Introduction
Bone defects caused by tumor surgery, infection or
congenital bone malformation, are usually treated by bone grafting
to increase bone regeneration (1).
Bone grafting is one of the most common surgical procedures
performed to increase bone regeneration via the surgical
implantation of materials and ranks second only to blood
transfusion and tissue transplantation (2). According to incomplete statistics,
more than two million bone grafts are performed worldwide each year
(3). At present, the materials
used for bone reconstruction are mainly obtained from autograft and
allograft materials (4,5). The use of autologous bone for bone
grafts offers several advantages, such as superior bone conduction,
inducibility and osteogenesis. As a result, autogenous bone grafts
have become the ‘gold standard’ for use in promoting bone
regeneration. However, the limited supply of autologous bone and
the difficulties with obtaining appropriate donors limit the use of
autogenous bone grafts in clinical practice (6).
Bone substitute materials are divided into synthetic
and biological materials, which are characterized by their
morphology, chemical composition, and crystallinity (7). These materials have to meet specific
criteria, such as having the proper surface configuration and
structure needed to simulate the desired effect in the body
(8). As calcium sulfate (CaS) is
biodegradable and biocompatible (9,10),
it is frequently used as an insulating material for bone defects,
where it serves to inhibit the growth of fibrous tissues and
promote bone growth (11,12). CaS serves as an external fixator
and can be used in distraction osteogenesis to cure osteomyelitis
and bone tumors (13). CaS
increases the time of distraction osteogenesis in the case of
synchronous biodegradation. Studies have also suggested that CaS
helps to maintain the connections between bone and periosteum,
which promotes osteogenesis (14–17);
in addition, CaS plays an essential role bone formation (18). However, a previous study showed
that CaS does not significantly alter CaS levels in the bone matrix
and CaS does not have good osteoconductive properties or stimulate
osteogenesis (19). Therefore, it
is beneficial to add stimulatory agents such as trace elements to
orthopedic implant materials to promote bone growth.
A growing body of evidence indicates that strontium
(Sr) promotes the differentiation of osteoblasts and inhibits the
formation and absorption of osteoclasts (20,21).
A previous study showed that Sr is beneficial for the synthesis of
collagen in vitro (22).
Therefore, the implantation of a bone substitute material followed
by its continuous treatment with Sr might help to stimulate bone
growth in a specific desired location. Bone implant materials
containing SR have been extensively used in bone tissue engineering
studies. For example, SR-containing mesoporous bioactive glass has
the advantages of providing good bone formation bioactivity,
enhanced mechanical strength and ion release regulation, and
therefore can expected to be widely used for stimulating bone
regeneration (23). Sr substituted
bioactive glass is more effective at accelerating bone formation
than is bioactive glass without Sr (24,25).
In the present study, co-precipitation and
hydrothermal techniques were used to prepare a novel Sr-CaS
material, and also established a tibia bone defect model in Sprague
Dawley (SD) rats. The biocompatibility and safety of different
concentrations of Sr-CaS in the model were investigated. the
effects and mechanisms of different Sr-CaS concentrations on bone
repair were also investigated in vitro and in
vivo.
Materials and methods
Animals
Healthy male specific pathogen free SD rats
(7-weeks-old, 180–230 g) were provided by the Experimental Animal
Center of Southern Medical University (Guangzhou, China). After the
SD rats were purchased, they were raised for 7–10 days according to
the national standard (freely available food and water, 50–60%
humidity, 21–25°C and a 12-h light and dark cycle) prior to being
used in experiments. All protocols used for animal studies were
approved by the Animal Ethics Committee of Southern Medical
University.
Sr-CaS extraction preparation
The method of extraction preparation was referred to
in a previous study by Li et al (26). The original extraction of Sr-CaS
material was kept at concentration of 3 g/ml and 25% dilution
extraction was generated by diluting with normal saline by 1:3.
Establishment of the animal model
The bone defect models were created as previously
described (27,28). The rats received general anesthesia
by an intravenous injection of 3% pentobarbital sodium (30 mg/kg
body weight). Next, the skin over the proximal tibia was incised
and the periosteum was cleared using a periosteal elevator. A
micro-burr with a 0.8 mm tip was used to create a defect (3 mm wide
and 5 mm long) in both tibias, starting at 10 mm below the
articular surface in the anteromedial cortex. The defects and
intramedullary canals were washed with physiological saline to
remove any residual bone and bone marrow.
Experimental groups
SD rats were assigned to four different groups based
on the materials that were used to fill their defects: i) A blank
group in which no material was implanted into the bone defects; ii)
a CaS group in which CaS was implanted into the bone defects; iii)
a 5% Sr-CaS group in which 5% Sr-CaS was implanted into the bone
defects and iv) a 10% Sr-CaS group in which 10% Sr-CaS was
implanted into the bone defects. The defects were filled flush to
the anterior cortex with the paste-form material prior to being
allowed to set in situ. After surgery, the skin was
carefully sutured and the rats were injected with an antibiotic (3%
penicillin) for 2 days. All mice recovered well from surgery and
were housed separately in plastic cages. Food and water were
available ad libitum.
Sample collection
The mice were closely observed and recorded data for
the following parameters: Postoperative animal spirit, food intake,
activity, wound drainage and swelling. At 8 h post-surgery,
specimens of tibia were obtained and analyzed by micro-computed
tomography (CT) scans. Consecutive micro-CT cross section images of
regions of interest were obtained and the values for various
parameters were calculated three-dimensional model visualization
software (CTVol ver. 2.0, Bruker). At 8 weeks after surgery, the
rats were sacrificed, the amount of osteotylus in the bone defects
was evaluated and decalcified specimens were prepared for routine
sectioning.
Cell culture and treatment
Bone marrow mesenchymal stem cells (BMSCs) were
isolated from cavities of the femurs and tibias of SD rats as
previously described (28). The
isolated BMSCs were maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 2 mM l-glutamine and penicillin (100
U/ml)/streptomycin (100 g/ml) at 37°C in a humidified atmosphere of
5% CO2. The Sr-CaS stock material was diluted 1:3 with
DMEM. The BMSCs were divided into the following four groups based
on the different materials used for treatment: Blank group (treated
with an equal amount of PBS), CaS group, 5% Sr-CaS group and 10%
Sr-CaS group, respectively.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) assay
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total RNA from the rat
tissues and treated BMSCs. A 50 ng sample of total RNA was used to
synthesize cDNA with incubation at 37°C for 15 min and then at 85°C
for 5 sec by using the reagents in a PrimeScript RT reagent kit
(Takara Bio, Inc., Otsu, Japan). The RT-qPCR assay was carried out
for 40 cycles with pre-denaturation at 95°C for 30 sec, and then
denaturation at 95°C for 5 sec, and finally annealing/extension at
60°C for 30 sec by using Bestar™ qPCR MasterMix (cat. no. 2043; DBI
Bioscience, Shanghai, China) on an ABI 7500 detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers
used in the present study are shown in Table I. GAPDH was used as internal
reference gene, and relative expression of candidate genes were
calculated using 2−ΔΔCt methods (29). All the primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Guangzhou, China).
 | Table I.The sequence of primers used in the
present study. |
Table I.
