Introduction
Pre-eclampsia is characterized by hypertension and
proteinuria during pregnancy, and is prevalent in 3–5% of all
pregnancies worldwide (1,2). Pre-eclampsia affects maternal health,
birth rate and neonatal mortality (3–5).
Pregnant women with pre-eclampsia are at risk of suffering a
stroke, pulmonary edema and renal failure, and have shown symptoms
of postpartum depression (6). They
are also more susceptible to a variety of chronic diseases,
including cardiovascular diseases later in life (6). Pre-eclampsia may also cause uterine
fetal developmental limitations and premature birth. Infants born
to mothers with pre-eclampsia have a higher incidence of cerebral
palsy and bronchopulmonary dysplasia (5). Despite previous studies, the
pathogenesis of pre-eclampsia remains elusive, with the most
effective treatment being the immediate termination of pregnancy
(7,8).
Although the pathogenesis of pre-eclampsia is not
fully understood, it is thought that abnormal placental development
plays a significant role in the disease (9). During the normal remodeling of the
maternal spiral artery, extravillous trophoblasts (EVTs)
differentiate and invade the maternal endometrium, thus causing
endovascular transformation (10,11).
Failure in the differentiation and invasion of trophoblastic cells
may cause inadequate remodeling of the maternal spiral arteries,
incomplete development of placental vessels and insufficient blood
perfusion, an important cause of pre-eclampsia (11,12).
However, the detailed molecular mechanisms involved in this process
have not been fully explored.
In the absence of the complete open reading frames
necessary for protein synthesis, long non-coding RNAs (lncRNAs) are
unable to encode proteins; however, they can carry out an array of
biological functions associated with the regulation of gene
expression (13). lncRNAs have
been associated with various types of cancer, cardiovascular and
neurodegenerative diseases, and are involved in the growth and
development of several organisms (14,15).
Previous studies have shown that lncRNAs are involved in the
invasion of trophoblast cells (9,11).
By determining the expression profile of lncRNAs in pre-eclamptic
and normal placentas, He et al (16) suggested that lncRNAs may be
involved in the pathogenesis of this condition. Previous studies
have discussed the role of the abnormal expression of maternally
expressed 3 (MEG3), metastasis associated lung adenocarcinoma
transcript 1 (MALAT-1), Hox transcript antisense RNA and SPRY4
intronic transcript 1 (SPRY4-IT1) lncRNAs in the pathogenesis of
pre-eclampsia (11,12,17,18).
In the present study, the lncRNA and mRNA expression
profiles were examined from tissues derived from the placentas of 3
patients with pre-eclampsia and 3 healthy patients. Thereafter, the
function of NR_002794 was investigated as its expression was found
to differ between the aforementioned samples with respect to
trophoblast biology. The present study suggested that high levels
of NR_002794 may be associated with the atypical conditions of
trophoblast cells in pre-eclampsia; as such, NR_002794 may be a
potential biomarker for the diagnosis of pre-eclampsia.
Materials and methods
Microarray assay and bioinformatics
analysis
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. To detect the expression profiles of
lncRNAs and mRNAs in the placental tissues of patients with
pre-eclampsia and in patients with normal pregnancies, microarray
analysis was performed on lncRNAs and mRNAs in 3 pre-eclamptic and
3 matched control subjects (Affymetrix; Thermo Fisher Scientific,
Inc.) (19). The placental tissues
of the patients (median age, 29; range, 25–36) with pre-eclampsia
and those with normal pregnancies were collected after parturition
at the People's Hospital of Guangxi Zhuang Autonomous Region
between April 2016 and September 2017. The population analyzed in
the present study were pregnant women who had a gestational age of
more than 20 weeks. Written consent was obtained from all donors.
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analyses were used to investigate differentially
expressed mRNAs and lncRNAs related to pre-eclampsia (20). DAVID version 6.8 (https://david.ncifcrf.gov/) was used to analyze large
gene lists. These analyses were applied to investigate the
functional enrichment and clustering of the differentially
expressed mRNAs and lncRNAs.
Cell culture
SWAN71 first-trimester human trophoblasts and 293T
cells were cultured in DMEM medium supplemented with 10% FBS (both
Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
atmosphere with 5% CO2. SWAN71 cells (derived using
telomerase-mediated transformation of a 7 weeks old cytotrophoblast
isolate) were kindly provided by Professor Ke Wu (School of Life
Sciences, Peking University, China) and 293T cells were purchased
from the American Type Culture Collection.
