Introduction
Periodontal ligament (PDL) is the connective tissue
located between the alveolar bone and tooth root. If PDL is
severely damaged by periodontitis, regeneration is difficult
(1). Periodontal ligament stem
cells (PDLSCs) are essential for periodontal tissue regeneration,
which often forms a cementum/PDL-like structure after
transplantation in vivo (1,2).
However, the mechanisms responsible for regulating PDLSC
differentiation have yet to be fully elucidated. PDL regeneration
involves fiber fabrication and hard tissue formation, such as
alveolar bone and cementum. It is significative for the stimulation
of PDLSCs to differentiate into osteoblast, cementoblast and
ligaments in damaged periodontal tissue regeneration. The use of
morphogens and growth factors have been shown to stimulate PDLSC
differentiation (3–5).
Transforming growth factor (TGF) β1 is a potent
stimulator of tissue regeneration, and is abundant in the bone
matrix (6,7). A previous study found that TGF-β1 can
improve bone regeneration in animal models of guided tissue
regeneration (8). However, a
previous study reported that TGF-β1 inhibited primary PDL cell
cementogenic and osteogenic differentiation via competition with
bone morphogenetic protein 2 (9).
The differential effects of TGF-β1 on fibrogenesis, chondrogenesis
and osteogenesis have been identified by comparing in vivo
and in vitro experiments, suggesting a controversial role
for TGF-β1 in periodontal differentiation (10,11).
Cellular senescence results in irreversible cell
cycle arrest, as well as a series of morphological and functional
changes, which may further inhibit the potential for self-renewal
in PDLSCs (12–14). The acquisition of a
senescence-associated secretory phenotype (SASP) reinforces
senescence and stimulates the immune system to clear the senescent
cells (15). Cellular senescence
can be induced by various stimuli and stressors, including DNA
damage, metabolic insults, oxidative stresses, oncogene activation
and epigenetic changes (16–18).
A number of studies suggest that TGF-β1 is also capable of inducing
senescence in tumor and other cells (19–21).
However, whether TGF-β1 induces PDLSC senescence has yet to be
clarified. Thus, the present study attempted to investigate the
effect of TGF-β1 on the senescence of PDLSCs and its association to
reactive oxygen species (ROS) generation, in order to elucidate the
function of TGF-β1 in periodontal differentiation.
Materials and methods
Human PDLSC culture and treatment
PDL tissues were collected from healthy third molar
teeth extracted from ten male and eight female dental surgery
patients aged between 18 and 25 years old who had visited the
Department of Periodontology in the Affiliated Hospital of Qingdao
University from February 2018 to April 2019. The study guidelines
were approved by the Review Board of the Affiliated Hospital of
Qingdao University. Normal periodontal tissues were digested using
dispase (4 mg/ml; Sigma-Aldrich; Merck KGaA) and collagenase (3
mg/ml; Sigma-Aldrich; Merck KGaA) for 1 h at 37°C. Cell suspensions
were cultured in α-MEM (HyClone; GE Healthcare Life Sciences)
supplemented with 20% fetal bovine serum (HyClone; GE Healthcare
Life Sciences) and 1% streptomycin and penicillin at 37°C in 5%
CO2. PDLSCs were isolated from third-passage periodontal
ligament cells using a cluster of differentiation (CD)146 microbead
kit (Miltenyi Biotec GmbH) according to the manufacturer's
instructions. For characterization of the isolated cells, the
PDLSCs were pre-incubated with Human TruStain FcX™ (Fc Receptor
Blocking Solution; cat. no. 422301; BioLegend, Inc.) for 10 min at
room temperature, and then stained with the following antibodies
for 30 min at 4°C: Anti-human PE-conjugated CD34 (cat. no.
12-0349-42), CD44 (cat. no. 12-0441-82), CD45 (cat. no.
12-9459-42), CD90 (cat. no. 12-0909-42) and CD105 (cat. no.
12-1057-42) antibodies (eBioscience; Thermo Fisher Scientific,
Inc.). Flow cytometry analysis was performed on a FACS Calibur flow
cytometer (BD Biosciences) and analyzed using WinMDI version 2.9
software (http://www.cyto.purdue.edu/flowcyt/software/Winmdi.htm).
For cell treatments, the isolated PDLSCs were
starved in serum-free α-MEM overnight and then cultured in fresh
serum-free α-MEM supplemented with TGF-β1 (10 ng/ml; PeproTech,
Inc.,) alone, or in combination with N-acetyl-L-cysteine (NAC; 10
mM; Beyotime Institute of Biotechnology) or LY364947 (2 µM; Selleck
Chemicals).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cultured PDLSCs with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
then reverse-transcribed to cDNA using a PrimeScript RT reagent kit
(Takara Bio, Inc.) according to the manufacturer's protocols. qPCR
analysis was performed on a Light Cycler 96 (Roche Diagnostics
GmbH) using SYBR Green mix (Takara Bio, Inc.). Samples were
initially denatured for 30 sec at 95°C, followed by 40 cycles of
denaturation for 5 sec at 95°C and annealing for 30 sec at 60°C.