The sequence of primers used in the
present study.
| ID | Sequence
(5′-3′) | Product length
(bp) |
|---|
| GAPDH F |
CCTCGTCTCATAGACAAGATGGT | 169 |
| GAPDH R |
GGGTAGAGTCATACTGGAACATG |
|
| ALP F |
TGTAGGTGCTGTGGTCAAGG | 177 |
| ALP R |
AGAGTGACGGTGTCGTAGCC |
|
| RUNX2 F |
GAATGATGAGAACTACTCTGCCG | 144 |
| RUNX2 R |
GGATTTGTGAAGACCGTTATGG |
|
| OCN F |
CAACAATGGACTTGGAGCCC | 133 |
| OCN R |
ATAGATGCGCTTGTAGGCGT |
|
| OPG F |
GCTCCTGGCACCTACCTAAA | 157 |
| OPG R |
ACTCCTGTTTCACGGTCTGC |
|
| BSP F |
CTGACCAGTTATGGCACCAC | 278 |
| BSP R |
TAATCCTGACCCTCGTAGCC |
|
Western blotting assay
Total proteins were extracted by using RIPA buffer
(P0013B; Beyotime Institute of Biotechnology) containing
phenylmethylsulfonyl fluoride and the protein concentration in each
extract was measured using a bicinchoninic acid kit (Pierce; Thermo
Fisher Scientific, Inc.). Aliquots of total protein (~20 µg) were
separated by 10% SDS-PAGE. The separated protein bands were
transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA), which were subsequently blocked by incubation
in 5% low fat dried milk for 2 h at room temperature. After being
washed, the membranes were incubated overnight with primary
antibodies at 4°C; after which, they were incubated with
horseradish peroxidase conjugated donkey-anti-rabbit secondary
antibodies at room temperature for 2 h. Immunostaining was detected
with the use of enhanced chemiluminescent reagents (P0018FS,
Beyotime Institute of Biotechnology). The primary antibodies used
in the study were Smad2/3 (Cell Signaling Technology, Inc., Danvers
MA, USA; cat. no. 3102, dilution 1:1,000), phosphorylated
(p)-Smad2/3 (Cell Signaling Technology, Inc.; cat. no. 8828,
dilution 1:1,000), TGF-β (Abcam, Cambridge, MA, USA; cat. no.
ab31013, dilution 1:1,000), β-catenin (Cell Signaling Technology,
Inc.; cat. no. 9562, dilution 1:1,000), p-β-catenin (Cell Signaling
Technology, Inc.; cat. no. 4270, dilution 1:1,000) and GAPDH
(Abcam; cat. no. ab9385, dilution 1:5,000). Then secondary
anti-rabbit antibodies (Abcam; cat. no. ab7097, dilution 1:10,000)
were used. Bands were quantified using Image-Pro Plus (ver. 6.0;
Media Cybernetics, Inc., USA).
Cell proliferation assay
Cell proliferation ability was evaluated by using a
Cell Counting Kit-8 assay (CCK-8; Sigma-Aldrich; Merck KGaA). In
brief, the treated BMSCs were seeded into 96-well plates. Next, 15
µl of CCK-8 solution was added to each well and incubated for 3 h.
The optical density of each well was measured with a microtiter
plate reader (SpectraMax; Molecular Devices, LLC, Sunnyvale, CA,
USA).
Colony formation assay
The treated BMSCs were seeded into 7 cm culture dish
at a density of 200 cell/well and maintained in complete medium for
14 days at 37°C in a humidified atmosphere with 5% CO2.
The cell colonies were fixed with 4% paraformaldehyde for 15 min at
room temperature and then stained with 0.5% crystal violet for 15
min at room temperature. The numbers of colonies were counted under
a light microscope.
ELISA
Cells were plated into a 96-well plate at a density
of 1×104 cells/well. Next, cell lysis buffer was added
to each well; after which, the plate was centrifuged and the
supernatant fractions were collected. Alkaline phosphatase (ALP)
concentrations were then measured using an ALP assay kit (cat. no.
A059-2; Nanjing Jiancheng Bioengineering Institute).
Cell apoptosis analysis
The treated BMSCs were harvested and washed with
PBS. The BMSCs were then fixed in 70% ethanol for 2 h at room
temperature and treated with Annexin V-fluorescein isothiocyanate
and PI (KeyGen Biotech Co., Ltd.) for 10 min in the dark. The
results were analyzed by flow cytometer (BD Biosciences;
Becton-Dickinson and Company, Franklin Lakes, NJ, USA) with FlowJo
software (ver. 7.6.1, FlowJo LLC).
Transwell assay
Treated BMSCs (1×105 cells/ml) were
re-suspended in 0.2 ml of culture medium and added to the upper
chambers of a Transwell plate, while 0.8 ml of culture medium
containing 20% FBS was added to the lower chambers. After
incubation for 48 h in a cell incubator at 37°C with 5%
CO2, the non-invading cells on the membrane surface were
removed and the migrated cells were fixed with 4% paraformaldehyde
for 15 min at room temperature and subsequently stained with 0.5%
crystal violet (Beyotime Institute of Biotechnology, Beijing,
China) for 10 min at room temperature. The number of migrated cells
was then counted under a light microscope (OLYMPUS CX41; Olympus
Corporation).
Alizarin Red staining
Treated BMSCs (5×105 cells/ml) were
seeded into 6-well plates and maintained in complete medium for 24
h at 37°C in a 5% CO2 atmosphere. Next, the cells were
washed with PBS, fixed with 95% ethanol for 15 min at room
temperature and then stained with 1% Alizarin Red for 5 min at room
temperature. After washing, the staining results were obtained by
using a light microscope (OLYMPUS CX41; Olympus Corporation).
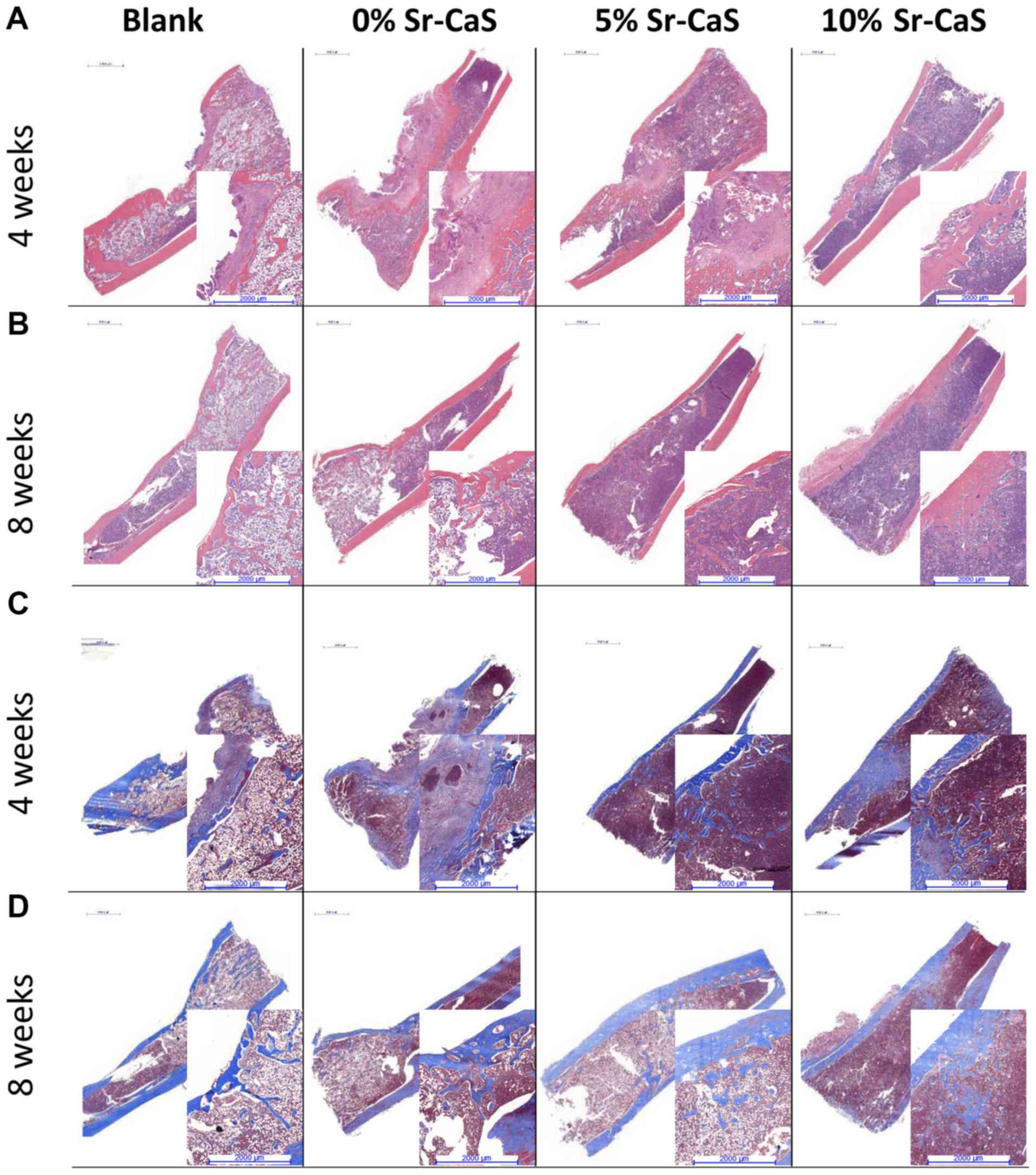
Hematoxylin and eosin (H&E)
staining and Masson staining
The tissues from each group were fixed in 4%
paraformaldehyde for 24 h at room temperature and then decalcified
using Quick Decalcifying Solution (cat. no. G1107; Servicebio,
Wuhan, China). Next, the tissues were embedded with paraffin and
5-µm sections were obtained for dehydration with different
concentrations of alcohol. For H&E staining, 5-µm sections were
stained with H&E solution (hematoxylin: 5 min; eosin: 2 min)
(cat. no. H8070; Beijing Solarbio Science & Technology Co.,
Ltd.). For Masson staining, 5-µm sections were first treated with
Ponceau solution for 5 min, then with phosphomolybdic acid solution
for 4 min and finally with aniline blue solution for 10 min. All
the procedures were performed at room temperature. The stained
sections were analyzed by light microscopy (OLYMPUS CX41; Olympus
Corporation).
