Introduction
Somatotroph adenomas cause disfiguring growths and
medical complications, including cardiovascular and pulmonary
complications, and increased mortality rates due to malignancy
(1). Acromegaly is mainly caused
by somatotroph adenomas that secrete excess growth hormone (GH) and
insulin-like growth factor 1 (IGF-1) (2). Somatotroph adenomas are initially
treated surgically, followed by radiation and/or medication, if
necessary (3). Large tumor sizes
and high post-operative IGF-1 levels are predictors for incomplete
remission and uncontrolled disease (4). In a complex multistep process,
genetic and epigenetic factors, hormonal stimulation, growth
factors and their receptors contribute to tumorigenesis and the
development of somatotroph adenomas. To date, no high-frequency
somatic genetic alterations have been identified in somatotroph
adenomas, and the somatic landscape shows Ca+2 and the
ATP signaling pathways to be involved in pituitary tumorigenesis
(5). However, there is a lack of
clinical evidence of phenotype-genotype correlations between the α
subunit of stimulatory G-protein (GNAS) in patients with mutated
and non-mutated somatotroph adenomas (6).
Inorganic phosphate (Pi) enters the cells via Na/Pi
cotransporters, which comprise solute carrier (SLC)20 and SLC34 in
mammals (7). The SLC20 family
consists of two members, namely SLC20A1 [also known as phosphate
transporter 1 (PiT-1)] and SLC20A2 (PiT2). SLC20A1 encodes
sodium-dependent phosphate transporter 1, whose gene is located on
chromosome 2q11-14, a log 10 odds peak score region (8). Overexpression of SLC20A1 increases
cell proliferation and colony formation in murine fibroblastic
NIH3T3 and pre-osteoblastic MC3T3-E1 cells. Furthermore,
non-proliferating cells exhibit the lowest SLC20A1 mRNA levels in
these cell lines (9).
Overexpression of SLC20A1 leads to faster adhesion and spreading
compared with control NIH3T3 cells (10). High SLC20A1 expression was
associated with worse survival in 401 patients with estrogen
receptor-positive breast tumors compared with low SLC20A1
expression (10-year survival rates, 17.16 vs. 68.45%) (11). The epidermal growth factor receptor
inhibitor gefitinib exhibits strong inhibition of cell
proliferation in glioblastoma cell lines by regulating SLC20A1
expression (12). In addition,
SLC20A1 modulates erythroid maturation by modulating the activity
of erythroid Kruppel-like factor in vivo and in vitro
(7).
The Wnt signaling pathway has been implicated in the
processes of tissue differentiation, proliferation, apoptosis and
tumorigenesis (12). The levels of
Wnt inhibitory factor 1 (Wif1) and secreted frizzled-related
protein 4 (sFRP4) are lower in invasive nonfunctional pituitary
adenomas (NFPAs) than in noninvasive NFPAs (13). The present study investigated the
expression signatures of SLC20A1 and the Wnt/β-catenin signaling
pathway in 52 patients with somatotroph adenomas. Furthermore, it
explored the possibility that SLC20A1 could be targeted by
chemotherapeutic agents or used as a molecular marker for the
diagnosis of patients with cancer via RNA interference (RNAi)
technology in the GH3 cell line.
Materials and methods
Patients and tissue specimens
The present study retrospectively reviewed 52
patients (mean age, 40.3 years; range 16–62 years) with somatotroph
adenomas at Beijing Tiantan Hospital affiliated to Capital Medical
University, between July 2012 and December 2016. The diagnosis of
somatotroph adenoma was based on clinical symptoms and
histopathological features, per the 2017 World Health Organization
Classification (14). All patients
were diagnosed with sporadic somatotroph adenomas. The inclusion
criteria for recruitment of patients were as follows: i) Serum GH
>1 ng/ml and IGF-1 above normal (115–307 ng/ml), or GH >2
ng/ml; ii) magnetic resonance imaging confirmed the existence of
the saddle area; iii) acromegaly or facial changes; and iv)
transmission electron microscopy revealed large secretory granules
(400–600 nm). Patients presenting familial pituitary adenoma were
excluded from the present study. In addition, three normal
pituitary glands were obtained from a donation program at Tianjin
First Central Hospital in China (two male and one female patients;
age, 21–45). None of the donors had a history of pituitary disease.
Progression-free survival (PFS) was defined as the interval between
the date of surgery and the date of tumor recurrence or tumor
regrowth. The protocols were approved by the Internal Review Board
of Beijing Tiantan Hospital affiliated to Capital Medical
University and the study was conducted according to the principles
of the Declaration of Helsinki (no. KY-2013-02). Informed consent
was obtained from all patients.
Immunohistochemistry (IHC)
Tissue microarray construction and the IHC protocol
were performed according to a previously described method (15). The following primary antibodies
were used: Anti-SLC20A1 (cat. no. ab237527; 1:1,000; Abcam),
anti-β-catenin (cat. no. ab32572; 1:2,000; Abcam), anti-Wif1 (cat.
no. ab155101; 1:500; Abcam) and anti-Ki 67 (cat. no. ACK02;
1:1,000; Leica Microsystems, Inc.). A secondary antibody (cat. no.
sc-2040; 1:5,000; Santa Cruz Biotechnology, Inc.) was used in
combination with the Bond Polymer Refine Detection system (cat. no.
DS9800; Leica Microsystems, Inc.). Staining intensity was
stratified on a scale of 0–3 as follows: 0, no staining; 1, weak
staining; 2, moderate staining; and 3, strong staining. The H-score
was obtained by multiplying the staining intensity by a constant to
adjust the mean to the strongest staining, to produce a score in
the range of 0–300. Specifically, H-score=scale × percentage of
strong staining (0–100%). H-score=1.0 indicated a weak percentage;
2.0 indicated a moderate percentage; and 3.0 indicated a strong
percentage.
Cell culture, proliferation and
migration
GH3 cells were purchased from the American Type
Culture Collection (ATCC) and were cultured in F12K (ATCC)
supplemented with 2.5% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 15% horse serum (Gibco; Thermo Fisher
Scientific, Inc.) under 37°C and 5% CO2.
The GH3 cells were plated onto 96-well dishes with
1×104 cells and 100 µl medium in each well, and
incubated overnight. SLC20A1 gene silencing in the GH3 cells was
established by transfection of short hairpin RNA (1 µg for
1×106 cells; OriGene Technologies, Inc.) or
non-targeting control shRNA (scramble) for 72 h using 5 µl Opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.) and 0.3 µl
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The sequences of the pGFP-C-shLenti plasmid were: Scramble,
TTCTCCGAACGTGTCACGT; A, CACCGAAGTATGACAATCTGTGGATGCTC; B,
GCAATGCTGTGTCTGACCTTCACTCAGAG; C, ATAGGAATCCTGTGTCTGAGGTAGTATGT;
and D, GCTTCTGCTCCACCGAAGTATGACAATCT. Then, 20 µl MTS solution was
added to each well and further incubated for 4 h. The absorbance at
490 nm of each well was measured using an ELISA plate reader
(Thermo Fisher Scientific, Inc.).
