Introduction
Cellular senescence was originally reported as
definite proliferative capacity in human fibroblasts culture, which
reflects one particular type of senescence produced by the absence
of endogenous telomere activities (1). Apart from telomere erosion, various
noxious stimuli such as DNA lesions and reactive oxygen species
(ROS) have also been shown to induce a senescent growth arrest
in vitro (2–5). Cellular senescence was originally
proposed to prevent the growth of damaged cells, indicating a
safeguard against cancer (6,7).
Subsequent studies during past decades convincingly demonstrated
that senescent cells are involved in aging, aging-related
dysfunction and chronic diseases (8,9).
Intriguingly, senescent cells have a complex senescence-associated
secretory phenotype (SASP). SASPs were postulated that they
contributed to the development and processing of age-associated
pathologies including malignant transformation by changing tissue
microenvironment (10–12).
In the brain, the aging process is associated with
neuronal degeneration and loss, which eventually lead to cognitive
impairment. Thus, aging is widely recognized as one the most
prominent risk factors for Alzheimer Disease (AD). Brain aging is
typically accompanied with the suppression of innate immunity,
favoring a pro-inflammatory status (13). Astrocytes in aging brains can
trigger SASP, presenting a typical proinflammatory phenotype,
suggesting that senescent astrocytes drives a low-level, chronic
inflammatory status in aged brains (14). Therefore, models of senescence may
permit investigations into potential cellular mechanisms of
astrocytic senescence, in which treatments for delaying or
preventing brain aging and aging-related neuroinflammation or
subsequent degeneration could be developed.
Chronic low dose D-galactose treatment leads to
accelerated aging in rodent and Drosophila models.
Interestingly, rodents chronically treated with D-galactose showed
progressive decline in learning and memory abilities,
neurodegeneration and a damaged immune system (15–17).
Drosophila models exhibited shortened lifespans and
increasing oxidative stress. While D-galactose-induced models have
been widely reported for the study of brain aging processes and
drug candidate screening, how galactose affects organismal brain
aging remains unclear. In the present study, we report evidence
indicating D-galactose-induced astrocytic senescence.
Materials and methods
Reagents and cell culture
D-galactose and Bay 11–7082 were purchased from
Sigma-Aldrich (Merck KGaA). Astrocytic CRT and U373-MG Uppsala
cells (kindly provided by Professor Chul-hee Choi, Korea Advanced
Institute of Science and Technology, South Korea) were maintained
in RPMI-1640 medium (Thermo Fisher Scientific, Inc.) with 10%
heat-inactivated fetal bovine serum (FBS), 100 U/ml of penicillin,
and 100 µg/ml of streptomycin (Thermo Fisher Scientific, Inc.) as
previously described (18). N2a
cells (ATCC® CCL-131™) were grown in Dulbecco's Modified
Eagles medium (DMEM; Thermo Fisher Scientific, Inc.) supplemented
with 1 or 10% heat-inactivated FBS (Thermo Fisher Scientific,
Inc.), 100 U/ml of penicillin and 100 µg/ml of streptomycin.
Primary rat astrocytes were maintained in 10% FBS-DMEM containing
1% nonessential amino acids (Gibco; Thermo Fisher Scientific,
Inc.). Stable cell line CRT-MG/IL-8p-d2EGFP cells were prepared and
maintained as previously described (19). All the cells were incubated at 37°C
in a 5% CO2 atmosphere.
Senescence induction and
senescence-associated β-galactosidase (SA-β-gal) staining
Human astrocytic CRT cells and rat primary
astrocytes were treated with varying doses of D-galactose resolved
in culture medium (0–60 g/l) at 37°C and the cell viability after
72 h exposure was determined. The half-maximal inhibitory
concentration, 50 g/l, of D-galactose was used for the subsequent
experiments. SA-β-gal staining was performed using an SA-β-gal kit
(cat. no. 9860, Cell Signaling Technology, Inc.) in accordance with
the manufacturer's protocols to confirm cellular senescence. The
cells were fixed for 10–15 min at room temperature, then rinsed
twice with PBS and stained with staining solution at a final pH of
6.0 overnight. The SA-β-gal-positive cells were seen as blue, and
were counted under a phase-contrast microscope (magnification,
×100; 5 fields per view analyzed). The experiment was repeated
three times in each group.
Cell viability assay
Cell viability was evaluated by a WST-1 assay, which
is based on the enzymatic cleavage of the tetrazolium salt WST-1 to
formazan by cellular mitochondrial dehydrogenase present in viable
cells. In brief, after 24 h following treatment, 20 µl of WST-1 was
added to each well (12-well plate with 1×105 cells per
well) and the plates were incubated at 37°C for 2 h. The plates
were employed for centrifugation (300 × g) at room temperature for
3 min and 100 µl of the medium was withdrawn and analyzed by
measuring the absorbance at a wavelength of 450 nm using microplate
reader (Tecan Group, Inc.).
