Introduction
Ischemic stroke is a type of acute cerebrovascular
disease that is usually caused by cerebral thrombosis and results
in irreversible effects, including microvascular damage, neuronal
death and destruction of the blood-brain barrier, in a short period
of time (1–5). At present, rapid thrombolytic therapy
is commonly used in the treatment of the disease (6). Compound tripeptide injection is used
clinically to treat acute and chronic cerebrovascular diseases,
including cerebral thrombosis, cerebral embolism and cerebral
palsy, as well as brain dysfunction caused by brain trauma and
cerebrovascular diseases (brain insufficiency, cerebral infarction)
(7–9). Compound porcine cerebroside and
ganglioside injection (CPCGI: Drug approval no. H22026472; Buchang
Pharmaceutical Group Ltd., Jilin, China), which was approved by the
China Food and Drug Administration in 2010, is used to treat
arteriosclerosis, thrombophlebitis, capillary hemorrhage and edema
caused by increased vascular permeability (10,11).
The cerebral microcirculation network refers to the
blood vessel system with a diameter of <200 µm in the brain,
consisting of arterioles, capillaries and venules, which can
regulate cerebral blood flow, transport nutrients to the neurons
and glial cells and remove waste products (12–14).
The blood circulation of the large blood vessels is powered by the
heart, whereas microcirculation depends upon small arteries,
arterioles and capillaries to perform the function of contraction
and relaxation (15).
Following the occurrence of ischemic stroke,
cerebral microcirculation is blocked, accompanied by changes in
multiple brain structures and functions (3). It primarily affects the function of
the blood-brain barrier (16). Due
to cerebral ischemia and hypoxia, the intimate connection between
cerebral vascular endothelial cells is destroyed, the microvascular
basement membrane is damaged and the permeability of the vascular
wall is increased, resulting in punctiform hemorrhage and brain
edema (17,18). Additionally, the vasomotion of
cerebral arterioles is inhibited and the blood cannot reach the
capillaries controlled by the arterioles, leading to the injury and
necrosis of the neurons (19,20).
Moreover, the structure of small blood vessels is damaged, which
provokes cerebral arterial stenosis or occlusion and a decrease in
collateral circulation, thereby further aggravating the disturbance
in microcirculation (21).
Previous studies have demonstrated that the
improvement of cerebral microcirculation by drug administration may
increase the blood flow of the infarcted side, which is beneficial
for the repair, regeneration and recovery of nerve functions
(22–25). The current study aimed to explore
the effects of CPCGI injection on brain ischemia/reperfusion injury
in the focal transient middle cerebral artery occlusion (MCAO) rat
models.
Materials and methods
Ethics statement
The current study was approved by the Ethics
Committee of Renji Hospital, South Campus, Shanghai Jiaotong
University School of Medicine (Shanghai, China).
Establishment of rat models of
permanent MCAO
A total of 24 male Sprague-Dawley rats (Shanghai
Laboratory Animal Center, Shanghai, China) aged 7–8 weeks and
weighing 250–300 g were housed under standard laboratory conditions
at a temperature of 20–22°C and 12-h light/dark cycles and were
provided with food and water ad libitum. Rats were
anesthetized by intraperitoneal injection of 7% chloral hydrate
(350 mg/kg; Bax & Company, Cambridge, UK) and then placed in a
supine position. After disinfection, the skin was cut open along
the midline of the neck and the muscle layers were separated by
blunt dissection. The right common carotid artery, the external
carotid artery and the internal carotid artery were carefully
separated, and the external carotid artery and the common carotid
artery were ligated. The pterygopalatine artery was isolated along
the internal carotid artery. The distal ends of the pterygopalatine
and internal carotid arteries were temporarily clamped with a rat
artery clip. A small incision was made at the bifurcation between
the external and internal carotid arteries using a pair of
ophthalmic scissors. A nylon suture with a spherical-shaped head
(0.265 mm in diameter with a mark made 18 mm from the head) was
inserted into the incision. The time that resistance was
encountered was recorded as the ischemia time, and the suture was
removed when resistance was no longer encountered the length of the
inserted nylon suture was 18–20 mm. The wound was sutured after
ligating the incision at the bifurcation between the external and
internal carotid arteries.
Study design
The rats were randomly divided into the sham
operation group (rats were anesthetized without the insertion of
nylon suture), MCAO rat model group, low dose and high dose groups
(0.83 and 2.5 ml/kg/day CPCGI, respectively; Jilin Buchang
Pharmaceutical Group Co., Ltd.) for 7 consecutive days. The
intraperitoneal injection was delivered immediately after the model
was established. Sham and model groups received an equivalent
volume of saline. All the animals were sacrificed after 7 days of
modeling.
Monitoring cerebral blood flow changes
in cortex by laser speckle
The PeriCam PSI system (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) is a blood perfusion imaging system based
on the laser speckle contrast analysis technology. The instrument
excites a laser with a certain frequency. Different degrees of
reflected waves are received after the laser beam is irradiated to
the tissues. Blood with different flow velocities induces different
reflected waves. Therefore, different color images are produced
according to the variations of blood flow volume and these can be
used to monitor the blood flow velocity and blood flow volume in
real-time. This technology has been widely applied to monitor the
blood flow changes following cerebral stroke (18). In the current study, the PeriCam
PSI system was adopted to monitor the changes of blood flow in the
right cerebral cortex before and 7 days after the establishment of
the MCAO model. After anesthetizing the rats, the fur over the top
of the skull was shaved, a 3 cm incision was created along the
sagittal suture, and the skin was pulled apart with the sutures. A
dental drill was used to evenly thin the skull until the blood
vessels were clearly visible. At this time, the PeriCam PSI system
was used to monitor the blood flow volume and this measurement was
used as the baseline level of the blood flow volume of the cerebral
cortex. After the MCAO model was established, the blood flow at the
same location was repeatedly monitored. After treatment for 7 days,
the blood flow in each group was repeatedly monitored. The duration
of each monitoring was 20 min. The image processing was performed
by the PimSoft data analysis software version 13.0 (Beyotime
Institute of Biotechnology, Haimen, China). The ischemic region was
manually delineated to statistically analyze the blood flow volume.
The rats were kept in the same position during the measurements to
facilitate data processing.
