Introduction
Glioblastoma isthe most common malignant tumor that
originates in the brain. Nowadays, glioblastoma becomes an
intractable refractory tumor with high mortality, owing to its high
degree of aggressiveness (1,2).
Investigating its pathogenesis, administering appropriate therapy
and improving the prognosis are essential but challenging in the
field of neurosurgery and cancer research (3). Despite the development of traditional
treatments, such as surgical tumor removal followed by the
postoperative 40Gy-whole-brain, 15Gy-regional radiotherapy and
chemotherapy (4), the resistance
against chemotherapy and radiotherapy leads to the failure in
obtaining satisfactory therapeutic benefits (5). Given the present data of clinical
trials using molecular targets including monoclonal antibodies such
as bevacizumab, or small molecule inhibitors such as bortezomib
(6), more effective therapies are
urgently neededto combat this disease. Such insight might be
provided through proteomics and biomarker approaches that would
simultaneously ascertain potential candidates for targeting therapy
(7).
The signal transducer and activators of
transcription (STAT) protein family is an ensemble of related
proteinreceptors that could be triggered by specific cytokines.
They serve as carriers in cytokine-receptor interaction and
maintain specific intrinsic intracellular signaling (8,9).
STAT3, as a member of STATs, could be phosphorylated by JAK.
Additionally, STAT3 plays a critical role in a number of cellular
processes such as cell growth and apoptosis. There exists an
intimate relationship between STAT3 and multiple cancers. STAT3 is
reported as being abnormally activated in glioblastoma, which
therefore further activates multiple downstream genes and affects
the growth, apoptosis and invasion of glioblastoma cells (10,11).
AZD0530 is a dual inhibitor of Src family
kinase/Abl. A previous study indicated that in prostate cancer cell
lines, AZD0530 suppressed the activation of Src and blocked cell
growth by inducing G1/S cell cycle arrest (12). Additionally, AZD0530 could reduce
the expression of c-Myc, cyclin D1 and β-catenin, and prevent the
phosphorylation of extracellular signal regulated kinase 1/2 and
glycogen synthase kinase 3b. AZD0530 is also known as a selective
and high efficacy Src-kinase inhibitor (11,13).
AZD0530 mechanistically features cooperation with the Src kinase
and competition of the kinase with the ATP binding site, thereby
inhibiting the kinase activity, and impeding tumor migration,
invasion, proliferation, and division (14). Upon the basis of the authors'
preliminary findings (15), the
present study used the STAT3 small interfering (si)RNA and Src
protein inhibitor AZD0530 to specifically target Src-Abl, and
evaluated the effects of combined therapy on glioblastoma.
Materials and methods
Ethics statement
Animal studies were approved by the Tianjin Huanhu
Hospital Institutional Animal Care and Use Committee.
Reagents and antibodies
AZD0530 was purchased from Selleck Chemicals and
dissolved in dimethyl sulfoxide (DMSO) as a stock solutions of 10
mg/ml, then stored at −20°C. The anti-β-actin, anti-STAT3,
anti-p-STAT3, anti-SRC and p-SRC antibodies were bought from Abcam,
the anti-B cell lymphoma-2 (Bcl-2) antibody was purchased from
Signalway Antibody and the SABC Immunohistochemistry assay kit was
provided by Abcam.
Cell lines and cell cultures
The human glioblastoma cell lines, U87 and U251, are
glioblastoma of unknown origin and were purchased from the American
type culture collection (catalogue number: ATCC HTB-14) and the
European Collection of Authenticated Cell Cultures (cat. no.
09063001), respectively. Both cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin and
0.25 µg/ml amphotericin B in a humidified atmosphere containing 5%
CO2 at 37°C. All reagents were obtained from Invitrogen;
Thermo Fisher Scientific, Inc.
STAT3 siRNA lentivirus vectors
Lentivirus vectors containing siRNA targeted STAT3
(LV-STAT3 siRNA) and an empty control of lentivirus vectortagged
with green fluorescent protein (GFP) and luciferase (LV-empty) were
purchased from Genbank. In brief, cells were infected with the
luciferase lentivirus and implanted into mice. The activity of
luciferase was measured using a small animal in vivo imaging
system (In-Vivo Xtreme, Bruker Corporation). The STAT3 Gene Bank
Accession Number is NC_000017 and the siRNA targeted sense sequence
was as follows (5′-CTTCAGACCCGTCAACAAA-3′).
Cell transfection
The human glioblastoma cell lines U87 and U251
(2×105) in serum-free DMEM medium were transfected with
LV-STAT3 siRNA, LV-empty using Lipofectamine RNAiMAX Reagent
(Thermo Fisher Scienific, Inc.) and treatment with 20 µM AZD0530,
respectively, or combined treatment with LV-STAT3 siRNA and
AZD0530. Cells treated with DMSO served as a negative control.
After 6 h, the serum-free culture medium was replaced with fresh
complete DMEM and continued incubation for 36 h. The expression of
GFP showed 80–90% infection efficiency.
Cell proliferation assay
Cell proliferation was measured by Cell Counting
Kit-8 (CCK-8) assay. Briefly, glioblastoma cell lines U87 and U251
were suspended and diluted to 5×104 cells/ml with DMEM,
and 100 µl cell solution (5,000 cells) were seeded into 96-well
plates, then incubated overnight at 37°C. Next day, cells were
transfected with LV, LV-STAT3 siRNA, LV-empty, treatment with
ADZ0530 or combined treatment with LV-STAT3 siRNA and ADZ0530,
respectively. During eight consecutive days, 10 µl CCK-8 solution
was added to each well for 3 h. The absorbance was measured at 450
nm using a microplate reader. For each group, three duplicate wells
were set up and the data were summarized as mean ± standard
deviation (SD).