Preparation of conditioned medium (CM)
from CRT cells
Astrocytic CRT cells (8×105 cells in 90
mm petri dish) were exposed to D-galactose (50 g/l) at 37°C for 10
days to induce premature secretory senescence. Then, 8 days
following senescence induction, cells were cultured with complete
culture medium (without 10% FBS) for the collection of CM. The CM
was collected from both pre-senescent (untreated cells) and
senescent cells after 48 h. CM were centrifuged for 20 min at 800 ×
g at 4°C, filtered through 0.22 µm bottle-top filters (Sartorius
Stedim Biotech) and used for subsequent experiments. CM was used to
treat U373-MG and N2a cells with different percentages (25, 50, and
100%) at 37°C for 24 h. Cells were then exposed with or without 0.5
mM Temozolomide (TMZ, Sigma-Aldrich; Merck KGaA) at 37°C for 48 h
followed by cell viability tests using the WST-1 method and
observations of morphological changes by microscopy (magnification,
×100; 6 fields per view analyzed).
Interleukin (IL)-6 and IL-8 levels in the CM were
quantified using the Human IL-6 and IL-8 ELISA kits (cat. nos.
D6050 and D8000C, R&D Systems, Inc.) according to the
manufacturer's protocols. Briefly, 100 µl of culture media
normalized by cell number was added in the wells with five
replicates and incubated for 2.5 h at room temperature with gentle
agitation. Then, the medium was discarded and wells were washed
with washing buffer for four times. After that, 100 µl of human
IL-6 or IL-8 conjugate were added to each well and plates was
incubated for 2 h at room temperature with gentle agitation.
Following washing with washing buffer, 100 µl of substrate solution
was added to each well and incubated for 20 min at room
temperature. Lastly, 50 µl of stop solution was added and wells
were assayed with a microplate reader set to 450 nm within 30
min.
Immunoblotting
Whole cell lysate (20 mg) was prepared from various
treatments. The samples (30 µg determined by Bradford Assay) were
loaded onto 10% SDS-PAGE and separated by electrophoresis for 2 h
at 100 V. Protein were transferred polyvinylidene difluoride
membrane for 1 h at 110 V. The following primary antibodies were
used: Antibody p16ink4a, p53, p21, PARP, Cleaved caspase 3, c-Myc
and Tju1 (rabbit polyclonal, 1:2,000, Abcam, cat. nos. ab51243;
ab32389; ab109520; ab74290; ab2302; ab39688 and ab18207,
respectively); senescence marker protein 30 (SMP30; mouse
monoclonal, 1:1,000, Santa Cruz Biotechnology, Inc., cat. no.
sc-390098), GAPDH as a loading control (mouse monoclonal, 1:4,000,
Santa Cruz Biotechnology, Inc., cat. no. sc-47724). Membranes were
washed and incubated with horseradish peroxidase-conjugated
secondary antibodies (1:5,000; Cell Signaling Technology, Inc.,
cat. nos. sc-2370 and sc-2380). The bands from the western blots
were densitometrically visualized by ECL detection and the signals
quantified using ImageJ software (version 1.5.1, National
Institutes of Health).
For the immunofluorescence assay, the cells were
first washed twice with PBS, then fixed with 4% paraformaldehyde
for 15 min and permeabilized with buffer (0.15% Triton X-100 in
PBS) for 20 min at room temperature. Cells were blocked with 3%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 30 min and
then incubated with primary antibodies against NF-κB p65 (rabbit
polyclonal, 1:200, Santa Cruz Biotechnology, Inc., cat.no.
sc-8008), and IL-6 (mouse monoclonal, 1:1,000, Abcam) overnight at
4°C. Following washing three times for 5 min each with PBS and
0.05% Triton X-100. Cells were incubated with appropriate Alexa
Fluor® secondary antibodies 488 or 594 (Invitrogen;
Thermo Fisher Scientific, Inc., cat. nos. A-11008; A21209) to
detect the signal at room temperature for 1.5 h. After another set
of washing, images were captured with a Nikon i2 U fluorescence
microscope (magnification, ×100; Nikon Corporation).
Optical density analysis was conducted to quantify
the fluorescence signal and was performed by using CellProfiler
Software (2.2.0, http://cellprofiler.org). Each evaluation was
conducted on five fields randomly selected for each of the target
proteins.
Statistical analysis
All data were expressed as the mean ± standard
deviation except unless otherwise indicated. P-values were generate
using a Student's t-test or one-way analysis of variance; P<0.05
was considered to indicate a statistically significant difference.
A protected Fisher's Least Significant Difference post hoc test was
used for multiple comparisons. All calculations were performed
using GraphPad Prism software (v7.0, GraphPad Software, Inc.).