TTC staining
Following reperfusion, rat brains were removed and
frozen at −80°C for 5 min. Sections (2 mm) were made using a rodent
brain matrix (HEAD Biotechnology Co., Ltd., Beijing, China) and
stained for 20 min at 37°C with 2% 2,3,5-triphenyltetrazolium
chloride monohydrate (TTC; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and were then fixed with 4% formaldehyde. The infarct
volume was calculated as previously described (26). The sections were scanned and the
infarct area was calculated using ImageJ analysis software version
1.46 (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Protein was extracted from brain tissues with
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) with protease inhibitors and
phosphatase inhibitors. Proteins were determined by BCA Protein
Assay kit (Pierce; Thermo Fisher Scientific, Inc.); 50 µg protein
was separated via SDS-PAGE on a 12% gel. The separated proteins
were subsequently transferred onto polyvinylidene fluoride
membrane. The membrane was blocked with 5% non-fat milk at room
temperature for 1.5 h. The membrane was incubated with rabbit
polyclonal primary antibodies purchased from Santa Cruz
Biotechnology, Inc. at 4°C overnight. Primary antibodies against
vascular endothelial growth factor (cat. no. 03265, VEGF; 1:1,000),
angiopoietin 1 (cat. no. 21783, ANGPT1; 1:1,000), TEK receptor
tyrosine kinase (cat. no. 25309, TEK; 1:1,000), fibroblast growth
factor (cat. no. 37021, FGF; 1:500), frizzled (cat. no. 22301, Fzd;
1:500), β-catenin (cat. no. 70225, 1:800), Wnt family member 7A
(cat. no. 13762, WNT7A; 1:500) and β-actin (cat. no. 24971, 1:800)
were used. The membrane was subsequently incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
2041; 1:1,000; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. ECL chromogenic substrates were used for quantitative
measurement using Quantity One software (version 4.6; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Neurological function assessment
Neurological function was graded on a scale of 0–12
(normal score, 0; maximal score, 12) as previously described by
Belayev et al (27). Tests
were conducted by an observer blinded to the treatment groups. The
rats were lifted by the tail and suspended in the air (2 points): 0
points, no obvious neurological function deficit; 1 point, slight
flexion of the contralateral limb of the infarcted limb; 2 points,
significant flexion of the contralateral limb of the infarcted limb
(28).
Limb placement was assessed by the visual sub-test
(anterior, lateral) and tactile sub-test (anterior, lateral; 8
points): 0 points, normal limb placement; 1 point, reaction delay
time ≤2 sec; 2 points, reaction delayed time >2 sec.
Proprioceptive sub-test (2 points): 0 points, equal strength to the
contralateral limb; 1 point, slightly stronger than the
contralateral limb; 2 points, weaker than the contralateral limb
(29).
Cerebral perfusion and brain
extraction in rats
Following the MCAO procedure, rats were anesthetized
with an intraperitoneal injection of 7% chloral hydrate (350 mg/kg;
Sigma-Aldrich; Merck KGaA). No animals exhibited signs of
peritonitis following the administration of chloral hydrate. The
rats were placed on the operating table in a supine position and
the abdominal muscle layer was cut open, and the ribs and diaphragm
were transected to fully expose the heart. Cardiac puncture was
performed in the apex of the left ventricle with a 10 ml syringe
filled with 0.9% physiological saline (Sigma-Aldrich, Merck KGaA).
A small incision was created at the right atrial appendage using a
pair of ophthalmic scissors. The rat liver became white and the
heartbeat slowed down. A portion of 200 ml of 2% paraformaldehyde
perfusion was performed at a fast and then slow speed. The signs of
successful perfusion were as follows: At the beginning of
perfusion, the rats were twitched violently and the tails tilted
upwards. After the perfusion, the hind limbs of the rats were
stretched straight, the tail was erected and the limbs were pale
and rigid.
The muscles of the back of the neck were removed and
the brain tissues were collected with curved forceps to maintain
the integrity of the brain. The brain tissues were placed in the
prepared 2% paraformaldehyde solution for 10 min and stored at 4°C.
Initially, the brain tissues floated on the surface, while one week
later they sank to the bottom of the solution. The fixation
solution was replaced every ~48 h, and subsequently the brain
tissues were prepared for frozen sections.
Latex perfusion procedures
The rats were anesthetized and placed on a plate in
a supine position. A syringe filled with 0.9% physiological saline
was used to puncture the apex of the left ventricle and a small
incision was created at the right atrial appendage with a pair of
ophthalmic scissors. A total of 50 ml of 0.9% physiological saline
was gently injected. The rat liver became white and the heartbeat
was slowed down. Approximately 20 ml of warm latex perfusion fluid
(Sigma-Aldrich; Merck KGaA) was injected into the heart at a speed
of 10 ml/min. The signs of perfusion were as follows: The forelimbs
of the rats were slightly gray. When the skull was cut open, the
cerebral vessels were filled with black latex perfusion fluid,
whereas the brain tissues remained white. After perfusion, the rat
whole brain was carefully collected and immediately observed under
an inverted microscope at ×10 magnification (Nikon Ti; Nikon
Corporation, Tokyo, Japan).
Immunofluorescence assay
Brains were fixed by perfusion with 4%
paraformaldehyde for 3 h at 4°C. Brains were embedded in paraffin
and sections (thickness, 20 µm) were blocked 4°C for 1.5 h by 1.5%
BSA in 0.01 M PBS, the sections were incubated with primary
antibodies overnight at 4°C. The antibodies used were as follows:
Anti-lectin (cat. no. ab64693; 1:200; Abcam), anti-Ki67 (cat. no.
ab833; 1:800; Abcam), anti-VE-cadherin (cat. no. ab231227; 1:200;
Abcam) or anti-phosphorylated-focal adhesion kinase (p-FAK; cat.
no. ab4792; 1:500; Abcam). Sections were then washed with 0.01 M
PBS three times, and incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
2041; 1:1,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. All sections were washed three times with 0.01 M PBS
for 10 min in the dark and mounted on a coverslip with mounting
medium containing DAPI at room temperature for 2 h (Santa Cruz
Biotechnology, Inc.).
The slides were visualized using a fluorescence
microscope (Olympus Corporation; magnification, ×20) and analyzed
by Image-Pro Plus software version 7.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18; SPSS, Inc., Chicago, IL, USA). Data were
presented as mean ± SD of at least three independent experiments.
