Introduction
Myocardial infarction (MI) is a disease with the
highest mortality rate, accounting for 1 in 5 deaths in the United
States (1); in the past decade,
17.6 million individuals have suffered from MI. According to the
American Heart Association, acute cardiac vascular occlusion and
poor prognosis are the main causes of mortality (2). At present, instantly restoring blood
flow to the ischemic area by percutaneous coronary intervention is
the best approach to saving lives (3). Along with the development of gene
technology and molecular biology, research into gene and genomic
regulation is being conducted to reveal the cause of MI and to
advance therapies (4). Cytokine
and gene therapy have been widely utilized for therapeutic
angiogenesis in myocardial ischemia (4). Various growth factors, such as
vascular endothelial growth factor (VEGF) and hepatocyte growth
factor (HGF), serve roles in balancing cell proliferation and
survival under stress (5–7). Furthermore, cardiac remodeling and
collateral angiogenesis improve early cardiac recovery (8). In our previous study, it was reported
that p27kip1 (p27) haploinsufficiency improved cardiac
function in the early stages of MI by protecting the myocardium and
increasing angiogenesis through IκB kinase activation (9). The protective effects of knocking
down the cell cycle inhibitor p27 to stimulate the autocrine and
paracrine effects of VEGF and HGF through the activation of
inflammation and angiogenesis in the myocardium.
p27 is a potent cell cycle inhibitor that maintains
cell proliferation by arresting the cell cycle in the G0/G1 phase;
a previous study reported that the regulation of p27 not only
affected cell cycle-related proliferation but was also involved in
multifunctional molecular mechanisms in vivo and in
vitro such as aging and inflammation regulating (10). The downstream activities of p27
include the regulation of inflammation and survival pathways
(9,11,12).
Through the regulation of p27 expression, development/tumorigenesis
and metabolism are altered, which are implicated in energy
modulation (13). Rossi et
al (14) reported that gene
knockdown may induce different biological effects than gene
deletion; it was found that knockdown was more effective than
knockout for mimicking pathological progression. In our previous
study, p27 haploinsufficient mice (p27+/−) exhibited a
different phenotype compared with wild-type (WT; p27+/+)
or homozygous (p27−/−) mice (9).
Macroautophagy, more commonly referred to as
autophagy, balances cell death and survival in response to stress
and starvation (15). It is an
important cellular homeostatic process that cells use to degrade
misfolded proteins and recycle damaged organelles, and is also
related to a number of diseases, including cancer and
neurodegeneration. The cup-shaped pre-autophagosomal
double-membrane structure containing cytoplasmic material
characterizes the formation of autophagosomes (16). As previously reported, the
cardiomyocyte-specific abrogation of basal autophagy that results
from a deficiency in autophagy protein 5 (Atg5) leads to
spontaneous cardiac hypertrophy (17). Accordingly, autophagy acts as a
protective mechanism that improves cardiomyocyte survival under
stress (18). Serum withdrawal and
hypoxia are common approaches used to mimic myocardial cell
ischemia in vitro. Ding et al (19) reported that p27 levels were
regulated by autophagy in serum-deprived mouse mesangial cells. It
also has been reported that, in relation to autophagy and
inflammation, cardiac remodeling and heart failure are promoted by
the upregulation of p27 in the long term (20). To the best of our knowledge, no
studies have investigated the relationship between p27 and
autophagy in the early stages of MI.
The present study aimed to determine the functional
role of p27 haploinsufficiency in the induction of autophagy by
hypoxia and serum withdrawal in vitro and in vivo for
cardiomyocyte survival, and protection from apoptosis. The results
demonstrated that p27+/− resulted in the increased
induction of Atg5-related autophagosomes and suppressed serum
withdrawal-induced caspase-3 activation and apoptosis in MI hearts.
It was demonstrated that p27 knockdown governed the regulation of
autophagy by increasing the expression of Atg5 in the early stages
of MI in mice and in MI mimic cells. In the present study,
3-methyladenine (3-MA) and rapamycin were used to investigate the
rescue of autophagy and cell survival following MI.
Materials and methods
Animals and establishment of an MI
model
The establishment of the MI animal model was
described in our previous study (9). p27+/− and strain-specific
C57BL/6 WT p27+/+ mice were bred from a breeding pair of
heterozygous p27 mice (kindly donated by Professor Dengshun Miao,
McGill University; Fig. S1A) and
housed in the Animal Research Center of Nanjing Medical University.
The mice were bred in a specific pathogen-free rodent feeding room
and kept at 15% humidity and 25°C conditions and a 12 h-light/dark
cycle. Tail fragment genomic DNA was used to genotype the mice; a
total of 40 adult male 3-month-old C57BL/6 (25±5 g) WT (n=20) and
p27+/− (n=20) mice were split into MI and Sham control
groups (10 mice/group). Left anterior descending (LAD) artery
ligation was performed to induce MI, as previously describe
(9). Briefly, the LAD coronary
artery was ligated permanently using an 8-0 polypropylene suture;
the Sham animals underwent the same procedure, but without ligation
of the LAD coronary artery. During the experimental trial, all mice
were allowed ad libitum access to food and water. The
protocols used in the present study were approved by the Ethics
Review of Lab Animal Use Application of Nanjing Medical University,
and the procedures complied with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (9).
Echocardiogram
Cardiac function was determined using a
high-frequency ultrasound system Vevo2100 (FUJIFILM VisualSonics,
Inc.) equipped with a 30-MHz transducer. The ejection fraction (EF)
and fractional shortening (FS) were calculated according to the
following formulas: EF=[(LVEDV-LVESV)/LVEDV] ×100, where LVEDV is
left ventricular end-diastolic volume and ESV is LV end-systolic
volume; and FS=[(LVEDd-LVESd)/LVEDd] ×100, where EDd is
end-diastolic dimension and ESd is end-systolic dimension. Each
parameter was the average of three values. Mice were anesthetized
with 4–5% chloral hydrate at 300–4550 mg/kg; no signs of
peritonitis, pain or discomfort were observed. The heart rates of
the mice were maintained at 250±35 beats/min under anesthesia.