Cell migration and invasion were measured using
fibronectin- and Matrigel-coated polycarbonate filters,
respectively, and modified transwell chambers (Corning Inc.). In
total, 5×104 GH3 cells in 200 µl F12K medium were added
into the upper chambers and 600 µl medium [F12K + 2.5% FBS (cat.
no. 10099-141; Gibco; Thermo Fisher Scientific, Inc.)+ 5% horse
serum (cat. no. sh30074; HyClone; GE Healthcare Life Sciences] was
added into lower chamber. After 24 h, migrating cells that adhered
to the lower membrane were fixed in 4% paraformaldehyde (for 30 min
at 4°C) and stained using hematoxylin (OriGene Technologies, Inc.)
for 5 min at room temperature. The average number of migrated cells
was quantified in five randomly selected fields of view under a
fluorescence microscope (Zeiss GmbH; magnification, ×100)
Experiments were performed in triplicate.
ELISA
GH level was detected using an ELISA kit (cat. no.
710685; Shanghai Enzyme-linked Biotechnology Co., Ltd.) according
to the manufacturer's protocol. The absorbance at 450 nm in each
well was measured using an ELISA plate reader (M200 Pro; Tecan
Group, Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from 24 frozen pituitary
adenoma samples (~10 mg) using the RNeasy® Mini Kit
(Qiagen GmbH). Purified total RNA (1 µg) was reverse transcribed
into complementary DNA (cDNA) using a Revert Aid First-Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), based on the
manufacturer's protocol (25°C for 10 min, 37°C for 2 h, 95°C for 5
min). The primer details are presented in Table I. GAPDH was used as an endogenous
control for normalizing the levels of target genes. RT-qPCR was
performed in an ABI 7500 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using
Platinum® SYBR® Green qPCR SuperMix-UDG
(Invitrogen; Thermo Fisher Scientific, Inc.). The comparative
quantification cycle (Cq) method was used to calculate the
fold-change in differential expression of each gene
(2−ΔΔCq method) (16)
the thermocycling conditions were the following: Initial
denaturation at 95°C for 15 followed by 40 cycles of 94°C for 15
sec, 55°C for 30 sec and 74°C for 34 sec.
 | Table I.Primers used in reverse
transcription-quantitative PCR. |
Table I.
Primers used in reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| SLC20A1 |
GCTTTATGGTGGTGTTGGCA |
GTGTTGTGCTGATGGGAAGG |
| E-CAD |
ACTTTGGTGTGGGTCAGGAA |
CACATGCTCAGCGTCTTCTC |
| N-CAD |
AGAACAGGGTGGACGTCATT |
ACCACTGTGACTAGCCCATC |
| MMP-2 |
TGCAACCACAACCAACTACG |
TAGAGCTCCTGGATCCCCTT |
| MMP-9 |
TGGGCAAGCAGTACTCTACC |
GTCTTCATGCAGAGGGGAGT |
| Snail |
CGAGCAGAGTTGTCTACCGA |
CTGCTGGAAGGTGAACTCCA |
| Vimentin |
ACTAATGAGTCCCTGGAGCG |
AGGTGGCGATCTCAATGTCA |
| VEGF |
CACCAAAGCCAGCACATAGG |
TTTAACTCAAGCTGCCTCGC |
| GAPDH |
AGTCTACTGGCGTCTTCACC |
CCACGATGCCAAAGTTGTCA |
SDS-PAGE and western blot
analyses
A total of 10 mg specimen was lysed to extract total
protein in TNE buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl and 1 mM
EDTA; Sigma-Aldrich; Merck KGaA) containing 1% Nonidet P-40
(Calbiochem; Merck KGaA) with protease and phosphatase inhibitor
cocktails (Roche Applied Science). The total protein extracted from
somatotroph adenomas or normal pituitary glands was centrifuged at
12,000 × g for 30 min at 4°C, and the protein concentration was
determined using a bicinchoninic acid protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). For western blot analysis, 40 µg
protein per lane was separated electrophoretically using 4–12%
Bis-Tris SDS-PAGE gels and blotted onto polyvinylidene fluoride
membranes. Each membrane was blocked for 1 h with 5% milk in TBS at
room temperature, incubated with antibodies against SLC20A1 (cat.
no. ab237527, 1:2,000; Abcam), Wif1 (cat. no. ab155101, 1:1,000;
Abcam), β-catenin (cat. no. ab32527, 1:5,000; Abcam), sFRP4 (cat.
no. ab154167, 1:2,000; Abcam) and GAPDH (cat. no. G5262, 1:8,000;
Sigma-Aldrich; Merck KGaA) at 4°C overnight, and then incubated 1 h
with horseradish peroxidase-tagged secondary antibodies (cat. no.
sc-2363, 1:5,000; Santa Cruz Biotechnology, Inc.) at room
temperature. Finally, the membranes were visualized by enhanced
chemiluminescence (cat. no. sc2048, Santa Cruz Biotechnology, Inc.)
using a Versadoc XL imaging system (Amersham Imager 600; GE
Healthcare Life Sciences), and densitometry was performed using the
ImageQuant TL software (version 7.0; GE Healthcare Life Sciences).
GAPDH levels were analyzed as a loading control.
Statistical analysis
The χ2 exact test was used to determine
the significance of clinicopathological characteristics and
variables. Pearson's correlation coefficient was used to analyze
the correlation between β-catenin/Wif1 expression and SLC20A1
score. Student's t-test was applied to examine the differential
expression of SLC20A1, β-catenin, Ki67 and Wif1 in patient samples.
Data are presented as the mean ± SD. One-way ANOVA was used in cell
growth, GH release and migration of GH3 cells experiments. All
P-values were two sided and P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
three times. Analyses were performed using SPSS (vesion 19.0; IBM
Corp.).
Results
Clinicopathological features
There were 28 males and 24 females in the present
study (Table SI) with a mean age
of 40.3 years (age range, 17–62 years). The median tumor volume was
6.25 cm3 (range, 2.24–18.7 cm3). The average
level of GH was 22.83±7.31 ng/ml, and ranged from 7.9 to 76.2
ng/ml. The number of total resections was 31/52 (59.6%), while the
number of partial resections was 21/52 (40.4%). The number of
recurrent cases was 11/52 (21.2%). Tissues from 52 patients were
stained with standard hematoxylin and eosin staining, and IHC was
performed with antibodies against SLC20A1, β-catenin, sFRP4, Ki67
and Wif1. According to the Knosp classification, the 52 patients
were classified into an invasive group (15 patients) and a
non-invasive group (37 patients). The patients in the invasive
group had a greater degree of headache and visual deficits than the
patients in the non-invasive group. The preoperative serum GH level
was 36.7±11.9 ng/ml in the invasive group and 17.2±6.3 ng/ml in the
non-invasive group (P<0.05). Follow-up data revealed that total
resection was carried out in 5/15 (33.3%) cases in the invasive
group and 26/37 (70.3%) cases in the non-invasive group
(χ2=4.611, P=0.032). Furthermore, recurrence in the
invasive group occurred in 6/15 (40%) cases and in 5/37 (13.5%)
cases in the non-invasive group (χ2=4.489, P=0.034)
(Table II).
 | Table II.Univariate analyses of
clinicopathological correlates in 52 patients with somatotroph
adenomas. |
Table II.