One-way analysis of variance and post hoc test were used to
determine the differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Protective effects of CPCGI on the
brain of rats with ischemic stroke
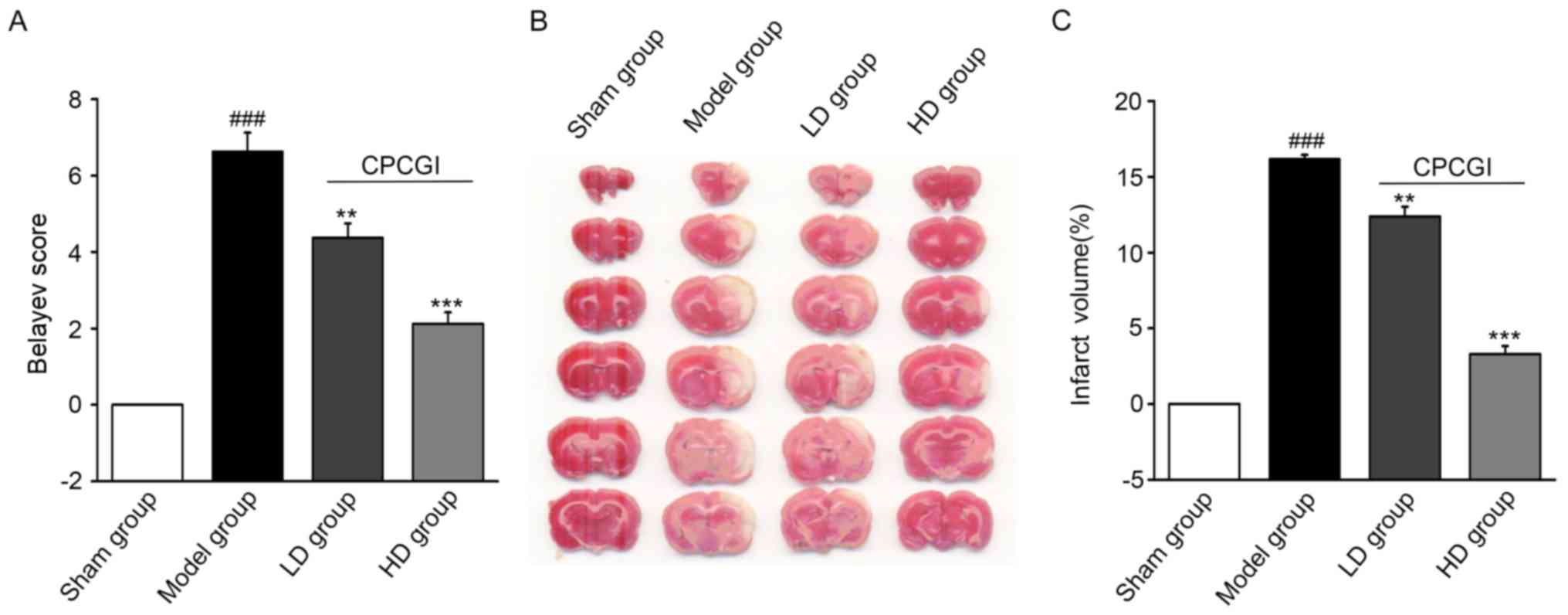
At 7 days following the establishment of MCAO, the
Belayev score (27) was
significantly different between the model and sham operation groups
(P<0.001; Fig. 1A). Compared
with the model group, the Belayev scores in the LD and HD groups
were significantly decreased (P<0.01 and P<0.001,
respectively; Fig. 1A), suggesting
that CPCGI effectively reduced the neurological impairment and
contributed to rehabilitation following ischemic stroke. The
infarction volume of the rat brains at 7 days after model
establishment was evaluated by TTC staining. The infarction volume
(white part) in the brains of the CPCGI-treated groups was smaller
compared with the model group (Fig.
1B). The percentages of the infarction volume in the whole
brain were 23.10% in the model group, 15.57% in the LD group and
11.57% in the HD group. Compared with the model group, there was a
significant decrease in the cerebral infarction volume in the LD
(P<0.01) and HD groups (P<0.001; Fig. 1C).
Effects of CPCGI administration on
cerebral vessels and blood flow in rats with cerebral stroke
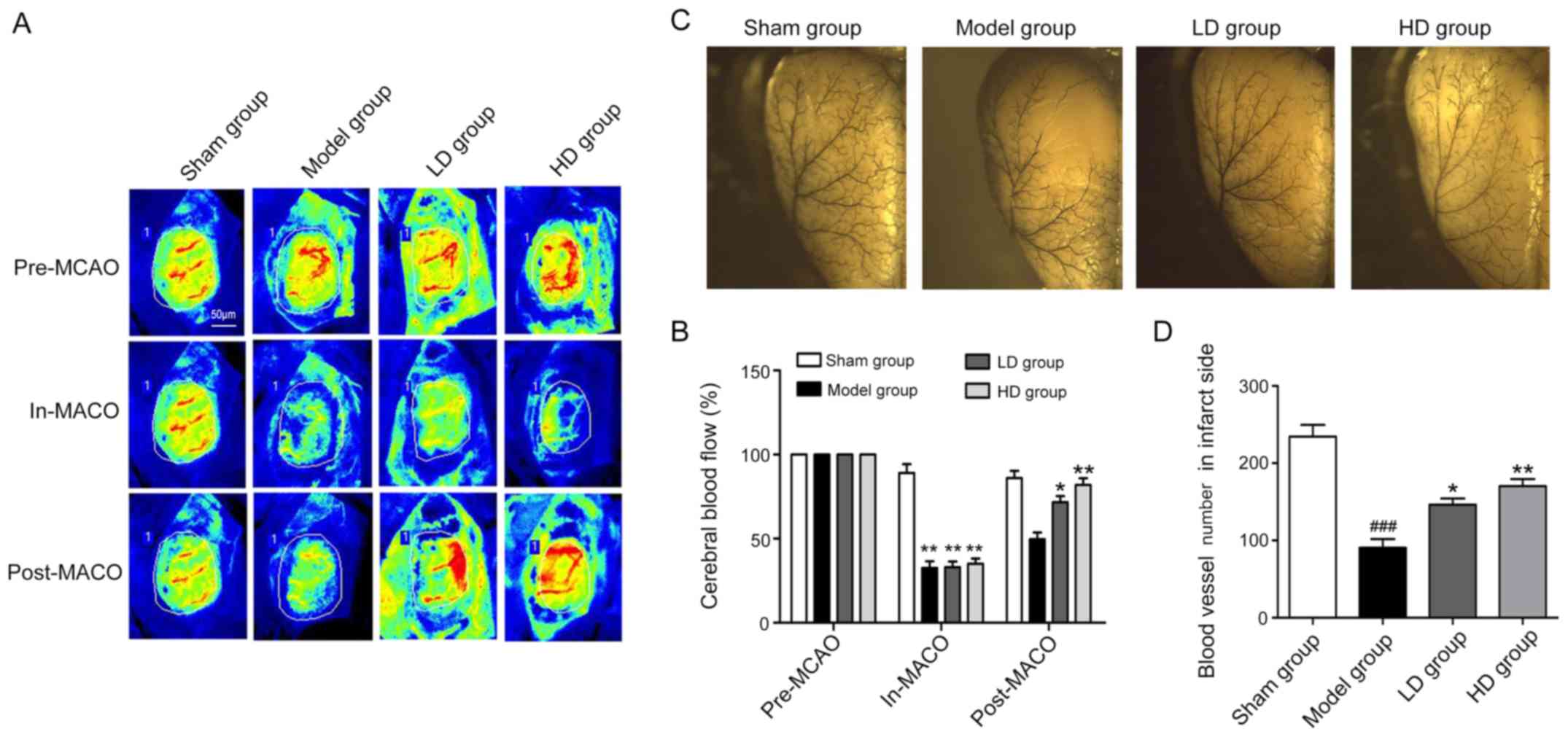
The PeriCam PSI system was used to monitor the blood
flow volume before and after model establishment and at 7 days
