Introduction
Prostate cancer (PC) remains the most commonly
diagnosed malignant disease in males (1). Increasing evidence has revealed that
the tumor microenvironment exerts a crucial role in the progression
of PC (2,3). Furthermore, the tumor
microenvironment exhibits a high degree of heterogeneity in
different individuals (4). In PC,
inflammatory cells, immune cells, the vasculature, stromal cells,
extracellular matrix and immune cells constitute the tumor
microenvironment (5). In terms of
its involvement in PC, the essential function of the tumor
microenvironment manifests via the recurrence of the disease,
metastasis and castration resistance (6).
In previous studies, the protein, collagen triple
helix repeat containing 1 (CTHRC1), has been proposed as a pivotal
tumor promoter (7–9). Additionally, CTHRC1 has been revealed
to activate the planar cell polarity pathway via stabilization of
the Wnt-receptor complex (10). In
certain solid tumors, including those of pancreatic ductal
adenocarcinoma (11), gastric
cancer (12), hepatocellular
carcinoma (13) and esophageal
squamous cell carcinoma (14),
CTHRC1 has been demonstrated to be markedly upregulated, although
its role in PC is yet to be fully elucidated.
Currently, therapeutic applications of the
immunological microenvironment in the treatment of PC have been
extensively studied (15). Among
them, immune checkpoint therapy has achieved impressive results in
certain types of neoplasms. In this case, typical immunotherapeutic
antibodies targeting programmed cell death protein 1
(PD-1)/programmed cell death 1 ligand 1 (PD-L1) have been applied
in clinical trials for a number of different types of cancer,
including PC (16,17). However, not all patients react
favorably towards the treatment; thus, further research is required
to elucidate the underlying mechanism.
At present, to the best of our knowledge, the
association between the expression levels of CTHRC1, PD-1
and PD-L1 in PC has not been studied. The premise of the present
study was therefore to use the PC dataset in the Tumor Immune
Estimation Resource (TIMER) database to determine the association
between the CTHRC1 network and PC recurrence. Additionally, the
expression levels of PD-1/PD-L1 and CTHRC1 in a cohort of PC
patients was analyzed, and their potential association with
recurrence was investigated.
Materials and methods
Data mining of the cancer genome Atlas
(TCGA)-prostate adenocarcinoma (PRAD) dataset
The correlation between the disease-free survival
(DFS) rate and the expression level of CTHRC1 in PRAD was
computed using the Gene Expression Profiling Interactive Analysis
(GEPIA) online database (18). The
TIMER web server was utilized to examine the correlation between
CTHRC1 mRNA expression and its clinical impact in dif-ferent immune
cells in PRAD (19), thereby
acquiring the immunity profile of TCGA-PRAD dataset.
Patient selection and tissue
microarray (TMA) construction
Prostate tissue samples and the associated clinical
pathology data from a total of 122 patients with PC were retrieved
from the archives of the Department of Pathology, Xiangya Hospital,
Central South University. All the enrolled patients underwent
radical prostatectomy between January 2003 and December 2010, and
they did not receive chemotherapy or radiation prior to surgery.
For the use of clinical materials for research purposes, written
informed consent was obtained from each patient prior to surgery.
The research program was approved by the Ethics Committee of The
First Affiliated Hospital of Hunan University of Chinese Medicine.
The clinical characteristics of patients enrolled in our study were
presented in Table I. The modified
Gleason and 2010 pathological tumor-node-metastasis classification
grading systems (20) were applied
to categorize the hematoxylin and eosin-stained tumor sections of
each sample (21). Ultimately, TMA
blocks containing the PRAD tissues from the 122 cases were
structured.
 | Table I.Summary of clinical characteristics
of PC (n=122). |
Table I.
Summary of clinical characteristics
of PC (n=122).
| Clinical
characteristics | Value |
|---|
| Age (years) |
|
|
Mean | 59.7 |
|
Range | 40-72 |
| Pre-operative
prostate-specific antigen (ng/ml) |
|
|
<4 | 2 |
|
4-10 | 86 |
|
>10 | 33 |
|
Unknown | 1 |
| Gleason grade |
|
| 5 | – |
| 6 | 18 |
| 7
(3+4) | 96 |
| 8 | 7 |
| 9 | 1 |
| 10 | 0 |
| Pathology
stage |
|
|
pT2 | 37 |
|
pT3a | 75 |
|
pT3b | 8 |
|
pT4 | 2 |
| Positive surgical
margins | 43 |
| Extra-capsular
extension | 85 |
Cell culture and western blot
assay
Human prostate cancer cell lines LNCaP and PC-3
cells, and human prostate normal cells RwPE-2 were obtained from
the American Type Culture Collection. All cells were propagated
standard cell culture conditions (5% CO2, 37°C) with
Dulbecco's Modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) and 10% fetal calf serum. After 3 days in
culture, cells were harvested and lysed. Cell extracts were
prepared in a lysis buffer (150 mM NaCl, 20 mM HEPES, 1% Triton
X-100, 2 mM EGTA, 20 mM glycerol phosphate, 1 mM EDTA, and 10%
glycerol with protease). Protein levels of cells were determined by
a Bradford's assay. A total of 50 µg protein were denatured and
loaded on 10% sodium dodecyl sulfate polyacrylamide gels. PVDF
membranes (EMD Millipore). After blocking with 5% milk in TBST, the
membranes were blocked for 1 h in Blocking Buffer (5% 1X TBST) for
90 min before incubation overnight (at 4°C) Buffer (5% 1X TBST) for
90 min before incubation overnight (4°C). The proteins were
transferred to PVDF membranes, and incubated with the corresponding
primary and secondary antibodies for 90 min at 37°C before
incubation then overnight at 4°C. The following primary antibodies
were used: Anti-CTHRC1 (polyclonal antibody 1:1,000; Novus
Biologicals, LLC, cat. no. AF5960). Anti-actin (cat. no.
SAB4200248; monoclonal antibody, 1:3,000; Sigma-Aldrich; Merck
KGaA) antibody was used as protein loading control. A horseradish
peroxidase-conjugated secondary antibody (polyclonal antibody
1:10,000, Santa Cruz Biotechnology, Inc.) was used for the assay.
The protocols of western blot assay were employed as described
previously (22).
