Introduction
Multiple myeloma (MM) is a hematological malignancy
characterized by the clonal proliferation of plasma cells in the
bone marrow and the presence of monoclonal immunoglobulin in the
blood and/or urine (1). MM
accounts for ~13% of hematologic cancers and is the second most
common hematological malignancy in adults worldwide (1,2).
According to age-adjusted rates between 2009 and 2013, the number
of new cases of myeloma was 6.5/100,000 people per year, whereas
the mortality rate was 3.3/100,000 people per year (3). The estimated 5-year prevalence is
~230,000 patients worldwide (4).
Although the introduction of novel agents (thalidomide,
lenalidomide and bortezomib) into clinical application has
remarkably improved response and survival rates in patients with
MM, the disease remains largely incurable due to relapse and drug
resistance (5). Therefore,
developing novel therapeutic strategies for MM is urgently
required.
Ribonucleotide reductase (RR) is a potential
therapeutic target for cancer since it catalyzes the conversion of
ribonucleoside 5′-diphosphates into 2′-deoxyribonucleoside
5′-triphosphates, which are necessary for DNA repair and
replication (6). Human RR
comprises two subunits: RRM1, and one of two RRM2 and p53R2
homologues (7). RRM1 protein
levels are relatively stable across the cell cycle; by contrast,
RRM2 is expressed during the late G1/early S phase, when DNA
replication occurs. RRM2 is a dimer comprising two 44-kDa proteins,
each of which contains a tyrosine free radical and non-heme iron
(8). As the RRM2 subunit is the
primary regulator of RR enzymatic activity, its effect on the
biological activities of RR protein has been extensively studied
(7,8). RRM2 has been reported to serve an
active role in tumor progression and identified as a predictor of
poor patient outcome for several types of cancer (9–11).
RRM2 overexpression is associated with cancer cell invasiveness,
metastasis, tumorigenesis and poor patient outcome; it may serve as
a prognostic biomarker for a number of types of cancer and it
regulates several oncogenes that control malignancy (12–16).
However, the significance of RRM2 in MM and the mechanisms
underlying its regulation of biological functions remain
unclear.
RNA interference (RNAi), which is induced by small
interfering RNAs (siRNAs), is a strategy used to suppress the
expression of individual genes with a high degree of specificity.
RNAi has become the method of choice for regulating gene
expression, as opposed to antisense ribozymes or DNAzyme technology
(17). Lin et al (18) reported that the suppression of RRM2
expression using RNAi sensitizes colon cancer cells to DNA damage
agents and RR inhibitors. Duxbury et al (19) demonstrated that siRRM2 enhances
pancreatic adenocarcinoma cell chemosensitivity to gemcitabine.
Wang et al (15) reported
that siRRM2 increased human cervical cancer cell apoptosis and
promoted cell cycle arrest at the G1 stage in vitro, and
inhibited tumor formation in vivo. These reports suggest
that further study of siRNA-mediated suppression of RRM2 as a
cancer therapeutic may provide positive results. Therefore, the aim
of the present study was to determine whether RRM2-specific siRNA
suppression may be an effective strategy to inhibit MM cell
proliferation in vitro.
The Wnt signaling pathway serves important roles in
the regulation of various biological processes, including cell
differentiation, cell proliferation, cell cycle and apoptosis.
Activation of the Wnt/β-catenin signaling pathway contributes to
the progression of human cancers including lung, colorectal and
liver cancer, as well as MM. The canonical Wnt/β-catenin signaling
pathway is involved in the pathogenesis of MM and regulates the
differentiation, proliferation, apoptosis and migration of MM cells
(20). Wnt responsiveness to MM
plasma cells may be a major factor in the progression of MM
(21). In addition, uncontrolled
Wnt signaling may contribute to the defects in apoptosis that
characterize MM (21). Therefore,
the Wnt pathway was examined in the present study to explore the
pathological mechanisms of MM.
The results of the present study demonstrated that
RRM2 was expressed at high levels in MM, and that RRM2 knockdown
inhibited RR activity and proliferation in MM cells. In addition,
RRM2 silencing enhanced apoptosis and activated the DNA-damage
response in MM cells. RRM2 downregulation may enhance apoptosis by
targeting the Wnt/β-catenin signaling pathway. The present study
aimed to provide a theoretical basis for the development of novel
therapeutic targets for MM.
Materials and methods
Oncomine database analysis
Oncomine (oncomine.org/)
was used to investigate the expression of RRM2 in MM, the search
conditions set as follows: i) Gene: RRM2; ii) Analysis Type: cancer
vs. normal analysis; iii) Cancer Type: MM; iv) Data Type: mRNA; v)
Threshold: P=0001, Fold Change: 2, Gene Rank: Top 10%.
Cell culture
The MM cell lines RPMI-8226, U266, NCI-H929 and
MM.1S were purchased from the Type Culture Collection of the
Chinese Academy of Sciences. The cells were cultured in RPMI-1640
medium (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin/streptomycin (Thermo Fisher Scientific, Inc.). Cells
were incubated at 37°C with 5% CO2.
RNAi assay
NCI-H929 cells were plated at a density of
1×106 cells/well in 6-cm dishes and incubated in
complete RPMI-1640 medium without antibiotics at 37°C for 24 h.
