Introduction
Human esophageal cancer is a commonly diagnosed
disease worldwide, with an increasing incidence estimated at more
than 450,000 new cases each year (1). Two important types of human
esophageal cancer have been identified, including squamous cell
carcinoma and adenocarcinoma, in which, esophageal squamous cell
carcinoma has a higher prevalence in China (2). Several therapeutic approaches have
been developed for esophageal cancer in recent decades, including
endoscopic resection and surgery; however, these approaches show
some efficacy only during the early stages of esophageal cancer
(3,4). Radiotherapy and chemotherapy are more
commonly used for advanced stages, but their efficacy remains
unsatisfactory due to the development of therapeutic resistance and
unavoidable side effects (4,5).
Recently, numerous studies have demonstrated that compounds
isolated from traditional Chinese medicines, such as osthole,
bufadienolides, matrine, and the ajoene analogue BisPMB, exhibit
anti-esophageal cancer activity through the induction of apoptosis,
cell cycle arrest and endoplasmic reticulum stress (6–9).
Consequently, the development of new treatment agents from
traditional Chinese medicine is becoming a promising strategy for
the treatment of esophageal cancer.
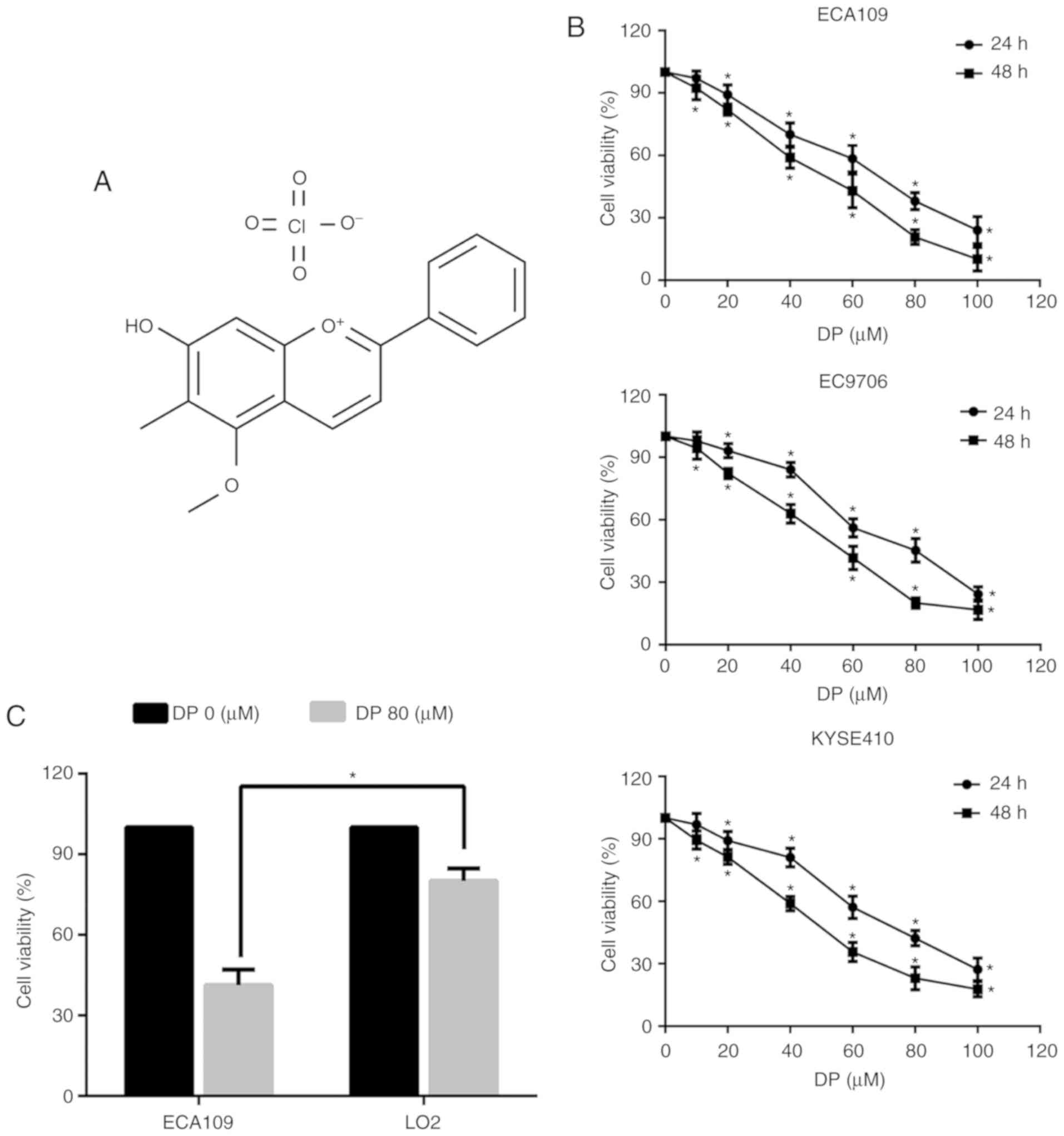
Dracorhodin perchlorate (DP) (Fig. 1A) is a synthetic analogue of the
anthocyanin red pigment dracorhodin, which is extracted from
exudates of the fruit of Daemonorops draco, also known as
‘dragon's blood’ in traditional Chinese medicine (10,11).
It has been reported to exert a variety of physiological and
pharmacological effects, such as antimicrobial and antifungal
activity and promotion of wound healing (11–14).
Recently, there has been increasing interest in the anticancer
properties of DP, which have been demonstrated in several studies
undertaken on various types of malignant cells. For example, DP was
reported to induce apoptosis in human gastric adenocarcinoma
through inactivation of the AKT/FOXO3a and NF-κB signaling pathways
(15) and through activation of
the p38/JNK MAPK signaling pathways in human melanoma cells
(16). In addition to apoptosis,
DP has also been shown to induce cell cycle arrest in various types
of cancer cells (15,17). However, the effect of DP on ESCC
remains unknown, and the molecular mechanisms underlying the
anticancer properties of DP warrant further investigation.
In our previous study, DP induced intrinsic
apoptosis and G1 phase arrest and upregulated p53 in human lung
squamous carcinoma cells (18). In
the present study, the potential antitumor effects of DP were
investigated on ESCC cells, as well as the associated underlying
mechanisms. The results showed that DP significantly inhibited the
proliferation of ESCC cells, while exerting a low cytotoxic effect
on normal human liver LO2 cells. Furthermore, DP induced apoptosis
and G2 phase arrest, and inhibited the activation of the JAK2/STAT3
and AKT/FOXO3a pathways in ESCC cells.
