Introduction
Polycystic ovary syndrome (PCOS) exhibits high
morbidity and affects ~4% of women of reproductive age worldwide
(1,2). PCOS is frequently accompanied by a
number of alterations in the reproductive, endocrine and metabolic
pathways. Patients with PCOS typically present with polycystic
ovaries, insulin resistance, chronic anovulation and infertility
(3,4). Previous studies revealed that PCOS
pathogenesis is due to environmental and genetic factors (5); however, efforts to understand the
underlying pathogenic mechanisms are complicated by the
heterogeneity of PCOS.
Prokineticin 1 (PROK1), also known as endocrine
gland-derived vascular endothelial growth factor (EG-VEGF), is
involved in ovarian physiology, endometrial receptivity, embryo
implantation and successful pregnancies, together with its
receptors [PROK1 receptors (PROKRs)] (6,7).
Previous research revealed that PROK1 is an angiogenic and
proliferative factor predominantly expressed in the organs of the
female reproductive system (8).
PROK1 is also involved in female reproductive pathophysiological
processes (8). In addition, PROK1
was reported to be differentially expressed during the cycle of
vascular morphogenesis in the primate ovary and polycystic human
ovaries, compared with VEGF (9–11).
There is also evidence that PROK1 mediates placental angiogenesis
directly or indirectly (11);
however, the precise mechanisms of PROK1 in PCOS require further
investigation.
MicroRNAs (miRNAs/miRs) are widely expressed small
RNAs that negatively regulate the post-transcriptional expression
of functional genes (12).
Aberrant expression of miRNAs has been broadly identified as an
important factor in the development of metabolic disorders, such as
insulin resistance and obesity (13,14).
A recent study reported that miRNAs serve important roles in PCOS,
and that the concentration of miR-155 in serum may be a biomarker
for monitoring estroprogestinic treatment (15). Dysregulated expression of another
miRNA, miR-28-5p, has been frequently reported in the development
of various types of cancers, including hepatocellular carcinoma,
colorectal and prostate cancer (16–18),
and most recently ovarian cancer (19). Shi and Teng (18) demonstrated that miR-28-5p
expression was downregulated in hepatocellular carcinoma, and that
cell proliferation and tube formation capacity were attenuated in
cells expressing miR-28-5p. Our previous research indicated that
hypermethylation of the miR-28-5p promoter reduces miR-28-5p
expression and promotes cell proliferation in rat ovary granulosa
cells (Meng et al, unpublished data); however, the
downstream mechanisms underlying the effects of miR-28-5p remain
unclear.
A previous study indicated that cell proliferation
and follicular growth are activated during all stages of ovarian
development in PCOS, indicating that cell death in the ovary may
mediate follicular atresia (20).
There is also evidence that granulosa cell death occurs following
oocyte apoptosis (21). Therefore,
cell proliferation and survival may be responsible for the
development of PCOS.
In the present study, decreased expression of PROK1
was reported in cells transfected with miR-28-5p mimics. A
dual-luciferase reporter assay indicated that miR-28-5p targeted
the 3′-untranslated region (3′-UTR) of PROK1, attenuating PROK1
expression. Finally, cell proliferation, apoptosis and cell cycle
distribution were evaluated following transfection with a
PROK1-overexpressing plasmid, in the presence or absence of
miR-28-5p mimics. The results of the present study indicated that
miR-28-5p inhibited the progression of PCOS by targeting PROK1,
which acts via the PI3K/AKT/mTOR signaling pathway, suggesting that
PROK1 may be a potentially useful target in the treatment of
PCOS.
Materials and methods
Cell culture and transfection
The rat ovary granulosa cells used in the present
study were primary cells prepared by Procell Life Science &
Technology Co., Ltd. (cat. no. CP-R050) via mechanical separation,
collagenase digestion and differential adhesion. The cells were
cultured in DMEM/F12 (HyClone; GE Healthcare Life Sciences) with
15% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.),
epidermal growth factor (10 ng/ml; cat. no. E5036; Sigma-Aldrich;
Merck KGaA), and penicillin and streptomycin (100 U/ml) at 37°C and
5% CO2. miR-28-5p mimics (50 nM), inhibitor (50 nM) and
NC (50 nM) were synthesized by Shanghai GenePharma Co., Ltd., and
pcDNA3.0-PROK1 plasmids (4 µg) were transfected into cells using
Lipofectamine® 2000 transfection reagent (Thermo Fisher
Scientific, Inc.) according the manufacturer's protocols. Briefly,
cells (1.5×105 cells/well) were seeded in six-well
plates containing complete medium without antibiotics for 24 h
prior to transfection. Cell transfection was conducted in six-well
plates, in which each well contains a total of 2 ml medium with 5
µl Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
and 10 µl mimics. Assays were performed 24 h following cell
transfection. Empty pcDNA3.0 vector was used as a negative control
(NC) for PROK1 overexpression. Nonsense sequences were used as the
NC for miR-28-5p mimics and inhibitors. The sequences of mimics,
inhibitor and NC are the as follows: miR-28-5p mimics,
5′-AAGGAGCTCACAGTCTATTGAG-3′; miR-28-5p inhibitor
5′-UUCCUCGAGUGUCAGAUAACUC-3′; NC, 5′-UUCUCCGAACGUGUCACGUTT-3′.
Animals
Female Sprague Dawley rats (age, 21 days; weight,
50±10 g; n=12) had free access to food and water under a controlled
temperature of 23±2°C and humidity (55–65%) with a 12-h light/dark
cycle. A PCOS model was established as previously described
(22).
