Introduction
The prevalence of diabetic nephropathy (DN)
worldwide has markedly increased and DN has become a leading cause
of end-stage renal disease (1). It
is characterized by the appearance of albuminuria, hypertension and
a gradual decline in the glomerular filtration rate (2,3).
Despite emerging strategies, no treatment has been able to reverse
disease progression (1).
Therefore, much effort has been devoted to unraveling the
mechanisms that promote glomerular damage in DN with the hope of
identifying promising therapeutic targets.
Podocytes are terminally differentiated visceral
epithelial cells located on the glomerular basement membrane
outside the glomerular capillaries; their injury or loss leads to
proteinuria and disease progression (4–6). A
large body of evidence has demonstrated that podocyte apoptosis
plays a vital role in the pathogenesis of DN (7–9) and
inhibition of podocyte apoptosis is a promising therapeutic target
for DN (10). However, the concert
mechanisms involved in DN have not been fully elucidated.
Src-associated substrate during mitosis of 68 KDa
(Sam68), also known as K homology (KH) domain-containing, RNA
binding, signal transduction associated 1 (KHDRBS1), is a member of
the signal transduction activator of RNA (STAR) family of
RNA-binding proteins (11,12). Sam68 is a versatile protein with
roles in various cellular processes including RNA metabolism,
apoptosis, and signal transduction (13). Previous results have suggested that
dysregulation of Sam68-regulated splicing events is a key event in
tumor development and progression (14,15).
In addition, some studies have also revealed that Sam68 modulates
nuclear transcription factor kappa B (NF-κB) activation, thus
inducing inflammation (16,17).
Recently, increasing evidence has implicated Sam68 in cell
apoptosis (17,18). However, Sam68 expression and its
biological functions in podocytes are unclear. The aim of this
study was to explore Sam68 expression in vitro in podocytes
treated with high glucose (HG) and its function in HG-induced
podocyte apoptosis.
Materials and methods
Podocyte culture and treatments
A conditionally immortalized mouse podocyte cell
line was kindly provided by Professor Jochen Reiser (Rush
University Medical Center, Chicago, USA). The mouse podocyte
clone-5 (MPC-5) is characterized by expression of T antigen
stimulated by γ-IFN and SV40 heat-sensitive variant gene (19). Cells were amplified at 33°C in
RPMI-1640 medium (Gibco BRL; Thermo Fisher Scientific, Inc.
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco
BRL; Thermo Fisher Scientific, Inc. Waltham, MA, USA) and
recombinant IFN-γ (CYT-358, ProSpec-Tany Technogene Ltd., Rehovot,
Israel). After passaging, the cells were cultured at 37°C under 5%
CO2 for 10–14 days in RPMI-1640 medium without IFN-γ to
induce differentiation. Differentiated podocytes were cultured for
24 h in serum-free DMEM basic (1X) medium (Thermo Fisher
Scientific, Inc.) before being exposed to various experimental
conditions. The cells were divided into the following groups: i) A
normal glucose group (NG, 5.3 mM glucose); ii) a high glucose group
(HG) incubated in basic DMEM (1X) containing various concentrations
of glucose (20, 30 or 40 mM); and iii) a mannitol group (MA)
incubated in NG (5.3 mM) medium supplemented with 24.7 mM
D-mannitol (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as an
osmotic control. The time-points of HG (30 mM) intervention were 6,
12, 24, 48 and 72 h. All the glucose used in this study was
D(+)glucose (Sigma Aldrich; Merck KGaA). All experiments were
performed in triplicate.
Transfection of small interfering
RNA
Three pairs of siRNA sequences that targeted Sam68
and one pair of control-siRNA were designed and synthesized by
RiboBio Co., Ltd. (Guangzhou, China). The siRNA sequences were as
follows: Sam68-siRNA1: GAAAGAACGCGTGCTGATA; Sam68-siRNA2:
GAGGAGAATTATTTGGATT; Sam68-siRNA3: TTACGAAGCCTACGGACAA; and the
product no. of Con-siRNA: siN05815122147. Transfection was carried
out in 6-well plates or 50 cm2 culture plates with
siRNAs (50 nM) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) transfection reagent. The transfection efficiency
was evaluated by assessing the mRNA expression of Sam68 using
qPCR.
Immunofluorescence staining
Podocytes cultured under different conditions (NG,
MA, HG, HG + Sam68-siRNA and Con-siRNA) were fixed with cold
methanol for 20 min at −20°C, and then blocked with 5% bovine serum
albumin for 30 min at room temperature. Then, the cells were
incubated with rabbit anti-Sam68 antibody (1:100; cat. no.
ab109197; Abcam, Cambridge, MA, USA) and goat anti-synaptopodin
antibody (1:100; cat. no. sc21536; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C. Subsequently, the cells were
incubated with donkey anti-goat Alexa Fluor 488 (1:250; cat. no.
A-11055) and donkey anti-rabbit Alexa Fluor 546 (1:250; cat. no.