Cells were transfected with RRM2 siRNA (siRRM2; 10 µM; cat. no.
sc-36338; Santa Cruz Biotechnology, Inc.) using
Lipofectamine® 3000 (Invitrogen, Thermo Fisher
Scientific, Inc.). The RRM2 siRNA is a pool of three
target-specific siRNAs (19–25 nucleotides long) designed to knock
down RRM2 gene expression. The sequences of siRNA fragments were as
follows: sc-36338A, sense 5′-CGAUGGCAUAGUAAAUGAAtt-3′ and antisense
5′-UUCAUUUACUAUGCCAUCGtt-3′; sc-36338B, sense
5′-CACCAUGAAUUGUCCGUAAtt-3′ and antisense
5′-UUACGGACAAUUCAUGGUGtt-3′; sc-36338C, sense
5′-CAAGGAGCUUCUUAAGUUAtt-3′ and antisense
5′-UAACUUAAGAAGCUCCUUGtt-3′. Cells transfected with scrambled siRNA
(10 µM; cat. no. sc-44231; Santa Cruz Biotechnology, Inc.) and
untreated cells were used as the siRNA control (siC) and blank
groups, respectively. The sequences of control siRNA fragments were
as follows: Sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. Transfection was performed according
to the manufacturer's protocol using Lipofectamine® 3000
reagent. Following co-culture for 12 h at 37°C, the medium was
replaced with culture media and the transfected cells were used for
subsequent experiments.
Cell viability assay
The effect of RRM2 silencing on the proliferation of
NCI-H929 cells was detected using Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc.). NCI-H929 cells
(1×103 cells/well) were seeded in 96-well plates and
transfected with siRRM2 and siC. After 24 and 48 h of culture, 20
µl CCK-8 was added to each well and the plate was incubated for 4 h
at 37°C. Optical density (OD) at 450 nm was analyzed using a
microplate reader (BioTek Instruments, Inc.). The experiment was
performed in triplicate. Cell viability was calculated by comparing
the OD of the transfected group with the blank group.
RR activity assay
RR activity was measured using a [3H]
cytidine diphosphate (CDP) reduction assay as previously described
(22). Briefly, transfected and
blank cells were lysed on ice in a low-salt homogenization buffer
(10 mM HEPES; pH 7.2), and the supernatants were collected
following centrifugation at 12,000 × g for 10 min at 4°C. A total
of 25 µg protein-containing supernatant was added to 100 µl
reaction mixture, which contained 0.125 µmol/l [3H] CDP,
50 mM HEPES (pH 7.2), 100 mM KCl, 6 mM dithiothreitol, 4 mM
magnesium acetate, 2 mM ATP and 0.05 mM CDP. Following incubation
at 37°C for 15–30 min and dephosphorylation, samples were analyzed
by high-performance liquid chromatography and liquid scintillation
counting (22). RR activity was
calculated as follows: RR activity (%) = dCDP/(CDP + dCDP) ×100,
where dCDP is the number of deoxyribonucleotides catalyzed from
ribonucleotides by RR and CDP is the number of the remaining
ribonucleotide substrates in the enzyme reaction system.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following 48 h of transfection, TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
isolate total RNA according to the manufacturer's instructions.
Purified RNA was reverse transcribed using oligonucleotide dT
primers (Takara Biotechnology Co., Ltd.). The incubation conditions
for RT was as follows: 37°C for 15 min; 85°C for 5 sec; and a hold
at 4°C. The reactions were performed using SYBR Green Mix kit
(Roche Molecular Diagnostics) using the LightCycler® 480
(Roche Diagnostics). The following thermocycling conditions were
used for the PCR: Denaturation at 95°C for 10 min, followed by 40
cycles of denaturation at 95°C for 15 sec and elongation at 60°C
for 1 min. Gene expression was normalized to the expression levels
of β-actin. The relative expression of genes was calculated using
the 2−ΔΔCq method as follows: ΔΔCq =
ΔCqexperimental group -ΔCqcontrol group, ΔCq
= Cqtarget gene - Cqinternal control;
2−ΔΔCq is the relative transcript level of the target
gene mRNA (23). Primer sequences
were as follows: RRM2, forward 5′-ATCCGGATCCACTATGCTCTCCCTCCGTGT-3′
and reverse 5′-GCTTAAGCTTATTTAGAAGTCAGCATCCAAG-3′; β-actin, forward
5′-TCCCTGGAGAAGAGCTACG-3′ and reverse
5′-GTAGTTTCGTGGATGCCACA-3′.
Western blot analysis
Cells were harvested and lysed in RIPA buffer
(Beyotime Institute of Biotechnology) with protease inhibitor
(Roche Diagnostics). Protein concentrations were determined using
the bicinchoninic acid protein quantitation kit (Thermo Fisher
Scientific, Inc.). Equal amounts of protein (20 µg) were separated
by SDS-PAGE on 12% gel and transferred to PVDF membranes (EMD
Millipore). Following blocking with 5% skimmed milk in TBS+0.1%
Tween-20 (TBST), the membranes were incubated with primary
antibodies against the following proteins: RRM2 (cat. no. sc-81850;
1:1,000; Santa Cruz Biotechnology, Inc.), Bcl-2 (cat. no. ab32124;
1:1,000; Abcam), Bax (cat. no. ab32503; 1:1,000; Abcam), caspase-3
(cat. no. ER30804; 1:1,000; Huabio, Inc.), cleaved caspase-3 (cat.
no. ET1602-47; 1:1,000; Huabio, Inc.), poly(ADP-ribose) polymerase
(PARP; cat. no. ET1608-56; 1:1,000; Huabio, Inc.), cleaved PARP
(cat. no. ET1608-10; 1:1,000; Huabio, Inc.), p53 (cat. no. EM20603;
1:1,000; Huabio, Inc.), phosphorylated (p)-p53 (cat. no. ET1609-14;
1:1,000; Huabio, Inc.), glucose synthase kinase 3b (GSK-3β,
cat.sc-81462; 1:1,000; Santa Cruz Biotechnology, Inc.), p-GSK-3β
(cat. no. sc-373800; 1:1,000; Santa Cruz Biotechnology, Inc.),
β-catenin (cat. no. ab32572; 1:1,000; Abcam), c-Myc (cat. no.