Plasmid construction
Total RNA was extracted using TRIzol reagent (Takara
Bio, Inc.) from ovary granulosa cells (cat. no. CP-R050; Procell
Life Science & Technology Co., Ltd.) according to the
manufacturer's protocols. Reverse transcription reaction was
performed using a Bester™ qPCR RT Kit (cat. no. DBI-2220; DBI
Bioscience). The reverse transcription protocol was as follows: RNA
denaturation at 65°C for 5 min; genomic DNA removal at 37°C for 5
min; reverse transcription at 37°C for 15 min; reverse
transcriptase inactivation at 98°C for 5 min. The vector pcDNA3.0
(Guangzhou Vipotion Biotechnology, Co., Ltd.) was used for the
overexpression of PROK1. The coding sequence of PROK1 was cloned
using the following primers: Forward
5′-CGGGATCCATGAGAGGTGCTGTGCAAGTCT-3′ and reverse,
5′-CCGCTCGAGCTAAAAGTTGACATTCTTCAAGTCC-3′. The PCR mixture
contained: 0,25 µl PrimeSTARRHS DNA Polymerase (Clontech
Laboratories, Inc.), 5 µl 5X PrimeSTAR buffer (Clontech
Laboratories, Inc.), 2 µl dNTP Mix (Clontech Laboratories, Inc.);
0.5 µl primers (Sangon Biotech Co., Ltd.), 1 µl cDNA and 15.75 µl
ddH2O, (Takara Bio, Inc.). The thermocycling conditions as follows:
Initial denaturation at 94°C for 5 min, followed by 30 cycles of
94°C for 30 sec; 58°C for 30 sec; 72°C for 30 sec; 72°C 5 min.
Then, amplification products were ligated into the vector using
BamHI and XhoI restriction sites and identified via
double enzyme digestion. Empty vector was used as a NC. Animal
experiments were performed in compliance with the ARRIVE guidelines
(23), once ethical approval from
the Nanfang Hospital Animal Ethics Committee had been obtained.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNAs were extracted using TRIzol®
reagent (Takara Bio, Inc.) according to the manufacturer's
protocols. Total RNA (~400 ng) was reverse transcribed into cDNA
using a Bestar qPCR RT kit (cat. no. 2200; Kay Biological
Technology Co., Ltd.). Samples were incubated at 37°C for 15 min
and at 85°C for 5 min. U6 small nuclear RNA was used as an internal
reference for miR-28-5p expression; primers were obtained from
Takara Bio, Inc. GAPDH was used as internal reference; primers were
synthesized by Sangon Biotech Co., Ltd. A SYBR Green qPCR kit (Kay
Biological Technology Co., Ltd.) was used to quantify miRNA or mRNA
expression using a Stratagene Real time PCR system (Mx3000P;
Agilent Technologies, Inc.). The amplification reactions were
performed under the following conditions: Initial denaturation at
95°C for 5 min, followed by 40 cycles of 95°C for 15 sec, 58°C for
20 sec, and 72°C for 30 sec. All qPCR reactions were repeated at
least three times. Relative mRNA or miRNA expressions were
calculated by 2−ΔΔCq method (24). The primers are listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene symbols | Sequence
(5′-3′) |
|---|
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGTCTCA |
| miR-28-5p | F:
AAGGAGCTCACAGTCTATTGAG |
| Universal
reverse | R:
ACTGGTGTCGTGGA |
| GAPDH | F:
CCTCGTCTCATAGACAAGATGGT |
|
| R:
GGGTAGAGTCATACTGGAACATG |
| PROK1 | F:
CAACTGTCTCTGACTGTGCG |
|
| R:
AAGGGATCTTGTGGCTTCCA |
Western blotting
Cells (~106) were lysed using protein
lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
and total protein was then extracted. Subsequently, a Pierce BCA
Protein Assay kit (cat. no. 23227; Pierce; Thermo Fisher
Scientific, Inc.) was used to determine the total protein
concentration. Equal amounts of total protein (10 µg) were
separated by 12% SDS-PAGE. The PVDF membrane was placed in a 5% BSA
blocking solution for 1.5 h at room temperature. The membranes were
incubated at 4°C for 12 h with primary antibodies against PROK1
(1:500; cat. no. ab72807; Abcam), Bcl-2 (1:1,000; cat. no.
ab196495; Abcam), Bax (1:1,000; cat. no. ab32503; Abcam), caspase-3
(1:500; cat. no. ab13847; Abcam), p62 (1:1,000; cat. no. 16177;
Cell Signaling Technology), cyclin D1 (1:200; cat. no. ab16663;
Abcam), PI3K (1:1,000; cat. no. 4255S; Cell Signaling Technology),
phosphorylated (p-)PI3K (1:1,000; cat. no. 4228T; Cell Signaling
Technology), AKT (1:1,000; cat. no. 4691S; Cell Signaling
Technology), p-AKT (1:2000; cat. no. 4060S; Cell Signaling
Technology), mTOR (1:1,000; cat. no. 2983S; Cell Signaling
Technology), p-mTOR (1:1,000; cat. no. 5536S; Cell Signaling
Technology) and GAPDH (1:1,000; cat. no. ab8245; Abcam). The PVDF
membrane was placed in a diluted horseradish peroxidase-conjugated
rabbit anti-mouse immunoglobulin G secondary antibody (1:10,000;
cat. no. ab6728; Abcam) solution and incubated for 1 h at room
temperature. The intensities of labeled proteins were visualized
using X-film (cat. no. 4741023951; Yestar Healthcare Holdings Co.,
Ltd.; http://www.yestarcorp.com/) with an ECL
chemiluminescence detection kit (BeyoECL Plus; cat. no. P0018M;
Beyotime Institute of Biotechnology) and ImageJ (version 1.8.0;
National Institutes of Health) was used to quantify expression by
densitometry.
Cell Counting Kit-8 (CCK-8)
Cells (2×104) transfected with exogenous
sequences were suspended in 200 µl culture medium and seeded into
96-well plates. The CCK-8 assay was conducted following culture
under standard conditions (37°C) for a further 48 h. Then, 10 µl
CCK-8 reagent (Beijing Solarbio Science & Technology Co., Ltd.)
was added to each well, followed by incubation for a further 1 h at
37°C. Finally, the absorbance at 450 nm was detected for
quantification of cell proliferation rates using a plate reader
(BioTek Instruments, Inc.).
5-Ethynyl-2′-deoxyuridine (EdU)
staining and flow cytometry
Cells (104) transfected with exogenous
sequences were plated into 96-well plates and cultured for 48 h.