Immunohistochemistry (IHC)
analysis
IHC was performed as previously described (23). Sections as thin as 4 µm were sliced
from paraffin-embedded tissue blocks. Antibody dilutions used were
1:500 for the CTHRC1 polyclonal antibody (cat. no. AF5960; Novus
Biologicals, LLC), 1:1,000 for the PD-L1 polyclonal antibody (cat.
no. PA5-18337; Thermo Fisher Scientific, Inc.), and 1:1,000 for the
PD-1 polyclonal antibody (cat. no. PA5-32543; Thermo Fisher
Scientific, Inc.) for 30 min at 37°C. The samples were incubated
with horseradish peroxidase-labeled polymer conjugated to goat
anti-rabbit immunoglobulin G (IgG; cat. no. K5007; the DAKO
EnVision™+ system; Agilent Technologies, Inc.) for 30 min at room
temperature, and subsequently developed using 3,3′-diaminobenzidine
as the color substrate for 1 min at room temperature.
Stained IHC sections were evaluated by two expert
pathologists using uniform criteria from the Department of
Pathology of Xiangya Hospital in Central South University; ~100
cells were randomly selected, and the positive cells were filtered
from five average isolated fields of each section. Protein
expression was evaluated according to the extent and intensity of
staining. The percentage of positive cells was measured on a scale
of 0–3: <5% scored 0; 6–25% scored 1; 25–50% scored 2; >50%
scored 3. The intensity of staining was measured on a scale of 0–3:
0, no staining; 1, weak staining; 2, moderate staining; 3, strong
staining. A median score was employed as the cut-off value to
differentiate between low and high expression by using the score of
positive staining multiplied by the grade of intensity
staining.
Statistical analysis
The categorical vari-ables were compared using a
χ2 test. Continuous parametric variables were analyzed
by Student's t-test or a Mann-Whitney test and data were presented
as the mean ± standard error of the mean. Correlation between two
groups was determined using Spearman correlation coefficient
analysis. In multivariate analysis, Cox's regression model was
employed to test the contribution of individual factors to
survival. P<0.05 was considered to indicate a statistically
significant value. GraphPad Prism software (GraphPad Software,
Inc.) was used for all statistical analyses.
Results
Patient characteristics
The validation cohort included 122 cases who
underwent radical prostatectomy, aged 45–73 years (median age, 64
years). A total of 42 patients experienced cancer recurrence, and
86 patients were determined to have a high preoperative serum
prostate-specific antigen levels (>5 ng/ml; 70.4%). In light of
the combined Gleason score, patients were assigned either to group
A (score ≤7; 79.7%) or B (score >7; the remaining 21.3%). The
patients' tumor stage was distributed either in pT2 (99/122; 81.4%)
or pT3 (23/122; 18.6%).
Upregulation of CTHRC1 expression in
PRAD
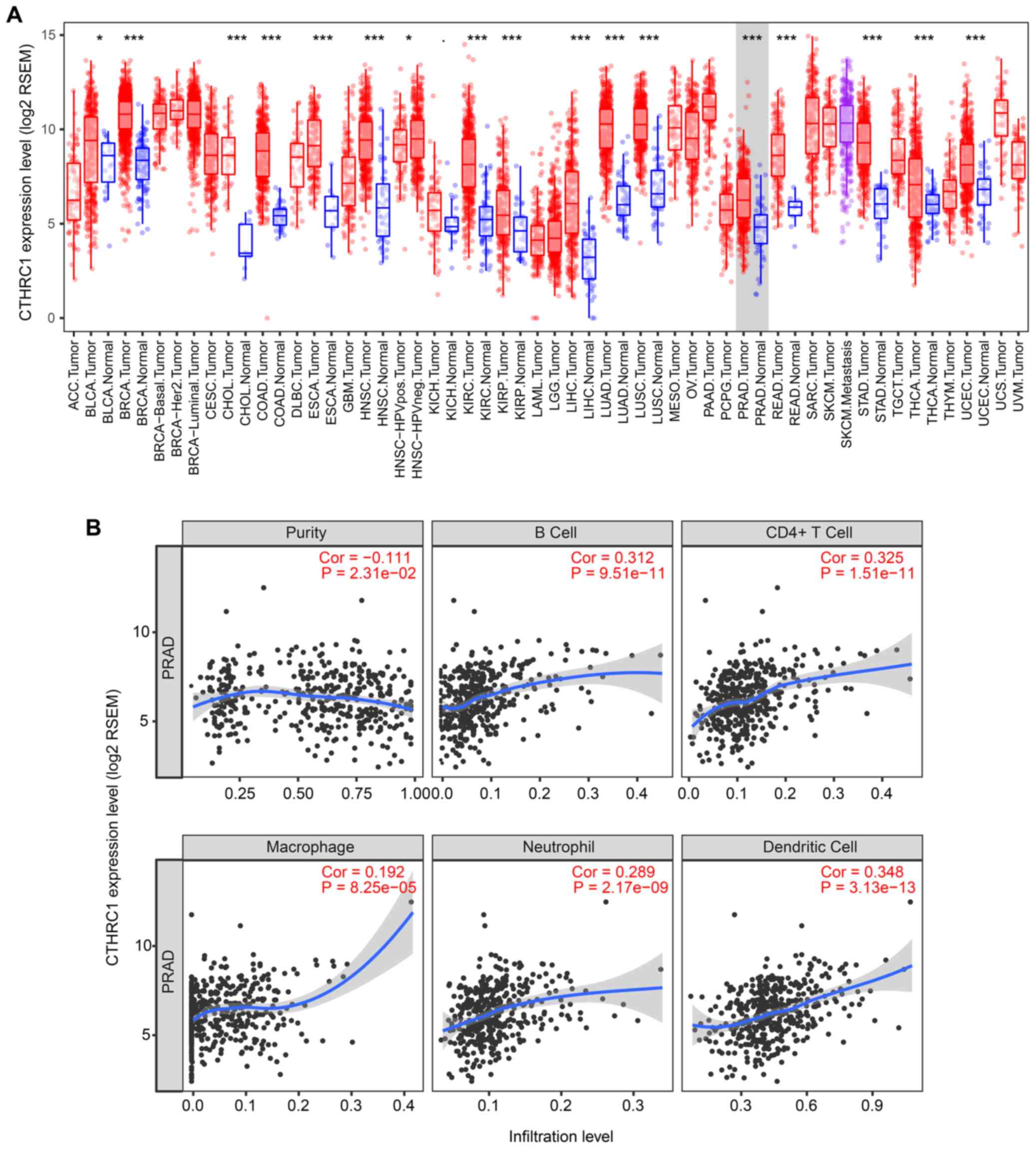
As shown in Fig.
