP-1 exerts cytoprotective effects in the
heart after I/R injury (9–11). Thus, in addition to treating
hyperglycemia, GLP-1 offers promising prospects in treating
reperfusion injury of the myocardium. However, the cardioprotective
effects of GLP-1 after MI/R injury are temporary (9). Therefore, in vivo imaging of
GLP-1R may be a useful tool to further study the role of GLP-1 and
GLP-1R in treating reperfusion injury.
GLP-1 cannot be used as an imaging tracer as it is
rapidly degraded by dipeptidyl-peptidase-IV (DPP-IV) upon being
released into the blood (12).
Exendin-4, an analog of GLP-1, has a 53% amino acid homology with
GLP-1, but is resistant to DPP-IV, thus prolonging its half-life
(T1/2) in plasma (13).
Numerous attempts have been made to attach exendin-4 to
radioisotopes, such as 111In, 99mTc for
single-photon emission computed tomography (SPECT) and
68Ga, 64Cu for positron emission tomography
(PET) (14–16). However, these combinations have
certain limits; SPECT has a lower density resolution compared with
PET, and 68Ga and 64Cu are not commonly used
in the clinic. Fluorine-18 (18F), however, has suitable
imaging properties (β+, 0.635 MeV 97%; T1/2,
110 min). Previously,
18F-4-fluorobenzamido-N-ethylamino-maleimide
(FBEM)-Cys40-exendin-4 was developed for GLP-1R imaging
by PET; 18F-FBEM-Cys40-exendin-4 exhibited
high affinity with GLP-1R in an INS-1 rat insulinoma xenograft
model and a rat MI/R model (17,18).
However, the radiosynthesis of
18F-FBEM-Cys40-exendin-4 is time-consuming,
which complicates the application of the tracer.
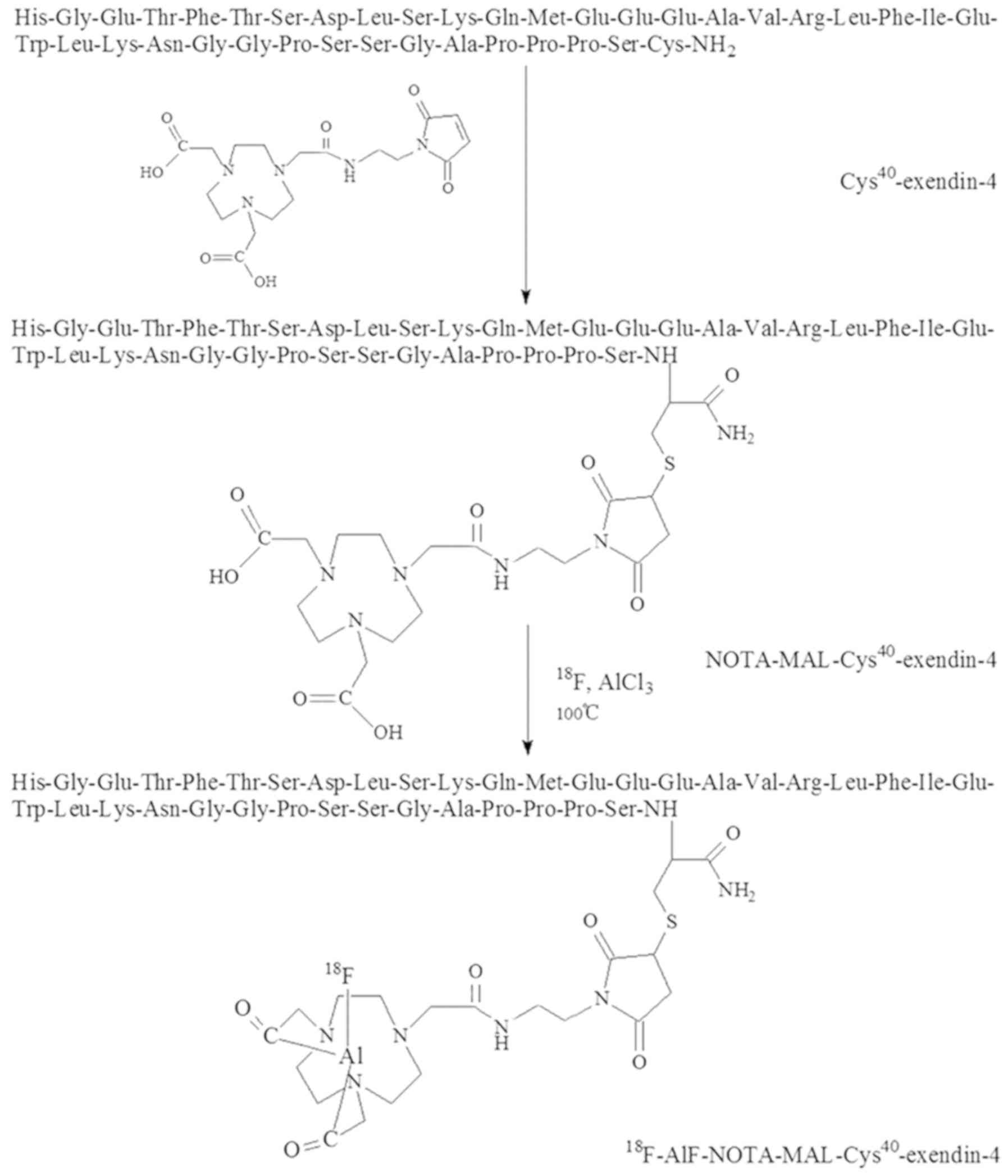
In the present study, a radiotracer of GLP-1R was
synthesized by conjugating 18F-labled aluminum fluoride
(18F-AlF) with 1,4,7-triazacyclononanetriacetic acid
(NOTA)-maleimide (MAL)-Cys40-exendin-4 in a one-step
procedure. Subsequently,
18F-AlF-NOTA-MAL-Cys40-Exendin-4 was
evaluated in a rat MI/R model for longitudinal PET imaging.
Materials and methods
Materials and high-performance liquid
chromatography (HPLC)
Cys40-exendin-4 was a generous gift from
Professor Min Yang (Jiangsu Institute of Nuclear Medicine, Nanjing,
China). NOTA-MAL was purchased from CheMatech.