Primers used for qPCR are listed in Table I. The 2−∆∆Cq method
normalized to GAPDH was used for quantification (22). Experiments were repeated three
times independently.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| GAPDH | Forward |
AGGGCTGCTTTTAACTCTGGT |
|
| Reverse |
CCCCACTTGATTTTGGAGGGA |
| IL-6 | Forward |
GATGAGTACAAAAGTCCTGATCCA |
|
| Reverse |
CTGCAGCCACTGGTTCTGT |
| IL-8 | Forward |
TTGGCAGCCTTCCTGATTTC |
|
| Reverse |
TGGTCCACTCTCAATCACTCTCA |
| IL-18 | Forward |
CACCCCGGACCATATTTATTATAAGT |
|
| Reverse |
TGTTATCAGGAGGATTCATTTCCTT |
| P21 | Forward |
GCCTGGACTGTTTTCTCTCG |
|
| Reverse |
ATTCAGCATTGTGGGAGGAG |
| P16 | Forward |
CACGGGTCGGGTGAGAGT |
|
| Reverse |
CCCAACGCACCGAATAGTTAC |
ROS measurement
ROS were detected using a MitoSOX Red Superoxide
Indicator (Invitrogen; Thermo Fisher Scientific, Inc.), or hydrogen
peroxide-specific peroxy orange 1 (PO1; APExBIO Technology LLC)
probe. Following treatment with TGF-β1, PDLSCs were incubated with
5 µM MitoSOX Red for 10 min, or 5 µM PO1 for 30 min at 37°C.
Fluorescence was detected by a FACSCalibur flow cytometer (BD
Biosciences) and analyzed using WinMDI version 2.9 software.
Western blot analysis
PDLSCs treated with TGF-β1 were lysed in 2% SDS
lysis buffer (Beyotime Institute of Biotechnology). The
concentration of protein lysates were assayed using a Pierce™ BCA
protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.).
A total of 20 µg of protein was resolved via 12% SDS-PAGE and
transferred to 0.45-µm PVDF membranes. Following 1 h of blocking