1A, the mRNA expression levels of CTHRC1 were
significantly decreased in PRAD compared with normal tissues by
using the TIMER web server (P<0.001). CTHRC1 has been
shown to serve an immune modulatory role (18). It is noteworthy that the increase
in tumor purity (i.e., the percentage of cancer cells in a solid
tumor sample) was inversely correlated with the expression of
CTHRC1 (Fig. 1B).
Specifically, this may have resulted from an increase in the
numbers of infiltrating B cells, CD4+ cells,
macrophages, neutrophils and dendritic cells.
Association between CTHRC1, recurrence
and poor survival
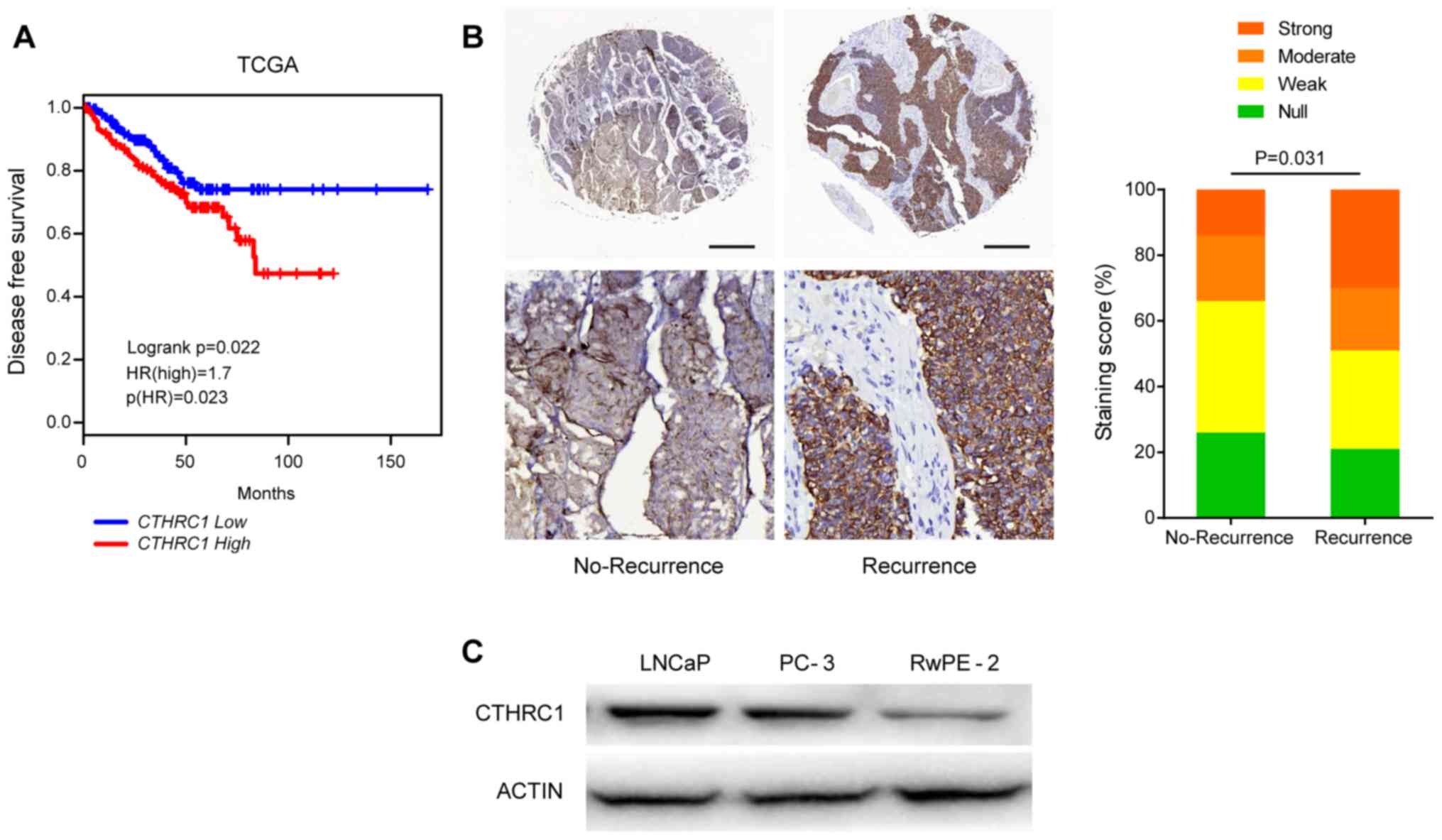
Furthermore, survival analysis based on the data
from the GEPIA web server also revealed that high levels of
CTHRC1 expression were associated with a lower DFS rate in
PRAD (P=0.022; Fig. 2A). In the
IHC cohort, CTHRC1 protein expression was detected in the
present study. These results indicated that CTHRC1
expression was markedly higher in recurrent PRAD tumor tissues
compared with recurrence-free tissues (P=0.031; Fig. 2B). Western blotting verified the
similar trend in elevated CTHRC1 protein expression in PRAD cell
lines (Fig. 2C).