Then, 100 µl EdU reagent (cat. no. B23151; Invitrogen; Thermo
Fisher Scientific, Inc.) was added to each well after washing with
PBS. The cells were incubated at 37°C for 2 h. 100 µl glycerol was
added to each well for neutralization after cells were fixed with
4% paraformaldehyde for 15 min at room temperature. Subsequently,
0.5% Triton X-100 was added into each well, and cells were
incubated for 10 min at room temperature, followed by washing with
PBS. Apollo reagents and Hoechst 33342 staining reagent included in
an EdU kit (cat. no. CA1170; Beijing Solarbio Science &
Technology Co., Ltd.) were used according to the manufacturer
protocols. Then, the cells were incubated for 30 min at room
temperature. Cells were analyzed via flow cytometry and the
software used for the analysis was Cytomics FC500 Flow Cytometry
CXP Software v2.0 (CytoFLEX; Beckman Coulter, Inc.).
Cell apoptosis and cell cycle
distribution
Following transfection, 104 cells were
cultured for 48 h, and the apoptosis of cells was then analyzed
using the Annexin V-FITC/propidium iodide (PI) cell apoptosis
detection kit (BD Biosciences) containing according to the
manufacturer's protocols, and cells were incubated at 4°C for 30
min. Cells with positive signals for Annexin V-FITC and PI were in
the late stage of apoptosis, whereas cells with positive signals
for Annexin V-FITC only were in the early apoptosis stage. The
total apoptosis ratio was defined by the summation of
late-apoptotic and early-apoptotic cells.
Following transfection of cells with exogenous
sequences, cells were stained with PI dye was incubated at 4°C for
30 min to determine the cell cycle distribution. A flow cytometer
was used to analyze the cells and the software used for the
analysis was Cytomics FC500 Flow Cytometry CXP Software v2.0
(Beckman Coulter, Inc.).
Dual-luciferase reporter assay
TargetScan 7.2 (http://www.targetscan.org) was used to identify the
putative targets of miR-28-5p, and PROK1 was found to be one of the
targets of miR-28-5p. The software was used according to previous
report (25). The 3′-UTR sequences
of the PROK1 gene were obtained using primers (forward,
5′-CCGCTCGAGTGGGTGACCTCTTGTGTTACATCTG-3′ and reverse,
5′-ATTTGCGGCCGCCCCAGCCCACCTCACACTCATA-3′; Sangon Biotech Co.,
Ltd.). Mutated (MUT) 3′-UTR sequences were amplified using paired
primers with a mismatched nucleotide introduced in the upstream
primer (MUT; forward, 5′-TTTCTTTCATTGGCTCCGATGATGTTTTAGGAGTAAG-3′
and reverse, 5′-CTTACTCCTAAAACATCATCGGAGCCAATGAAAGAAA-3′; Sangon
Biotech Co., Ltd.). Amplification products including the wild-type
(WT) and MUT 3′-UTRs of PROK1 were then separately ligated into
Psi-CHECK2 plasmids (cat. no. C8021; Promega Corporation).
Psi-CHECK2 plasmids were co-transfected with miR-28-5p mimics or NC
into cells using Lipofectamine 2000™ and incubated for 48 h at
37°C. The luciferase activities of firefly and Renilla were
measured with the Dual-Luciferase Reporter Assay system (cat. no.
C8021; Promega Corporation) according to the manufacturer's
protocol. Firefly luciferase activity was normalized to Renilla
luciferase activity. Cell lysates were collected using a GloMax
machine (Promega Corporation).
Statistical analysis
Data from >3 biological replicates for each assay
were presented as the mean ± standard deviation, and data were
analyzed using SPSS 16.0 software (SPSS, Inc.). Student's t-test
was used to analyze differences between two groups, and one-way
analysis of variance followed by Tukey's test was performed to
compare differences between three or more groups.
Results
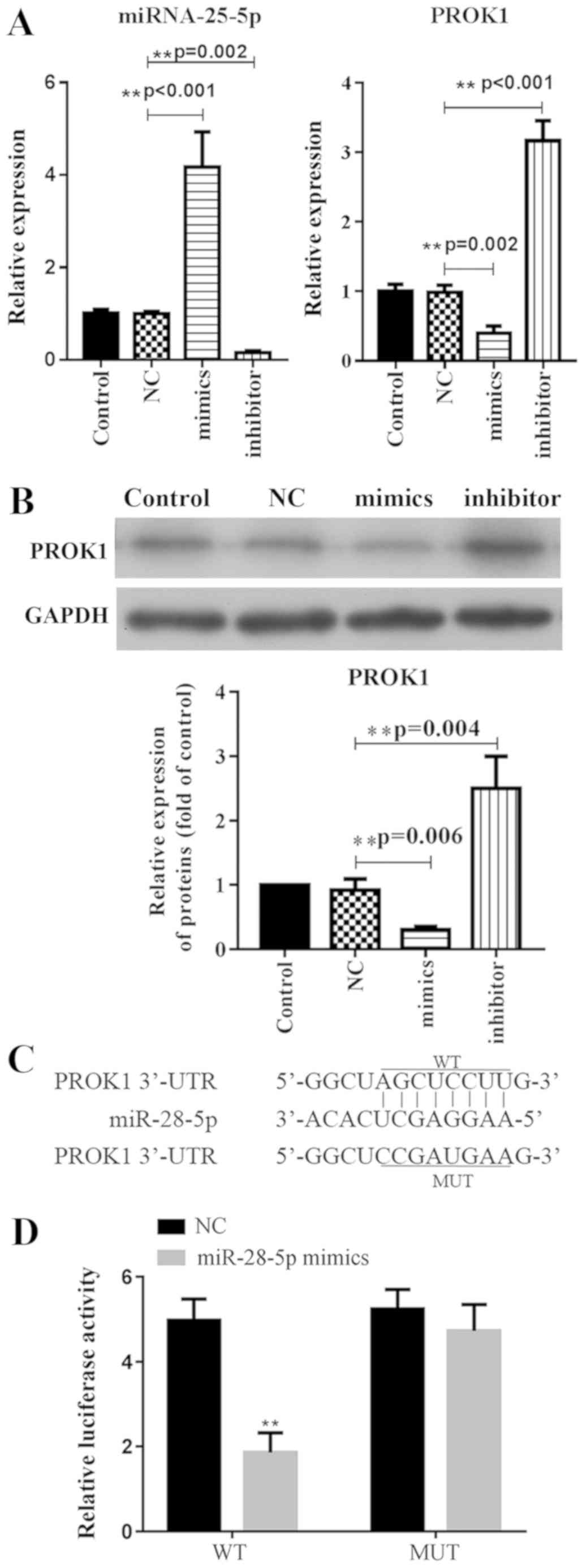
miR-28-5p suppresses PROK1 expression
by targeting its 3′-UTR
To investigate the relationship between miR-28-5p
and PROK1, the mRNA expression levels of PROK1 were analyzed by
qPCR, and the protein expression was evaluated via western blotting
following transfection of ovary granule cells with miR-28-5p
mimics. It was demonstrated that mRNA expression of PROK1 was
significantly suppressed in miR-28-5p overexpressing cells compared
with the NC (Fig. 1A). Similarly,
the protein expression of PROK1 was significantly downregulated
following the transfection of ovary granule cells with miR-28-5p
mimics compared with the NC (Fig.