A10040; both from Life Technologies; Thermo Fisher Scientific,
Inc.) at room temperature. Cells were stained for 10 min with
4′-6-diamidino-2-phenylindole (DAPI; 1:1,000; Sigma Aldrich; Merck
KGaA) for 10 min to visualize the nuclei. Images were captured with
confocal microscopy (LCSM, Zeiss KS 400; Zeiss AG, Oberkochen,
Germany).
RNA extraction and
quantitative-PCR
Total RNA of cultured podocytes under different
conditions (NG, MA, HG, HG + Sam68-siRNA and Con-siRNA) was
extracted using TRIzol Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Then, RNA (1 µg) was reverse-transcribed at 37°C
for 30 min and 85°C for 5 sec using the PrimeScript™ RT reagent Kit
(Takara Biotechnology Co., Ltd., Dalian, China). Quantitative-PCR
was performed using an ABI PRISM7900 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with SYBR
Green Real-Time PCR Master Mix (Takara Biotechnology Co., Ltd.).
Samples were amplified at 95°C for 2 min, 40 cycles at 95°C for 30
sec, 95°C for 5 sec, 60°C for 5 sec, and 72°C for 10 min. The
primers sequences were as follows: Sam68 upstream,
5′-TTATGGCCCATGCTATGGAAGA-3′ and downstream,
5′-AGGTACTCCGTTCAAGTAGGAC-3; GAPDH upstream
5′-AGGTCGGTGTGAACGGATTTG-3′ and downstream,
5′-TGTAGACCATGTAGTTGAGGTCA-3′. In order to confirm amplification
specificity, the PCR products from each primer pair were subjected
to melting curve analysis and subsequent agarose gel
electrophoresis. All reactions were performed in triplicate and
were normalized to GAPDH. Ratio results were calculated based on
the 2−ΔΔCq method (20).
Western blotting
Differentiated podocytes were collected with a cold
plastic cell scraper and lysed with lysis buffer (Beyotime
Institute of Biotechnology) containing 20% SDS, glycerol and 1mM
PMSF. A nuclear and cytoplasmic extraction kit (Nanjing KeyGEN
Biotech Co., Ltd., Nanjing, China) was used to obtain nuclear
proteins from the cultured podocytes. Protein concentrations were
measured with a Pierce bicinchoninic protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Cellular proteins (40 µg) were
separated by 10% SDS-polyacrylamide denaturing gel and transferred
to nitrocellulose membranes, which were then blocked in 5% non-fat
dry milk for 1 h, then, incubated overnight at 4°C with primary
antibodies against Sam68 (1:10,000; cat. no. ab109197; Abcam),
Histone H3 (1:1,000; cat. no. 9715S; Cell Signaling Technology,
Inc., Danvers, MA, USA), Bax (1:500; cat. no. sc7480; Santa Cruz
Biotechnology, Inc.), Bcl-2 (1:1,000; cat. no. ab59348; Abcam) and
GAPDH (1:1,000; cat. no. AP0063; Biogot Technology Co., Ltd.,
Nanjing, China). After being washed in TBS-Tween buffer, the
membranes were incubated with anti-rabbit IgG (1:5,000; cat. no.
7074; Cell Signaling Technology, Inc.) for 1 h at room temperature.
Protein bands were visualized with ECL reagent (Advansta, Inc.,
Menlo Park, CA, USA). The intensities of the identified bands were
quantified with ImageJ 1.47 software (National Institutes of
Health, Bethesda, MD, USA). GAPDH or Histone H3 was used as the
internal control to standardize protein expression.
Analysis of apoptosis by flow
cytometry
Apoptosis of the podocytes was assessed 48 h after
exposure to various treatments (NG, MA, HG, HG + Sam68-siRNA and
Con-siRNA). Apoptosis was determined with an Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit according to
the manufacturer's instructions (Nanjing KeyGEN Biotech. Co.,
Ltd.). Briefly, podocytes were trypsinized and centrifuged at 878 ×
g for 3 min at room temperature. The cells were then suspended in
1X binding buffer and incubated with 5 µl Annexin V-FITC and 5 µl
PI in the dark for 10 min. Cell fluorescence was then assessed by
using a Cell Lab Quanta™ SC flow cytometer (Beckman Coulter, Inc.,
Brea, CA, USA). Cells that stained positive for Annexin V-FITC and
negative for PI were considered apoptotic.
Statistical analysis
The data were analyzed with SPSS17.0 (SPSS, Inc.,
Chicago, IL, USA). All values are presented as the means ± SEM.
