Introduction
Advanced glycation end products (AGEs) are produced
during the irreversible Maillard reaction between carbohydrates and
proteins (1). The accumulation of
AGEs may lead to myocardial fibrosis, thereby facilitating
myocardial injury (2,3). The progression of myocardial injury
can lead to numerous events associated with cardiovascular disease,
including myocardial ischemia, heart failure and myocardial
infarction (4). Therefore, early
and appropriate treatments are required to inhibit the progression
of myocardial injury; however, the treatment of myocardial injury
in the early stage remains a major challenge.
Halofuginone (HF,
7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl) is a
halogenated derivative of febrifugine, which is the major active
ingredient of Dichroa febrifuga. Several studies have
suggested that HF exerts beneficial effects on various diseases
models, including rheumatoid arthritis (5), radiation-induced lung injury
(6), and burn-induced hepatic and
renal damage in rats (7). A
previous study indicated that the amino acid response pathway may
be activated by HF in cardiac tissue (8). Additionally, HF may also alleviate
the structural and functional effects of cardiac stress (8). The present study aimed to demonstrate
the potential effects of HF in the treatment of myocardial
injury.
Evidence has demonstrated that an increase in
autophagy may protect against myocardial dysfunction in mice
(9,10). In addition, endoplasmic reticulum
(ER) stress-associated cardiomyocyte apoptosis is a major
contributor to myocardial injury, according to published research
(11). The ER is a vital organelle
responsible for numerous cellular processes; for example, the ER
can maintain calcium homeostasis, protein folding and
post-translational modifications, and can detect oxidative stress
(12,13). Furthermore, the ER has been
reported to be involved in the regulation of apoptosis and the
inflammatory response via various pathways (14). Therefore, myocardial injury may be
alleviated by the regulation of autophagy and ER stress-associated
apoptosis. Coincidentally, previous studies have indicated that HF
serves an important role in regulating cell apoptosis and autophagy
(15). Therefore, whether HF may
affect ER stress to reduce cardiomyocyte injury requires further
investigation.
In the present study, the possible protective
effects and underlying molecular mechanisms of HF on cardiomyocyte
injury were investigated. AGEs-induced H9C2 cell lines were
incubated with HF at various concentrations. The results of the
present study suggested that HF may protect AGEs-induced H9C2 cells
against damage via the suppression of ER stress-associated
apoptosis and the induction of autophagy.
Materials and methods
Cell culture and treatment
The rat cardiomyocyte cell line H9C2 was obtained
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 10 U/ml penicillin-streptomycin at 37°C in an
atmosphere containing 5% CO2. The medium was replaced
every 3 days. When cell confluence reached 70–80%, cells were
passaged. Bovine serum albumin (BSA)-AGEs were prepared as
previously described (1,2,16,17).
Briefly, 0.3% BSA (cat. no. 9048-46-8; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was incubated with 50 mM D-glucose in 5%
CO2/95% air at 37°C for 12 weeks. AGE-BSA specific fluorescence
determinations were performed by measuring emission at 440 nm on
excitation at 370 nm using a fluorescence spectrophotometer
(Hitachi, Japan). AGE-BSA was stored at −70°C until use. The H9C2
cellular damage model was established via pretreatment with AGEs
(400 µg/ml) for 12 h at 37°C.
Cell grouping
H9C2 cells were randomly divided into five groups:
Control group, H9C2 cells without any treatment; AGEs group, H9C2
cells treated with 400 µg/ml AGEs; three experimental groups,
AGEs-treated cells plus different concentrations of HF (0.5, 2 and
8 nM) for 24 h. For autophagy assay, the H9C2 cells were randomly
divided into 4 groups: AGEs group, AGEs-induced cells; AGEs + 3-MA
group, AGEs-induced cells treated with 3-MA (5 mM) for 24 h; AGEs +
HF group, AGEs-induced cells treated with HF (8 nM) for 24 h and
AGEs + 3-MA + HF group, AGEs-induced cells treated with 3-MA (5 mM)
and HF (8 nM) for 24 h.
Cell viability
The viability of H9C2 cells was evaluated via an MTT
assay (Sigma-Aldrich; Merck KGaA). Cells were seeded into 96-well
plates at a concentration of 6×103 cells/well and were
cultured for 12 h at 37°C under 5% CO2. Subsequently,
the culture medium was replaced with fresh medium containing HF
(CAS no. 64924-67-0; Sigma-Aldrich; Merck KGaA) at different
concentrations (0, 0.5, 1, 2, 4, 8, 10, 20, 40, 80, 100 and 200
nM), and cells were cultured for 48 h at 37°C. The MTT solution was
added to the cells and formazan crystals were dissolved using
dimethyl sulfoxide. The optical density values were detected using
a spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
at 550 nm.
Western blot analysis
H9C2 cells were lysed in lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) and the protein
concentration was determined using a Pierce™ BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.). A total of 20 µg
protein was separated via 10% SDS-PAGE, after which, they were
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). After blocking with 5% BSA for 1 h at room
temperature, the samples were probed with primary antibodies
against myoglobin (Mb; 1:1,000; cat. no. 25919; Cell Signaling
Technology, Inc., Danvers, MA, USA), creatine kinase MB (CK-MB;
1:1,000; cat. no. ab31832; Abcam), cardiac troponin I (cTnI;
1:1,000; cat. no. ab47003; Abcam), CCAAT/enhancer-binding protein
homologous protein (CHOP; 1:1,000; cat. no. 2895; Cell Signaling
Technology, Inc.), cleaved caspase-12 (1;1,000; cat. no. 2202; Cell
Signaling Technology, Inc.), growth arrest and DNA damage-inducible
protein GADD34 (GADD34; 1:2,000; cat. no. ab9869; Abcam), binding
immunoglobulin protein (BiP; 1:1,000; cat. no. 3177; Cell Signaling
Technology, Inc.), cleaved caspase-3 (1:1,000; cat. no. 9661; Cell
Signaling Technology, Inc.), cleaved caspase-9 (1:1,000; cat. no.
9509; Cell Signaling Technology, Inc.), microtubule-associated
proteins 1A/1B light chain 3B (LC3; 1:1,000; cat. no. 4108; Cell
Signaling Technology, Inc.), Beclin 1 (1:1,000; cat. no. 3495; Cell
Signaling Technology, Inc.), P62 (1:1,000; cat. no. 88588; CST) and
GAPDH (1:200; cat. no. ab9485; Abcam) overnight at 4°C. The next
day, membranes were incubated with a horseradish peroxidase goat
anti-mouse immunoglobulin G secondary antibody (1:2,000; cat. no.
ab205719; Abcam) for 1.5 h at room temperature. Protein bands were
visualized using an enhanced chemiluminescent (ECL) kit (Bio-Rad
Laboratories, Inc.). Densitometric analysis was performed using
ImageJ software version 1.48 (National Institutes of Health,
Bethesda, MD, USA).