18F-labled fluoride was obtained using the HM-67
cyclotron (Sumitomo Heavy Industries, Ltd.) at the Jiangsu
Institute of Nuclear Medicine by proton irradiation of
18O-enriched water. All other commercially obtained
reagents were of analytical grade. For semi-preparative HPLC, an
Xbridge C18 HPLC column (5 µm, 250×9 mm; Waters Corporation) and a
Waters HPLC system with a Q1 Waters 2998 photodiode array detector
(Waters Corporation) were used. The system was maintained at room
temperature and the sample quantity was 1 ml. No internal standards
were used. The flow rate was 5 ml/min. The linear gradient started
at 95% A [0.1% trifluoroacetic acid (TFA) in water] and 5% B (0.1%
TFA in acetonitrile) for 2 min, and increased to 35% A and 65% B at
32 min.
Preparation of
NOTA-MAL-Cys40-exendin-4 analogs
The sequence of Cys40-exendin-4 is
His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Cys-NH2.
Cys40-exendin-4 was conjugated with NOTA-MAL as
previously described (19).
Briefly, NOTA-MAL (2.16 mg, 3.2 µmol) was mixed with
Cys40-exendin-4 (6.77 mg, 1.57 µmol) and dissolved in 6
ml 0.4 M ammonium acetate. Subsequently, 400 µl acetonitrile was
added. Following stirring at 40°C overnight, the mixture was
purified by semi-preparative HPLC, aforementioned. The desired
product was collected and lyophilized.
Radiosynthesis of
18F-AlF-NOTA-MAL-Cys40-exendin-4
In a 2 ml centrifuge tube, 70 µg
NOTA-MAL-Cys40-exendin-4 and 10 µl double-distilled
water (ddH2O) were charged, and 10 µl glacial acetic
acid (CH3COOH) was added. Cyclotron target water
containing 18F (~50 µl containing 90 mCi) was added,
followed by 280 µl acetonitrile (CH3CN) and 3 µl
aluminum chloride (2 mM) in 0.5 M NaOAc (pH=4). After complete
mixing, the resulting solution was incubated in oil at 100°C for 10
min. Following cooling, the reaction mixture was diluted with 15 ml
ddH2O and eluted through a C18 column. The product was
subsequently re-eluted with 20% aqueous ethanol containing 10 mM
HCl. The elute was treated with 100 µl of 0.1 M NaOAc (pH 4) and
concentrated to ~200 µl total volume under a stream of argon. The
sample was diluted with PBS for animal injections.
Quality control of
18F-AlF-NOTA-MAL-Cys40-exendin-4
A sample of the elute (10 µl) was analyzed by
reversed-phase HPLC on a C18 column (5 µm, 250×4.6 mm; Phenomenex)
with linear gradient at 1 ml/min, as previously described (19). UV absorption and radioactivity were
monitored by a Waters 2487 dual λ absorbance detector (Waters
corporation) and a Radiomatic 610TR flow scintillation analyzer
(PerkinElmer, Inc.), respectively. Mass spectra were obtained using
a Q1 Waters LC-MS system (Waters corporation) featuring an Acquity
UPLC system coupled with a Waters Q-Tof Premier high-resolution
mass spectrometer (Waters Corporation). Positive mode was used with
a vaporizer temperature of 300°C, a nebulizer pressure of 20 psi
and a gas flow rate of 5 l/min. Positive ions were acquired in the
multiple reaction monitoring mode (collision energy, 10 eV).
Animal model of myocardial ischemia
and reperfusion
All animal studies were conducted in accordance with
the principles and procedures outlined in the Guide for the Care
and Use of Laboratory Animals, and the protocols were approved by
the Laboratory Animal Ethics Committee of The Fourth Military
Medical University (Xi'an, China). A total of 36 adult male
Sprague-Dawley rats (6–8 weeks old, 200–250 g) obtained from
Laboratory Animal Center of the Fourth Military Medical University
were used. The animals were housed under a 12:12-h light/dark cycle
at 25°C and 50% relative humidity, with access to food and water
ad libitum. Intraperitoneal injection of 3% pentobarbital
sodium was conducted for anesthesia at a dose of 50 mg/kg initially
with modifications according to the depth of anesthesia (20), and the animals were intubated for
mechanical ventilation. MI/R models were established by ligation of
the left anterior descending coronary artery 2–3 mm from the tip of
the auricle with a 6-0 polypropylene suture. Reperfusion was
achieved thirty minutes after the coronary artery occlusion by
removal of the suture. In the sham-operated group, a suture was
placed in the myocardium without ligation of the left coronary
artery. The occlusion and reperfusion were confirmed by ST-segment
elevation on an electrocardioscope and by
18F-fluorodeoxyglucose (FDG) imaging at post-surgery day
1 (see the ‘Micro-PET imaging’ section below for details).
Echocardiographic assessment of left
ventricular (LV) contractility
Echocardiography was performed at day 1 post-MI/R.
Gaseous anesthesia was conducted using 3% isoflurane, and the rats
were placed on the scanning table. Echocardiographic images were
obtained using a small-animal high-resolution imaging unit and a
30-MHz linear transducer (Vevo 770; FUJIFILM VisualSonics, Inc.).
LV end-diastolic diameter (LVEDD) and LV end-systolic diameter
(LVESD) were measured using a parasternal short-axis view, and LV
fractional shortening was calculated as [(LVEDD-LVESD)/LVEDD]
×100%. All measurements were averaged over three successive cardiac
cycles.
Micro-PET imaging
PET scans and image analysis were performed using an
Inveon micro-PET (Siemens Medical Solutions USA, Inc.). At 8 h, 1
day, 3 days, and 1 week post-MI/R, each rat was injected in the
tail vein with 18.5 MBq (500 µCi)
18F-AlF-NOTA-MAL-Cys40-exendin-4 under 3%
isoflurane anesthesia (n=6 rats/group). Ten-minute static PET scans
were acquired 1 h post-injection. For the blocking experiment, 8
mg/kg of unlabeled Cys40-exendin-4 was pre-injected 10
min prior to the injection of labeled Cys40-exendin-4 at
post-MI/R day 1 (n=6). The images were reconstructed using a
three-dimensional ordered subsets expectation maximum algorithm,
and no correction was applied for attenuation or scatter. For each
scan, regions of interest (ROIs) were drawn around the ischemic
myocardium, lung and surgical wounds using the vendor software ASI
Pro 6.7.1.1 (Siemens AG) on whole-body coronal images. The
radioactivity concentration (accumulation) within myocardium was
obtained from mean pixel values within the multiple ROI volume,
which was converted to MBq/ml/min using a conversion factor. The
conversion to MBq/g/min assumed tissue density of 1 g/ml. The
values were divided by the administered activity to obtain an image
ROI-derived percentage of injected dose per gram (%ID/g). Following
18F-AlF-NOTA-MAL-Cys40-exendin-4 scanning,
rats were kept on the scanning gantry for image co-registration for
1 h and injected with 18.5 MBq (500 µCi) of 18F-FDG.