Association of CTHRC1 with PD-1/PD-L1
expression in PRAD
Similarly to CTHRC1, high levels of
expression of PD-1/PD-L1 were associated with tumor progression,
including PRAD (24); therefore,
we proposed that our contradictory findings were due to the
presence of an increased number of tumor-infiltrating leukocytes,
with concomitantly increased levels of PD-1/PD-L1 expression in
PRAD. The association between CTHRC1 expression and immune
infiltrates was analyzed, as indicated by the decreased tumor
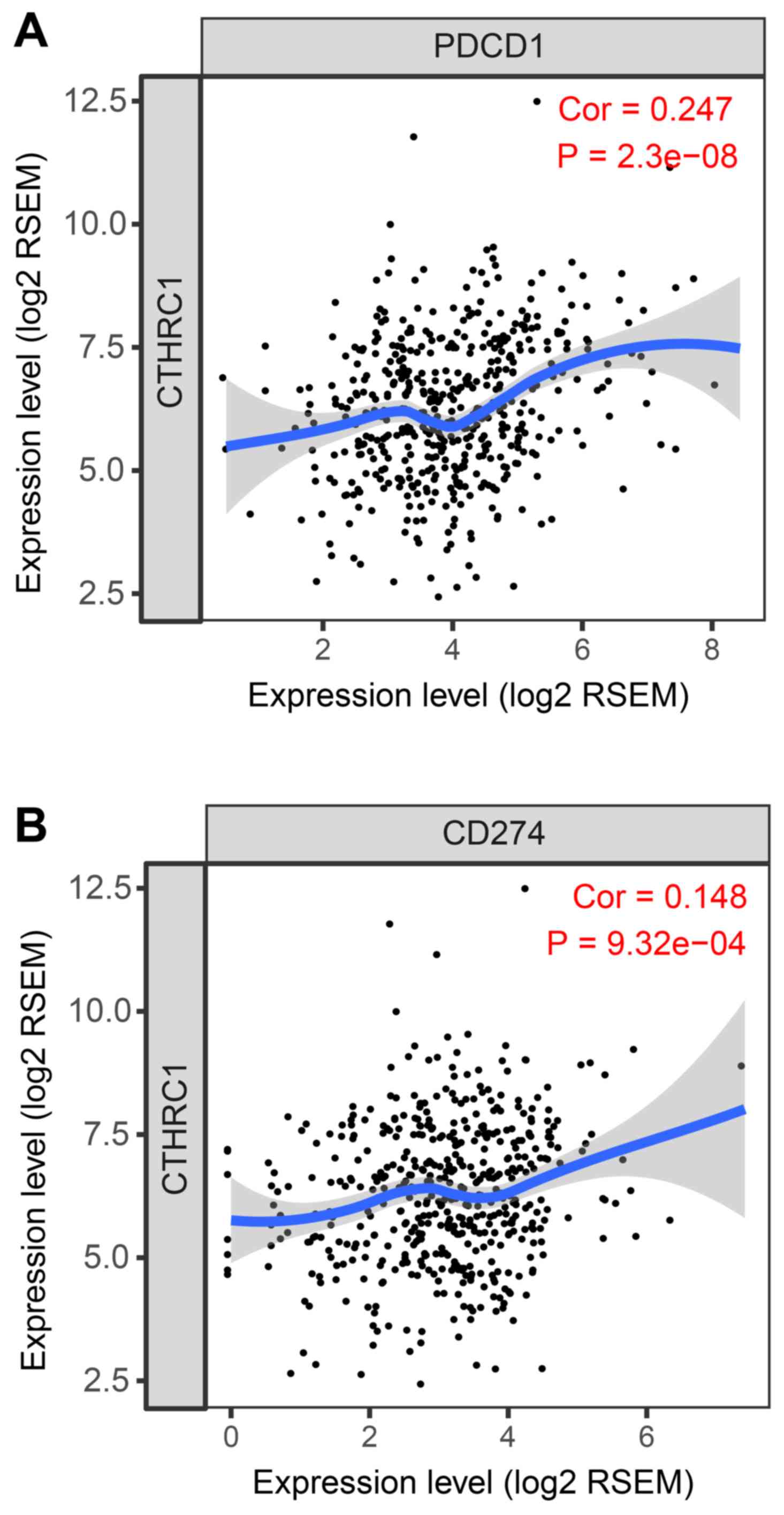
purity. CTHRC1 mRNA expression was positively correlated
with increased levels of PD-1 and PD-L1 expression in the TCGA
cohort (Fig. 3). In our validation
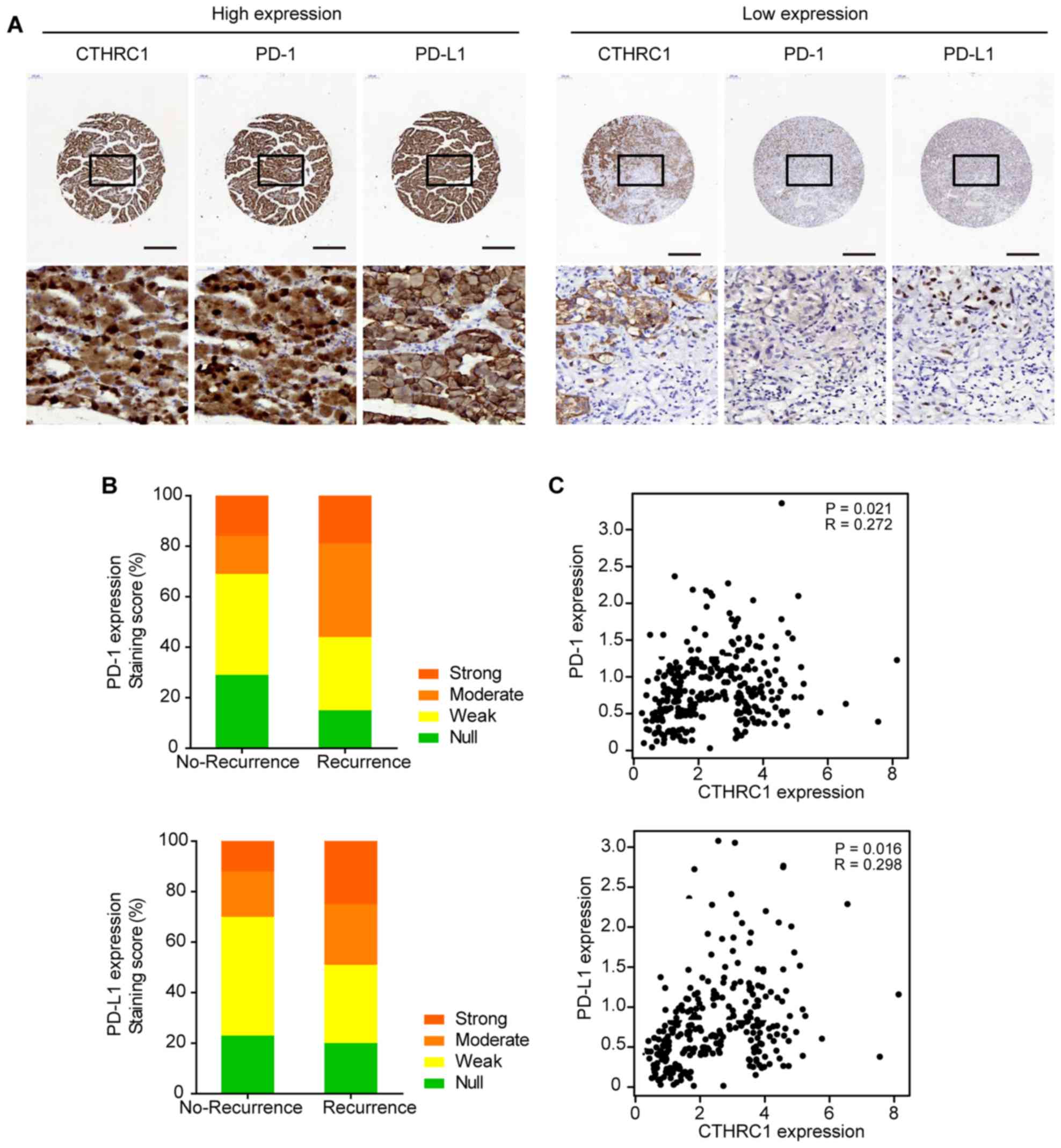
cohort, CTHRC1-positive staining was detected in 43.6%
(53/122) of the tumor samples. PD-1 was assessed to have positive
staining reactivity in 30.3% (36/122) of tumors, whereas PD-L1 was
assessed to have positive staining reactivity in 38.5% (47/122) of
the tumors. Of note, cases with higher levels of CTHRC1
expression exhibited significantly increased levels of PD-1/PD-L1
in PRAD (Fig. 4A). IHC staining
score analysis showed that PD-1/PD-L1 expression was notably higher
in patients with recurrence of PRAD than those with no-recurrence
(Fig. 4B). Furthermore,
CTHRC1 expression did not only correlate with PD-1 (R=0.272,
P=0.021), but also with PD-L1 expression (R=0.298, P=0.016)
(Fig. 4C).
CTHRC1 expression is associated with