ab32072; 1:1,000; Abcam), cyclin D1 (cat. no. ab16663; 1:1,000;
Abcam) and β-actin (cat. no. M1210-2; 1:1,000; Huabio, Inc.) at 4°C
overnight. Following three washes with TBST, the PVDF membranes
were incubated with horseradish peroxidase-conjugated antibodies
rabbit anti-mouse immunoglobulin G (IgG; cat. no. HA1008;1:5,000,
Hangzhou HuaAn Biotechnology Co., Ltd.) or goat anti-rabbit IgG
(cat. no. HA1008; 1:5,000; Hangzhou HuaAn Biotechnology Co., Ltd.)
for 1 h at room temperature. Enhanced chemiluminescence (ECL;
Origene Technologies, Inc.) and GE ECL Start detection system
reagent (GE Healthcare) were used to visualize the bands. The
signals were analyzed using the Bio-Rad Gel Imaging System (Bio-Rad
Laboratories, Inc.) and densitometry was performed using ImageJ
version 1.8.0 software (National Institutes of Health).
Apoptosis assay
Following transfection for 48 h, an Annexin
V-FITC/PI kit (Beyotime Institute of Biotechnology) was used to
measure apoptosis according to the manufacturer's protocol.
Briefly, 105 cells transfected with siRRM2 or siC or
those treated with medium only were collected and washed with PBS.
A total of 50 µl binding buffer, 5 µl Annexin V-FITC and 5 µl PI
were added into the cell suspension and mixed at room temperature
in the dark for 10 min. Apoptosis was measured and analyzed by flow
cytometry (NovoCyte Flow Cytometer; ACEA Biosciences, Inc.) within
1 h. The flow cytometry data was analyzed using NovoExpress™
(version no. 1.2.1; ACEA Biosciences, Inc.)
Statistical analysis
Data are presented as the mean ± SD, and all
experiments were repeated at least three times. One-way ANOVA
followed by a Dunnett's post-hoc test was applied to compare
differences between groups using GraphPad Prism 6 software
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
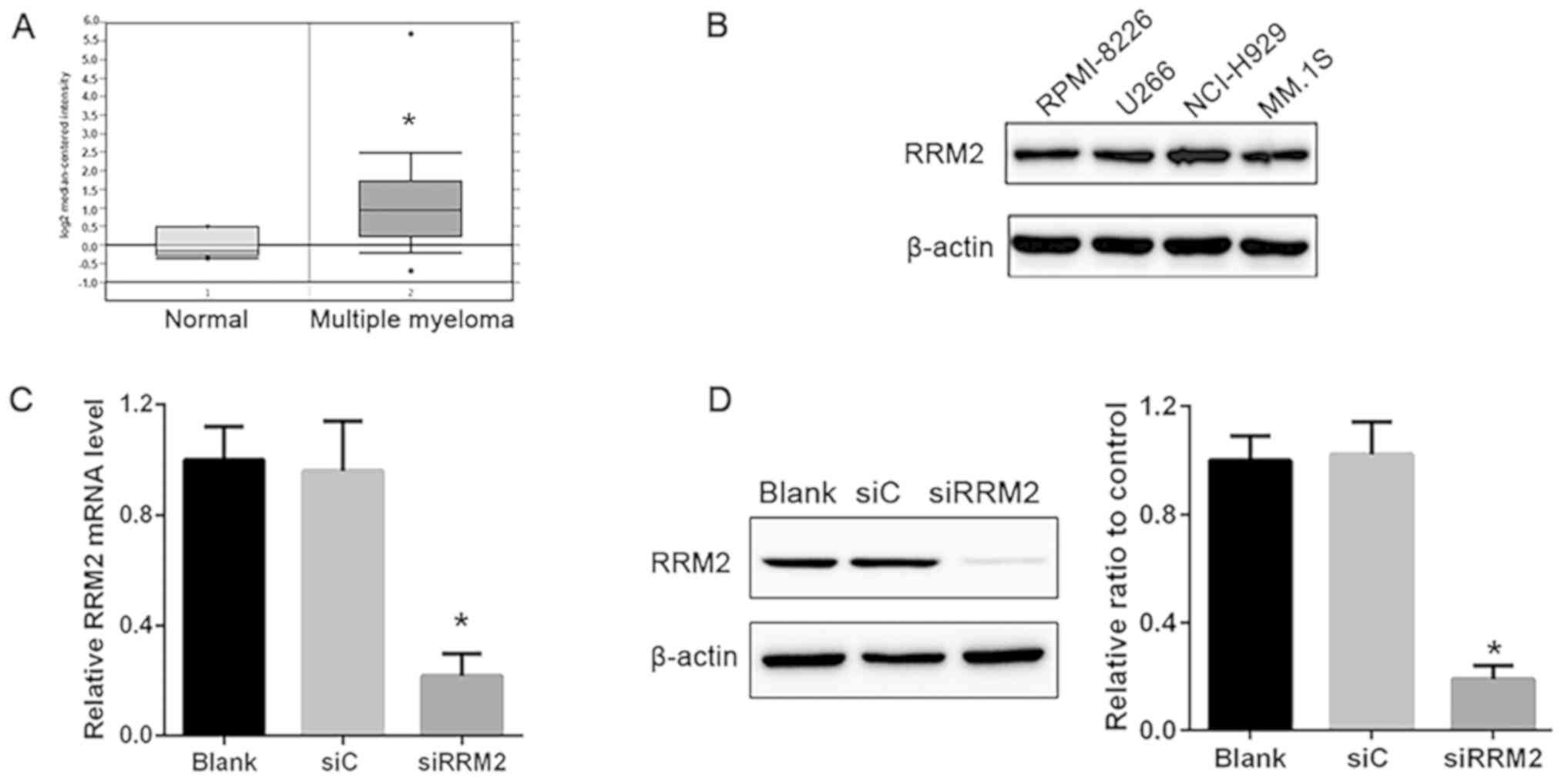
RRM2 is significantly upregulated in
patients with MM
To determine the expression profile of RRM2 in
patients with MM, the Oncomine (oncomine.org/)
online collection of microarrays was used. Using the Agnelli
Myeloma microarray dataset (24),
RRM2 expression was higher in MM compared with that in the healthy
subjects (Fig. 1A).