Measurement of reactive oxygen species
(ROS)
The production of ROS in the H9C2 cell line was
detected by measuring 2′,7′-dichlorodihydrofluorescein
(DCFH)-derived fluorescence via flow cytometry. Cells were cultured
with 10 µM DCFH-diacetate (CAS no. 4091-99-0; Sigma-Aldrich; Merck
KGaA) in serum-free DMEM at 37°C under 5% CO2 for 1 h.
Subsequently, the cells were routinely collected and suspended in
PBS. Cells were analyzed via flow cytometry by measuring DCF
fluorescence at an excitation wavelength of 488 nm and an emission
wavelength of 519 nm. FACSDiva software version 5.0.2 (BD
Biosciences, San Jose, CA, USA) was used to analyze fluorescence
intensities and determine ROS production.
Detection of cell apoptosis by flow
cytometry
Cells (1.5×105−1×106) were
suspended and immobilized in 75% cold ethanol (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) for 12 h at
4°C. Subsequently, cells were stained with 5 µl Annexin
V-fluorescein isothiocyanate (FITC) and 5 µl propidium iodide (PI)
for 15 min at room temperature in the dark. Cell apoptotic index
was determined by Annexin V-FITC/PI ratio, as detected by flow
cytometry (BD Biosciences, Franklin Lakes, NJ, USA). FACSDiva
software version 5.0.2 (BD Biosciences) was used for data
acquisition and analysis.
Immunofluorescence staining of
LC3
H9C2 cells from each group were washed with PBS
three times and fixed with 4% paraformaldehyde for 15 min at 4°C.
Cells were permeabilized with Tris-buffered saline (TBS) containing
0.25% Triton X-100 (TBSX) three times for 10 min each at 4°C, and
then blocked with 10% horse serum (cat. no. H8890; Sigma-Aldrich;
Merck KGaA) in TBSX for 1 h at 4°C. Subsequently, cells were
incubated with a primary antibody against LC3 (1: 500; cat. no.
NB100-2220; Novus Biologicals Canada ULC, Oakville, ON, Canada)
overnight at 4°C, followed by incubation with a swine anti-rabbit
FITC-conjugated secondary antibody (1:50; cat. no. F0205; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) for 30 min at
4°C. Cells were then stained with 1 µg/ml DAPI at 37°C for 5 min.
Cells were mounted with anti-fading medium. Three randomly chosen
microscopic fields were analyzed under a confocal laser scanning
microscope (Carl Zeiss AG, Oberkochen, Germany) and quanitified
using ImageJ software version 1.48 (National Institutes of
Health).
Statistical analysis
Experiments were repeated at least three times and
the data are presented as the means ± standard deviation.
Statistical comparisons between different groups were conducted
using one-way analysis of variance followed by Bonferroni post hoc
test using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
HF alleviates AGEs-induced H9C2
cellular damage
The structure of HF is presented in Fig. 1A. An MTT assay was used to detect
the viability of H9C2 cells treated with HF at various
concentrations (0, 0.5, 1, 2, 4, 8, 10, 20, 40, 80, 100 and 200
nM), for 48 h. As presented in Fig.
1B, the cell viability was significantly decreased in H9C2
cells treated with HF at concentrations >8 nM. To exclude cell
toxicity, concentrations of 0.5, 2 and 8 nM were selected for
subsequent analysis. The expression levels of the markers of
myocardial injury (Mb, CK-MB and cTnI) were analyzed by western
blotting. The results suggested that the expression levels of the
three markers were significantly increased in the AGEs group
compared with in the control group. Conversely, Mb, CK-MB and cTnI
expression levels were significantly reduced in the experimental
groups treated with HF compared with in the AGEs-only group
(Fig. 2A). These results indicated
that HF may mitigate AGEs-induced myocardial injury.
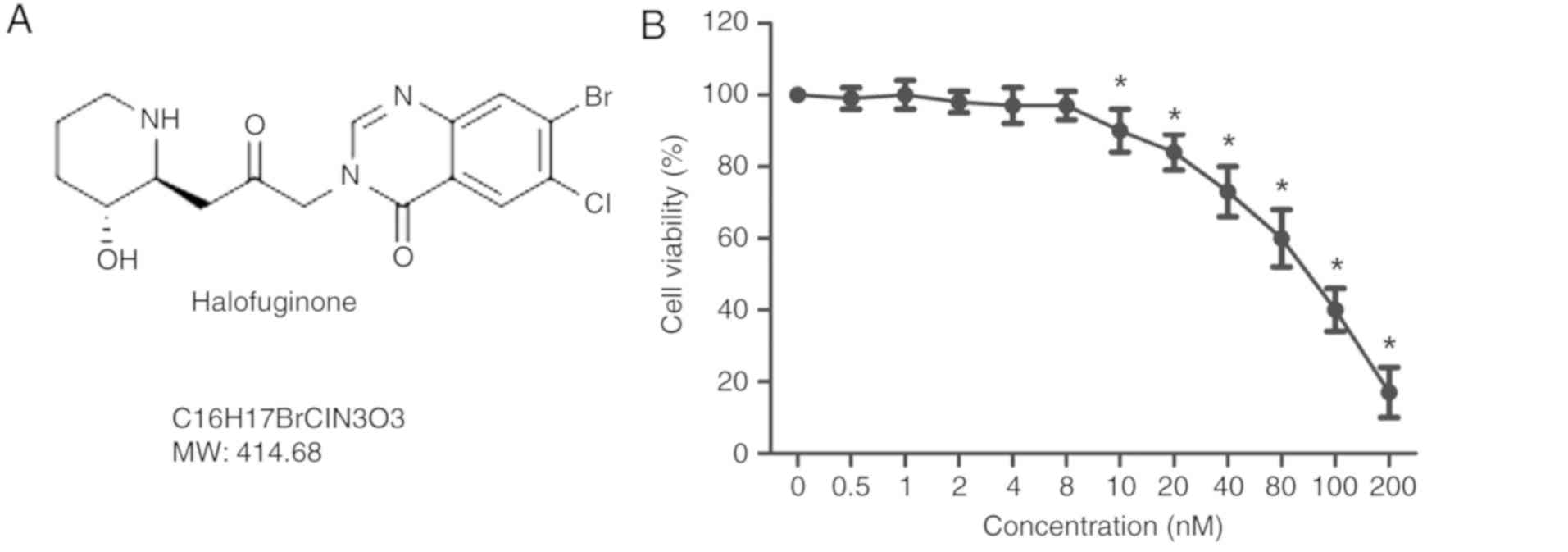 | Figure 1.Effects of HF on H9C2 cell viability.