the signaling molecules of the tumor microenvironment
As presented in Fig.
5, in the TIMER web server, the mRNA expression of
CTHRC1 was positively correlated with certain genes in PRAD,
including the expression of matrix metalloproteinase (MMP)9
(25), mucin 1 (26), solute carrier organic anion
transporter family member 2B1 (27), phosphatase and tensin homolog
(28,29), E-cadherin and CD44 (30). The expression of these genes has
been suggested to be associated with functions that support tumor
growth, invasion and the microenvironment of PRAD (31–33).
However, further investigation in CTHRC1 in PRAD is required
to determine its critical role in tumor growth and invasion.
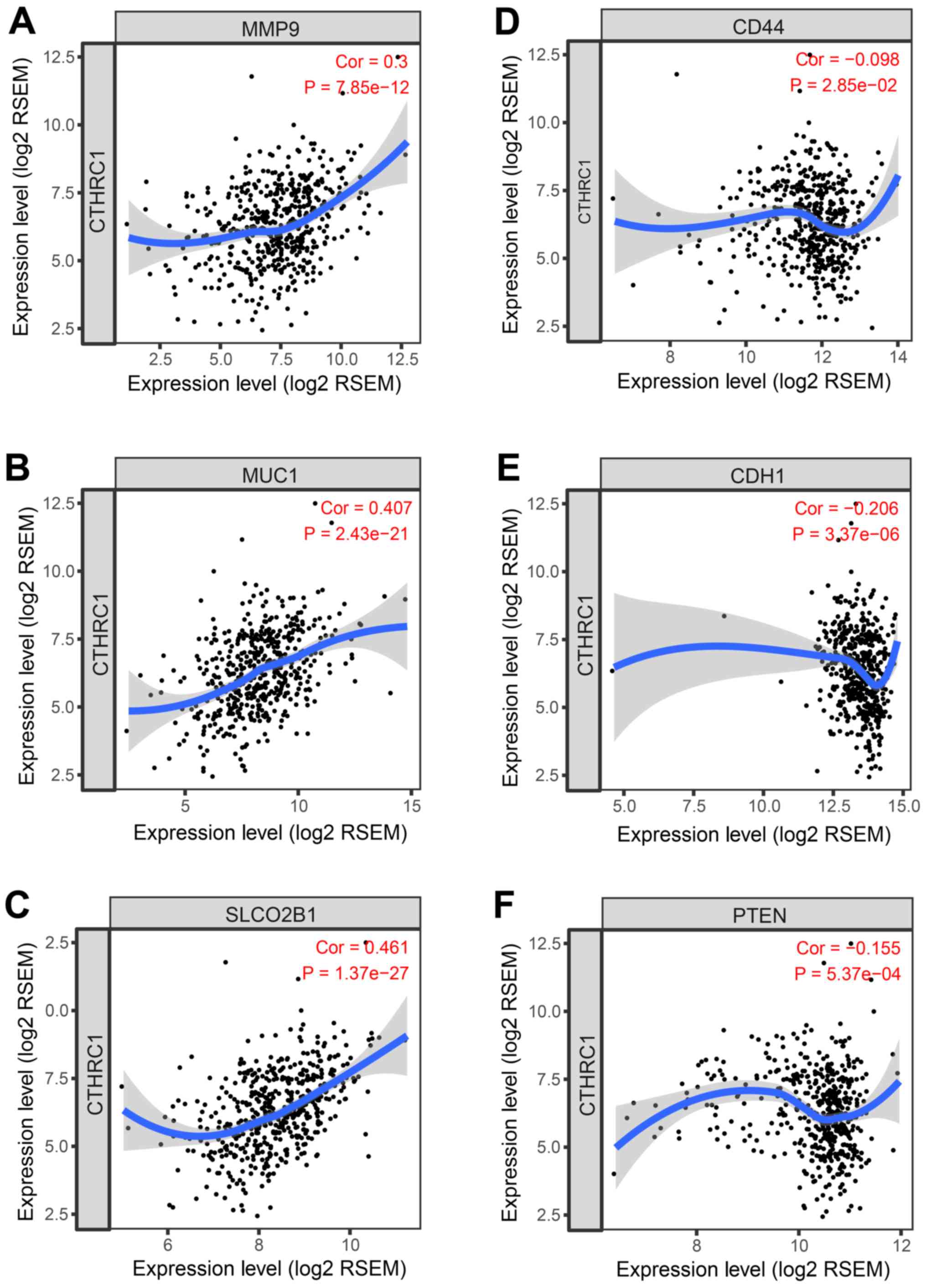 | Figure 5.Spearman's correlation analysis of
the tumor markers, (A) MMP9, (B) MUC1, (C) SLCO2B1, (D) CD44, (E)
CDH1, and (F) PTEN with CTHRC1 expression in the PRAD profiles of
The Cancer Genome Atlas dataset. MMP9, matrix metalloproteinase 9;
MUC1, mucin 1; SLCO2B1, solute carrier organic anion transporter
family member 2B1; CTHRC1, collagen triple helix repeat containing
1; CDH1, E-cadherin; PTEN, phosphatase and tensin homolog; RSEM,
RNA-Seq by expectation-maximization. |
Discussion
Although our understanding of the prognostic role of
cancer-associated CTHRC1 gene expression in solid tumors has
improved in light of several recently studies (34,35),
CTHRC1 function in cancer immune modulation remains unclear.