Univariate analyses of
clinicopathological correlates in 52 patients with somatotroph
adenomas.
|
| Invasiveness | Univariate
analysis |
|---|
|
|
|
|
|---|
| Variable | Yes (n=15) | No (n=37) | χ2 | P-value |
|---|
| Age, years |
|
| 2.342 | 0.126 |
|
≤40.3 | 10 | 16 |
|
|
|
>40.3 | 5 | 21 |
|
|
| Sex |
|
| 0.843 | 0.358 |
|
Male | 9 | 17 |
|
|
|
Female | 6 | 20 |
|
|
| Tumor size,
cm3 |
|
| 5.996 | 0.014 |
|
≤6.25 | 12 | 14 |
|
|
|
>6.25 | 3 | 23 |
|
|
| GH level,
ng/ml | 36.7±11.9 | 17.2±6.3 | – | <0.05 |
| Resection |
|
| 4.611 | 0.032 |
|
Total | 5 | 26 |
|
|
|
Partial | 10 | 11 |
|
|
| Recurrence |
|
| 4.489 | 0.034 |
|
Yes | 6 | 5 |
|
|
| No | 9 | 37 |
|
|
| SLC20A1 |
|
| 7.589 | 0.006 |
|
Higha | 12 | 14 |
|
|
|
Low | 3 | 23 |
|
|
| Wif1 |
|
| 9.369 | 0.002 |
|
High | 2 | 24 |
|
|
|
Low | 13 | 13 |
|
|
| β-catenin |
|
| 4.591 | 0.032 |
|
High | 11 | 15 |
|
|
|
Low | 4 | 22 |
|
|
SLC20A1, Wif1, β-catenin and Ki67
expression signatures in 52 somatotroph adenomas
IHC was used to evaluate the levels of SLC20A1,
Wif1, β-catenin and Ki67 in the 52 somatotroph adenoma samples
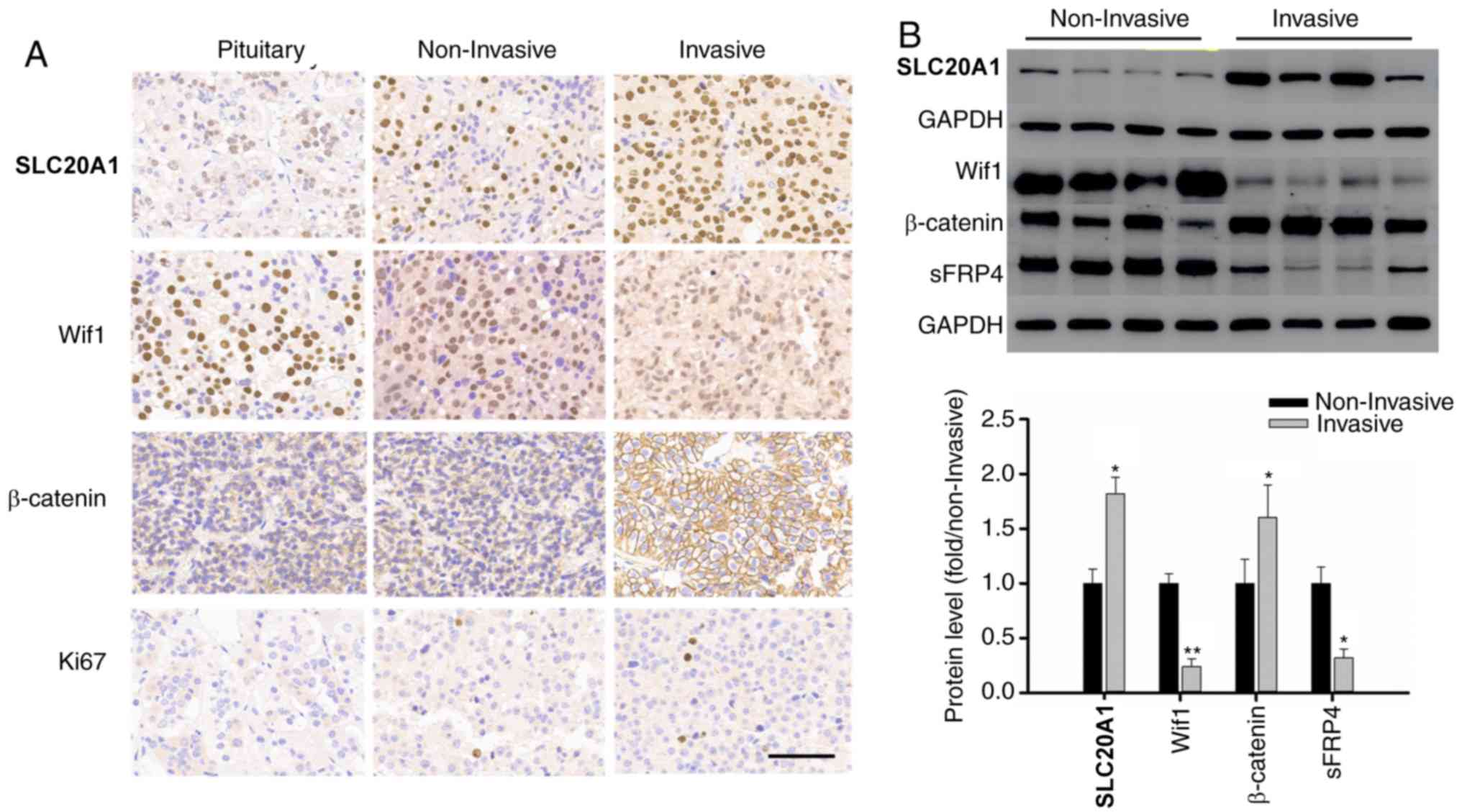
(Fig. 1A). The H-score of SLC20A1
was 222.6±15.2 in the invasive group and 144.5±30.4 in the
non-invasive group (P<0.01), while the H-scores of β-catenin
were 210.1±21.4 and 134.9±32.7 in these two groups, respectively
(P<0.05). The H-scores of Wif1 presented the opposite trend
compared with β-catenin or SLC20A1, with values of 134.5±22.7 and
253.6±14.8 in the invasive and non-invasive groups, respectively
(P<0.01). The Ki67 index was 6.3±2.2 in the invasive group and
1.9±1.3 in the non-invasive group. Western blotting also confirmed
the same tendency, as depicted in Fig.
1B. In the present study, a high-level case was defined as
having a H-score higher than the median value.