Vector construction and lentiviral
infection
In order to overexpress NR_002794, a pcDNA3.1
plasmid (Promega Corporation) was constructed expressing
full-length NR_002794 (213 bp); the empty pcDNA3.1 plasmid was used
as the control. The two plasmids (100 nM) were co-transfected into
293T cells with a packaging plasmid (psPAX2; Promega Corporation)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). NR_002794 overexpression lentivirus
(lenti-NR_002794) and a negative control lentivirus (lenti-control)
were obtained after 3 days. Thereafter, the SWAN71 trophoblast
cells were infected with 2×106 transducing units of
lenti-NR_002794 and lenti-control. The cells were collected to
determine infection efficiency at 48 h post-infection following
incubation at 37°C.
RNA extraction and reverse
transcription-quantitative PCR
Total RNA was extracted from placental tissues or
cultured cells using RNA-ISOPLUS (Takara Bio, Inc.), according to
the manufacturer's instructions. Complementary DNA (cDNA) was
produced using a PrimeScript™ RT Reagent with gDNA Eraser (Perfect
Real Time) kit (Takara Bio, Inc.) and qPCR was performed using SYBR
Green (Takara Bio, Inc.). RT reactions were performed as follows:
42°C for 30 min and 95°C for 5 min. The total volume of the
reaction was 25 µl and the following components were used: cDNA (2
µl), SYBR Premix Ex Taq (2X; 10 µl), forward primer (10 µΜ; 1 µl),
reverse primer (10 µM; 1 µl), ROX Reference Dye II (1:50 in
double-distilled water; 1 µl used) and double-distilled water (10
µl). The reaction was performed with an initial denaturation step
of 30 sec at 95°C, followed by 40 cycles of denaturation at 95°C
for 5 sec, annealing at 60°C for 30 sec and extension at 72°C for
30 sec. For quantification, all samples were normalized to GAPDH.
The mRNA expression levels of each gene were calculated using the
2−ΔΔCq method (21).
The primer sequences were as follows: GAPDH forward,
5′-ATGGCCTTCCGTGTTCCTAC-3′ and reverse, 5′-CTTTACAAAGTTGTCGTTGA-3′;
ARPC3 forward, 5′-TGCAATTCCAAAAGCCAAGGT-3′ and reverse,
5′-AGGCTCTCATCACTTCATCTTCC-3′; HCK forward,
5′-GCAACCGCTGTCATGAGTTAC-3′ and reverse,
5′-CTGGTGTGTTGCTGTTGTGG-3′; PIK3CG forward,
5′-GCACTTCCTTCTCGGCTAGAT-3′ and reverse,
5′-AATATGAAGCCTGGCTGCCG-3′; PRKCB forward,
5′-GACCAAACACCCAGGCAAAC-3′ and reverse, 5′-GATGGCGGGTGAAAAATCGG-3′;
CD209 forward, 5′-TGCTGAGGAGCAGAACTTC-3′ and reverse,
5′-GTTGGGCTCTCCTCTGTTCC-3′; CLEC4M forward,
5′-CTCCTGGGGTGTCTTGGC-3′ and reverse, 5′-GCGTCTTGCTCGGATTGTTC-3′;
FCGR1A forward, 5′-CGTGAGCACTGCGTACAAAC-3′ and reverse,
5′-TCAGGCTAGGCTTGACTTGT-3′; PTPRC forward,
5′-ACCAGGAATGGATGTCGCTA-3′ and reverse, 5′-TGGGGCCTGTAAAAGTGTCC-3′;
CYBB forward, 5′-TGTCAAGTGCCCAAAGGTGT-3′ and reverse,
5′-CCCAACGATGCGGATATGGA-3′; NCF4 forward,
5′-GAGAGGTGAACTCAGCCTGG-3′ and reverse, 5′-TTCAAAGTCACTCTCGGCCC-3′;
n337689 forward, 5′-CAGGTAGGCTGGGCCATTAC-3′ and reverse,
5′-CCAATGCCCTTATCCCCCTC-3′; n340778 forward,
5′-GCAGGCATCTGATCCAAGGT-3′ and reverse, 5′-AGGGCTAGGCTCAGAATGGA-3′;
n341675 forward, 5′-GGCTCCCGGTCTTTCAGAAT-3′ and reverse,
5′-TACCCTGTATTGGCCACGTT-3′; n342887 forward,
5′-TCTGTCTGTGCAGTGCTTCTG-3′ and reverse,
5′-GTCGTCCTGCAGCAAGTAGC-3′; n345093 forward,