Immunohistochemistry (IHC) and
Masson's trichrome staining
Mouse hearts were collected and left ventricular
tissues fixed in 4% buffered paraformaldehyde at room temperature
for 24 h and embedded in paraffin at 60°C for 1 h. The paraffin
embedded tissues were cut into 5 µm-thick sections, which were
subsequently deparaffinized and rehydrated with a graded xylene and
ethanol series. Antigen retrieval was performed by high temperature
and pressure: 1 mM EDTA (Beijing Solarbio Science & Technology
Co., Ltd.) was heated to 100°C, the sections were soaked in EDTA
and heated for 5 min at a pressure of 110 kPa. Sections were
incubated in 3% H2O2 at room temperature for
10 min. Subsequently, the sections were blocked in 10% BSA (Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature
for 30 min. IHC was performed using the SuperPicture™ 3rd Gen IHC
Detection kit (cat. no. 878973; Invitrogen; Thermo Fisher
Scientific, Inc.), following manufacturer's protocol. Briefly, the
sections were incubated with a primary antibody against
microtubule-associated proteins 1A/1B light chain (LC3) (1:500;
cat. no. ab128025; Abcam) overnight at 4°C. The sections were
subsequently incubated with a horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody [1:500: cat.
no. 7074/6; Cell Signaling Technology, Inc. (CST)] for 2 h at 25°C.
A two-step technique was used for visualization using components
from the SuperPicture™ 3rd Gen IHC Detection kit, and hematoxylin
was used as a counterstain. The heart tissue sections from the left
ventricle of mice 28 days post-MI and fixed, embedded, cut into
5-µm-thick sections were also stained using Masson's trichrome to
confirm successful model establishment by infarction and fibrotic
areas (Fig. S1B). A total of six
fields were analyzed per sample and images were captures using an
Olympus CX41 light microscope (CX41; Olympus Corporation, Tokyo,
Japan) and Image-Pro Plus 6.0 (Media Cybernetics, Inc.) was used to
detect LC3 expression.
Cell culture and treatment
The rat H9c2 cell line (cat. no. GNR5; Cell Bank of
the Chinese Academy of Science) was seeded at 1.25×104
cells/well in DMEM (Gibco; Thermo Fisher Scientific, Inc.) in
6-well culture plates and were allowed to grow for 2 days in
incubated box at 37°C, 5% CO2, pH 7.2–7.4. FBS (10%;
Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin and
100 µg/ml streptomycin were added to the culture medium before cell
culture. When the cells reached 80–85% confluency the media was
replaced with DMEM containing 1% FBS, to mimic ischemia, and the
cells were placed into an anoxic box (95% nitrogen, 5%
CO2 at 37°C) to induce hypoxia for 3 or 12 h.
Confirmation of hypoxia was determined by western blotting for
increased protein expression levels of hypoxia-inducible factor-1α
(HIF-1α; Fig. S1C).
Western blot analysis
Tissues and cells were lysed using RIPA buffer [20
mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM
EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 µg/ml leupeptin]. Protein
concentrations were determined by BCA; 20 µg of protein lysates
were separated by 10% SDS-PAGE and transferred to PVDF membranes.
Membranes were washed with PBS, blocked with 5% non-fat dry milk
for 1 h at room temperature, and subsequently incubated over night
at 4°C with primary antibodies (all 1:1,000) against p27 (cat. no.
610241; BD Biosciences), Cleaved-caspase-3 (cat. no. 9664; CST),
Bcl-2 (cat. no. 2870; CST), Beclin 1 (cat. no. 3495; CST), Atg5
(cat. no. 12994; CST), LC3 (cat. no. ab128025; Abcam), HIF-1α (cat.
no. 36169; CST) and GAPDH (cat. no. 2118; CST). Subsequently, the
membranes were incubated with HRP-conjugated goat anti-mouse (cat.
no. sc-2005; Santa Cruz Biotechnology, Inc.) or goat anti-rabbit
HRP (cat.no. sc-2004; Santa Cruz Biotechnology, Inc.) secondary
antibodies overnight at 4°C (both 1:2,000). Protein bands were
visualized using the Tanon 4600 High-Sig ECL Western Blotting
Substrate (Tanon Science and Technology Co., Ltd.) then Gel-Pro
Analyzer 4.0 used for the densitometric analyses; GAPDH was used as
a loading control and for normalization.
Cell transduction
Lentiviral vectors for
pLV-p27kip1-inhibitor (5′-AAGGTTGCATACTGAGCCAAG-3′),
pLV-Atg5-inhibitor (5′-CAUCUGAGCUACCCGGAUAUU-3′), Scrambled shRNA
negative control (NC) (5′-GGGTGATTCACTTTCAGGTCAGTAA-3′) (all from
Shanghai GeneChem Co., Ltd.) were used to establish stable
knockdown cell lines (Fig. S1D).
Briefly, 1×105 H9c2 cells were incubated with
lentiviruses (10 µg/ml; 20 multiplicity of infection) in the
presence of 2 µg/ml polybrene (cat. no. sc-134220; Santa Cruz
Biotechnology, Inc.) overnight at 37°C. Stably transduced cells
were selected using 2 µg/ml puromycin (Sigma-Aldrich; Merck
KGaA).
Transmission electron microscopy
(TEM)
The tissues obtained from the ischemic area of the
isolated hearts were cut into 1–2 mm3 cubes and fixed
with 2% glutaraldehyde in 0.1 M cacodylate buffer at 4°C overnight.
The excised tissues were embedded in epoxy resin, cut into 60–70-nm
sections with an ultramicrotome and placed on TEM grids for
examination using TEM at the public laboratory of Nanjing Medical
University, gold was used to increase contrast for imaging.