Results
D-galactose induces the senescence of
astrocytic CRT cells and rat primary astrocytes
To determine whether D-galactose can induce the
senescence of astrocytic cells, we first cultured astrocytic CRT
cells and rat primary astrocytes in the absence or presence of
varying doses of D-galactose (0–60 g/l) and observed the cell
viability after 72 h treatment (Fig.
1A). Treatment with D-galactose significantly suppressed
cellular viability in a dose-dependent manner from 40 g/l of
D-galactose upwards. Importantly, a higher dose of D-galactose (60
g/l) induced a considerable cell death (profound apoptotic bodies)
as observed under microscopy. Then, we investigated further the
classic markers of the senescence-related phenotype. The astrocytic
CRT and rat primary cells exhibited flattened morphology and
significantly increased levels of SA-β-gal activity compared with
the control (Fig. 1B).
Additionally, the expression of hallmark regulatory proteins, such
as p16, p53, p21 and SMP30 were markedly elevated after D-galactose
exposure (Fig. 1C). Taken
together, these observations demonstrated that, in a relative
long-term of 3 days with D-galactose, astrocytic cells were
susceptible to the stress, thereby inducing a characteristic
senescent phenotype.
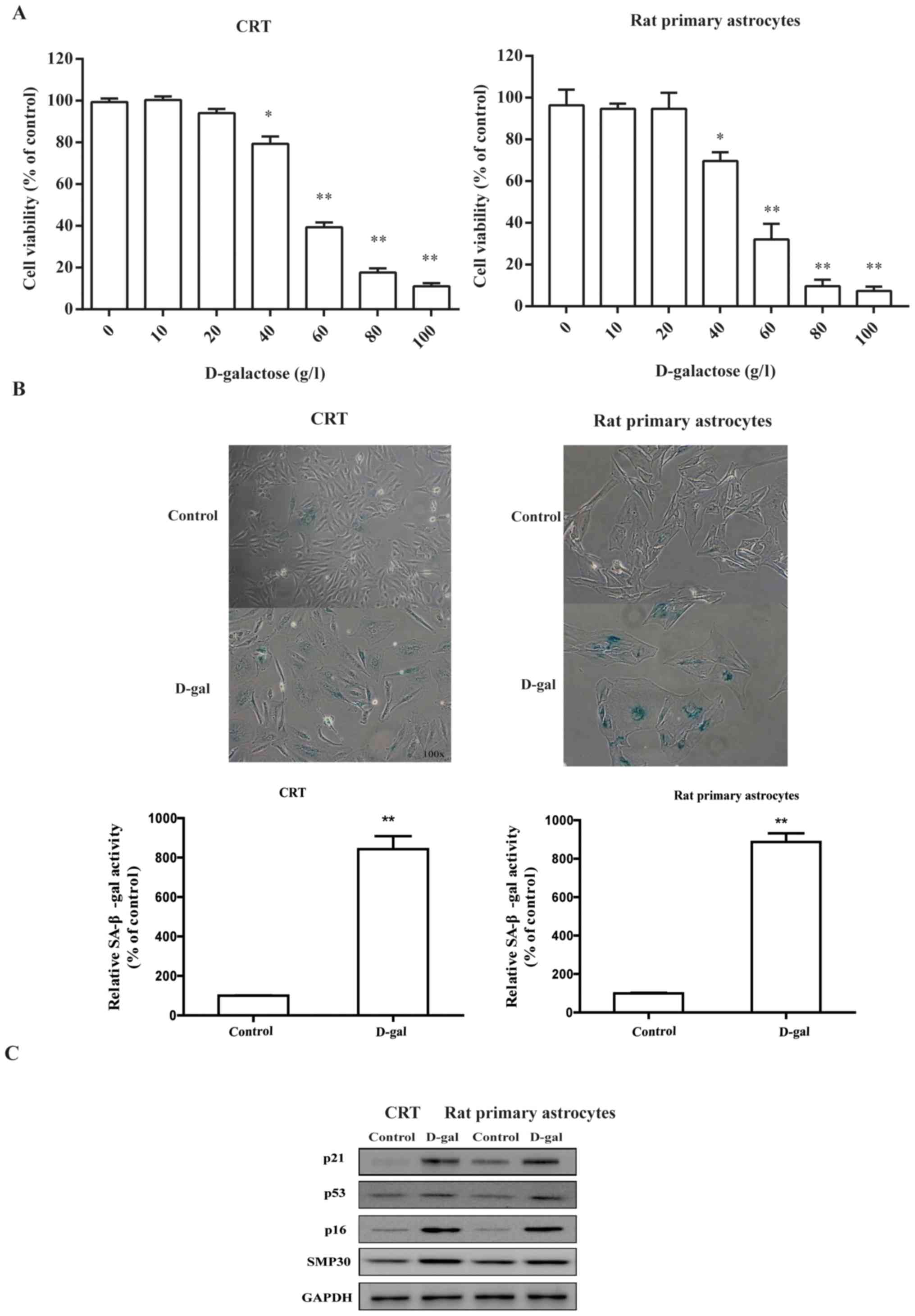 | Figure 1.D-gal treatment suppresses cell