After 1 h, 10-min static scans were performed, and images were
reconstructed and analyzed using the same procedure.
GLP-1R immunohistochemical
staining
Heart samples from the rats were harvested after the
rats were fully anesthetized and immediately placed into 10%
formalin for fixation at room temperature for 24 h. Formalin-fixed
samples were embedded in paraffin. Tissue sections (5-µm) were
dried for 1 h at 90°C prior to deparaffinization in xylene,
rehydrated by a series of descending ethanol concentrations and
subsequently washed with PBS. Antigen retrieval was achieved by
heat treatment at 95°C in a pressure cooker for 2 min in 10 mM
citrate buffer (pH 6.5). Endogenous peroxidase was blocked at room
temperature using 3% hydrogen peroxide for 20 min. The tissue
section was incubated at 4°C overnight with a rabbit anti-rat
polyclonal GLP-1R antibody (1:200; cat. no. ab188602; Abcam). After
washing with PBS, the slide was incubated with a horseradish
peroxidase (HRP)-labeled goat anti-rabbit IgG antibody (1:50; cat.
no. ab6721; Abcam) for 30 min at room temperature. Diaminobenzidine
(Sangon Biotech Co., Ltd.) was used for staining according to the
manufacturer's protocols, and the analysis was performed using an
Olympus BX41 optical microscope (magnification, ×20; Olympus
Corporation).
Western blot analysis of GLP-1R
The area of ischemia was defined as the region
appearing pale. The corresponding areas of the heart were divided
into different samples. The samples of each group (n=3) were
homogenized, suspended in RIPA buffer (Sigma-Aldrich, Merck KGaA)
and protease inhibitor cocktail (Roche Applied Science), and
centrifuged at 14,000 × g for 10 min at 4°C. Protein concentration
of the supernatant was measured by the bicinchoninic acid method;
50 µg of protein was separated by Tris-glycine 10% SDS-PAGE and
transferred to a nitrocellulose membrane. Blocking was performed
with 5% skimmed milk and 0.1% bovine serum albumin (Gibco; Thermo
Fisher Scientific, Inc.) at room temperature for 1 h. The membrane
was incubated with rabbit anti-rat polyclonal GLP-1R primary
antibody (1:500; cat. no. ab188602; Abcam) at 4°C overnight, washed
with PBS, and incubated with HRP-labeled goat anti-rabbit IgG
antibody (1:300; cat. no. ab6721; Abcam) at room temperature for 1
h. Chemiluminescence was performed with chemiluminescent HRP
substrate using a SuperSignal West Pico Chemiluminescence Kit
Detection System (Pierce, Thermo Fisher Scientific, Inc.). β-actin
(1:500; cat. no. ab8227; Abcam) was used as internal control; each
protein band was quantified using ImageJ software (v1.8.0, National
Institutes of Health) and normalized to β-actin.
Statistical analysis
Data are expressed as mean ± SD. Statistical
analysis was performed by one-way analysis of variance and a
Student-Newman-Keuls multiple comparison post hoc test was
performed to identify differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Chemistry and radiochemistry
NOTA-MAL-Cys40-exendin-4 was synthesized
by NOTA-MAL and Cys40-exendin-4. Following purification
by semi-preparative HPLC, the yield of
NOTA-MAL-Cys40-exendin-4 was 68%. Mass spectrometry
results indicated a mass-to-charge ratio of 4,712.83 for
MH+
(C205H314N56O68S2;
calculated molecular weight, 4,713; Fig. 1). Radiosynthesis of
18F-AlF-NOTA-MAL-Cys40-exendin-4 was
completed in 30 min, and the yield was 18.5±3.4% (no decay
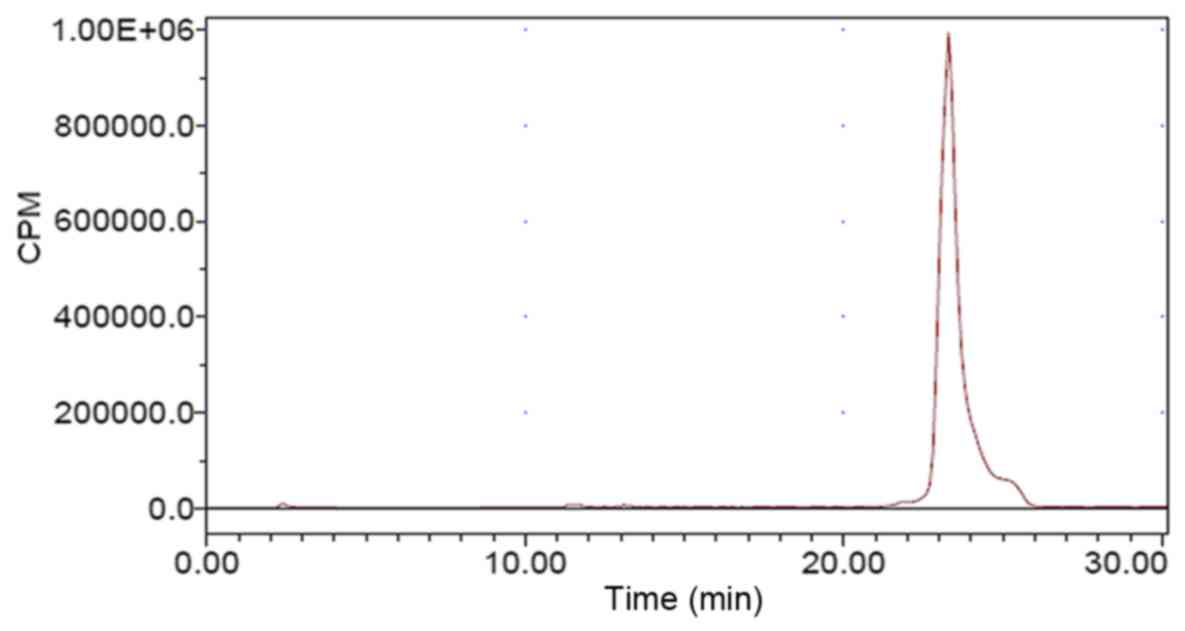
corrected) with a purity of >95%. The retention time of
18F-AlF-NOTA-MAL-Cys40-exendin-4 obtained by
analytical HPLC was 23 min (Fig.
2).