Co-immunoprecipitation (Co-IP)
Tissues were lysed in RIPA buffer, aforementioned,
and incubated with primary antibodies (all 1:300) against Atg5
(cat. no. 12994; CST) and p27 (cat. no. 610241; BD Biosciences) and
with protein G-agarose beads (cat. no. sc-2002; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The next day, beads were
collected by centrifugation (4°C; 3,000 × g; 5 min) and 60 µl of
SDS sample buffer was used to wash the beads while heating at 100°C
for 10 min. The proteins collected were utilized for western
blotting, following the aforementioned protocol.
Determination of apoptosis using flow
cytometry
The level of apoptosis in the treated H9c2 cells was
determined using an Annexin V-APC Apoptosis Detection kit (BD
Biosciences) according to the manufacturer's instructions. The data
were analyzed using a flow cytometer and Image-Pro Plus 6.0 used to
analyze.
Autophagy detection using monomeric
red fluorescent protein (mRFP)-green fluorescent protein (GFP)
vector
H9c2 cells were plated in 6-well plates and allowed
to reach a confluency of 70–85%. An mRFP-GFP-LC3 adenoviral vector
(Hanbio Biotechnology Co., Ltd.) was used to infect the cells,
according to the manufacturer's instructions. Cells were incubated
in growth medium with the adenoviruses at a multiplicity of
infection of 100 for 2 h at 37°C. Cells were subsequently incubated
in fresh medium for a further 24 h at 37°C. After infection, cells
were exposed to the aforementioned hypoxic/ischemic conditions for
3 h. Autophagy was observed using an Olympus BX51 fluorescent
microscope (Olympus Corporation) as previously described (16).
Statistical analysis
Data are presented as the mean ± SEM from three
independent experiments. All statistical analysis was conducted
using SPSS 19.0 statistical software (IBM Corp.). Statistical
significance between groups was determined using one-way ANOVA and
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
p27 haploinsufficiency improves
cardiac function after MI
p27+/− has been shown to have either a
proangiogenic or an antiapoptotic effect depending on cell cycle
re-entry (9). LAD artery ligation
was used to mimic MI in WT and p27+/− mice. In our
previous study, it was reported that p27−/− and WT mice
exhibited high mortality following this procedure, but that
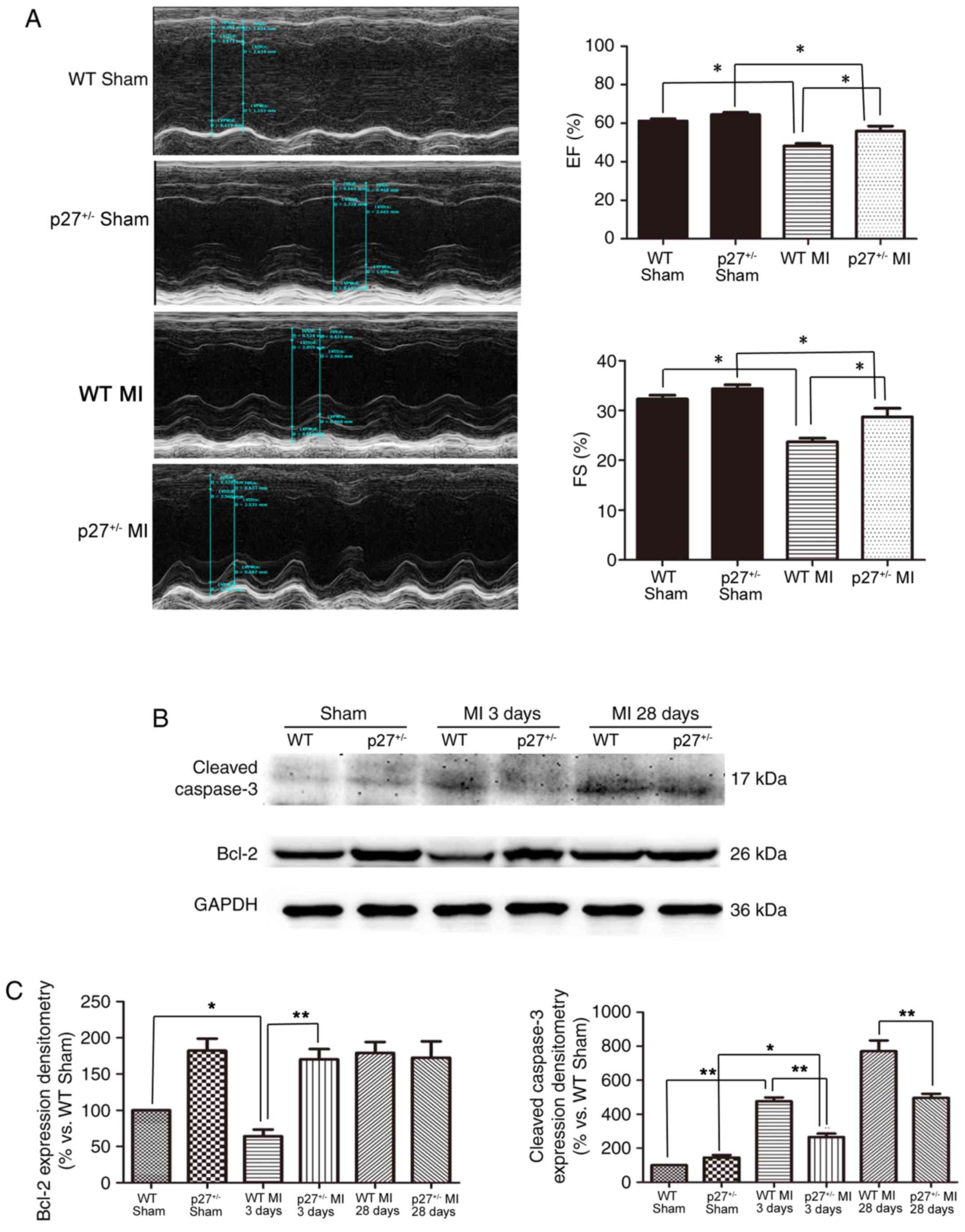
p27+/− mice had a moderate mortality (9). Significant systolic dysfunction was
observed in WT MI compared with WT Sham (P<0.05),
p27+/− MI compared with p27+/− Sham
(P<0.05) at 28 days following MI and in WT MI compared with
p27+/− MI as assessed using the percentage of LV FS and
EF in the MI and control groups (Fig.
