Introduction
Spinal epidural adhesions and scars are implicated
as key factors of failed back surgery syndrome, which may cause
duramater compression or epidural tethering, resulting in
persistent backache and leg pain (1). Epidural fibrosis (EF) is thought to
be the main cause of spinal epidural adhesion and scar formation in
patients undergoing laminectomy (2). Therefore, the prevention of fibrosis
and scar formation is key to reducing postoperative recurrence and
sequelae and improving patient quality of life. EF regulation is a
complex mechanism. Hypernomic proliferation of fibroblasts,
accumulation of collagens and the production of pro-inflammatory
factors have been considered to be the primary causes of EF
formation (3,4).
MicroRNAs (miRs) are relatively conserved tiny
non-coding RNA molecules of 18–25 nucleotides in length, which
negatively regulate gene expression by targeting specific motifs at
the 3′-untranslated region (UTR) of mRNA molecules (5). miRs serve important roles in diverse
biological processes including carcinogenesis (6,7),
immune reactions (8,9), intervertebral disc degeneration
(10) and EF (5). A previous study demonstrated that
miR-519d overexpression is associated with the accumulation of
lipids (11). A high fat diet
induces fibrosis of the heart in mice. It was demonstrated that
miR-519d is associated with transforming growth factor (TGF)β
signaling; a master gene in the regulation of fibrosis (12). However, the association between
miR-519d and EF remains unclear.
Bone morphogenetic protein and activin
membrane-bound inhibitor (BAMBI) exhibited an inverse expression
pattern with TGF-β1 and collagen I (col I) mRNAs, suggesting an
inhibitory role of BAMBI in the expression of these two genes
(13). BAMBI overexpression and
injection experiments suppress the growth of human keloid and
excessive accumulation of col I in vitro and in vivo
(13). BAMBI is a component of a
rheostat-like mechanism that is controlled by TGF-β and interleukin
(IL)-2 signaling strength (14).
In lung disease, the overexpression of BAMBI has been observed and
hypothesized to control local inflammation (15). However, the function of BAMBI in EF
formation and its regulation have not yet been comprehensively
studied.
In the present study it was demonstrated that
miR-519d-3p was highly expressed in postoperative patients
suffering from epidural scarring, compared with patients without
scars. Up- and downregulation of miR-519d-3p altered secretion of
inflammatory factors and expression of fibrotic markers in
fibroblasts from postoperative epidural scar patients. It was also
demonstrated that the effect of miR-519d-3p on epidural scar
formation was associated with the BAMBI-mediated TGF-β signaling
pathway.
Materials and methods
Patients and sampling
A total of 64 post-laminectomy patients with lumbar
disc herniation were recruited for the present study, of which 40
patients developed epidural scars and the other 24 patients were
without scars. Their lumbar disc tissues were sampled during the
laminectomy and the removed tissues were collected, labeled and
saved at −80°C for future use. The procedures were conducted at the
Central Hospital of Zibo Mining Refco Group Ltd. (Zibo, China) and
the mean age of the patients was 49 years old (ranging between 38
and 60 years) with a 50:50 sex ratio. The samples were collected
from the patients between May 2016 and May 2017. Patients who had
received chemotherapy or radiotherapy, or had other systemic
disease were excluded. The present study was approved by the Ethics
Committee of Central Hospital of Zibo Mining Refco Group Ltd.
(Zibo, China). All participants gave written consent.
Cell culture
Samples of epidural scar tissues were taken and
human primary epidural fibroblasts were isolated as previously
described (16). The primary
fibroblasts were obtained from the epidural scars using the
enzymatic digestion method. A total of 5 mg obtained tissues were
cut into small pieces and mixed with 3 ml 0.2% collagenase II. An
equal volume of Dulbecco's modified Eagle's medium (DMEM)/F12
supplemented with fetal bovine serum (FBS) (both from Invitrogen,
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added to
terminate the digestion. The mixture was subsequently centrifuged
at 500 × g for 7 min at 37°C. The precipitate was subsequently
resuspended with 2 ml medium, and centrifugation repeated, followed
by further resuspension. The isolated cells were incubated in DMEM
supplemented with 10% FBS, 100 µg/ml streptomycin and penicillin.
Cells were incubated in a humidified incubator with an atmosphere
of 95% air and 5% CO2 at 37°C.
BAMBI overexpression and
transfection
The BAMBI overexpression vectors were obtained from
Addgene, Inc., (Cambridge, MA, USA). The cDNA for BAMBI was
amplified by polymerase chain reaction (PCR). The thermocycling
conditions were set as the following: Denaturation for 60 sec at
95°C, annealing for 45 sec at 56°C and elongation for 60 sec at
72°C. The obtained BAMBI cDNA was subcloned into the pBABE-puro
vector (Cell Biolabs, Inc., San Diego, CA, USA) to generate the
overexpression vector of BAMBI, named pBABE-BAMBI, which was used
at 1.2 µg/ml. Transfection was performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The empty pBABE-puro vector was used as a
control. Cells (2×105/well) were plated in a 6-well
plate in DMEM supplemented with 10% FBS for 24 h to yield 70%
confluency.
Small interfering (si)RNA-mediated
BAMBI silencing and transfection
The cells were then transiently transfected with 30
nM BAMBI siRNA or nontargeting (random) siRNA (Ambion; Thermo
Fisher Scientific, Inc.) using a Silencer™ siRNA Transfection kit
II (Ambion; Thermo Fisher Scientific, Inc.) for 48 h. Scrambled
siRNA was used as a control. Following transfection, total RNA was
extracted for quantification of BAMBI gene expression, viable cells
were counted with a hemocytometer and apoptotic analysis was
conducted using a Cell Counting Kit-8 (CCK-8) assay and flow
cytometry analysis. A light microscope (magnification, ×100) was
used to count viable cells. The sequences of the siRNA used in the
present study were BAMBI siRNA (5′-AAUAAAGUUCAUGUAUGGCAA-3′) and
scrambled siRNA (5′-AACCCCGUUCAUGUAUGGCAA-3′).
Reverse transcription-quantitative
(RT-q)PCR assay
Total RNA was isolated from transfected cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and treated with DNaseI (Promega Corporation, Madison, WI,
USA). RT was performed by using the Multiscribe RT kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and random hexamers or
oligo(dT). The RT conditions were 10 min at 25°C, 30 min at 48°C
and a final step of 5 min at 95°C. qPCR was performed with SYBR
Premix Ex Taq II (Tli RNaseH Plus; Takara Biotechnology, Dalian,
China) by the ABI7500 Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The PCR thermocycling conditions
were as follows: 95°C for 30 sec, 1 cycle; 94°C for 15 sec, 55°C
for 60 sec, 30 cycles. The expression of genes in all groups was
calculated using the 2−ΔΔCq method (17). All experiments were repeated three
times independently. The primers used were as follows: miR-519d-3p
forward, (5′-GCGGAAAAGTGCTTACAGTG-3′), miR-519d-3p reverse,
(5′-ATCCAGTGCAGGGTCCGAGG-3′); U6 forward,
(5′-GCGGAAAAGTGCTTACAGTG-3′), U6 reverse,
(5′-ATCCAGTGCAGGGTCCGAGG-3′).