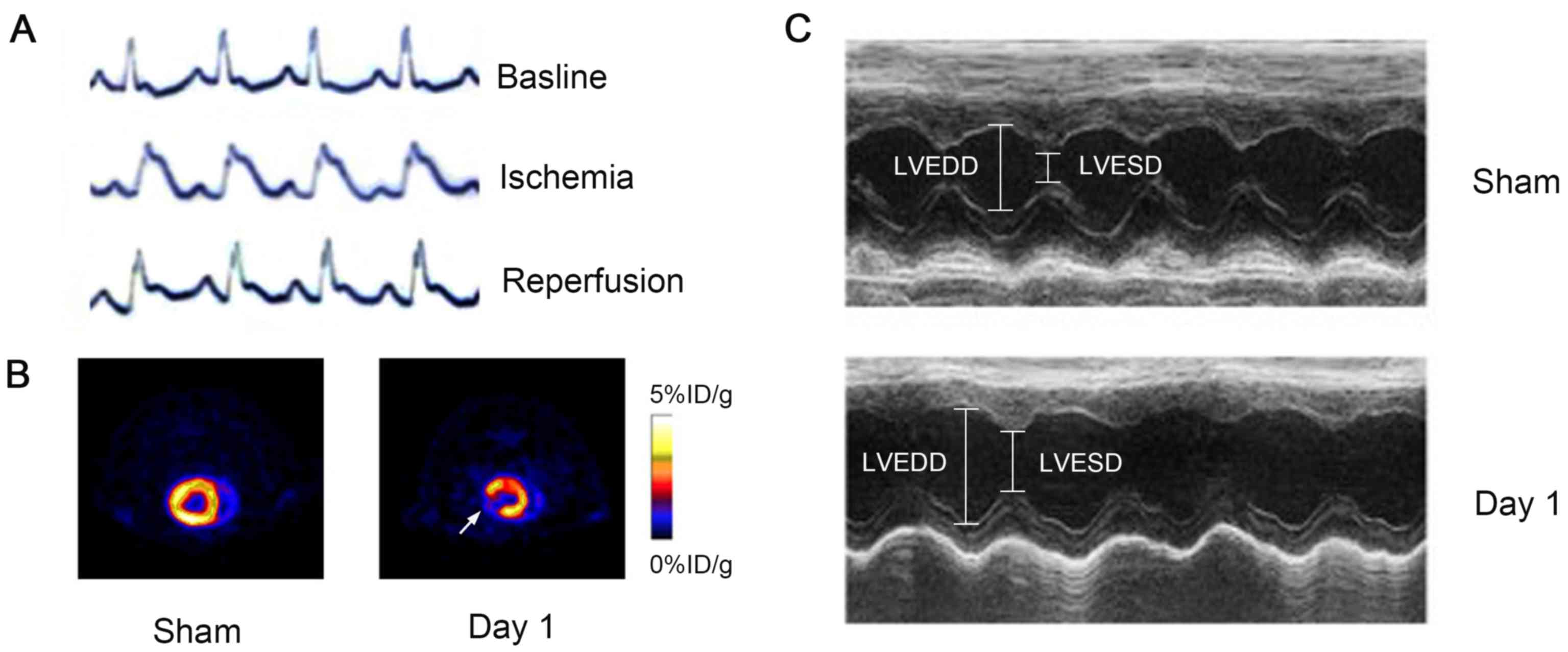
Electrocardioscope monitoring during establishment
of the MI/R models was used to confirm I/R conditions by ST-T
segment change (Fig. 3A). At MI/R
day 1, 18F-FDG PET scanning demonstrated a significant
defect in the anterolateral wall of the LV (white arrow), whereas
no uptake defect was observed in the sham-operated 1 day group
(n=6; Fig. 3B). On the same day,
M-mode high-resolution ultrasound was performed and revealed a
decrease in the amplitude of the anterolateral wall of LV (Fig. 3C). Fractional shortening was
decreased from 69±5.2 to 44±3.6% in rats following surgery; no
compromised cardiac function was observed by M-mode high-resolution
ultrasound in sham-operated animals (Fig. 3C).
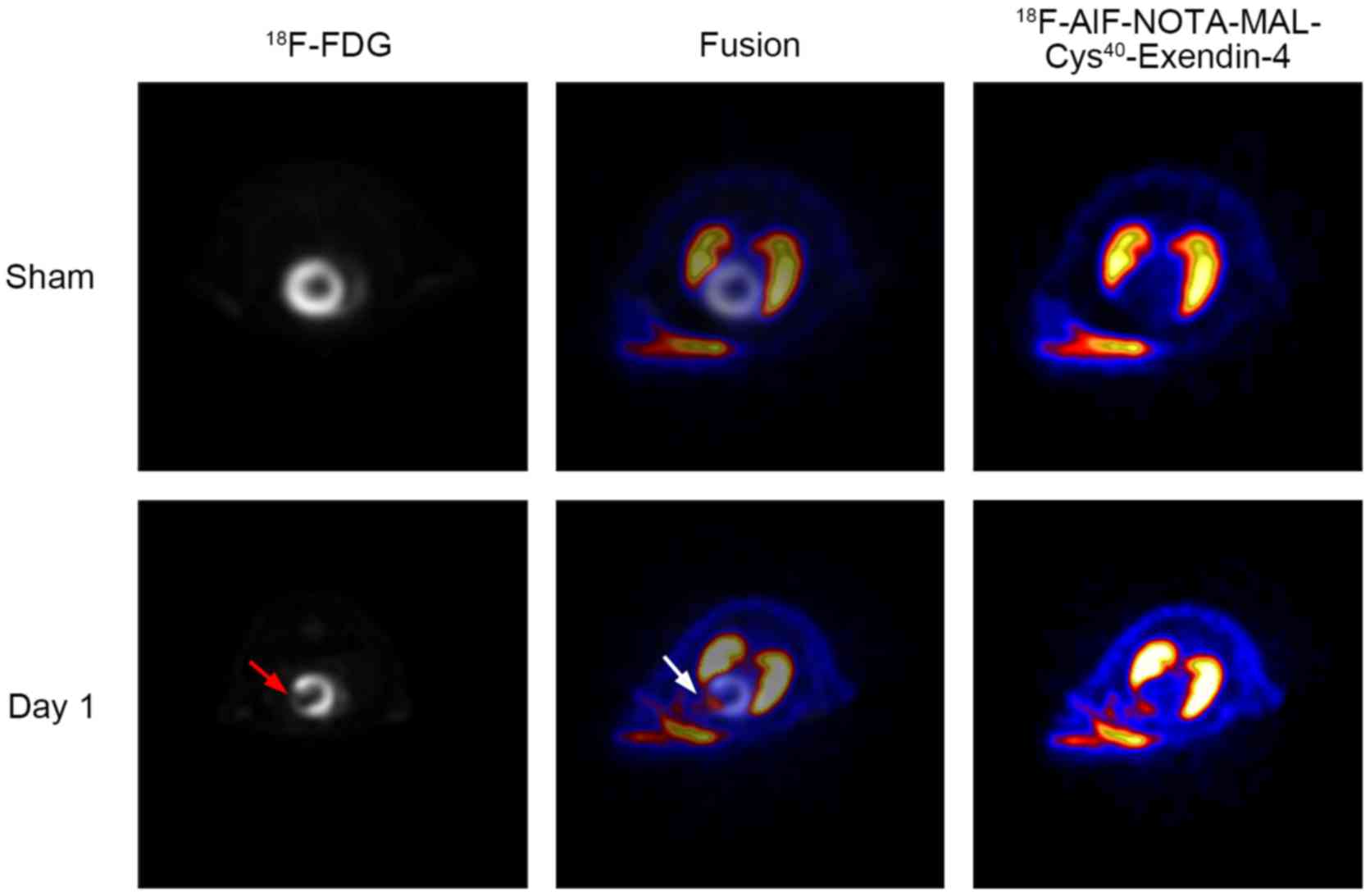
Image colocalization
18F-FDG PET imaging was used to identify
the area of ischemia. Co-registration of
18F-AlF-NOTA-MAL-Cys40-exendin-4 PET and
18F-FDG PET provided a visual method for anatomically
localizing the high-radioactivity area of
18F-AlF-NOTA-MAL-Cys40-exendin-4 in hearts.