1A). However, LV dysfunction was lower in p27+/−
mice compared with the WT mice after infarction (P<0.05;
Fig. 1A). These data suggested
that the haploinsufficiency of p27 may preserve cardiac function
following MI. p27+/− mice exhibited reduced cardiac
injury after MI by promoting the expression of the anti-apoptotic
protein Bcl-2 at 3 days post-MI compared with WT MI at day 3
(P<0.01) and by reducing the protein expression level of cleaved
caspase-3 at 3 and 28 days post-MI compared with the WT MI
counterpart (P<0.05 and P<0.01, respectively) (Fig. 1B and C).
p27 haploinsufficiency attenuates
ischemic injury through a pro-autophagy-induced antiapoptotic
pathway in vivo
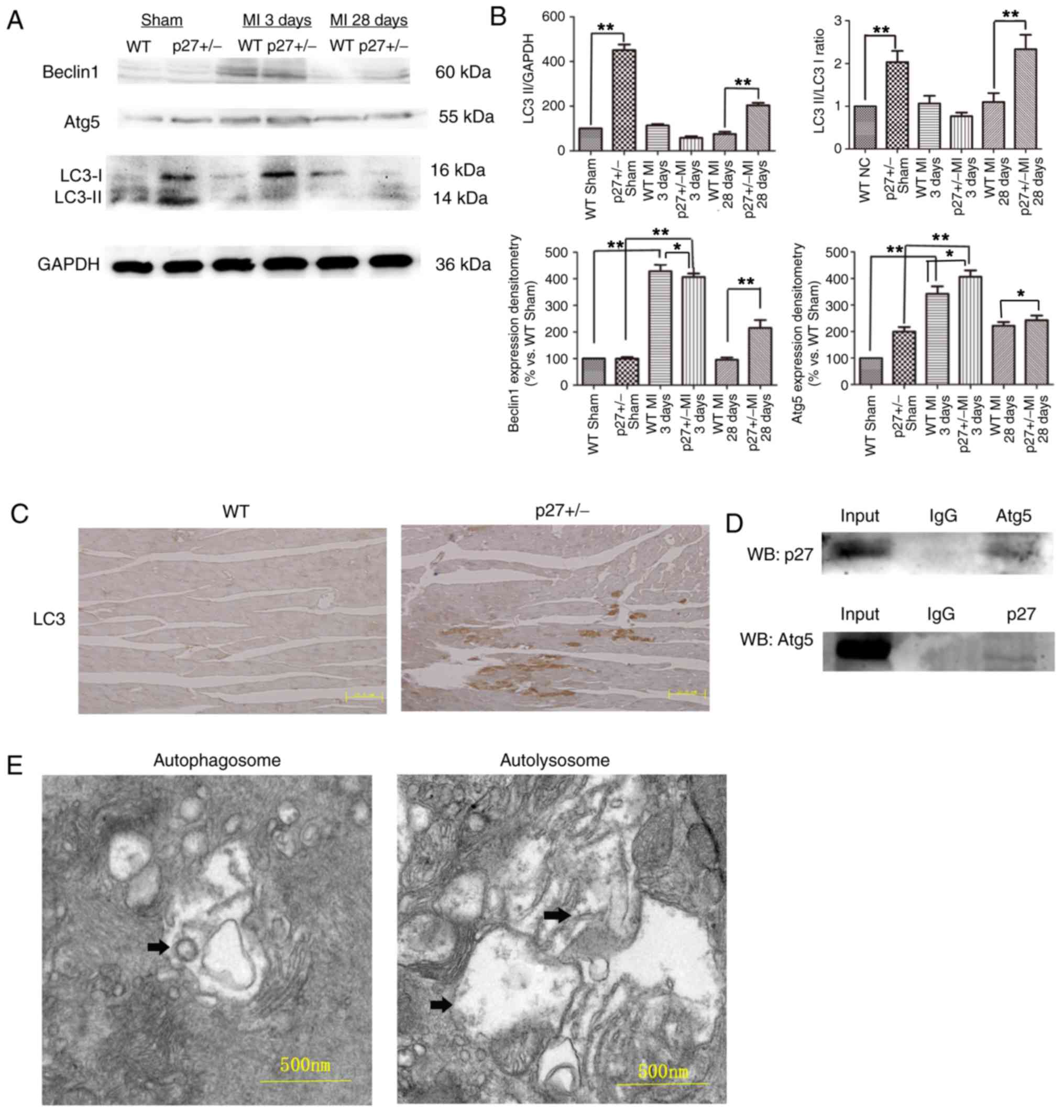
As shown in Fig.
2A, MI induced the expression of the autophagic
vesicle-associated form of LC3-II within 28 days after LAD artery
ligation. It was observed that in both the Sham and MI groups at 28
days the autophagy marker LC3 was expressed at significantly higher
levels in the p27+/− groups compared with expression
levels in the WT group (P<0.01; Fig. 2A and B). In addition, Beclin 1 and
downstream Atg5 expression levels were affected. Beclin 1 was
expressed at significantly higher levels in the p27+/−
and WT MI groups compared with expression levels in the respective
p27+/− Sham and WT MI groups at 3 days (P<0.01), and
at 28 days Beclin 1 expression was significantly higher in the
p27+/− MI group compared with the WT MI 28 days group
(P<0.01) (Fig. 2A and B). Atg5
was also expressed at significantly higher levels in the
p27+/− groups compared with expression levels in the
respective WT groups (P<0.05 or P<0.01; Fig. 2A and B). These data suggested that
p27 haploinsufficiency contributes to the observed increased levels
of autophagy in the Sham and MI groups. Furthermore, IHC was used
to detect the levels of LC3 in WT and p27+/− mice at 28
days post-surgery; fewer LC3-positive areas were observed in the WT
mice compared with the p27+/− mice (Fig. 2C). Co-IP experiments were performed
on WT mouse heart tissue lysates. Binding was detected between Atg5
and p27 in heart tissue (Fig. 2D).