5′-TGGGAGAGGCACACCAACAA-3′ and reverse, 5′-CTCCTCGTCCATTTCCGGC-3′;
n346352, forward: 5′-GATAAGGGCATTGGGGAGCTG-3′ and reverse,
5′-TGTTTTGGGCTTTGCCCCTG-3′; n411602 forward,
5′-TGGGTCTAGCCCACCCAAT-3′ and reverse, 5′-CCACTTGGCTCAGATCCACC-3′;
NR_002794 forward, 5′-TCTGTCTGTGCAGTGCTTCTG-3′ and reverse,
5′-GTCGTCCTGCAGCAAGTAGC-3′; NR_038877 forward,
5′-GGGTGGGATTGGGAGTGTTC-3′ and reverse, 5′-TCGGTCCCCTGTTTGAGGTA-3′;
NR_039741 forward, 5′-AGGGAGAAGGGTCGGGGC-3′ and reverse,
5′-GGGGAGACGTGGGCAGAG-3′.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay was used to
determine cell proliferation. Lenti-control and lenti-NR_002794
cells were seeded in 96-well plates (2×103 cells/well).
The cells were incubated in medium with CCK-8 solution (10 µl/well)
for 2 h at 37°C following 24, 48 and 72 h of incubation at 37°C.
Absorbance was measured at 450 nm using a spectrometer (Thermo
Fisher Scientific, Inc.).
In vitro cell migration assay
The migratory and invasive abilities of cells were
evaluated using the transwell assay in different cell lines.
Initially, all transwell chambers were placed in DMEM/Ham's F-12
(1:1; DF-12) medium (Sangon Biotech Co., Ltd.) without serum and
were incubated at 37°C for 1 h. Blank, lenti-control cells and
lenti-NR_002794 cells were harvested and resuspended in DF/12
medium containing 2% FBS. Subsequently, 1×104 cells were
seeded in each upper chamber, while DF/12 medium containing 10% FBS
was added to the lower chambers. Following incubation at 37°C for
24 h, the remaining cells on the membrane of the upper chamber
surface were gently removed and all chambers were washed with PBS.
Cells that had migrated to the lower side of the membrane were
treated with 95% ethanol and stained with crystal violet for 10
min. A fluorescent microscope (magnification, ×200; Olympus CX2;
Olympus Corporation) was used to examine the number of cells that
had migrated, which was calculated from five random fields from
each chamber. The fields were used to calculate the average
value.
Apoptosis analysis using flow
cytometry
To increase the levels of oxidative stress in the
cells, 0.01% H2O2 was used; this resulted in
the induction of apoptosis. Following culture with medium
containing 0.01% H2O2 for 24 h 37°C, the
cells were harvested with EDTA-free trypsin. Following washing with
PBS, the cells were resuspended in Annexin binding buffer (Thermo
Fisher Scientific, Inc.) and double-stained with Annexin V (Nanjing
KeyGen Biotech Co., Ltd.) and propidium iodide (PI; Nanjing KeyGen
Biotech Co., Ltd.) in the dark for 15 min at room temperature.
Subsequently, the cells were analyzed using a BD FACSCalibur™ (BD
Biosciences). The percentage of apoptotic cells was analyzed using
FCAP Array Software (v3.1; BD Biosciences). The analysis resulted
in the identification of the four following types of cells: Viable
cells (PI negative, Annexin V negative; lower left quadrant),
necrotic cells (PI positive, Annexin V negative; upper left
quadrant), early apoptotic cells (PI negative, Annexin V positive;
lower right quadrant) and late apoptotic cells (PI positive,
Annexin V positive; upper right quadrant). The rate of apoptosis
was calculated as the sum of the early apoptotic cells and the late
apoptotic cells.