viability and induces cellular senescence of astrocytic CRT cells
and rat primary astrocytes. (A) Astrocytic CRT cells and rat
primary cells were incubated with different concentrations of D-gal
(0, 10, 20, 40 and 60 g/l) for 72 h. Then, cells were analyzed for
their viability. (B) Astrocytic CRT cells and rat primary
astrocytes were treated with 50 g/l D-galactose for 5 days, and the
percentage of cells expressing
senescence-associated-β-galactosidase was determined by light
microscopy (top panel) and counting (bottom panel; both
magnification, ×100). (C) Extracts from non-senescent or senescent
(D-gal) astrocytic CRT cells and rat primary astrocytes were
assayed for the indicated senescence marker proteins (p16, p21,
SMP30 and p53) by western blotting. GAPDH served as loading
control. *P<0.05 and **P<0.01 vs. control group. Three
independent experiments were conducted. D-gal, D-galactose; SMP30,
senescence marker protein 30. |
Senescent astrocytic CRT cells develop
the SASP phenotype
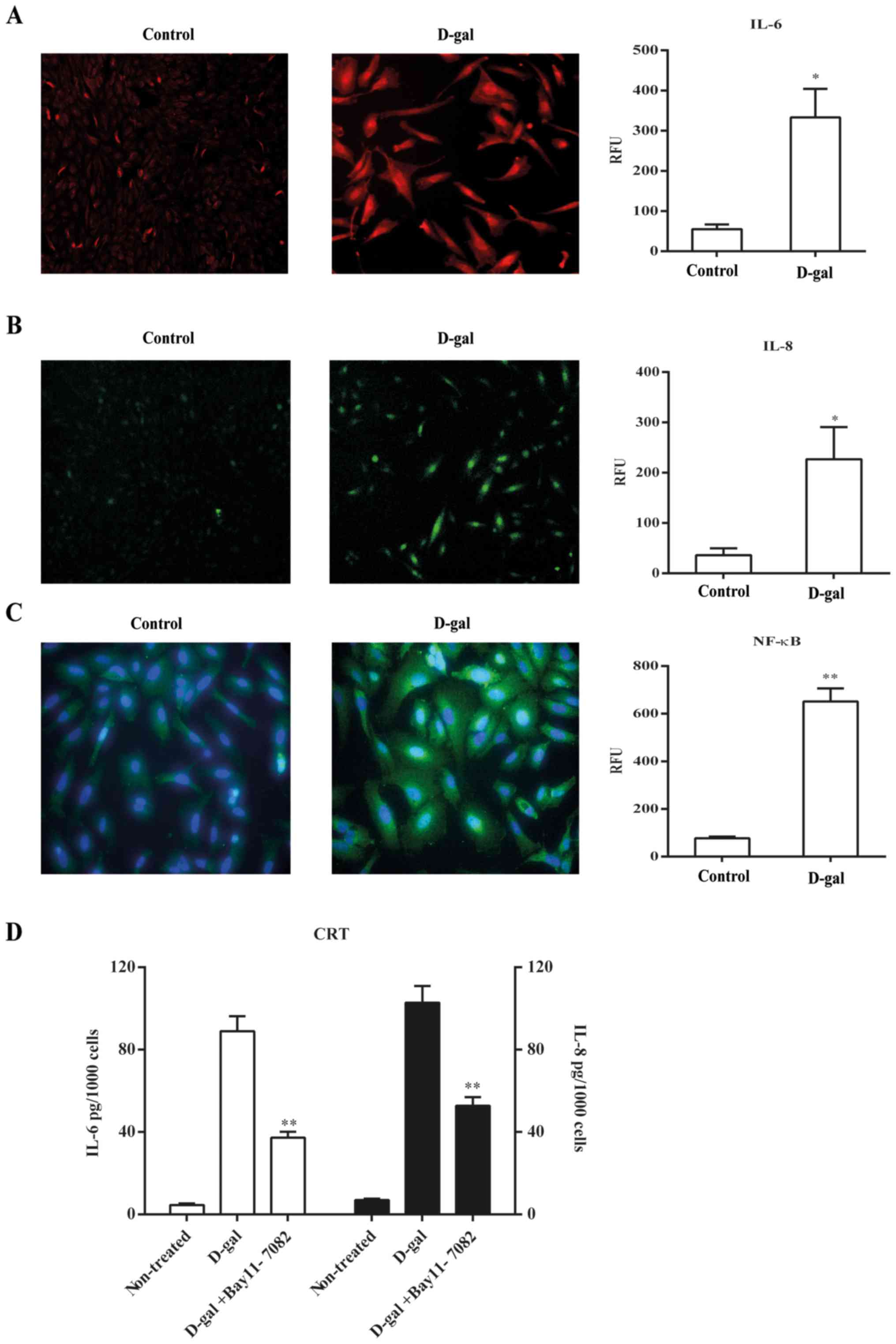
Since the treatment of D-galactose induced typical
the senescent phenotype in CRT cells, we next examined at the
intracellular level the expression of proinflammatory cytokines
IL-6 and IL-8, the major SASP components in rodent and human cells
(20). As expected, D-galactose
significantly increased IL-6 expression by astrocytic CRT-cells
compared with the control (Fig.