In addition, TEM also detected the formation of autophagosomes and
autolysosomes in WT tissue 28 days after MI (Fig. 2E). These results suggested that p27
haploinsufficiency attenuated ischemic injury by mitigating
apoptosis via Atg5-related autophagy.
p27KD restores autophagy
flux in the early stages of hypoxia/ischemia in vitro
The lentiviral-mediated stable p27KD H9c2
cell line (~50% efficiency; Fig.
S1D) was used to mimic the effect of hypoxia/ischemia in
cardiomyocytes. Western blotting was used to determine the
expression of the autophagy-related proteins Beclin 1, Atg5 and LC3
in H9c2 cells. The results demonstrated that both Beclin 1 and Atg5
expression were higher in the p27KD H9c2 cells compared
with WT cells after 3 h of hypoxia/ischemia, but attenuated after
12 h (Fig. 3A and B). The ratio of
LC3-II/I, a hallmark of autophagosomes, coincided with the
expression of Beclin 1 and Atg5 (Fig.
3B). Western blotting revealed that after 3 h of
hypoxia/ischemia, Beclin 1 and Atg5 protein expression levels
increased to a peak expression level compared with WT NC
(P<0.01), and the levels in p27KD with 3 h of hypoxia
were significantly higher compared with WT with 3 h of hypoxia
(P<0.01) (Fig. 3A and B). H9c2
cells were infected with the mRFP-GFP-LC3 adenovirus and then
exposed to 3 h of hypoxia; the results demonstrated that, after 3
h, there was a significantly increased ratio of GFP and mRFP puncta
per cell in p27KD cells compared with WT (P<0.01;
Fig. 3C), whereas the effects were
reversed by Atg5KD co-knockdown (P<0.01; Fig. 3C). Flow cytometry results revealed
that total early+late apoptotic rates in Atg5KD cells
were significantly increased after 12 h of hypoxia and serum
deprivation compared with WT cells (P<0.05; Fig. 3D). p27KD cells had lower