Construction of an in vitro RRM2
knockdown MM model
MM cell lines, RPMI-8226, U266, NCI-H929 and MM.1S,
were used to examine the role of RRM2 in MM, and samples from
healthy subjects were taken as the control cell lines (data not
shown). The results demonstrated that RRM2 was highly expressed in
these cell lines and no differences were observed among the cell
lines (Fig. 1B). Based on this
result, NCI-H929 cells were selected for further experiments.
NCI-H929 cells were transfected with siRNA targeting RRM2; RT-qPCR
results demonstrated that the mRNA expression of RRM2 was
significantly decreased in the siRRM2 group compared with that in
the siC group, demonstrating 74% interference efficacy (P<0.05;
Fig. 1C). In addition, protein
levels detected by western blotting were significantly reduced in
the siRRM2 group compared with the siC group (Fig. 1D). These results confirmed that
transfection with siRRM2 induced a significant decrease in RRM2
expression.
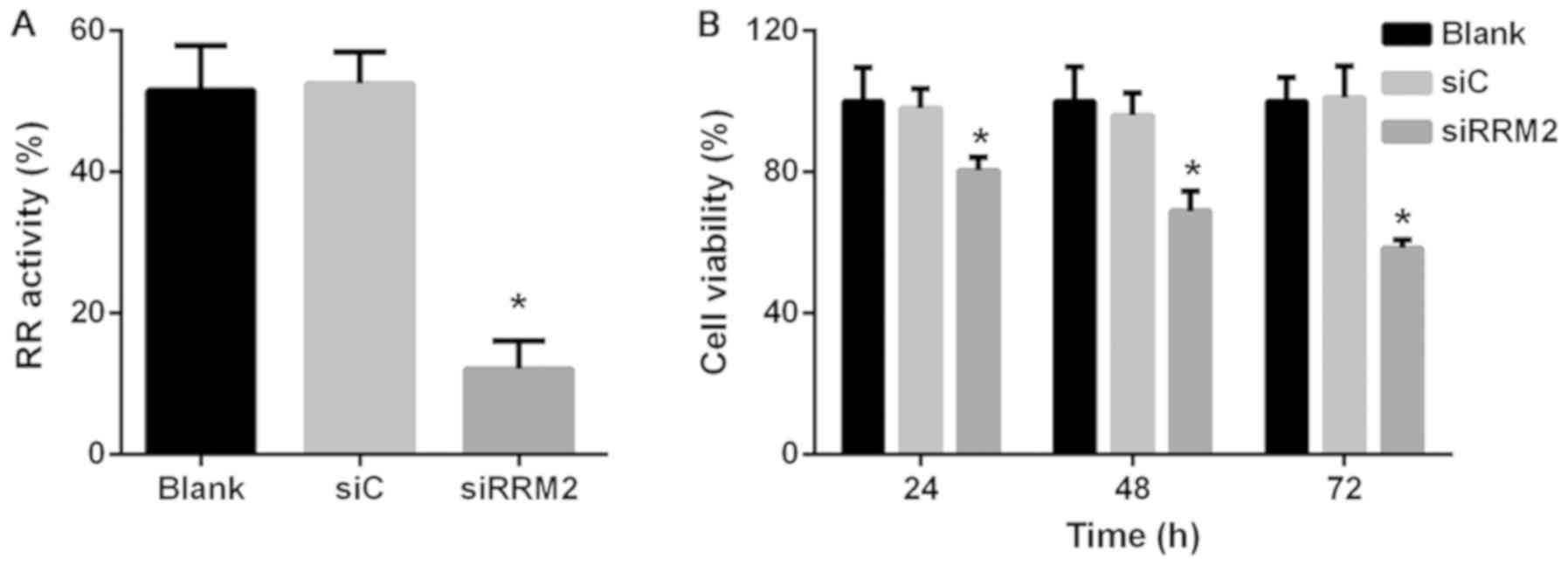
Reduced expression of RRM2 inhibits
cell RR activity and proliferation in NCI-H929 cells
Following the construction and confirmation of the
RRM2 knockdown model, RR activity was analyzed in the transfected
cells; the results revealed that enzymatic activity was markedly
decreased following downregulation of RRM2 expression compared with
the siC group (Fig. 2A). The
viability of transfected cells was investigated using CCK-8 assay,
which demonstrated that downregulation of RRM2 significantly
inhibited cell proliferation in a time-dependent manner in NCI-H929
cells. Cell viability in the siRRM2 group was reduced to 80.4, 68.9
and 58.4% of siC group levels at 24, 48 and 72 h, respectively
(P<0.05; Fig. 2B).