Transfection of mimic and
inhibitor
miR-519d-3p mimic (5′-CAAAGTGCCTCCCTTTAGAGTG-3′) and
antago-miR-519d-3p (5′-GTTTCACGGAGGGAAATCTCAC-3′) were synthesized
by Invitrogen (Thermo Fisher Scientific, Inc.), and respectively
compounded into concentrations of 20, 40 and 80 nM and 10, 20 and
40 nM using RNase-free water (Takara Biotechnology, Dalian, China),
as well as NC mimic and antago-NC. One day before transfection,
cultured cells were plated in 96-well plate, trypsin digesting and
counting at a density of 1×105. Transfection was
performed at 90% yield using Lipofectamine® 3000 (Thermo
Fisher Scientific, Inc.) for 24 h.
CCK-8 assay
The cells pretreated with different concentrations
of miR-519d-3p mimic (20, 40 or 80 nM) or antago-miR-519d-3p (10,
20 or 40 nM) or BAMBI vector or BAMBI siRNA as described previously
were used for the CCK-8 assay. Cells (2×105 well) were
plated in a 96-well plate and proliferation was determined using a
CCK-8 kit (Vazyme, Piscataway, NJ, USA) according to the
manufacturer's protocol, and subsequently detected the absorbance
at 450 nm wavelength.
Flow cytometry
Flow cytometry was performed to observe the numbers
of living cells. Cells (2×105/ml) cultured as above were
trypsinized and washed with PBS. This pellet was resuspended in 70%
ethanol and kept at −20°C until staining. The samples were then
treated with 1 µl RNase for 30 min at room temperature and stained
with 0.5 ml propidium iodide (PI/Annexin V Cell Apoptosis Detection
kit; Sigma-Aldrich; Merck KGaA) for 1 h at 4°C. Following the
incubation, the cells were washed one time and then analyzed using
a flow cytometer (Accuri C6; BD Biosciences, San Jose, CA,
USA).
ELISA assay
Tumor necrosis factor (TNF)-α and IL-1 production in
cell supernatants was measured using the Rat Betacellulin ELISA kit
(Wuhan Boster Biological Technology, Ltd., Wuhan, China; cat. no.
EK1297) according to the manufacturer's protocol.
Western blotting
The protein used for western blotting was extracted
using a radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Guangzhou, China) supplemented with
protease inhibitors (Beyotime Institute of Biotechnology). The
supernatant was collected and the protein concentration was
measured using a bicinchoninic acid commercial kit (Thermo Fisher
Scientific, Inc.). Equal amounts of protein (25 µg) were separated
on 12–15% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). Membranes were
blocked with 5% non-fat dry milk buffer overnight at 4°C and were
incubated with primary antibodies of 1:500 dilution, including
those against human phosphorylated (p) mothers against
decapentaplegic homolog (Smad)2/3 (cat. no. 5678) and α-smooth
muscle actin (α-SMA; cat. no. 68463) from Cell Signaling
Technology, Inc. (Danvers, MA, USA), and fibronectin (FN; cat. no.
ab158459), col I (cat. no. ab90395) and BAMBI (cat. no. ab200737)
from Abcam (Cambridge, UK) and GAPDH (cat. no. KC-5G4; 1:800;
KangChen Bio-tech, Ltd., Shanghai, China), at 4°C overnight.
Subsequently, membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. ab205718;
1:1,000; Abcam) for 1 h at room temperature and then detected using
a Gel imaging system (Thermo Fisher Scientific, Inc.). ImageJ
software (v1.8.0; National Institutes of Health, Bethesda, MD, USA)
was used to analyze the protein band intensities.
Vector construction and luciferase
reporter assays
The miR-519d-3p targets were predicted by TargetScan
(http://www.targetscan.org/vert_72/),
Microcosm Targets (https://omictools.com/microcosm-targets-tool) and
Miranda Database Tool Software (https://www.winsite.com/miranda/miranda+database+tool/).
BAMBI was used as the research target. The 3′-UTR of BAMBI,
containing the putative miR-519d-3p binding site (BAMBI-3′-UTR-WT),
was amplified from human genomic DNA by PCR. The 3′-UTR of BAMBI
containing a mutant miR-519d-3p binding side (BAMBI-3′-UTR-MUT),
was created by overlap extension of PCR. The BAMBI-3′-UTR-WT and
the BAMBI-3′-UTR-MUT were cloned into pmirGLO Dual-Luciferase miRNA
Target Expression Vector (Promega Corporation) at the SacI
and XhoI site (BAMBI-WT and BAMBI-MUT, respectively). Cells
were seeded into 24-well plates and co-transfected with 200 ng
BAMBI-WT or BAMBI-MUT vector, miR-519d-3p mimic or control mimic
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, cells were harvested and lysed using
lysis buffer (Promega Corporation). The luciferase reporter gene
assay was implemented using the Dual-Luciferase Reporter Assay
System (Promega Corporation) according to the manufacturer's
protocol. Comparison with Renilla luciferase was used for
normalization.
Bioinformatics references
Based on the results of a previous study that
miR-519d-3p is negatively associated with BAMBI (18), the interaction between miR-519d-3p
and BAMBI mRNA was evaluated with the online bioinformatics tools
TargetScan (http://www.targetscan.org/vert_71/) and RNAhybrid
(https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/).