(A) Structure of HF. (B) H9C2 cells were treated with HF at various
concentrations (0, 0.5, 1, 2, 4, 8, 10, 20, 40, 80, 100 and 200 nM)
for 48 h; cell viability was measured using an MTT assay. HF,
halofuginone; MW, molecular weight. *P<0.05 vs. the 0 nM
group. |
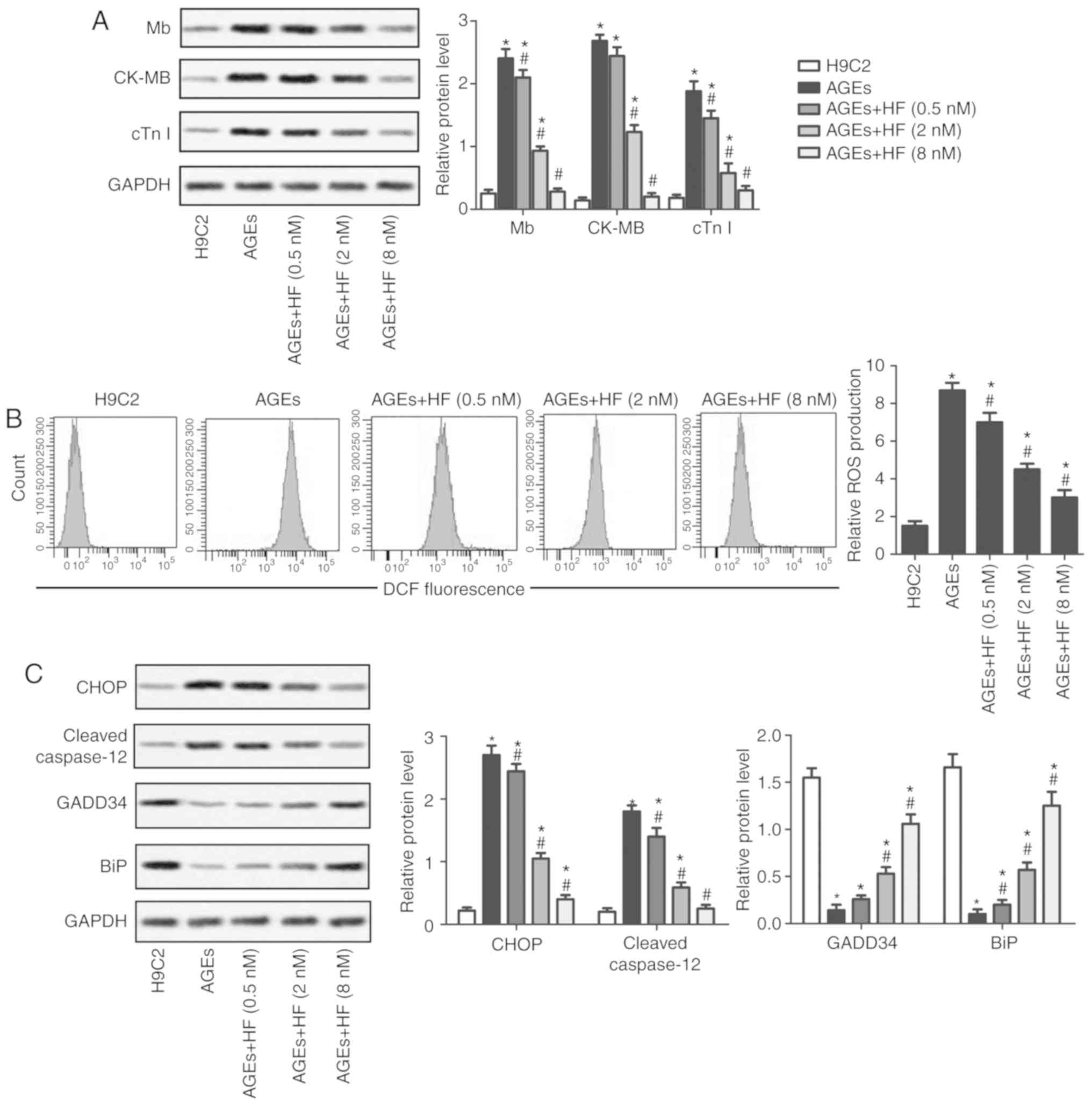 | Figure 2.HF alleviates AGEs-induced H9C2 cell
injury and ER stress. H9C2 cells were randomly divided into five
groups: Control group, normal H9C2 cells; AGEs group, AGEs-induced
cells; and three experimental groups, AGEs-induced cells treated
with HF at different concentrations (0.5, 2 and 8 nM) for 24 h. (A)
Expression levels of myocardial injury markers (Mb, CK-MB and cTnI)
were detected using western blotting. (B) Relative ROS production
was measured by flow cytometry. (C) Expression levels of ER
stress-associated proapoptotic proteins (CHOP and cleaved
caspase-12) and prosurvival proteins (GADD34 and BiP) were
investigated by western blotting. GAPDH was used as an endogenous
reference. Experiments were repeated at least three times, and data
are presented as the means ± standard deviation. *P<0.05 vs. the
H9C2 group; #P<0.05 vs. the AGEs group. AGEs,
advanced glycation end products; BiP, binding immunoglobulin
protein; CK-MB, creatine kinase-muscle/brain; CHOP,
CCAAT/enhancer-binding protein homologous protein; cTnI, cardiac
troponin I; DCF, 2.7-dihydrochlorofluorescein; GADD34, growth
arrest and DNA damage-inducible protein GADD34; HF, halofuginone;
Mb, myoglobin; ROS, reactive oxygen species. |
HF mitigates ER stress
To determine whether HF may affect ROS-mediated ER
stress, the production of ROS was examined using flow cytometry in
H9C2 cells. As presented in Fig.