To facilitate image co-registration, the rats were kept in the
scanning gantry under anesthesia during tracer injection and
imaging acquisition. Representative myocardial transaxial slices
from one rat following in vivo PET imaging of
18F-AlF-NOTA-MAL-Cys40-exendin-4 and
18F-FDG are presented in Fig. 4. Compared with sham-operated rats,
the hearts of MI/R day 1 rats exhibited decreased
18F-FDG uptake and increased
18F-AlF-NOTA-MAL-Cys40-exendin-4 uptake in
the anterolateral wall of the LV. The area of ischemic myocardium
was matched between 18F-FDG and
18F-AlF-NOTA-MAL-Cys40-exendin-4 uptake
(Fig. 4).
Dynamic change in GLP-1R in PET
imaging
Representative transaxial micro-PET images of
18F-AlF-NOTA-MAL-Cys40-exendin-4 at different
time points following MI/R are presented in Fig. 5A. In the lung, the area of ischemia
and surgery wound exhibited higher uptake of
18F-AlF-NOTA-MAL-Cys40-exendin-4 compared
with surrounding tissues (Fig.
5A). Tracer uptake in the ROI of ischemic myocardium (white
arrow) reached a peak (0.35±0.053 %ID/g) at 8 h post-MI/R, which
was not observed in the sham-operated group (0.06±0.012 %ID/g; n=6;
Fig. 5B). In addition, the
radioactivity level remained high at post-MI/R day 1 (0.20±0.032
%ID/g; n=6) and decreased on day 3 (0.16±0.017 %ID/g; n=4; Fig. 5B). Minimal tracer uptake persisted
at 1 week post-MI/R (0.08±0.006 %ID/g; n=6), but the levels were
not significantly different (Fig.
5B).
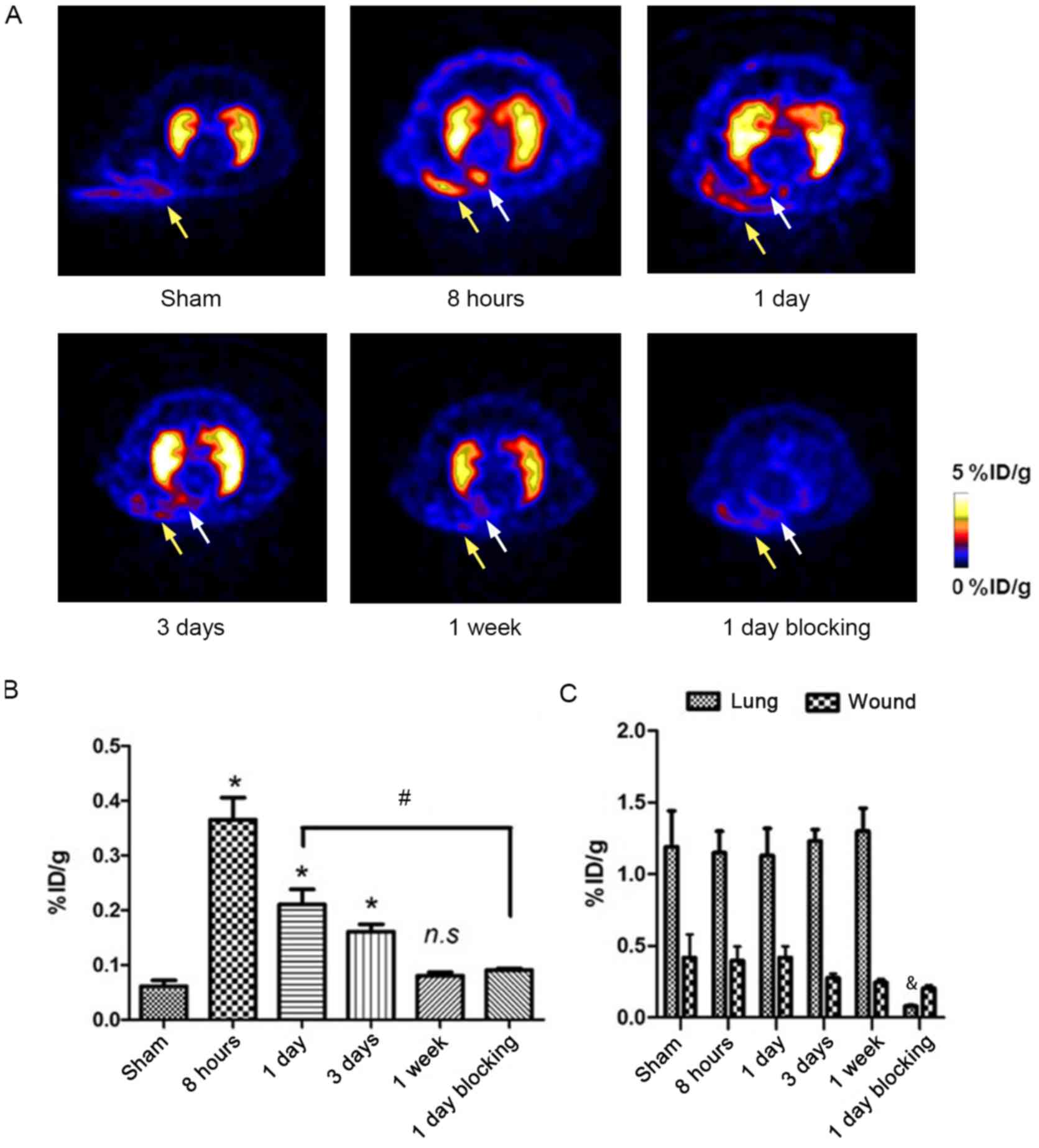 | Figure 5.Longitudinal PET imaging of
18F-AlF-NOTA-MAL-Cys40-exendin-4 in rats
following MI/R. (A) Representative transaxial positron emission
tomography images using
18F-AlF-NOTA-MAL-Cys40-exendin-4 at different
time points following MI/R. High
18F-AlF-NOTA-MAL-Cys40-exendin-4 accumulation
region in infarcted/ischemic myocardium is indicated by white
arrows. High tracer uptake was detected in the lungs and did not
change over time. In surgical wounds (yellow arrows), tracer uptake
decreased over time. (B) Quantification of
18F-AlF-NOTA-MAL-Cys40-exendin-4 accumulation
in infarcted/ischemic myocardium following MI/R over time,
expressed as %ID/g of tissue. (C) Quantification of
18F-AlF-NOTA-MAL-Cys40-exendin-4 accumulation
in lung and surgical wound region following MI/R over time,
expressed as %ID/g of tissue. *P<0.05 vs. sham;
#P<0.05 vs. 8 h; &P<0.05 vs. 1 day.