apoptosis compared with WT (P<0.01), and co-knockdown of Atg5
reversed this effect of p27 haploinsufficiency (P<0.05). These
data indicated that the mechanisms by which p27 insufficiency
protects against hypoxia/ischemia may be through restoring the
autophagy flux.
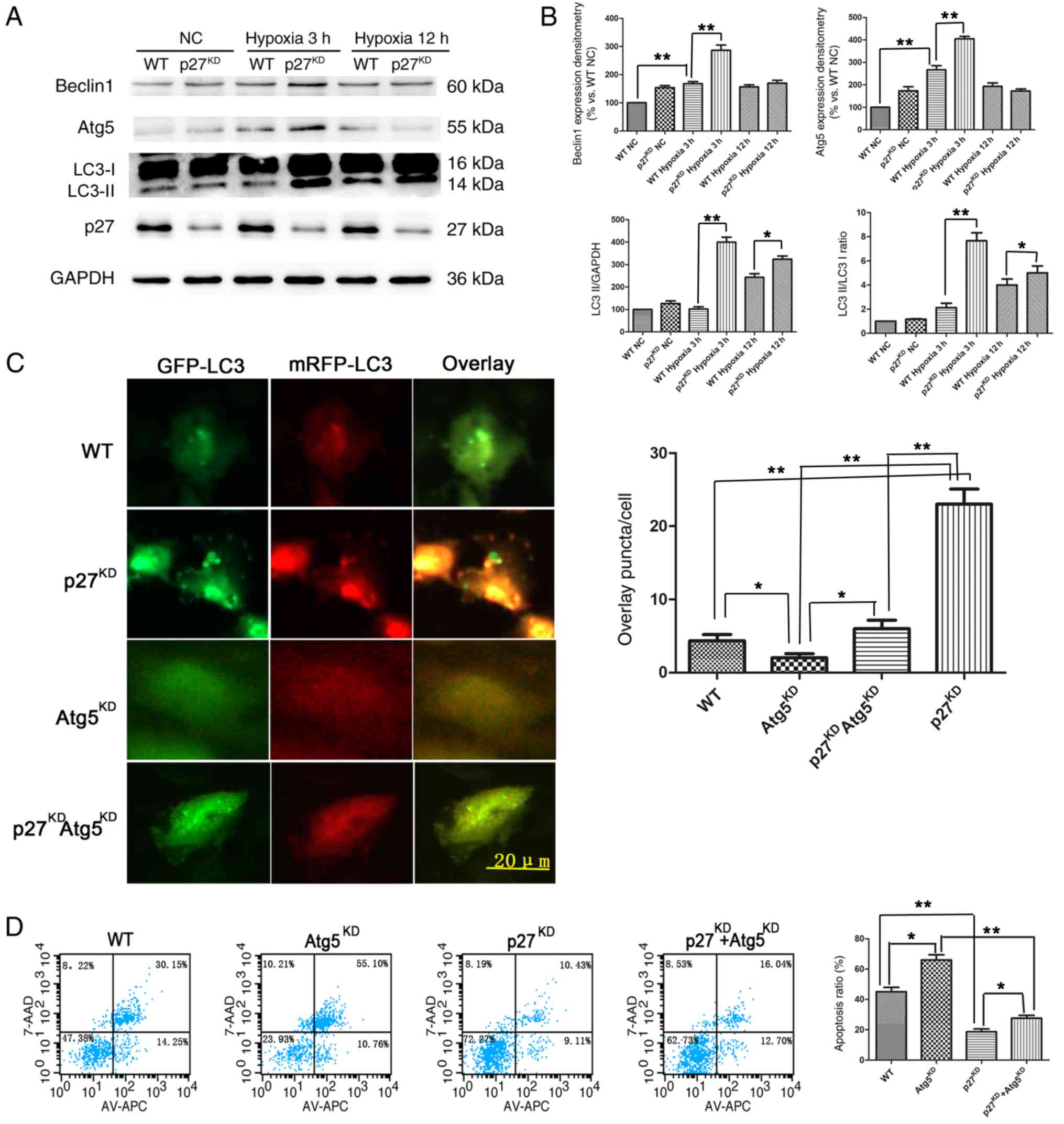 | Figure 3.Effects of p27KD on the
expression of autophagy markers in H9c2 cells under
ischemia/hypoxia. (A) Representative western blotting and (B)
quantitative analysis of Beclin 1, Atg5, LC3 and p27 protein
expression levels in H9c2 cells exposed for various times to
ischemic/hypoxic conditions; GAPDH served as a loading control;
n=3. (C) H9c2 cells transfected with mRFP-GFP-LC3 adenovirus and
exposed to hypoxic/ischemic conditions for 3 h, the ratio of GFP to
mRFP puncta/cell was calculated. (D) Representative plots of
apoptotic cardiomyocytes using flow cytometry, quantification is
shown as total apoptosis. *P<0.05; **P<0.01. Atg5, autophagy
protein 5; GFP, green fluorescent protein; KD, knockdown; LC3,
microtubule-associated proteins 1A/1B light chain; mRFP, monomeric
red fluorescent protein; NC, negative control; p27,
p27kip1. |
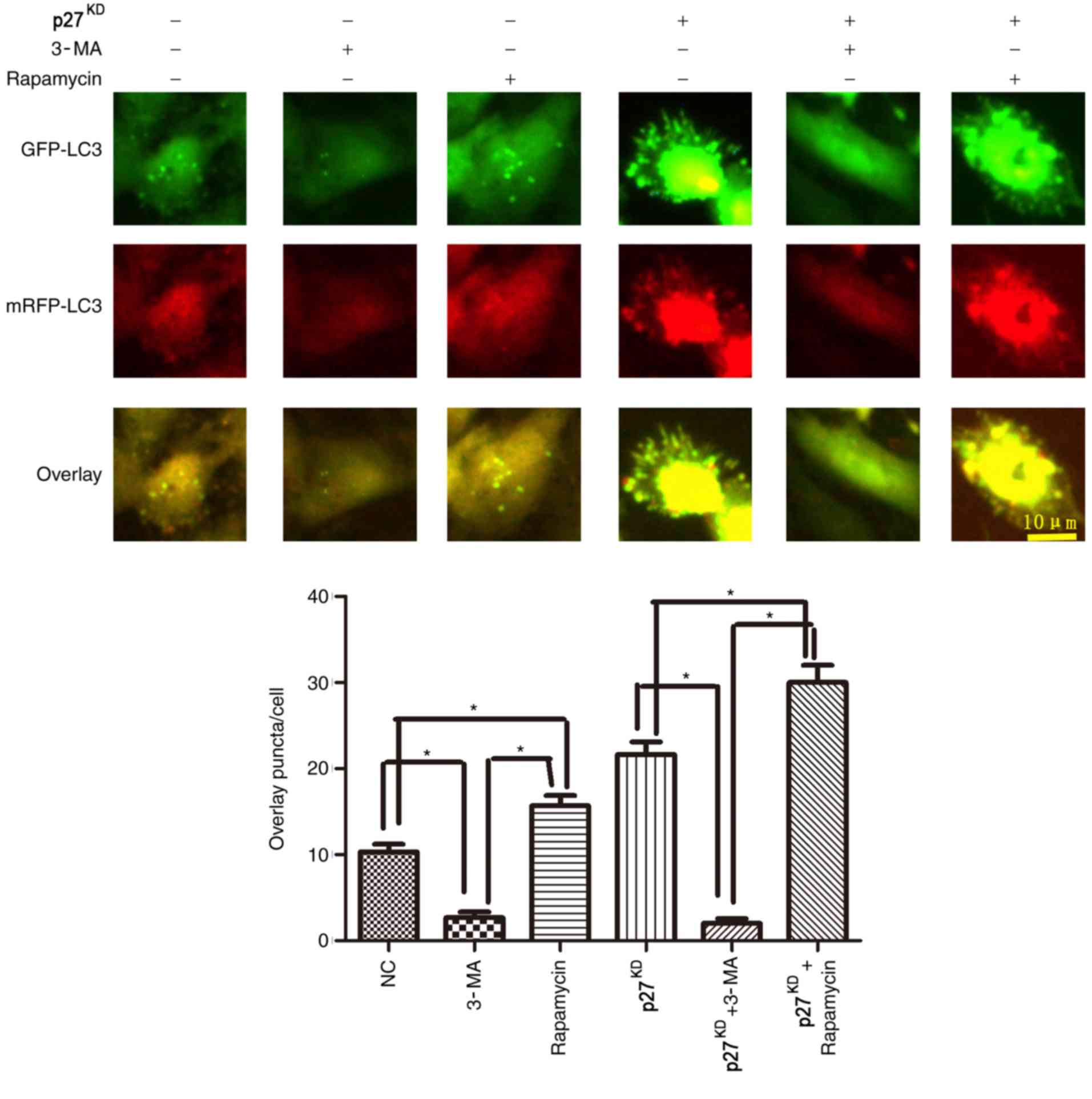
p27-mediated autophagy flux is
affected by 3-MA/rapamycin
To further investigate whether p27KD
improved autophagy flux, cells were treated with the autophagy
promoter rapamycin and the autophagy inhibitor 3-MA. Rapamycin
treatment resulted in an increased level of GFP and mRFP
puncta/cell compared with untreated cells, whereas 3-MA treatment
significantly decreased the number of puncta/cell (Fig. 4). In addition, p27KD
increased expression of the autophagic vesicle-associated form of
LC3II following either rapamycin after 3 h of hypoxia/ischemia
(P<0.05; Fig. 4).