2A). In addition, significantly increased IL-8 expression at
the transcriptional level was also exhibited by the stable cell
line CRT-MG/IL-8p-d2EGFP cells compared with the control (Fig. 2B). To explore the molecular
mechanism, we examined the expression of NF-κB by
immunofluorescence, which has been revealed to stimulate the
transcription of many SASP genes (10), and the results indicated a
significant increase in the nuclear translocation after D-galactose
exposure compared with the control (Fig. 2C). Our findings suggested that
D-galactose increased the expression of SASP-related proteins
through activating the NF-κB pathway as early as 6 days after
treatment as determined by elevated secretions of IL-6 and IL-8,
which was abrogated by a NF-κB pathway inhibitor, Bay 11–7082
(Fig. 2D), suggesting that it may
act as a potential target for inhibition on development of SASP in
astrocytes.
CM from senescent astrocytes promotes
brain tumor cell viability and survival from chemotherapy agent
treatment
To explore the role of CM in the process of cellular
senescence, we used two different brain tumor cell lines: U373-MG
and N2a. CM was used to treat U373-MG and N2a cells at various
concentrations (25–100% of culture medium; Fig. 3A and D). Treatment of U373-MG and
N2a cells (with 10% FBS) promoted cell viability, suggesting a
pro-tumoral action. Furthermore, U373 cells treated with different
concentrations of CM were exposed to 0.5 mM temozolomide, and
strong chemotherapy resistance was observed with sustained cell
viability compared with the control treated with temozolomide
(Fig. 3B). This resistance was
further confirmed by the decreased levels of activated
apoptosis-associated molecules cleaved caspase-3 and PARP (Fig. 3C). Intriguingly, N2a cell-derived
neurons (with 1% FBS) were observed as tumor-like neuroblasts after
treatment with CM. The results herein showed that neuron-specific
class III β-tubulin expression levels were markedly decreased, and
tumor-like alterations were observed with reduced dendritic
extensions. In addition, we also observed the increased expression
of c-Myc protein (Fig. 3E), which
is important for cancer cell proliferation (21). Therefore, these studies suggest a
potential key role of CM derived from senescent astrocytic cells in