Immunohistochemistry
Slides with 4-µm thick tissue sections were treated
with 3% H2O2 for 25 min at room temperature
in the dark and then washed 3 times with PBS. The slides were then
incubated in 3% bovine serum albumin (AR1006, Wuhan Boster
Biological Technology, Ltd.) at room temperature for 30 min,
followed by an overnight incubation with the primary antibodies
(ab13418, dilution: 1:100; Abcam) at 4°C. Next, the slides were
incubated with the secondary antibodies (Abcam; cat. no. ab150113,
dilution 1:1,000), followed by treated with chromogenic agent, DAB
(K5007, Dako; Agilent Technologies, Inc.) at room temperature for
50 min and then incubated with 3,3′-diaminobenzidine. Finally, the
slides were stained with hematoxylin for 3 min at room temperature
and dehydrated with different concentrations of alcohol. The
stained tissues were observed under a light microscope (Nikon
Eclipse TI-SR; Nikon Corporation, Tokyo, Japan).
Statistical analysis
All data in this study was presented as mean ±
standard deviation. One-way analysis of variance followed by
Tukey's test was used to analyze the data. P<0.05 was considered
to indicate a statistically significant difference. All the
experiments in this study were repeated in triplicate.
Results
Sr-CaS does not alter the
proliferation and apoptosis of BMSCs
To explore the effect of Sr-CaS on BMSCs, compounds
containing different levels of calcium and strontium ions were
prepared [0% Sr (pure CaS), 5% Sr-CaS, and 10% Sr-CaS,
respectively]. These compounds were then used to treat BMSCs for
periods for 1, 3 and 7 days, respectively. The results from the
CCK-8 and soft agar colony formation assays revealed that none of
the compounds significantly affected the proliferative ability of
the BMSCs (Fig. 1A-C). These
results were further confirmed by flow cytometry assays (Fig. 1D and E). Therefore, 5 and 10%
Sr-CaS (25% dilution ratio) had no significant cytotoxicity when
administered to BMSCs.
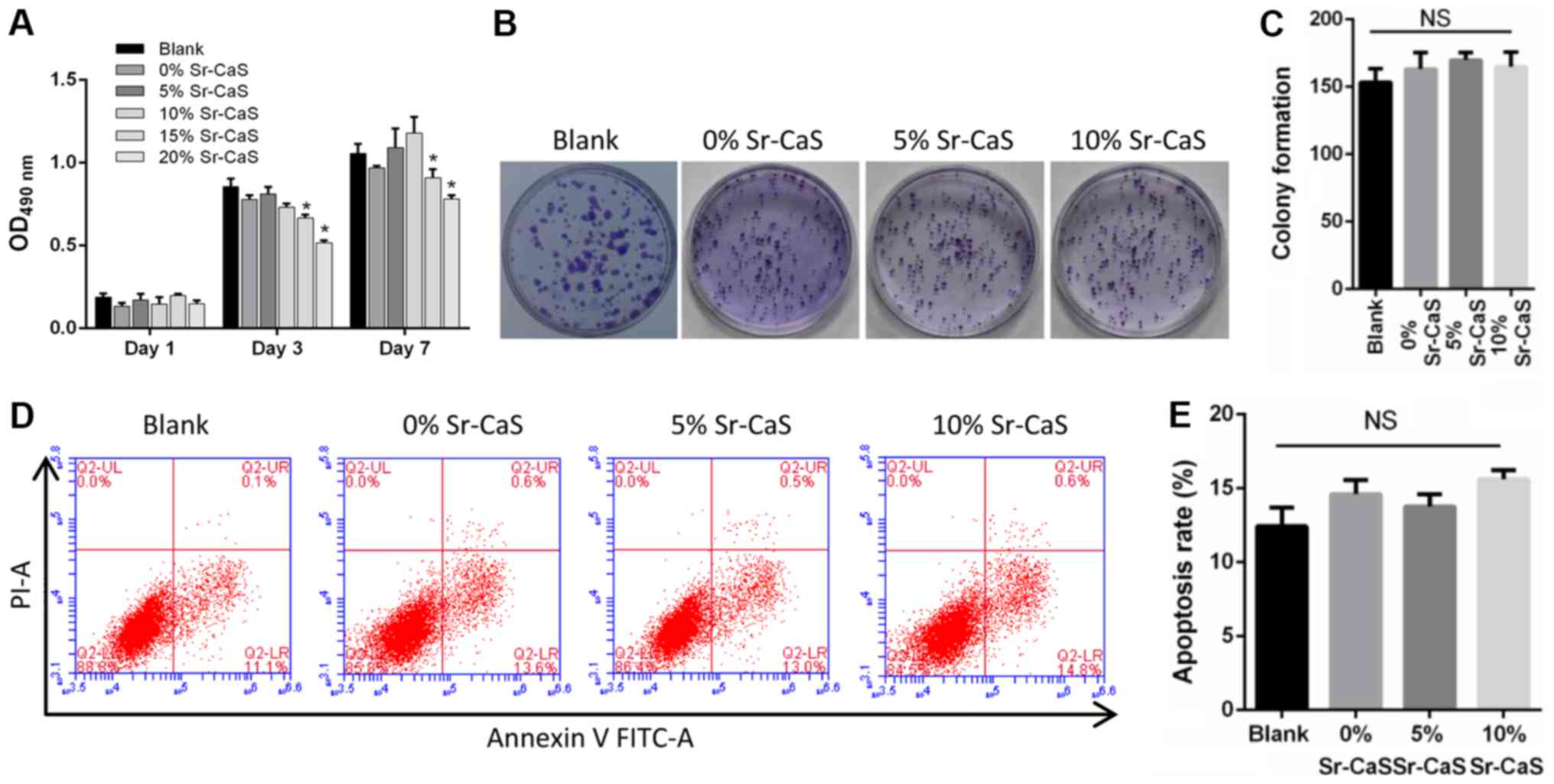 | Figure 1.Effects of Sr-CaS on BMSC
proliferation and apoptosis at 7 days after treatment. (A) The
proliferative ability of BMSCs treated with 0, 5 or 10% Sr-CaS at
1, 3, and 7 days after treatment, respectively. *P<0.05 vs.