1B). Conversely, transfection with miR-28-5p inhibitor induced
the opposite results in PROK1 expression in ovary granule cells
(Fig. 1A and B).
To determine whether miR-28-5p binds to PROK1, the
3′-UTR of PROK1 was cloned and inserted into psi-CHECK2 plasmids.
For comparison, the binding site predicted by the online database
TargetScan was mutated, and then also inserted into psi-CHECK2
plasmids (Fig. 1C). Cells
co-transfected with WT 3′-UTR and miR-28-5p exhibited significantly
reduced luciferase activity compared with cells transfected with NC
or MUT 3′-UTR (Fig. 1D). The
results indicated that miR-28-5p negatively regulated PROK1
expression via direct binding to its 3′-UTR.
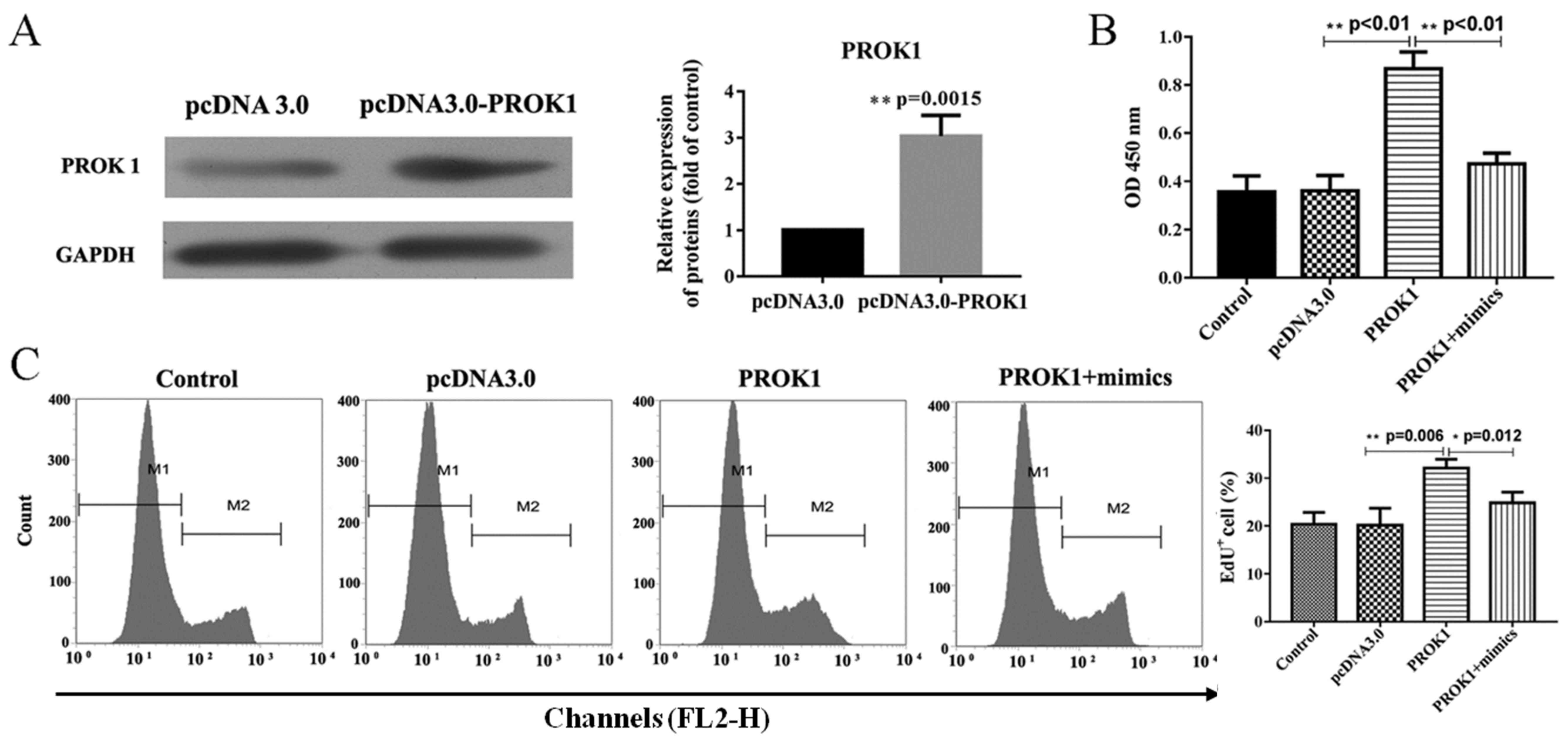
PCOS development promoted by PROK1
overexpression is inhibited by miR-28-5p via the PI3K/AKT/mTOR
signaling pathway
PCOS is a common endocrine-reproductive disease in
females and it has been demonstrated that ovary granular cells in
PCOS patients showed increased apoptotic rates (17,26).