Analysis of apoptosis
Cell apoptosis was detected using Annexin V-PE
Apoptosis Detection kit (cat. no. 559763, BD Biosciences). After
treatment 96 h, cells from each group were harvested and adjusted
concentration to 1×106/ml, then 5 µl Annexin V-Alexa
Fluor 647, and 10 µl propidium iodide(PI) were added. After 15 min
in darkness under room temperature, 300 µl PBS was added and
analyzed with FACS Calibur (BD Pharmingen; BD Biosciences). Flow
cytometry data were analyzed with CellQuest software (1998, BD
Pharmingen; BD Biosciences).
Immunoblot assays
Glioblastoma cells were lysed in RIPA buffer (cat.
no. 9800; Cell Signaling, Inc.). Then total proteins (30 µg) were
quantified by the BCA method and analyzed by 8% SDS-PAGE.
Subsequently the polyvinylidene fluoride (PVDF) membranes were
blocked with 5% milk buffer for 2 h at room temperature and then
incubated with the primary antibodies (All1:500 dilution) at room
temperature for 2 h. Then the PVDF membranes were incubated with
HRP-conjugate secondary antibodies (Goat anti Rabbit and goat anti
mice, cat. nos. 31430 and 31460, Thermo Fisher Scientific, Inc.
1:5,000 dilution) at room temperature for 45 min. Blots were
detected with an ECL kit (ECL Substrate Kit, ab133406, Abcam).
Image Pro software 6.0 (Media Cybernetics, Inc.) was used in this
assay to calculate the intensity of each blot.
Establishment of animal model
A total of 130 BALB/c nude mice (half male and
female; 10 weeks old; 20 g) were randomly assigned into five groups
(n=26 for each group), including the negative control group, the
empty vector group, the LV-STAT3 siRNA group, AZD0530 group and the
LV-STAT3 siRNA-AZD0530 combination-therapy group. The mice were
maintained in the place where environment temperature was 25°C, the
humidity was 50%, the light and darkness were alternated for 12 h,
and free water and food were given. Three group's mice were
respectively subjected to LV, LV-STAT3 siRNA transfected U87 cells
and LV-empty transfected U87 cells differential intracranial
injection through the stereotactic approach (16,17).
The AZD0530 group and for the combination groups, after LV-STAT3
siRNA transfected cell intracranial injection, 50 mg/kg AZD0530 was
received via oral gavage 5 times per week for 3 weeks. Tumor sizes
were measured by fluorescent images of whole mice at 7, 14 and 21
days. In view of humane endpoints, the mice whose tumors grew to a
size >1,000 mm3 were sacrificed. The volume (V) of
tumors was calculated using the formula: V=0.5×W2xL.
Additionally, the largest diameter of tumor was measured using a
vernier caliper.
Nuclear magnetic reasonance (NMR)
scanning
Mice were imaged by a small animal in vivo
imaging system. After the final luciferase imaging, the mice were
anesthetized with isoflurane and situated in a magnetic coil in 3T
NMR (GE Healthcare) for two-dimensional scans with T2-weighted
imaging to further assess the position of the tumor and the extent
of its spread. The tumor size was calculated according to previous
studies (18,19). Scanning parameters: 1.2 mm
isotropic spatial resolutions, repetition time=2,500 msec, band
width=203 Hz/px, matrix=256×256×128. The first echo time=72 msec,
echo spacing=12 msec, flip angle=1,200, the scan time=5 min 34 sec.
Three days after the experiment, animals were sacrificed to extract
tumor tissue for immunohistochemical analysis.
Immunohistochemistry
Non-necrotic and cystic tumor tissues of mice models
were selected to make pathological sections (5 µm thickness) and
the sections were subsequently deparaffinized and rehydrated, then
3% hydrogen peroxide was used to remove endogenous peroxide
activity. Antigen retrieval was performed using 0.01 mol/l sodium
citrate buffer at 92–98°C. The sections were blocked with 5% fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.) in PBS at room
temperature for 30 min to avoid non-specific binding, then
anti-p-STAT3 (Tyr705; 0.5 mg/ml; BioLegend, Inc.) and anti-Bcl-2
(1:50; 1 mg/ml; BioLegend, Inc.) were added to sections,
respectively and incubated overnight at 4°C. After washing with
phosphate buffer, the sections were incubated in alkaline
phosphatase-labeled streptavidin working solution (1:200) for 30
min at 37°C. The sections were visualized with DAB, finally, the
sections were counterstained with hematoxylin for 2 min at room
temperature, dehydrated, then mounted. For negative controls,
normal rabbit serum replaced the primary antibody.
Semi-quantitative analysis: Under high magnification (CX23, ×400,
Olympus Corporation), tumor regions were randomly selected for gray
area examination (ImageJ 1.8.0, National Institutes of Health),
with possible values ranging from 0 (black) to 255 (white) and a
greater gray value denotes a higher protein expression, and the
relative protein level was normalized to combinated group.
Statistical analysis
Results were analyzed using SPSS software 13.0 and
compared using one-way analysis of variance. Multi-group
comparisons in this study were carried out by one-way analysis of
variance test followed by a Student-Newman-Keuls post hoc test.
Comparisons between the parametric groups were done using the LSD-t
test. The data was presented as the mean ± SD of three or six
independent experiments was defined to be appropriate. P<0.05
was considered to inidicate a statistically significant
difference.