postoperatively. The changes in blood flow values revealed that at
the same ischemic degree, the cerebral blood flow of the infarcted
side was improved in the LD group compared with the model group at
7 days following CPCGI administration (P<0.05; Fig. 2A and B). The blood flow in the HD
group was also significantly improved compared with the model group
(P<0.01; Fig. 2A and B). The
quantity and morphology of the vessels in the infarcted cerebral
parietal cortex in each group were then detected by latex
perfusion. The number of vessels in the model, LD and HD groups was
significantly decreased compared with the sham operation group
(Fig. 2C and D). The number of
vessels in the LD and HD groups was significantly increased
compared with the model group (Fig. 2C
and D), indicating that CPCGI was beneficial for the
preservation of the cerebral cortex vessels following stroke.
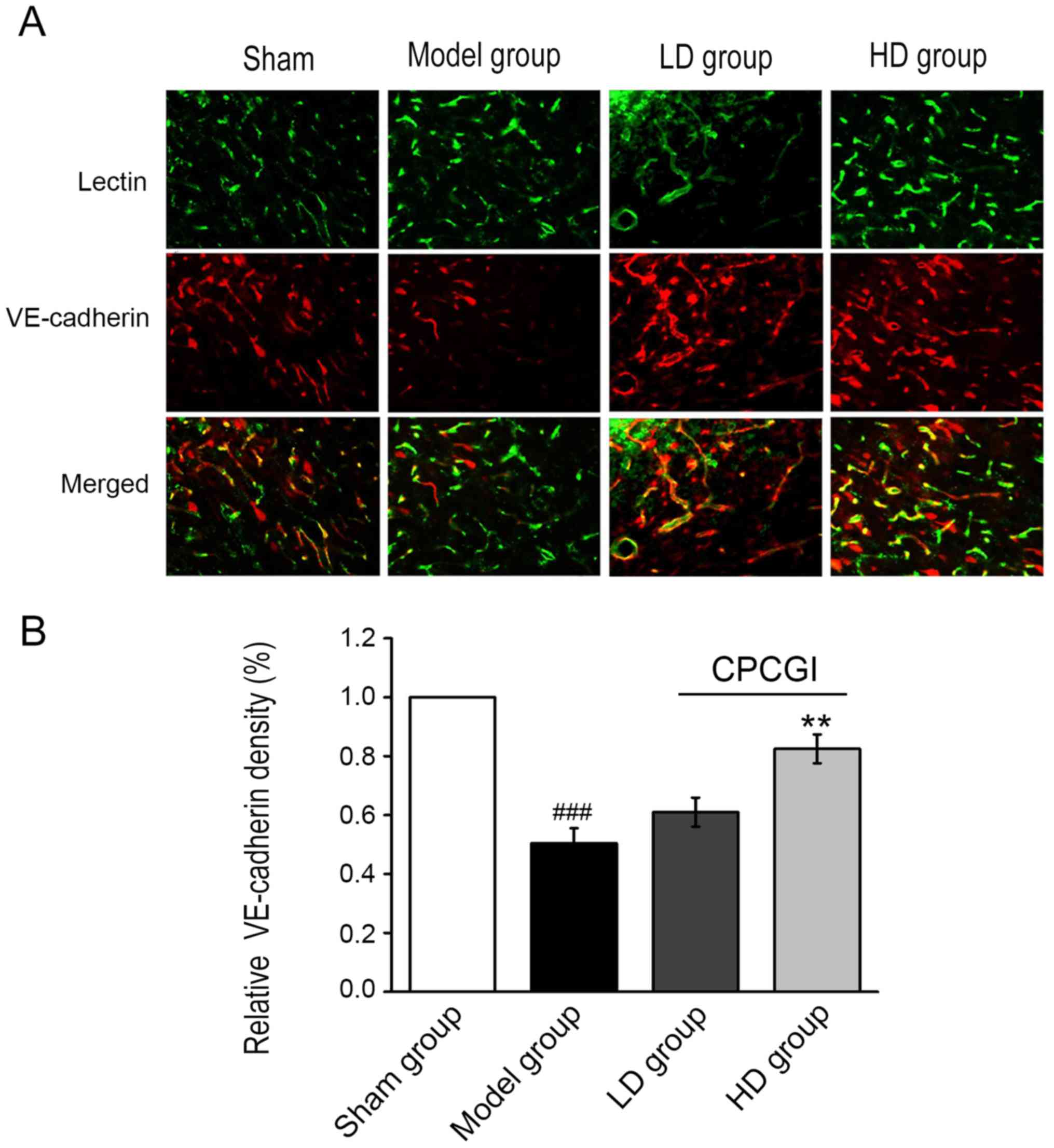
Next, the expression levels of vascular endothelial
(VE)-cadherin in microvessels adjacent to the ischemic cerebral
infarction were examined by
lectin+/VE-cadherin+ immunofluorescent
staining. The expression of VE-cadherin in the CPCGI-treated groups
was markedly upregulated compared with the model group (Fig. 3A). Quantification of the
lectin+/VE-cadherin+ double-positive staining
revealed that VE-cadherin expression in microvessels was
significantly increased in the HD group compared with the model
group (P<0.01; Fig. 3A and B).