To verify whether PROK1 promotes PCOS in rat ovary granule cells, a
PROK1 overexpression plasmid was constructed. Additionally, a
rescue experiment series was designed to further demonstrate the
influence of the interaction between miR-28-5p and PROK1 on rat
ovary granule cell survival. As presented in Fig. 2A, a western blot assay verified the
overexpression of the PROK1 plasmid, revealing that PROK1 was
significantly upregulated in cells transfected with the PROK1
overexpression plasmid compared with empty vector. The
overexpression of PROK1 protein was accompanied by significantly
increased cell proliferation rates in rat ovary granule cells
(Fig. 2B and C); however, the
effects of PROK1 upregulation on proliferation were attenuated by
co-expression of PROK1 and miR-28-5p mimics, compared with cells
transfected with pcDNA-PROK1 alone.
Additionally, apoptosis was significantly suppressed
in rat ovary granule cells overexpressing PROK1 compared with empty
vector (Fig. 3A and B), and a
significantly increased percentage of these cells were in S phase
(Fig. 3C and D). These tendencies
were attenuated by co-transfection with miR-28-5p mimics, which
elevated cell apoptosis, and redistributed cells to the G0/G1 phase
compared with pcDNA-PROK1 alone. The expression of apoptosis- and
cell cycle-associated proteins was investigated via western
blotting (Fig. 3E). Compared with
empty pcDNA3.0 vector, transfection with pcDNA3-PROK1 significantly
upregulated Bcl-2 and cyclin D1 expression, whereas caspase-3, Bax
and p62 expression levels were downregulated; however,
co-transfection with miR-28-5p mimics attenuated these effects.
Additionally, it was revealed via western blotting that
PI3K/AKT/mTOR activation was also potentiated in
PROK1-overexpressing cells compared with the control (Fig. 3F). Conversely, this activation was
reversed by co-transfection with miR-28-5p mimics. These results
indicated that PROK1 promoted the proliferation and suppressed the
apoptosis of rat ovary granule cells via the PI3K/AKT/mTOR
signaling pathway.
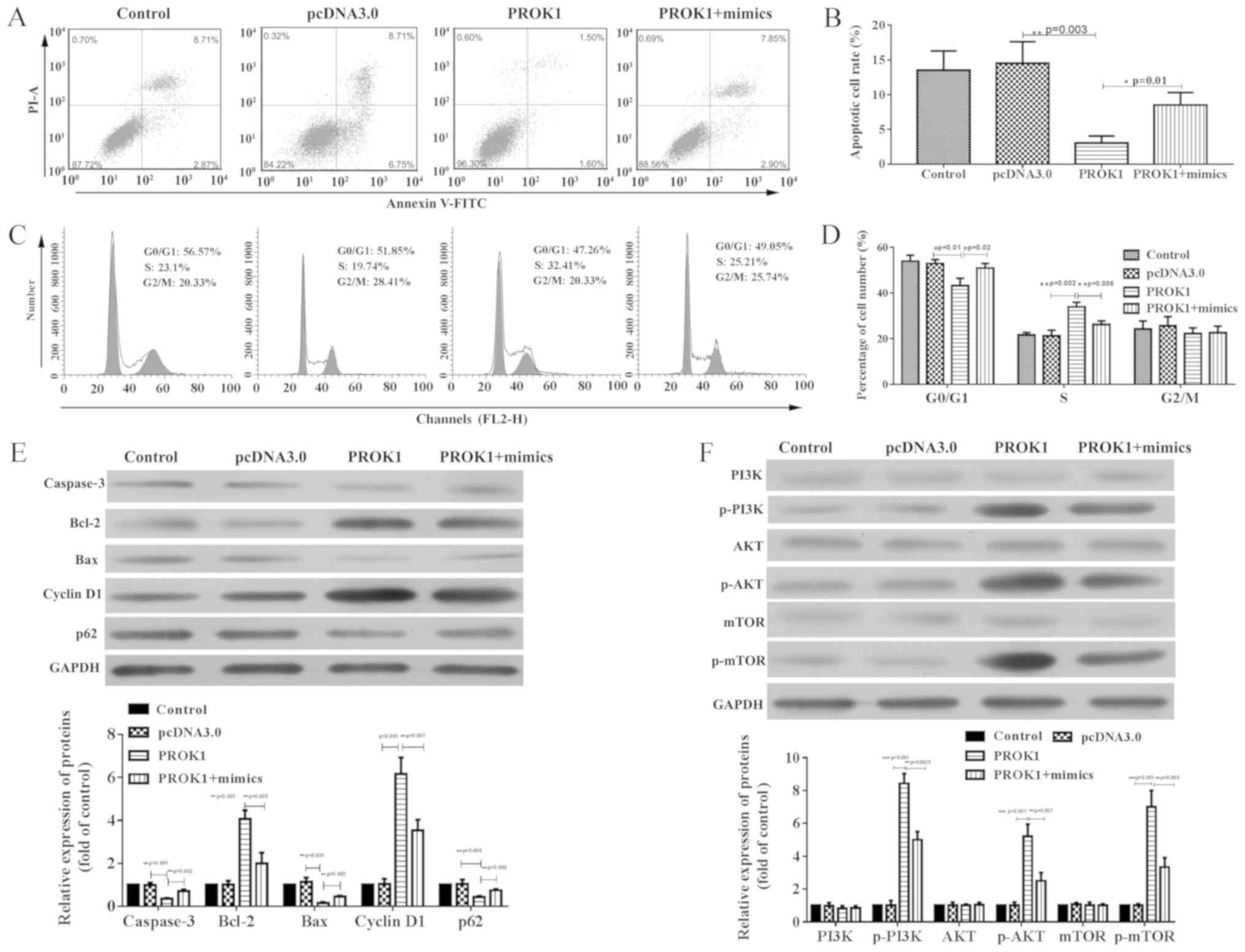 | Figure 3.PROK1-induced apoptosis suppression
and cell cycle distribution shift are reversed by miR-28-5p mimics
in rat ovary granulosa cells. (A and B) Decreased apoptosis in
cells co-transfected with miR-28-5p and PROK1 was observed when
compared with cells transfected with PROK1 alone, as determined by
flow cytometry. (C and D) Cell cycle distribution in cells
co-transfected with mimics and PROK1 compared with PROK-transfected
cells alone, as determined by flow cytometry. (E) Altered
expression of Bax, Bcl-2, Caspase 3, p21 and cyclin D protein
induced by PROK1 overexpression were reversed by miR-28-5p. (F)
Activation of the PI3K/AKT/mTOR signaling pathway following
transfection with pcDNA-PROK1, or co-transfection with pcDNA-PROK1
and mimics. All data are presented as the mean ± SD. *P<0.05,
**P<0.01 and ***P<0.001, as indicated (n=3). miR-28-5p,
microRNA-28-5p; PROK1, prokineticin 1; mimics, miR-28-5p mimics;
p-, phosphorylated; PI, propidium iodide. |
Discussion
Abnormal expression of miRNAs has been reported in a
variety of cancers, resulting in increased attention regarding the
potential pathogenic effects of miRNAs on cancer initiation and
progression (27,28). miR-28-5p has been reported to be
closely associated with tumor carcinogenesis. For example,
hepatocellular carcinoma progression was suppressed by miR-28-5p
via the targeting of insulin-like growth factor-1 (IGF-1) (18); miR-28-5p also exerts a number of
antitumor effects in renal cell carcinoma via the regulation of
Ras-related protein Rap-1B (29).