Materials and methods
Reagents
Dracorhodin perchlorate (DP) was purchased from
ShangHai YuanYe Biotechnology Co., Ltd. (Shanghai, China) and
dissolved in dimethyl sulfoxide (DMSO). Fetal bovine serum (FBS)
and the enhanced chemiluminescence (ECL) kit were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Phosphate-buffered saline (PBS), RPMI-1640, and
penicillin-streptomycin were purchased from HyClone/GE Healthcare
Life Sciences (Victoria, Australia). The Giemsa stain kit was
purchased from Beijing Solarbio Science & Technology (Beijing,
China). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Labortories (Kumamoto, Japan). Hoechst 33342 and the Cell Cycle
Analysis kit were purchased from Beyotime Institute of
Biotechnology (Shanghai, China). The FITC/Annexin V Apoptosis
Detection kit was purchased from BD Biosciences (Franklin Lakes,
NJ, USA). The broad-spectrum caspase inhibitor (Z-VAD-FMK) was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Primary antibodies against β-actin (1:2,000; cat. no. 4970T), Bcl-2
(1:1,000; cat. no. 4223T), caspase-9 (1:500; cat. no. 9508S.),
cleaved caspase-3 (1:500; cat. no. 9661T), cleaved caspase-7
(1:500; cat. no. 8438T), total caspase-3 (1:1,000; cat. no. 9662S),
total caspase-7 (1:1,000; cat. no. 12827T), DR4 (1:1,000; cat. no.
42533S), DR5 (1:1,000; cat. no. 8074T), cyclin B1 (1:1,000; cat.
no. 12231T), Cdc2 (1:1,000; cat. no. 9116T), AKT (1:1,000; cat. no.
4691T), and p-AKT (Ser473; (1:500; cat. no. 4060T) were purchased
from Cell Signaling Technology (Danvers, MA, USA). Primary
antibodies against PARP (1:1,000; cat. no. ab32138), caspase-10
(1:1,000; cat. no. ab32155), p21 (1:1,000; cat. no. ab188224), p27
(1:1,000; cat. no. ab92741), JAK2 (1:1,000; cat. no. ab108596),
p-JAK2 (Tyr1007/1008; 1:500; cat. no. ab32101), STAT3 (1:1,000;
cat. no. ab68153), p-STAT3 (Tyr705; 1:500; cat. no. ab76315),
FOXO3a (1:1,000; cat. no. ab109629), and p-FOXO3a (Ser253; (1:500;
cat. no. ab154786) were purchased from Abcam (Cambridge, MA, USA).
Goat Anti-rabbit (1:5,000; cat. no. sc-2004) and goat anti-mouse
(1:5,000; cat. no. sc-2005) secondary antibodies were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cells and cell culture
Human ESCC cell lines (ECA109, EC9706 and KYSE410)
were purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China).
Normal human liver LO2 cells were a kind gift from Dr Yan Jiao from
Jilin University (Jilin, China). All the cells were maintained in a
humidified atmosphere with 5% CO2. Cells were cultured
in RPMI-1640 medium supplemented with 10% FBS.
Cell viability assay
Cell viability was evaluated using a CCK-8 assay.
Briefly, 5×103 cells were plated in a 96-well plate and
cultured for 1 day for cell attachment. Next, the cells were
treated with various concentrations of DP (10–100 µM) for 24 h, and
then 10 µl CCK-8 was added to the cells, followed by a 2-h
incubation at 37°C. Finally, the absorbance was detected at 450 nm
using a microplate reader.
Colony formation assay
A total of 4×105 cells/well were seeded
in a 6-well plate and cultured overnight. The cells were then
exposed to various concentrations of DP (0–80 µM) for 24 h.
Subsequently, the cells were harvested and counted, and then plated
at 400 cells/well in a 6-well plate and cultured for 2 weeks for
colony formation. The cells were then washed twice with PBS, fixed
with 4% paraformaldehyde for 20 min, and stained with Giemsa stain
for 30 min at room temperature followed by two washes with PBS.
Finally, positive colonies containing more than 50 cells were
counted under an optical microscope (magnification, ×100).
Analysis of apoptosis by flow
cytometry
The effect of DP on cell apoptosis was measured by
Annexin V-FITC/propidium iodide (PI) staining. Briefly,
4×105 cells/well were seeded in a 6-well plate. After
overnight culture and attachment, the cells were treated with 0, 40
and 80 µM DP for 24 h. The cells were then harvested, washed twice
with PBS and resuspended in 400 µl binding buffer. Next, 5 µl
Annexin V-FITC/PI was added to the cell suspension, and the cells
were incubated in the dark at 37°C for 15 min. Finally, apoptosis
was measured by flow cytometry.
Morphological evaluation of
apoptosis
The morphological detection of apoptosis was
performed with Hoechst 33342 staining. Briefly, 4×105
cells/well were seeded in a 6-well plate. After overnight culture
and attachment, the cells were treated with 0 and 80 µM DP for 24
h. The cells were then collected in a centrifuge tube and washed
twice with PBS. Subsequently, 200 µl of Hoechst 33342 was added,
followed by incubation at 37°C for 30 min. Finally, the cells were
mounted on a glass slide for morphological analysis of apoptosis
under a fluorescence microscope.
Cell cycle analysis by flow
cytometry
The effect of DP on the cell cycle was measured by
flow cytometry using PI staining. Briefly, 4×105
cells/well were seeded in a 6-well plate and incubated at 37°C
overnight for cell attachment. The cells were then treated with 0,
40 and 80 µM DP for a further 24 h. The cells were subsequently
harvested in a centrifuge tube and fixed with 500 µl of 70%
ice-cold ethanol at 4°C for 4 h. The cells were washed twice with
PBS and then incubated in the dark with 10 µl RNase A and 25 µl PI
staining solution at 37°C for 30 min. Finally, the cell samples
were analyzed by flow cytometry.
Western blotting
Protein expression was detected by western blotting.
Briefly, 1×106 cells were seeded in 60-mm dishes and
cultured overnight for attachment. After treatment with 0, 40, 60
and 80 µM DP for 24 h, the cells were harvested and washed twice
with PBS. The cells were then suspended in protein extraction
buffer containing protease inhibitors, and lysed on ice for 30 min.
The supernatant was collected after centrifugation at 13,000 × g
for 10 min, and the protein content was measured using a
bicinchoninic acid protein assay kit. Equal amounts of protein
lysates (20 µg) were separated by electrophoresis on a 10-15%
sodium dodecyl sulphate-polyacrylamide gel at 120 V. The proteins
were then transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA), which were then soaked in blocking
buffer (5% skimmed milk) for 1 h. The membranes were then incubated
with the relevant primary antibodies overnight at 4°C, followed by
incubation with the appropriate horseradish peroxidase-conjugated
secondary antibodies for 1 h at room temperature. Finally, ECL
detection was performed. β-actin was used as reference protein.
Grey values of the protein bands were measured by Image J version
1.5.1 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All the experiments were repeated 3 times. Data were
analyzed using SPSS version 19.0 statistical software (IBM Corp.,
Armonk, NY, USA) and expressed as the mean ± standard deviation.