These results suggested that CPCGI may maintain the integrity of
blood vessels by upregulating the expression of VE-cadherin in the
microvessels surrounding the infarction region, preserving the
blood supply of small blood vessels and contributing to the
protection and reconstruction of blood-brain barrier.
Effects of CPCGI administration on
cerebral angiogenesis following stroke
Immunofluorescent staining revealed that the number
of Ki67-labeled new vessels at 7 days postoperatively in the HD
group was significantly higher compared with the model group
(P<0.05; Fig. 4A and B). The
pseudopodia of the sprouting vessels were labeled by
lectin+/p-FAK+ immunofluorescent dual
staining. p-FAK was highly expressed in the axonal structure of the
microvessels compared with the blood vessels. The expression levels
of p-FAK in the peripheral cortex of the infarcted region in the LD
and HD groups were significantly upregulated compared with the
model group (P<0.05 and P<0.001, respectively; Fig. 4C and D).
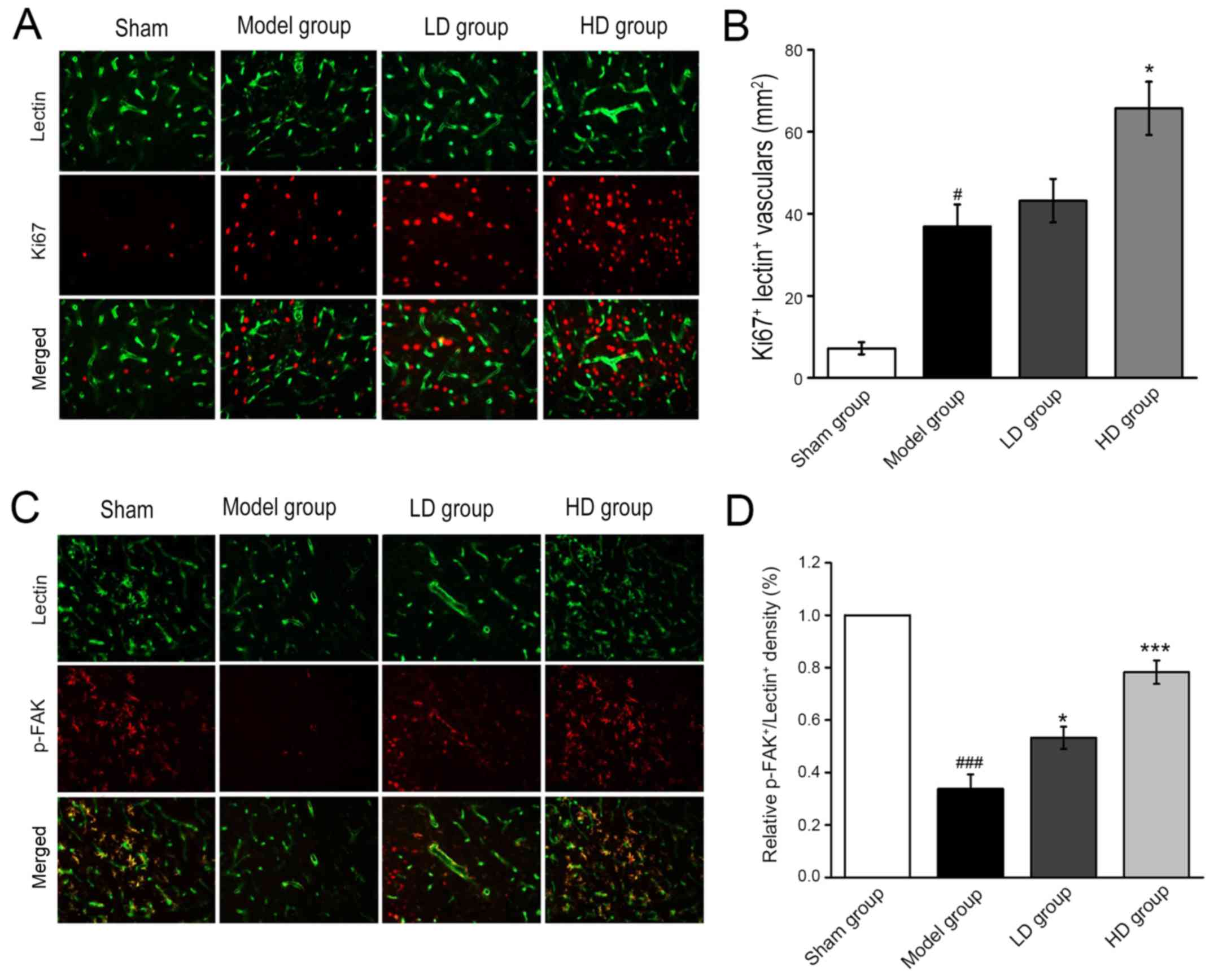 | Figure 4.Immunofluorescent staining reveals
Ki67- and p-FAK-positive newly formed vessels after 7 days of CPCGI
administration. (A) Representative images (magnification, ×20) and
(B) quantification of newly sprouting vessels, labeled by
ki67+/lectin+ immunofluorescent dual staining
(magnification, ×20) (C) Representative images and (D)
quantification of pseudopodia of the sprouting vessels in the
peripheral cortex of the infarcted region, by
p-FAK+/lectin+ immunofluorescent dual
staining. #P<0.05, ###P<0.001 vs. sham
operation group; *P<0.05, ***P<0.001 vs. model group. CPCGI,
compound porcine cerebroside and ganglioside injection; p-FAK,
phosphorylated focal adhesion kinase; LD, low dose; HD, high
dose. |
Effects of CPCGI administration on
VEGF, ANGPT1, TEK, FGF and Wnt signaling proteins following
stroke
The relative protein expression levels of VEGF,
ANG1, TEK and FGF were detected by western blot analysis in each
group at 7 days following the stroke. In the model group, the
expression levels of VEGF, ANGPT1, TEK and FGF were significantly
upregulated compared with the sham operation group (Fig. 5A). The expression levels of each
protein in the LD and HD groups were significantly increased
compared with the model group (P<0.05 and P<0.001,
respectively; Fig. 5A). In
addition, following CPCGI treatment for 7 days, the expression
levels of WNT7A, Fzd and β-catenin were slightly upregulated,
however the differences were not statistically significant
(Fig. 5B).