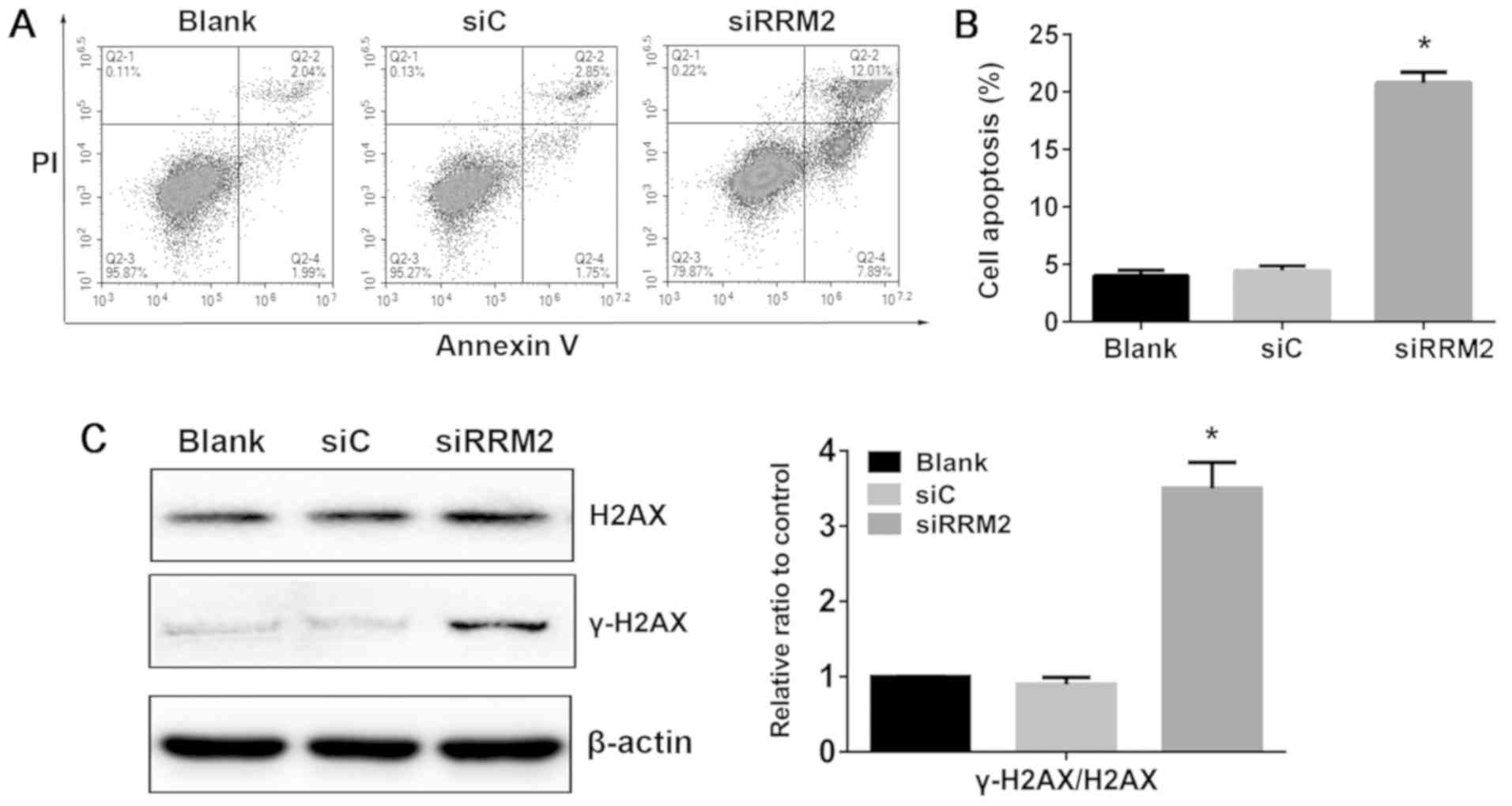
Reduced expression of RRM2 induces
apoptosis in NCI-H929 cells
Although RRM2 has been demonstrated to be involved
in cell cycle arrest and apoptosis in a number of cancer cells, its
mechanism in MM cell apoptosis was unclear. In the present study,
apoptosis induction upon knockdown of RRM2 was observed in NCI-H929
cells at 48 h. Following knockdown of RRM2 expression, the
proportion of apoptotic cells was increased to 19.9%, whereas the
apoptotic rate in the blank and siRNA control groups was 4.03 and
4.60%, respectively (P<0.05; Fig.
3A and B). These results suggested that reduced RRM2 expression
may promote MM cell apoptosis.
Reduced expression of RRM2 activates
the DNA-damage response in NCI-H929 cells
To determine whether the cytotoxicity induced upon
RRM2 knockdown was associated with DNA damage, the phosphorylation
level of histone H2AX at serine 139 (γ-H2AX) in NCI-H929 cells was
evaluated. The results demonstrated that γ-H2AX levels were
increased following siRRM2 transfection in NCI-H929 cells compared
with siC (Fig. 3C). This result
indicated that reduced expression of RRM2 may induce apoptosis with
a profound increase in markers of DNA damage in NCI-H929 cells.