with 5% skimmed milk at room temperature, PVDF membranes were
incubated with the following primary antibodies: Anti-α-tubulin
(1:5,000; cat. no. 11224-1; ProteinTech Group, Inc.); anti-p21
(1:1,000; cat. no. ab109520; Abcam); anti-p16 (1:1,000; cat. no.
ab108349; Abcam); and anti-superoxide dismutase2 (SOD2; 1:1,000;
cat. no. ab13533; Abcam), and then reacted with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:5,000; cat. no.
31460; Invitrogen; Thermo Fisher Scientific, Inc.). α-tubulin was
used as internal reference. Signals were visualized using an
enhanced chemiluminescent HRP substrate (cat. no. WBKLS0050; EMD
Millipore) and ImageJ (version 1.50i; National Institutes of
Health) was used for densitometry.
ELISA
An ELISA kit (cat. no. KHC0081; Invitrogen; Thermo
Fisher Scientific, Inc.) was used to detect levels of interleukin
(IL)-8 in the cell culture supernatants, according to the
manufacturer's instructions.
Senescence-associated
beta-galactosidase (SA-β-Gal) staining
The PDLSCs were treated with TGF-β1 alone or
combined with NAC or LY364947 for 48 h. SA-β-gal activity was
detected with the SA-β-gal staining kit (cat. no. RG0039; Beyotime
Institute of Biotechnology), in accordance with the manufacturer's
protocols.
Statistical analysis
Data are shown as mean ± standard deviation. One-way
analysis of variance (ANOVA) followed by Tukey's post-hoc test was
used for comparisons between >2 groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