Results
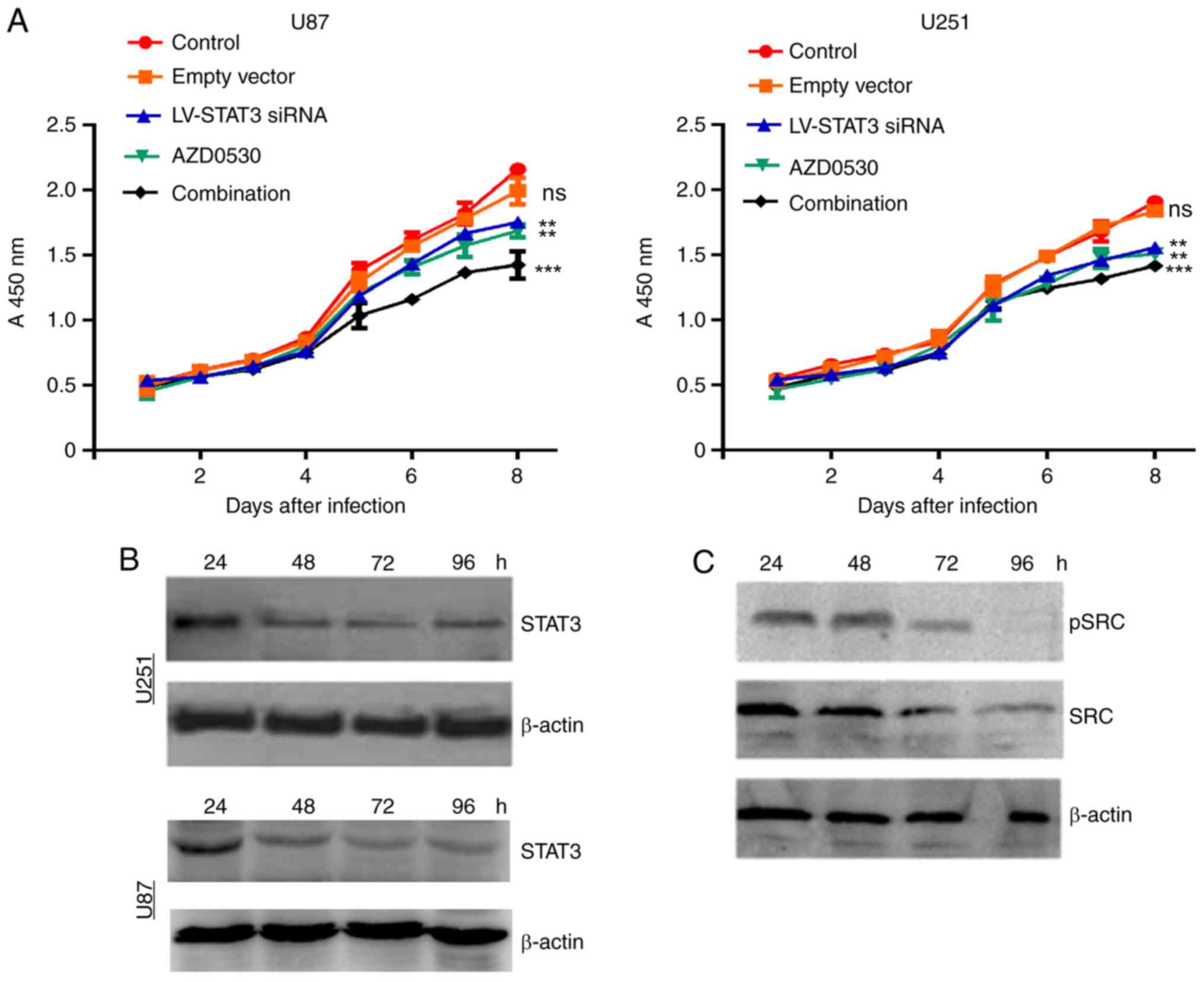
Combined treatment of STAT3 siRNA and
AZD0530 inhibits the proliferation of glioblastoma cells
The CCK-8 assays produced a stable growth curve for
the transfected cell (Fig. 1). The
expression of STAT3, SRC, pSRC decreased gradually within four days
in AZD0530-treated U87 and U251 cells. The AZD0530 gave noticeable
inhibition compared with the control groups.
Similarly, LV-STAT3 siRNA treatment had an obvious
inhibition effect on the proliferation of U87 and U251 cells,
compared with control groups. Interestingly, combination therapy
had a significant inhibitory effect on U87 and U251 cell
proliferation compared with other groups (P<0.001), and the mean
inhibition of tumor growth rate were 28.60 and 36.36%,
respectively. As a comparasion, the curves for the negative control
group and the empty vector cell growth were similar, displaying a
modest effect on tumor growth. Based on this, the present study
further detected the level of both STAT3 and phosphorylated STAT3
after siRNA and AZD0530 combined treatment. It was noticed that the
expression levels of STAT3 was decreased in both U251 and U87 cells
(Fig. 1B). The expression level of
SRC and its phosphorylation level were also detected, and found
that both was decreased in U251 cells (Fig. 1C). Therefore, these results suggest
that the combined treatment inhibited the proliferation of
glioblastoma cells in a SRC-dependent manner.