Comparisons between groups were analyzed using the unpaired
Student's t-test or one-way analysis of variance (ANOVA) followed
by Tukey's post hoc tests. P<0.05 was considered statistically
significant.
Results
DP reduces the viability of ESCC
cells
To determine the effect of DP on ESCC cells, a Cell
Counting Kit-8 (CCK-8) assay was performed to evaluate the
viability of three ESCC cell lines (ECA109, EC9706 and KYSE410). As
shown in Fig. 1B, exposure to DP
at concentrations ranging from 0 to 100 µM for 24 and 48 h
significantly decreased the viability of all three ESCC cell lines
in a concentration- and time-dependent manner. Furthermore, DP
cytotoxicity was compared between the ECA109 and normal human liver
LO2 cell lines. As shown in Fig.
1C, after treatment for 24 h, DP at 80 µM showed reduced
toxicity towards LO2 cells compared with the ECA109 cells. These
results indicated that DP has increased selective cytotoxicity
towards ESCC cells compared to normal human cells.
DP inhibits the colony-forming ability
of ESCC cells
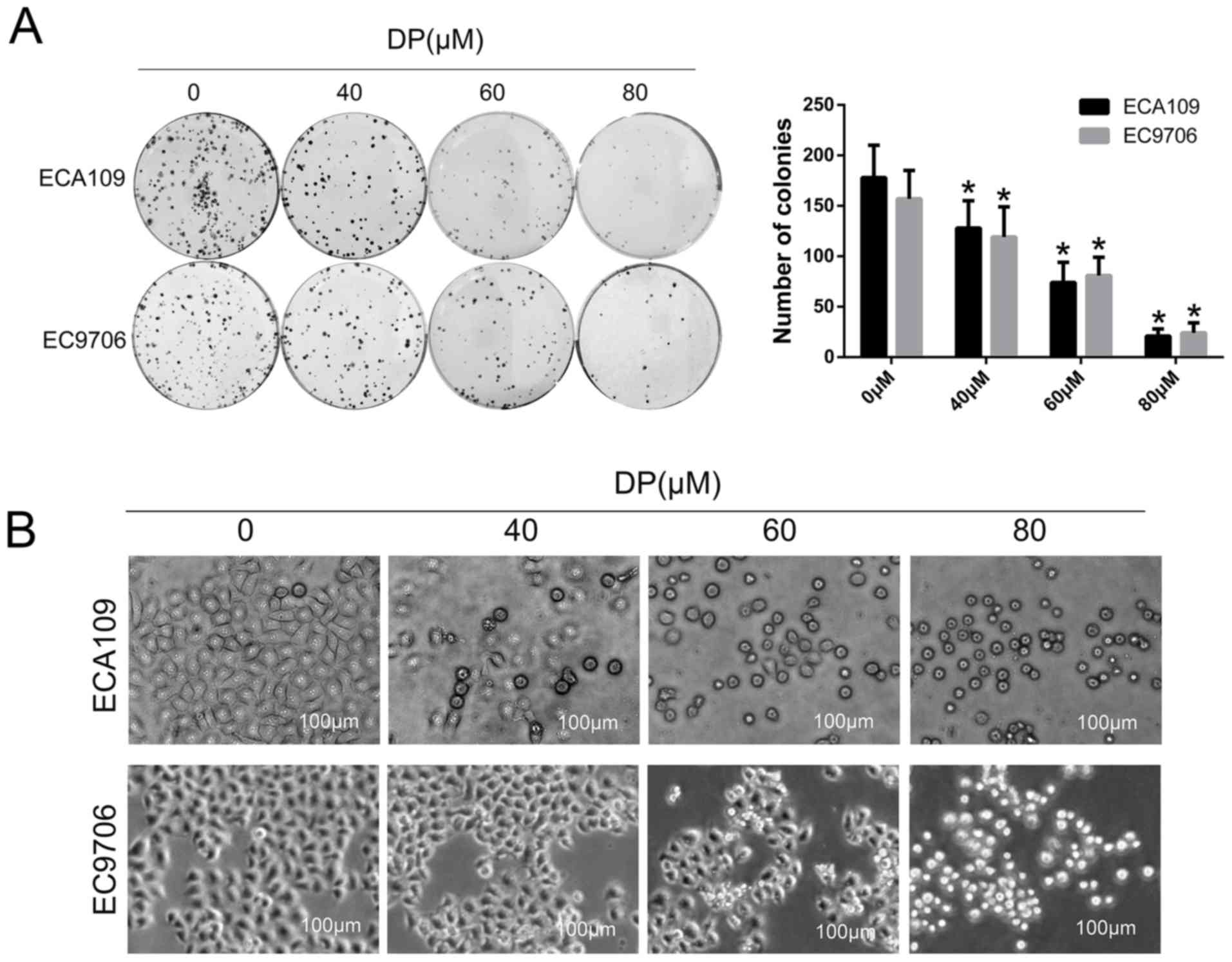
To investigate the effect of DP on long-term
proliferation of ESCC cells, a colony formation assay was
performed. After treatment with DP (0, 40, 60 and 80 µM) for 24 h,
the treated cells were reseeded in a 6-well plate with 400
cells/well and cultured for 14 days. As shown in Fig. 2A, the colony-forming ability of the
ECA109 and EC9706 cells decreased significantly in a
concentration-dependent manner compared with control group. In
addition, morphological changes in ECA109 and EC9706 cells after
treatment with DP (0, 40, 60 and 80 µM) were observed under an
optical microscope. As shown in Fig.
2B, the cells became round and small, and floating cells were
also observed with increasing concentrations.