Phagocytosis
The phagocytic capacity of SWAN71 cells following
NR_002794 overexpression was determined using a fluorescently
labeled pHrodo™ phagocytic indicator (Thermo Fisher Scientific,
Inc.). Cells were incubated with medium containing 1 µg/ml pHrodo™,
after a 24 h incubation at 37°C the fluorescence intensity was
measured using BD FACSCalibur™ and FCAP Array Software. The
fluorescence intensity of the cells (peak area) was also observed
using a fluorescent microscope (magnification, ×200).
Statistical analysis
All data were analysed with SPSS 23.0 (IBM Corp.)
and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
The data are presented as the mean ± SD. All experiments were
repeated three times. Comparisons between two groups were analyzed
using independent sample t-tests. One-way ANOVA followed by
Dunnett's test was used for the comparison of multiple samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Differentially expressed lncRNAs and
mRNAs in human trophoblast cells
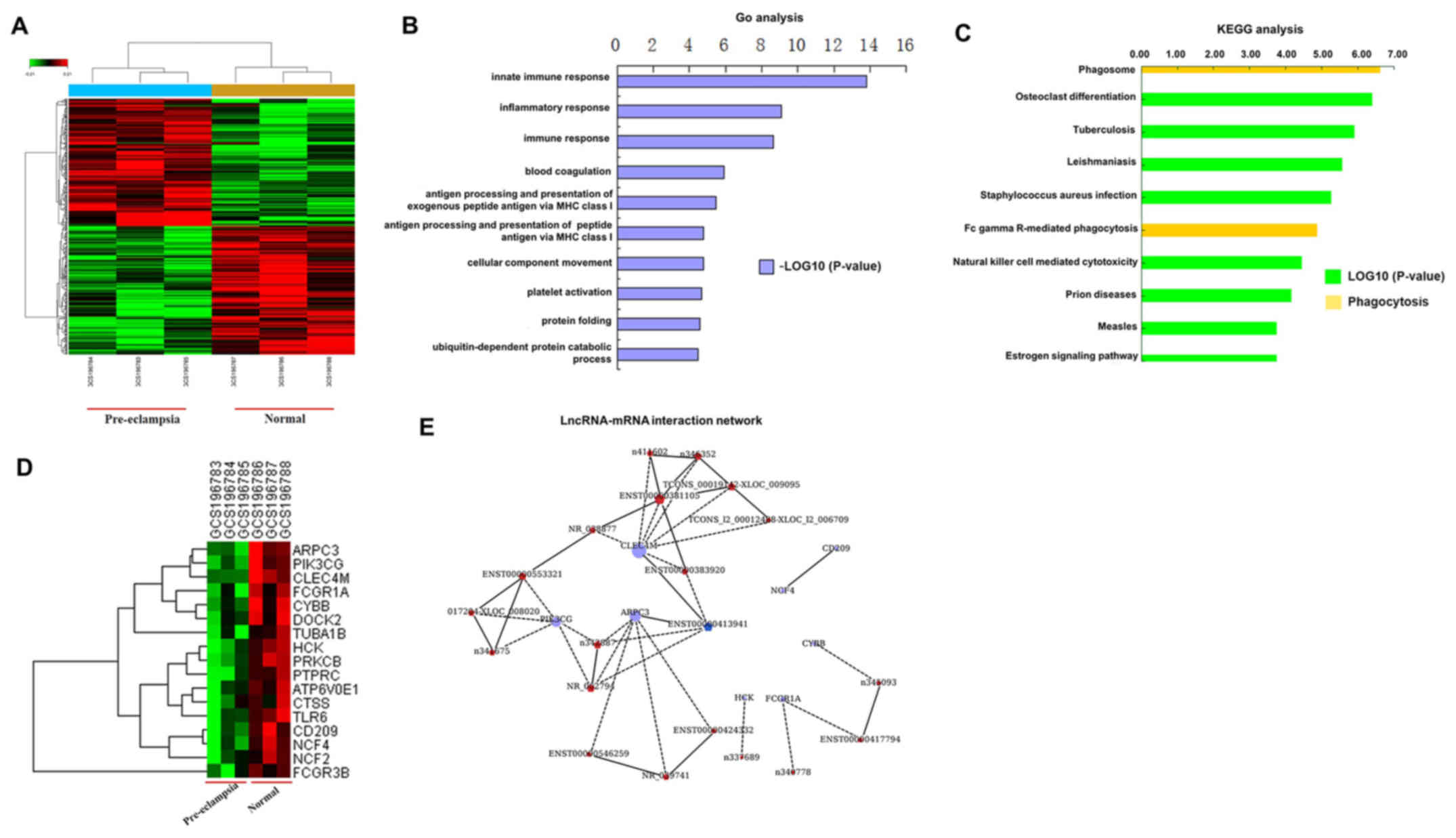
Based on microarray and computational analysis, 16
lncRNAs were identified as being differentially expressed between
human trophoblast cells from patients with pre-eclampsia and those
without pre-eclampsia, including 9 that were upregulated and 17
that were downregulated (Table I).