Statistical analysis
All experiments were repeated three times. The
results of multiple experiments are presented as the mean ±
standard deviation. Statistical analyses were performed using SPSS
19.0 statistical software (IBM, Corp., Armonk, NY, USA). The
P-values were calculated using one-way analysis of variance with
Bonferroni's correction. The association of miR-519d-3p level and
the mass of scar was assessed by the Pearson's correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-519d-3p is upregulated in patients
developing epidural scars post laminectomy and levels are
positively associated with mass of the scar
As previously mentioned, following laminectomy, the
removed lumbar disc tissues were collected, labeled and preserved
at −80°C. A total of two years later, an investigation was
undertaken to confirm whether each patient had an epidural scar and
whether there had been a relapse of lumbar disc herniation. A total
of 24 scar-free patients and 40 patients who developed an epidural
scar were randomly selected. The basic information and
clinicopathological parameters of the patients were respectively
listed in Tables I and II. Expression of miR-519d-3p in their
lumbar disc tissues sampled previously was detected using RT-qPCR
analysis and the results demonstrated that miR-519d-3p was
significantly upregulated in patients who had developed an epidural
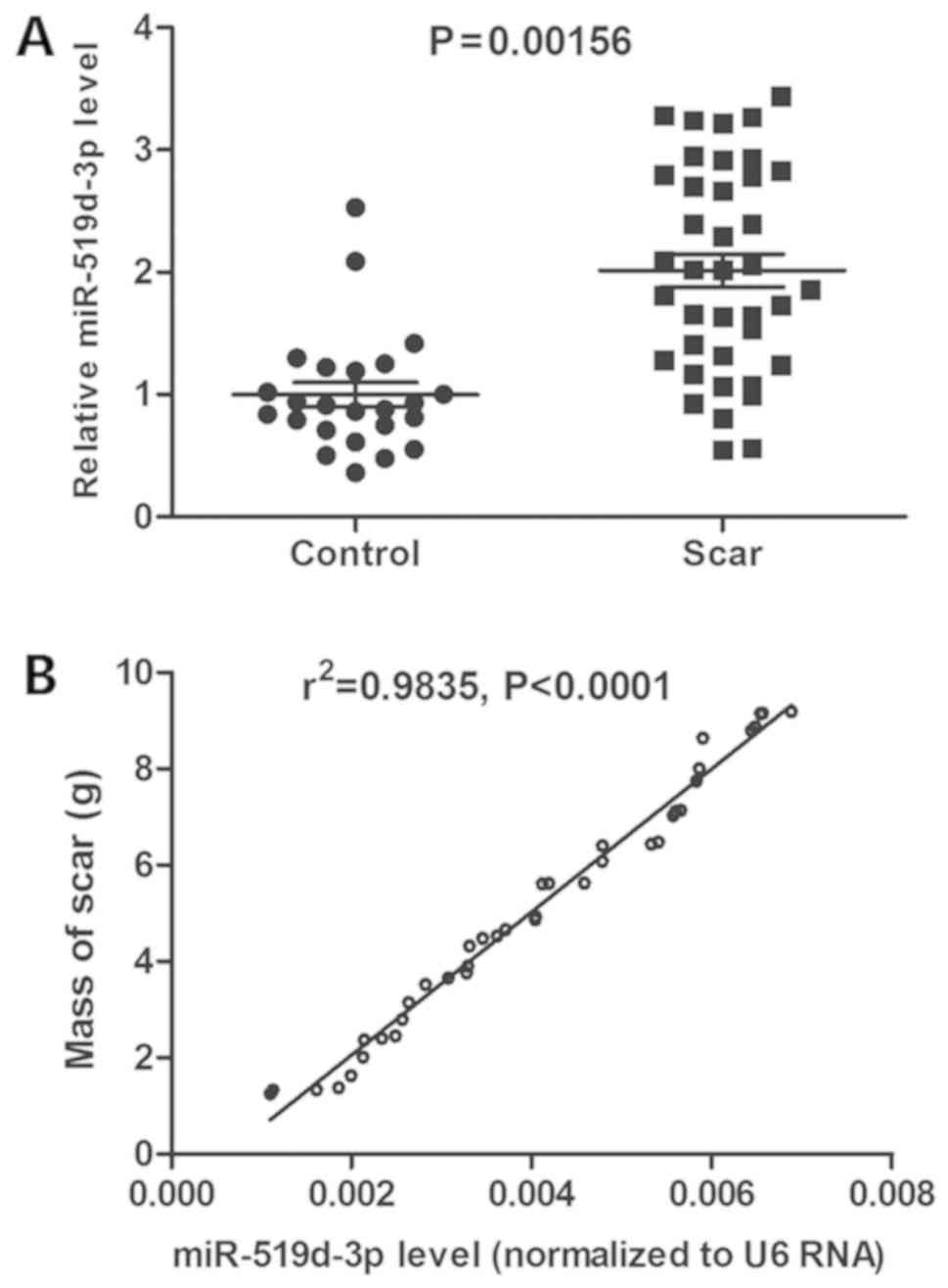
scar, compared with scar-free patients (P<0.01; Fig. 1A). Furthermore, miR-519d-3p level
was positively associated with the mass of scar by Pearson's
correlation coefficient (P<0.0001, r2=0.9835;
Fig. 1B). These results suggested
that lumbar disc miR-519d-3p expression is associated with epidural
scar formation.
 | Table I.Basic information of the patients
with epidural scars. |
Table I.
Basic information of the patients
with epidural scars.
| Indices | Total no. of
patients | No. of patients
with high miR-519d |
|---|
| Age
distribution | 40 (41.2±11.4) | 33 (40.1±10.9) |
| Sex |
|
Male | 23 | 20 |
|
Female | 17 | 13 |
| Location |
|
L3-4 | 11 | 8 |
|
L4-5 | 29 | 25 |
| Enhanced extent (by
CT) |
| I | 8 | 6 |
| II | 12 | 10 |
|
III | 13 | 11 |
| IV | 7 | 6 |
| Duration of
disease |
| <26
weeks | 13 | 10 |
| 26-104
weeks | 22 | 18 |
| >104
weeks | 5 | 5 |
 | Table II.Clinicopathological parameters of
patients with epidural scars (n=40). |
Table II.
Clinicopathological parameters of
patients with epidural scars (n=40).
| Parameters | Prior to
operation | Post operation |
|---|
| High shear rate
blood viscosity | 4.39±0.74 |
5.65±0.56b |
| Low shear rate
blood viscosity | 8.63±1.03 |
11.21±1.00b |
| Plasma
viscosity | 1.27±0.18 |
1.55±0.21a |
| Red cell
aggregation index | 1.88±0.32 |
2.99±0.51b |
| Prostaglandin E2
(pg/ml) | 62.24±16.31 |
50.12±15.72a |
| Urine
hydroxyproline (µg/ml) | 9.55±1.30 | 10.87±1.84 |
| Serum
interleukin-1β (ng/ml) | 155.11±21.62 |
69.33±13.58b |
| Serum
interleukin-10 (ng/l) | 27.34±5.89 |
78.90±26.61b |
miR-519d-3p promotes fibroblast
proliferation, col I accumulation and inflammation
In order to investigate the role of miR-519d-3p in
scar formation in vitro, epidural scar samples were taken
from the patients and primary fibroblasts were isolated. Then,
fibroblasts were transfected with miR-519d-3p mimic at the
concentrations of 20, 40 and 80 nM and miR-519d-3p inhibitor at the
concentrations of 10, 20 and 40 nM, respectively. Following
incubation for 24 and 48 h, cell proliferation was analyzed with a
CCK-8 assay and flow cytometry. It was demonstrated that 20 nM
mimic transfection significantly increased the expression of
miR-519d-3p, while 40 and 80 nM mimic further increased its level
(Fig. 2A). Analysis demonstrated
that upregulation of miR-519d-3p significantly increased cell
viability and proliferation (P<0.05; Fig. 2B and C). The concentration of
inflammatory cytokines, TNF-α and IL-1, were additionally increased
significantly (P<0.05; Fig.
2D). The expression levels of FN, α-SMA and col I proteins,
which were connected with fibroblast formation, were determined by
western blotting. The data demonstrated that miR-519d-3p
overexpression resulted in a significant increase in the expression
levels of FN, α-SMA and col I (P<0.05; Fig. 2E). However, the miR-519d-3p
inhibitor significantly decreased the expression of miR-519d-3p
(Fig. 3A). Furthermore, inhibiting
miR-519d-3p restrained cell viability, proliferation and TNF-α and
IL-1 secretion (P<0.05; Fig.
3B-D). However, the protein levels of FN, α-SMA and col I were
decreased significantly, following inhibition of miR-519d-3p
(P<0.05; Fig. 3E). These data
indicated that miR-519d-3p controlled fibroblast proliferation,
TNF-α and IL-1 secretion, and protein levels of FN, α-SMA and col
I.