According to the H-score median of SLC20A1, Wif1 and
β-catenin, 52 samples were divided into high and low H-score
groups. The tumor volume in patients with high SLC20A1 H-scores
(high SLC20A1 group) was 8.34±2.64 and 4.16±2.39 cm3 in
patients with low SLC20A1 H-scores (low SLC20A1 group) (P<0.05),
and the rate of tumor recurrence was 8/26 (30.8%) compared with
3/26 (11.5%) in these two groups, respectively. Patients with high
Wif1 H-scores (high Wif1 group) had less tumor volume (3.14±2.35
vs. 9.36±3.64 cm3) than those with low Wif1 H-scores
(low Wif1 group) (P<0.05) and lower recurrence rates compared
with patients in the low Wif1 group, data not shown (2/26 vs. 9/26,
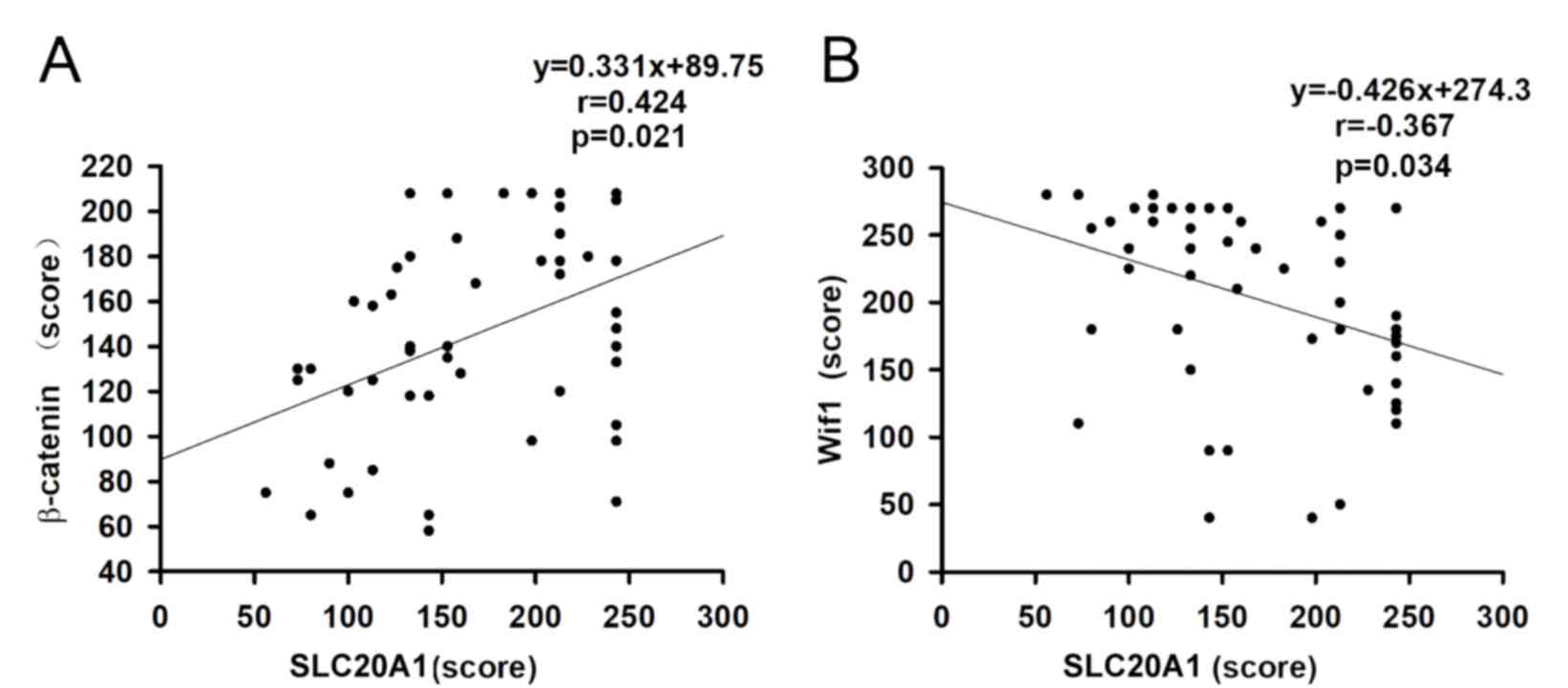
χ2=5.65, P=0.017). The H-scores of SLC20A1 were
negatively associated with the H-scores of β-catenin in somatotroph
adenomas, with a correlation coefficient of 0.366 (Fig. 2A). The correlation coefficient was
−0.424 between Wif1 and SLC20A1 (Fig.
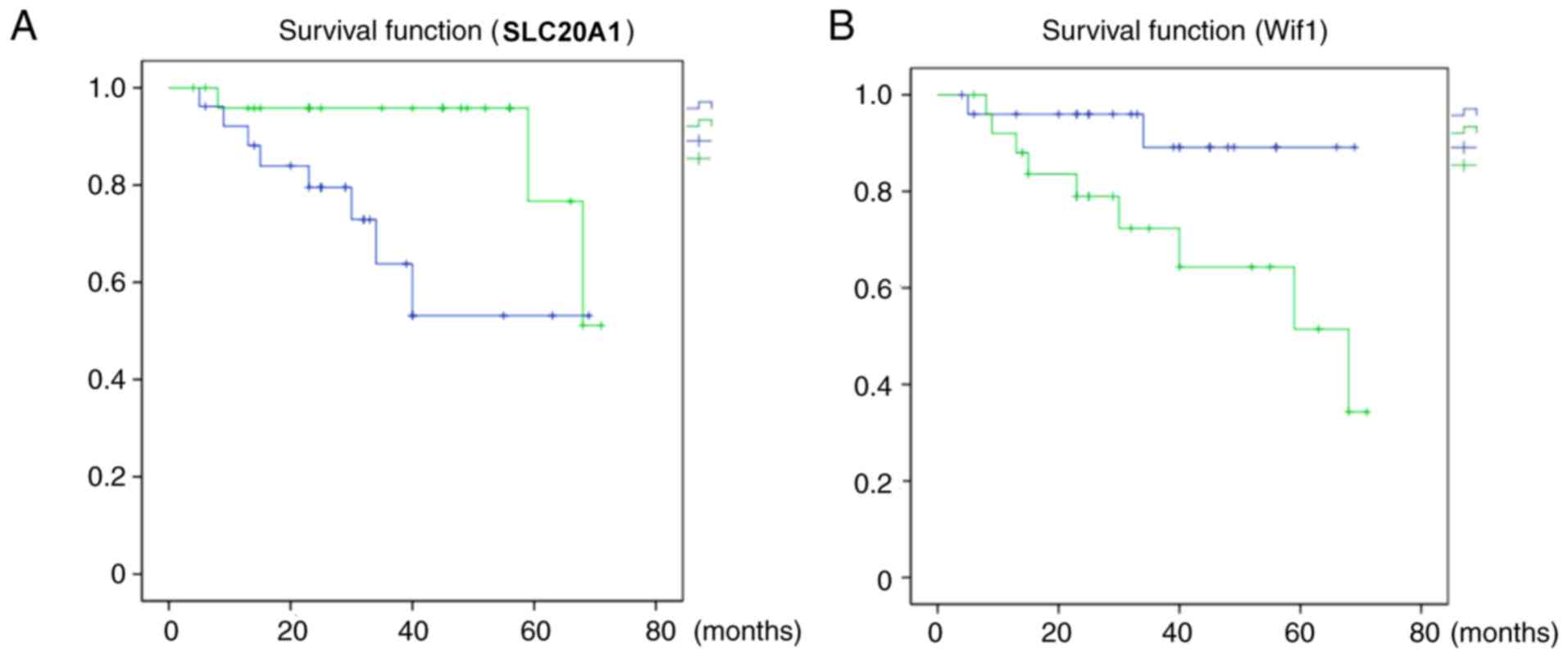
2B). PFS associated with low SLC20A1 H-scores was longer than
that associated with high SLC20A1 H-scores (P=0.033, Fig. 3A), while the PFS in the high Wif1
group was longer than that in the low Wif1 group (P=0.043), as
shown in Fig. 3B.