The present study investigated the aberrant expression of
CTHRC1 in PC and its potential role. Higher levels of
expression of CTHRC1 were shown to be associated not only
with poor DFS, but also with poor immune response in a TCGA-PRAD
dataset and validation cohort in the present study. A previous
study has also confirmed the potential role of CTHRC1 in
cancer cells (36). However,
little is currently known about the role of this gene in human
immune cells. The most important finding of the current study was
the identification of a positive correlation between CTHRC1
and increased numbers of infiltrating B cells, CD4+
cells, macrophages, neutrophils, and dendritic cells. This is a
potential novel feature of PRAD in the context of the immune
response, driven by CTHRC1.
Immune checkpoint therapies have demonstrated broad
antitumor activity in cancer treatment. Monoclonal antibodies
targeting PD-L1/PD-1 have also been approved as antitumor
immunotherapy in clinical trials (NCT00730639) for PC (37). Since blocking PD-1/PD-L1 should
lead to an improvement in anticancer immunity and immunosuppression
of the tumor microenvironment, predictive biomarkers to test the
efficacy are urgently needed. Data from the aforementioned clinical
trials of 122 patients with PC revealed that the expression of
PD-L1 as determined by IHC and suppressed immunity were able to
predict more effective therapies of anti-PD-1/PD-L1 in patients of
numerous cancer types (38).
Recently, Shalapour et al (39) revealed that eradication of the
immunosuppressive IgA+ IL-10+
PD-L1+ plasmocytes reactivated the killing capacity of
CD8+ cytotoxic T cells to tumor cells in aggressive
mouse prostate cancer models. Another intriguing finding of the
present study was that CTHRC1 expression was increased in
PRAD, and PD-1/PD-L1 expression were also upregulated. PD-L1,
together with CTHRC1, an inflammation-associated factor,
have been demonstrated to be an independent factor for predicting
prognosis in tumors and autoimmune diseases (35). However, the R value of correlation
analysis on the CTHRC1 and PD-1/PD-L1 was <0.3. As genes highly
expressed in the microenvironment are expected to have negative
associations with tumor purity, the opposite is expected for genes
highly expressed in the tumor cells; the abundance of immune cells
in tumor tissues is extremely low (40). As the TIMER database contains
microarray expression values of glioblastoma multiforme/ovarian
serous cystadenocarcinoma) for calculation; the results have
statistical significance and correlation although R is <0.3. In
addition, one study meets the criteria (40); patients with high expression of
CTHRC1 and PD-L1 had poor clinical outcome. The findings of
the present study were consistent with those studies, and are, to
the best of our knowledge, the first to propose a predictive role
for CTHRC1 and PD-L1 in the prognosis of PC.
The role served by CTHRC1 in cancer, and the
underlying mechanism, at present remain unknown. CTHRC1 has
often been shown to be aberrantly expressed in human solid tumors,
promoting cancer cell invasion and metastasis (41). Recent studies have suggested that
it functions in modulating the tumor microenvironment, particularly
the extracellular matrix via the E6/E7-p53-POU2F1 axis or focal
adhesion kinase signaling (7,8). In
addition, numerous mRNAs have been revealed to target CTHRC1, and
directly regulate cell proliferation and metastasis (42–44).
CTHRC1 also promoted the infiltration of M2-like tumor-associated
macrophages by upregulating Fractalkine chemokine receptor
expression (45). The precise
mechanism underlying the CTHRC1-mediated tumor progression
of PC, however, remains to be elucidated. Recent studies have
indicated that B cells are likely to have a dual role in regulating
cancer immunity (46,47). The tumor microenvironment may
enable a population of tumor cells to escape the immune response by
impairing the migration and homing of dendritic cells (48). Since tissue invasion and metastasis
are both associated with cancer cell migration (49), it is possible that CTHRC1
contributes to these processes by increasing tumor cell migration.
The present study suggests that an increased number of B cells may
not be a contributory factor towards prognosis in patients
overexpressing CTHRC1. Although it appears that B cells do
exert a pro-tumorigenic role in PRAD, the results of the present
study only fulfil an observational capacity in a cross-sectional
perspective.
A previous study demonstrated that prostaglandin E2
also led to an increase in MMP9 expression, and activated Notch1
signaling on dendritic cells (50). In the present study, CTHRC1
was revealed to correlate with MMP9 expression. Therefore, our
findings may suggest a novel perspective for therapeutic
interventions in terms of PC immune escape. There are limitations
of the present study. First, a novel prognostic marker for the
pro-tumorigenic pathway with CTHRC1 upregulation in PRAD has
been proposed, although how CTHRC1 is associated with the
mechanism of immune escape requires further investigation.