Multiple comparisons among the groups were performed with one-way
analysis of variance (ANOVA) with a Bonferroni/Tukey's test or
Dunnett's T3-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sam68 is increased in HG-treated
podocytes in vitro
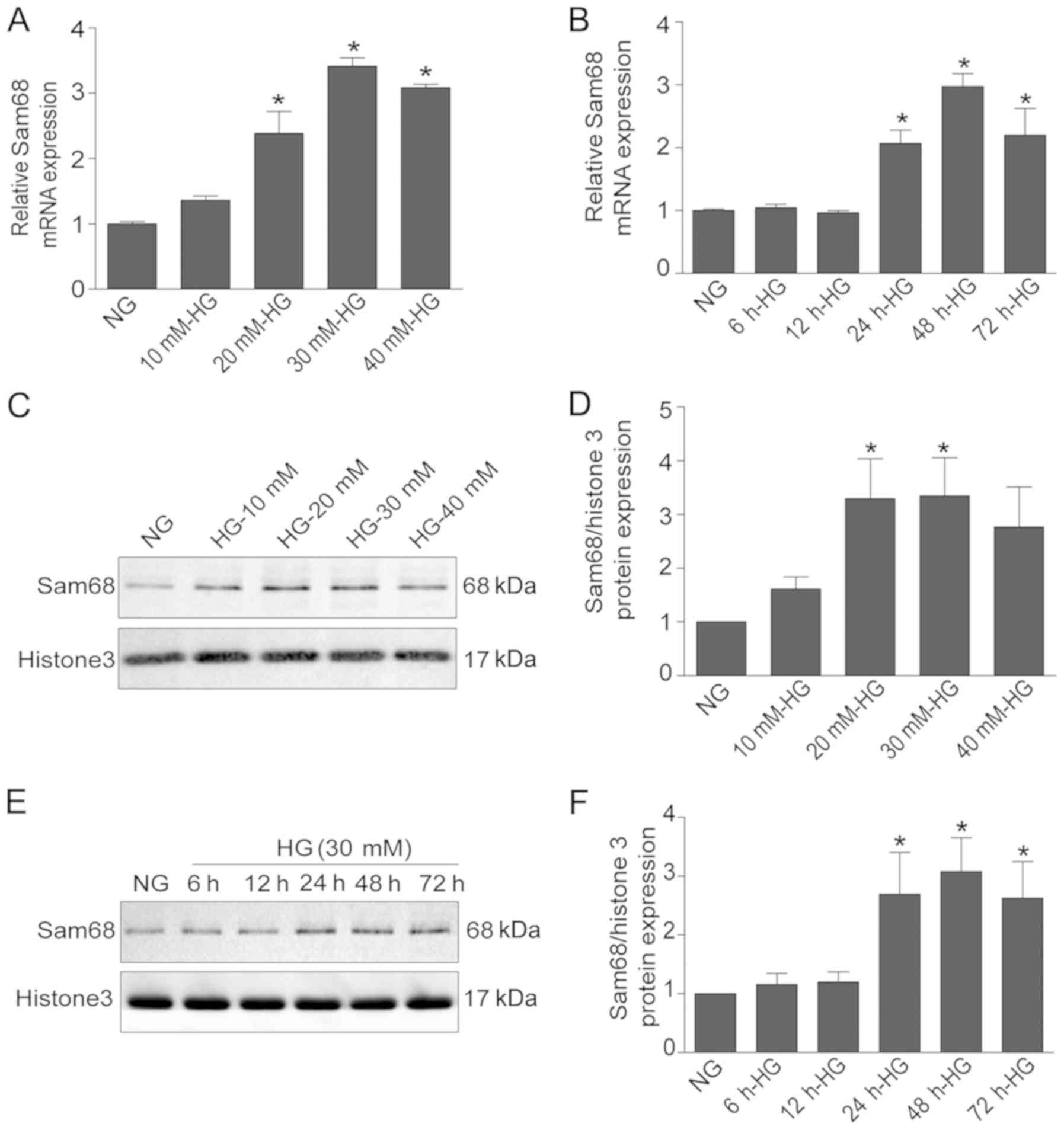
To evaluate the effect of HG on Sam68 expression,
Sam68 expression was assessed under different treatments (NG, MA,
HG, HG + Sam68-siRNA and Con-siRNA) by using real-time PCR,
immunoblotting and immunofluorescence staining. As revealed in
Fig. 1A and B, real-time PCR
revealed that HG stimulation significantly increased the mRNA
levels of Sam68 in a dose- and time-dependent manner (P<0.05).
Western blot analysis also demonstrated that the protein expression
of Sam68 was increased in HG-cultured podocytes after 48 h in a
dose-dependent manner (Fig. 1C).
In a time-response experiment, higher expression of Sam68 protein
was observed in the nuclear extracts of podocytes treated with HG
(30 mM) for 48 h (Fig. 1D).
Densitometric analysis of the immunoblots is presented in Fig. 1E and F. Similar results from
confocal microscopy also demonstrated that Sam68 protein expression
was markedly increased in cultured podocytes treated with 30 mM HG
for 48 h, and this effect was blocked by concurrent treatment with
Sam68 siRNA (Fig. 2).
Collectively, these data indicated that HG treatment increased
Sam68 expression in differentiated mouse podocytes, and this effect
was prevented by concurrent treatment with Sam68 knockdown.