human brain tumor progression.
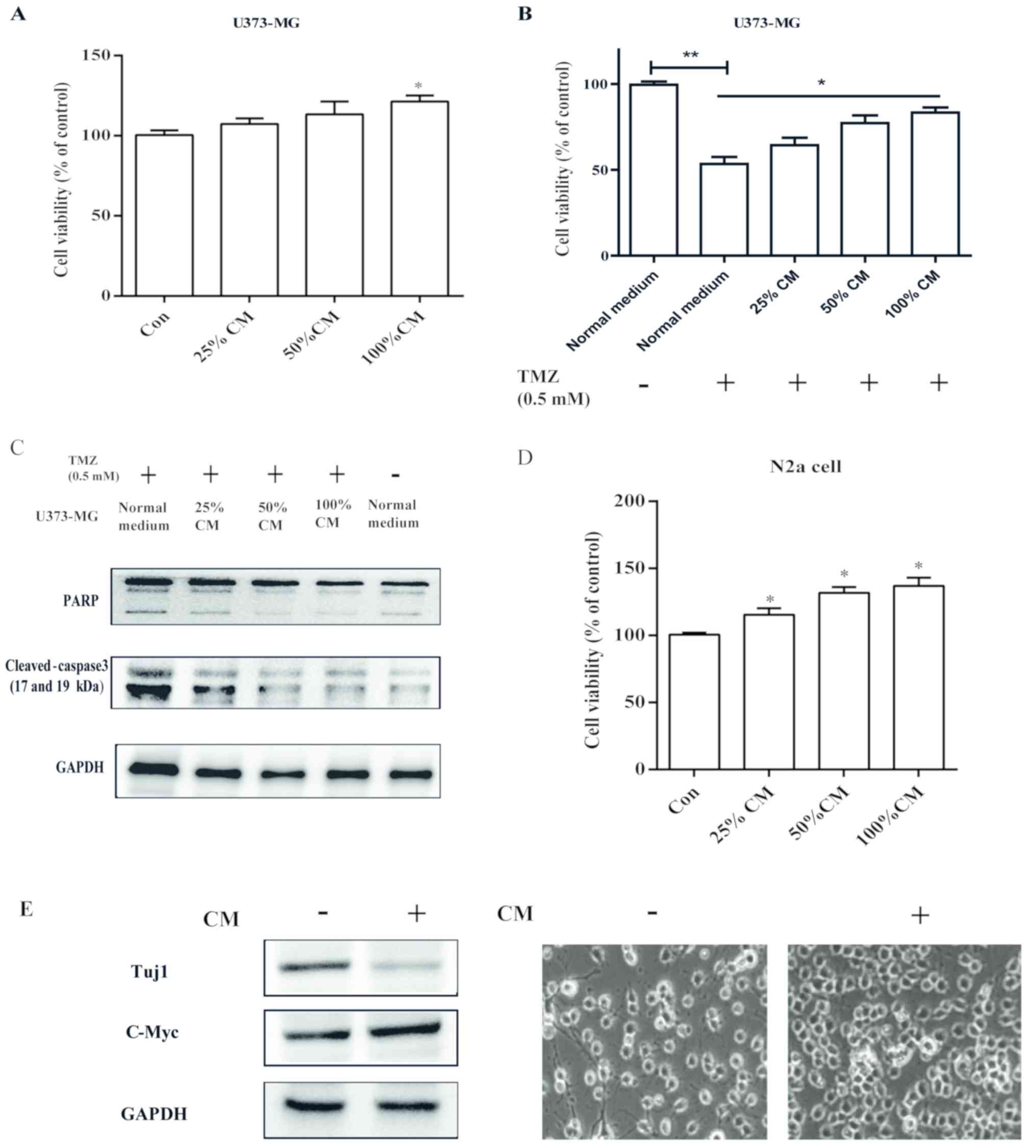 | Figure 3.SASP of astrocytic CRT cells promotes
brain tumor viability and chemotherapy resistance. (A) U373-MG
cells were treated with the indicated concentrations of CM from
senescent astrocytic CRT cells, after which cell viability was
analyzed by a WST-1 assay. (B) U373-MG cells were treated with
normal medium or indicated concentrations of CM for 24 h, after
which cells were plated with fresh medium supplemented with 0.5 mM
TMZ for 48 h. Finally, the cell viability was determined by a WST-1
assay. (C) Extracts from non-treated or TMZ treated, CM-treated
U373-MG cells and TMZ-treated, were assay for cleaved-caspase3 and
PARP by western blotting. (D) N2a cells were treated with the
indicated concentrations of CM from senescent astrocytic CRT cells,
then the viability was analyzed by a WST-1 assay. (E) After
treatment with CM, protein extracts from N2a cells were analyzed
for Tuj1 and c-Myc expression by western blotting for the indicated
tumor and neuronal markers; microscopic analysis was also conducted
(magnification, ×100). GAPDH served as the loading control.
*P<0.05 and **P<0.01 vs. control group. Con, control; CM,
conditioned medium; PARP, poly (ADP-ribose) polymerase; TMZ,
temozolomide; Tuj1, neuron-specific class III β-tubulin. |
Discussion
Our findings suggest that treatment with D-galactose
might induce astrocytic cellular senescence and promote brain aging
pathologies, including the onset of brain cancer by the mechanism
that comprises the SASP. Senescent cells are not limited to
irreversible cell-cycle arrest. Of note, senescent cells have
undergone various changes in protein expression and secretion,
ultimately leading to SASP(11). Coppe et al (22), Orjalo et al (23) and Davalos et al (24) first discovered that senescent cells
could trigger tumorigenesis in neighboring malignant cells, and
disrupt normal tissue structure and function. Additionally, the
accumulation of senescent cells after chemotherapy gives rise to
chemoresistance and cancer recurrence (25). Our study proposed that senescent
astrocytes induced by D-galactose develop SASP via activating the
NF-κB pathway, suggesting a potential mechanism of cellular
senescence in the normal brain aging process.