Blank. (B) The soft agar colony formation assay was used to analyze
the effect of 0, 5 and 10% Sr-CaS on the proliferative ability of
BMSCs. (C) The number of cell colonies was counted. (D) BMSCs were
treated with 0, 5 or 10% Sr-CaS for 7 days, and their apoptosis
rates were determined by flow cytometry performed with Annexin V/PI
staining. (E) The percentage of apoptotic cells was calculated. Sr,
strontium; BMSCs, bone marrow stem cells; CaS, calcium sulphate;
PI, propidium iodide; OD, optical density; NS, not significant. |
Sr-CaS promotes the migration and
osteogenic differentiation of BMSCs after 7 days after
treatment
To further investigate the effect of Sr-CaS on BMSC
migration and osteogenic differentiation, BMSCs that had been
treated with 0, 5, or 10% Sr-CaS for 7 days were used in Transwell
and Alizarin Red staining assays. The results showed that the
migratory ability of BMSCs in the 5 Sr-CaS and 10% Sr-CaS groups
were significantly increased when compared with BMSCs in the blank
group (P<0.05). Furthermore, the migratory ability of BMSCs in
the 10% Sr-CaS group was significantly increased compared with
BMSCs in the 5% Sr-CaS group (P<0.01; Fig. 2A and B). The results of Alizarin
Red staining indicated that Sr-CaS could promote the osteogenic
differentiation of BMSCs and this effect increased in conjunction
with the increase in Sr concentration. ELISA was then used to
assess the ALP levels in the treated BMSCs. The results showed that
Sr-CaS significantly increased ALP levels and those levels
gradually increased in conjunction with the increase in Sr
concentration (P<0.05; Fig.
2D).
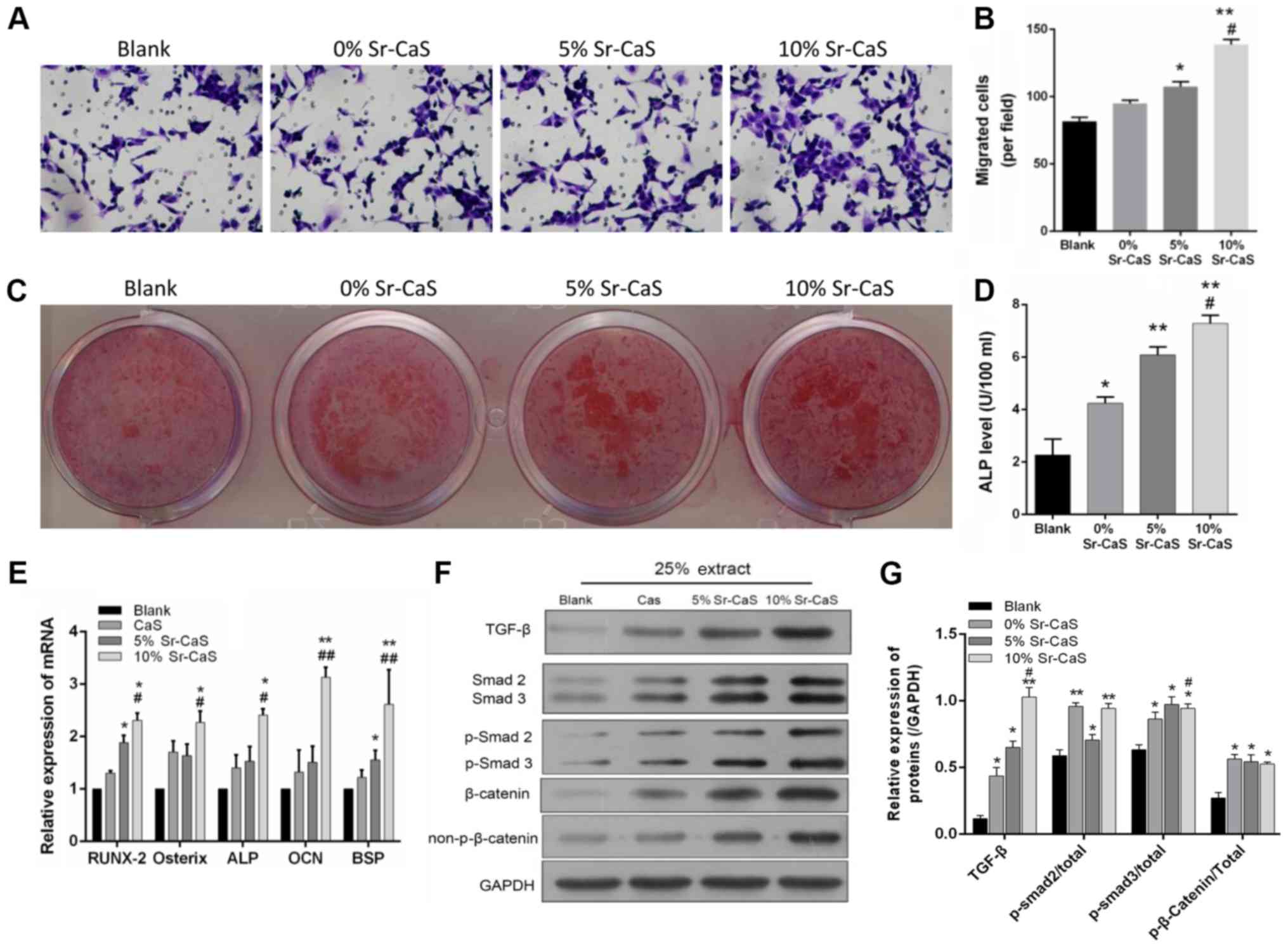 | Figure 2.Effects of Sr-CaS on BMSC migration
and osteogenic differentiation at 7 days after treatment. (A) The
effects of 0, 5, and 10% Sr-CaS on the invasive ability of BMSCs
were measured by a Transwell assay at 7 days after treatment. (B)
The numbers of migrated cells are shown. *P<0.05 and **P<0.01
vs. blank group; #P<0.05 vs. 5% Sr-CaS group. (C) The
osteoblastic differentiation ability of BMSCs treated with 0, 5 or
10% Sr-CaS for 7 days was assessed by Alizarin Red staining. (D)
ALP levels were detected by ELISA. *P<0.05 and **P<0.01 vs.
blank group; #P<0.05 vs. 5% Sr-CaS group. (E) BMSCs
were treated with 0, 5 or 10% Sr-CaS for 7 days, and their levels
of RUNX2, Osterix, ALP, OCN, and BSP mRNA expression were analyzed
by the reverse transcription-quantitative PCR assay. TGF-β,
Smad2/3, and β-catenin protein expression was measured by (F)
western blotting and (G) densitometry analysis. *P<0.05 and
**P<0.01 vs. blank group; #P<0.05,
##P<0.01 vs. CaS group. ALP, alkaline phosphatase;
Sr, strontium; BMSCs, bone marrow stem cells; CaS, calcium
sulphate; OCN, osteocalcin; TGF, transforming growth factor; BSP,
bone sialoprotein; p-Smad, phosphorylated mothers against
decapentaplegic homolog; RUNX2, runt-related transcription factor
2. |
In addition, the influence of Sr-CaS on the
expression of various genes associated with the osteogenic
differentiation of BMSCs was analyzed. The results of the present
study demonstrated that the levels of Runt-Related Transcription
Factor 2 (RUNX2), Osterix, ALP, OCN and bone sialoprotein
(BSP) expression were significantly elevated in the 10%
Sr-CaS group when compared with those in the blank group
(P<0.05). Furthermore, those levels of gene expression were
directly associated with the Sr concentration (Fig. 2E). It was also found that Sr-CaS
upregulated TGF-β, Smad2/3 and β-catenin expression related to the
Sr content (P<0.05; Fig. 2F and
G).
Sr-CaS, as bone-rebuilding material,
promotes bone repair
SD rats with shin defects were treated with 0, 5 or
10% Sr-CaS for 2 months and their bone reconstructive states were
recorded. As shown in Fig. 3A, the
tibia bone defects in the blank treatment group continued to be
severe, while the tibia bone defects in the 0, 5 and 10% Sr-CaS
treatment groups showed improvement when compared with those in the
blank group. Moreover, the tibia bone defects showed their greatest
improvement in 10% Sr-CaS group.