Furthermore, the profiles of differentially expressed mRNAs are
shown in Table II. The data
indicated that 121 mRNAs were downregulated and 87 mRNAs were
upregulated in the placental tissue of patients with pre-eclampsia
compared with the expression of these molecules in the placental
tissue from normal pregnancies; Table
II presents the top 10 upregulated and 10 downregulated mRNAs.
GO and KEGG analyses were performed to reveal the relationship
between the differentially expressed mRNAs and their biological
functions (Fig. 1A-D). These
results indicated that these genes identified were involved in
invasion, metastasis and phagocytosis. In addition, according to
the interactions between lncRNAs and mRNAs, NR_002794 was
identified as an upregulated lncRNA with a role in the induction of
apoptosis and migration (Fig. 1E).
Thus, the function of NR_002794 in pre-eclampsia was investigated
in the present study.
 | Table I.Differential expression of lncRNA in
the placental tissues of patients with pre-eclampsia compared with
normal pregnancies using microarray analysis. |
Table I.
Differential expression of lncRNA in
the placental tissues of patients with pre-eclampsia compared with
normal pregnancies using microarray analysis.
| A, lncRNAs
upregulated in patients with pre-eclampsia |
|---|
|
|---|
| Gene symbol | Accession no. | Fold-change |
|---|
| GSTT1 | n336841 | 3.212795 |
| TLT5 | n342887 | 2.190874 |
| SNORA2A | NR_002950 | 2.156267 |
| linc-ADORA2B-1 |
TCONS_l2_00010577-XLOC_l2_005672 | 2.080765 |
| TREML5P | NR_002794 | 2.053127 |
| − |
ENST00000381105 | 1.7945 |
| − |
ENST00000450426 | 1.746861 |
| − | n340625 | 1.649479 |
| LOC100506453 |
ENST00000424415 | 1.517736 |
|
| B, lncRNAs
downregulated in patients with pre-eclampsia |
|
| Gene
symbol | Accession
no. |
Fold-change |
|
| SNORA38 | NR_002971 | −1.51867 |
| TMSB4XP8 |
OTTHUMT00000253552 | −1.5274 |
| FCAR | n336109 | −1.54679 |
| IL17RA | n332742 | −1.55508 |
| linc-MED12L |
TCONS_00006273-XLOC_002874 | −1.57362 |
| − |
ENST00000515960 | −1.59832 |
| SNORA11 | NR_002953 | −1.63376 |
| GIMAP4 | n334779 | −1.63512 |
| CD309 | n407224 | −1.69438 |
| VNN2 | n409772 | −1.71248 |
| SNORD14E |
ENST00000364009 | −1.71758 |
| − |
ENST00000363189 | −1.80291 |
| SNORD113-2 | NR_003230 | −1.82711 |
| SNORA31 |
ENST00000534033 | −1.82752 |
| SNORA38B | NR_003706 | −1.85379 |
| IL1RL1 | n333421 | −2.09845 |
| − |
ENST00000384564 | −3.09063 |
 | Table II.Differential expression of mRNA in in
the placental tissues of patients with pre-eclampsia compared with
normal pregnancies using microarray analysis. |
Table II.