Of note, low-dose administration of D-galactose is a
widely recognized model for brain aging in rodents. Increasing
evidence has been reported that D-galactose treatment induces
memory impairment, synaptic dysfunction, oxidative stress and
neurodegeneration (26,27); however, little is known about the
underlying mechanism. Elevated levels of D-galactose trigger ROS
accumulation in the brain, ultimately inducing oxidative stress and
the production of advanced glycation end products (AGEs). These
changes have been shown to be involved in physiologically aging and
in neurodegenerative diseases, such as AD and Parkinson's disease,
indicated by the presence in senile plaques and neurofibrillary
tangles (28,29). Additionally, the accumulation of
ROS activates the mitochondrial apoptotic pathway via cytochrome
c release (30) and
markedly decreased glutamine synthetase expression in the brain of
mice (31). Collectively, the
majority of studies have reported on mitochondrial damage by ROS
and the interaction of AGE-receptor for AGE in the brain (32). Our study herein addressed a novel
possibility in vitro: D-galactose might induce brain
inflammation caused by senescent astrocytes and the SASP. The SASP
involves potent inflammatory cytokines such as IL-6 and IL-8, which
may alter tissue environments. This is in agreement with previous
reports in epithelial cell models (33,34).
In summary, our findings reported astrocytic SASP as a potential
mechanism by which D-galactose treatment induces brain aging and
aging-related pathologies, including the development of brain
cancer. Understanding how the SASP is regulated and how it may be
prevented is useful in clinical practice. The model described in
the present study provided a basis for the screening and
development of potential therapeutic strategies to reduce
deleterious effects of astrocytic senescence during the normal
process of aging.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Intelligent
Synthetic Biology Center of the Global Frontier Project, funded by
the Ministry of Education, Science and Technology (grant no.
2011-0031967).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JH wrote the paper. JH and YY performed the
experiments. JH, JX, MS and SK designed the experiments and
improved the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy and integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allsopp RC and Harley CB: Evidence for a
critical telomere length in senescent human fibroblasts. Exp Cell
Res. 219:130–136. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito T, Teo YV, Evans SA, Neretti N and
Sedivy JM: Regulation of cellular senescence by polycomb chromatin
modifiers through distinct DNA damage-and histone
methylation-dependent pathways. Cell Rep. 22:3480–3492. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson AR, Yousefzadeh MJ, Rozgaja TA,
Wang J, Li X, Tilstra JS, Feldman CH, Gregg SQ, Johnson CH, Skoda
EM, et al: Spontaneous DNA damage to the nuclear genome promotes
senescence, redox imbalance and aging. Redox Biol. 17:259–273.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Li P, Xu J, Wu X, Guo Z, Fan L,
Song R, Wang J, Wei L and Teng H: Resveratrol attenuates high
glucose-induced nucleus pulposus cell apoptosis and senescence
through activating the ROS-mediated PI3K/Akt pathway. Biosci Rep.
38:BSR201714542018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Shi D, Wu Q, Zhang Z, Qu H and Jiang
Y: Sodium para-aminosalicylate delays pericarp browning of litchi
fruit by inhibiting ROS-mediated senescence during postharvest
storage. Food Chem. 278:552–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Myrianthopoulos V, Evangelou K, Vasileiou
PVS, Cooks T, Vassilakopoulos TP, Pangalis GA, Kouloukoussa M,
Kittas C, Georgakilas AG and Gorgoulis VG: Senescence and
senotherapeutics: A new field in cancer therapy. Pharmacol Ther.
193:31–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sieben CJ, Sturmlechner I, van de Sluis B
and van Deursen JM: Two-step senescence-focused cancer therapies.
Trends Cell Biol. 28:723–737. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ovadya Y, Landsberger T, Leins H, Vadai E,
Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, et al:
Impaired immune surveillance accelerates accumulation of senescent
cells and aging. Nat Commun. 9:54352018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shakeri H, Lemmens K, Gevaert AB, De Meyer
GRY and Segers VF: Cellular senescence links aging and diabetes in
cardiovascular disease. Am J Physiol Heart Circ Physiol.
315:H448–H462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeon OH, David N, Campisi J and Elisseeff
JH: Senescent cells and osteoarthritis: A painful connection. J
Clin Investig. 128:1229–1237. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baar MP, Perdiguero E, Munoz-Canoves P and
de Keizer PL: Musculoskeletal senescence: A moving target ready to
be eliminated. Curr Opin Pharm. 40:147–155. 2018. View Article : Google Scholar
|
|
12
|
Gonzalez-Meljem JM, Apps JR, Fraser HC and
Martinez-Barbera JP: Paracrine roles of cellular senescence in
promoting tumourigenesis. Br J Cancer. 118:1283–1288. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corlier F, Hafzalla G, Faskowitz J, Kuller
LH, Becker JT, Lopez OL, Thompson PM and Braskie MN: Systemic
inflammation as a predictor of brain aging: Contributions of
physical activity, metabolic risk, and genetic risk. Neuroimage.
172:118–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salminen A, Ojala J, Kaarniranta K,
Haapasalo A, Hiltunen M and Soininen H: Astrocytes in the aging
brain express characteristics of senescence-associated secretory
phenotype. Eur J Neurosci. 34:3–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Wu Dm, Zheng Y, Hu B and Zhang Zf:
Purple Sweet Potato Color Alleviates D-galactose-induced brain
aging in old mice by promoting survival of neurons via PI3K pathway
and inhibiting cytochrome c-mediated apoptosis. Brain Pathol.