DP induces G2 phase arrest in ESCC
cells
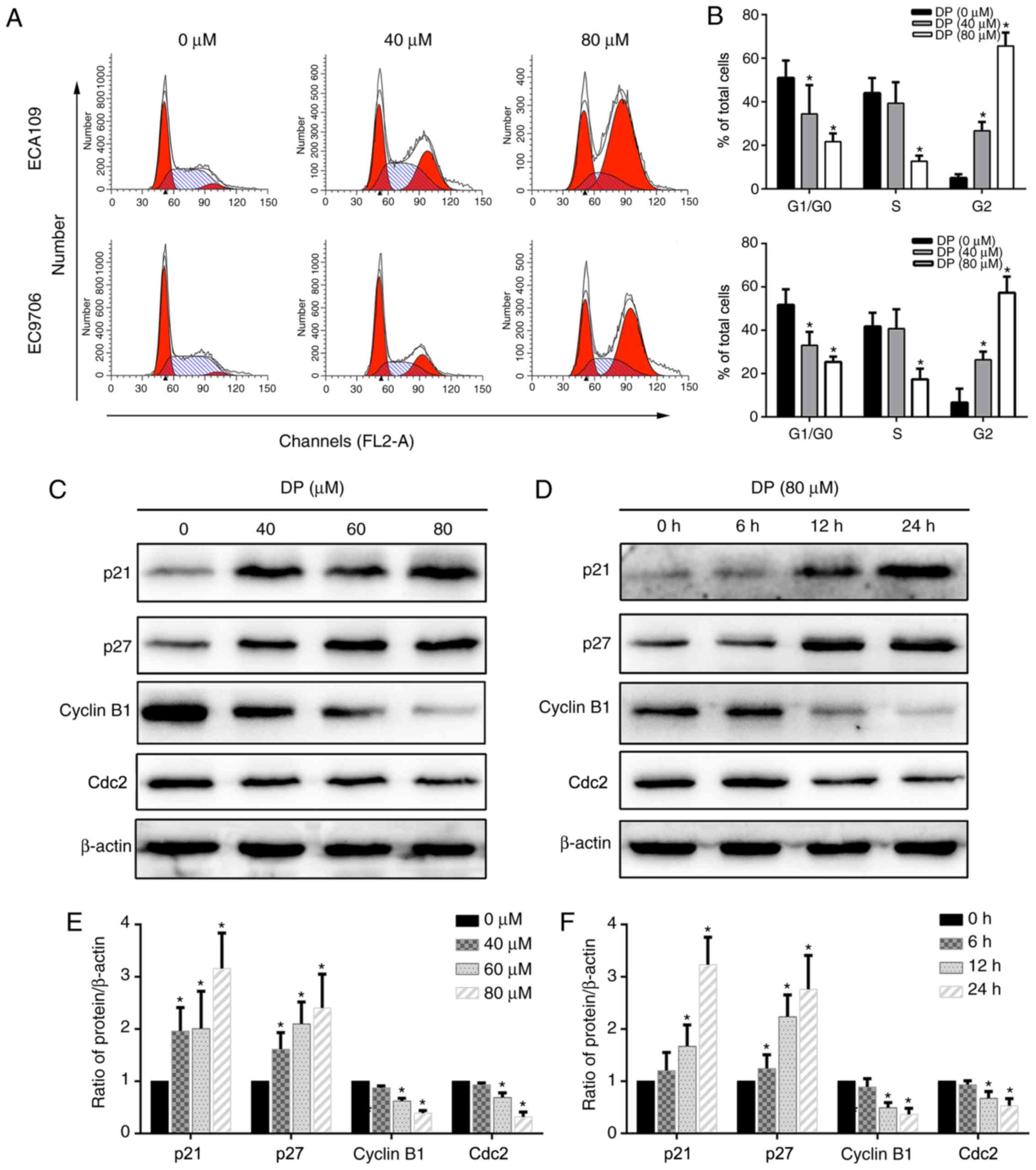
Cell cycle arrest is a mechanism that potentially
mediates the anticancer effect of many drugs. To determine the
effect of DP on cell cycle progression, DP-treated ECA109 and
EC9706 cells stained with PI were analyzed by flow cytometry. As
shown in Fig. 3A and B, after
treatment with DP for 24 h, the proportion of cells in the G2/M
phase significantly increased compared with the control group,
accompanied by a corresponding decrease in G0/G1 and S phase
populations. Furthermore, the underlying molecular mechanism was
explored by detecting the expression of G2/M phase-related proteins
using western blotting. As shown in Fig. 3C-F, after DP treatment, p21 and p27
were upregulated and cyclin B1 and Cdc2 were downregulated in a
concentration- and time-dependent manner compared with the control
group in ECA109 cells. Taken together, our results showed that DP
induced G2 phase arrest in ESCC cells through upregulation of p21
and p27, and downregulation of cyclin B1 and Cdc2.
DP induces caspase-dependent apoptosis
in ESCC cells
Apoptosis is an important target for the cancer
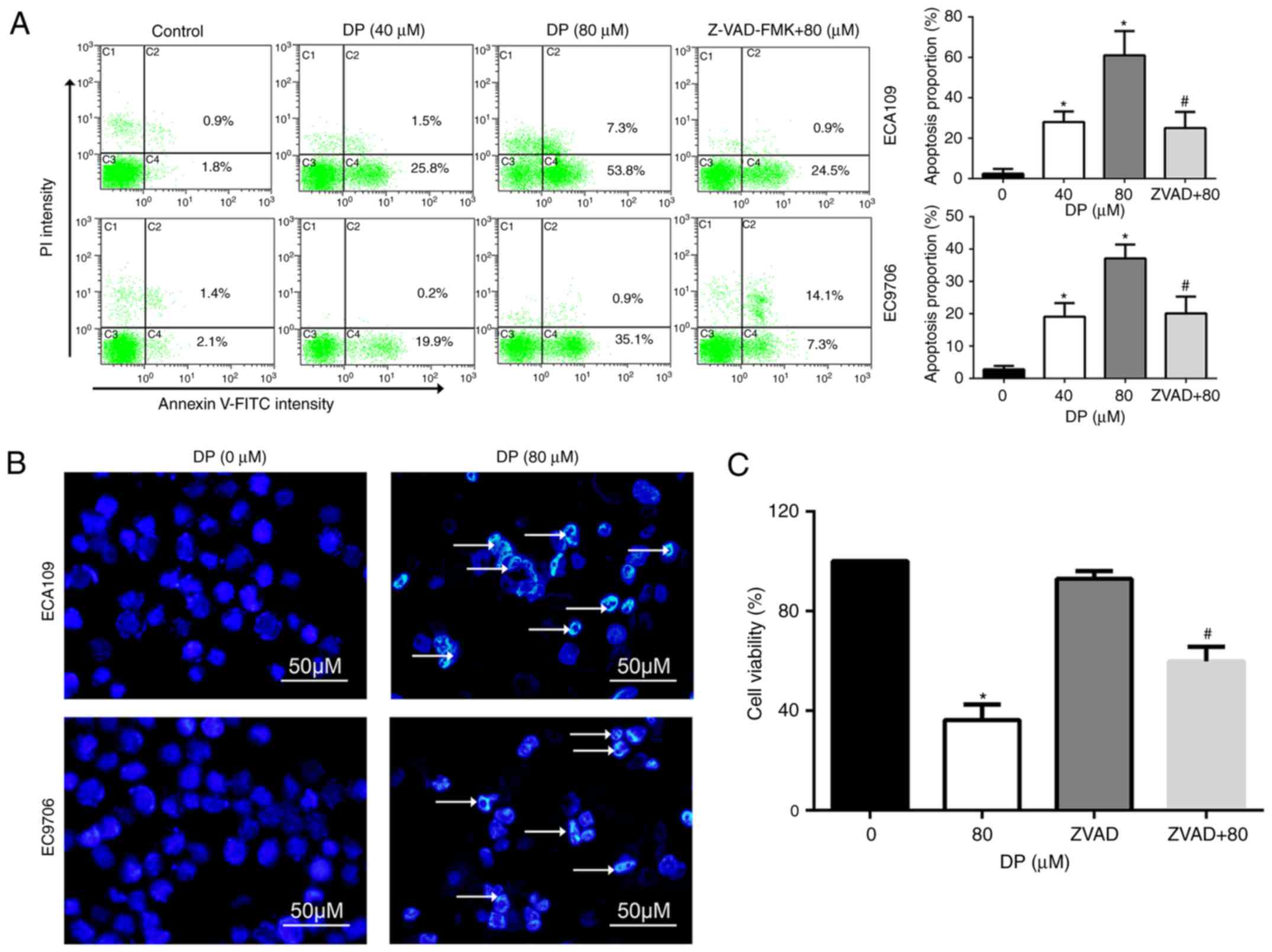
inhibitory effects of chemotherapeutic drugs. To determine whether
apoptosis is induced by DP treatment, Annexin V-FITC/PI double
staining was performed, followed by flow cytometry. As shown in
Fig. 4A, DP treatment (0, 40 and
80 µM) for 24 h significantly increased the proportion of apoptotic
ECA109 and EC9706 cells. In addition, pretreatment for 1 h with
Z-VAD-FMK, a broad-spectrum caspase inhibitor, significantly
reversed apoptosis induced by DP (80 µM) compared to 80 µM DP
treatment alone.