18F, fluorine-18; %ID/g, percentage of injected dose per
gram; AlF, aluminum fluoride; Cys, cysteine; MAL, maleimide; MI/R,
myocardial ischemia and reperfusion; NOTA,
1,4,7-triazacyclononanetriacetic acid. |
To further test the GLP-1R binding specificity of
18F-AlF-NOTA-MAL-Cys40-exendin-4, unlabeled
exendin-4 was used as a blocking agent. Tracer uptake was
significantly lower compared with unblocked rats at post-MI/R day 1
(0.09±0.041 vs. 0.20±0.02 %ID/g, respectively; P<0.05; Fig. 5A and B).
Ischemic operation did not affect lung uptake of the
radiotracer. The %ID/g of the lung remained high throughout the
experiment (from 8 h to 1 week following MI/R) at 1.19±0.25 (n=6;
Fig. 5C).
18F-AlF-NOTA-MAL-Cys40-exendin-4 accumulation
within the surgical wound (yellow arrows) was 0.42±0.12 %ID/g at 8
h following MI/R (Fig. 5C).
Western blotting and
immunohistochemical staining
To verify the direct relation between GLP-1R
upregulation and high uptake of
18F-ALF-NOTA-MAL-Cys40-exendin-4, western
blot analysis and immunohistochemical staining were preformed using
a GLP-1R-specific antibody on myocardial samples from MI/R and
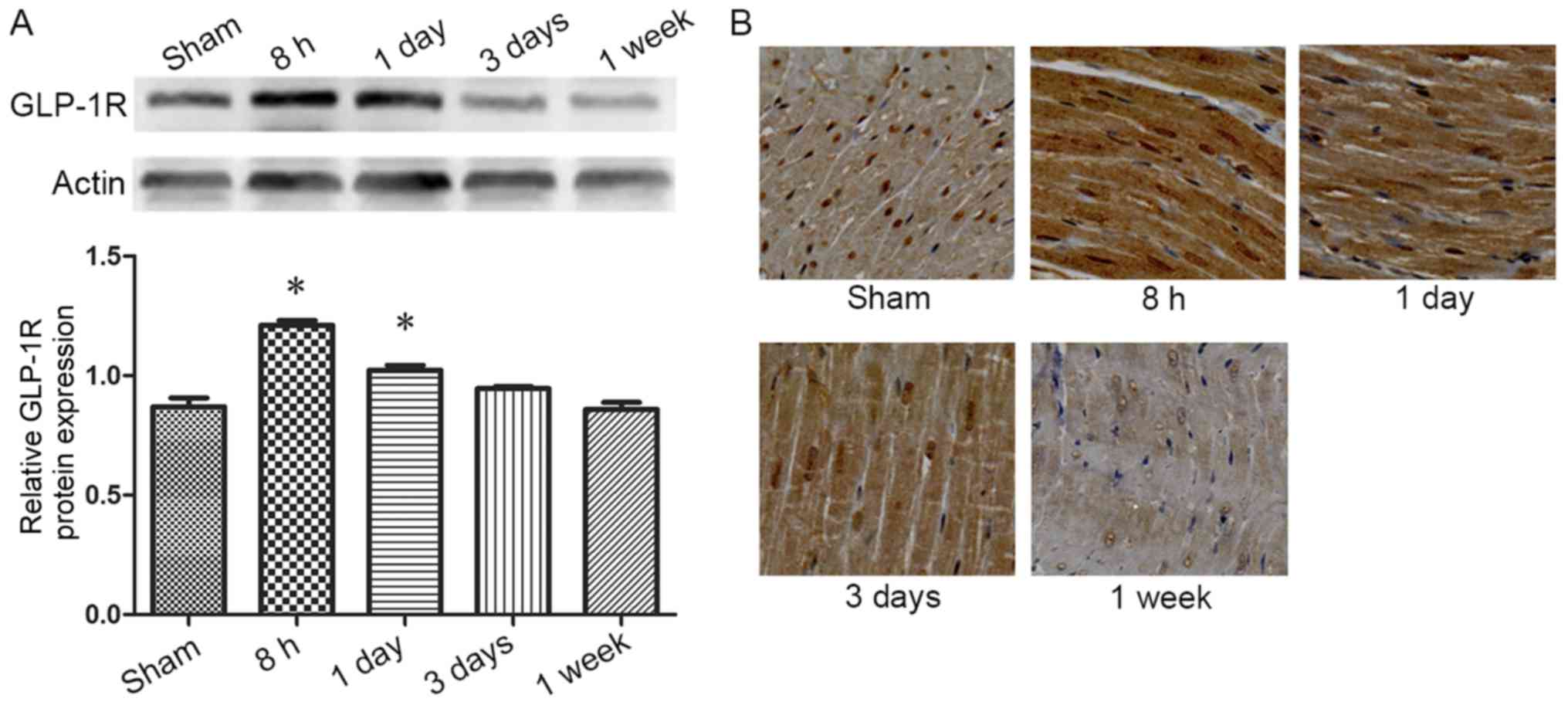
sham-operated rats. GLP-1R protein level increased at 8 h post-MI/R
compared with the sham-operated group (P<0.05; Fig. 6A), and the dynamic change observed
later was consistent with that of tracer uptake (Fig. 5B). The immunostaining results
revealed notably enhanced cardiomyocyte staining of GLP-1R at 8 h
and 1 day post-MI/R compared with the sham-operated group. The
staining density decreased with time and returned to control levels
in 1 week (Fig. 6B).