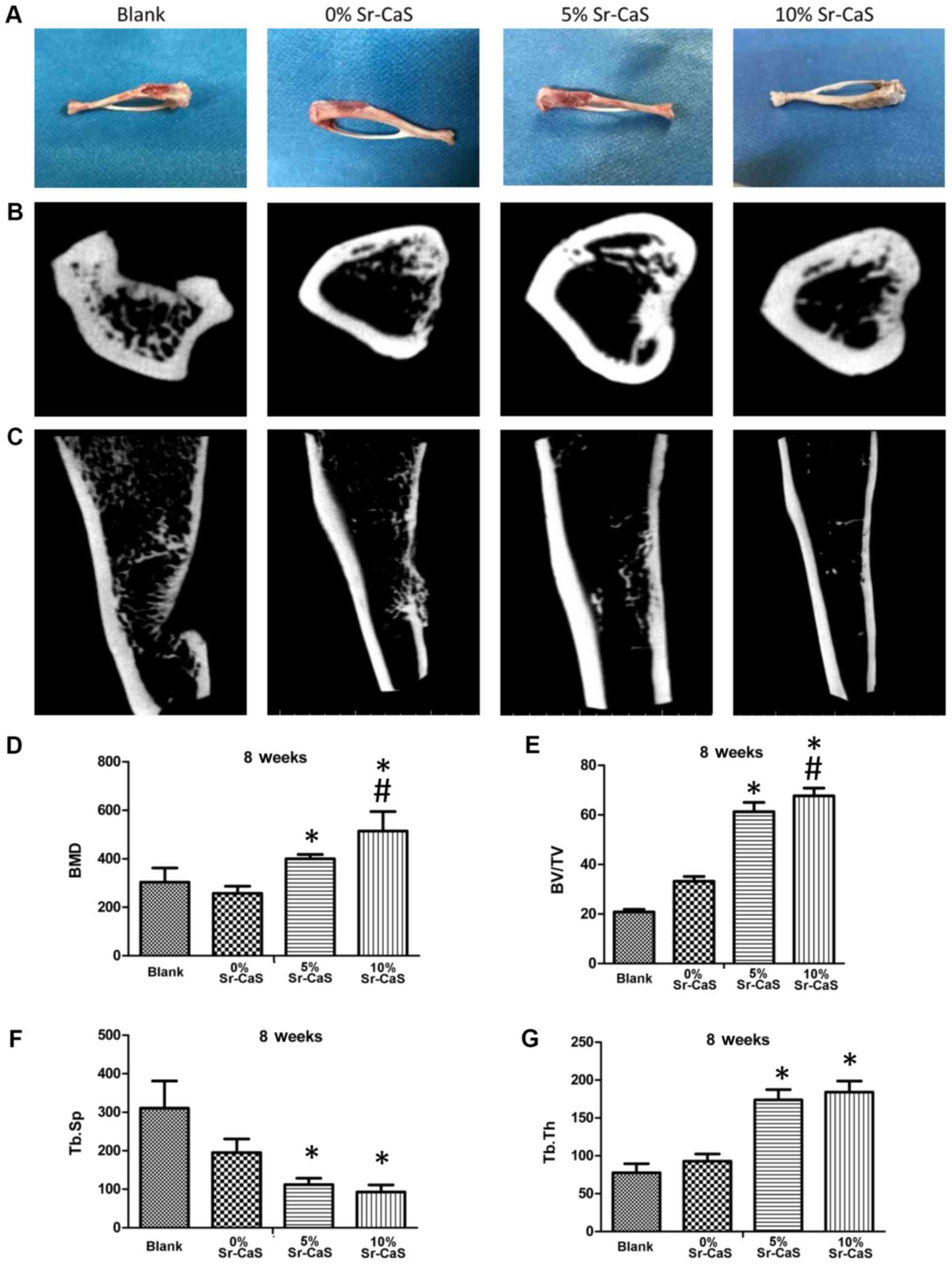 | Figure 3.Sr-CaS, as a bone-rebuilding
material, promotes bone repair. (A) After treatment of the rat shin
defects with 0, 5 or 10% Sr-CaS, the bone reconstructive states
were observed. (B and C) The micro-computed tomography method was
used to detect the effect of 0, 5, and 10% Sr-CaS on bone
rebuilding in rats with a shin defect. (B) aerial view, (C)
parallel perspective. The values for BMD (D) BV/TV (E) Tb.Sp (F)
and Tb.Th (G) were calculated. *P<0.05 vs. blank group;
#P<0.05 vs. 5% Sr-CaS group. BMD, bone mineral
density; BV/TV, relative bone volume; Tb.Sp, trabecular separation;
Tb.Th, trabecular thickness; Sr, strontium; CaS, calcium sulphate;
FITC, fluorescein isothiocyanate; OD, optical density. |
The results of microCT examinations also showed
obvious tibia bone defects in the blank group. Moreover, those
defects were severely depressed, indicating their poor degree of
restoration. In the Sr-CaS groups, the bone-marrow cavity at the
defect site was closed, but the periosteum was still thin and the
connection between the two ends remained incomplete. In the 5 and
10% Sr-CaS groups, the periosteum was markedly thickened when
compared with periostea in the 0% Sr-CaS group (Fig. 3B and C).
Values were also obtained for BMD, BV/TV, Tb.Sp and
Tb.Th. Those data revealed that the values for BMD, BV/TV, and
Tb.Th were significantly increased, while the values for Tb.Sp were
significantly decreased in the 5 Sr-CaS and 10% Sr-CaS groups when
compared with those values in the blank group. Furthermore, the
values for BMD and BV/TV in the 10% Sr-CaS group were significantly
increased compared with those in the 5% Sr-CaS group (P<0.05;
Fig. 3D-G).
Sr-CaS improves the tibia bone
defects
To further explore the effect of Sr-CaS on tibia
bone defects, specimens of defective bone tissue were obtained from
the SD rats that had been implanted with 0, 5 or 10% Sr-CaS for 4
and 8 weeks, respectively. The H&E and Masson staining results
showed poor recovery of the bone defects in the blank group, where
there were few new collagen fibers, and the bone calcium content
was low. After the implantation of Sr-CaS, the bone remodeling
process significantly improved, and the bone defects showed signs
of recovery. At the same time, the rate of collagen fiber synthesis
and the bone calcium content increased in conjunction with the
increase in Sr content (Fig.
4).
Sr-CaS accelerates bone repair via the
TGF-β/Smad signaling pathway
To further explore the effects of Sr-CaS on the
expression levels of bone-related factors after creation of a tibia
defect, the defective tissues from the bone defect animal models at
8 weeks post-implantation surgery were collected. IHC assays were
performed to detect the levels of OCN expression in those
tissues. The results showed that OCN expression was
increased in both the 5 Sr-CaS group and 10% Sr-CaS group relative
to its expression in the blank group, and OCN expression was
highest in 10% Sr-CaS group (Fig.
5A). Data from RT-qPCR assays showed that RUNX2, Osterix,
ALP, OCN and BSP expression were significantly
upregulated in the 5 Sr-CaS group and 10% Sr-CaS group relative to
their expression levels in the blank group (P<0.05). OCN
expression was highest in the 10% Sr-CaS group, where it was
significantly increased compared with the 5% Sr-CaS group
(P<0.05; Fig. 5B).