Apoptotic cells often exhibit typical morphological
changes, characterized by chromatin condensation and DNA
fragmentation. To further confirm DP-induced apoptosis, Hoechst
33342 staining assay was used to assess cell morphological changes.
After 80 µM DP treatment for 24 h, chromatin condensation and DNA
fragmentation were observed in ECA109 and EC9706 cells, as
indicated by the arrow in Fig.
4B.
The role of DP-induced apoptosis in the
anti-proliferative effect of this compound was analyzed through
pretreatment of ECA109 cells with Z-VAD-FMK before co-incubation
with 80 µM DP for 24 h. The results showed that Z-VAD-FMK
significantly attenuated the DP-induced reduction in cell viability
to 59.8% in ECA109 cells, compared with 36.2% for DP (80 µM)
treatment alone (Fig. 4C). These
results indicate that DP induced apoptosis in a caspase-dependent
manner and consequently exhibited a cytotoxic effect in ESCC
cells.
DP induces apoptosis through the
extrinsic and intrinsic pathways in ECA109 cells
Caspase-dependent apoptosis is characterized by the
activation of a caspase cascade, in which caspase-3 and caspase-7
function as executioner caspases. When caspase-3 and caspase-7 are
activated, the active forms cleave several cellular substrates,
including PARP, the cleavage of which is also regarded as a typical
molecular apoptotic marker. To study the molecular mechanisms
involved in DP-induced apoptosis, the levels of total PARP, total
caspase-3/7, cleaved PARP, and cleaved caspase-3/-7 were determined
by western blotting. As shown in Fig.
5A and B, DP treatment for 24 h significantly decreased total
PARP and total caspase-3/7 expression levels and increased the
expression levels of cleaved PARP and cleaved caspase-3/-7 in a
concentration-dependent manner in ECA109 cells.
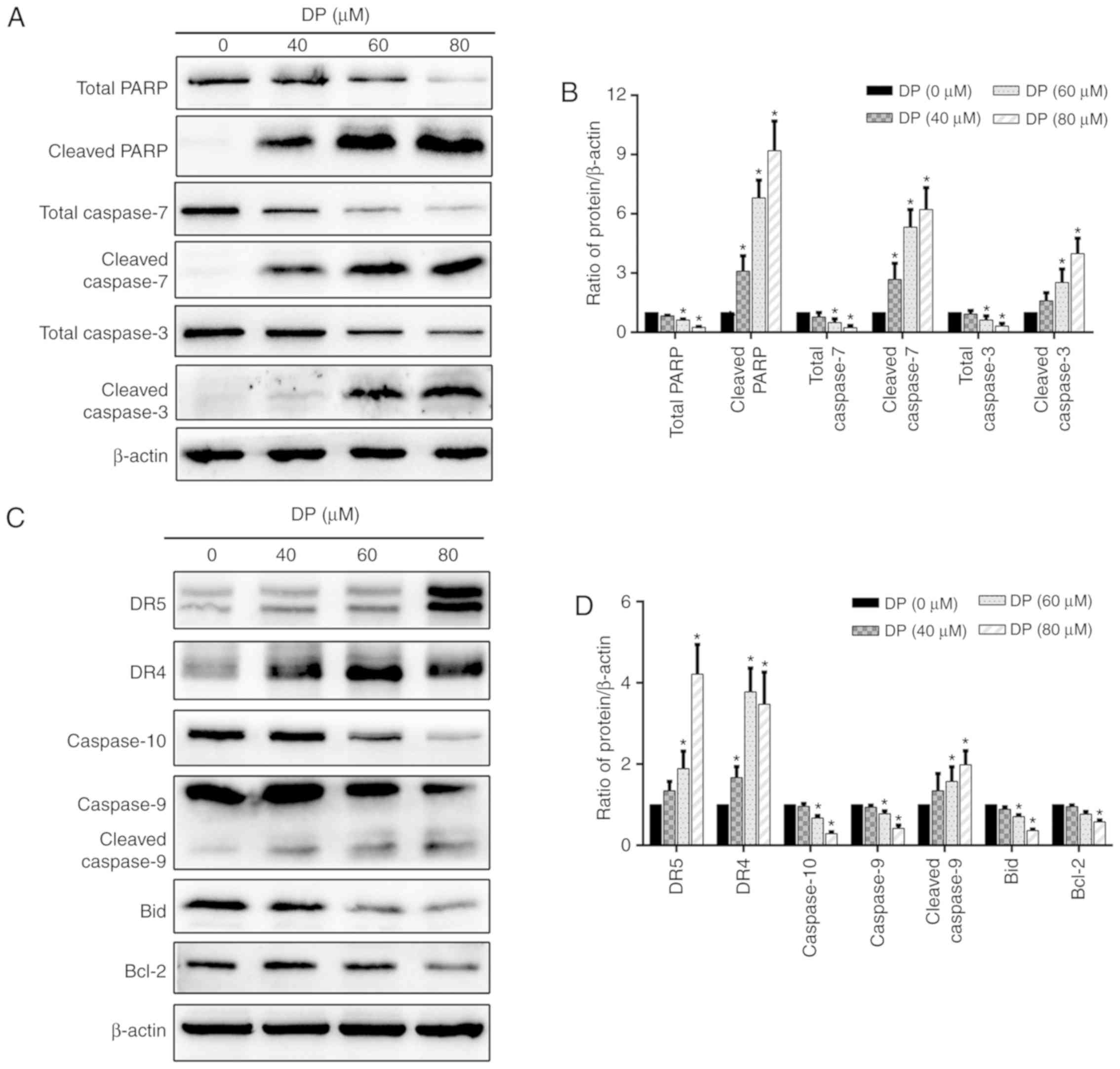 | Figure 5.DP induces extrinsic and intrinsic
apoptosis in ECA109 cells. ECA109 cells were treated DP (0, 40, 60
and 80 µM) for 24 h. (A and B) The expression of PARP, caspase-3/7,
cleaved PARP and cleaved-3/7 was detected by western blot analysis.