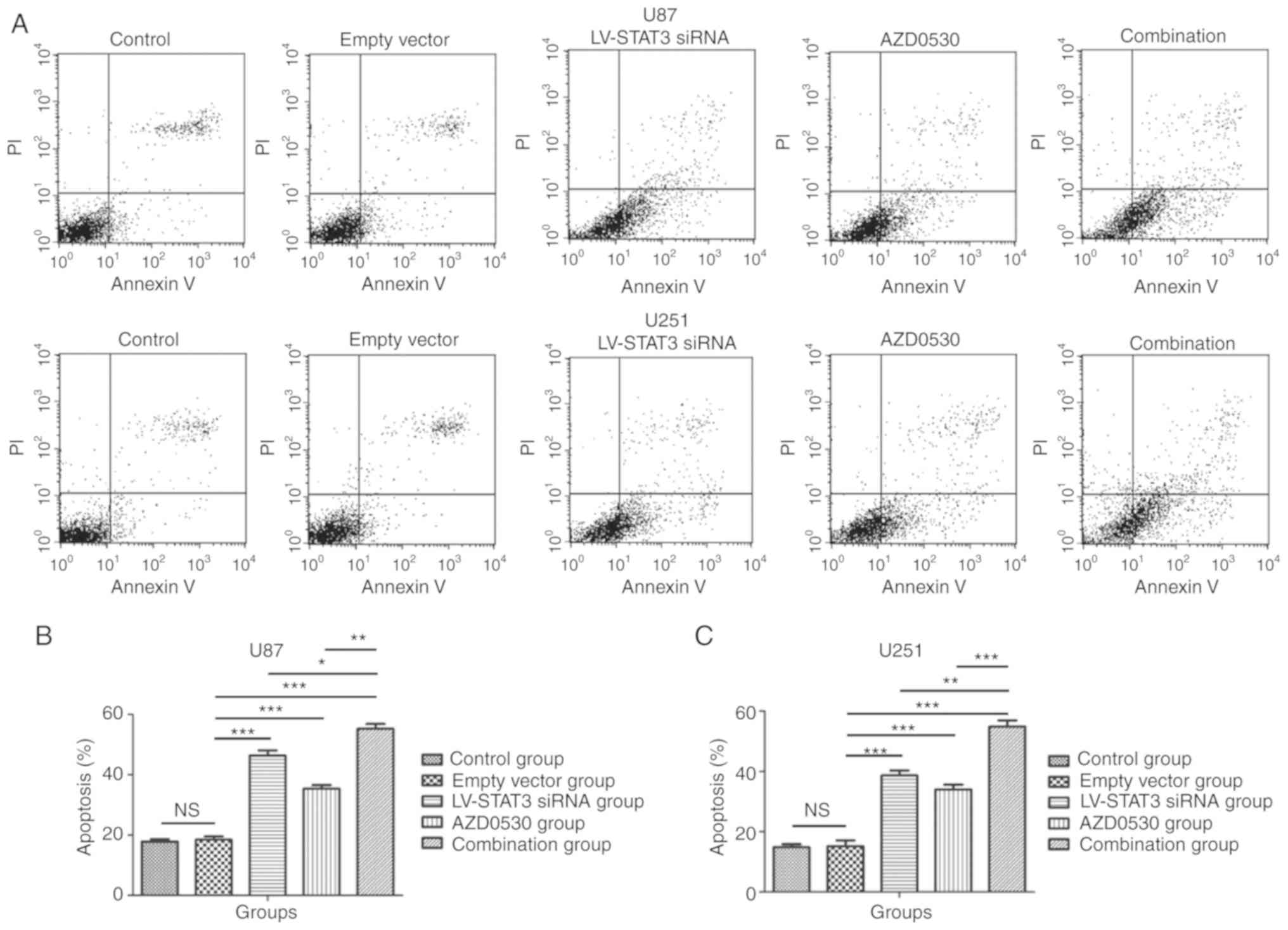
Combined treatment shows pro-apoptotic
effects on glioblastoma cells in vitro
Through flow cytometry assays, it was demonstrated
that the combined treatment with STAT3 siRNA and AZD0530 induced
the apoptosis of glioblastoma cells. After 96 h incubation,
glioblastoma U87 and U251 cells with AZD0530 and STAT3 siRNA
treatment were analyzed through Annexin V-Alexa Fluor 647 and PI
double staining flow cytometry assays, an increased rate of
apoptosis was observed in combined treatment group compared with
the STAT3 siRNA and AZD0530 alone group (P=0.029), and the rate of
apoptosis in the STAT3 siRNA group was increased compared with
AZD0530 treatment alone. There was a statistically significant
difference among STAT3 siRNA group, AZD0530 treated group, and the
combined treatment group (P<0.05). The combined treatment group,
STAT3 siRNA and AZD0530 treated group exhibited a significantly
increasedlevel of apoptosis compared with the empty-vector group
and control group (P<0.05). Whereas no significant difference
was found between the empty vector group and thecontrol group
(P=0.554), and there were similar results between U87 and U251 cell
lines (Fig. 2). All these results
indicate that the STAT3 siRNA and AZD0530 treatment groups showed
significant effectson the apoptosis of glioblastoma cells,
respectively. However, the effect granted by the combined treatment
group was even more notable.
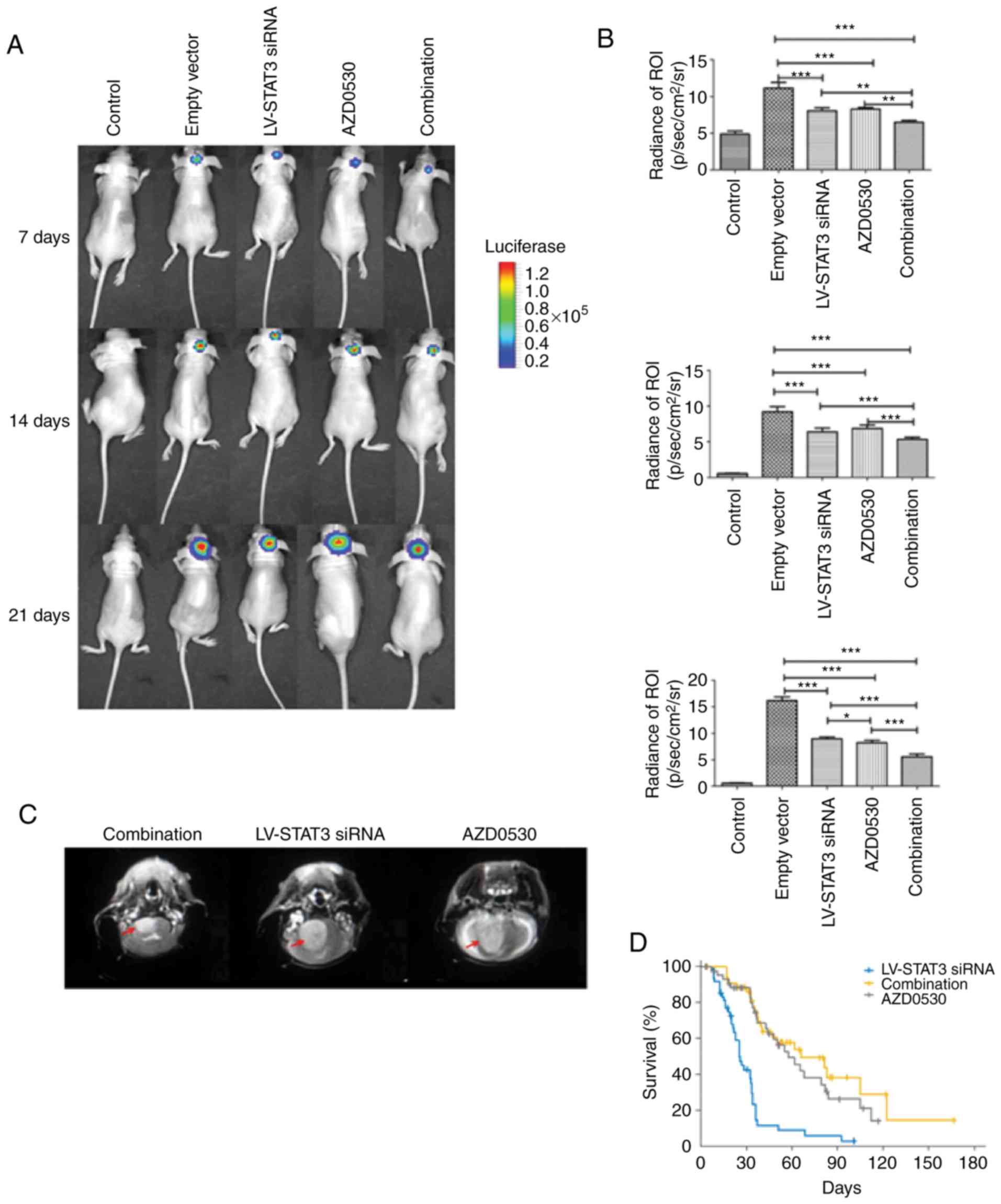
Combined treatment with STAT3 siRNA
and AZD0530 inhibits tumor growth in mice
To further explore the potential synergistic role of
LV-STAT3 siRNA and AZD0503 on glioblastoma, their effects on tumor
growth were assessed in vivo. Aside from the negative
control group, all mice showed xenograft formation in the right
brain caudate nucleus (Fig. 3A-C).
In the first week after the inoculation, tumor formation was
detected and after 2 weeks, a period of accelerated growth was
noted with the degree of growth proportional to time. Larger tumors
may invade contralaterally via the corpus callosum (Fig. 3C). A comparison of the tumor
fluorescence value (Fig. 3B)
showed that for the first week, the LV-STAT3 siRNA group, the
AZD0530 group and the combined treatment group had significantly
lower luminosity compared with the empty vector group (P<0.001).
However, between any two of the three groups, there was no
significant difference (P>0.05). The luminosity was the greatest
for the empty vector group, bearing the largest tumor. Notably,
this luminosity may reflect cellular activity but not tumor growth.
The values of the first week and the second week were similar, but
along with the enlargement of the tumor, the combinational therapy
group showed the least luminescence, followed by the LV-STAT3 siRNA