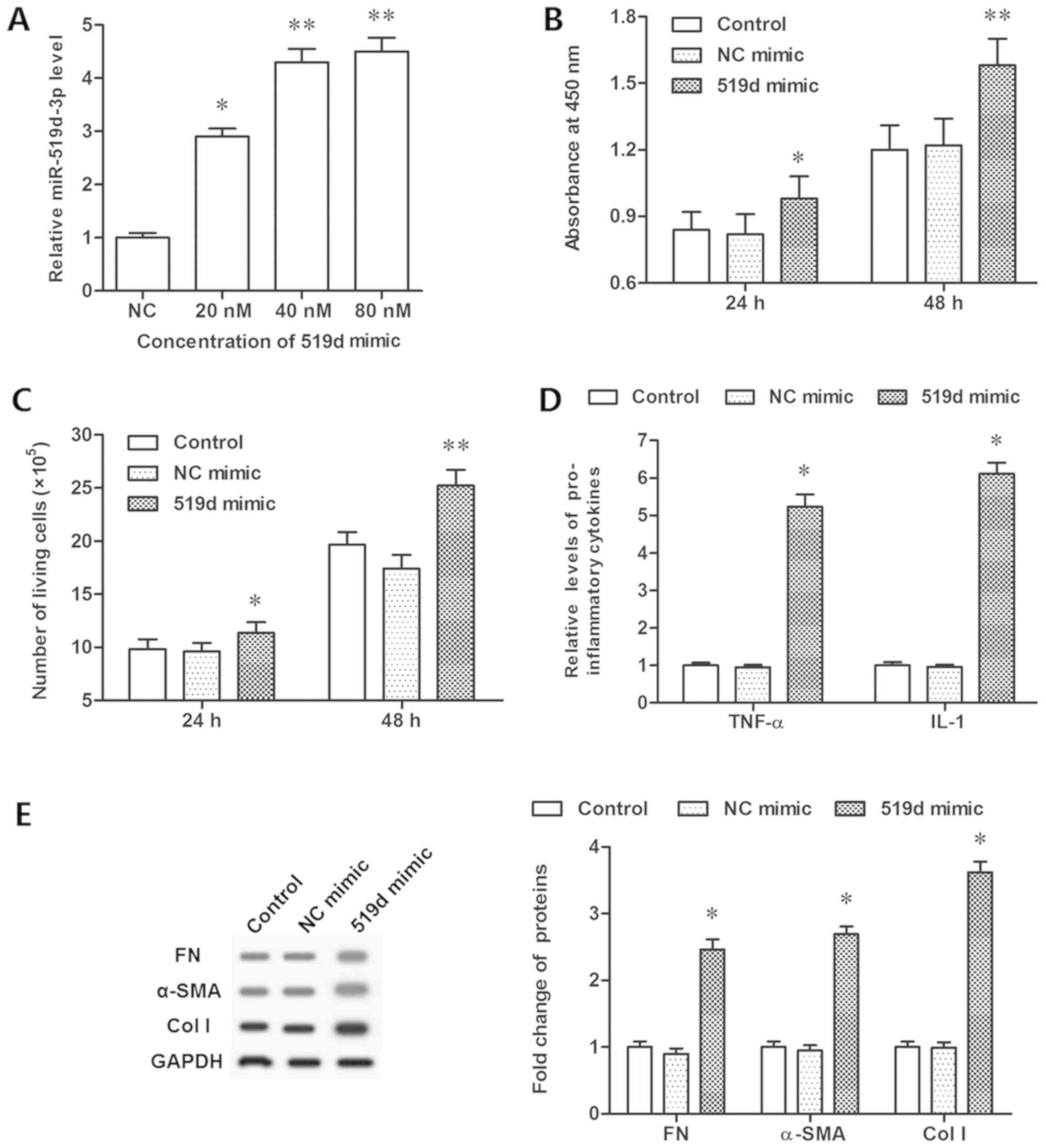 | Figure 2.Overexpression of miR-519d-3p
promotes epidural fibrosis in vitro. (A) Overexpression
efficiencies of miR-519d-3p by different concentrations of the
miR-519d-3p mimic. (B) Cell viability was detected by a Cell
Counting Kit-8 assay following the miR-519d-3p mimic transfection
for 24 and 48 h. (C) Cell proliferation detected with flow
cytometry following miR-519d-3p mimic transfection for 24 and 48 h.
(D) Secretion of TNF-α and IL-1 detected via ELISA following the
miR-519d-3p mimic transfection for 48 h. (E) Expression of FN,
α-SMA, col I proteins detected with western blotting following
miR-519d-3p mimic transfection for 48 h. *P<0.05, **P<0.01
vs. the NC mimic group. NC mimic group, fibroblasts transfected
with negative control mimic. NC, negative control; FN, fibronectin;
TNF, tumor necrosis factor-α; IL, interleukin; SMA, smooth muscle
actin; col I, collagen I; miR, microRNA. |
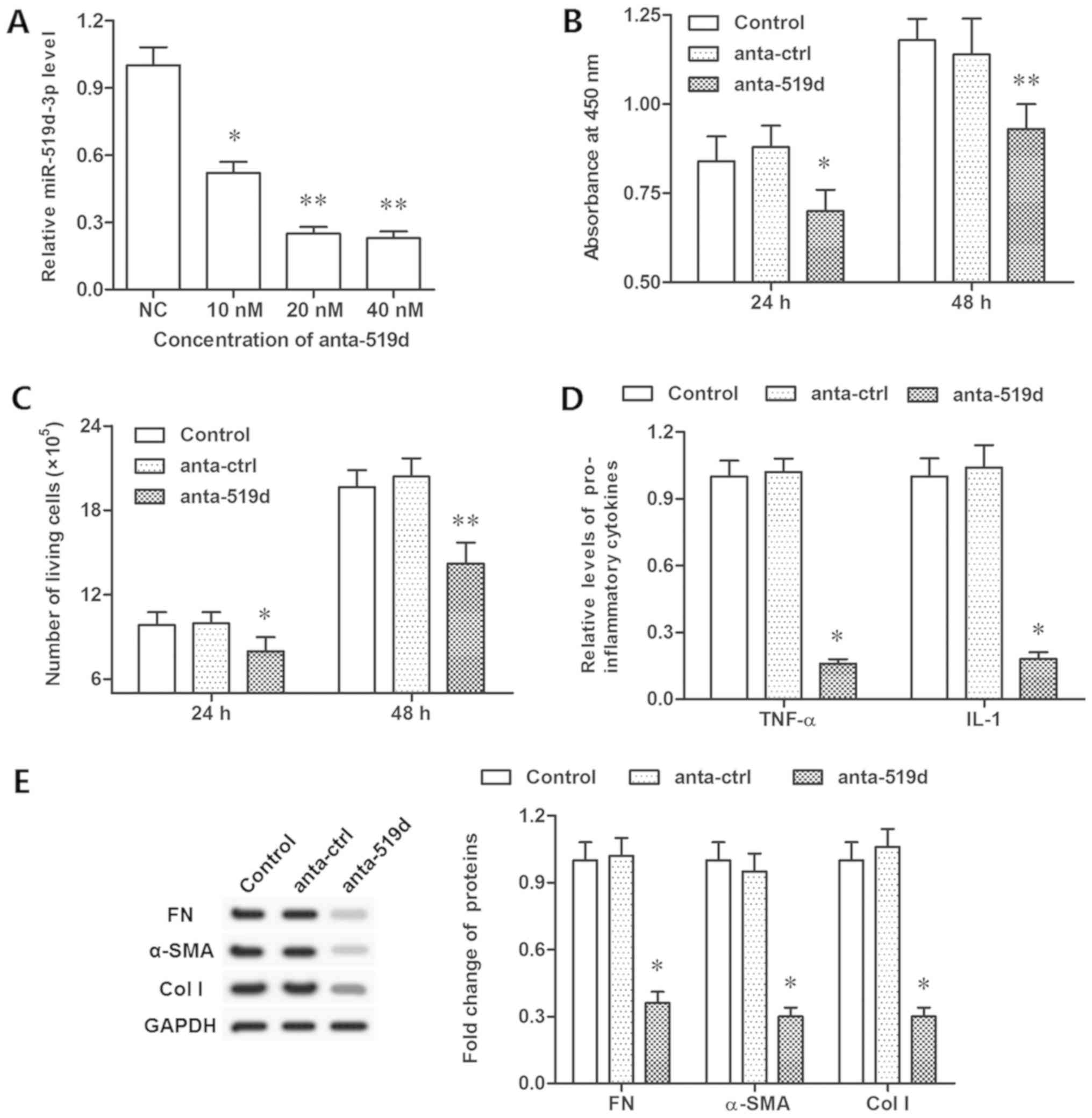 | Figure 3.Inhibition of miR-519d-3p suppresses
epidural fibrosis in vitro. (A) Knockdown efficiencies of
miR-519d-3p by different concentrations of miR-519d-3p antagomir.