Effects of RNAi-SLC20A1 on cell
growth, GH release and migration of GH3 cells
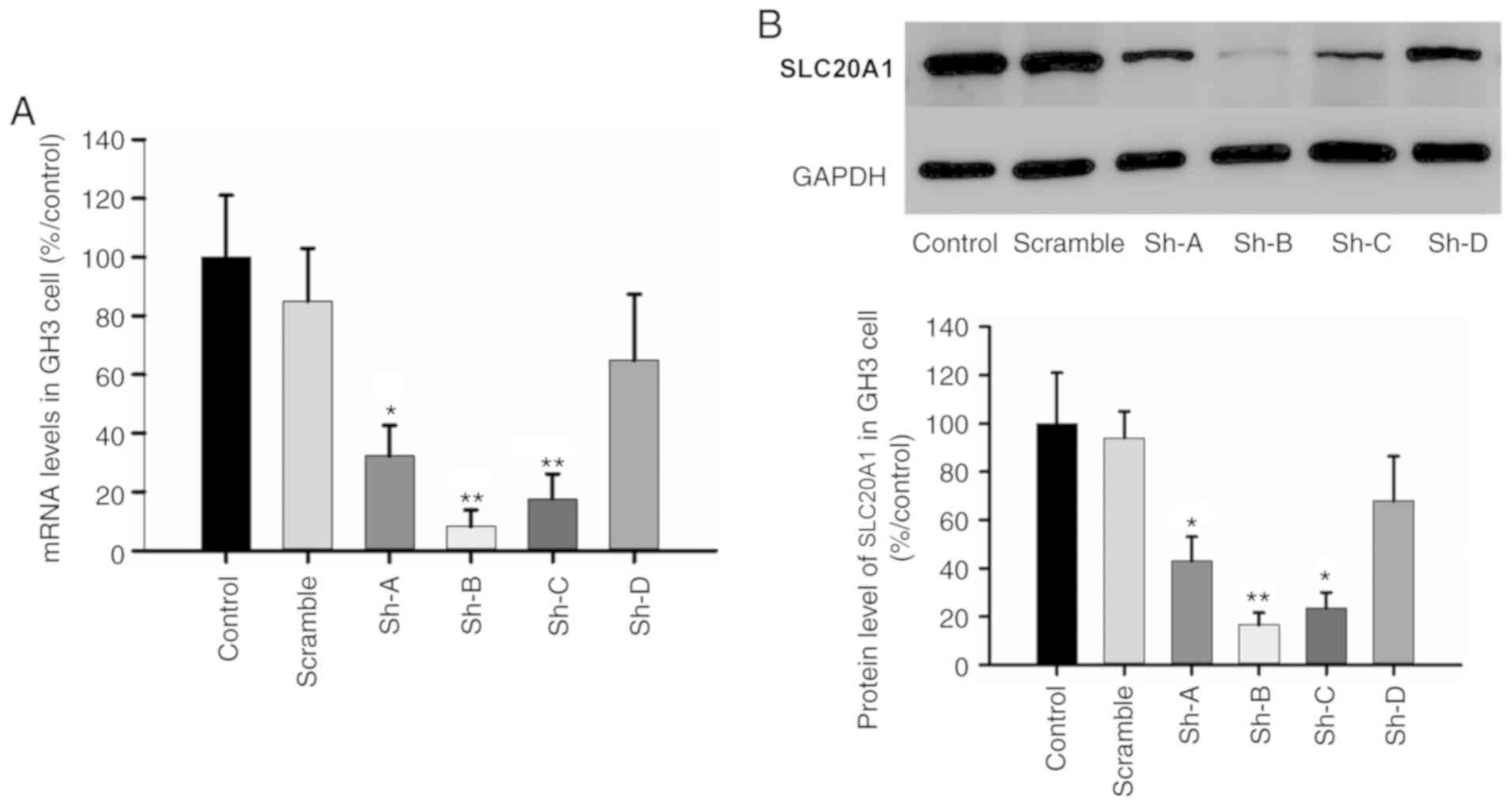
The efficiency of four shRNA segments
(Sh-A/B/C/D-SLC20A1) was measured in GH3 cells. RT-qPCR
demonstrated that the relative percentages of SLC20A1 mRNAs were
32.3±10.4, 8.2±5.7, 17.7±8.4 and 64.9±22.4%, respectively, after 72
h of transfection (Fig. 4A).
Western blotting also confirmed that the interference efficiency of
segment Sh-B was 83.4% and that of Sh-C was 76.4% compared with the
SLC20A1 protein levels in GH3 cells after 72 h of transfection
(Fig. 4B). Cell proliferation
experiments revealed that the cell viability of the Sh-B group was
76.3±4.5, 65.7±3.7 and 53.1±3.2% of control GH3 cells after 24, 48
and 72 h of transfection, respectively, and that of the Sh-C group
was 86.4±5.7, 75.6±4.4 and 67.5±3.8% of the control group,
respectively, after the same time (P<0.05; Fig. 5A). ELISA was used to measure the
effect of SLC20A1 on GH secretion in GH3 cells. The GH levels in
the Sh-B and Sh-C groups were 34.7±10.4 and 54.6±14.4% of that in
control GH3 cells, respectively (P<0.05; Fig. 5B). The transmembrane invasion assay
revealed that RNAi-SLC20A1 significantly suppressed cell invasion
in the Sh-B and Sh-C groups (Fig.
5C).
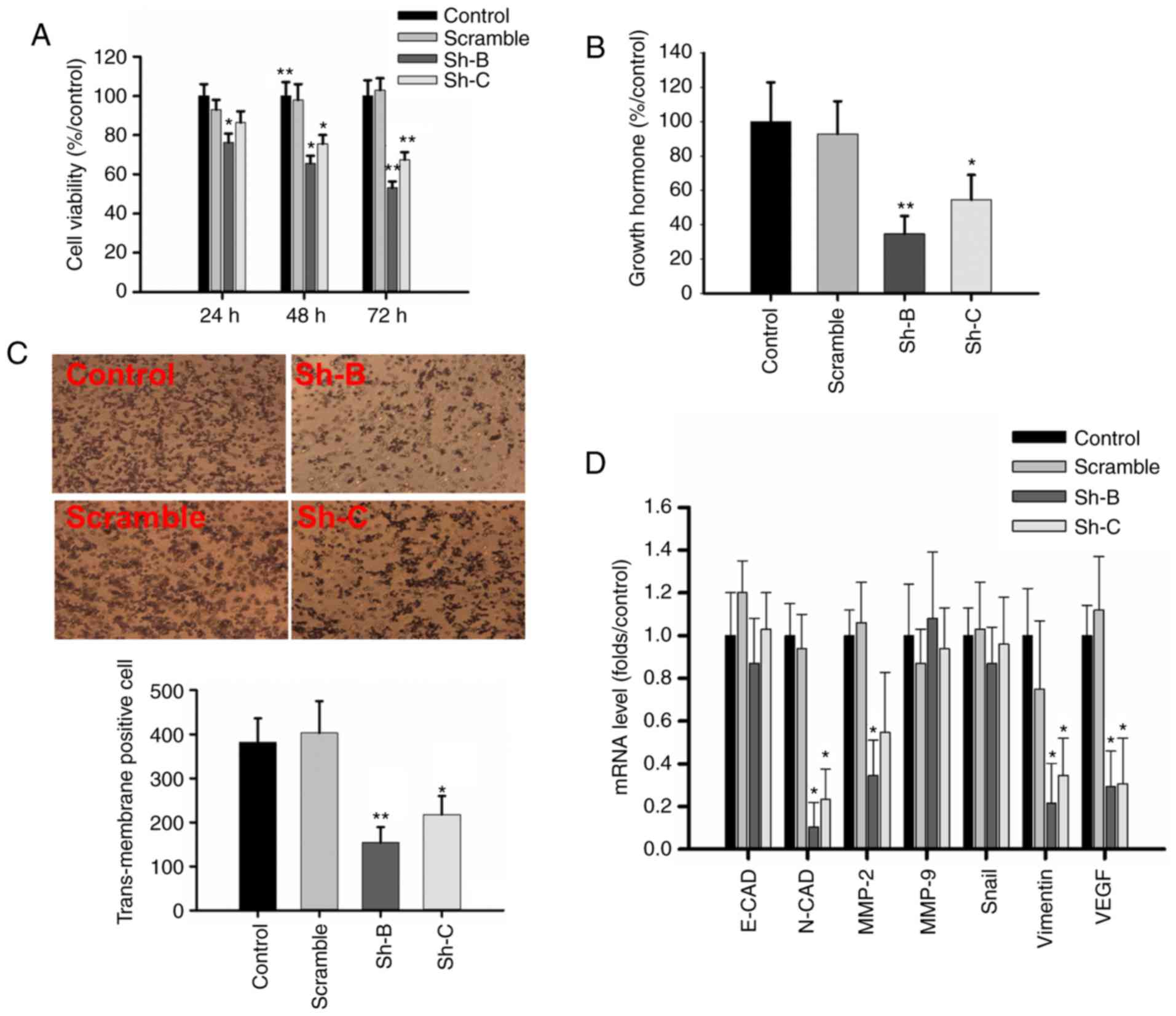 | Figure 5.RNAi-SLC20A1 suppresses cell
proliferation, GH secretion and migration of GH3 cells. (A) Effect
on cell viability, as shown by MTS assay. (B) ELISA revealed that
RNAi-SLC20A1 could reduce GH secretion. (C) The effect on invasion,
as shown by the transwell experiment. Magnification, ×200. (D) The
mRNA level of genes associated with migration and invasion upon
RNAi-SLC20A1 in GH3 cells. n=3. *P<0.05, **P<0.01 vs.