Differential expression of mRNA in in
the placental tissues of patients with pre-eclampsia compared with
normal pregnancies using microarray analysis.
| A, Genes
upregulated in patients with pre-eclampsia |
|---|
|
|---|
| Gene symbol | Accession no. | Fold-change |
|---|
| TREML2 | NM_024807 | 2.548855 |
| ALAS2 | NM_000032 | 2.513751 |
| IFIT1B | NM_001010987 | 2.513519 |
| GTSF1 | NM_144594 | 2.506887 |
| KIAA1199 | NM_018689 | 1.724283 |
| LY6G6C | NM_025261 | 1.611992 |
| SLC4A1 | NM_000342 | 1.605567 |
| GSTT1 | NM_000853 | 1.438739 |
| DERL3 | NM_001002862 | 1.41813 |
| HOOK1 | NM_015888 | 1.36205 |
|
| B, Genes
downregulated in patients with pre-eclampsia |
|
| Gene
symbol | Accession
no. |
Fold-change |
|
| CYTL1 | NM_018659 | −1.95817 |
| IL1RL1 | NM_003856 | −1.87074 |
| MRC1 | NM_002438 | −1.79006 |
| MRC1 | NM_002438 | −1.76872 |
| CD209 | NM_001144893 | −1.71566 |
| VSIG4 | NM_001100431 | −1.58222 |
| FKBP5 | NM_004117 | −1.55752 |
| CLEC4M | NM_001144904 | −1.53693 |
| DPYD | NM_000110 | −1.49611 |
| CYBB | NM_000397 | −1.49244 |
Validation using RT-qPCR
The lncRNA and mRNA expression levels were compared
in placental tissues from 3 patients with pre-eclampsia and in
tissues from 3 normal pregnancies using RT-qPCR. According to the
GO and KEGG analyses, the expression levels of 10 mRNAs and 10
lncRNAs were detected in placental samples. As shown in Fig. 2A, the expression levels of several
mRNAs (actin related protein 2/3 complex subunit 3,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit γ,
C-type lectin domain family 4 member M, Fc fragment of IgG receptor
1a, cytochrome b-245 β chain and neutrophil cytosolic factor 4)
were downregulated in patients with pre-eclampsia, whereas the
expression levels of several lncRNAs (n340778, n342887, n345093,
n346352, NR_002794, NR_038877 and NR_039741) were increased in
patients with pre-eclampsia (Fig.
2B).
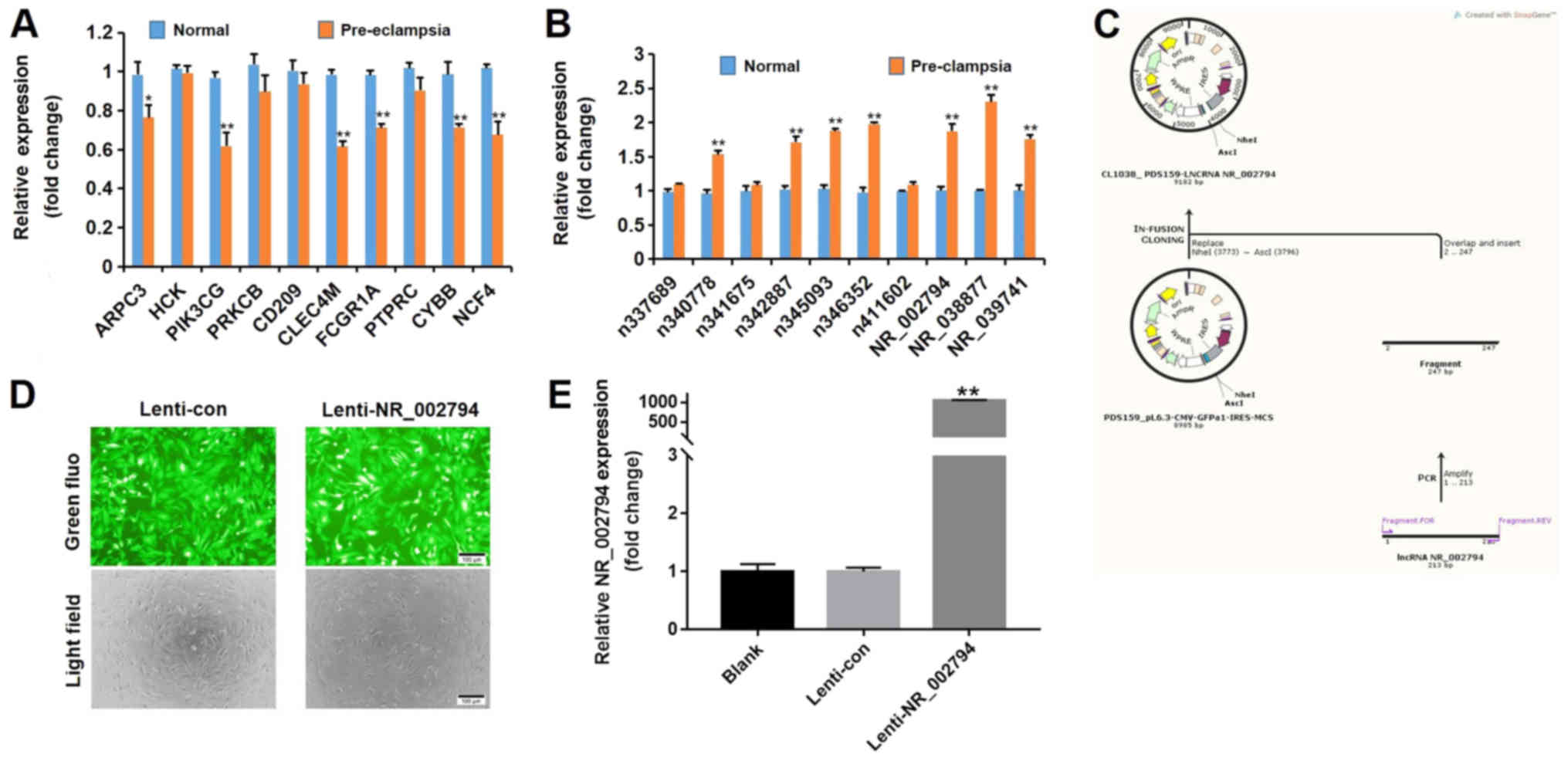 | Figure 2.RT-qPCR verification of differentially
expressed genes identified using microarray analysis and the
overexpression of NR_002794 in SWAN71 cells. (A) The expression
levels of 10 mRNAs (ARPC3, HCK, PIK3CG, PRKCB, CD209, CLEC4M,