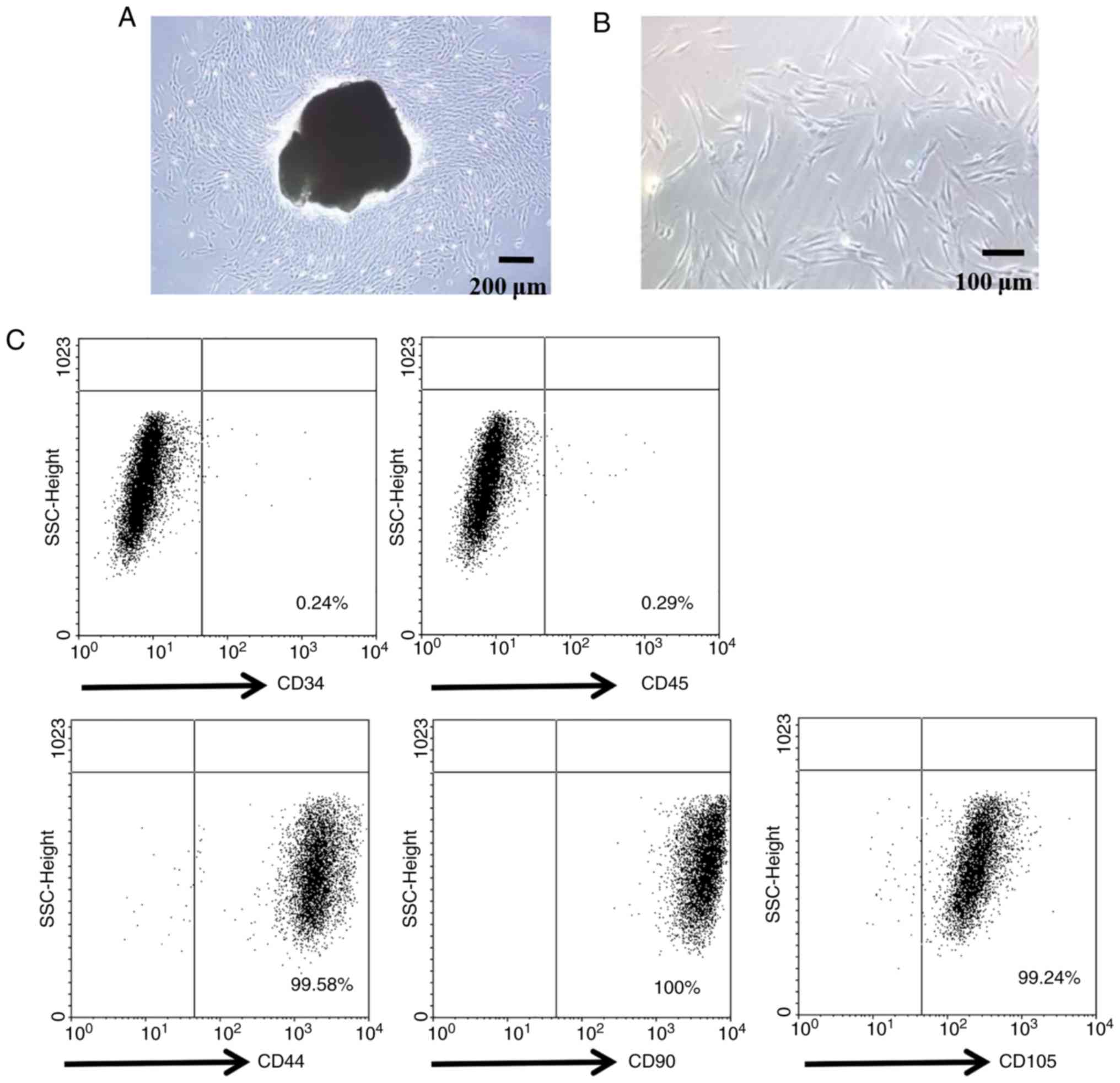
Isolation and characterization of
PDLSCs
PDLSCs were isolated using CD146 microbeads. The
majority of PDLSCs exhibited a fibroblastic spindle morphology, and
a small round or triangular shape (Fig. 1A and B). The mesenchymal stem cell
(MSC) properties of PDLSCs were then characterized, by detecting
their expression levels for MSC-specific cell surface antigens by
flow cytometry. As presented in Fig.
1C, PDLSCs highly expressed the MSC-specific markers CD44, CD90
and CD105. In addition, the cells were negative for the
hematopoietic stem cell marker CD34 and the pan-leukocyte marker
CD45.
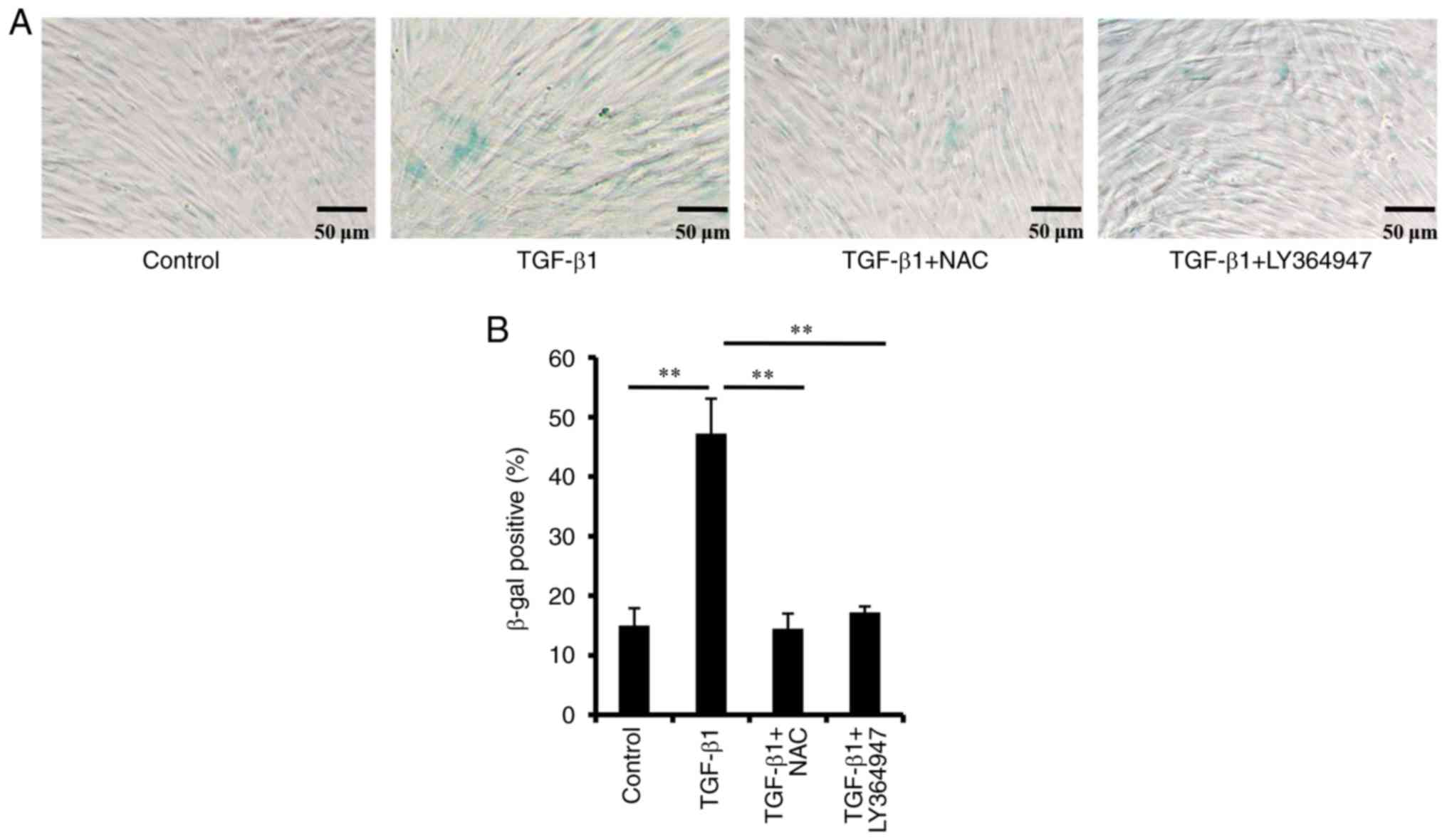
TGF-β1 induces PDLSC senescence
β-Galactosidase activity is a specific marker for
cellular senescence, and is observed only in senescent cells
(23). As shown in Fig. 2, following treatment with TGF-β1,
the percentage of positive PDLSCs for SA-β-gal staining was
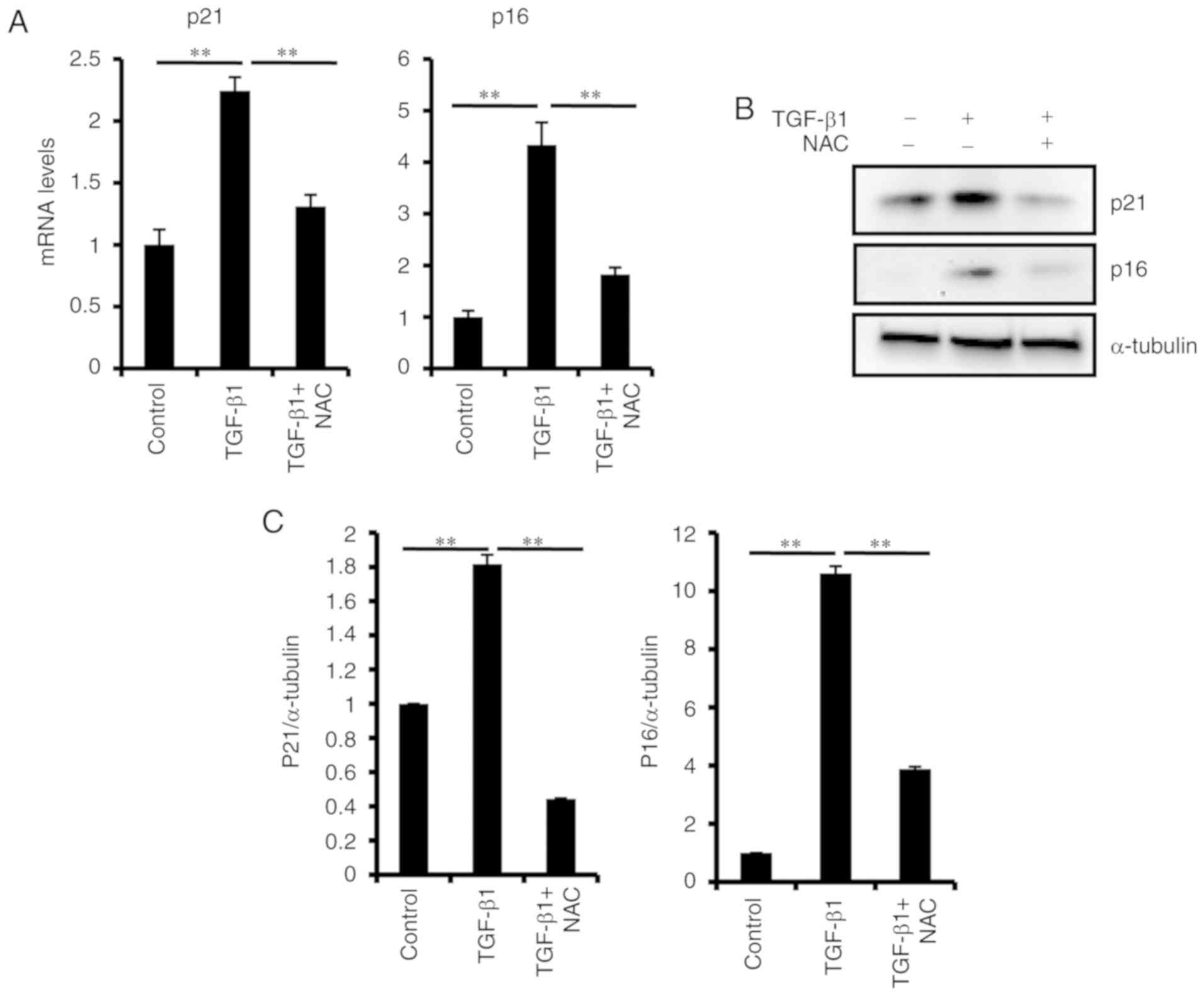
significantly increased. TGF-β1 treatment also significantly
increased the mRNA and protein expression levels of p16 and p21
(Fig. 3). To confirm the effect of
TGF-β1 on PDLSC senescence, the TGF-β signaling pathway was blocked
with a specific TGFβR inhibitor, LY364947. It was identified that
the percentage of SA-β-gal positive cells decreased significantly
(Fig. 2), and the expression
levels of p21 and p16 were also downregulated (Fig. S1), following treatment with
LY364947. These results indicated that TGF-β1 treatment induced
PDLSC senescence.