Additionally, miR-28-5p was reported to be downregulated in
colorectal cancer tissue compared with in normal colon tissue, and
to suppress colorectal cancer cell proliferation, migration and
invasion (30). Conversely,
certain studies have suggested that miR-28-5p may also serve as a
potential marker for the development and progression of cancers
(19,31). For example, it was reported that
miR-28-5p may promote the development and progression of renal cell
carcinoma by increasing the risk of chromosomal instability and
promoting checkpoint weakness (31). Additionally, miR-28-5p acted as a
carcinogenic biomarker to enhance ovarian cancer cell
proliferation, inhibit cell apoptosis, and contribute to migration
and invasion (19). In the present
study, it was demonstrated that miR-28-5p suppressed cell
proliferation, and induced cell cycle arrest and apoptosis in PCOS.
Therefore, miR-28-5p regulate PCOS and inhibit PCOS
progression.
It has been previously reported that miRNAs
contribute to the regulation of various physiological processes by
targeting mRNAs (32,33). And the activation or suppression of
the mRNA by these miRNAs could induce positive or negative effects
on the progression of various diseases, including PCOS (34–36).
For example, miR-324-3p expression was observed to be reduced in
PCOS; miR-324-3p inhibited the proliferation, and promoted the
apoptosis of granulosa cells by targeting Wnt-2B (37). In addition, miR-93 promoted ovarian
granulosa cell proliferation by targeting cyclin-dependent kinase
inhibitor 1A in PCOS (38).
miR-483 suppressed cell proliferation in PCOS by targeting IGF-1
(39). In the present study, it
was demonstrated that miR-28-5p negatively regulated PROK1
expression by binding to the 3′-UTR of PROK1.
The present findings suggested that miR-28-5p was
involved in PCOS by regulating PROK1. PROK1 serves an important
role in angiogenesis, and it was observed that the concentration of
VEGF in the ovaries of females with PCOS was increased compared
with healthy females (40). In
addition, previous studies also demonstrated that PROK1 promoted
human umbilical vein endothelial cell proliferation,
differentiation and survival (7,41).
Furthermore, PROK1 also promoted the morphogenesis of vascular
endothelial cells into tube-like structures, as observed in
2D-model experiments (42). In the
present study, it was demonstrated that the overexpression of PROK1
promoted cell proliferation, and inhibited cell cycle arrest and
apoptosis in PCOS. The present findings further indicated that
PROK1 may accelerate PCOS progression.
The cell cycle is a series of important cellular
events that occurs during various physiological and pathological
processes, and the initiation of G1 phase is critical during cell
cycle progression (43,44). Cyclins are important regulators of
cell cycle progression. Cyclin D1, as a key regulatory protein of
G1 phase progression, has been established as a major indicator of
cell cycle progression, with increased sensitivity when compared
with other cyclins (45).
Specifically, cyclin D1 promotes the transition from G1 to S phase
by binding and activating cyclin-dependent kinase 4 (CDK4), which
accelerates cell proliferation (46). Previous studies have reported that
p62 serves important roles in cell cycle progression by reducing
the ubiquitinylation-mediated degradation of CDK1 and accelerating
G2/M phase progression (47,48).
In the present study, it was demonstrated that PROK1 upregulated
cyclin D1 expression and downregulated p62 expression in ovary
granule cells, whereas miR-28-5p mimics reversed these effects,
which indicated that miR-28-5p promoted cell cycle arrest by
targeting PROK1 in PCOS.
Apoptosis is an important biological death process
that is genetically controlled, which serves as an important
mechanism for maintaining a stable internal environment (49). Caspase-3, Bcl-2 and Bax are central
functional proteins in apoptosis pathways (50). Caspases are a class of cysteine
aspartic acid-specific proteases in the cytoplasm; caspase-3, also
known as 32 kDa cysteine protease, is a member of the
interleukin-lp-converting enzyme family (51). Caspase-3 is a common downstream
factor in various apoptotic pathways. Bcl-2 exhibits anti-apoptotic
effects, whereas Bax protein is structurally similar to Bcl-2 but
induces opposing effects on cell apoptosis (52). In the present study, PROK1
decreased caspase-3 and Bax expression, and increased Bcl-2
expression, whereas treatment with miR-28-5p mimics attenuated
these alterations in protein expression. Therefore, it was further
indicated that miR-28-5p promoted cell apoptosis by targeting PROK1
in PCOS.
The PI3K/AKT pathway serves an important role in
mediating ovarian granular cell function (53–56).
PI3K activation induces the phosphorylation of AKT proteins and
mTOR gene expression, leading to the activation of downstream
signaling associated with cell proliferation, survival and
angiogenesis (57). Patients with
PCOS show decreased ovary granular cells caused by decreasing
viability of cell proliferation or survival (20). Previous studies showed that the
PI3K/AKT signaling pathway is activated in PCOS (53,54).
Upregulation of Wnt-5a, which acts as a proinflammatory factor, is
a signal to PI3K/AKT, activating inflammation and oxidative stress
in the granulosa cells of patients with PCOS via NF-κB (53). Additionally, Xin et al
(55) reported that patients with
insulin resistance underwent significant alterations to the
PI3K/AKT/GSK3 signaling pathway. The present study demonstrated
that PROK1 affect the progression of PCOS involved in the
PI3K/AKT/mTOR signaling pathway.