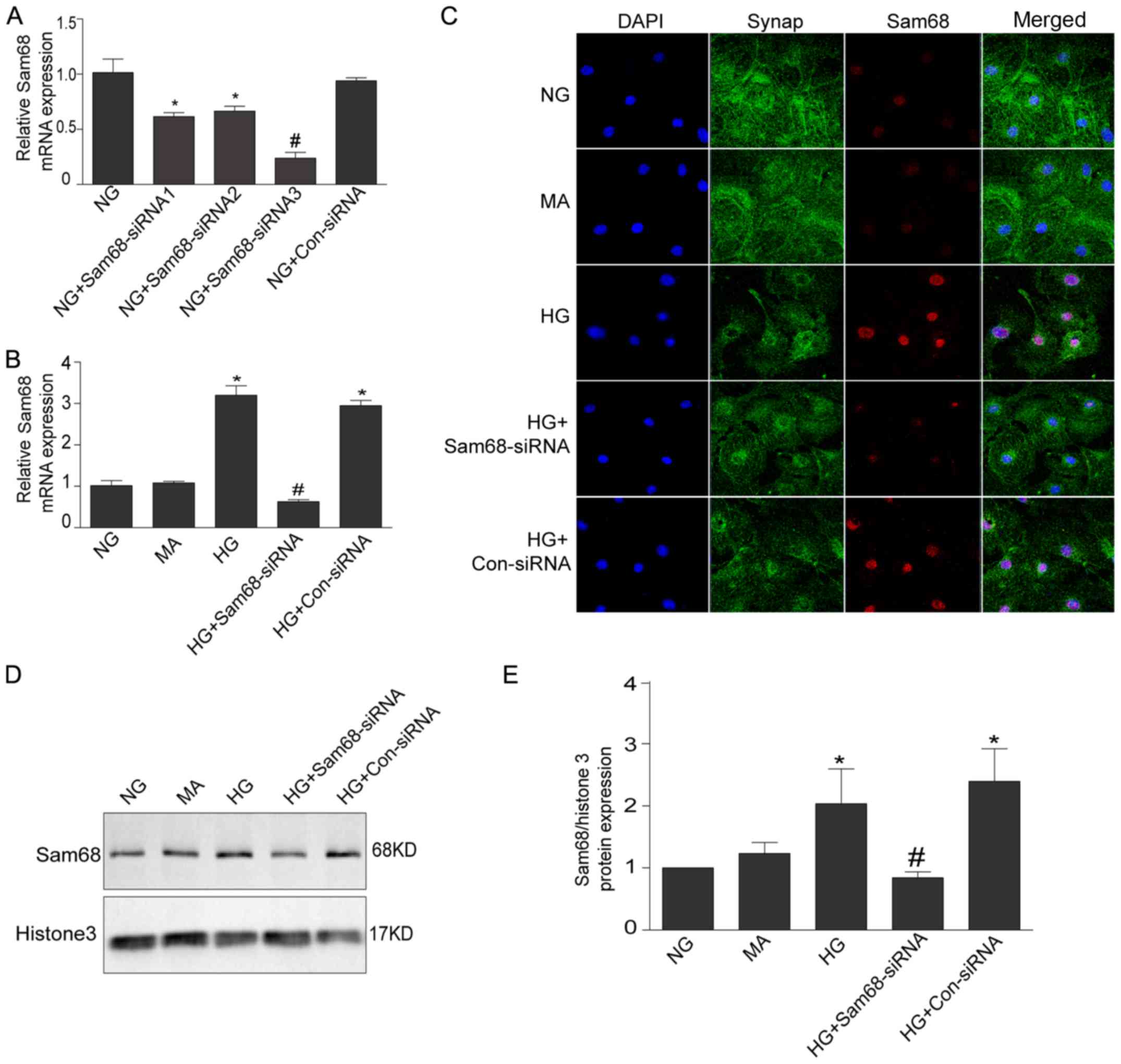 | Figure 2.Increased Sam68 expression in cultured
podocytes with HG is inhibited by Sam68 knockdown. (A) The effects
of siRNA sequences targeting Sam68 on the mRNA expression of Sam68
in in vitro cultured podocytes. Three pairs of siRNA
sequences that targeted Sam68 and one pair of control-siRNA were
designed and synthesized. The third pair of siRNA sequence was
selected for further experiments based on the interference effect.
NG (5.3 mM) group. Values are expressed as the mean ± SEM.
*P<0.05 NG+Sam68-siRNA1 or NG+Sam68-siRNA2 vs. NG;
#P<0.01, NG+Sam68-siRNA1-3 vs. NG+Con-siRNA. (B) The
effects of Sam68 siRNA on the mRNA expression of Sam68 in
HG-cultured podocytes. The mRNA level of Sam68 was examined with
real-time-PCR, and was calculated with the 2−ΔΔCq
method. (C) Double immunofluorescence staining of Sam68 (red),
synaptopodin-identified podocytes (green), DAPI-stained nuclei
(blue) and merged images (purple) in cultured podocytes treated
with HG for 48 h. (D) The protein expression of Sam68 was detected
by immunoblotting in the nuclear extract of podocytes incubated
with HG (30 mM) for 48 h. Histone H3 was used as the nuclear
protein loading control. (E) Densitometric analysis of three
independent experiments displayed in D. NG (5.3 mM) group; HG (30
mM) group; MA, NG (5.3 mM)+MA (24.7 mM) group, as an osmolality
control; HG+Sam68-siRNA, HG (30 mM)+Sam68-siRNA (50 nM) group;
HG+Con-siRNA, HG (30 mM)+non target-siRNA (50 nM) group. Values are
expressed as the mean ± SEM. *P<0.05, HG or HG+Con-siRNA vs. NG;
#P<0.05, HG+Sam68-siRNA vs. HG. Sam68, Src-associated
substrate during mitosis of 68 kDa; HG, high glucose; PCR,
polymerase chain reaction; NG, normal glucose; HG, high glucose;
MA, mannitol. |
Sam68 mediates HG-induced podocyte
apoptosis
In agreement with previous results, the number of
Annexin V-FITC+/PI− (apoptotic) cells in the
HG group (16.18±1.09%) was significantly greater than that in the
NG group (9.2±0.44%) and the mannitol group (10.20±0.29%) at 48 h
(Fig. 3; all P<0.01). As
anticipated, mannitol treatment did not have this effect, thus
indicating that the apoptosis effect under HG did not result from
high osmolarity.