group and the AZD0530 group; the empty vector group had the maximum
luminosity. The results were statistically significant
(P<0.001). However, the effects of the LV-STAT3 siRNA group and
the AZD0530 group were not statistically different (P=0.517).
Combination therapy results in greater
tumor inhibition
According to the results of the in vivo
assays, the present study found that the luminosity for LV-STAT3
siRNA, AZD0530, or combined treatment groups were decreased
compared with the control (Fig. 3A and
B). Additionally, with tumor enlargement, the luminosity for
LV-STAT3 siRNA and AZD0530 treatment alone group was significantly
decreased compared with the control, and the luminosity for the
combined treatment group was significantly lower than that in other
groups (P<0.001).
As treatment continued, the STAT3 targeted siRNA
loaded lentivirus had a more pronounced and sustained anti-tumor
effect. From the observation of weight changes in mice, the
LV-STAT3 siRNA group had little influence on body weight (data not
shown). While the negative control group had a significantly
greater luminosity relative to the other groups (P<0.05;
Fig. 3C). Interestingly, the data
of K-M survival analysis further confirmed the improved survival of
mice in the combined treatment group (Fig. 3D).
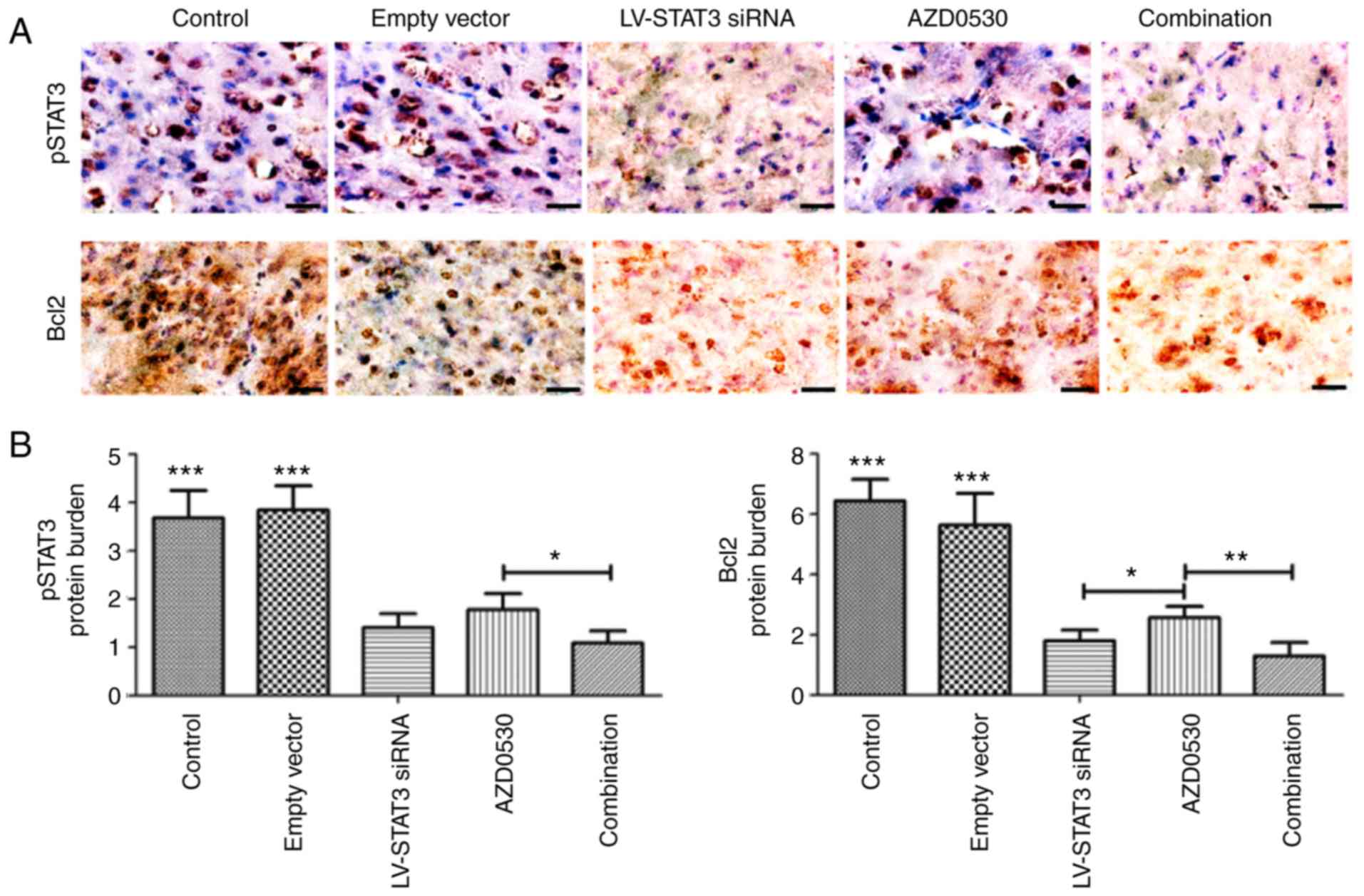
Combined therapy decreases STAT3
phosphorylation and the expression of Bcl-2 in vivo
According to the results of IHC assays, p-STAT3 and
Bcl-2was mainly localized in the cytoplasm of tumor cells,
demonstrated by the light yellow, yellow, or tantint. There were
sometimes mild coloration in the nuclei (Fig. 4A). The p-STAT3 was highly expressed
in the control and empty vector group, but expression was less in
the AZD0530 group, LV-STAT3 siRNA group and the combined treatment
group. The p-STAT3 protein expression value for the negative
control group and the empty vector group was ~three times that of
the AZD0530 alone group, LV-STAT3 siRNA alone group and the
combined treatment group. There were also a significant difference
of values among the AZD0530 group and combined treatment group
(Fig. 4B).
Bcl-2expression was associated withp-STAT3, which
suggested that lentiviral transfection with STAT3-targeted siRNA
produced a significant reduction of p-STAT3 and Bcl-2expression in
tumor tissues (P<0.05).
Discussion
STAT3 abnormal activation has a close relationship
to cancer. Therefore, the abnormal activation of STAT3 could
encourage the genetic transcription of cyclin D1, Bcl-xl, c-Myc,
interleukin (IL)-10, vascular endothelial growth factor (VEGF)
(20), assisting the tumor in
cellular survival, proliferation, angiogenesis and mitochondrial
respiratory chain transfer (21).
STAT3 is abnormally expressed in human malignant
glioblastoma tissues. Suppressing the expression of the STAT3