In conclusion, these findings suggested that
miR-28-5p could attenuate the progression of PCOS by targeting the
3′-UTR of PROK1which may involved in the PI3K/AKT/mTOR pathway,
indicating that the miR-28-5p/PROK1 axis may be a potential
therapeutic target for patients with PCOS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM made substantial contributions to the conception
and design of the study. HY contributed to data acquisition, data
analysis and data interpretation. CJ performed some experiments,
analyzed the data and drafted the article. SQ was involved in the
design of the experiments and critically revised the manuscript for
important intellectual content in the submission process. All
authors agree to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of the
work are appropriately investigated and resolved. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Nanfang
Hospital Animal Ethic Committee, and conducted in accordance with
the ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hull MG: Epidemiology of infertility and
polycystic ovarian disease: Endocrinological and demographic
studies. Gynecol Endocrinol. 1:235–245. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knochenhauer ES, Key TJ, Kahsar-Miller M,
Waggoner W, Boots LR and Azziz R: Prevalence of the polycystic
ovary syndrome in unselected black and white women of the
southeastern United States: A prospective study. J Clin Endocrinol
Metab. 83:3078–3082. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wajid S and Rashid N: miRNA signatures and
their potential role in PCOS. Int J Gen Cancer. 1:28–36. 2015.
|
|
4
|
Eser A, Hizli D, Namuslu M, Haltas H,
Kosus N, Kosus A and Kafali H: Protective effect of curcumin on
ovarian reserve in a rat ischemia model: An experimental study.
Clin Exp Obstet Gynecol. 44:453–457. 2017.PubMed/NCBI
|
|
5
|
Diamanti-Kandarakis E, Piperi C, Spina J,
Argyrakopoulou G, Papanastasiou L, Bergiele A and Panidis D:
Polycystic ovary syndrome: The influence of environmental and
genetic factors. Hormones (Athens). 5:17–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brouillet S, Hoffmann P, Thomas-Cadi C,
Bergues U, Feige JJ, Alfaidy N and Hennebicq S: PROK1, prognostic
marker of embryo implantation? Gynecol Obstet Fertil. 41:562–565.
2013.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lecouter J, Kowalski J, Foster J, Hass P,
Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller
GA, et al: Identification of an angiogenic mitogen selective for
endocrine gland endothelium. Nature. 412:877–884. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brouillet S, Hoffmann P, Feige JJ and
Alfaidy N: EG-VEGF: A key endocrine factor in placental
development. Trends Endocrinol Metab. 23:501–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fraser HM and Duncan WC: Vascular
morphogenesis in the primate ovary. Angiogenesis. 8:101–116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrara N, Frantz G, Lecouter J,
Dillard-Telm L, Pham T, Draksharapu A, Giordano T and Peale F:
Differential expression of the angiogenic factor genes vascular
endothelial growth factor (VEGF) and endocrine gland-derived VEGF
in normal and polycystic human ovaries. Am J Pathol. 162:1881–1893.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alfaidy N, Hoffmann P, Boufettal H, Samouh
N, Aboussaouira T, Benharouga M, Feige JJ and Brouillet S: The
multiple roles of EG-VEGF/PROK1 in normal and pathological
placental angiogenesis. Biomed Res Int. 2014:4519062014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voorhoeve PM, le Sage CL, Schrier M,
Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J,
Griekspoor A, et al: A genetic screen implicates miRNA-372 and
miRNA-373 as oncogenes in testicular germ cell tumors. Cell.
124:1169–1181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takanabe R, Ono K, Abe Y, Takaya T, Horie
T, Wada H, Kita T, Satoh N, Shimatsu A and Hasegawa K: Up-regulated
expression of microRNA-143 in association with obesity in adipose
tissue of mice fed high-fat diet. Biochem Biophys Res Commun.
376:728–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murri M, Insenser M, Fernández-Durán E,
San-Millán JL and Escobar-Morreale HF: Effects of polycystic ovary
syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21,
miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol
Metab. 98:E1835–E1844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arancio W, Calogero Amato M, Magliozzo M,
Pizzolanti G, Vesco R and Giordano C: Serum miRNAs in women
affected by hyperandrogenic polycystic ovary syndrome: The
potential role of miR-155 as a biomarker for monitoring the
estroprogestinic treatment. Gynecol Endocrinol. 34:704–708. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Almeida MI, Nicoloso MS, Zeng L, Ivan C,
Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F, et al:
Strand-specific miR-28-5p and miR-28-3p have distinct effects in
colorectal cancer cells. Gastroenterology. 142:886–896. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rizzo M, Berti G, Russo F, Evangelista M,
Pellegrini M and Rainaldi G: The miRNA pull out assay as a method
to validate the mir-28-5p targets identified in other tumor
contexts in prostate cancer. Int J Genomics. 2017:52148062017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi X and Teng F: Down-regulated miR-28-5p
in human hepatocellular carcinoma correlated with tumor
proliferation and migration by targeting insulin-like growth
factor-1 (IGF-1). Mol Cell Biochem. 408:283–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Jiang N, Shi H, Zhao S, Yao S and
Shen H: miR-28-5p promotes the development and progression of
ovarian cancer through inhibition of N4BP1. Int J Oncol. 2017.(Epub
ahead of print). View Article : Google Scholar
|
|
20
|
Hughesdon PE: Morphology and morphogenesis
of the Stein-Leventhal ovary and of so-called ‘hyperthecosis’.
Obstet Gynecol Surv. 37:59–77. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morita Y and Tilly JL: Oocyte apoptosis:
Like sand through an hourglass. Dev Biol. 213:1–17. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim EJ, Jang M, Choi JH, Park KS and Cho
IH: An improved dehydroepiandrosterone-induced rat model of
polycystic ovary syndrome (PCOS): Post-pubertal improve PCOS's
features. Front Endocrinol (Lausanne). 9:7352018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS One.
8:10004122010. View Article : Google Scholar
|
|
24
|
Schmittgen TD: Real-time quantitative PCR.