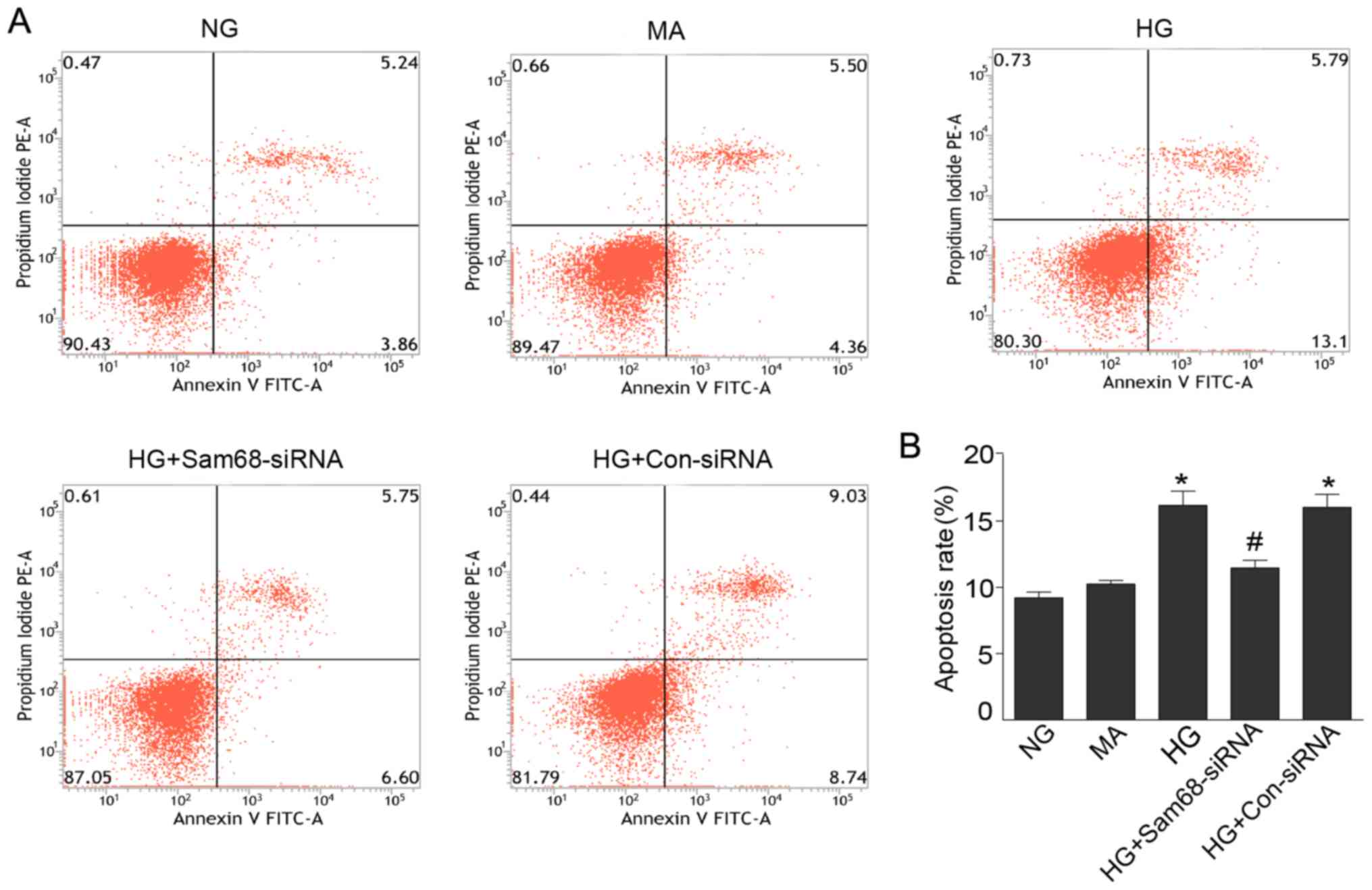 | Figure 3.Sam68 mediates HG-induced podocyte
apoptosis. (A) Podocytes cultured under different conditions were
stained with Annexin V-FITC/PI for flow cytometric analysis. Cells
that stained positive for Annexin V-FITC and negative for PI were
considered apoptotic. (B) Histogram revealing changes in the
percentage of apoptotic cells. NG (5.3 mM) group; HG group (30 mM);
MA, NG (5.3 mM)+MA (24.7 mM) group, as an osmolality control;
HG+Sam68-siRNA, HG (30 mM)+Sam68-siRNA (50 nM) group; HG+Con-siRNA,
HG (30 mM)+non target-siRNA (50 nM) group. Data are expressed as
the mean ± SEM. The experiments were performed in triplicate.