2B, ROS production was significantly increased in the AGEs
group compared with in the control group. In addition, the
production of ROS was significantly decreased in the experimental
groups compared with in the AGEs group. Western blotting was
applied to investigate the expression of ER stress-associated
proapoptotic (CHOP and cleaved caspase-12) and prosurvival proteins
(GADD34 and BiP). The findings indicated that HF may inhibit the
upregulation of proapoptotic proteins and increase the expression
of prosurvival proteins in AGEs-treated cells (Fig. 2C). These results suggested that HF
may alleviate ER-associated stress.
HF inhibits AGEs-induced ER-associated
H9C2 apoptosis
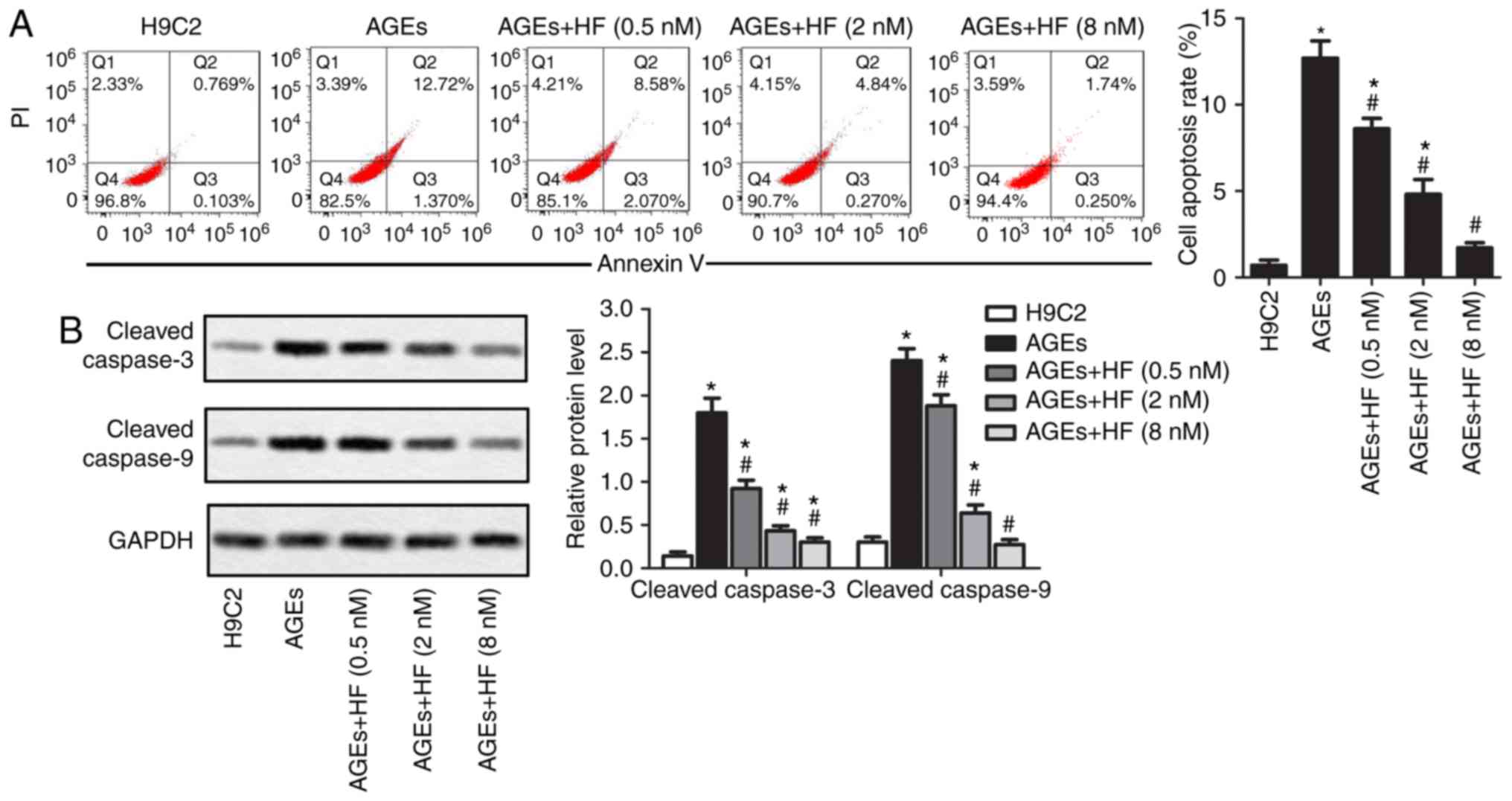
To detect the effects of HF on ER-associated H9C2
cell apoptosis induced by AGEs, the present study investigated cell
apoptosis and the expression of apoptosis-associated proteins
within H9C2 cells. As presented in Fig. 3A, AGEs-induced cell apoptosis may
be suppressed by HF. Apoptotic markers (cleaved caspase-3 and
cleaved caspase-9) were detected via western blotting. The results
demonstrated that HF inhibited the overexpression of apoptotic
markers induced by AGEs (Fig. 3B).
These results indicated that HF may inhibit AGEs-induced
ER-associated H9C2 apoptosis.
HF promotes autophagy of H9C2 cells
under AGEs-induced ER stress
To determine the survival of H9C2 cells under
AGEs-induced ER stress, the expression levels of
autophagy-associated proteins were investigated in the present
study. As presented in Fig. 4A,
AGEs-induced cells exhibited reduced cytoplasmic staining of LC3
compared with in non-AGEs-induced cells. In addition, AGEs-induced
H9C2 cells demonstrated increased LC3 staining in the cytoplasm in
response to increasing concentrations of HF. To further verify
these observations, the expression levels of the
autophagy-associated proteins (LC3II/LC3I, Beclin 1 and P62) were
analyzed using western blotting (Fig.
4B). The results revealed that AGEs significantly upregulated
the expression levels of P62, but reduced the expression levels of
LC3II/LC3I and Beclin 1 compared with in normal cells. Conversely,
the expression levels of P62 were significantly decreased, whereas
the expression levels of LC3II/LC3I and Beclin 1 were significantly
increased in AGEs-induced cells treated with HF compared with in
cells induced only with AGEs. These results suggested that HF may
promote AGEs-induced ER-associated H9C2 cell autophagy.