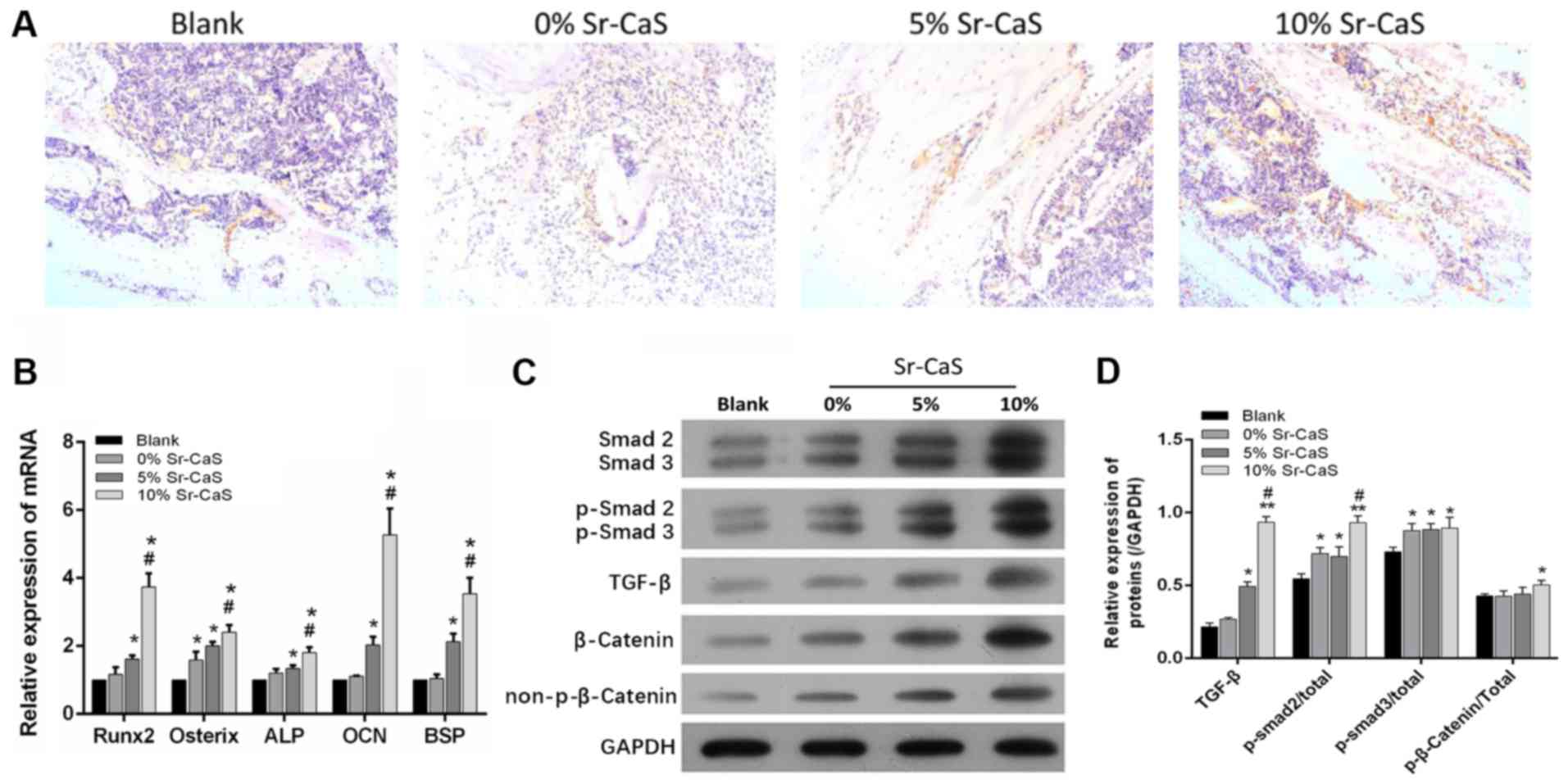 | Figure 5.Sr-CaS accelerates bone repair via
the TGF-β/Smad signaling pathway. (A) The levels of OCN expression
in bone defects at 8 weeks after implantation surgery were assessed
by immunohistochemistry. Magnification, ×100. (B) The levels of
RUNX2, Osterix, ALP, OCN, and BSP mRNA expression in
the defective bone tissues implanted with 0, 5 or 10% Sr-CaS for 8
weeks were analyzed by the reverse transcription-quantitative PCR.
*P<0.05 vs. blank group; #P<0.05 vs. 5% Sr-CaS
group. Smad2/3, p-Smad2/3, TGF-β, β-catenin, and p-β-catenin
expression in bone defects treated with 0, 5 or 10% Sr-CaS for 8
weeks was examined using (C) western blotting and (D) densitometric
analysis. *P<0.05 and **P<0.01 vs. blank group;
#P<0.05 vs. CaS group. Sr, strontium; CaS, calcium
sulphate; TGF, transforming growth factor; ALP, alkaline
phosphatase; p-Smad, phosphorylated-mothers against decapentaplegic
homolog; OCN, osteocalcin; BSP, bone sialoprotein; RUNX2,
runt-related transcription factor 2. |
A previous study has suggested involvement of the
TGF-β/Smad signaling pathway in the bone reconstruction process
(30). Therefore, whether Sr-CaS
might affect the TGF-β/Smad signaling pathway to promote bone
repair was investigated. As shown in Fig. 5C and D, the levels of Smad2/3,
p-Smad2/3, TGF-β, β-catenin, and p-β-catenin protein expression
were all upregulated in the tissues implanted with CaS compared
with tissues in the blank group. Furthermore, the expression levels
of proteins involved in the TGF-β/Smad signaling pathway gradually
increased in conjunction with the increase in Sr content (Fig. 5C and D).
Discussion
In recent years, a number of advances in cytobiology
have been incorporated into the new discipline of bioengineering.
At the same time, tissue engineering performed with diverse
scaffolds has been widely applied in medical science (31–33).
However, more efficient techniques are needed to accelerate
osteogenic differentiation and bone formation in clinical practice.
BMSCs are a class of osteoprogenitor cells (34). Research has suggested that BMSCs
can differentiate into osteoblasts and be used to treat diseases
caused by insufficient bone formation (35).
A previous study has shown that Sr activates the
calcium-sensing receptor of osteoblasts, stimulates the production
of osteoprotein (36,37) and then induces the production of
osteoclasts by inhibiting the expression of receptor activator of
nuclear factor-κB (38). In
addition, Sr inhibits BMSC proliferation and promotes bone
differentiation in a dose-dependent manner (39).
At the same time, when rat osteoclasts were
co-cultured with primary mature rabbit osteoclasts, Sr was shown to
reduce bone resorption and osteoclast formation (40). Numerous clinical and animal studies
have demonstrated that strontium ranelate suppresses bone
absorption and promotes bone formation (41–43).
At present, strontium ranelate has been used in Europe as a
prescribed treatment for senile menopausal osteoporosis (44).
A number of studies have suggested that Sr can
improve the osteoconductive properties of CaS (44,45).
Sr-CaS is a novel bone substitute material with high levels of
biocompatibility and biological activity (26,45).
Sr-enriched biomaterials have shown good efficacy and safety when
used to promote bone formation and remodeling in animal models
(46). In the present study, BMSCs
were treated with 0, 5 or 10% Sr-CaS. The results showed that 5 and
10% Sr-CaS did not adversely affect the proliferation and apoptosis
rates of BMSCs, suggesting the low toxicity of those formulations.
However, 5 and 10% Sr-CaS did promote BMSC migration and osteogenic
differentiation. In addition, bone defect animal models were
created using a previously described method (27,28)
and filled the defects with different materials. When used as a
bone-rebuilding material, Sr-CaS improved the bone defects in
tibias by promoting bone repair, which is consistent with results
in previous studies (27,28). The present study further confirmed
that 10% Sr-CaS could effectively promote bone repair.
RUNX2 is a transcription factor that functions
during osteoblast differentiation. Previous studies showed that
silencing of RUNX2 blocked the differentiation of osteoblasts in
mice (47,48). Patients with cleidocranial
dysplasia caused by heterozygous mutations in the RUNX2 gene
are characterized by having an underdeveloped collarbone, short
stature, excess teeth, a patent fontanelle and other bone
growth-related defects (49).
Osterix is a zinc finger transcription factor present in
osteoblasts and plays a leading role in the osteoblast
differentiation process (49). A
previous study proved that RUNX2 can regulate the levels of Osx
(osterix) (50). ALP is widely
distributed in human skeletal, kidney, liver, intestinal and
placental tissues. OCN is a vitamin k-dependent calcium binding
protein. ALP and OCN are two typical biomarkers of osteoblasts, and
are involved in osteogenic differentiation and the mineralization
of extracellular matrix during bone formation and repair (51,52).
Previous studies have shown that RUNX2 stimulates the
differentiation of mesenchymal stem cells into osteoblasts by
regulating OCN and ALP activity (47,53).