*P<0.01, HG or HG+Con-siRNA vs. NG; #P<0.01,
HG+Sam68-siRNA vs. HG. Src-associated substrate during mitosis of
68 kDa; HG, high glucose; NG, normal glucose; HG, high glucose; MA,
mannitol. |
To assess the effect of Sam68 on apoptosis
regulation, Sam68-siRNA was further used to determine the role of
Sam68 in podocyte apoptosis induced by HG treatment. As presented
in Fig. 3, the apoptosis-inducing
effect of HG was abolished by Sam68-siRNA knockdown (16.18±1.09%
vs. 11.45±0.60%, P<0.01). These results demonstrated that Sam68
mediates HG-induced podocyte apoptosis and may play a proapoptotic
role in HG-induced podocyte apoptosis.
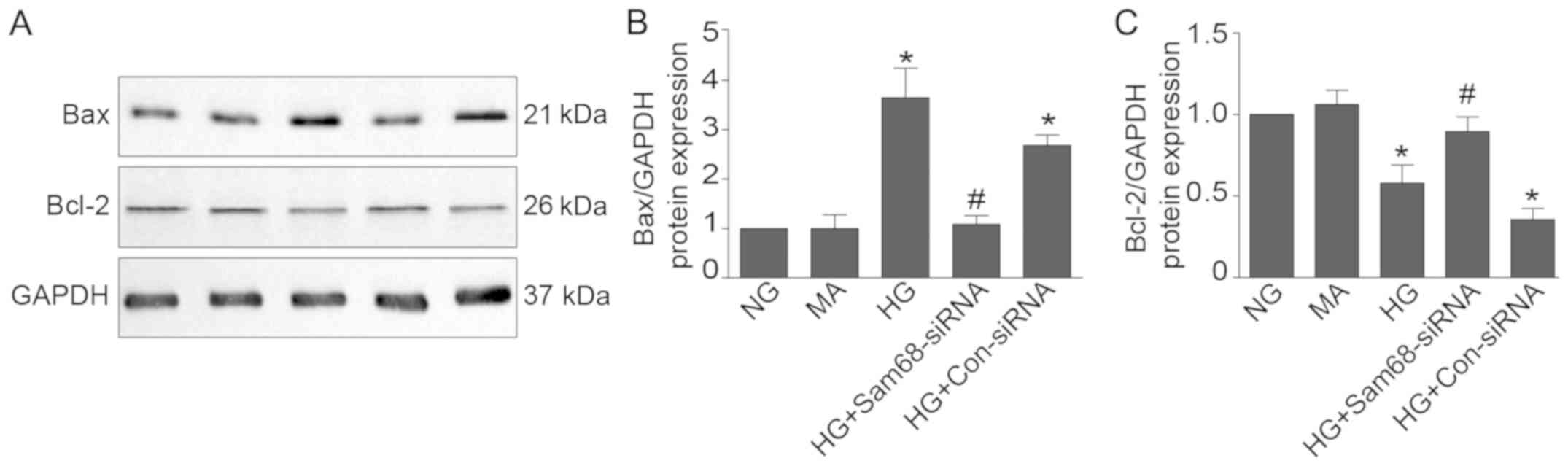
Sam68 regulates Bax and Bcl-2
expression in podocytes under HG
To further investigate the probable mechanism of
Sam68-mediated apoptosis in podocytes treated with HG, the effect
of Sam68 on the protein expression of proapoptotic Bax and
antiapoptotic Bcl-2 was investigated. As revealed in Fig. 4, HG increased the pro-apoptotic Bax
protein and decreased the anti-apoptotic Bcl-2 protein in in
vitro cultured podocytes, in contrast, in Sam68-siRNA-knockdown
podocytes, the pro-apoptotic Bax protein was decreased while the
anti-apoptotic Bcl-2 protein was increased (P<0.01). According
to these results, Sam68 is implicated in the regulation of Bax and
Bcl-2 expression in HG-treated podocytes.
Discussion
Podocytes are highly differentiated cells that play
a crucial role in the function of the glomerular filtration barrier
(4,5). Podocyte depletion due to apoptosis
plays a pivotal role in the initiation and development of DN
(9,21). Previous results have demonstrated
that a decrease in podocyte number due to apoptosis or detachment
contributes to the development of proteinuria and
glomerulosclerosis in DN (8,9,22,23).
Therefore, preventing or inhibiting podocyte apoptosis may be a
promising therapeutic target for treatment of DN.