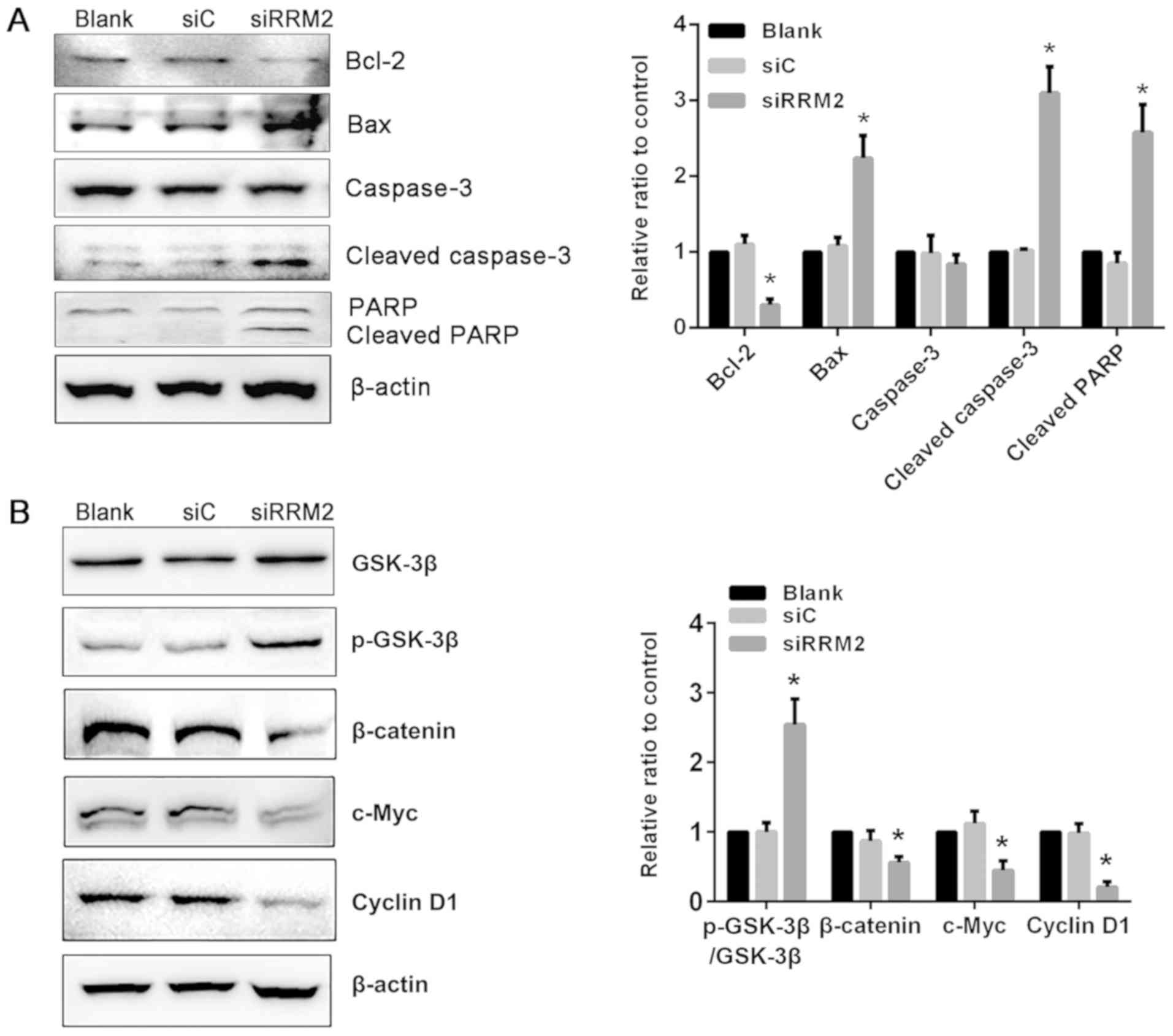
Reduced expression of RRM2 alters
apoptosis-associated protein expression in NCI-H929 cells
To delineate the molecular mechanism of RRM2 in
apoptosis in MM, proteins that are activated during the repair of
DNA strand breaks and upon apoptosis were detected. The levels of
apoptosis-associated proteins such as Bcl-2, Bax, caspase-3, and
cleaved caspase-3 were evaluated; Bcl-2 levels were reduced in the
siRRM2 transfection group compared with the siC group. By contrast,
Bax and cleaved caspase-3 levels were increased following RRM2
knockdown compared with the siC group. Western blotting of PARP-1,
which catalyzes single-strand DNA break repair, demonstrated that
RRM2 knockdown induced an increase in cleaved PARP-1 compared with
that in the siC group (Fig.
4A).
siRNA-mediated RRM2 knockdown
suppresses Wnt/β-catenin signaling through a phosphorylated
GSK-3β-dependent mechanism
To further investigate the molecular mechanism
associated with the induction of apoptosis in NCI-H929 cells
following RRM2 knockdown, the expression of downstream target genes
of Wnt/β-catenin signaling pathway was examined. Total β-catenin
levels were significantly reduced by siRNA-mediated RRM2 knockdown
compared with the siC group in NCI-H929 cells. The downstream
effectors of Wnt/β-catenin signaling, including cyclin D1 and
c-Myc, which serve important roles in tumor progression, were also
decreased following RRM2 knockdown compared with the siC group.
Additionally, GSK-3β phosphorylation expression, which promotes
β-catenin degradation, was upregulated compared with that in the
siC and blank groups (Fig. 4B).
These results suggested that inhibition of Wnt/β-catenin signaling
through enhanced GSK-3β phosphorylation may be one of the
underlying molecular mechanisms through which RRM2 knockdown
induces apoptosis in MM cells.
Discussion
The pathogenesis of MM is affected by alterations,
aberrations, and/or dysregulation of endocrine, genetic and
metabolic factors (25). Advances
in MM treatment include chemotherapy and autologous hematopoietic
stem cell transplantation; however, the prognosis for patients with
MM remains poor (26). Thus,
discovery of effective therapeutic methods and novel molecular
biomarkers for MM detection is urgently required.
The optimization of cellular dNTP concentration is
crucial for high-fidelity DNA replication and repair (7). RR is an enzyme that is involved in
cell proliferation by providing materials required for DNA
synthesis. RRM2, which is a small subunit of RR, is overexpressed
in a number of tumors and its overexpression is associated with
tumor aggressiveness, poor prognosis and poor overall survival
(12,13). A study by Li et al (27) reported that the suppression of RRM2
inhibits cell proliferation, induces cell cycle arrest and promotes
apoptosis in human neuroblastoma cells. In addition, Li et
al (28) demonstrated that
RRM2 promotes the progression of human glioblastoma. RRM2
inhibitors, such as hydroxyurea, GIT-2040 and tripine, are viable
treatment options, either as a monotherapy or in combination with
cancer chemotherapy, based on the outcome of clinical trials in
various cancer types, including myeloid leukemia, renal cell
carcinoma, advanced non-small cell lung cancer and prostate cancer
(29). These reports suggested
that therapies targeting RRM2 may serve as useful strategies for
controlling cancer progression.