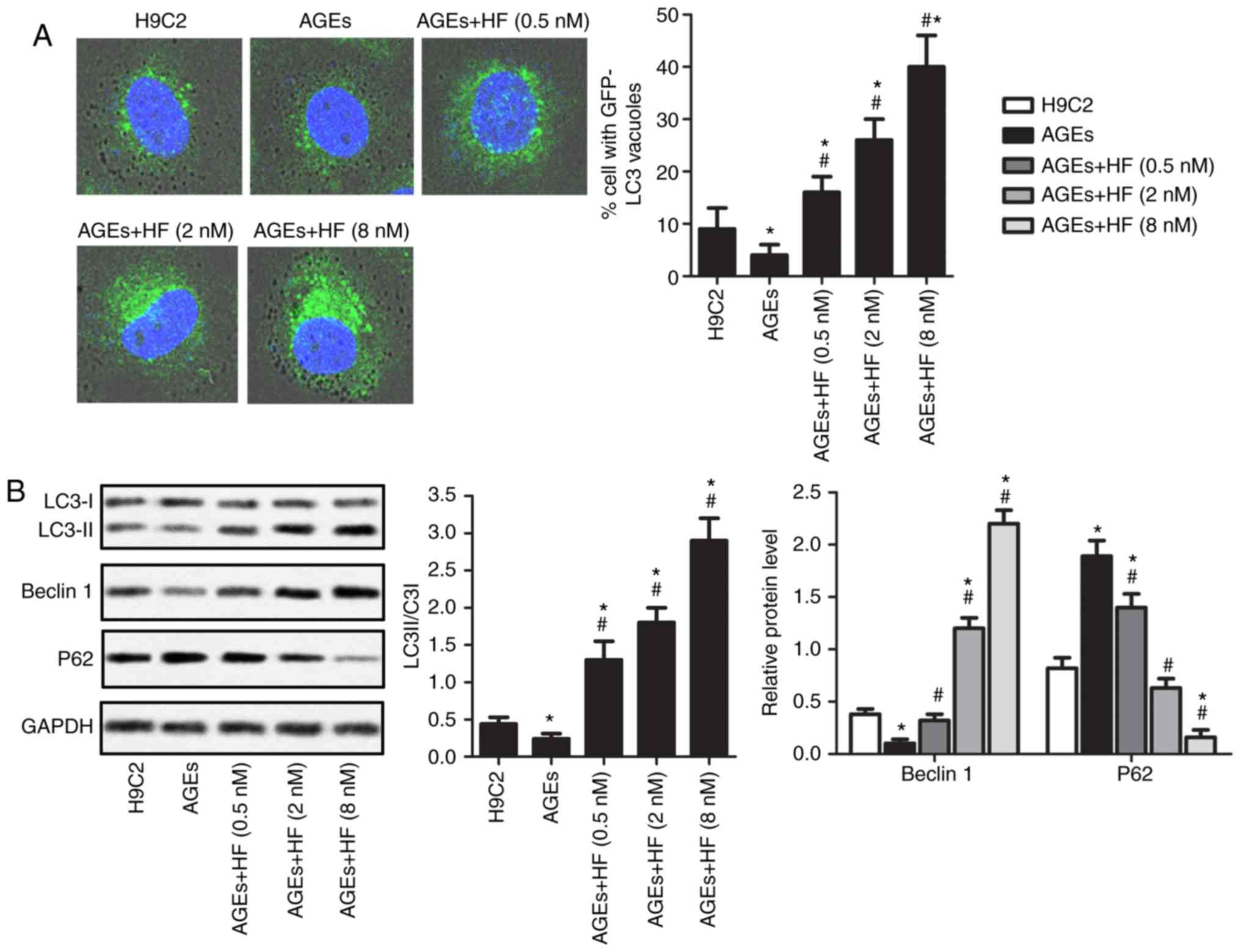 | Figure 4.HF promotes the autophagy of H9C2
cells under AGEs-induced ER stress. H9C2 cells were randomly
divided into five groups: Control group, normal H9C2 cells; AGEs
group, AGEs-induced cells; and three experimental groups,
AGEs-induced cells treated with HF at different concentrations
(0.5, 2 and 8 nM) for 24 h. (A) Expression levels of LC3 were
detected via immunofluorescence (magnification, ×400). (B)
Expression levels of autophagy-associated proteins (LC3, Beclin 1
and P62) were measured by western blotting. Experiments were
repeated at least three times, and data are presented as the means
± standard deviation. *P<0.05 vs. the H9C2 group;
#P<0.05 vs. the AGEs group. AGEs, advanced glycation
end products; GFP, green fluorescent protein; HF, halofuginone;
LC3, microtubule-associated proteins 1A/1B light chain 3B. |
HF protects H9C2 cells from
AGEs-induced damage via inducing autophagy
To investigate the protective effects of HF on H9C2
cells, the expression of autophagy-associated (LC3, Beclin 1 and
P62), apoptosis-associated (cleaved caspase-3 and cleaved
caspase-9) and ER stress-associated (CHOP, cleaved caspased-12,
GADD34 and BiP) proteins were detected in AGEs-induced cells
treated with 3-MA. As presented in Fig. 5, the LC3II/LC3I ratio, and the
expression levels of Beclin 1, BiP and GADD34 were significantly
reduced, whereas ROS production, and P62, caspase-3, caspase-9,
CHOP and caspase-12 expression levels were upregulated in the AGEs
+ 3-MA group compared with in the AGEs-only group. Conversely, the
LC3II/LC3I ratio, and the expression levels of Beclin 1, BiP and
GADD34 were significantly increased, whereas ROS production, and
P62, caspase-3, caspase-9, CHOP and caspase-12 expression levels
were significantly suppressed in the AGEs + HF group compared with
in the AGEs-only group. Furthermore, the LC3II/LC3I ratio, and the
expression levels of Beclin 1, BiP and GADD34 were significantly
decreased, whereas ROS production, and P62, caspase-3, caspase-9,
CHOP and caspase-12 expression levels were increased in the AGEs +
3-MA + HF group compared with in the AGEs + HF group. These results
indicated that 3-MA reversed the protective effects of HF in H9C2
cells.