(B) Cell viability was detected by a Cell Counting Kit-8 assay
following antago-miR-519d-3p transfection for 24 and 48 h. (C) Cell
proliferation detected with flow cytometry following
antago-miR-519d-3p transfection for 24 and 48 h. (D) Secretion of
TNF-α and IL-1 detected via ELISA following antago-miR-519d-3p
transfection for 48 h. (E) Expression of FN, α-SMA, col I proteins
detected with western blotting following antago-miR-519d-3p
transfection for 48 h. *P<0.05, **P<0.01 vs. the anta-ctrl
group. Anta-ctrl group, fibroblasts transfected with negative
control antagomir; NC mimic group, fibroblasts transfected with
negative control mimic. NC, negative control; FN, fibronectin; TNF,
tumor necrosis factor-α; IL, interleukin; SMA, smooth muscle actin;
col I, collagen I; miR, microRNA. |
BAMBI is a negatively regulated target
of miR-519d
Using bioinformatics tools BAMBI was identified as a
putative target of miR-519d-3p (Fig.
4A). Furthermore, by analyzing the BAMBI protein level and mass
of scar tissue, the BAMBI protein level was demonstrated to be
inversely associated with the mass of scar tissue in patients, as
exhibited in Fig. 4B. In order to
validate the association, luciferase reporter vectors were
constructed containing wt or mut potential binding sequences in the
BAMBI 3′-UTR. The wild-type BAMBI (BAMBI-wt) and mutant BAMBI
(BAMBI-mut) vectors were co-transfected with miR-519d-3p mimic,
into the primary fibroblasts. The results in Fig. 4C revealed that luciferase activity
in BAMBI-wt and miR-519d-3p mimic co-transfected cells was
significantly reduced, compared with the NC mimic group
(P<0.05), whereas, cells co-transfected with BAMBI-mut and
miR-519d-3p mimic demonstrated similar fluorescence intensity
compared with the NC mimic group with no significant difference.
BAMBI-mut has four different bases with BAMBI-wt; CUUU alters to
AAAA (Fig. 4D). In primary
fibroblasts, miR-519d-3p mimic transfection caused a significant
reduction in BAMBI protein expression, however p-smad2/3 were
significantly increased (P<0.05; Fig. 4E). However, antago-miR-519d-3p
transfection significantly upregulated BAMBI protein expression
level and significantly inhibited smad2/3 phosphorylation
(P<0.05; Fig. 4E). These data
demonstrated that BAMBI is a target of miR-519d-3p and miR-519d-3p
negatively regulates BAMBI expression and fibrosis.
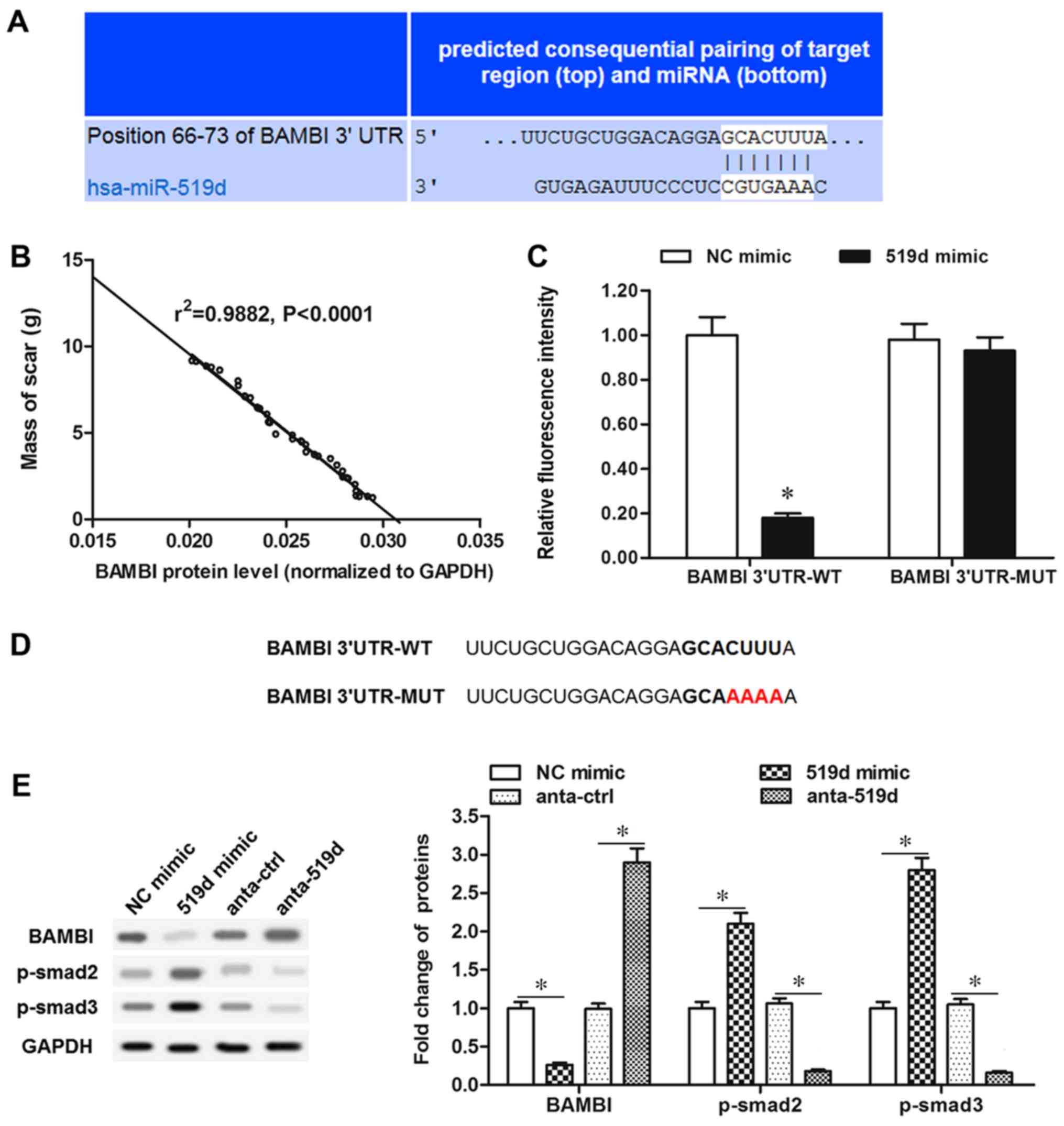 | Figure 4.Bioinformatics and luciferase
reporter gene assays for targeting the association of miR-519d-3p
and BAMBI. (A) Output of bioinformatic prediction demonstrating the
complementary base-pairing of the miR-519d-3p seed sequence and the
3′UTR of BAMBI mRNA. (B) Proportion of BAMBI/total scar. (C)
Luciferase reporter gene assay for targeting the association of
miR-519d-3p and BAMBI. *P<0.05 vs. NC mimic. (D) BAMBI 3′UTR of
the WT and MUT sequence. (E) BAMBI, p-smad2 and p-smad3 protein
levels were detected with western blotting, following miR-519d-3p
mimic or antago-miR-519d-3p transfection. *P<0.05. miR,
microRNA; UTR, untranslated region; NC, negative control; MUT,
mutant; WT, wild-type; BAMBI, bone morphogenetic protein and
activin membrane-bound inhibitor; p-smad, phosphorylated mothers
against decapentaplegic homolog 9 pathway; anta-ctrl, angonist
control. |
BAMBI negatively regulates epidural
scar formation
In order to further clarify the mechanism of
miR-519d-3p, the role of BAMBI in scar formation was investigated.