signal pathway or silencing STAT3 (22) could induce glioblastoma cell
apoptosis and differentiation, and inhibit the proliferation and
angiogenesis (23). siRNA through
a DNA-directed RNA interference (RNAi) approach, enables gene
silencing and expression attenuation, thereby creating a sustained
block of the expression of a particular protein. Therefore, siRNA
has become a potential new nucleic-acid drug (24). RNAi can be used to reduce STAT3
expression in glioblastoma cells and facilitate apoptosis (25). This study used
STAT3-siRNA-lentivirus vectors with glioblastoma transfection and
the subsequent luciferase imaging to monitor the dynamic changes of
the tumor in vivo.
The JAK/STAT pathway is a major signaling pathway
for relaying external signals into the nucleus (26). Cytokines IL-6, IL-10, growth
factors epidermal growth factor, hepatocyte growth factor,
HER2/Neu, VEGF and other mediators such as erythropoietinand
granulocyte-macrophage-colony stimulating factor can induce STAT3
tyrosine phosphorylation and activation. STAT3 tyrosine can be
phosphorylated by three kinds of protein kinases, such as Src, a
non-receptor tyrosine kinases. After tyrosine phosphorylation,
STAT3 forms homo- or heterodimers, enters the nucleus and binds to
specific DNA sequences, and initiates the expression of the target
gene (18). Therefore, blocking
the Src signaling pathway can indirectly compromise the STAT3
signaling pathway (27).
AZD0530 is a Src-Abl dual inhibitor that produces
promising results in preclinical and clinical studies on solid
tumors and osteolytic lesions (28). This study is the first time to the
best of our knowledge that direct siRNA and the indirect AZD0530
was jointly used to block the STAT3 signaling pathway as a part of
a therapeutic strategy.
AZD0530 is an inhibitor of SRC and therefore it
could affect the phosphorylation of STAT3. However, the present
study found that AZD0530 not only can affect the phosphorylation
level of STAT3, but also has an effect on the expression of STAT3.
In general, AZD0530 has a more significant effect on the
phosphorylation of STAT3. The present study hypothesized that
AZD0530 may lead to the ubiquitination degradation of STAT3 to some
extent, but the finer molecular mechanism needs to be confirmed by
further experiments.
Mitochondria are the crucial integrators and
coordinators of both intracellular and extracellular signals that
mediate caspase-dependent and caspase-independent cell death
(29). Loss of the mitochondrial
membrane potential is a prerequisite for mitochondrial-mediated
apoptosis (27). In addition,
members of Bcl-2 family are major regulators of Cytochrome C
release from mitochondria. They participate in downstream caspase
activation and play an important role in the regulation of
glioblastoma cell apoptosis (30,31).