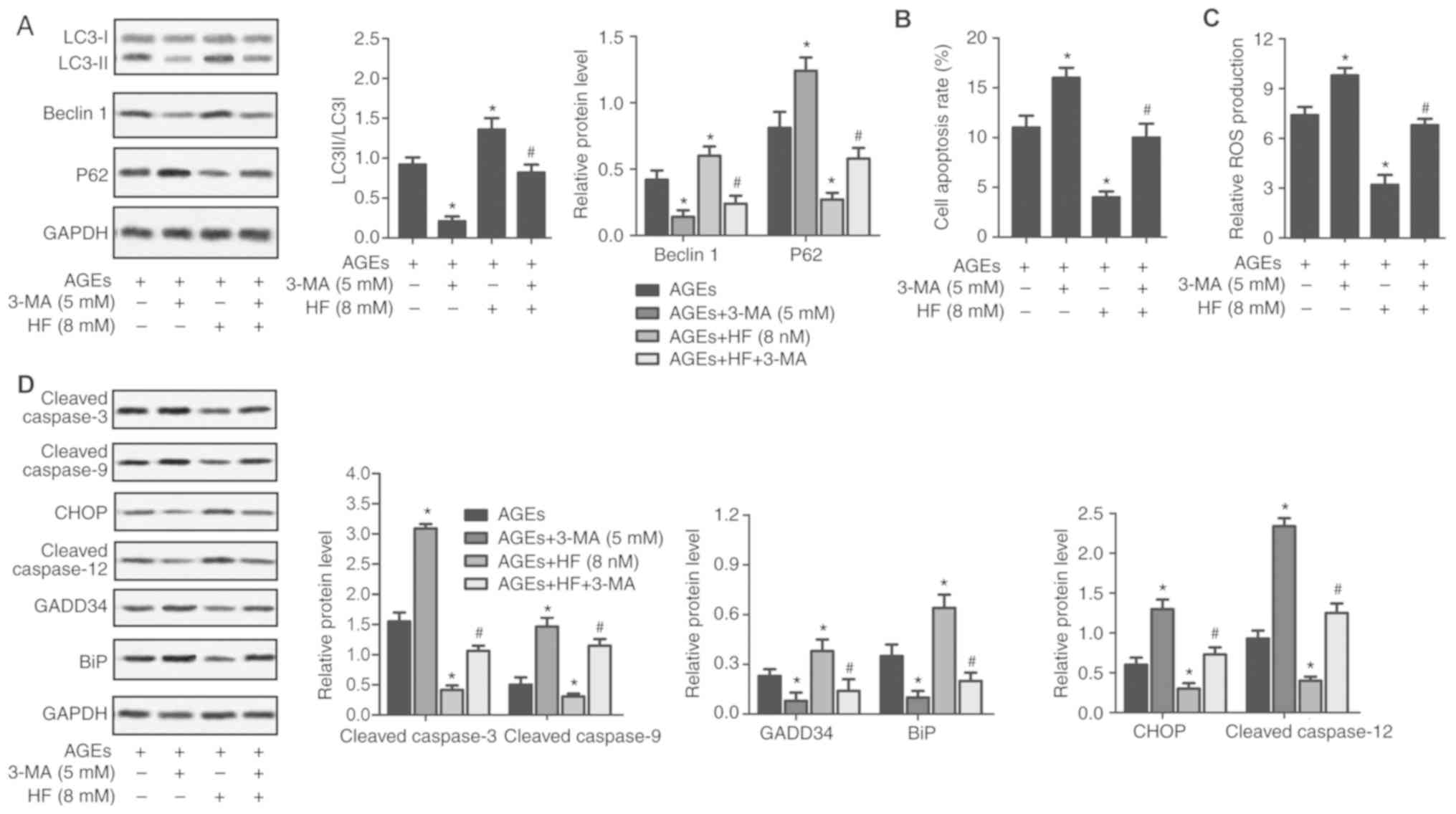 | Figure 5.HF protects H9C2 cells from
AGEs-induced damage by inducing autophagy. H9C2 cells were randomly
divided into four groups: AGEs group, AGEs-induced cells; AGEs +
3-MA group, AGEs-induced cells treated with 3-MA (5 mM) for 24 h;
AGEs + HF group, AGEs-induced cells treated with HF (8 nM) for 24 h
and AGEs + 3-MA + HF group, AGEs-induced cells treated with 3-MA (5
mM) and HF (8 nM) for 24 h. (A) Western blot analysis of the
autophagy-associated proteins (LC3, Beclin 1 and P62). GAPDH was
used as an endogenous reference. Flow cytometry was used to
determine (B) cell apoptosis rate and (C) relative ROS production.
(D) Expression levels of apoptosis-associated proteins (cleaved
caspase-3 and cleaved caspase-9), ER stress-associated proapoptotic
proteins (CHOP and cleaved caspase-12) and prosurvival proteins
(GADD34 and BiP) were detected via western blot analysis. GAPDH was
used as an endogenous reference. Experiments were repeated at least
three times, and data are presented as the means ± standard
deviation. *P<0.05 vs. the AGEs group; #P<0.05 vs.
the AGEs + HF group. 3-MA, 3-methyladenine; AGEs, advanced
glycation end products; BiP, binding immunoglobulin protein; CHOP,
CCAAT/enhancer-binding protein homologous protein; ER, endoplasmic
reticulum; GADD34, growth arrest and DNA damage-inducible protein
GADD34; HF, halofuginone; LC3, microtubule-associated proteins
1A/1B light chain 3B; ROS, reactive oxygen species. |
Discussion
Uncontrolled myocardial cell injury has been
reported to be the major cause of various cardiovascular diseases,
including myocardial infarction, myocarditis and heart failure
(18). Patients suffering from
heart disease are administered various medical treatments; however,
the rates of morbidity and mortality remain high and continue to
rise (19). Therefore, an advanced
therapeutic method is urgently required for the treatment of
myocardial cell injury prior to the onset of severe effects.
Previous studies have demonstrated that numerous
Chinese herbs exert a positive effect on myocardial cell injury
(20–22). For example, polydatin defends
cardiomyocytes against myocardial infarction injury via
upregulation of sirtuin-3 expression (20). In addition, the apoptosis of
cultured neonatal cardiomyocytes induced by daunorubicin is
prevented following treatment with Astragalus membranaceus
(21). Andrographolide has been
reported to increase the levels of cellular-reduced glutathione and
defend cardiomyocytes against hypoxia/reoxygenation damage
(22). These reports indicate the
potential of Chinese herbs in the treatment of myocardial cell
injury.
HF, which has been used as an antiprotozoal for 20
years, has been identified to possess notable anti-inflammatory and
antifibrotic effects (23). At
present, HF has been applied in certain models of cardiovascular
disease. According to Qin et al (8), HF not only activates the amino acid
response pathway in the heart, but also attenuates the structural
and functional effects of cardiac stress. Mb, CK-MB and cTnI are
the most widely established and useful biomarkers of myocardial
injury. In the present study, HF protected H9C2 cardiomyocytes
against AGEs-induced damage. The expression of myocardial injury
markers (Mb, CK-MB and cTnI) was significantly decreased in
response to by HF; therefore, the present study aimed to understand
the mechanisms underlying HF-regulated cardiomyocyte survival.