(C and D) The expression of extrinsic apoptosis-related proteins
DR5, DR4 and caspase-10, and intrinsic apoptosis-related proteins
caspase-9, Bax and Bid were detected by western blot analysis. The
data are expressed as the mean ± standard deviation (n=3).
*P<0.05 compared with the control group. DP, dracorhodin
perchlorate; PARP, cleaved poly (ADP-ribose) polymerase; DR4, death
receptor 4; DR5, death receptor 5. |
To further investigate which apoptotic pathway is
involved in DP-induced apoptosis, the expression of several
extrinsic and intrinsic apoptosis-related proteins was determined
by western blotting. After treatment of ECA109 cells with
increasing concentrations of DP (0–80 µM) for 24 h, extrinsic and
intrinsic apoptosis were both activated, as demonstrated by the
upregulation of DR4, DR5 and cleaved caspase-9, and the
downregulation of caspase-9, caspase-10 and Bcl-2 (Fig. 5C and D). In addition, the levels of
the Bid protein, which can transduce the extrinsic apoptotic signal
to mitochondria to trigger intrinsic apoptosis when activated by
cleavage, was decreased after DP treatment (Fig. 5C and D), suggesting the presence of
a crosstalk between extrinsic and intrinsic apoptosis in DP-treated
ECA109 cells.
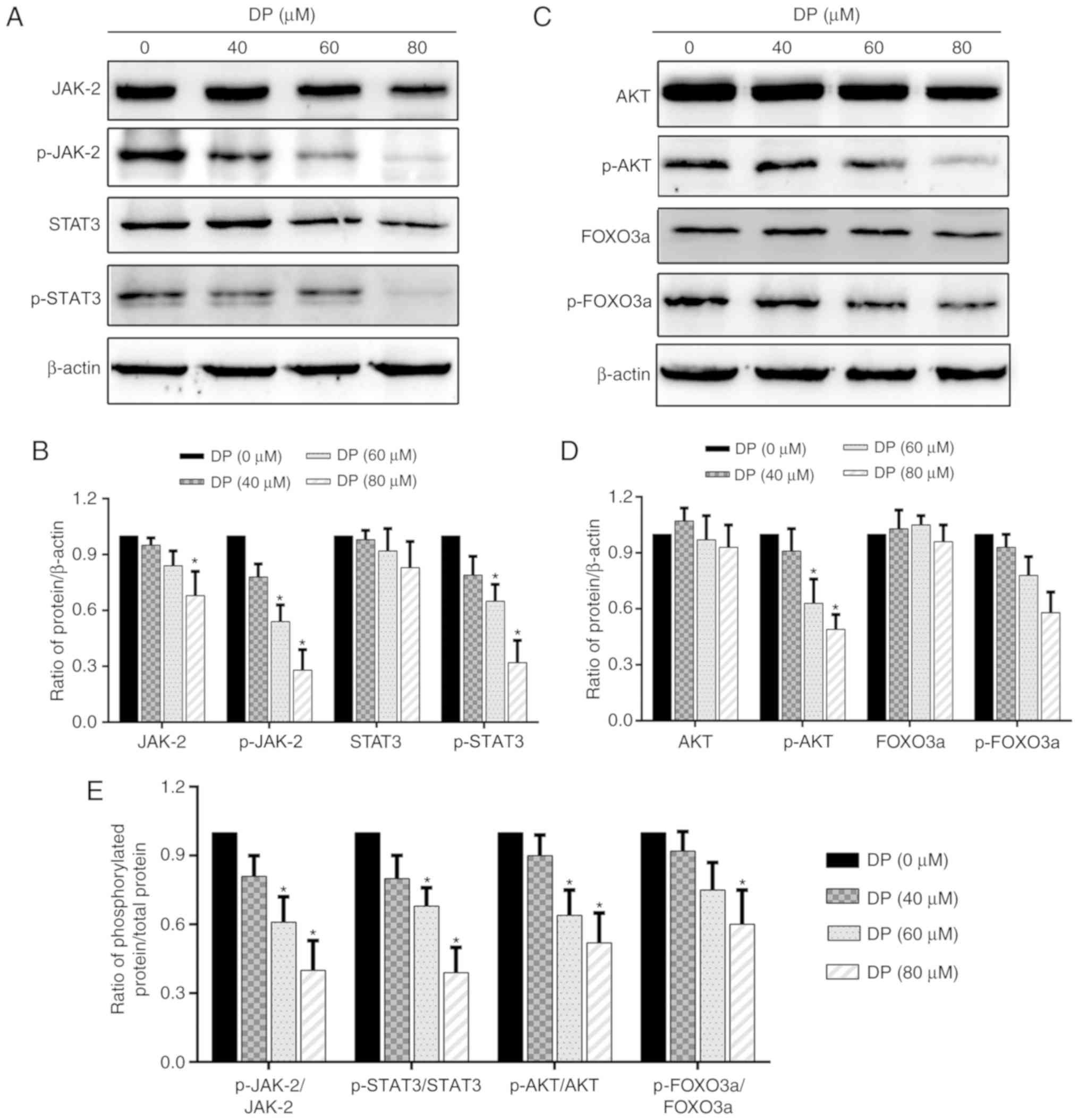
DP inhibits the JAK2/STAT3 and
AKT/FOXO3a signaling pathways in ECA109 ESCC cells
Research has shown that the STAT3 and AKT signaling
pathways are closely associated with cell proliferation and are
overactivated in a variety of cancers. Therefore, in the present
study, we investigated whether these two signaling pathways were
affected by DP treatment. After treatment of ECA109 cells with DP
for 24 h, western blot analysis was performed to assess the
expression and phosphorylation of the related proteins. As shown in
Fig. 6A, B and E, the expression
of JAK2 and STAT3 was decreased slightly, while their
phosphorylation and the ratio of phosphorylated protein/total
protein of JAK2 and STAT3 was significantly reduced. In addition,
DP treatment also decreased the phosphorylation and the ratio of
phosphorylated protein/total protein of AKT and FOXO3a, but had
little effect on the expression of these proteins (Fig. 6C-E). These data suggest that
apoptosis and cell cycle arrest induced by DP in ESCC cells were
partly due to the inactivation of JAK2/STAT3 and AKT/FOXO3a.
Discussion
The development of new anticancer agents derived
from traditional Chinese medicine is becoming an attractive
strategy for the treatment of various types of cancer. Dracorhodin
perchlorate (DP), a synthetic analogue of the anthocyanin red
pigment dracorhodin isolated from exudates of the fruit of
Daemonorops draco, has previously been shown to exert a
robust anticancer effect in various cancer cell lines (15,17–19).