Secondly, the association between CTHRC1 and immunity
supports the application of appropriate PD-1/PD-L1 inhibitors in
PRAD; however, further investigation is required to provide further
insight into the role of CTHRC1 in PC cell proliferation and
growth in vitro and in vivo for PRAD.
In conclusion, the present study has identified a
link between CTHRC1 and PD-1/PD-L1 expression in PRAD.
CTHRC1 may serve as a candidate target in treating PRAD. Our study
has an additional limitation. The characteristics of CTHRC1
expression were found in PC samples from a Chinese population;
thus, validation is required from prostate cancer patient cohorts
from other regions.
Acknowledgements
The authors would like to thank Dr Xiong Cai of the
Technology Innovation Center of Hunan University of Chinese
Medicine for discussions and guidance with the manuscript.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81573988,
81704093 and 81473617), the Hunan Science and Technology Department
of China (grant no. 2015JC3075), and the Innovation Platform Open
Foundation of Hunan Educational office (grant no. 16K066).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The manuscript was written through contributions of
all authors. XFT conceived and designed the study. QZ, WX, XZ, RSG,
QFL, HYL and JNL performed the experiments. XFT and QZ reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Hunan University of
Chinese Medicine; patients provided written informed consent.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
R, Torre L and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiao SL, Chu GC and Chung LW: Regulation
of prostate cancer progression by the tumor microenvironment.
Cancer Lett. 380:340–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai J, Lu Y, Roca H, Keller JM, Zhang J,
McCauley LK and Keller ET: Immune mediators in the tumor
microenvironment of prostate cancer. Chin J Cancer. 36:292017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lalonde E, Ishkanian A, Sykes J, Fraser M,
Ross-Adams H, Erho N, Dunning M, Halim S, Lamb AD, Moon NC, et al:
Tumour genomic and microenvironmental heterogeneity for integrated
prediction of 5-year biochemical recurrence of prostate cancer: A
retrospective cohort study. Lancet Oncol. 15:1521–1532. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiao S, Chu G and Chung L: Regulation of
prostate cancer progression by the tumor microenvironment. Cancer
Lett. 380:340–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bregni G, Rebuzzi S and Fornarini G:
Enzalutamide in castration-resistant prostate cancer. N Engl J Med.
379:1380–1381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang R, Lu H, Lyu YY, Yang XM, Zhu LY,
Yang GD, Jiang PC, Re Y, Song WW, Wang JH, et al:
E6/E7-P53-POU2F1-CTHRC1 axis promotes cervical cancer metastasis
and activates Wnt/PCP pathway. Sci Rep. 7:447442017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo B, Yan H, Li L, Yin K, Ji F and Zhang
S: Collagen triple helix repeat containing 1 (CTHRC1) activates
Integrin β3/FAK signaling and promotes metastasis in ovarian
cancer. J Ovarian Res. 10:692017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang XM, You HY, Li Q, Ma H, Wang YH,
Zhang YL, Zhu L, Nie HZ, Qin WX, Zhang ZG and Li J: CTHRC1 promotes
human colorectal cancer cell proliferation and invasiveness by
activating Wnt/PCP signaling. Int J Clin Exp Pathol. 8:12793–12801.
2015.PubMed/NCBI
|
|
10
|
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H,
Zhang WM, You H, Qin W, Gu J, Yang S, et al: CTHRC1 acts as a
prognostic factor and promotes invasiveness of gastrointestinal
stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia.
16:265–278, 278.e1-e13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Fu X, Yang J, Yang M, Tao L, Liu D,
Huo Y, Zhang JF, Hua R and Sun YW: Elevated expression of CTHRC1
predicts unfavorable prognosis in patients with pancreatic ductal
adenocarcinoma. Am J Cancer Res. 6:1820–1827. 2016.PubMed/NCBI
|
|
12
|
Gu L, Liu L, Zhong L, Bai Y, Sui H, Wei X,
Zhang W, Huang P, Gao D, Kong Y and Lou G: Cthrc1 overexpression is
an independent prognostic marker in gastric cancer. Hum Pathol.
45:1031–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tameda M, Sugimoto K, Shiraki K, Yamamoto
N, Okamoto R, Usui M, Ito M, Takei Y, Nobori T, Kojima T, et al:
Collagen triple helix repeat containing 1 is overexpressed in
hepatocellular carcinoma and promotes cell proliferation and
motility. Int J Oncol. 45:541–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Li Z, Shao F, Yang X, Feng X, Shi
S, Gao Y and He J: High expression of Collagen triple helix repeat
containing 1 (CTHRC1) facilitates progression of oesophageal
squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 activation. J
Exp Clin Cancer Res. 36:842017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Antonarakis E: Cyclin-dependent kinase 12,
immunity, and prostate cancer. N Engl J Med. 379:1087–1089. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taube J: Unleashing the immune system:
PD-1 and PD-Ls in the pre-treatment tumor microenvironment and
correlation with response to PD-1/PD-L1 blockade. Oncoimmunology.
3:e9634132014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi X, Zhang X, Li J, Zhao H, Mo L, Shi X,
Hu Z, Gao J and Tan W: PD-1/PD-L1 blockade enhances the efficacy of
SA-GM-CSF surface-modified tumor vaccine in prostate cancer. Cancer
Lett. 406:27–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Z, Li C, Kang B, Gao G and Zhang Z:
GEPIA: A web server for cancer and normal gene expression profiling
and interactive analyses. Nucleic Acids Res. 45:W98–W102. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
J, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pierorazio PM, Walsh PC, Partin AW and
Epstein JI: Prognostic Gleason grade grouping: Data based on the
modified Gleason scoring system. BJU Int. 111:753–760. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
international society of urological pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a New Grading System. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI
|
|
22
|
Chaiswing L, Zhong W and Oberley TD:
Distinct redox profiles of selected human prostate carcinoma cell
lines: Implications for rational design of redox therapy. Cancers
(Basel). 3:3557–3584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haffner MC, Guner G, Taheri D, Netto GJ,
Palsgrove DN, Zheng Q, Guedes LB, Kim K, Tsai H, Esopi DM, et al:
Comprehensive evaluation of programmed death-ligand 1 expression in
primary and metastatic prostate cancer. Am J Pathol. 188:1478–1485.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hahn E, Liu SK, Vesprini D, Xu B and
Downes MR: Immune infiltrates and PD-L1 expression in
treatment-naïve acinar prostatic adenocarcinoma: An exploratory
analysis. J Clin Pathol. 71:1023–1027. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He W, Zhang H, Wang Y, Zhou Y, Luo Y, Cui
Y, Jiang N, Jiang W, Wang H, Xu D, et al: CTHRC1 induces non-small
cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9.