To the best of our knowledge, the role of RRM2 in MM
has not been reported. Therefore, further studies on the mechanism
associated with RRM2 in MM are required. In the present study, the
overexpression of RRM2 in MM tumor tissues was demonstrated using
the publicly accessible microarray database, Oncomine. The result
indicated that RRM2 may serve an important role in the progression
of MM. The effects of RRM2 on the NCI-H929 MM cell line
proliferation and apoptosis were also examined. Apoptosis is
involved in maintaining tissue homeostasis in multicellular
organisms in various physiological and pathological situations
(30). Resistance to apoptosis is
an indication of human cancer and promotes its development and
progression (31). Resistance to
apoptosis is one of the leading causes of failure of leukemia
therapy, as a number of anticancer treatments mediate apoptosis
(32). The present study
demonstrated that RRM2 downregulation led to reduced NCI-H929 cell
proliferation and increased apoptosis. DNA damage is a
characteristic of apoptosis, and γ-H2AX is best known for its role
in DNA double-strand break repair (33). The present study revealed that RRM2
downregulation induced an increase in γ-H2AX levels, which
suggested that it induced DNA damage in NCI-H929 cells. These
results were further confirmed by the upregulation of Bax and
downregulation of Bcl-2 protein expression following siRRM2
transfection. Caspase-3 is an important member of the caspase
family and represents a key step in the induction of apoptosis
(34); cells transfected with
siRNA targeting RRM2 induced activation of caspase-3 and
inactivation of downstream PARP, which a nuclear protein involved
in DNA repair and cell apoptosis (35).
The induction of apoptosis by RRM2 knockdown in
NCI-H929 cells may be a result of its inhibitory effect on the
Wnt/β-catenin signaling pathway. This pathway has been described to
manage embryonic development, and accumulating evidence indicates
that abnormal activation promotes cancer progression through the
modulation of downstream genes (36). Therefore, the Wnt/β-catenin
signaling pathway is involved in cancer cell proliferation and
apoptosis (37). In addition, it
participates in the pathogenesis of MM by regulating MM cell
differentiation, proliferation, apoptosis and migration (38). The present study demonstrated that
RRM2 knockdown may activate the phosphorylation of GSK-3β and
reduce the expression of β-catenin. Downstream effectors of the
Wnt/β-catenin pathway, c-Myc and cyclin D1, are associated with
cancer cell proliferation and differentiation (36); in the present study, RRM2 knockdown
significantly downregulated c-Myc and cyclin D1 expression levels.
Therefore, the results of the present study demonstrated that
downregulation of RRM2 may induce apoptosis through the suppression
of the Wnt/β-catenin signaling pathway.
The present study revealed a possible role of RRM2
in MM, which may provide new insights for the diagnosis and
treatment of MM. However, there were limitations to this study, for
instance, the interactions between the RRM2 and Wnt/β-catenin
signaling need to be investigated to determine the underlying role
of RRM2 in MM development.
In conclusion, the results of the present study
indicated that RRM2 upregulation occurred in MM tumors, and that
RRM2 knockdown inhibited MM cell proliferation. In addition, RRM2
downregulation may promote apoptosis and activate DNA damage,
possibly through the suppression of Wnt/β-catenin signaling via a
p-GSK-3β-dependent mechanism. These results suggest that RRM2 may
be involved in MM tumorigenesis, and may serve as a biomarker and
potential therapeutic target for MM.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science
Foundation of Zhejiang Province (grant nos. LQ17H030006 and
LY18H280006), the Health and Family Planning Commission Science
Foundation of Zhejiang (grant no. 2017KY122) and the Scientific
Research Fund project of Zhejiang Chinese Medical University (grant
no. 2016ZG07).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XL and JW designed the experiments. XL wrote the
manuscript. JP and YZ performed the experiments. BX analyzed the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Becker N: Epidemiology of multiple
myeloma. Recent Results Cancer Res. 183:25–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin T and Huff CA: Multiple myeloma:
Current advances and future directions. Clin Lymphoma Myeloma Leuk.
19:255–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Howlader N NA, Krapcho M, Miller D, Bishop
K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al: SEER
Cancer Statistics Review, 1975–2013, National Cancer Institute
Bethesda, MD, based on November 2015 SEER data submission, posted
to the SEER website, 2016. https://seer.cancer.gov/archive/csr/1975_2013/April
20–2016
|
|
4
|
Cid Ruzafa J, Merinopoulou E, Baggaley RF,
Leighton P, Werther W, Felici D and Cox A: Patient population with
multiple myeloma and transitions across different lines of therapy
in the USA: An epidemiologic model. Pharmacoepidemiol Drug Saf.
25:871–879. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varga C, Maglio M, Ghobrial IM and
Richardson PG: Current use of monoclonal antibodies in the
treatment of multiple myeloma. Br J Haematol. 181:447–459. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crona M, Codó P, Jonna VR, Hofer A,
Fernandes AP and Tholander F: A ribonucleotide reductase inhibitor
with deoxyribonucleoside-reversible cytotoxicity. Mol Oncol.
10:1375–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aye Y, Li M, Long MJ and Weiss RS:
Ribonucleotide reductase and cancer: Biological mechanisms and
targeted therapies. Oncogene. 34:2011–2021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao J, Liu X, Zhu L and Yen Y: Targeting
ribonucleotide reductase for cancer therapy. Expert Opin Ther
Targets. 17:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morikawa T, Hino R, Uozaki H, Maeda D,
Ushiku T, Shinozaki A, Sakatani T and Fukayama M: Expression of
ribonucleotide reductase M2 subunit in gastric cancer and effects
of RRM2 inhibition in vitro. Hum Pathol. 41:1742–1748. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morikawa T, Maeda D, Kume H, Homma Y and
Fukayama M: Ribonucleotide reductase M2 subunit is a novel
diagnostic marker and a potential therapeutic target in bladder
cancer. Histopathology. 57:885–892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Meng L, Wang XW, Ma GY and Chen
JH: Expression of RRM1 and RRM2 as a novel prognostic marker in
advanced non-small cell lung cancer receiving chemotherapy. Tumour
Biol. 35:1899–1906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Angiolella V, Donato V, Forrester FM,
Jeong YT, Pellacani C, Kudo Y, Saraf A, Florens L, Washburn MP and
Pagano M: Cyclin F-mediated degradation of ribonucleotide reductase
M2 controls genome integrity and DNA repair. Cell. 149:1023–1034.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai L, Lin Z, Qiao J, Chen Y, Flemington
EK and Qin Z: Ribonucleotide reductase represents a novel
therapeutic target in primary effusion lymphoma. Oncogene.