Discussion
Myocardial infarction is induced by cardiac coronary
vascular and branch occlusion, resulting in nutrient and oxygen
insufficiency (21,22). As a result, the ischemic myocardium
dies and is replaced by fibrotic scar tissue (23). Fibroblast cells are implicated in
the dysfunction of contractility, leading to heart failure
(24). Furthermore, in
cardiomyocytes, our previous study reported that 3 and 28 days of
hypoxia in vivo, and 3 and 12 h of hypoxia in vitro
induces ischemia/hypoxia that can be used to mimic the early stages
of natural heart attack, according to immunodetection and
ultrasonic cardiograms (9).
Recently, an association between autophagy and cardiac disorders
such as myocardial infarction has been demonstrated (25). p27 is a potent cell cycle inhibitor
implicated in survival, proliferation, antiapoptotic functions and
migration (10). Cardiomyocytes
undergo ischemia in the early stages of MI, and p27KD
may activate immunoreactivity and autophagy. It has been
hypothesized that by promoting the cell cycle, p27
haploinsufficiency could improve cell survival and basic autophagy
naturally (26). Previously, it
was reported that the knockdown of p27 preserved cardiac function
in the early stage of MI by regulating VEGF and HGF, and their
receptors, promoting cardiomyocyte survival and leading to
proangiogenic effects (9).
Similarly, results from the present study demonstrated that
p27+/− mice exhibited improved cardiac function and
higher cardiomyocyte viability following acute MI compared with WT
mice. The reduced survival found in p27−/− mice in the
earliest stages after MI, but not in WT and p27+/− mice,
has also been described (9). The
present study revealed that p27+/− mice had augmented
autophagy in the early stages after MI.
Autophagy can be stimulated by various forms of
cellular stress, including serum withdrawal, nutrient deprivation,
damaged organelles, hypoxia, DNA damage and protein misfolding,
which resist apoptosis and necrosis (27,28).
Proper autophagy levels are required to maintain cell homeostasis
and function. In the heart, uncontrolled autophagy has been
demonstrated to contribute to pathological cardiac remodeling and
subsequent heart failure (18).
Although p27 has been reported to be involved in regulating the
process of autophagy (20), few
studies have investigated the relationship between p27 and
autophagy in the heart. In the present study, it was found that
p27KD in cardiomyocytes increased LC3 protein expression
in vivo and in vitro. Furthermore, restored autophagy
flux in p27KD ischemic H9C2 cells were observed by
GFP/mRFP LC3 fluorescence and IHC. Therefore, the results indicated
that p27 may inhibit the autophagy process of cardiomyocytes in the
early stages after MI, which may lead to subsequent cardiac
dysfunction. In addition, to evaluate the potential contribution of
changes in the autophagic flux in p27KD cardiomyocytes,
the tandem fluorescence reporter mRFP-GFP-LC3 was used to measure
flux. The data from the present study revealed a significantly
increased number of GFP and mRFP puncta/cell ratios in
p27KD cells, indicating that flux is blocked in the
early stages of MI.
Atg5 is an important upstream mediator of autophagy
through its binding to LC3, which blocks the formation of
autophagosomes (26). It has also
been found to serve an important role in the maintenance of cardiac
function (29). In the present
study, co-IP using Atg5 was used to analyze the relationship
between Atg5 and LC3. The results demonstrated that p27 can bind to
the Atg5 protein in cardiomyocytes. p27KD increased Atg5
expression and promoted autophagy in the early stages of MI.
Furthermore, it was found that Atg5KD reversed the
effects of increased autophagy and prevented apoptosis in cultured
cardiomyocytes that underwent hypoxia/ischemia. Pathways such as
AMP-activated protein kinase/mTOR/ErB/AKT regulate autophagy and
apoptosis in cardiomyocytes (16);
however, a limited number of studies have attempted to reveal a
relationship with p27. The results from the present study provide a
new insight of autophagy and apoptosis in cardiomyocytes under
hypoxic/ischemic conditions and indicate mechanisms of immunologic
injury protection and autophagy flux restoration. The present study
indicated that p27 may serve as an inhibitor of Atg5-mediated
autophagy activation.
In conclusion, the results from the present study
revealed that the p27/Atg5/LC3 pathway modulates the regulation of
autophagy in cardiomyocytes under hypoxic/ischemic conditions,
providing an insight into the regulation of cardiomyocyte autophagy
through p27. The present study paves the way for further studies
investigating therapeutic targets of p27 in the early stages of
MI.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was granted financial support from
a project supported by the National Natural Science Foundation of
China (grant nos. 81670328, 81441011 and 81570328).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
NZ and JW wrote the manuscript and edited the
figures. NZ, WC, QH, JW, DL and YG carried out the molecular
laboratory work and participated in the data analysis. DL and QH
revised the manuscript and acquired funding. DL, JW and YG
supervised all experimental process. QH prepared the supplementary
data.
Ethics approval and consent to
participate
The protocols of the present study were approved by
the Ethics Review of Lab Animal Use Application of Nanjing Medical
University (Nanjing, China), and the procedures complied with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saleh M and Ambrose JA: Understanding
myocardial infarction. F1000Res. 7:F10002018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magnoni M, Berteotti M, Norata GD, Limite
LR, Peretto G, Cristell N, Maseri A and Cianflone D: Applicability
of the 2013 ACC/AHA risk assessment and cholesterol treatment
guidelines in the real world: Results from a multiethnic
case-control study. Ann med. 48:282–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramanathan K, Abel JG, Park JE, Fung A,
Mathew V, Taylor CM, Mancini GBJ, Gao M, Ding L, Verma S, et al:
Surgical versus percutaneous coronary revascularization in patients
with diabetes and acute coronary syndromes. J Am Coll Cardiol.