respective control. RNAi, RNA interference; SLC20A1, solute carrier
family 20 member 1; GH, growth hormone; sh, short hairpin; E-CAD,
E-cadherin; N-CAD, N-cadherin; MMP, matrix metalloproteinase; VEGF,
vascular endothelial growth factor. |
RT-qPCR revealed that SLC20A1 silencing reduced the
mRNA levels to 0.105±0.114 and 0.233±0.141-fold for N-cadherin
(N-CAD), 0.216±0.185 and 0.346±0.172-fold for vimentin, 0.294±0.155
and 0.387±0.212-fold for vascular endothelial growth factor (VEGF),
and 0.344±0.165 and 0.547±0.282-fold for matrix metalloproteinase-2
compared with the control group. There was no significant
difference in the expression levels of E-cadherin, matrix
metalloproteinase-9 and Snail. In additional, no significant
difference was observed between the control group and scramble
group (Fig. 5D).
SLC20A1 silencing inhibits the
activation of the Wif1/β- catenin signaling pathway
Aberrant expression of the Wif1 and
sFRP genes has been reported to be associated with the
malignant and invasive characteristics of certain human tumors,
including somatotroph adenomas (17,18).
RT-qPCR revealed that the mRNA levels of Wif1 in the Sh-B and Sh-C
groups were 4.3±1.2 and 3.6±0.7-fold, respectively, of those in the
control GH3 cells, while the mRNA levels of sFRP4 were 5.8±1.3 and
4.6±0.9-fold of those in the control GH3 cells (Fig. 6). Western blotting detected that
the Sh-B and Sh-C segments of SLC20A1 could evidently increase the
levels of Wif1 and sFRP4, and significant decrease the β-catenin
level in the Sh-B group (Fig.
6).
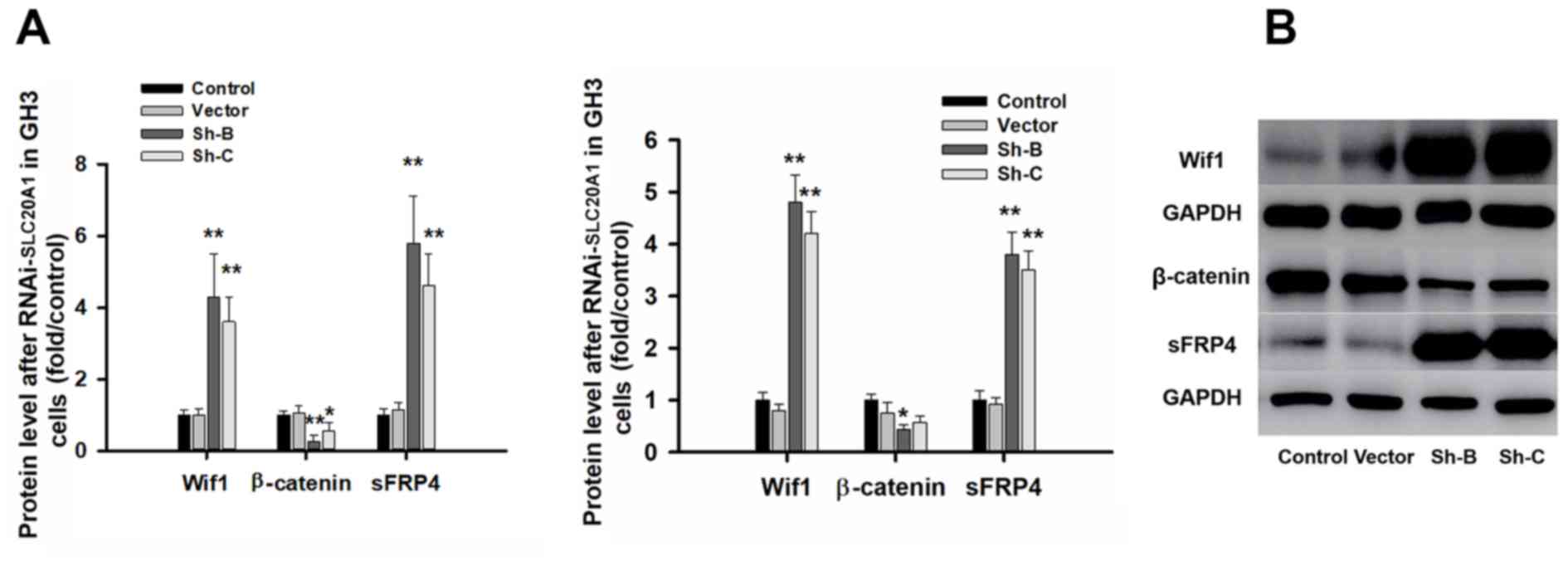 | Figure 6.RNAi-PIT inhibits the Wnt/β-catenin
pathway. (A) Reverse transcription-quantitative PCR analysis
revealed significant changes in Wif1, β-catenin and sFRP4 mRNA
levels in GH3 cells. (B) Western blot analysis revealed a
significant change in Wif1, β-catenin and sFRP4 proteins in the
Sh-B and Sh-C groups compared with the control group. The protein
levels of Wif1 in the Sh-B and Sh-C groups were 4.8±0.52 and
4.2±0.42-fold greater, respectively, than those in the control GH3
cells, while the protein levels of sFRP4 in the Sh-B and Sh-C
groups were 3.8±0.43 and 3.5±0.37-fold greater, respectively, than
those in the control GH3 cells. n=3. *P<0.05, **P<0.01 vs.
respective control. RNAi, RNA interference; SLC20A1, solute carrier
family 20 member 1; Wif1, Wnt inhibitory factor 1; sFRP4, secreted
frizzled-related protein 4; sh, short hairpin. |
Discussion
Somatotroph adenomas are pituitary tumors that
secrete GH and IGF-1, resulting in chronic systemic disease with
complications and increased mortality when not adequately treated.