36:5068–5074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang W, Tong JH, Chan AW, Zhao J, Wang S,
Dong Y, Sin FM, Yeung S, Cheng AS, Yu J and To K: Targeting
ribonucleotide reductase M2 subunit by small interfering RNA exerts
anti-oncogenic effects in gastric adenocarcinoma. Oncol Rep.
31:2579–2586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang N, Li Y and Zhou J: Downregulation of
ribonucleotide reductase subunits M2 induces apoptosis and G1
arrest of cervical cancer cells. Oncol Lett. 15:3719–3725.
2018.PubMed/NCBI
|
|
16
|
Shah KN, Wilson EA, Malla R, Elford HL and
Faridi JS: Targeting ribonucleotide reductase M2 and NF-κB
activation with didox to circumvent tamoxifen resistance in breast
cancer. Mol Cancer Ther. 14:2411–2421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin ZP, Belcourt MF, Cory JG and
Sartorelli AC: Stable suppression of the R2 subunit of
ribonucleotide reductase by R2-targeted short interference RNA
sensitizes p53(−/-) HCT-116 colon cancer cells to DNA-damaging
agents and ribonucleotide reductase inhibitors. J Biol Chem.
279:27030–27038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: RNA interference targeting the M2 subunit of
ribonucleotide reductase enhances pancreatic adenocarcinoma
chemosensitivity to gemcitabine. Oncogene. 23:1539–1548. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang P, Yan R, Zhang X, Wang L, Ke X and
Qu Y: Activating Wnt/β-catenin signaling pathway for disease
therapy: Challenges and opportunities. Pharmacol Ther. 196:79–90.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Andel H, Kocemba KA, Spaargaren M and
Pals ST: Aberrant Wnt signaling in multiple myeloma: Molecular
mechanisms and targeting options. Leukemia. 33:1063–1075. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu L, Zhou B, Chen X, Jiang H, Shao J and
Yen Y: Inhibitory mechanisms of heterocyclic carboxaldehyde
thiosemicabazones for two forms of human ribonucleotide reductase.
Biochem Pharmacol. 78:1178–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agnelli L, Forcato M, Ferrari F, Tuana G,
Todoerti K, Walker BA, Morgan GJ, Lombardi L, Bicciato S and Neri
A: The reconstruction of transcriptional networks reveals critical
genes with implications for clinical outcome of multiple myeloma.
Clin Cancer Res. 17:7402–7412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Furukawa Y and Kikuchi J: Molecular
pathogenesis of multiple myeloma. Int J Clin Oncol. 20:413–422.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mimura N, Hideshima T and Anderson KC:
Novel therapeutic strategies for multiple myeloma. Exp Hematol.
43:732–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Pang J, Liu Y, Zhang J, Zhang C,
Shen G and Song L: Suppression of RRM2 inhibits cell proliferation,
causes cell cycle arrest and promotes the apoptosis of human
neuroblastoma cells and in human neuroblastoma RRM2 is suppressed
following chemotherapy. Oncol Rep. 40:355–360. 2018.PubMed/NCBI
|
|
28
|
Li C, Zheng J, Chen S, Huang B, Li G, Feng
Z, Wang J and Xu S: RRM2 promotes the progression of human
glioblastoma. J Cell Physiol. 233:6759–6767. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mannarqudi MB and Deb S: Clinical
pharmacology and clinical trials of ribonucleotide reductase
inhibitors: Is it a viable cancer therapy? J Cancer Res Clin Oncol.
143:1499–1529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahler M, Miyachi K, Peebles C and
Fritzler MJ: The clinical signifcance of autoantibodies to the
proliferating cell nuclear antigen (PCNA). Autoimmun Rev.
11:771–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Solier S and Pommier Y: The nuclear γ-H2AX
apoptotic ring: Implications for cancers and autoimmune diseases.
Cell Mol Life Sci. 71:2289–2297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khalilzadeh B, Shadjou N, Kanberoglu GS,
Afsharan H, de la Guardia M, Charoudeh HN, Ostadrahimi A and
Rashidi MR: Advances in nanomaterial based optical biosensing and
bioimaging of apoptosis via caspase-3 activity: A review. Mikrochim
Acta. 185:4342018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morales J, Li L, Fattah FJ, Dong Y, Bey
EA, Patel M, Gao J and Boothman DA: Review of poly (ADP-ribose)
polymerase (PARP) mechanisms of action and rationale for targeting
in cancer and other diseases. Crit Rev Eukaryot Gene Expr.
24:15–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tammela T, Sanchez-Rivera FJ, Centinbas
NM, Wu K, Joshi NS, Helenius K, Park Y, Azimi R, Kerper NR,
Wesselhoeft RA, et al: A Wnt-producing niche drives proliferative
potential and progression in lung adenocarcinoma. Nature.
545:355–359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren Z, van Andel H, de Lau W, Hartholt RB,
Maurice MM, Clevers H, Kersten MJ, Spaargaren M and Pals ST:
Syndecan-1 promotes Wnt/β-catenin signaling in multiple myeloma by
presenting Wnts and R-spondins. Blood. 131:982–994. 2018.
View Article : Google Scholar : PubMed/NCBI
|