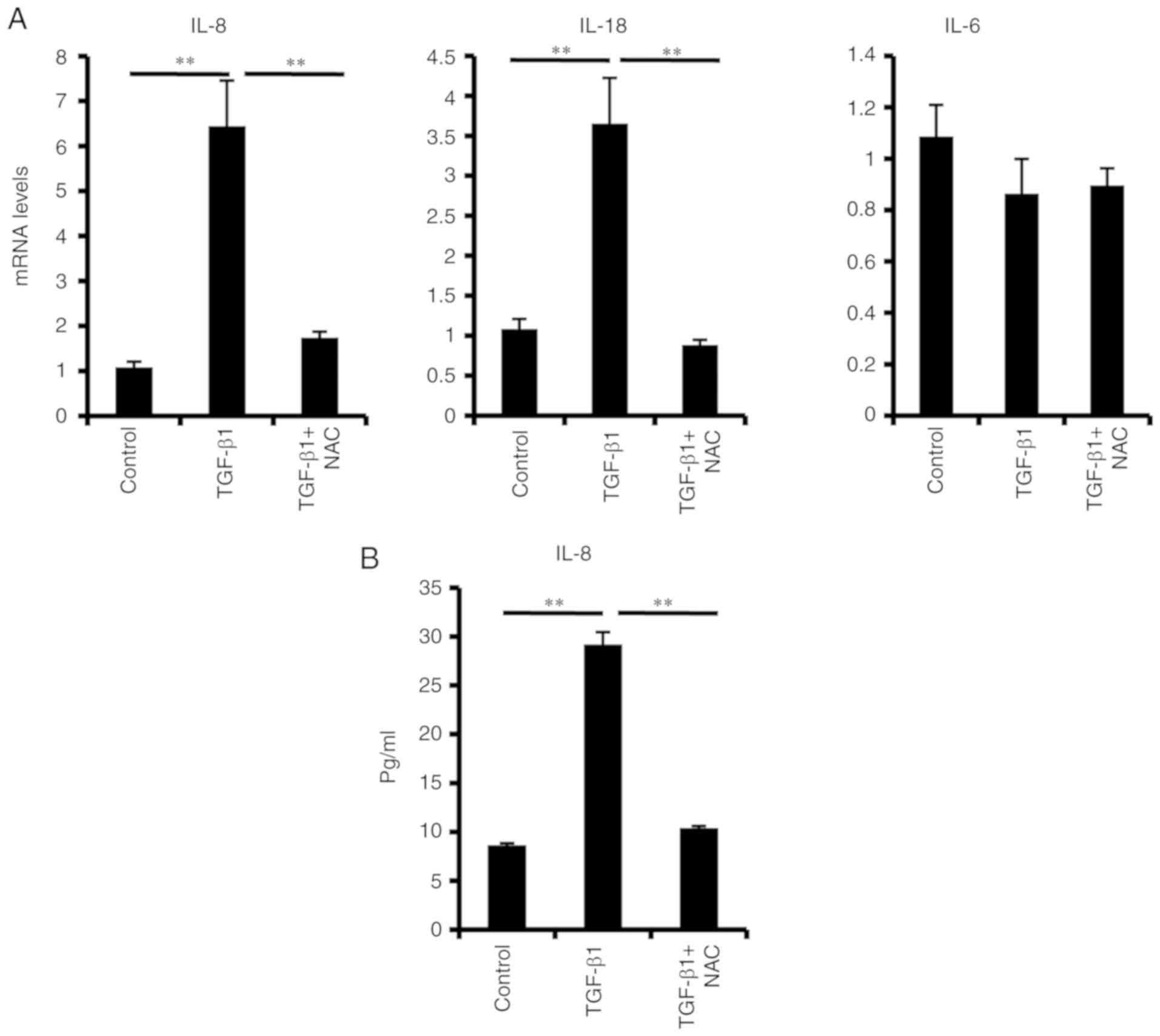
It has been reported that senescent cells release a
series of inflammatory cytokines, a process that further reinforces
cellular senescence (15). To
investigate whether senescent PDLSCs develop a complex SASP, the
expression of several key inflammatory mediators was investigated
in the present study. Elevated IL-18 and IL-8 mRNA levels (Fig. 4A) and secreted IL-8 protein levels
(Fig. 4B) were observed in
TGF-β1-induced senescent PDLSCs; by contrast, IL-6 mRNA expression
levels were unchanged (Fig.
4A).
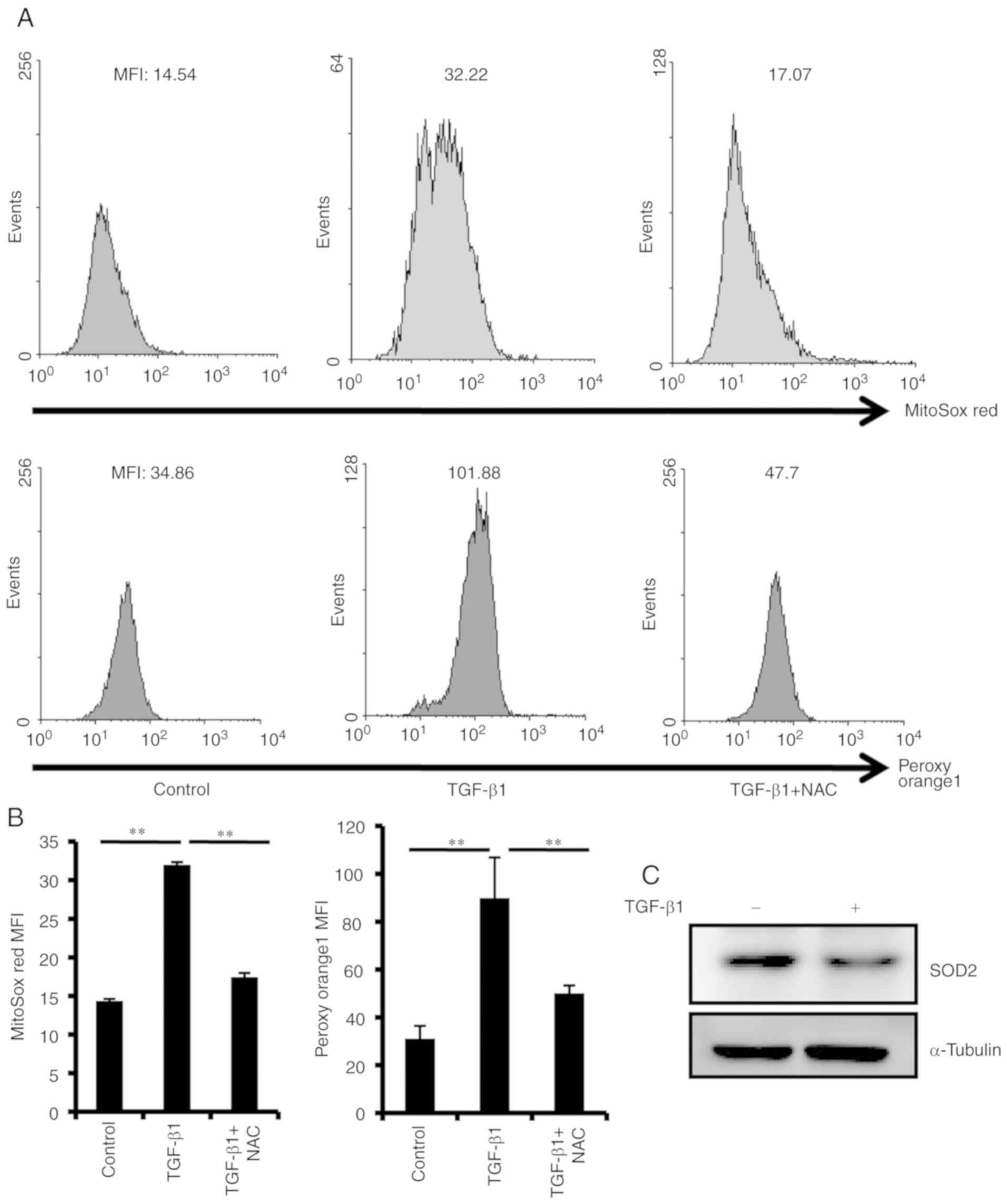
TGF-β1 treatment increases ROS
production in PDLSCs
ROS was detected using the MitoSox Red probe, and
the results demonstrated that treatment with TGF-β1 for 48 h
markedly increased ROS production in PDLSCs (Fig. 5A and B).
H2O2was also detected with a PO1 probe.
Detection of PO1 further confirmed the TGF-β1-induced ROS
production (Fig. 5A and B).
Additionally, the protein expression levels of SOD2, which is a
mitochondrial matrix enzyme and protects mitochondria against ROS
insult (24), were obviously
downregulated following TGF-β1 treatment (Fig. 5C), suggesting the involvement of
SOD2 expression levels in cellular senescence.
Senescence of PDLSCs is rescued by
NAC
To evaluate the role of ROS in TGF-β1-induced PDLSC
senescence, the physiological mitochondrial ROS scavenger NAC was
used in the senescence assay. The results demonstrated that NAC
effectively impaired TGF-β1-induced ROS production in PDLSCs
(Fig. 5A and B). NAC treatment
significantly suppressed TGF-β1-induced SA-β-Gal activity (Fig. 2), p16 and p21 expression (Fig. 3), as well as IL-8 and IL-18
production (Fig. 4). These results
indicated that ROS were an important mediator of the TGF-β1-induced
PDLSC senescence.
Discussion
PDLSCs which are positive for CD146 or STRO-1 have
greater osteogenic potential and colony-forming ability than CD146
or STRO-1-negative PDLSCs (25,26).
The present study investigated the effect of TGF-β1 on