Through flow cytometry assays, it was found that the
LV-STAT3 siRNA group, AZD0530 group and the combined treatment
group had an inhibitory effect in vitro, with the combined
treatment being the most effective. Meanwhile, apoptotic rates for
these groups increased 2.51, 1.92 and 2.99 times in their
respective orders. STAT3-targeting siRNA and Scr-blocker AZD0530
lowered the expression of STAT3 in U87 and U251 cells, and in turn,
promoted apoptosis in glioblastoma. Although currently there are
multiple theories concerning the mechanism of apoptosis initiation,
the present study revealed that LV-STAT3 siRNA might cause
apoptosis through the mitochondrial pathway (15).
In this study, in vivo imaging was used after
tumor inoculation to non-invasively detect the continuous growth of
tumors. A week following the inoculation, obvious tumor formation
and comparable luminescence values were found for the LV-STAT3
siRNA group, the AZD0530 group and the combined treatment group. In
the second week, the empty vector group exhibited the greatest
luminescence, while the combined approach showed the least. The Src
signaling pathway inhibitor AZD0530 of the tumor had its effect
dampened. Moreover, the third week had similar outcomes, with the
combined treatment group giving greater cancer inhibition, while
the influence by AZD0530 diminished further. Although the effect of
STAT3 blocking is noticeable by dynamically observing tumor growth,
the block of STAT3 is weakened owing to in vivo drug
metabolism and tumor drug resistance, whereas STAT3-directed RNAi
elicited a sustained inhibition on tumor growth. This calls for a
stronger emphasis on the underlying mechanisms for AZD0530
resistance in future research endeavors. Immunohistochemistry
assays indicated that p-STAT3was highly expressed in the negative
control group, the empty vector group and the AZD0530 group. The
LV-STAT3 siRNA group and the combined treatment group in turn
showed a decline of expression. LV-STAT3 siRNA continued to block
the STAT3 expression and achieved the purpose of undermining tumor
growth. As the central target molecules of the mitochondrial
apoptosis pathway, Bcl-2 protein (32) and STAT3 protein shared similar
trends in expression. From a different standpoint, it validates the
results from the authors' earlier study on the apoptosis effects of
LV-STAT3 siRNA via the mitochondrial pathway (21).
Recent studies demonstrated that IL-8 derepression
represents an critical and functionally-relevant consequence of
deregulation of the PTEN-STAT3 tumor suppressive pathway in tumor
cells (33,34). The downregulation of STAT3-IL-8
signaling pathway contributes to the proliferation and invasion of
glioblastoma cells. Additionally, STAT3 is known to be activiated
in glioblastoma. These studies further confirmed the present
study's hypothesis.
Integrated results from in vitro and in
vivo studies suggest that in vivo, LV-STAT3 siRNA and
AZD0530 individually caused significant clearance of glioblastoma
while triggering apoptosis. However, a combination-therapeutic
approach was able to render a more striking effect in eliminating
cancer cells. LV-STAT3 siRNA in orthotopic glioblastoma was capable
of continual expression by blocking the STAT3 expression. STAT3 was
one of the targets for cancer therapy, and a recombinant lentivirus
carrying siRNA that silences the STAT3 gene would yield a desired
and sustained inhibition on tumor, and its combination with AZD0530
could augment the cancer inhibition even further. This method
provides a novel and effective way to combatglioblastoma.
In conclusion, this study showed combined therapy of
LV-STAT3 siRNA with AZD0530 could enhance the therapeutic effect
against glioblastoma. Combined treatment inhibited proliferation
and promoted apoptosis of glioblastoma cells in vitro, and
inhibited tumor growth in mice. Mechanistic studies confirmed that
the combined therapy decreased STAT3 phosphorylation and the
expression of Bcl-2 in vivo. Taken together, this study
supports the idea that combination of LV-STAT3 siRNA and AZD0530
could provide a novel and effective strategy to combat
glioblastoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by Tianjin Public
Health Bureau grant (grant no. 2013KG124).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL designed the study; DL, JZ, SC, JL and LW
searched and selected the articles, conducted the data extraction
and did the statistical analysis; QL and LW wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee at
Tianjin Huanhu Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
STAT
|
signal transducer and activators of
transcription
|
|
siRNA
|
small interfering RNA
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
RNAi
|
RNA interference
|
|
Bcl-2
|
B cell lymphoma-2
|
References
|
1
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gray GK, McFarland BC, Nozell SE and
Benveniste EN: NF-κB and STAT3 in glioblastoma: Therapeutic targets
coming of age. Expert Rev Neurother. 14:1293–1306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roa W, Brasher PM, Bauman G, Anthes M,
Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, et al:
Abbreviated course of radiation therapy in older patients with
glioblastoma multiforme: A prospective randomized clinical trial. J
Clin Oncol. 22:1583–1588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hide T, Takezaki T, Nakamura H, Kuratsu J
and Kondo T: Brain tumor stem cells as research and treatment
targets. Brain Tumor Pathol. 25:67–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang F, Brown C, Buettner R, Hedvat M,
Starr R, Scuto A, Schroeder A, Jensen M and Jove R: Sorafenib
induces growth arrest and apoptosis of human glioblastoma cells
through the dephosphorylation of signal transducers and activators
of transcription 3. Mol Cancer Ther. 9:953–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Li C and Lin J: STAT3 as a
therapeutic target for glioblastoma. Anticancer Agents Med Chem.
10:512–519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koskela HL, Eldfors S, Ellonen P, van
Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ,
Olson T, Jalkanen SE, et al: Somatic STAT3 mutations in large
granular lymphocytic leukemia. N Engl J Med. 366:1905–1913. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang XP, Zheng G, Zou L, Liu HL, Hou LH,
Zhou P, Yin DD, Zheng QJ, Liang L, Zhang SZ, et al: Notch
activation promotes cell proliferation and the formation of neural
stem cell-like colonies in human glioma cells. Mol Cell Biochem.