Methods. 25:383–385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
26
|
Ma HX, Xie J and Lai MH: Effects of
yangling zhongyu decoction on the secretion of ovarian granule
cells in polycystic ovarian syndrome rat model. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 32:54–57. 2012.(In Chinese). PubMed/NCBI
|
|
27
|
Chi XX, Zhang T, Chu XL, Zhen JL and Zhang
DJ: The regulatory effect of genistein on granulosa cell in ovary
of rat with PCOS through Bcl-2 and Bax signaling pathways. J Vet
Med Sci. 80:1348–1355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tutar Y: miRNA and cancer; Computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang WT and Chen YQ: Circulating miRNAs in
cancer: From detection to therapy. J Hematol Oncol. 7:862014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang C, Wu C, Yang Q, Ding M, Zhong J,
Zhang CY, Ge J, Wang J and Zhang C: miR-28-5p acts as a tumor
suppressor in renal cell carcinoma for multiple antitumor effects
by targeting RAP1B. Oncotarget. 7:73888–73902. 2016.PubMed/NCBI
|
|
31
|
Hell MP, Thoma CR, Fankhauser N,
Christinat Y, Weber TC and Krek W: miR-28-5p promotes chromosomal
instability in VHL-associated cancers by inhibiting Mad2
translation. Cancer Res. 74:2432–2443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amirkhah R, Schmitz U, Linnebacher M,
Wolkenhauer O and Farazmand A: MicroRNA-mRNA interactions in
colorectal cancer and their role in tumor progression. Genes
Chromosomes Cancer. 54:129–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giza DE, Vasilescu C and Calin GA:
MicroRNAs and ceRNAs: Therapeutic implications of RNA networks.
Expert Opin Boil Ther. 14:1285–1293. 2014. View Article : Google Scholar
|
|
34
|
Devaraj S and Natarajan J: miRNA-mRNA
network detects hub mRNAs and cancer specific miRNAs in lung
cancer. In Silico Boil. 11:281–295. 2011.
|
|
35
|
Huang X, Liu C, Hao C, Tang Q, Liu R, Lin
S, Zhang L and Yan W: Identification of altered microRNAs and mRNAs
in the cumulus cells of PCOS patients: MiRNA-509-3p promotes
oestradiol secretion by targeting MAP3K8. Reproduction.
151:643–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song J, Luo S and Li SW: miRNA-592 is
downregulated and may target LHCGR in polycystic ovary syndrome
patients. Reprod Boil. 15:229–237. 2015. View Article : Google Scholar
|
|
37
|
Jiang YC and Ma JX: The role of MiR-324-3p
in polycystic ovary syndrome (PCOS) via targeting WNT2B. Eur Rev
Med Pharmacol Sci. 22:3286–3293. 2018.PubMed/NCBI
|
|
38
|
Jiang L, Huang J, Li L, Chen Y, Chen X,
Zhao X and Yang D: MicroRNA-93 promotes ovarian granulosa cells
proliferation through targeting CDKN1A in polycystic ovarian
syndrome. J Clin Endocrinol Metab. 100:E729–E738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiang Y, Song Y, Li Y, Zhao D, Ma L and
Tan L: miR-483 is down-regulated in polycystic ovarian syndrome and
inhibits KGN cell proliferation via targeting insulin-like growth
factor 1 (IGF1). Med Sci Monit. 22:3383–3393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agrawal R, Jacobs H, Payne N and Conway G:
Concentration of vascular endothelial growth factor released by
cultured human luteinized granulosa cells is higher in women with
polycystic ovaries than in women with normal ovaries. Fertil
Steril. 78:1164–1169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin R, Lecouter J, Kowalski J and Ferrara
N: Characterization of endocrine gland-derived vascular endothelial
growth factor signaling in adrenal cortex capillary endothelial
cells. J Biol Chem. 277:8724–8729. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Murray JD: On the mechanochemical theory
of biological pattern formation with application to vasculogenesis.
C R Biol. 326:239–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bertoli C, Skotheim JM and de Bruin RA:
Control of cell cycle transcription during G1 and S phases. Nat Rev
Mol Cell Boil. 14:518–528. 2013. View Article : Google Scholar
|
|
44
|
Karamysheva Z, Diaz-Martinez LA,
Warrington R and Yu H: Graded requirement for the spliceosome in
cell cycle progression. Cell Cycle. 14:1873–1883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aleem E and Arceci RJ: Targeting cell
cycle regulators in hematologic malignancies. Front Cell Dev Biol.
3:162015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li H, Peng X, Wang Y, Cao S, Xiong L, Fan
J, Wang Y, Zhuang S, Yu X and Mao H: Atg5-mediated autophagy
deficiency in proximal tubules promotes cell cycle G2/M arrest and
renal fibrosis. Autophagy. 12:1472–1486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang J, Yang Z and Dong J: P62: An
emerging oncotarget for osteolytic metastasis. J Bone Oncol.
5:30–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Breckenridge DG and Xue D: Regulation of
mitochondrial membrane permeabilization by BCL-2 family proteins
and caspases. Curr Opin Cell Biol. 16:647–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao Y, Zhang C, Huang Y, Yu Y, Li R, Li
M, Liu N, Liu P and Qiao J: Upregulated expression of WNT5a
increases inflammation and oxidative stress via PI3K/AKT/NF-kB
signaling in the granulosa cells of PCOS patients. J Clin
Endocrinol Metab. 100:201–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao Y, Qiao J, Zhang C, Huang Y and Liu
P: Increased WNT5a expression upregulates inflammation via
PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients.
Fertility Sterility. 102 (Suppl 3):e27–e28. 2014. View Article : Google Scholar
|
|
55
|
Xin MA and Wang R: Endometrial lesions
mechanisms for insulin to promote PCOS through PI3K/AKT/GSK3
pathways in PCOS patient. Labeled Immunoassays Clin Med.
22:3302015.
|
|
56
|
Weickert MO, Hodges P, Tan BK and Randeva
HS: Neuroendocrine and endocrine dysfunction in the
hyperinsulinemic PCOS patient: The role of metformin. Minerva
Endocrinol. 37:25–40. 2012.PubMed/NCBI
|
|
57
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|