CD146+ PDLSC senescence. PDLSCs also possess
characteristics of MSCs, such as cell surface MSC-specific marker
expression (CD44+, CD73+, CD90+,
CD105+, CD45−, CD31− and
CD34−) (27,28). In accordance with that, the present
study characterized the isolated PDLSCs by flow cytometry, and
found that the isolated PDLSCs successfully expressed the
MSC-specific markers CD44, CD90 and CD105, and were negative for
CD34 and the pan-leukocyte marker CD45. The data from the present
study demonstrated that TGF-β1 increased SA-β-Gal activity and ROS
production in PDLSCs. The expression levels of the aging-related
p16 and p21 proteins were also significantly increased following
TGF-β1 treatment. In addition, expression of the mitochondrial
matrix enzyme SOD2 was decreased in TGF-β1-treated PDLSCs. Addition
of the ROS scavenger NAC repressed the ROS production and PDLSC
senescence induced by TGF-β1. The data from the present study thus
demonstrated that TGF-β1 induced PDLSC senescence through the
increase of ROS production.
Periodontal disease (PD) is characterized by
inflammation and tissue destruction in the periodontal apparatus.
PDLSCs are critical to the PDL tissue reconstruction process and
are known to differentiate into osteoblastic or cementoblastic
cells to form calcified tissue (29,30).
Previous studies have indicated that TGF-β1 could cause tumor cell
senescence (19,20). The present study demonstrated that
TGF-β1 significantly increased SA-β-Gal activity in PDLSCs,
suggesting that PDLSCs were undergoing senescence. Cellular
senescence can result in phenotype changes and low proliferation of
PDLSCs (12–14), which may further attenuate the
efficiency of PDLSC-based transplantation. PDLSC expression of
aging regulators p16 and p21 was also upregulated significantly
following TGF-β1 treatment.
Senescent cells usually produce a series of
cytokines, chemokines, and other soluble factors, a phenotype
called SASP, which is believed to reinforce senescence (13,15).