20:598–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W
and Liu J: D-Galactose-caused life shortening in Drosophila
melanogaster and Musca domestica is associated with oxidative
stress. Biogerontology. 5:317–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shwe T, Pratchayasakul W, Chattipakorn N
and Chattipakorn SC: Role of D-galactose-induced brain aging and
its potential used for therapeutic interventions. Exp Gerontol.
101:13–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi C, Xu X, Oh JW, Lee SJ, Gillespie GY,
Park H, Jo H and Benveniste EN: Fas-induced expression of
chemokines in human glioma cells: Involvement of extracellular
signal-regulated kinase 1/2 and p38 mitogen-activated protein
kinase. Cancer Res. 61:3084–3091. 2001.PubMed/NCBI
|
|
19
|
Choi C, Kutsch O, Park J, Zhou T, Seol DW
and Benveniste EN: Tumor necrosis factor-related apoptosis-inducing
ligand induces caspase-dependent interleukin-8 expression and
apoptosis in human astroglioma cells. Mol Cell Biol. 22:724–736.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhat R, Crowe EP, Bitto A, Moh M, Katsetos
CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C and Torres C:
Astrocyte senescence as a component of alzheimer's disease. PLoS
One. 7:e450692012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akinyeke T, Matsumura S, Wang X, Wu Y,
Schalfer ED, Saxena A, Yan W, Logan SK and Li X: Metformin targets
c-MYC oncogene to prevent prostate cancer. Carcinogenesis.
34:2823–2832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coppe JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orjalo AV, Bhaumik D, Gengler BK, Scott GK
and Campisi J: Cell surface-bound IL-1alpha is an upstream
regulator of the senescence-associated IL-6/IL-8 cytokine network.
Proc Natl Acad Sci USA. 106:17031–17036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davalos AR, Coppe JP, Campisi J and
Desprez PY: Senescent cells as a source of inflammatory factors for
tumor progression. Cancer Metastasis Rev. 29:273–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le Duff M, Gouju J, Jonchère B, Guillon J,
Toutain B, Boissard A, Henry C, Guette C, Lelièvre E and Coqueret
O: Regulation of senescence escape by the cdk4-EZH2-AP2M1 pathway
in response to chemotherapy. Cell Death Dis. 9:1992018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J,
Packer L and Liu J: Chronic systemic D-galactose exposure induces
memory loss, neurodegeneration, and oxidative damage in mice:
Protective effects of R-alpha-lipoic acid. J Neurosci Res.
84:647–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu J, Zheng YL, Wu DM, Luo L, Sun DX and
Shan Q: Ursolic acid ameliorates cognition deficits and attenuates
oxidative damage in the brain of senescent mice induced by
D-galactose. Biochem Pharmacol. 74:1078–1090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu R, Zhang TT, Zhou D, Bai XY, Zhou WL,
Huang C, Song JK, Meng FR, Wu CX, Li L and Du GH: Quercetin
protects against the Aβ(25–35)-induced amnesic injury: Involvement
of inactivation of rage-mediated pathway and conservation of the
NVU. Neuropharmacology. 67:419–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xing Z, He Z, Wang S, Yan Y, Zhu H, Gao Y,
Zhao Y and Zhang L: Ameliorative effects and possible molecular
mechanisms of action of fibrauretine from Fibraurea recisa Pierre
on d-galactose/AlCl3-mediated Alzheimer's disease. RSC
Advances. 8:31646–31657. 2018. View Article : Google Scholar
|
|
30
|
Kumar A, Prakash A and Dogra S: Naringin
alleviates cognitive impairment, mitochondrial dysfunction and
oxidative stress induced by D-galactose in mice. Food Chem Toxicol.
48:626–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang XL, An LJ, Bao YM, Wang JY and Jiang
B: D-galactose administration induces memory loss and energy
metabolism disturbance in mice: Protective effects of catalpol.
Food Chem Toxicol. 46:2888–2894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ali T, Badshah H, Kim TH and Kim MO:
Melatonin attenuates D-galactose-induced memory impairment,
neuroinflammation and neurodegeneration via RAGE/NF-κB/JNK
signaling pathway in aging mouse model. J Pineal Res. 58:71–85.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parrinello S, Coppe JP, Krtolica A and
Campisi J: Stromal-epithelial interactions in aging and cancer:
Senescent fibroblasts alter epithelial cell differentiation. J Cell
Sci. 118:485–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laberge RM, Awad P, Campisi J and Desprez
PY: Epithelial-mesenchymal transition induced by senescent
fibroblasts. Cancer Microenviron. 5:39–44. 2012. View Article : Google Scholar : PubMed/NCBI
|