ER fulfills various cellular functions, including
Ca2+ homeostasis, protein synthesis and maturation, and
stress responses. The dysregulation of any of these functions may
induce ER stress. Studies have suggested that ER stress is often
induced by increases in ROS generation within the myocardium
(24,25); the overexpression of ROS may
further result in the dysregulation of the ER, complex unfolded
protein response signaling pathway and cell apoptosis. Increasing
evidence has indicated that ER stress-induced apoptosis may exert a
vital effect on myocardial injury (26). Therefore, reducing ROS-mediated ER
stress and apoptosis may be considered a therapeutic target for the
treatment of myocardial injury. HF has been reported to suppress
the generation of ROS, lipid peroxidation and myeloperoxidase
activity to reduce oxidative ischemia/reperfusion injury (27). This was supported by the findings
of the present study, in which ROS production was significantly
suppressed by HF in AGEs-induced H9C2 cells.
Cell apoptosis may be induced by chronic or
unresolved ER stress by activating the CHOP and caspase-12
signaling pathway (28). The
results obtained from the present study demonstrated the
anti-apoptotic effects of HF. According to Bodanovsky et al
(29), the abundance of apoptotic
nuclei decreases in response to HF in the diaphragm of mdx mice.
Furthermore, it has been reported that the expression levels of the
proapoptotic marker B-cell lymphoma 2 (Bcl-2)-associated X are
reduced, whereas those of the anti-apoptotic marker Bcl-2 are
increased in myofibers following treatment with HF (15). In the present study, ER stress was
inhibited via the suppression of proapoptotic proteins (CHOP and
cleaved caspase-12) and the upregulation of prosurvival proteins
(GADD34 and BiP) in AGEs-treated H9C2 cells induced by HF.
Furthermore, cell apoptosis was also reduced in response to HF;
decreased expression levels of caspase-3 and caspase-9 were
detected in the present study. These results suggested that HF may
alleviate AGEs-induced myocardial injury via inhibiting
ER-associated apoptosis.
In the heart, autophagy contributes to cell
homeostasis via degrading excessive proteins and aged organelles,
as observed in numerous heart disease models (30,31).
Inhibiting autophagy can cause adverse effects in cardiomyocytes
(32,33). According to Chen et al
(15), HF serves a dual regulatory
role in the initiation stage of autophagy via the liver kinase
B1-5′AMP-activated protein kinase-unc-5 like autophagy activating
kinase 1 (ULK1) or protein kinase B-mammalian target of rapamycin
complex 1-ULK1 signaling pathways. The effect of this regulation
always depends on the nutritional conditions of cells (15). In addition, Qin et al
(8) observed that HF treatment
alone, or in combination with endothelin-1, suppresses the
expression of P62, but increases the number of LC3B-positive
vesicles in cardiomyocytes, thus suggesting that HF may induce
autophagy in cardiomyocytes. Additionally, the present study
demonstrated that HF may protect H9C2 cells against AGEs-induced
injury via the induction of autophagy, as observed by the increased
LC3II/LC3I ratio and Beclin 1 expression, and decreased P62
expression in HF-treated cells.
3-MA is an autophagy antagonist, which is widely
used for its anti-autophagic activity in various cell types. In the
present study, 3-MA reversed the protective effects of HF in H9C2
cells; 3-MA suppressed autophagy, and increased apoptosis,
production and the ER stress response. These findings indicated
that autophagy may serve a vital role in myocardial cell
injury.
In conclusion, the present study revealed that HF
may protect H9C2 cells from AGEs-induced damage at concentrations
of 0.5, 2 and 8 nM. In addition, ROS- mediated ER stress was
suppressed by HF in AGEs-induced H9C2 cells. Therefore,
ER-stress-associated cell apoptosis was inhibited, leading to the
survival of H9C2 cardiomyocytes. Furthermore, HF induced autophagy,
which is a beneficial process for cardiomyocyte homeostasis;
conversely, 3-MA inhibited the effects of HF on AGEs-induced H9C2
cell damage. The present study revealed the protective effects of
HF against AGEs-induced myocardial cell injury; however further
investigation is required to understand the mechanism underlying
the effects of HF on myocardial injury. The results of the present
study may contribute towards the further analysis of HF in clinical
trials.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YHL performed cell culture and treatments. WLZ was
responsible for cell viability and ROS measurements. HYZ was
involved in western blot and flow cytometry analysis. DWY conducted
the immunofluorescence staining and autophagy assay. XNS collected
the data and performed statistical analysis. QH was involved in
designing the study and drafting the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HF
|
halofuginone
|
|
AGEs
|
advanced glycation end products
|
|
ROS
|
reactive oxygen species
|
|
ER stress
|
endoplasmic reticulum stress
|
|
Mb
|
myoglobin
|
|
CK-MB
|
creatine kinase-MB
|
|
3-MA
|
3-methyladenine
|
References
|
1
|
Basta G, Schmidt AM and De Caterina R:
Advanced glycation end products and vascular inflammation:
Implications for accelerated atherosclerosis in diabetes.
Cardiovasc Res. 63:582–592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corman B, Duriez M, Poitevin P, Heudes D,
Bruneval P, Tedgui A and Levy BI: Aminoguanidine prevents
age-related arterial stiffening and cardiac hypertrophy. Proc Natl
Acad Sci USA. 95:1301–1306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rani N, Bharti S, Bhatia J, Nag TC, Ray R
and Arya DS: Chrysin, a PPAR-γ agonist improves myocardial injury
in diabetic rats through inhibiting AGE-RAGE mediated oxidative
stress and inflammation. Chem Biol Interact. 250:59–67. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swirski FK and Nahrendorf M: Leukocyte
behavior in atherosclerosis, myocardial infarction, and heart
failure. Science. 339:161–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng S, Wang K, Huang M, Qiu Q, Xiao Y,
Shi M, Zou Y, Yang X, Xu H and Liang L: Halofuginone inhibits
TNF-α-induced the migration and proliferation of fibroblast-like
synoviocytes from rheumatoid arthritis patients. Int
Immunopharmacol. 43:187–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calik M, Yavas G, Calik SG, Yavas C, Celik
ZE, Sargon MF and Esme H: Amelioration of radiation-induced lung
injury by halofuginone: An experimental study in Wistar-Albino
rats. Hum Exp Toxicol. 36:638–647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cerit KK, Karakoyun B, Yüksel M, Ercan F,
Tuğtepe H, Dagli TE and Yeğen BÇ: Halofuginone alleviates
burn-induced hepatic and renal damage in rats. J Burn Care Res.