A previous study has also indicated that BSP significantly affects
bone matrix and bone tumor growth (54). In the present study, it was
verified that Sr-CaS dramatically upregulates RUNX2, Osterix, ALP,
OCN and BSP expression, suggesting that it accelerates bone
repair.
TGF-β plays an important role in keeping bone
formation and resorption in balance, and thereby promotes the
differentiation of BMSCs into bone tissue and inhibits osteoclast
formation (55). Smads are
directly involved in the signal transduction processes mediated by
members of the TGF-β superfamily (56). A previous study indicated that
TGF-β receptor conducted by TGF-β can recruit Smads, like Smad2/3
and finally enhance the factor related to bone mineralization,
including RUNXs and osterix (57).
However, TGF-β also has been reported as a double-edged sword in
maintenance of articular cartilage metabolic homeostasis and the
pathogenesis of arthritis (58).
Research suggests that TGF-β participated functions with Smad2/3
(59). Li et al (60) indicate that Smad2/3 can be
activated by TGF-β and then osteogenesis has demonstrated to be
strengthen. Furthermore, β-catenin is one of the most important
factors in osteogenesis (61).
Also, it demonstrated to be modulated by TGF-β (62). In the present study, it was proved
that Sr-CaS upregulated the levels of Smad2/3, p-Smad2/3, TGF-β,
β-catenin and p-β-catenin expression, suggesting that it promotes
bone repair via the TGF-β/Smad signaling pathway.
The present study suggest that Sr-CaS can promote
osteogenic differentiation via the TGF-β/Smad2/3 molecular
signaling pathway both in vitro and in vivo. The
implantation material used in the present study, which contained
10% Sr-CaS as a new component significantly promoted the
improvement and healing of bone defects.
Acknowledgements
Not applicable.
Funding
This research was supported by the Key Projects of
Science and Technology plan of Guangdong province (grant no.
2016B90913004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL and BY designed the experiments, ZL, ZY and HC
conducted the experiments; ZL, YW, HX and XZ analyzed the data; BY
and XZ validated the data analysis. ZL drafted the manuscript, and
BY and XZ revised the manuscript. All authors read and approved the
final version.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Animal Ethics Committee of Southern Medical University. All
experiments on animals were performed according the Animal Care and
Use guidelines established by the committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ishack S, Mediero A, Wilder T, Ricci JL
and Cronstein BN: Bone regeneration in critical bone defects using
three-dimensionally printed beta-tricalcium
phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds
with either dipyridamole or BMP-2. J Biomed Mater Res B Appl
Biomater. 105:366–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia-Gareta E, Coathup MJ and Blunn GW:
Osteoinduction of bone grafting materials for bone repair and
regeneration. Bone. 81:112–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Houdt CI, Tim CR, Crovace MC, Zanotto
ED, Peitl O, Ulrich DJ, Jansen JA, Parizotto NA, Renno AC and van
den Beucken JJ: Bone regeneration and gene expression in bone
defects under healthy and osteoporotic bone conditions using two
commercially available bone graft substitutes. Biomed Mater.
10:0350032015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lazar MA, Rotaru H, Baldea I, Bosca AB,
Berce CP, Prejmerean C, Prodan D and Campian RS: Evaluation of the
biocompatibility of new fiber-reinforced composite materials for
craniofacial bone reconstruction. J Craniofac Surg. 27:1694–1699.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sahoo N, Roy ID, Desai AP and Gupta V:
Comparative evaluation of autogenous calvarial bone graft and
alloplastic materials for secondary reconstruction of cranial
defects. J Craniofac Surg. 21:79–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W and Yeung KW: Bone grafts and
biomaterials substitutes for bone defect repair: A review.
Bioactive Mater. 2:224–247. 2017. View Article : Google Scholar
|
|
7
|
Kenley RA, Yim K, Abrams J, Ron E, Turek
T, Marden LJ and Hollinger JO: Biotechnology and bone graft
substitutes. Pharm Res. 10:1393–1401. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrere F, van Blitterswijk CA and de
Groot K: Bone regeneration: Molecular and cellular interactions
with calcium phosphate ceramics. Int J Nanomedicine. 1:317–332.
2006.PubMed/NCBI
|
|
9
|
Scarano A, Orsini G, Pecora G, Iezzi G,
Perrotti V and Piattelli A: Peri-implant bone regeneration with
calcium sulfate: A light and transmission electron microscopy case
report. Implant Dent. 16:195–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaffer CD and App GR: The use of plaster
of paris in treating infrabony periodontal defects in humans. J
Periodontol. 42:685–690. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hing KA, Wilson LF and Buckland T:
Comparative performance of three ceramic bone graft substitutes.
Spine J. 7:475–490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frenkel SR, Simon J, Alexander H, Dennis M
and Ricci JL: Osseointegration on metallic implant surfaces:
Effects of microgeometry and growth factor treatment. J Biomed
Mater Res. 63:706–713. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu T, Zhang X, Li Z and Peng D:
Management of combined bone defect and limb-length discrepancy
after tibial chronic osteomyelitis. Orthopedics. 34:e363–e367.
2011.PubMed/NCBI
|
|
14
|
Villar CC and Cochran DL: Regeneration of
periodontal tissues: Guided tissue regeneration. Dent Clin North
Am. 54:73–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Z, Li B, Li Q, Huang Z, Yin B, Ma P,
Xu D, Wu Z and Qiu G: Effect of injectable composites of calcium
sulfate and hyaluronate in enhancing osteogenesis. Zhongguo Xiu Fu
Chong Jian Wai Ke Za Zhi. 31:730–737. 2017.(In Chinese). PubMed/NCBI
|
|
16
|
Cao L, Weng W, Chen X, Zhang J, Zhou Q,
Cui J, Zhao Y, Shin JW and Su J: Promotion of in vivo
degradability, vascularization and osteogenesis of calcium
sulfate-based bone cements containing nanoporous lithium doping
magnesium silicate. Int J Nanomedicine. 12:1341–1352. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Liu HY, Lian X, Shi XL, Wang W, Cui
FZ and Zhang Y: Osteogenesis of mineralized collagen bone graft
modified by PLA and calcium sulfate hemihydrate: In vivo study. J
Biomater Appl. 28:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin W, Sun Q and Ma L: Study on the
effects of curculigoside on proliferation, differentiation, and
calcification of mouse osteoblastic MC3T3-E1 cells. World Sci
Technol. 13:852–855. 2011. View Article : Google Scholar
|
|
19
|
Beuerlein MJ and Mckee MD: Calcium
sulfates: What is the evidence? J Orthop Trauma. 24 (Suppl
1):S46–S51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonnelye E, Chabadel A, Saltel F and
Jurdic P: Dual effect of strontium ranelate: Stimulation of
osteoblast differentiation and inhibition of osteoclast formation
and resorption in vitro. Bone. 42:129–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marie PJ, Hott M, Modrowski D, De Pollak
C, Guillemain J, Deloffre P and Tsouderos Y: An uncoupling agent
containing strontium prevents bone loss by depressing bone
resorption and maintaining bone formation in estrogen-deficient
rats. J Bone Miner Res. 8:607–615. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Canalis E, Hott M, Deloffre P, Tsouderos Y
and Marie PJ: The divalent strontium salt S12911 enhances bone cell
replication and bone formation in vitro. Bone. 18:517–523. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Zhao S, Zhu Y, Huang Y, Zhu M,
Tao C and Zhang C: Three-dimensional printing of
strontium-containing mesoporous bioactive glass scaffolds for bone
regeneration. Acta Biomater. 10:2269–2281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poh PS, Hutmacher DW, Stevens MM and
Woodruff MA: Fabrication and in vitro characterization of bioactive
glass composite scaffolds for bone regeneration. Biofabrication.