70:2995–3006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scimia MC, Gumpert AM and Koch WJ:
Cardiovascular gene therapy for myocardial infarction. Expert Opin
Biol Ther. 14:183–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang N, Tong G, Yang J, Zhou Z, Pan H, Huo
Y, Xu J, Zhang X, Ling F and Li P: Effect of hepatocyte
growth-promoting factors on myocardial ischemia during exercise in
patients with severe coronary artery disease. Int Heart J.
50:291–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen J, Xie Y, Liu Z, Zhang S, Wang Y, Jia
L, Wang Y, Cai Z, Ma H and Xiang M: Increased myocardial stiffness
activates cardiac microvascular endothelial cell via VEGF paracrine
signaling in cardiac hypertrophy. J Mol Cell Cardiol. 122:140–151.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaminsky SM, Rosengart TK, Rosenberg J,
Chiuchiolo MJ, Van de Graaf B, Sondhi D and Crystal RG: Gene
therapy to stimulate angiogenesis to treat diffuse coronary artery
disease. Human Gene Ther. 24:948–963. 2013. View Article : Google Scholar
|
|
8
|
Zhao Q, Huang J, Wang D, Chen L, Sun D and
Zhao C: Endothelium-specific CYP2J2 overexpression improves cardiac
dysfunction by promoting angiogenesis via Jagged1/Notch1 signaling.
J Mol Cell Cardiol. 123:118–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou N, Fu Y, Wang Y, Chen P, Meng H, Guo
S, Zhang M, Yang Z and Ge Y: p27(kip1) haplo-insufficiency improves
cardiac function in early-stages of myocardial infarction by
protecting myocardium and increasing angiogenesis by promoting IKK
activation. Sci Rep. 4:59782014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koff A and Polyak K: p27KIP1, an inhibitor
of cyclin-dependent kinases. Prog Cell Cycle Res. 1:141–147. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Ding X, Fan P, Guo J, Tian X, Feng
X, Zheng J, Tian P, Ding C and Xue W: Inactivation of p27(kip1)
promoted nonspecific inflammation by enhancing macrophage
proliferation in islet transplantation. Endocrinology.
157:4121–4132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marone M, Bonanno G, Rutella S, Leone G,
Scambia G and Pierelli L: Survival and cell cycle control in early
hematopoiesis: Role of bcl-2, and the cyclin dependent kinase
inhibitors P27 and P21. Leuk Lymphoma. 43:51–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rehman G, Shehzad A, Khan AL and Hamayun
M: Role of AMP-activated protein kinase in cancer therapy. Arch
Pharm (Weinhim). 347:457–468. 2014. View Article : Google Scholar
|
|
14
|
Rossi A, Kontarakis Z, Gerri C, Nolte H,
Holper S, Kruger M and Stainier DY: Genetic compensation induced by
deleterious mutations but not gene knockdowns. Nature. 524:230–233.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deter RL, Baudhuin P and De Duve C:
Participation of lysosomes in cellular autophagy induced in rat
liver by glucagon. J Cell Biol. 35:C11–C16. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Liu J, Tao Z, Wu P, Cheng W, Du Y,
Zhou N, Ge Y and Yang Z: Exogenous HGF prevents cardiomyocytes from
apoptosis after hypoxia via up-regulating cell autophagy. Cell
Physiol Biochem. 38:2401–2413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai SN, Hou AJ, Zhao SM, Chen XM, Huang
HT, Chen BH and Kong HL: Ginsenoside Rb1 ameliorates autophagy of
hypoxia cardiomyocytes from neonatal rats via AMP-activated protein
kinase pathway. Chin J Integr Med. 25:521–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ponnusamy M, Li PF and Wang K:
Understanding cardiomyocyte proliferation: An insight into cell
cycle activity. Cell Mol Life Sci. 74:1019–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee
SJ and Choi ME: TGF-{beta}1 protects against mesangial cell
apoptosis via induction of autophagy. J Biol Chem. 285:37909–37919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Momen A, Wu J, Noyan H, Li R, von
Harsdorf R and Husain M: p27 protein protects metabolically
stressed cardiomyocytes from apoptosis by promoting autophagy. J
Biol Chem. 289:16924–16935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arya R and White K: Cell death in
development: Signaling pathways and core mechanisms. Semin Cell Dev
Biol. 39:12–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stegehuis VE, Wijntjens GW, Piek JJ and
van de Hoef TP: Fractional flow reserve or coronary flow reserve
for the assessment of myocardial perfusion: Implications of FFR as
an imperfect reference standard for myocardial ischemia. Curr
Cardiol Rep. 20:772018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lopez B, Gonzalez A, Ravassa S, Beaumont
J, Moreno MU, San Jose G, Querejeta R and Diez J: Circulating
biomarkers of myocardial fibrosis: The need for a reappraisal. J Am
Coll Cardiol. 65:2449–2456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delgado V, van Bommel RJ, Bertini M,
Borleffs CJ, Marsan NA, Arnold CT, Nucifora G, van de Veire NR,
Ypenburg C, Boersma E, et al: Relative merits of left ventricular
dyssynchrony, left ventricular lead position, and myocardial scar
to predict long-term survival of ischemic heart failure patients
undergoing cardiac resynchronization therapy. Circulation.
123:70–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun T, Li MY, Li PF and Cao JM: MicroRNAs
in cardiac autophagy: Small molecules and big role. Cells.
7:E1042018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Peng X, Wang Y, Cao S, Xiong L, Fan
J, Wang Y, Zhuang S, Yu X and Mao H: Atg5-mediated autophagy
deficiency in proximal tubules promotes cell cycle G2/M arrest and
renal fibrosis. Autophagy. 12:1472–1486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaminskyy VO and Zhivotovsky B: Free
radicals in cross talk between autophagy and apoptosis. Antioxid
Redox Signal. 21:86–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang WQ, Wen JL, Lin RQ, Wei P and Huang
F: Effects of mTOR/NF-κB signaling pathway and high thoracic
epidural anesthesia on myocardial ischemia-reperfusion injury via
autophagy in rats. J Cell Physiol. 233:6669–6678. 2018. View Article : Google Scholar : PubMed/NCBI
|