The pcDNA-BAMBI vector or BAMBI siRNA were transfected into the
primary fibroblasts. Then, the effect of BAMBI on the functions of
fibroblasts, including cell proliferation, secretion of
inflammatory cytokines and associated protein expression levels
were investigated. The results demonstrated that pcDNA-BAMBI
transfection significantly inhibited cell viability and
proliferation (P<0.05; Fig. 5A and
B), TNF-α and IL-1 secretion (P<0.05; Fig. 5C) and significantly reduced
fibrosis associated protein levels, including FN, α-SMA, col I,
p-smad2/3 (P<0.01; Fig. 5D).
Conversely, transfection with the BAMBI siRNA increased cell
viability and proliferation, TNF-α and IL-1 secretion, and enhanced
fibrosis associated protein levels, suggesting an increased degree
of fibrosis. These data indicated that BAMBI possessed a
suppressive effect on scar formation.
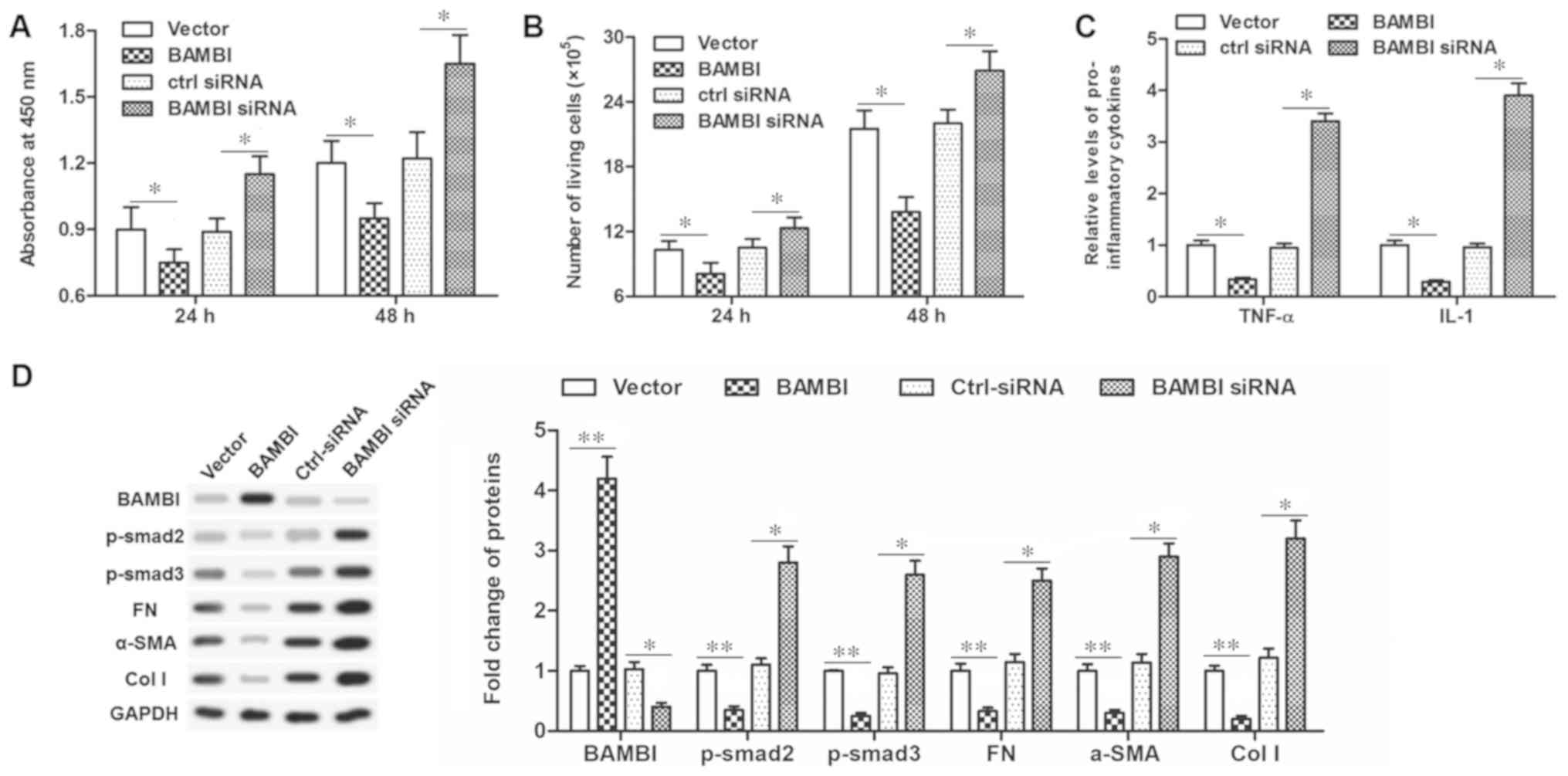 | Figure 5.BAMBI is an inhibitor in human
primary epidural fibroblasts. (A) Cell viability was detected by a
Cell Counting Kit-8 assay after pcDNA-BAMBI or BAMBI-siRNA
transfection for 24 and 48 h. (B) Cell proliferation detected with
flow cytometry after pcDNA-BAMBI or BAMBI-siRNA transfection for 24
and 48 h. (C) Secretion of TNF-α and IL-1 detected via ELISA after
pcDNA-BAMBI or BAMBI-siRNA transfection for 48 h. (D) Protein
expression of BAMBI, FN, α-SMA, col I, p-smad2/3 detected with
western blotting after transfection for 48 h. *P<0.05;
**P<0.01. BAMBI, bone morphogenetic protein and activin
membrane-bound inhibitor; p-smad, phosphorylated mothers against
decapentaplegic homolog 9; FN, fibronectin; SMA, smooth muscle
actin; col I, collagen I; miR, microRNA; TNF, tumor necrosis
factor-α; IL, interleukin; si, small interfering; ctrl-siRNA,
scrambled siRNA sequence. |
Discussion
Lumbar laminectomy is one of the most common
treatments for lumbar disc herniation and other lumbar disorders
with serious complications (19,20),
including failed back surgery syndrome (21,22),
mainly caused by EF. EF causes spinal epidural adhesion and
scarring (23). EF is demonstrated
in the epidural space and contributes greatly to postoperative pain
and recurrent lumbar disc herniation (24). Extensive EF often results in
negative effects on patients. Unsatisfactory clinical outcomes
include radiculopathy, persistent low back pain and disability
(25). Various materials or drugs
have been used to inhibit formation of epidural fibrosis and reduce
the compressive effect on neural structures (26,27).
miRs comprise a broad class of small non-coding RNAs
that control the expression of complementary target messenger RNAs.