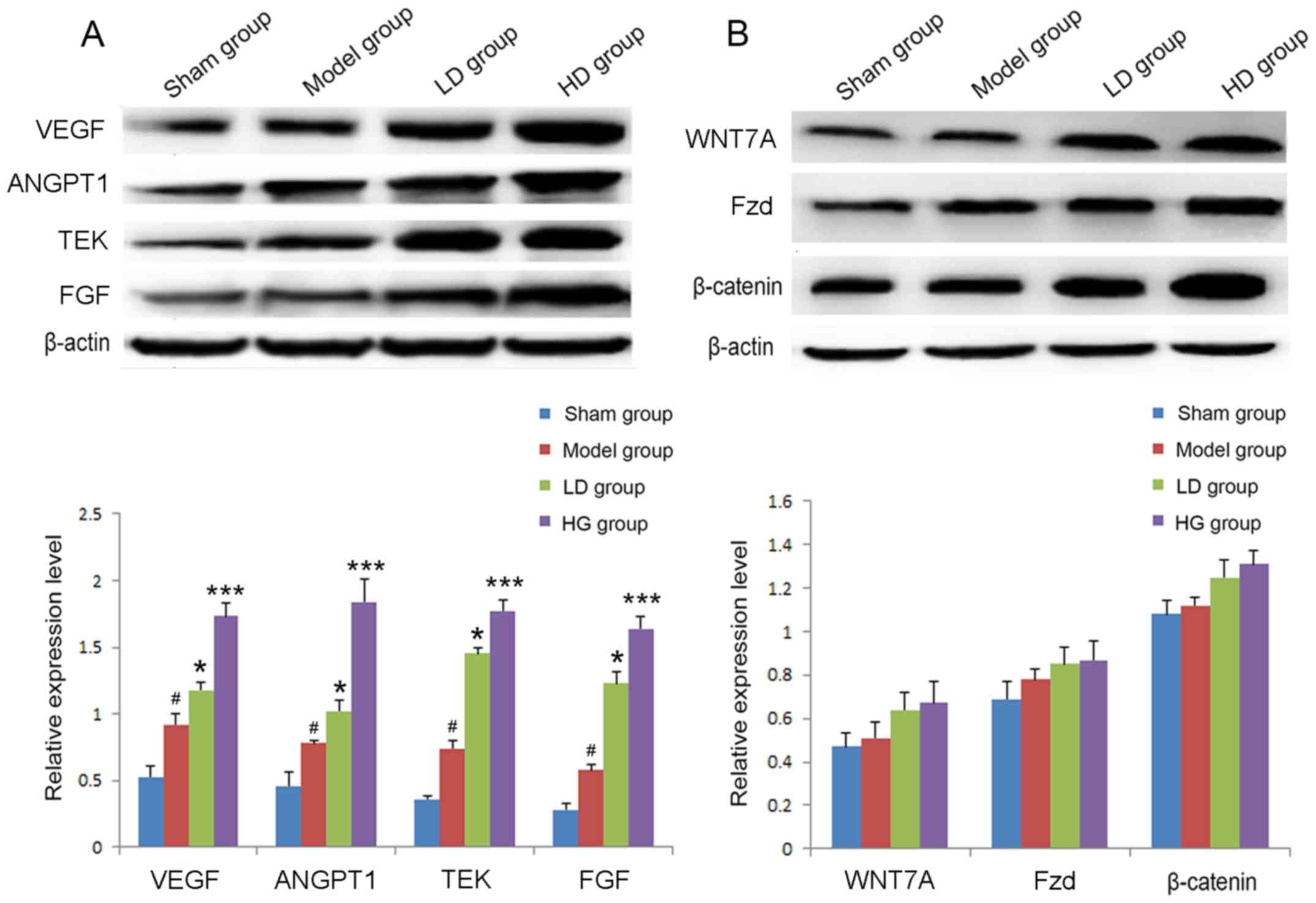 | Figure 5.Relative expression levels of VEGF,
ANGPT1, TEK and FGF 7 days after stroke in each group. (A) The
expression levels of VEGF, ANGPT1, TEK and FGF were detected by
western blot analysis. (B) The expression levels of WNT7A, Fzd and
β-catenin were detected by western blot analysis.
#P<0.05 vs. sham operation group; *P<0.05,
***P<0.001 vs. model group. VEGF, vascular endothelial growth
factor; ANGPT1, angiopoietin 1; TEK, TEK receptor tyrosine kinase;
FGF, fibroblast growth factor; WNT7A Wnt family member 7A; Fzd,
frizzled. |
Discussion
As a major challenge in geriatric medicine
worldwide, cerebral stroke is characterized by high morbidity,
recurrence and mortality rates, as well as severe irreversible
injury which is difficult to treat (30). Although the treatment and
rehabilitation of patients with stroke is actively researched,
rapid thrombolysis remains the mainstay of treatment following
stroke (31,32).
In the current study, the neurological function of
rats with ischemia-reperfusion following 7 days of intraperitoneal
injection of CPCGI was evaluated. Compared with the model group,
the administration of CPCGI for 7 consecutive days gradually
restored function in rats with ischemic cerebral injury. TTC
staining demonstrated that the volume of cerebral infarction in the
CPCGI group was significantly decreased compared with the control
group, suggesting that CPCGI may ameliorate cerebral infarction and
contribute to post-stroke recovery. In the present investigation,
laser speckle was adopted to monitor the variations of the cerebral
cortex parietal blood flow before and after model establishment,
and at 7 days following CPCGI administration. CPCGI improved the
blood flow of the infarcted side in rats with stroke. Additionally,
a higher dose of CPCGI exerted more significant effects compared
with a lower dose, suggesting that administration of CPCGI
following stroke contributes to the maintenance of blood flow.