Discussion
Reperfusion injury is a complex process that
involves a series of signaling pathways (21). From extracellular ligands to
intracellular signal cascades, receptors serve a key role in
delivering signals. GLP-1 and its analogs have been reported to
exert cardioprotective effects in several experimental models and
preliminary clinical trials, but the underlying mechanism has yet
to be studied (22,23). Therefore, an effective method for
monitoring in vivo dynamic changes in GLP-1R expression may
be of great value.
PET imaging is a promising method for receptor
imaging for research use; however, the synthesis of radiotracers
for PET requires consideration of biological and chemical issues.
Several positron-emitting radionuclides have been used for PET
imaging in the laboratory (64Cu, 68Ga,
18F, 11C), among which 18F stands
out for its ideal imaging properties and may have the potential to
be translated into clinical practice (14,24).
The T1/2 of 18F (109.8 min) allows
long-duration, multistep radiosynthesis, and the relatively low
positron emission energy (0.64 MeV) provides a short tissue range,
thus allowing generation of high-resolution images (25). However, combining 18F
with target peptides has remained a challenge. In our previous
study, 18F-FBEM-Cys40-exendin-4 was
developed, which exhibited high and receptor-specific accumulation
in INS-1 rat insulinoma models (17). However, the synthesis of
18F-FBEM-Cys40-exendin-4 requires a multistep
procedure, specialized equipment and well-trained personnel, which
may counteract the advantages that enable the tracer to translate
into clinical practice.
In the present study, a novel GLP-1R specific
radiotracer was synthesized by conjugating 18F-AlF to
NOTA-MAL-Cys40-exendin-4; the entire process required
only one step and was completed in 30 min, which was notably less
compared with the time required to synthesize
18F-FBEM-Cys40-exendin-4 (17) and other GLP-1R-based radiotracers
such as 18F-FBEM-EM3106B (26) and
18F-FBEM-Cys39-exendin-4 (27). The yield of
18F-AlF-NOTA-MAL-Cys40-exendin-4 was moderate
at 18.5±3.4% (not decay corrected), with a purity of >95%. No
HPLC purification was required, thus simplifying the synthesis
process.
Longitudinal PET in a rat model of MI/R with
18F-AlF-NOTA-MAL-Cys40-exendin-4 was
performed. Ischemic myocardium, lung and surgical wounds showed
higher tracer accumulation compared with other tissues.
Pre-injected unlabeled Cys40-exendin-4 significantly
blocked tracer accumulation, which indicated that the accumulation
of 18F-AlF-NOTA-MAL-Cys40-exendin-4 was
GLP-1R-specific. Preliminary western blotting experiments
demonstrated that GLP-1R expression was highest at 8 h following
ischemia/reperfusion injury; therefore, 8 h was indicated as a
critical time point. Compared with focal tracer uptake in ischemic
myocardium at 8 h, 1 day, 3 days, and 1 week following the onset of
ischemia, tracer uptake reached the highest level at 8 h post-MI/R;
tracer accumulation then decreased over time. At 1 week post-MI/R,
tracer uptake within ischemic myocardium was restored to the
near-normal level. The performance of
18F-AlF-NOTA-MAL-Cys40-exendin-4 as a
radiotracer in rat hearts was similar to that of
18F-FBEM-Cys40-exendin-4 (18). The results of western blotting and
immunohistochemical staining were consistent with PET. Thus, a
non-invasive method for molecular imaging was established to
monitor dynamic changes in GLP-1R expression in ischemic myocardium
in vivo. However, the experiments conducted in the present
study offered only indirect evidence of association of the analog
with GLP-1R, and the mechanism by which reperfusion induces GLP-1R
upregulation remains unclear and requires further
investigation.
Lung tissue exhibited higher tracer accumulation
compared with ischemic myocardium at all time points owing to high
levels of GLP-1R expression, which may compromise the value of
18F-AlF-NOTA-MAL-Cys40-exendin-4 in clinical
use. However, considering that human lung tissue expresses
significantly lower levels of GLP-1R compared with rat lung tissue,
the ischemic myocardium-to-lung ratio of tracer uptake may be
higher in humans (28). The analog
may be eliminated through the lungs in a pharmacokinetic pathway,
which requires further investigation. Surgical wounds exhibited
increased tracer accumulation that could not be completely blocked
with unlabeled Cys40-exendin-4. Thus, the increased
accumulation of tracer in surgical wounds may have been due to
increased vascular permeability caused by inflammatory
reaction.
The results of the present preliminary evaluation of
18F-AlF-NOTA-MAL-Cys40-exendin-4 revealed a
dynamic pattern of GLP-1R expression following MI/R in rats, which
suggested that GLP-1 may exert its cardioprotective effects early
following MI/R, when GLP-1R expression is relatively high. After 1
week, the therapeutic effect of GLP-1 may be compromised owing to
low receptor expression. Consistent with the present study,
previous studies demonstrated that the administration of GLP-1R
agonists during the early stages of acute cardiac ischemia
protected against MI/R injury (9,29).
In conclusion,
18F-AlF-NOTA-MAL-Cys40-exendin-4 was
synthesized in a time-efficient and effective way. PET imaging
using 18F-AlF-NOTA-MAL-Cys40-exendin-4
displayed high ischemic myocardium-to-background radioactivity
ratios, and further analysis revealed dynamic changes in GLP-1R
expression in rats following MI/R. The radiotracer offers a new
approach to observe the dynamic changes of GLP-1R expression
following MI/R injury, which may help clinicians make decisions
regarding the exact time points of GLP-1R agonist administration
during MI/R injury treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81570210).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XP established the myocardial ischemia/reperfusion
rat models, performed positron emission tomography imaging and
wrote the manuscript. QX synthesized the radiotracer and
contributed to the acquisition of data. JC established the animal
models. TW performed western blot analysis and immunohistochemical
staining. MZ analyzed the data and revised the manuscript. HW and
HG designed the experiments and critically revised the
manuscript.
Ethics approval and consent to
participate
All animal studies were conducted in accordance with
the principles and procedures outlined in the Guide for the Care
and Use of Laboratory Animals, and the protocols were approved by
the Laboratory Animal Ethics Committee of The Fourth Military
Medical University (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the American Heart Association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jennings RB, Sommers HM, Smyth GA, Flack
HA and Linn H: Myocardial necrosis induced by temporary occlusion
of a coronary artery in the dog. Arch Pathol. 70:68–78.