However, the underlying mechanism of this effect has not been fully
elucidated, and the effect of DP on esophageal squamous cell
carcinoma (ESCC) cells remains unknown. In the present study, DP
was observed to inhibit cell proliferation through the induction of
apoptosis and G2/M phase cell cycle arrest and to inhibit the
activation of the JAK2/STAT3 and AKT/FOXO3a signaling pathways.
It is well known that abnormal cell cycle
progression, with a consequent loss of key cell cycle checkpoints,
results in over-proliferation of cancer cells (20–22).
Therefore, we explored whether induction of cell cycle arrest was
one of the mechanisms involved in the inhibitory effect of DP on
the proliferation of ESCC cells. In the present study, it was found
that DP treatment significantly induced cell cycle arrest at the
G2/M phase in a concentration-dependent manner. Moreover, our
results also showed that DP induced G2/M phase arrest by regulating
the expression of various proteins that control the transition from
the G2/M phase to the G0/G1 phase (23,24),
such as by upregulating p21 and p27 and downregulating cyclin B1
and Cdc2. Previous studies have also reported that DP induced cell
cycle arrest at the G0/G1 phase in human lung squamous carcinoma
cells and human gastric adenocarcinoma cells (15,18).
Collectively, together with the results from the present study,
these findings indicate that, depending on cell type, induction of
cell cycle arrest at different phases is one potential anticancer
mechanism of DP.
Apoptosis is the most common target in the
development of new anticancer drugs (25,26).
There are two major apoptotic pathways, namely the extrinsic
pathway mediated by DRs, and the intrinsic pathway regulated by the
Bcl-2 family (27). The caspase
cascade is important for the initiation and execution of apoptosis,
where caspase-10 and −8 initiate extrinsic apoptosis, caspase-9
initiates intrinsic apoptosis, and caspase-3 and −7 are the
executioner caspases (28). In the
present study, both extrinsic and intrinsic apoptosis were induced
by DP treatment in ESCC cells, evidenced by the increased
expression of DR4, DR5, cleaved caspase-3/-7/-9, and cleaved PARP,
and decreased expression of total PARP, total caspase-3/7, Bcl-2
and caspase-9/-10. Bid is an important protein that transduces the
apoptotic signal from the extrinsic to the intrinsic pathway by
translocation of the truncated form (tBid) to mitochondria after
cleavage by caspase-8/-10, and it was demonstrated that Bid
expression was reduced at the protein level after treatment with
DP, indicating the presence of a crosstalk between extrinsic and
intrinsic apoptosis (29).
Furthermore, in the present study, DP-induced apoptosis of ESCC
cells was found to occur in a caspase-dependent manner, as
evidenced by the reversal of apoptosis mediated by Z-VAD-FMK, a
caspase inhibitor. Our results are in accordance with previous
studies reporting that DP induced apoptosis in multiple types of
cancer cells (15,17,18,30,31).
However, which apoptotic pathway plays the most significant role in
DP-induced apoptosis remains unknown and requires further
investigation.
There is evidence that numerous signaling pathways
are involved in the regulation of tumorigenesis, and the JAK2/STAT3
and AKT/FOXO3a signaling axes have been shown to play important
roles in this process (32–34).
The STAT3 transcription factor has been reported to be
constitutively activated through phosphorylation by upstream JAK
kinases in various cancer types, including lung cancer, melanoma,
and esophageal cancer, in response to various stimuli such as
cytokines and growth factors (32,35).
Activated STAT3 translocates to the nucleus where it promotes
proliferation and cell cycle progression and inhibits apoptosis by
activating the transcription of downstream oncogenes such as p21
and Bcl-2 (36,37). The AKT/FOXO3a signaling pathway is
also involved in the regulation of multiple cellular functions.
Once activated, AKT phosphorylates a variety of substrates,
resulting in cell cycle progression and inhibition of apoptosis
(38,39); FOXO3a, meanwhile, is a
transcription factor acting downstream of AKT, and its activity is
inhibited by AKT-mediated phosphorylation, resulting in reduced
transcription of key genes related to induction of apoptosis and
cell cycle arrest (40,41). Therefore, targeting these two
signaling pathways has potential benefits for the development of
new drugs.
In the present study, DP treatment was found to
significantly inhibit the phosphorylation of JAK2, STAT3, AKT and
FOXO3a. In addition, at the protein level, DP decreased JAK2 and
STAT3 expression, whereas it had no obvious effect on AKT and
FOXO3a expression. Treatment with DP also reduced Bcl-2 expression
and increased p21 expression, both of which are regulated by AKT
and STAT3. These results suggest that the JAK2/STAT3 and AKT/FOXO3a
signaling pathways were involved, at least partly, in the
regulation of apoptosis and cell cycle arrest induced by DP in ESCC
cells. Our results are consistent with the findings of a previous
study reporting that DP induced apoptosis in human gastric
adenocarcinoma SGC-7901 cells via inhibition of AKT activity
(15). Increasing evidence has
shown that the STAT3 and AKT pathways exert their function
interactively or independently in different cellular contexts
(42,43). Therefore, further experiments are
needed to determine whether there is a crosstalk between the STAT3
and AKT pathways in DP-treated ESCC cells.
The ideal drug for the treatment of cancer should
efficiently inhibit cancer cell proliferation but have little
cytotoxicity on normal cells. In the present study, DP showed a
more potent cytotoxic effect on ESCC cells compared with human
normal liver cell, yet one limitation of our study was that the
cytotoxicity of DP on esophageal epithelial cells was not
confirmed.
In conclusion, in the present study, DP was found to
reduce the viability and inhibit the proliferation of ESCC cells.
In addition, DP treatment induced extrinsic and intrinsic
apoptosis, and resulted in cell cycle arrest at the G2/M phase.
Moreover, the JAK2/STAT3 and AKT/FOXO3a signaling pathways were
inhibited by DP treatment. Together, our results indicate that DP
is a promising agent for use in the development of new drugs to
treat ESCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81702975).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL wrote the paper. ZL, CLi, CLu and YJ performed
the experiments. ZL, YL and CLu analyzed the data. GZ designed the
experiments and improved the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohashi S, Miyamoto S, Kikuchi O, Goto T,
Amanuma Y and Muto M: Recent advances from basic and clinical
studies of esophageal squamous cell carcinoma. Gastroenterology.
149:1700–1715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang H, Shin SK, Cho BC, Lee CG, Kim CB,
Kim DJ, Lee JG, Hur J, Lee CY, Bae MK, et al: A prospective phase
II trial of S-1 and cisplatin-based chemoradiotherapy for
locoregionally advanced esophageal cancer. Cancer Chemother
Pharmacol. 73:665–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu X, Li Z, Li T, Long F, Lv Y, Liu L,
Liu X and Zhan Q: Osthole inhibits the PI3K/AKT signaling pathway
via activation of PTEN and induces cell cycle arrest and apoptosis
in esophageal squamous cell carcinoma. Biomed Pharmacother.