However, whether this phenomenon results from small vascular
protection or angiogenesis remains to be investigated.
The latex perfusion results in the current study
revealed that the number of blood vessels in the ischemic cerebral
hemisphere cortex was significantly increased compared with the
model group following 7 days of CPCGI injection, indicating that
CPCGI has a protective effect on the blood vessels of the infarcted
side in rats with stroke.
Lectin+/VE-cadherin+ immunofluorescent dual
staining revealed that the expression levels of VE-cadherin in the
microvessels of rats with cerebral ischemia were significantly
upregulated compared with the model group, suggesting that CPCGI
may maintain the vascular integrity in the infarcted area and the
blood supply of microvessels and may contribute to the protection
and reconstruction of the blood-brain barrier.
Multiple studies have demonstrated that angiogenesis
adjacent to the infarcted area serves a significant role in the
recovery of neurological function in patients with stroke (33–35).
A large quantity of new microvessels gradually forms a new vascular
network through growth, adhesion and fusion. The new vascular
system is formed after the maturation of the new microvessels and
their network reconstruction (36). The newly-formed vascular system
gradually substitutes the damaged microvascular system, supplying
blood to the infarcted-penumbral brain tissues to provide the
nutrients required to promote neural repair (37). In the current study,
Ki67+/lectin+ immunofluorescent staining
demonstrated that administration of CPCGI for 7 days may
effectively promote angiogenesis and contribute to enhancing the
blood supply surrounding the infarcted area.
Taken together, intraperitoneal injection of CPCGI
accelerated the recovery of neurological function and reduced the
volume of cerebral infarction in rats with ischemic stroke,
indicating that CPCGI may be conducive to the recovery of
neurological function and cerebral microcirculation following
stroke. Furthermore, CPCGI exerted significant effects on
angiogenesis, and this was associated with increased expression of
VEGF, ANGPT1, TEK, FGF and Wnt signaling proteins. WNT7A, ANGPT1
and TEK may regulate a variety of cellular and developmental
pathways, and increase cell proliferation via activation of the
canonical WNT/β-catenin pathway (38,39).
In conclusion, the results obtained in the current
study demonstrated that CPCGI exerted positive effects on cerebral
microcirculation following ischemic stroke, suggesting that it may
be a promising therapeutic option for patients with cerebral stroke
in clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YQ conceived the study. YM and SY designed and
performed the experiments. RW participated in the experiments and
drafted the manuscript. HW performed statistical analysis and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Renji Hospital, South Campus, Shanghai Jiaotong
University School of Medicine (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chikuda H: Response: RE: Ischemic stroke
after cervical spine injury: Analysis of 11,005 patients using the
Japanese Diagnosis Procedure Combination database. Spine J.
15:2593–2594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arauz A, Romano JG, Ruiz-Franco A, Shang
T, Dong C, Rundek T, Koch S, Hernández-Curiel B, Pacheco J, Rojas
P, et al: Differences in lipid profiles in two Hispanic ischemic
stroke populations. Int J Stroke. 9:394–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Behrouz R: The risk of ischemic stroke in
major rheumatic disorders. J Neuroimmunol. 277:1–5. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu NN, Dong ZL and Han LL: MicroRNA-410
inhibition of the TIMP2-dependent MAPK pathway confers
neuroprotection against oxidative stress-induced apoptosis after
ischemic stroke in mice. Brain Res Bull. 143:45–57. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuo QZ, Liuyang ZY, Lei P, Yan X, Shentu
YP, Liang JW, Zhou H, Pei L, Xiong Y, Hou TY, et al: Zinc induces
CDK5 activation and neuronal death through CDK5-Tyr15
phosphorylation in ischemic stroke. Cell Death Dis. 9:8702018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ní Chróinín D, Asplund K, Åsberg S,
Callaly E, Cuadrado-Godia E, Díez-Tejedor E, Di Napoli M, Engelter
ST, Furie KL, Giannopoulos S, et al: Statin therapy and outcome
after ischemic stroke: Systematic review and meta-analysis of
observational studies and randomized trials. Stroke. 44:448–456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dmitrieva VG, Povarova OV, Skvortsova VI,
Limborska SA, Myasoedov NF and Dergunova LV: Semax and Pro-Gly-Pro
activate the transcription of neurotrophins and their receptor
genes after cerebral ischemia. Cell Mol Neurobiol. 30:71–79. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terent A and Ronquist G: Cerebrospinal
fluid markers of disturbed brain cell metabolism in patients with
stroke and global cerebral ischemia. Acta Neurol Scand. 62:327–335.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang M, Zhang Y, Feng L, Zheng J, Fan S,
Liu J, Yang N, Liu Y and Zuo P: Compound porcine cerebroside and
ganglioside injection attenuates cerebral ischemia-reperfusion
injury in rats by targeting multiple cellular processes.
Neuropsychiatr Dis Treat. 13:927–935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doronin II, Vishnyakova PA, Kholodenko IV,
Ponomarev ED, Ryazantsev DY, Molotkovskaya IM and Kholodenko RV:
Ganglioside GD2 in reception and transduction of cell death signal
in tumor cells. BMC Cancer. 14:2952014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Novak A, Režić Mužinić N, Cikeš Čulić V,
Božić J, Tičinović Kurir T, Ferhatović L, Puljak L and Markotić A:
Renal distribution of ganglioside GM3 in rat models of types 1 and
2 diabetes. J Physiol Biochem. 69:727–735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dostal P, Schreiberova J, Dostalova V,
Dostalova V Jr, Tyll T, Paral J, Abdo I, Cihlo M, Astapenko D and
Turek Z: Effects of hypertonic saline and mannitol on cortical
cerebral microcirculation in a rabbit craniotomy model. BMC
Anesthesiol. 15:882015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morikawa T, Kajimura M, Nakamura T,
Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori
K, Takenouchi T, et al: Hypoxic regulation of the cerebral
microcirculation is mediated by a carbon monoxide-sensitive
hydrogen sulfide pathway. Proc Natl Acad Sci USA. 109:1293–1298.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Li Q, Tang J, Feng H and Zhang JH:
The evolving roles of pericyte in early brain injury after
subarachnoid hemorrhage. Brain Res. 1623:110–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bragin DE, Bush RC and Nemoto EM: Effect
of cerebral perfusion pressure on cerebral cortical microvascular
shunting at high intracranial pressure in rats. Stroke. 44:177–181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Engelhardt B and Liebner S: Novel insights
into the development and maintenance of the blood-brain barrier.