To date, no novel recurrent mutated genes have been identified in
sporadic adenomas except for GNAS (19,20).
By allowing the cells to move more quickly out of the
G0/G1 phase of the cell cycle, overexpression
of SLC20A1 could confer faster adhesion and migration to the NIH3T3
cell line (10). The present study
evaluated the levels of SLC20A1 and the Wnt/β-catenin signaling
pathway, and their role in the tumorigenesis and clinical features
of 52 somatotroph adenomas.
Compared with other subtypes of pituitary adenomas,
somatotroph adenomas are associated with higher morbidity and
mortality. Main risk factors include histological subtype and tumor
invasion as well as the presence of molecular biomarkers, including
Ki67, p53 and IGF-1 (21,22). Epidermal growth factor-like domain
multiple 7 protein and sparsely granulated adenomas may serve as
useful biomarkers of somatotroph adenoma invasion and prognosis
(23). However, several predictive
biomarkers have failed in clinical research trials of
neuroendocrine tumors (24). There
is an urgent unmet need to incorporate novel biomarker candidates
in order to obtain robust prospective validation. The present IHC
results indicated that high expression of SLC20A1 may promote the
malignant potential of somatotroph adenomas. High levels of
expression of SLC20A1 and low levels of expression of Wif1 were
observed in somatotroph adenomas, as well as a longer PFS in
patients with low SLC20A1 and/or high Wif1. There are few cell
lines used for the study of pituitary adenomas. The MMQ cell line
(25) is used for prolactinomas,
the GH3 cell line (26) is used
for somatotroph adenomas and the Att20 cell line (27) is used for corticotroph adenomas.
GT1-1 cells (28) mainly secrete
luteinizing hormone and low levels of GH. In vitro, the
Wnt/β-catenin signaling pathway may be involved in the cell
proliferation, migration and GH secretion of GH3 cells via
overexpression of SLC20A1.
SLC20A1 is transcribed in response to the activation
of nuclear factor-κB (NF-κB) during cancer cell progression
(29). Furthermore, SLC20A1
inhibits tumor necrosis factor-induced apoptosis through the main
antiapoptotic signals triggered by the mitogen-activated protein
kinase-Jun N-terminal kinase signaling pathway (30,31).
In fact, NF-κB and β-catenin are constitutively activated by
upstream serine/threonine kinases implicated in malignant
transformation, including apoptosis evasion (32). There was a significant positive
correlation reported between the H-scores of β-catenin and c-myc
expression and aggressiveness in 212 patients with NFPAs (33). In a previous study, RT-qPCR
confirmed the lower mRNA levels of Wif1 and sFRP2/4 in all
pituitary adenoma subtypes, including somatotroph adenomas,
compared with normal pituitary specimens (34). In the present study, SLC20A1 and
β-catenin were increased in the invasive somatotroph adenomas
compared with the non-invasive somatotroph adenomas or normal
pituitary glands. A positive correlation between SLC20A1 and
β-catenin, and a negative correlation between SLC20A1 and Wif1, was
identified by IHC. Follow-up data also revealed the potential of
SLC20A1 and Wif1 as predictive factors for somatotroph adenoma
recurrence due to high recurrence rate and shorter time to
recurrence in patients with high SLC20A1 or low Wif1.
The Wnt/β-catenin signaling pathway plays an
important role in the adhesion, invasion and metastasis of
pituitary tumors, as well as development of these tumors and
tumorigenesis (35). The
activation of this pathway is an important factor for VEGF
production during the process of angiogenesis. Wif1 overexpression
plays an important role in the suppression of cell migration, and
Wif1 knockdown enhances the migratory potential of glioblastomas
through Wnt family member 5A activation in vitro and in an
orthotopic brain tumor model (36,37).
In vitro, silencing of SLC20A1 inhibits the proliferation
and migration of GH3 cells. Western blotting revealed that SLC20A1
could reduce the Wif1 and sFRP4 protein levels due to higher Wif1
and sFRP4 in the Sh-B or Sh-C groups compared with the control or
scramble groups. Epithelial-mesenchymal transition (EMT) markers
such as N-cad, vimentin and Snail are important indicators of the
appearance of cystic lesions, tumor progression, bone destruction
and endocrine functions in somatotroph adenomas (38). The present data revealed that
SLC20A1 evidently regulated the mRNA levels of N-CAD, VEGF and
vimentin, which are associated with cell invasion and migration,
after 72 h of RNAi. Taken together, these data suggest a novel
mechanism for the role of SLC20A1 in cell proliferation through the
Wnt/β-catenin signaling pathway in GH3 cells.
In conclusion, the present study demonstrated that a
high SLC20A1 level is positively associated with the tumor size,
invasive behavior and tumor recurrence of somatotroph adenomas.
Furthermore, SLC20A1 may be associated with activation of the
Wnt/β-catenin signaling pathway. Furthermore, in vitro
experiments demonstrated that silencing SLC20A1 inhibited cell
proliferation and invasion through inhibition of genes associated
with EMT. However, the present study had certain limitations. Due
to the small sample size and possible selection bias in the present
study, it is necessary to confirm and validate the conclusions in
further studies with larger sample sizes.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Beijing Natural
Science Foundation of China (grant no. 7162035), Beijing High Level
Program (grant no. 2015-3-040) and the National Natural Science
Foundation of China (grant no. 81601205).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL contributed to the writing and the IHC
experiment. WD contributed to the IHC experiment. ZL performed the
WB experiment and follow up. HW performed the cell culture. HG was
involved in the cell function experiments and revising the
manuscript. YZ designed the experiment and performed the
statistical analysis.
Ethics approval and consent to
participate
The protocols were approved by the Internal Review
Board of Beijing Tiantan Hospital affiliated to Capital Medical
University and the study was conducted according to the principles
of the Declaration of Helsinki (no. KY-2013-02). Informed consent
was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
AlDallal S: Acromegaly: A challenging
condition to diagnose. Int J Gen Med. 11:337–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inoshita N and Nishioka H: The 2017 WHO
classification of pituitary adenoma: Overview and comments. Brain
Tumor Pathol. 35:51–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abreu A, Tovar AP, Castellanos R,
Valenzuela A, Giraldo CM, Pinedo AC, Guerrero DP, Barrera CA,
Franco HI, Ribeiro-Oliveira A Jr, et al: Challenges in the
diagnosis and management of acromegaly: A focus on comorbidities.