102:502–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin S, Lv J, Peng P, Cai C, Deng J, Deng
H, Li X and Tang X: Bufadienolides induce p53-mediated apoptosis in
esophageal squamous cell carcinoma cells in vitro and in
vivo. Oncol Lett. 15:1566–1572. 2018.PubMed/NCBI
|
|
8
|
Jiang JH, Pi J, Jin H, Yang F and Cai JY:
Chinese herb medicine matrine induce apoptosis in human esophageal
squamous cancer KYSE-150 cells through increasing reactive oxygen
species and inhibiting mitochondrial function. Pathol Res Pract.
214:691–699. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siyo V, Schäfer G, Hunter R, Grafov A,
Grafova I, Nieger M, Katz AA, Parker MI and Kaschula CH: The
cytotoxicity of the ajoene analogue BisPMB in WHCO1 oesophageal
cancer cells is mediated by CHOP/GADD153. Molecules. 22:E8922017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brockmann H and Junge H: Die konstitution
des dracorhodins, eines neuen farbstoffes aus dem ‘Drachenblut’.
Berl Dtsch Chem Ges. 76:751–846. 1943. View Article : Google Scholar
|
|
11
|
Rao GS, Gerhart MA, Lee RT III, Mitscher
LA and Drake S: Antimicrobial agents from higher plants. Dragon's
blood resin. J Nat Prod. 45:646–648. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li F, Jiang T, Liu W, Hu Q and Yin H: The
angiogenic effect of dracorhodin perchlorate on human umbilical
vein endothelial cells and its potential mechanism of action. Mol
Med Rep. 14:1667–1672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang XW, Qiao L, Liu L, Zhang BQ, Wang
XW, Han YW and Yu WH: Dracorhodin perchlorate accelerates cutaneous
wound healing in wistar rats. Evid Based Complement Alternat Med.
2017:89505162017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang LF, Liu X, Lv LL, Ma ZM, Feng XC and
Ma TH: Dracorhodin perchlorate inhibits biofilm formation and
virulence factors of Candida albicans. J Mycol Med. 28:36–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rasul A, Ding C, Li X, Khan M, Yi F, Ali M
and Ma T: Dracorhodin perchlorate inhibits PI3K/Akt and NF-κB
activation, up-regulates the expression of p53, and enhances
apoptosis. Apoptosis. 17:1104–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia M, Wang M, Tashiro S, Onodera S,
Minami M and Ikejima T: Dracorhodin perchlorate induces A375-S2
cell apoptosis via accumulation of p53 and activation of caspases.
Biol Pharm Bull. 28:226–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Luo J, Meng L, Pan T, Zhao B, Tang
ZG and Dai Y: Dracorhodin perchlorate induces the apoptosis of
glioma cells. Oncol Rep. 35:2364–2372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang G, Sun M, Zhang Y, Hua P, Li X, Cui
R and Zhang X: Dracorhodin perchlorate induces G1/G0 phase arrest
and mitochondria-mediated apoptosis in SK-MES-1 human lung squamous
carcinoma cells. Oncol Lett. 10:240–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu JH, Zheng GB, Liu CY, Zhang LY, Gao HM,
Zhang YH, Dai CY, Huang L, Meng XY, Zhang WY and Yu XF: Dracorhodin
perchlorate induced human breast cancer MCF-7 apoptosis through
mitochondrial pathways. Int J Med Sci. 10:1149–1156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Molinari M: Cell cycle checkpoints and
their inactivation in human cancer. Cell Prolif. 33:261–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diaz-Moralli S, Tarrado-Castellarnau M,
Miranda A and Cascante M: Targeting cell cycle regulation in cancer
therapy. Pharmacol Ther. 138:255–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaltiel IA, Krenning L, Bruinsma W and
Medema RH: The same, only different-DNA damage checkpoints and
their reversal throughout the cell cycle. J Cell Sci. 128:607–620.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hochegger H, Takeda S and Hunt T:
Cyclin-dependent kinases and cell-cycle transitions: Does one fit
all? Nat Rev Mol Cell Biol. 9:910–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wood SJ, Goldufsky JW, Bello D, Masood S
and Shafikhani SH: Pseudomonas aeruginosa ExoT induces
mitochondrial apoptosis in target host cells in a manner that
depends on its GTPase-activating protein (GAP) domain activity. J
Biol Chem. 290:29063–29073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen JT and Wells JA: Direct activation
of the apoptosis machinery as a mechanism to target cancer cells.
Proc Natl Acad Sci USA. 100:7533–7538. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia MY, Wang MW, Cui Z, Tashiro SI,
Onodera S, Minami M and Ikejima T: Dracorhodin perchlorate induces
apoptosis in HL-60 cells. J Asian Nat Prod Res. 8:335–343. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He Y, Ju W, Hao H, Liu Q, Lv L and Zeng F:
Dracorhodin perchlorate suppresses proliferation and induces
apoptosis in human prostate cancer cell line PC-3. J Huazhong Univ
Sci Technolog Med Sci. 31:2152011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huynh J, Etemadi N, Hollande F, Ernst M
and Buchert M: The JAK/STAT3 axis: A comprehensive drug target for
solid malignancies. Semin Cancer Biol. 45:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prabhu VV, Allen JE, Dicker DT and
El-Deiry WS: Small-molecule ONC201/TIC10 targets
chemotherapy-resistant colorectal cancer stem-like cells in an
Akt/Foxo3a/TRAIL-dependent manner. Cancer Res. 75:1423–1432. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
36
|
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L,
Zhang Q, Wu Y, Jiang H and Sun X: STAT3 interacts with Skp2/p27/p21
pathway to regulate the motility and invasion of gastric cancer
cells. Cell Signal. 25:931–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma J, Song X, Xu X and Mou Y:
Cancer-associated-fibroblasts promote the chemo-resistance in
gastric cancer through secreting IL-11 targeting JAK/STAT3/Bcl2
pathway. Cancer Res Treat. 51:194–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Steelman LS, Pohnert SC, Shelton JG,
Franklin RA, Bertrand FE and McCubrey JA: JAK/STAT, Raf/MEK/ERK,
PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis.
Leukemia. 18:189–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
New DC, Wu K, Kwok AW and Wong YH: G
protein-coupled receptor-induced Akt activity in cellular
proliferation and apoptosis. FEBS J. 274:6025–6036. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rathbone CR, Booth FW and Lees SJ: FoxO3a
preferentially induces p27Kip1 expression while impairing muscle
precursor cell-cycle progression. Muscle Nerve. 37:84–89. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Cui N, Zheng PS and Yang WT: BMX/Etk
promotes cell proliferation and tumorigenicity of cervical cancer
cells through PI3K/AKT/mTOR and STAT3 pathways. Oncotarget.
8:49238–49252. 2017.PubMed/NCBI
|
|
43
|
Steelman LS, Abrams SL, Whelan J, Bertrand
FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A,
et al: Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and
Jak/STAT pathways to leukemia. Leukemia. 22:686–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|