Cell Tissue Res. 355:687–699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Wang J, Gao L, Wang R, Liu X, Gao
Z, Tao Z, Xu C, Song J, Ji X and Luo Y: MiRNA-424 protects against
permanent focal cerebral ischemia injury in mice involving
suppressing microglia activation. Stroke. 44:1706–1713. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Niu W, He Z, Zhang Q, Wu Y, Jiang
C, Tang C, Hu Y and Jia J: Autophagy suppression by exercise
pretreatment and p38 inhibition is neuroprotective in cerebral
ischemia. Brain Res. 1587:127–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao HF, Liu LM, Liu YY, Liu J, Yan L, Pan
CS, Wang MX, Wang CS, Fan JY, Gao YS and Han JY: Inhibitory effect
of rhynchophylline on contraction of cerebral arterioles to
endothelin 1: Role of rho kinase. J Ethnopharmacol. 155:147–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lapi D, Scuri R and Colantuoni A:
Trigeminal cardiac reflex and cerebral blood flow regulation. Front
Neurosci. 10:4702016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Wu B, Zhu G, Wintermark M, Wu X,
Su Z, Xu X, Tian C, Ma L, Zhang W and Lou X: Permeability imaging
as a biomarker of leptomeningeal collateral flow in patients with
intracranial arterial stenosis. Cell Biochem Biophys. 71:1273–1279.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong P, Zhao S, Wang J, Yang Z, Qian J, Wu
X, Cahoon J and Tang W: Mild hypothermia preserves cerebral cortex
microcirculation after resuscitation in a rat model of cardiac
arrest. Resuscitation. 97:109–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cabrales P, Zanini GM, Meays D, Frangos JA
and Carvalho LJ: Nitric oxide protection against murine cerebral
malaria is associated with improved cerebral microcirculatory
physiology. J Infect Dis. 203:1454–1463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KJ and Filosa JA: Advanced in vitro
approach to study neurovascular coupling mechanisms in the brain
microcirculation. J Physiol. 590:1757–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Q, Qi L, Li H, Mao L, Yang M, Xie R,
Yang X, Wang J, Zhang Z, Kong J and Sun B: Roflumilast reduces
cerebral inflammation in a rat model of experimental subarachnoid
hemorrhage. Inflammation. 40:1245–1253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen HJ, Shen YC, Shiao YJ, Liou KT, Hsu
WH, Hsieh PH, Lee CY, Chen YR and Lin YL: Multiplex brain proteomic
analysis revealed the molecular therapeutic effects of Buyang
Huanwu decoction on cerebral ischemic stroke mice. PLoS One.
10:e01408232015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belayev L, Alonso OF, Busto R, Zhao W and
Ginsberg MD: Middle cerebral artery occlusion in the rat by
intraluminal suture. Neurological and pathological evaluation of an
improved model. Stroke. 27:1616–1623. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Ryck M, Van Reempts J, Borgers M,
Wauquier A and Janssen PA: Photochemical stroke model: Flunarizine
prevents sensorimotor deficits after neocortical infarcts in rats.
Stroke. 20:1383–1390. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang JB, Jü XH, Wang J, Sun HR and Li F:
Serum cystatin C and cerebral microbleeds in patients with acute
cerebral stroke. J Clin Neurosci. 21:268–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakr SA, El-Rasheedy WA, Ramadan MM,
El-Menshawy I, Mahfouz E and Bayoumi M: Association between left
atrial appendage morphology evaluated by trans-esophageal
echocardiography and ischemic cerebral stroke in patients with
atrial fibrillation. Int Heart J. 56:329–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liutkiene G, Stropus R, Dabuzinskiene A
and Pilmane M: Structural changes of the human superior cervical
ganglion following ischemic stroke. Medicina (Kaunas). 43:390–398.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chin Y, Kishi M, Sekino M, Nakajo F, Abe
Y, Terazono Y, Hiroyuki O, Kato F, Koizumi S, Gachet C and
Hisatsune T: Involvement of glial P2Y(1) receptors in cognitive
deficit after focal cerebral stroke in a rodent model. J
Neuroinflammation. 10:952013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pavlidis E, Spagnoli C, Duca M, Ormitti F,
Magnani C and Pisani F: Neonatal forearm compartment syndrome: Look
for cerebral stroke. J Pediatr. 164:427.e12014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tamiya S, Yoshida Y, Harada S, Nakamoto K
and Tokuyama S: Establishment of a central post-stroke pain model
using global cerebral ischaemic mice. J Pharm Pharmacol.
65:615–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu Q, Chu M, Wang H, Lu S, Gao H, Li P,
Gan Y, Shi H, Liang W, Chen J and Gao Y: Sevoflurane
preconditioning protects blood-brain-barrier against brain
ischemia. Front Biosci (Elite Ed). 3:978–988. 2011.PubMed/NCBI
|
|
37
|
Lapi D, Vagnani S, Sapio D, Mastantuono T,
Sabatino L, Paterni M and Colantuoni A: Long-term remodeling of rat
pial microcirculation after transient middle cerebral artery
occlusion and reperfusion. J Vasc Res. 50:332–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pazhohan A, Amidi F, Akbari-Asbagh F,
Seyedrezazadeh E, Farzadi L, Khodarahmin M, Mehdinejadiani S and
Sobhani A: The Wnt/β-catenin signaling in endometriosis, the
expression of total and active forms of β-catenin, total and
inactive forms of glycogen synthase kinase-3β, WNT7a and
DICKKOPF-1. Eur J Obstet Gynecol Reprod Biol. 220:1–5. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katoh Y and Katoh M: Comparative
integromics on Angiopoietin family members. Int J Mol Med.
17:1145–1149. 2006.PubMed/NCBI
|