Pituitary. 19:448–457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kempf J, Schmitz A, Meier A, Delfs N,
Mueller B, Fandino J, Schuetz P and Berkmann S: Adenoma size and
postoperative IGF-1 levels predict surgical outcomes in acromegaly
patients: Results of the Swiss Pituitary Registry (SwissPit). Swiss
Med Wkly. 148:w146532018.PubMed/NCBI
|
|
5
|
Välimäki N, Demir H, Pitkänen E, Kaasinen
E, Karppinen A, Kivipelto L, Schalin-Jäntti C, Aaltonen LA and
Karhu A: Whole-genome sequencing of growth hormone (GH)-secreting
pituitary adenomas. J Clin Endocrinol Metab. 100:3918–3927. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Picard C, Silvy M, Gerard C, Buffat C,
Lavaque E, Figarella-Branger D, Dufour H, Gabert J, Beckers A, Brue
T, et al: Gs alpha overexpression and loss of Gs alpha imprinting
in human somatotroph adenomas: Association with tumor size and
response to pharmacologic treatment. Int J Cancer. 121:1245–1252.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forster IC, Hernando N, Biber J and Murer
H: Phosphate transporters of the SLC20 and SLC34 families. Mol
Aspects Med. 34:386–395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ravera S, Virkki LV, Murer H and Forster
IC: Deciphering PiT transport kinetics and substrate specificity
using electrophysiology and flux measurements. Am J Physiol Cell
Physiol. 293:C606–C620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byskov K, Jensen N, Kongsfelt IB, Wielsøe
M, Pedersen LE, Haldrup C and Pedersen L: Regulation of cell
proliferation and cell density by the inorganic phosphate
transporter PiT-1. Cell Division. 7:72012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kongsfelt IB, Byskov K, Pedersen LE and
Pedersen L: High levels of the type III inorganic phosphate
transporter PiT-1 (SLC20A1) can confer faster cell adhesion. Exp
Cell Res. 326:57–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato K and Akimoto K: Expression levels of
KMT2C and SLC20A1 identified by information-theoretical analysis
are powerful prognostic biomarkers in ER-positive breast cancer.
Clin Breast Cancer. 17:e135–e142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song W, Qian L, Jing G, Jie F, Xiaosong S,
Chunhui L, Yangfang L, Guilin L, Gao H and Yazhuo Z: Aberrant
expression of the sFRP and WIF1 genes in invasive non-functioning
pituitary adenomas. Mol Cell Endocrinol. 474:168–175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricardo VL, Robert YO, Gunter K and Juan
R: WHO classification of tumours of endocrine organ. 4th.
International agency for research on cancere; France, Lyon: pp.
19–23. 2018
|
|
15
|
Liu C, Gao H, Cao L, Gui S, Liu Q, Li C,
Li D, Gong L and Zhang Y: The role of FSCN1 in migration and
invasion of pituitary adenomas. Mol Cell Endocrinol. 419:217–224.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pez F, Lopez A, Kim M, Wands JR, Caron de
Fromentel C and Merle P: Wnt signaling and hepatocarcinogenesis:
Molecular targets for the development of innovative anticancer
drugs. J Hepatol. 59:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueland T, Olarescu NC, Jørgensen AP,
Otterdal K, Aukrust P, Godang K, Lekva T and Bollerslev J:
Increased serum and bone matrix levels of the secreted Wnt
antagonist DKK-1 in patients with growth hormone deficiency in
response to growth hormone treatment. J Clin Endocrinol Metab.
100:736–743. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hage M, Viengchareun S, Brunet E, Villa C,
Pineau D, Bouligand J, Teglas JP, Adam C, Parker F, Lombès M, et
al: Genomic alterations and complex subclonal architecture in
sporadic GH-secreting pituitary adenomas. J Clin Endocrinol Metab.
103:1929–1939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caimari F and Korbonits M: Novel genetic
causes of pituitary adenomas. Clin Cancer Res. 22:5030–5042. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mete O and Lopes MB: Overview of the 2017
WHO Classification of pituitary tumors. Endocr Pathol. 28:228–243.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park HH, Kim EH, Ku CR, Lee EJ and Kim SH:
Outcomes of aggressive surgical resection in growth
hormone-secreting pituitary adenomas with cavernous sinus invasion.
World Neurosurg. 117:e280–e289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Liu Q, Gao H, Wan D, Li C, Li Z
and Zhang Y: EGFL7 participates in regulating biological behavior
of growth hormone-secreting pituitary adenomas via Notch2/DLL3
signaling pathway. Tumour Biol. 39:10104283177062032017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barriuso J, Custodio A, Afonso R, Alonso
V, Astudillo A, Capdevila J, García-Carbonero R, Grande E,
Jimenez-Fonseca P, Marazuela M, et al: Prognostic and predictive
biomarkers for somatostatin analogs, peptide receptor radionuclide
therapy and serotonin pathway targets in neuroendocrine tumours.
Cancer Treat Rev. 70:209–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su Z, Cai L, Lu J, Li C, Gui S, Liu C,
Wang C, Li Q, Zhuge Q and Zhang Y: Global expression profile of
tumor stem-like cells isolated from MMQ rat prolactinoma cell.
Cancer Cell Int. 17:152017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Occhi G, Losa M, Albiger N, Trivellin G,
Regazzo D, Scanarini M, Monteserin-Garcia JL, Fröhlich B, Ferasin
S, Terreni MR, et al: The glucose-dependent insulinotropic
polypeptide receptor is overexpressed amongst GNAS1
mutation-negative somatotropinomas and drives growth hormone
(GH)-promoter activity in GH3 cells. J Neuroendocrin. 23:641–649.
2011. View Article : Google Scholar
|
|
27
|
Bergeron F, Sirois F and Mbikay M: ACTH
secretion by mouse corticotroph AtT20 cells is negatively modulated
by the intracellular level of 7B2. FEBS Lett. 512:259–262. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng H, Fan J, Wu J, Lang J, Wang J, Liu
H, Zhao S and Liao J: Silencing of HEPN1 is responsible for the
aggressive biological behavior of pituitary somatotroph adenomas.
Cell Physiol Biochem. 31:379–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salaün C, Leroy C, Rousseau A, Boitez V,
Beck L and Friedlander G: Identification of a novel
transport-independent function of PiT-1/SLC20A1 in the regulation
of TNF-induced apoptosis. J Biol Chem. 285:34408–34418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kavitha K, Kowshik J, Kishore TK, Baba AB
and Nagini S: Astaxanthin inhibits NF-κB and Wnt/β-catenin
signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to
induce intrinsic apoptosis in a hamster model of oral cancer.
Biochim Biophys Acta. 1830:4433–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu C, Wu Y, Yu S, Bai J, Li C, Wu D and
Zhang Y: Increased β-catenin and c-myc expression predict
aggressive growth of non-functioning pituitary adenomas: An
assessment using a tissue microarray-based approach. Mol Med Rep.
5:1793–1799. 2017. View Article : Google Scholar
|
|
34
|
Elston MS, Gill AJ, Conaglen JV, Clarkson
A, Shaw JM, Law AJ, Cook RJ, Little NS, Clifton-Bligh RJ, Robinson
BG and McDonald KL: Wnt pathway inhibitors are strongly
down-regulated in pituitary tumors. Endocrinology. 149:1235–1242.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chambers TJ, Giles A, Brabant G and Davis
JR: Wnt signalling in pituitary development and tumorigenesis.
Endocr Relat Cancer. 20:R101–R111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vassallo I, Zinn P, Lai M, Rajakannu P,
Hamou MF and Hegi ME: WIF1 re-expression in glioblastoma inhibits
migration through attenuation of non-canonical WNT signaling by
downregulating the lncRNA MALAT1. Oncogene. 35:12–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Sang A, Zhu M, Zhang G, Guan H, Ji
M and Chen H: Tissue factor induces VEGF expression via activation
of the Wnt/β-catenin signaling pathway in ARPE-19 cells. Mol Vis.
22:886–897. 2016.PubMed/NCBI
|
|
38
|
Shan XS, Liu Q, Li ZY, Li CZ, Gao H and
Zhang YZ: Epithelial-mesenchymal transition induced by SMAD4
activation in invasive growth hormone-secreting adenomas. Open
Chem. 16:571–582. 2018. View Article : Google Scholar
|