In the present study, elevated IL-8 and IL-18 expression was
detected following TGF-β1 treatment, suggesting that TGF-β1 was
capable of instigating inflammation in PDLSCs. IL-8 can cause
extensive infiltration of neutrophils, which serve an essential
role in the inflammatory process (31). Additionally, the levels of IL-8 in
periapical lesions has been reported to correlate with pain in
patients with apical periodontitis (32). In a recent study, IL-18 was
demonstrated to promote matrix metallopeptidase secretion by
activating the NF-κB signaling pathway in human periodontal
ligament fibroblasts, indicating that IL-18 is involved in chronic
periodontitis development (33).
The findings of the present study reveal a new role
for TGF-β1 signaling in PDLSC senescence and SASP production. It
should also be noted that TGF-β1 serves important roles in cell
growth, differentiation and transformation (34). In addition, the complete deletion
of TGF-β1 is developmentally lethal (34). Concerning the periodontal
regeneration, TGF-β1 signaling deficiency affects cementoblast
differentiation and periodontal wound-healing, indicating that a
normal level of TGF-β1 is biologically required and thereby
protective of the periodontium (35,36).
Therefore, appropriate TGF-β1 suppression can present a therapeutic
basis for treating periodontitis. Cellular senescence was initially
considered a fail-safe mechanism for tumor suppression (37). However, several studies have shown
that senescent cells promote the growth of premalignant or
malignant cells, at least partly via SASP (38,39).
A specific SASP component, IL-6 also serves an established role in
liver regeneration and repair (40). In addition, during periodontal
regeneration and wound repair, a phase of inflammation and
granulation is indispensable (41), thus making TGF-β1-induced SASP a
likely contributor to the process of regeneration.
Our previous study found that TGF-β1 treatment
enhanced ROS production in corneal endothelium cells and further
induced cellular senescence (42),
which raised the hypothesis that ROS could be involved in PDLSC
senescence. The results of the present study demonstrated that ROS
production was significantly upregulated in TGF-β1-treated PDLSCs.
The results also demonstrated that the expression levels of SOD2,
which can protect mitochondria against ROS insult (24), were significantly decreased in
PDLSCs following TGF-β1 treatment. To further examine the role of
ROS in TGF-β1 induced PDLSC senescence, PDLSCs were treated with
the ROS scavenger NAC in combination with TGF-β1. The results
revealed that NAC significantly inhibited the TGF-β1 induced ROS
production and SA-β-Gal activity, suggesting that TGF-β1-induced
PDLSCs senescence depended on ROS production.
In conclusion, the present study demonstrated that
TGF-β1, a potent stimulator for tissue regeneration, promoted PDLSC
senescence and this effect was dependent on ROS induction.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81701381).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CF, HS and ZL designed the study and analyzed the
data. CF, QJ, and CZ performed cell culture, SA-β-Gal staining,
RT-qPCR and western blotting. CF and SX performed the flow
cytometry analysis. CF, HS and ZL wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Review Board
of the Affiliated Hospital of Qingdao University. Written informed
consent was obtained from patients prior to procedure.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Zheng Y, Ding G, Fang D, Zhang C,
Bartold PM, Gronthos S, Shi S and Wang S: Periodontal ligament stem
cell-mediated treatment for periodontitis in miniature swine. Stem
Cells. 26:1065–1073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang GT, Gronthos S and Shi S:
Mesenchymal stem cells derived from dental tissues vs. those from
other sources: Their biology and role in regenerative medicine. J
Dent Res. 88:792–806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lekic P and McCulloch CA: Periodontal
ligament cell population: The central role of fibroblasts in
creating a unique tissue. Anat Rec. 245:327–341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kao RT, Murakami S and Beirne OR: The use
of biologic mediators and tissue engineering in dentistry.
Periodontol 2000. 50:127–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Yang H, Zhang Z, Yan Z, Lv H, Zhang
Y and Wu B: Concentrated growth factor exudate enhances the
proliferation of human periodontal ligament cells in the presence
of TNF-α. Mol Med Rep. 19:943–950. 2019.PubMed/NCBI
|
|
6
|
Hyun SY, Lee JH, Kang KJ and Jang YJ:
Effect of FGF-2, TGF-β-1, and BMPs on Teno/Ligamentogenesis and
Osteo/Cementogenesis of human periodontal ligament stem cells. Mol
Cells. 40:550–557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y and Massague J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wikesjo UM, Razi SS, Sigurdsson TJ,
Tatakis DN, Lee MB, Ongpipattanakul B, Nguyen T and Hardwick R:
Periodontal repair in dogs: Effect of recombinant human
transforming growth factor-beta1 on guided tissue regeneration. J
Clin Periodontol. 25:475–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawahara T, Yamashita M, Ikegami K,
Nakamura T, Yanagita M, Yamada S, Kitamura M and Murakami S:
TGF-beta negatively regulates the BMP2-dependent early commitment
of periodontal ligament cells into hard tissue forming cells. PLoS
One. 10:e01255902015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Gorter DJ, van Dinther M, Korchynskyi O
and ten Dijke P: Biphasic effects of transforming growth factor β
on bone morphogenetic protein-induced osteoblast differentiation. J
Bone Miner Res. 26:1178–1187. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lorda-Diez CI, Montero JA, Martinez-Cue C,
Garcia-Porrero JA and Hurle JM: Transforming growth factors beta
coordinate cartilage and tendon differentiation in the developing
limb mesenchyme. J Biol Chem. 284:29988–29996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baker DJ, Wijshake T, Tchkonia T,
LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL and van
Deursen JM: Clearance of p16Ink4a-positive senescent cells delays
ageing-associated disorders. Nature. 479:232–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lopez-Otin C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burova E, Borodkina A, Shatrova A and
Nikolsky N: Sublethal oxidative stress induces the premature
senescence of human mesenchymal stem cells derived from
endometrium. Oxid Med Cell Longev. 2013:4749312013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Acosta JC, Banito A, Wuestefeld T,
Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka
F, Andrulis M, et al: A complex secretory program orchestrated by
the inflammasome controls paracrine senescence. Nat Cell Biol.