Notable features of miR include their redundancy with respect to
their target binding sequences in the 3′-UTR of mRNA and their
relatively small total number, which is speculated to range from
500 to 1,000 (10). Using various
computational and experimental approaches, hundreds of miRs have
been identified in numerous animal species. Dysregulation of miRs
by several mechanisms has been described in various disease states
(7,8). As demonstrated in previous studies,
miR-519d-3p is an extracellular matrix and circulating miR that is
abnormally expressed in fibrotic tissue and serum (28,29).
miR-519d-3p has been proposed as a potential pro-fibrotic miR
during heart regeneration and has been revealed to be positively
associated with the degree of hepatic fibrosis in patients
(28,30). Recent years have witnessed an
increase in the number of studies on the dysregulation and role of
miRs. Evidence has also indicated that miR-519d-3p serves an
inhibitory role in the invasion and migration of trophoblast cells
(31). miR-519d-3p was identified
to be increased and its level was proportional to the mass of scar
in the present study. To further investigate miR-519d-3p in EF and
scar formation, the regulated function of miR-519d-3p in
fibroblasts was studied. Abnormal expression of miR-519d-3p
affected fibroblast proliferation, secretion of inflammatory
cytokines, and protein expression, which were associated with
fibrosis. To the best of the authors' knowledge, this is the first
report investigating miR-519d-3p and its regulation of spinal EF
and scar formation.
Using the bioinformatics software programs RNAhybrid
and TargetScan, BAMBI was identified as a putative target of
miR-519d-3p. BAMBI is a protein, which in humans is encoded by the
BAMBI gene. BAMBI is regarded as a pseudo-receptor of TGF-β, for
they share a common transmembrane glycoprotein responsible for
signal receiving (32,33), however BAMBI lacks the
intracellular serine/threonine kinase domain required for signal
transduction (34). In addition,
studies also revealed that BAMBI restrains TGF-β signaling and
serves a negative role in fibrosis of the liver and several other
tissues (13,35). It has previously been noted that
BAMBI has a negative impact on the growth of human keloid cells
(13). However, studies on the
role of BAMBI in epidural fibrosis and related diseases,
particularly in fibroblasts and scarring, have not yet been
reported. In the present study, it was demonstrated that expression
of the BAMBI protein was decreased in lumbar disc tissues from
patients and its level was inversely proportional to mass of scar.
It was demonstrated in the present study that miR-519d-3p directly
binds to the 3′-UTR of BAMBI. miR-519d-3p overexpression in
vitro indicated that miR-519d-3p significantly inhibited BAMBI
expression, however phosphorylation of smad2/3 and TGF-β were
increased. Inhibition of miR-519d-3p had the opposite effect on
BAMBI expression and smad2/3 phosphorylation. The targeting
association between miR-519d-3p and BAMBI is a novel finding of the
present study.
Following a thorough review of the literature and
the aforementioned experimental results, the authors hypothesize
that miR-519d-3p may regulate postoperative epidural scar formation
via BAMBI. This conjecture was verified and the results
demonstrated that upregulation of the BAMBI level reduced cell
proliferation, inflammatory cytokine secretion, and decreased
fibrosis associated protein expression, suggesting a reduced degree
of fibrosis. Conversely, reducing BAMBI increased cell
proliferation, TNF-α and IL-1 secretion, and enhanced fibrosis
associated protein levels. BAMBI is postulated to modulate
TGF-β/Smad signaling. Activation of the TGF-β/Smad signaling
pathway mediates postoperative epidural scar formation (36,37).
The results of the present study demonstrated that BAMBI inhibited
smad2/3 phosphorylation, suggesting depression of TGF-β/Smad
signaling and inhibition of epidural scar formation.
In conclusion, the role and mechanism of miR-519d-3p
and BAMBI in suppressing postoperative epidural scar formation was
examined. miR-519d-3p was a direct negative regulator of BAMBI and
served a crucial role in regulating cell proliferation,
inflammatory factors TNF-α, and IL-1 and fibrosis-associated
protein expression. The results raise the possibility of using
miR-519d-3p as a potential therapeutic agent to suppress the
TGFβ/Smad mediated postoperative epidural scar formation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY analyzed the data and prepared the manuscript. QG
and FL performed the experiments. MG was involved in data
interpretation and the critical revision of the manuscript for
important intellectual content. DZ designed the study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Central Hospital of Zibo Mining Refco Group Ltd.
(Zibo, China). All participants gave written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Epter RS, Helm S II, Hayek SM, Benyamin
RM, Smith HS and Abdi S: Systematic review of percutaneous
adhesiolysis and management of chronic low back pain in post lumbar
surgery syndrome. Pain physician. 12:361–378. 2009.PubMed/NCBI
|
|
2
|
Manchikanti L and Singh V: Epidural lysis
of adhesions and myeloscopy. Curr Pain Headache Rep. 6:427–435.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sae-Jung S, Jirarattanaphochai K,
Sumananont C, Wittayapairoj K and Sukhonthamarn K: Interrater
reliability of the postoperative epidural fibrosis classification:
A histopathologic study in the rat model. Asian Spine J. 9:587–594.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larionov SN, Sorokovikov VA, Erdyneyev KC,
Lepekhova SA and Goldberg OA: Experimental model of intervertebral
disk mediated postoperative epidural fibrosis. Ann Neurosci.
23:76–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi Z, Zhou H, Lu L, Li X, Fu Z, Liu J,
Kang Y, Wei Z, Pan B, Liu L, et al: The roles of microRNAs in
spinal cord injury. Int J Neurosci. 127:1104–1115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vanacore D, Boccellino M, Rossetti S,
Cavaliere C, D'Aniello C, Di Franco R, Romano FJ, Montanari M, La
Mantia E, Piscitelli R, et al: Micrornas in prostate cancer: An
overview. Oncotarget. 8:50240–50251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Liu X, Wu Y, Wu Q, Wang Q, Yang Z
and Li L: MicroRNAs in biofluids are novel tools for bladder cancer
screening. Oncotarget. 8:32370–32379. 2017.PubMed/NCBI
|
|
8
|
Dai R and Ahmed SA: MicroRNA, a new
paradigm for understanding immunoregulation, inflammation, and
autoimmune diseases. Transl Res. 157:163–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stickel N and Zeiser R: The role of
microRNAs for immunoregulation after allogeneic hematopoietic cell
transplantation. Dtsch Med Wochenschr. 139:1673–1678. 2014.(In
German). PubMed/NCBI
|
|
10
|
Wang C, Wang W, Yang W, Yu X, Yan Y, Zhang
J and Jiang Z: MicroRNAs: A type of novel regulative factor for
intervertebral disc degeneration. Zhejiang Da Xue Xue Bao Yi Xue
Ban. 45:170–178. 2016.(In Chinese). PubMed/NCBI
|
|
11
|
Martinelli R, Nardelli C, Pilone V,
Buonomo T, Liguori R, Castanò I, Buono P, Masone S, Persico G,
Forestieri P, et al: miR-519d overexpression is associated with
human obesity. Obesity (Silver Spring). 18:2170–2176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou JY, Zheng SR, Liu J, Shi R, Yu HL and
Wei M: MiR-519d facilitates the progression and metastasis of
cervical cancer through direct targeting Smad7. Cancer Cell Int.