5:0450052013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren J, Blackwood KA, Doustgani A, Poh PP,
Steck R, Stevens MM and Woodruff MA: Melt-electrospun
polycaprolactone strontium-substituted bioactive glass scaffolds
for bone regeneration. J Biomed Mater Res A. 104:21092016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Xu CP, Hou YL, Song JQ, Cui Z, Wang
SN, Huang L, Zhou CR and Yu B: A novel resorbable
strontium-containing α-calcium sulfate hemihydrate bone substitute:
A preparation and preliminary study. Biomed Mater. 9:0450102014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Chen SK, Li L, Qin L, Wang XL and
Lai YX: Bone defect animal models for testing efficacy of bone
substitute biomaterials. J Orthop Translat. 3:95–104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Del Rosario C, Rodriguez-Evora M, Reyes R,
Delgado A and Evora C: BMP-2, PDGF-BB, and bone marrow mesenchymal
cells in a macroporous beta-TCP scaffold for critical-size bone
defect repair in rats. Biomed Mater. 10:0450082015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu J, Xu L, Li K, Xie N, Xi Y, Wang Y,
Zheng X, Chen X, Wang M and Ye X: Zinc-modified calcium silicate
coatings promote osteogenic differentiation through TGF-β/smad
pathway and osseointegration in osteopenic rabbits. Sci Rep.
7:34402017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamada Y, Ueda M, Hibi H and Nagasaka T:
Translational research for injectable tissue-engineered bone
regeneration using mesenchymal stem cells and platelet-rich plasma:
From basic research to clinical case study. Cell Transplant.
13:343–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bajada S, Harrison PE, Ashton BA,
Cassar-Pullicino VN, Ashammakhi N and Richardson JB: Successful
treatment of refractory tibial nonunion using calcium sulphate and
bone marrow stromal cell implantation. J Bone Joint Surg Br.
89:1382–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quarto R, Mastrogiacomo M, Cancedda R,
Kutepov SM, Mukhachev V, Lavroukov A, Kon E and Marcacci M: Repair
of large bone defects with the use of autologous bone marrow
stromal cells. N Engl J Med. 344:385–386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muschler GF, Nakamoto C and Griffith LG:
Engineering principles of clinical cell-based tissue engineering. J
Bone Joint Surg Am. 86:1541–1558. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guanghua Chen, Guizhi Huang, Hao Lin,
Haojun Wu and Chen H: Bone marrow mesenchymal stem cell
transplatation increases bone mineral density of an ovariectomized
rat model of osteoporosis. Chin J Tissue Engineering Res. 21:49–53.
2017.
|
|
36
|
Cesareo R, Napolitano C and Iozzino M:
Strontium ranelate in postmenopausal osteoporosis treatment: A
critical appraisal. Int J Womens Health. 2:1–6. 2010.PubMed/NCBI
|
|
37
|
Kostenuik PJ and Shalhoub V:
Osteoprotegerin: A physiological and pharmacological inhibitor of
bone resorption. Curr Pharm Des. 7:613–635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen QY, Liang GQ, Lin Y, Liu BL and
Zhao-Hui LI: Effects of strontium ranelate on titanium particles
stimulating mononuclear macrophage to secrete osteolysis factor and
its RANK expression. Rheum Arthritis. 2015.
|
|
39
|
Li Y, Li J, Zhu S, Luo E, Feng G, Chen Q
and Hu J: Effects of strontium on proliferation and differentiation
of rat bone marrow mesenchymal stem cells. Biochem Biophys Res
Commun. 418:725–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gentleman E, Fredholm YC, Jell G,
Lotfibakhshaiesh N, O'Donnell MD, Hill RG and Stevens MM: The
effects of strontium-substituted bioactive glasses on osteoblasts
and osteoclasts in vitro. Biomaterials. 31:3949–3956. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nahass HE, Din NNE and Nasry SA: The
effect of strontium ranelate gel on bone formation in calvarial
critical size defects. Open Access Maced J Med Sci. 5:994–999.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao S, Wang X, Li N, Chen Y, Su Y and
Zhang J: Effects of strontium ranelate on bone formation in the
mid-palatal suture after rapid maxillary expansion. Drug Des Devel
Ther. 9:2725–2734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karatas OH, Toy E, Demir A, Toy H and
Kozacioglu S: Effects of strontium ranelate on sutural bone
formation: A histological and immunohistochemical study. Aust
Orthod J. 32:139–147. 2016.PubMed/NCBI
|
|
44
|
Meunier PJ, Roux C, Seeman E, Ortolani S,
Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM,
Pors-Nielsen S, et al: The effects of strontium ranelate on the
risk of vertebral fracture in women with postmenopausal
osteoporosis. N Engl J Med. 350:459–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Y, Zhou Y, Yang S, Li JJ, Li X, Ma Y,
Hou Y, Jiang N, Xu C, Zhang S, et al: Novel bone substitute
composed of chitosan and strontium-doped α-calcium sulfate
hemihydrate: Fabrication, characterisation and evaluation of
biocompatibility. Mater Sci Eng C Mater Biol Appl. 66:84–91. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Neves N, Linhares D, Costa G, Ribeiro CC
and Barbosa MA: In vivo and clinical application of
strontium-enriched biomaterials for bone regeneration: A systematic
review. Bone Joint Res. 6:366–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang D, Okamura H and Qiu L: Upregulated
osterix expression elicited by Runx2 and Dlx5 is required for the
accelerated osteoblast differentiation in PP2A Cα-knockdown cells.
Cell Biol Int. 42:403–410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Komori T: Runx2, an inducer of osteoblast
and chondrocyte differentiation. Histochem Cell Biol. 149:313–323.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mundlos S, Otto F, Mundlos C, Mulliken JB,
Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, et
al: Mutations involving the transcription factor CBFA1 cause
cleidocranial dysplasia. Cell. 89:773–779. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou H, Choong P, McCarthy R, Chou ST,
Martin TJ and Ng KW: In situ hybridization to show sequential
expression of osteoblast gene markers during bone formation in
vivo. J Bone Miner Res. 9:1489–1499. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van Leeuwen JP, van Driel M, van den Bemd
GJ and Pols HA: Vitamin D control of osteoblast function and bone
extracellular matrix mineralization. Crit Rev Eukaryot Gene Expr.
11:199–226. 2001.PubMed/NCBI
|
|
53
|
Takahashi T, Kato S, Suzuki N, Kawabata N
and Takagi M: Autoregulatory mechanism of Runx2 through the
expression of transcription factors and bone matrix proteins in
multipotential mesenchymal cell line, ROB-C26. J Oral Sci.
47:199–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Riminucci M, Corsi A, Peris K, Fisher LW,
Chimenti S and Bianco P: Coexpression of bone sialoprotein (BSP)
and the pivotal transcriptional regulator of osteogenesis,
Cbfa1/Runx2, in malignant melanoma. Calcif Tissue Int. 73:281–289.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rahman MS, Akhtar N, Jamil HM, Banik RS
and Asaduzzaman SM: TGF-β/BMP signaling and other molecular events:
Regulation of osteoblastogenesis and bone formation. Bone Res.
3:150052015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Leah E: Osteoarthritis: TGF-β overload at
bones of cartilage degeneration. Nat Rev Rheumatol. 9:3822013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Saito M, Ichikawa J, Ando T, Schoenecker
JG, Ohba T, Koyama K, Suzuki-Inoue K and Haro H: Platelet-derived
TGF-β induces tissue factor expression via the smad3 pathway in
osteosarcoma cells. J Bone Miner Res. 33:2048–2058. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li H, Fan J, Fan L, Li T, Yang Y, Xu H,
Deng L, Li J, Li T, Weng X, et al: MiRNA-10b reciprocally
stimulates osteogenesis and inhibits adipogenesis partly through
the TGF-β/SMAD2 signaling pathway. Aging Dis. 9:1058–1073. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen X, Hu C, Wang G, Li L, Kong X, Ding Y
and Jin Y: Nuclear factor-κB modulates osteogenesis of periodontal
ligament stem cells through competition with β-catenin signaling in
inflammatory microenvironments. Cell Death Dis. 4:e5102013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Amini Nik S, Ebrahim RP, Van Dam K,
Cassiman JJ and Tejpar S: TGF-beta modulates beta-Catenin stability
and signaling in mesenchymal proliferations. Exp Cell Res.
313:2887–2895. 2007. View Article : Google Scholar : PubMed/NCBI
|