15:978–990. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burton DG and Faragher RG: Cellular
senescence: From growth arrest to immunogenic conversion. Age.
37:272015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, Tchkonia T, Ding H, Ogrodnik M,
Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera
V, et al: JAK inhibition alleviates the cellular
senescence-associated secretory phenotype and frailty in old age.
Proc Natl Acad Sci USA. 112:6301–6310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou J, Lei T, Guo P, Yu J, Xu Q, Luo Y, Ke
R and Huang D: Mechanisms shaping the role of ERK1/2 in cellular
senescence (Review). Mol Med Rep. 19:759–770. 2019.PubMed/NCBI
|
|
19
|
Senturk S, Mumcuoglu M, Gursoy-Yuzugullu
O, Cingoz B, Akcali KC and Ozturk M: Transforming growth
factor-beta induces senescence in hepatocellular carcinoma cells
and inhibits tumor growth. Hepatology. 52:966–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hubackova S, Krejcikova K, Bartek J and
Hodny Z: IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA
damage response are shared features of replicative,
oncogene-induced, and drug-induced paracrine ‘bystander
senescence’. Aging. 4:932–951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li ZY, Chen ZL, Zhang T, Wei C and Shi WY:
TGF-β and NF-κB signaling pathway crosstalk potentiates corneal
epithelial senescence through an RNA stress response. Aging (Albany
NY). 8:2337–2354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Debacq-Chainiaux F, Erusalimsky JD,
Campisi J and Toussaint O: Protocols to detect
senescence-associated beta-galactosidase (SA-betagal) activity, a
biomarker of senescent cells in culture and in vivo. Nat Protoc.
4:1798–1806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Velarde MC, Flynn JM, Day NU, Melov S and
Campisi J: Mitochondrial oxidative stress caused by Sod2 deficiency
promotes cellular senescence and aging phenotypes in the skin.
Aging (Albany NY). 4:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Wang Y, Tang W, Wang X, Pang Y,
Yang S, Wei Y, Gao H, Wang D and Cao Z: Bone morphogenetic
protein-9 enhances osteogenic differentiation of human periodontal
ligament stem cells via the JNK pathway. PLoS One. 12:e01691232017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wada N, Menicanin D, Shi S, Bartold PM and
Gronthos S: Immunomodulatory properties of human periodontal
ligament stem cells. J Cell Physiol. 219:667–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gronthos S, Mrozik K, Shi S and Bartold
PM: Ovine periodontal ligament stem cells: Isolation,
characterization, and differentiation potential. Calcif Tissue Int.
79:310–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagata M, Iwasaki K, Akazawa K, Komaki M,
Yokoyama N, Izumi Y and Morita I: Conditioned medium from
periodontal ligament stem cells enhances periodontal regeneration.
Tissue Eng Part A. 23:367–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iwasaki K, Komaki M, Yokoyama N, Tanaka Y,
Taki A, Honda I, Kimura Y, Takeda M, Akazawa K, Oda S, et al:
Periodontal regeneration using periodontal ligament stem
cell-transferred amnion. Tissue Eng Part A. 20:693–704.
2014.PubMed/NCBI
|
|
31
|
Nair PN: Pathogenesis of apical
periodontitis and the causes of endodontic failures. Crit Rev Oral
Biol Med. 15:348–381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim AR, Ahn KB, Kim HY, Seo HS, Kum KY,
Yun CH and Han SH: Streptococcus gordonii lipoproteins induce IL-8
in human periodontal ligament cells. Mol Immunol. 91:218–224. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Guan M, Wei L and Yan H: IL18
promotes the secretion of matrix metalloproteinases in human
periodontal ligament fibroblasts by activating NF-κB signaling. Mol
Med Rep. 19:703–711. 2019.PubMed/NCBI
|
|
34
|
Bottinger EP, Letterio JJ and Roberts AB:
Biology of TGF-beta in knockout and transgenic mouse models. Kidney
Int. 51:1355–1360. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nokhbehsaim M, Winter J, Rath B, Jäger A,
Jepsen S and Deschner J: Effects of enamel matrix derivative on
periodontal wound healing in an inflammatory environment in vitro.
J Clin Periodontol. 38:479–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bosshardt DD, Stadlinger B and Terheyden
H: Cell-to-cell communication-periodontal regeneration. Clin Oral
Implants Res. 26:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:2853–2868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Serra MP, Marongiu F, Sini M and Laconi E:
Hepatocyte senescence in vivo following preconditioning for liver
repopulation. Hepatology. 56:760–768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sculean A, Chapple IL and Giannobile WV:
Wound models for periodontal and bone regeneration: The role of
biologic research. Periodontol 2000. 68:7–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li ZY, Liu T, Ma JW, Guo Q, Ma L, Lv QL,
Jiang Y, Wei C and Zhang JS: TGF-β induces corneal endothelial
senescence via increase of mitochondrial reactive oxygen species in
chronic corneal allograft failure. Aging (Albany NY). 10:3474–3485.
2018. View Article : Google Scholar : PubMed/NCBI
|