307:101–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan QW, Cheng C, Knight ZA, Haas-Kogan D,
Stokoe D, James CD, McCormick F, Shokat KM and Weiss WA: EGFR
signals to mTOR through PKC and independently of Akt in glioma. Sci
Signal. 2:ra42009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molina JR, Foster NR, Reungwetwattana T,
Nelson GD, Grainger AV, Steen PD, Stella PJ, Marks R, Wright J and
Adjei AA: A phase II trial of the Src-kinase inhibitor saracatinib
after four cycles of chemotherapy for patients with extensive stage
small cell lung cancer: NCCTG trial N-0621. Lung Cancer.
85:245–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin M, Yao Z, Zhao N and Zhang C: TLK2
enhances aggressive phenotypes of glioblastoma cells through the
activation of SRC signaling pathway. Cancer Biol Ther. 20:101–108.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nygaard HB, Wagner AF, Bowen GS, Good SP,
MacAvoy MG, Strittmatter KA, Kaufman AC, Rosenberg BJ, Sekine-Konno
T, Varma P, et al: A phase Ib multiple ascending dose study of the
safety, tolerability, and central nervous system availability of
AZD0530 (saracatinib) in Alzheimer's disease. Alzheimers Res Ther.
7:352015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Luca A, D'Alessio A, Gallo M, Maiello
MR, Bode AM and Normanno N: Src and CXCR4 are involved in the
invasiveness of breast cancer cells with acquired resistance to
lapatinib. Cell Cycle. 13:148–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Xu X, Feng X, Zhang B and Wang J:
Adenovirus-mediated delivery of bFGF small interfering RNA reduces
STAT3 phosphorylation and induces the depolarization of
mitochondria and apoptosis in glioma cells U251. J Exp Clin Cancer
Res. 30:802011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baumann BC, Dorsey JF, Benci JL, Joh DY
and Kao GD: Stereotactic intracranial implantation and in vivo
bioluminescent imaging of tumor xenografts in a mouse model system
of glioblastoma multiforme. J Vis Exp:. (pii): 40892012.PubMed/NCBI
|
|
17
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wadghiri YZ, Li J, Wang J, Hoang DM, Sun
Y, Xu H, Tsui W, Li Y, Boutajangout A, Wang A, et al: Detection of
amyloid plaques targeted by bifunctional USPIO in Alzheimer's
disease transgenic mice using magnetic resonance microimaging. PLoS
One. 8:e570972013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alvarez JV and Frank DA: Genome-wide
analysis of STAT target genes: Elucidating the mechanism of
STAT-mediated oncogenesis. Cancer Biol Ther. 3:1045–1050. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leslie K, Gao SP, Berishaj M, Podsypanina
K, Ho H, Ivashkiv L and Bromberg J: Differential
interleukin-6/Stat3 signaling as a function of cellular context
mediates Ras-induced transformation. Breast Cancer Res. 12:R802010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanseverino I, Purificato C, Gauzzi MC and
Gessani S: Revisiting the specificity of small molecule inhibitors:
The example of stattic in dendritic cells. Chem Biol. 19:1213–1216.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barre B, Vigneron A and Coqueret O: The
STAT3 transcription factor is a target for the Myc and riboblastoma
proteins on the Cdc25A promoter. J Biol Chem. 280:15673–15681.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao DD, Vorhies JS, Senzer N and
Nemunaitis J: siRNA vs. shRNA: Similarities and differences. Adv
Drug Deliv Rev. 61:746–759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao LF, Xu DQ, Wen LJ, Zhang XY, Shao YT
and Zhao XJ: Inhibition of STAT3 expression by siRNA suppresses
growth and induces apoptosis in laryngeal cancer cells. Acta
Pharmacol Sin. 26:377–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Firer MA and Gellerman G: Targeted drug
delivery for cancer therapy: The other side of antibodies. J
Hematol Oncol. 5:702012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Formisano L, Nappi L, Rosa R, Marciano R,
D'Amato C, D'Amato V, Damiano V, Raimondo L, Iommelli F, Scorziello
A, et al: Epidermal growth factor-receptor activation modulates
Src-dependent resistance to lapatinib in breast cancer models.
Breast Cancer Res. 16:R452014. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Wispelaere M, LaCroix AJ and Yang PL:
The small molecules AZD0530 and dasatinib inhibit dengue virus RNA
replication via Fyn kinase. J Virol. 87:7367–7381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bourke LT, Knight RA, Latchman DS,
Stephanou A and McCormick J: Signal transducer and activator of
transcription-1 localizes to the mitochondria and modulates
mitophagy. JAKSTAT. 2:e256662013.PubMed/NCBI
|
|
30
|
Chan SL and Yu VC: Proteins of the bcl-2
family in apoptosis signalling: From mechanistic insights to
therapeutic opportunities. Clin Exp Pharmacol Physiol. 31:119–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gielen PR, Aftab Q, Ma N, Chen VC, Hong X,
Lozinsky S, Naus CC and Sin WC: Connexin43 confers Temozolomide
resistance in human glioma cells by modulating the mitochondrial
apoptosis pathway. Neuropharmacology. 75:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Coleman CB, McGraw JE, Feldman ER, Roth
AN, Keyes LR, Grau KR, Cochran SL, Waldschmidt TJ, Liang C, Forrest
JC and Tibbetts SA: A gammaherpesvirus Bcl-2 ortholog blocks B cell
receptor-mediated apoptosis and promotes the survival of developing
B cells in vivo. PLoS Pathog. 10:e10039162014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdalla F, Nookala A, Padhye SB, Kumar A
and Bhat HK: 4-(E)-{(p-tolylimino)-methylbenzene-1,2-diol} (TIMBD)
suppresses HIV1-gp120 mediated production of IL6 and IL8 but not
CCL5. Sci Rep. 7:81292017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo Y, Zang Y, Lv L, Cai F, Qian T, Zhang
G and Feng Q: [Corrigendum] IL-8 promotes proliferation and
inhibition of apoptosis via STAT3/AKT/NF-κB pathway in prostate
cancer. Mol Med Rep. 19:29702019.PubMed/NCBI
|