In the present study, it was first revealed that the
mRNA and protein expression of Sam68 were significantly increased
in HG-cultured podocytes in vitro. Moreover, the effect of
apoptosis induced by HG was abolished by Sam68 knockdown. The first
evidence that HG mediates podocyte apoptosis was provided through
activation of Sam68, a specific target of the Src tyrosine kinase
in mitosis. It was also further determined that Sam68 may mediate
HG-induced apoptosis by significantly increased Bax expression and
decreased Bcl-2 expression (P<0.01). These data indicated that
the Sam68/Bax/Bcl-2 signaling pathway may play a proapoptotic role
in HG-induced podocyte apoptosis.
Sam68, which was originally identified as a
substrate for Src kinase phosphorylation, is a member of the STAR
family of KH domain-containing RNA-binding proteins, which link
signaling pathways to RNA processing (11,12).
Sam68 is broadly expressed in various tissues and cell types, and
it regulates a variety of cellular processes, such as alternative
splicing, gene transcription and signal transduction (14,15,18).
Sam68 has also been identified as an important signaling molecule
that regulates NF-κB activation and apoptosis mediated by the tumor
necrosis factor receptor (17).
Moreover, several tumor-associated proteins, including Bcl-x,
cyclin D1b and CD44 are targets of Sam68 (18,24,25).
Previous studies have demonstrated the importance of Sam68 in
carcinogenesis and have provided evidence that Sam68 promotes tumor
development (26,27), whereas other studies have reported
a tumor-suppressive function of Sam68 (28). The present study demonstrated that
the expression of Sam68 was increased in podocytes treated with HG
in vitro. Knockdown of Sam68 with siRNA in HG-treated
podocytes attenuated apoptosis, as assessed by flow cytometry, thus
indicating that Sam68 may exert a proapoptotic function in
podocytes. Podocyte apoptosis has been reported to play a vital
role in the pathophysiology of DN. Bax and Bcl-2 are two key
markers of cell apoptosis that play critical roles in a variety of
cell systems, including podocytes (29,30).
The present study investigated whether Bax and Bcl-2 are also
involved in Sam68-induced apoptosis in podocytes treated with HG.
The present study demonstrated that the expression of the
pro-apoptotic protein Bax increased, whereas that of the
anti-apoptotic protein Bcl-2 decreased in in vitro
HG-cultured podocytes, and this effect was attenuated by Sam68
knockdown, whereas mannitol had no such effect.
Several limitations of this study must be taken into
account. Firstly, the concrete mechanism on Sam68
expression-triggered apoptosis is still lacking. Does NF-κB or
other signaling pathways contribute to the changes, through
transcriptional induction or inhibition of Bax or Bcl-2? Secondly,
apoptosis was evaluated with only one method (flow cytometry based
on Annexin V staining). Thirdly, only apoptosis was studied, cell
viability was not investigated. These issues aforementioned should
be further studied in future research.
In conclusion, the expression of Sam68 was
investigated in in vitro cultured mouse podocytes with HG
stimulation and it was determined whether this expression led to
podocyte apoptosis. The mechanism of Sam68-mediated apoptosis in
podocytes treated with HG was also further explored. The results
revealed that the apoptosis-promoting effect of HG was abolished by
Sam68 knockdown. HG also significantly increased Sam68 expression
in a time and dose-dependent manner, and this effect was completely
blocked by Sam68 knockdown. Moreover, it was revealed that HG
increased Bax, and decreased Bcl-2, protein expression. These
results indicated that preventing or inhibiting the Sam68/Bax/Bcl-2
pathway may be a promising therapeutic target for treating podocyte
apoptosis under hyperglycemic conditions.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81470930).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LZ and WS conceived the present study. YC performed
the experiments and drafted the manuscript. SLiu, BY, HZ, SLia, JM
and XL interpreted and analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saran R, Robinson B, Abbott KC, Agodoa
LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A,
Eggers PW, et al: US renal data system 2017 annual data report:
Epidemiology of kidney disease in the United States. Am J Kidney
Dis. 71:A72018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Afkarian M, Zelnick LR, Hall YN, Heagerty
PJ, Tuttle K, Weiss NS and de Boer IH: Clinical manifestations of
kidney disease among US adults with diabetes, 1988–2014. JAMA.
316:602–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zelnick LR, Weiss NS, Kestenbaum BR,
Robinson-Cohen C, Heagerty PJ, Tuttle K, Hall YN, Hirsch IB and de
Boer IH: Diabetes and CKD in the United States Population,
2009–2014. Clin J Am Soc Nephrol. 12:1984–1990. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lal MA and Patrakka J: Understanding
podocyte biology to develop novel kidney therapeutics. Front
Endocrinol (Lausanne). 9:4092018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fogo AB: The targeted podocyte. J Clin
Invest. 121:2142–2145. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shankland SJ: The podocyte's response to
injury: Role in proteinuria and glomerulosclerosis. Kidney Int.