BMC Cancer. 18:4002018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin X, Gu Y, Kapoor A, Wei F, Aziz T, Ojo
D, Jiang Y, Bonert M, Shayegan B, Yang H, et al: Overexpression of
MUC1 and genomic alterations in its network associate with prostate
cancer progression. Neoplasia. 19:857–867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Harshman LC, Xie W, Nakabayashi M,
Qu F, Pomerantz MM, Lee GS and Kantoff PW: Association of SLCO2B1
genotypes with time to progression and overall survival in patients
receiving Androgen-deprivation therapy for prostate cancer. J Clin
Oncol. 34:352–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gillard M, Lack J, Pontier A, Gandla D,
Hatcher D, Sowalsky AG, Rodriguez-Nieves J, Vander Griend D, Paner
G and VanderWeele D: Integrative genomic analysis of coincident
cancer foci implicates CTNNB1 and PTEN alterations in ductal
prostate cancer. Eur Urol Focus. 5:433–442. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jamaspishvili T, Berman DM, Ross AE, Scher
HI, De Marzo AM, Squire JA and Lotan TL: Clinical implications of
PTEN loss in prostate cancer. Nat Rev Urol. 15:222–234. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kallakury BV, Sheehan CE and Ross JS:
Co-downregulation of cell adhesion proteins alpha- and
beta-catenins, p120CTN, E-cadherin, and CD44 in prostatic
adenocarcinomas. Hum Pathol. 32:849–855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woodson K, Hayes R, Wideroff L, Villaruz L
and Tangrea J: Hypermethylation of GSTP1, CD44, and E-cadherin
genes in prostate cancer among US Blacks and Whites. Prostate.
55:199–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rajabi H, Ahmad R, Jin C, Joshi MD, Guha
M, Alam M, Kharbanda S and Kufe D: MUC1-C oncoprotein confers
androgen-independent growth of human prostate cancer cells.
Prostate. 72:1659–1568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baspinar S, Bircan S, Ciris M, Karahan N
and Bozkurt KK: Expression of NGF, GDNF and MMP-9 in prostate
carcinoma. Pathol Res Pract. 213:483–489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang L, Dai DL, Su M, Martinka M, Li G and
Zhou Y: Aberrant expression of collagen triple helix repeat
containing 1 in human solid cancers. Clin Cancer Res. 12:3716–3722.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Q, Yang Q and Sun H: Role of collagen
triple helix repeat containing-1 in tumor and inflammatory
diseases. J Cancer Res Ther. 13:621–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duarte CW, Stohn JP, Wang Q, Emery IF,
Prueser A and Lindner V: Elevated plasma levels of the pituitary
hormone Cthrc1 in individuals with red hair but not in patients
with solid tumors. PLoS One. 9:e1004492014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shalapour S, Font-Burgada J, Di Caro G,
Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M,
Strasner A, Hansel DE, et al: Immunosuppressive plasma cells impede
T-cell-dependent immunogenic chemotherapy. Nature. 521:94–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pan JH, Zhou H, Cooper L, Huang JL, Zhu
SB, Zhao XX, Ding H, Pan YL and Rong L: LAYN is a prognostic
biomarker and correlated with immune infiltrates in gastric and
colon cancers. Front Immunol. 10:62019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ni S, Ren F, Xu M, Tan C, Weng W, Huang Z,
Sheng W and Huang D: CTHRC1 overexpression predicts poor survival
and enhances epithelial-mesenchymal transition in colorectal
cancer. Cancer Med. 7:5643–5654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan L, Yu J, Tan F, Ye GT, Shen ZY, Liu H,
Zhang Y, Wang JF, Zhu XJ and Li GX: SP1-mediated microRNA-520d-5p
suppresses tumor growth and metastasis in colorectal cancer by
targeting CTHRC1. Am J Cancer Res. 5:1447–1459. 2015.PubMed/NCBI
|
|
43
|
Lai YH, Chen J, Wang XP, Wu YQ, Peng HT,
Lin XH and Wang WJ: Collagen triple helix repeat containing-1
negatively regulated by microRNA-30c promotes cell proliferation
and metastasis and indicates poor prognosis in breast cancer. J Exp
Clin Cancer Res. 36:922017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen G, Wang D, Zhao X, Cao J, Zhao Y,
Wang F, Bai J, Luo D and Li L: miR-155-5p modulates malignant
behaviors of hepatocellular carcinoma by directly targeting CTHRC1
and indirectly regulating GSK-3β-involved Wnt/β-catenin signaling.
Cancer Cell Int. 17:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li LY, Yin KM, Bai YH, Zhang ZG, Di W and
Zhang S: CTHRC1 promotes M2-like macrophage recruitment and
myometrial invasion in endometrial carcinoma by integrin-Akt
signaling pathway. Clin Exp Metastasis. 36:351–363. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Largeot A, Pagano G, Gonder S, Moussay E
and Paggetti J: The B-side of cancer immunity: The underrated tune.
Cells. 8(pii): E4492019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu M, Sun Q, Wang J, Wei F, Yang L and
Ren X: A new perspective: Exploring future therapeutic strategies
for cancer by understanding the dual role of B lymphocytes in tumor
immunity. Int J Cancer. 144:2909–2917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Youlin K, Weiyang H, Simin L and Xin G:
Prostaglandin E inhibits prostate cancer progression by
countervailing tumor microenvironment-induced impairment of
dendritic cell migration through LXR/CCR7 pathway. J Immunol Res.
2018:58089622018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Prahl LS and Odde DJ: Modeling cell
migration mechanics. Adv Exp Med Biol. 1092:159–187. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao E, Wang L, Dai J, Kryczek I, Wei S,
Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET and Zou W:
Regulatory T cells in the bone marrow microenvironment in patients
with prostate cancer. Oncoimmunology. 1:152–161. 2012. View Article : Google Scholar : PubMed/NCBI
|