1960.PubMed/NCBI
|
|
3
|
Jordan JE, Zhao ZQ and Vinten-Johansen J:
The role of neutrophils in myocardial ischemia-reperfusion injury.
Cardiovasc Res. 43:860–878. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lesnefsky EJ, Chen Q, Tandler B and Hoppel
CL: Mitochondrial dysfunction and myocardial ischemia-reperfusion:
Implications for novel therapies. Annu Rev Pharmacol Toxicol.
57:535–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferdinandy P, Hausenloy DJ, Heusch G,
Baxter GF and Schulz R: Interaction of risk factors, comorbidities,
and comedications with ischemia/reperfusion injury and
cardioprotection by preconditioning, postconditioning, and remote
conditioning. Pharmacol Rev. 66:1142–1174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drucker DJ: The biology of incretin
hormones. Cell Metab. 3:153–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Theodorakis MJ, Carlson O, Michopoulos S,
Doyle ME, Juhaszova M, Petraki K and Egan JM: Human duodenal
enteroendocrine cells: Source of both incretin peptides, GLP-1 and
GIP. Am J Physiol Endocrinol Metab. 290:E550–E559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holst JJ, Deacon CF, Vilsboll T, Krarup T
and Madsbad S: Glucagon-like peptide-1, glucose homeostasis and
diabetes. Trends Mol Med. 14:161–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonne DP, Engstrom T and Treiman M:
Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36)
amide against ischemia-reperfusion injury in rat heart. Regul Pept.
146:243–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM,
Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M and Drucker
DJ: GLP-1R agonist liraglutide activates cytoprotective pathways
and improves outcomes after experimental myocardial infarction in
mice. Diabetes. 58:975–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bose AK, Mocanu MM, Carr RD, Brand CL and
Yellon DM: Glucagon-like peptide 1 can directly protect the heart
against ischemia/reperfusion injury. Diabetes. 54:146–151. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deacon CF, Johnsen AH and Holst JJ:
Degradation of glucagon-like peptide-1 by human plasma in vitro
yields an N-terminally truncated peptide that is a major endogenous
metabolite in vivo. J Clin Endocrinol Metab. 80:952–957. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eng J, Kleinman WA, Singh L, Singh G and
Raufman JP: Isolation and characterization of exendin-4, an
exendin-3 analogue, from Heloderma suspectum venom. Further
evidence for an exendin receptor on dispersed acini from guinea pig
pancreas. J Biol Chem. 267:7402–7405. 1992.PubMed/NCBI
|
|
14
|
Mikkola K, Yim CB, Fagerholm V, Ishizu T,
Elomaa VV, Rajander J, Jurttila J, Saanijoki T, Tolvanen T, Tirri
M, et al: 64Cu- and 68Ga-labelled
[Nle(14),Lys(40)(Ahx-NODAGA)NH2]-exendin-4 for pancreatic beta cell
imaging in rats. Mol Imaging Biol. 16:255–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wild D, Behe M, Wicki A, Storch D, Waser
B, Gotthardt M, Keil B, Christofori G, Reubi JC and Mäcke HR:
[Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for
glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med.
47:2025–2033. 2006.PubMed/NCBI
|
|
16
|
Sowa-Staszczak A, Pach D, Mikolajczak R,
Mäcke H, Jabrocka-Hybel A, Stefańska A, Tomaszuk M, Janota B,
Gilis-Januszewska A, Małecki M, et al: Glucagon-like peptide-1
receptor imaging with [Lys40(Ahx-HYNIC-99mTc/EDDA)NH2]-exendin-4
for the detection of insulinoma. Eur J Nucl Med Mol Imaging.
40:524–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kiesewetter DO, Gao H, Ma Y, Niu G, Quan
Q, Guo N and Chen X: 18F-radiolabeled analogs of exendin-4 for PET
imaging of GLP-1 in insulinoma. Eur J Nucl Med Mol Imaging.
39:463–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao H, Kiesewetter DO, Zhang X, Huang X,
Guo N, Lang L, Hida N, Wang H, Wang H, Cao F, et al: PET of
glucagonlike peptide receptor upregulation after myocardial
ischemia or reperfusion injury. J Nucl Med. 53:1960–1968. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiesewetter DO, Guo N, Guo J, Gao H, Zhu
L, Ma Y, Niu G and Chen X: Evaluation of an [(18)F]AlF-NOTA analog
of exendin-4 for imaging of GLP-1 receptor in insulinoma.
Theranostics. 2:999–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Silverman J, Mark A and Murthy S: The
Iacuc Handbook. Iacuc Handbook; 2000
|
|
21
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Timmers L, Henriques JP, de Kleijn DP,
Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek
JJ, Doevendans PA, et al: Exenatide reduces infarct size and
improves cardiac function in a porcine model of ischemia and
reperfusion injury. J Am Coll Cardiol. 53:501–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dawwas GK, Smith SM and Park H: Risk of
heart failure hospitalization among users of dipeptidyl peptidase-4
inhibitors compared to glucagon-like peptide-1 receptor agonists.
Cardiovasc Diabetol. 17:1022018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller PW, Long NJ, Vilar R and Gee AD:
Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron
emission tomography. Angew Chem Int Ed Engl. 47:8998–9033. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McBride WJ, D'Souza CA, Sharkey RM,
Karacay H, Rossi EA, Chang CH and Goldenberg DM: Improved 18F
labeling of peptides with a fluoride-aluminum-chelate complex.
Bioconjug Chem. 21:1331–1340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao H, Niu G, Yang M, Quan Q, Ma Y, Murage
EN, Ahn JM, Kiesewetter DO and Chen X: PET of insulinoma using
(1)(8)F-FBEM-EM3106B, a new GLP-1 analogue. Mol Pharm. 8:1775–1782.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Y, Pan D, Xu Q, Zhu C, Wang L, Chen F,
Yang R, Luo S and Yang M: Insulinoma imaging with glucagon-like
peptide-1 receptor targeting probe (18)F-FBEM-Cys (39)-exendin-4. J
Cancer Res Clin Oncol. 140:1479–1488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korner M, Stockli M, Waser B and Reubi JC:
GLP-1 receptor expression in human tumors and human normal tissues:
Potential for in vivo targeting. J Nucl Med. 48:736–743. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nikolaidis LA, Mankad S, Sokos GG, Miske
G, Shah A, Elahi D and Shannon RP: Effects of glucagon-like
peptide-1 in patients with acute myocardial infarction and left
ventricular dysfunction after successful reperfusion. Circulation.
109:962–965. 2004. View Article : Google Scholar : PubMed/NCBI
|