69:2131–2147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sifuentes-Franco S, Padilla-Tejeda DE,
Carrillo-Ibarra S and Miranda-Díaz AG: Oxidative stress, apoptosis,
and mitochondrial function in diabetic nephropathy. Int J
Endocrinol. 2018:18758702018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng Y, Chen S, Xu J, Zhu Q, Ye X, Ding D,
Yao W and Lu Y: Dysregulation of lncRNAs GM5524 and GM15645
involved in high-glucose-induced podocyte apoptosis and autophagy
in diabetic nephropathy. Mol Med Rep. 18:3657–3664. 2018.PubMed/NCBI
|
|
9
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim D, Lim S, Park M, Choi J, Kim J, Han
H, Yoon K, Kim K, Lim J and Park S: Ubiquitination-dependent CARM1
degradation facilitates Notch1-mediated podocyte apoptosis in
diabetic nephropathy. Cell Signal. 26:1774–1782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burd CG and Dreyfuss G: Conserved
structures and diversity of functions of RNA-binding proteins.
Science. 265:615–621. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lukong KE and Richard S: Sam68, the KH
domain-containing superSTAR. Biochim Biophys Acta. 1653:73–86.
2003.PubMed/NCBI
|
|
13
|
Najib S, Martin-Romero C, Gonzalez-Yanes C
and Sánchez-Margalet V: Role of Sam68 as an adaptor protein in
signal transduction. Cell Mol Life Sci. 62:36–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frisone P, Pradella D, Di Matteo A,
Belloni E, Ghigna C and Paronetto MP: SAM68: Signal transduction
and RNA metabolism in human cancer. Biomed Res Int.
2015:5289542015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sánchez-Jiménez F and Sánchez-Margalet V:
Role of Sam68 in post-transcriptional gene regulation. Int J Mol
Sci. 14:23402–23419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun W, Qin R, Wang R, Ding D, Yu Z, Liu Y,
Hong R, Cheng Z and Wang Y: Sam68 promotes invasion, migration, and
proliferation of fibroblast-like synoviocytes by enhancing the
NF-κB/P65 pathway in rheumatoid arthritis. Inflammation.
41:1661–1670. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramakrishnan P and Baltimore D: Sam68 is
required for both NF-κB activation and apoptosis signaling by the
TNF receptor. Mol Cell. 43:167–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paronetto MP, Achsel T, Massiello A,
Chalfant CE and Sette C: The RNA-binding protein Sam68 modulates
the alternative splicing of Bcl-x. J Cell Biol. 176:929–939. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mundel P, Reiser J, Zúñiga Mejía Borja A,
Pavenstädt H, Davidson GR, Kriz W and Zeller R: Rearrangements of
the cytoskeleton and cell contacts induce process formation during
differentiation of conditionally immortalized mouse podocyte cell
lines. Exp Cell Res. 236:248–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pagtalunan ME, Miller PL, Jumping-Eagle S,
Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L and Meyer TW:
Podocyte loss and progressive glomerular injury in type II
diabetes. J Clin Invest. 99:342–348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anil Kumar P, Welsh GI, Saleem MA and
Menon RK: Molecular and cellular events mediating glomerular
podocyte dysfunction and depletion in diabetes mellitus. Front
Endocrinol (Lausanne). 5:1512014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen W, Jiang Y, Han J, Hu J, He T, Yan T,
Huang N, Zhang Q, Mei H, Liao Y, Huang Y, Chen B, et al: Atgl
deficiency induces podocyte apoptosis and leads to glomerular
filtration barrier damage. FEBS J. 284:1070–1081. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paronetto MP, Cappellari M, Busa R,
Pedrotti S, Vitali R, Comstock C, Hyslop T, Knudsen KE and Sette C:
Alternative splicing of the cyclin D1 proto-oncogene is regulated
by the RNA-binding protein Sam68. Cancer Res. 70:229–239. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng C and Sharp PA: Regulation of CD44
alternative splicing by SRm160 and its potential role in tumor cell
invasion. Mol Cell Biol. 26:362–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bielli P, Busà R, Paronetto MP and Sette
C: The RNA-binding protein Sam68 is a multifunctional player in
human cancer. Endocr Relat Cancer. 18:R91–R102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song L, Wang L, Li Y, Xiong H, Wu J, Li J
and Li M: Sam68 up-regulation correlates with, and its
down-regulation inhibits, proliferation and tumourigenicity of
breast cancer cells. J Pathol. 222:227–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taylor SJ, Resnick RJ and Shalloway D:
Sam68 exerts separable effects on cell cycle progression and
apoptosis. BMC Cell Biol. 5:52004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin T, Zhang L, Liu S, Chen Y, Zhang H,
Zhao X, Li R, Zhang Q, Liao R, Huang Z, et al: WWC1 promotes
podocyte survival via stabilizing slit diaphragm protein dendrin.
Mol Med Rep. 16:8685–8690. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian X, Tan J, Liu L, Chen S, You N, Yong
H, Pan M, You Q, Ding D and Lu Y: MicroRNA-134-5p promotes high
glucose-induced podocyte apoptosis by targeting bcl-2. Am J Transl
Res. 10:989–997. 2018.PubMed/NCBI
|