38:e384–e394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin P, Arabacilar P, Bernard RE, Bao W,
Olzinski AR, Guo Y, Lal H, Eisennagel SH, Platchek MC, Xie W, et
al: Activation of the amino acid response pathway blunts the
effects of cardiac stress. J Am Heart Assoc. 6(pii):
e0044532017.PubMed/NCBI
|
|
9
|
Zhao P, Kuai J, Gao J, Sun L, Wang Y and
Yao L: Delta opioid receptor agonist attenuates
lipopolysaccharide-induced myocardial injury by regulating
autophagy. Biochem Biophys Res Commun. 492:140–146. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giricz Z, Mentzer RM Jr and Gottlieb RA:
Autophagy, myocardial protection, and the metabolic syndrome. J
Cardiovasc Pharmacol. 60:125–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashem SI, Perry CN, Bauer M, Han S, Clegg
SD, Ouyang K, Deacon DC, Spinharney M, Panopoulos AD, Izpisua
Belmonte JC, et al: Brief report: Oxidative stress mediates
cardiomyocyte apoptosis in a human model of danon disease and heart
failure. Stem Cells. 33:2343–2350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: Disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao Y, Lu Q, Hu Z, Yu Y, Chen Q and Wang
QK: A non-canonical pathway regulates ER stress signaling and
blocks ER stress-induced apoptosis and heart failure. Nat Commun.
8:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marchi S, Patergnani S and Pinton P: The
endoplasmic reticulum-mitochondria connection: One touch, multiple
functions. Biochim Biophys Acta. 1837:461–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen GQ, Gong RH, Yang DJ, Zhang G, Lu AP,
Yan SC, Lin SH and Bian ZX: Halofuginone dually regulates
autophagic flux through nutrient-sensing pathways in colorectal
cancer. Cell Death Dis. 8:e27892017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge J, Jia Q, Liang C, Luo Y, Huang D, Sun
A, Wang K, Zou Y and Chen H: Advanced glycosylation end products
might promote atherosclerosis through inducing the immune
maturation of dendritic cells. Arterioscler Thromb Vasc Biol.
25:2157–2163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, Feng L, Wang S, Zhu Q, Lin J, Lou C,
Xiang P, He B, Zheng Z, Tang D and Zuo G: Phytoestrogen
calycosin-7-O-β-D-glucopyranoside ameliorates advanced glycation
end products-induced HUVEC damage. J Cell Biochem. 112:2953–2965.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hippisley-Cox J and Coupland C: Diabetes
treatments and risk of heart failure, cardiovascular disease, and
all cause mortality: Cohort study in primary care. BMJ.
354:i34772016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heusch G, Libby P, Gersh B, Yellon D, Böhm
M, Lopaschuk G and Opie L: Cardiovascular remodelling in coronary
artery disease and heart failure. Lancet. 383:1933–1943. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Zhao Z, Shen M, Zhang Y, Duan J,
Guo Y, Zhang D, Hu J, Lin J, Man W, et al: Polydatin protects
cardiomyocytes against myocardial infarction injury by activating
Sirt3. Biochim Biophys Acta. 1863:1962–1972. 2017. View Article : Google Scholar
|
|
21
|
Luo Z, Zhong L, Han X, Wang H, Zhong J and
Xuan Z: Astragalus membranaceus prevents daunorubicin-induced
apoptosis of cultured neonatal cardiomyocytes: Role of free radical
effect of Astragalus membranaceus on daunorubicin cardiotoxicity.
Phytother Res. 23:761–767. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woo AY, Waye MM, Tsui SK, Yeung ST and
Cheng CH: Andrographolide up-regulates cellular-reduced glutathione
level and protects cardiomyocytes against hypoxia/reoxygenation
injury. J Pharmacol Exp Ther. 325:226–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yavas G, Calik M, Calik G, Yavas C, Ata O
and Esme H: The effect of Halofuginone in the amelioration of
radiation induced-lung fibrosis. Med Hypotheses. 80:357–359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu ZW, Zhu HT, Chen KL, Dong X, Wei J,
Qiu C and Xue JH: Protein kinase RNA-like endoplasmic reticulum
kinase (PERK) signaling pathway plays a major role in reactive
oxygen species (ROS)-mediated endoplasmic reticulum stress-induced
apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol.
12:1582013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan X, Xun M, Dou X, Wu L, Han Y and Zheng
J: Regulation of Na+-K+-ATPase effected high
glucose-induced myocardial cell injury through c-Src dependent
NADPH oxidase/ROS pathway. Exp Cell Res. 357:243–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyazaki Y, Kaikita K, Endo M, Horio E,
Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, et
al: C/EBP homologous protein deficiency attenuates myocardial
reperfusion injury by inhibiting myocardial apoptosis and
inflammation. Arterioscler Thromb Vasc Biol. 31:1124–1132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karadeniz Cerit K, Karakoyun B, Yüksel M,
Özkan N, Cetinel Ş, Tolga Dağli E, Yeğen BÇ and Tuğtepe H: The
antifibrotic drug halofuginone reduces ischemia/reperfusion-induced
oxidative renal damage in rats. J Pediatr Urol. 9:174–183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu D, Zhang M and Yin H: Signaling
pathways involved in endoplasmic reticulum stress-induced neuronal
apoptosis. Int J Neurosci. 123:155–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bodanovsky A, Guttman N, Barzilai-Tutsch
H, Genin O, Levy O, Pines M and Halevy O: Halofuginone improves
muscle-cell survival in muscular dystrophies. Biochim Biophys Acta.
1843:1339–1347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishida K, Yamaguchi O and Otsu K:
Crosstalk between autophagy and apoptosis in heart disease. Circ
Res. 103:343–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pattison JS, Osinska H and Robbins J: Atg7
induces basal autophagy and rescues autophagic deficiency in
CryABR120G cardiomyocytes. Circ Res. 109:151–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maron BJ, Roberts WC, Arad M, Haas TS,
Spirito P, Wright GB, Almquist AK, Baffa JM, Saul JP, Ho CY, et al:
Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy.
JAMA. 301:1253–1259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gottlieb RA and Mentzer RM: Autophagy
during cardiac stress: Joys and frustrations of autophagy. Annu Rev
Physiol. 72:45–59. 2010. View Article : Google Scholar : PubMed/NCBI
|