16:212016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin L, Wang Y, Liu W and Huang Y: BAMBI
inhibits skin fibrosis in keloid through suppressing TGF-Β1-induced
hypernomic fibroblast cell proliferation and excessive accumulation
of collagen I. Int J Clin Exp Med. 8:13227–13234. 2015.PubMed/NCBI
|
|
14
|
Postigo J, Iglesias M, Álvarez P, Jesús
Augustin J, Buelta L, Merino J and Merino R: Bone bone
morphogenetic protein and activin membrane-bound inhibitor, a
transforming growth factor β rheostat that controls murine treg
Cell/Th17 cell differentiation and the development of autoimmune
arthritis by reducing interleukin-2 signaling. Arthritis Rheumatol.
68:1551–1562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Drömann D, Rupp J, Rohmann K, Osbahr S,
Ulmer AJ, Marwitz S, Röschmann K, Abdullah M, Schultz H, Vollmer E,
et al: The TGF-beta-pseudoreceptor BAMBI is strongly expressed in
COPD lungs and regulated by nontypeable Haemophilus influenzae.
Respir Res. 11:672010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Ge Y, Fu Y, Yan L, Cai J, Shi K,
Cao X and Lu C: Mitomycin C induces fibroblasts apoptosis and
reduces epidural fibrosis by regulating miR-200b and its targeting
of RhoE. Eur J Pharmacol. 765:198–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Wang D, Li YJ, Ding N, Wang JY, Yang Q,
Yang YR, Li YM, Fang XD and Zhao H: Molecular networks and
mechanisms of epithelial-mesenchymal transition regulated by miRNAs
in the malignant melanoma cell line. Yi Chuan. 37:673–682. 2015.(In
Chinese). PubMed/NCBI
|
|
19
|
Bailey JC, Kurklinsky S, Sletten CD and
Osborne MD: The effectiveness of an intensive interdisciplinary
pain rehabilitation program in the treatment of post-laminectomy
syndrome in patients Who have failed spinal cord stimulation. Pain
Med. 19:385–392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng MX and Hong D: Update on prevention
of epidural adhesion after lumbar laminectomy. Zhongguo Gu Shang.
28:1064–1068. 2015.(In Chinese). PubMed/NCBI
|
|
21
|
Akbaş M, Yeğin MA, Özdemir İ, Göksu E and
Akyüz M: Subcutaneous stimulation as additional therapy to spinal
cord stimulation in a post-laminectomy syndrome patient. Agri.
28:49–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Assaker R and Zairi F: Failed back surgery
syndrome: To re-operate or not to re-operate? A retrospective
review of patient selection and failures. Neurochirurgie. 61 (Suppl
1):S77–S82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Y, Zhao S, Li X, Yan L, Wang J, Wang
D, Chen H, Dai J and He J: Local application of rapamycin reduces
epidural fibrosis after laminectomy via inhibiting fibroblast
proliferation and prompting apoptosis. J Orthop Surg Res.
11:582016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai J, Li X, Yan L, Chen H, He J, Wang S,
Wang J and Sun Y: The effect of suramin on inhibiting fibroblast
proliferation and preventing epidural fibrosis after laminectomy in
rats. J Orthop Surg Res. 11:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gottschalk A, Freitag M, Tank S,
Burmeister MA, Kreil S, Kothe R, Hansen-Algenstedt N, Weisner L,
Staude HJ and Standl T: Quality of postoperative pain using an
intraoperatively placed epidural catheter after major lumbar spinal
surgery. Anesthesiology. 101:175–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu ZC, Li Y, Zang Y, Cui G, Sang HX, Ma
ZS, Kong L, Lei W and Wu ZX: Clinical assessment of a CMC/PEO gel
to inhibit postoperative epidural adhesion formation after lumbar
discectomy: A randomized, control study. Arch Orthop Trauma Surg.
133:295–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bora H, Aykol SV, Akyurek N, Akmansu M and
Ataoglu O: Inhibition of epidural scar tissue formation after
spinal surgery: External irradiation vs. spinal membrane
application. Int J Radiat Oncol Biol Phys. 51:507–513. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prathipati P, Nandi SS and Mishra PK: Stem
cell-derived exosomes, autophagy, extracellular matrix turnover,
and miRNAs in cardiac regeneration during stem cell therapy. Stem
Cell Rev. 13:79–91. 2017. View Article : Google Scholar :
|
|
29
|
Fornari F, Ferracin M, Trerè D, Milazzo M,
Marinelli S, Galassi M, Venerandi L, Pollutri D, Patrizi C, Borghi
A, et al: Circulating microRNAs, miR-939, miR-595, miR-519d and
miR-494, identify cirrhotic patients with HCC. PLoS One.
10:e01414482015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao P, Sun D, Wang L, Fan R and Gao Z:
Deep sequencing and comprehensive expression analysis identifies
several molecules potentially related to human poorly
differentiated hepatocellular carcinoma. FEBS Open Bio.
7:1696–1706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding J, Huang F, Wu G, Han T, Xu F, Weng
D, Wu C, Zhang X, Yao Y and Zhu X: MiR-519d-3p suppresses invasion
and migration of trophoblast cells via targeting MMP-2. PLoS One.
10:e01203212015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pfeifer CG, Karl A, Berner A, Zellner J,
Schmitz P, Loibl M, Koch M, Angele P, Nerlich M and Mueller MB:
Expression of BMP and actin membrane bound inhibitor is increased
during terminal differentiation of MSCs. Stem Cells Int.
2016:26851472016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka H, Leung PS, Kenny TP, Gershwin ME
and Bowlus CL: Immunological orchestration of liver fibrosis. Clin
Rev Allergy Immunol. 43:220–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Z, Han Z, Tao J, Wang J, Liu X, Zhou
W, Xu Z, Zhao C, Wang Z, Tan R and Gu M: Role of
endothelial-to-mesenchymal transition induced by TGF-β1 in
transplant kidney interstitial fibrosis. J Cell Mol Med.
21:2359–2369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wermuth PJ, Carney KR, Mendoza FA,
Piera-Velazquez S and Jimenez SA: Endothelial cell-specific
activation of transforming growth factor-β signaling in mice
induces cutaneous, visceral, and microvascular fibrosis. Lab
Invest. 97:806–818. 2017. View Article : Google Scholar : PubMed/NCBI
|