FCGR1A, PTPRC, CYBB and NCF4) were determined by RT-qPCR in tissues
derived from pre-eclamptic pregnancies and normal pregnancies. (B)
The expression levels of 10 lncRNAs (n337689, n340778, n341675,
n342887, n345093, n346352, n411602, NR_002794, NR_038877 and
NR_039741) were determined by RT-qPCR in tissues derived from
pre-eclamptic pregnancies and normal pregnancies. *P<0.05;
**P<0.01 vs. respective normal group. (C) Schematic
representation of the construction of the overexpression plasmid
containing the full-length lncRNA NR_002794. (D) Lentiviral
infection of SWAN71 cells. Cells that were infected with
lenti-NR_002794 and lenti-con exhibited a consistent infection
efficiency. Magnification, ×200. (E) SWAN71 cells were infected
with lenti-NR_002794 or lenti-con and the infection efficiency was
assessed using RT-qPCR. The data are presented as the mean ± SD.
**P<0.01 vs. lenti-con. RT-qPCR, reverse
transcription-quantitative PCR; ARPC3, actin related protein 2/3
complex subunit 3; HCK, HCK proto-oncogene Src family tyrosine
kinase; PIK3CG, phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit γ; PRKCB, protein kinase C β; CD209, CD209
molecule; CLEC4M, C-type lectin domain family 4 member M; FCGR1A,
Fc fragment of IgG receptor 1a; PTPRC, protein tyrosine phosphatase
receptor type C; CYBB, cytochrome b-245 β chain; NCF4, neutrophil
cytosolic factor 4; lenti-NR_002794, lentivirus expressing
NR_002794; lenti-con, control lentivirus; fluo, fluorescence. |
Overexpression of NR_002794 in human
SWAN71 trophoblast cells
To further examine the function of NR_002794 in the
behavior of trophoblast cells, an increase in the expression of
NR_002794 was achieved using an expression vector in SWAN71 cells
(Fig. 2C). Cells that were
infected with lenti-NR_002794 or lenti-control exhibited a
consistent infection efficiency (Fig.
2D). Following infection with lenti-NR_002794 or lenti-control,
the cells were harvested to determine the level of overexpression
using RT-qPCR. Significant overexpression of NR_002794 was detected
in lenti-NR_002794 cells compared with the lenti-control cells
(Fig. 2E).
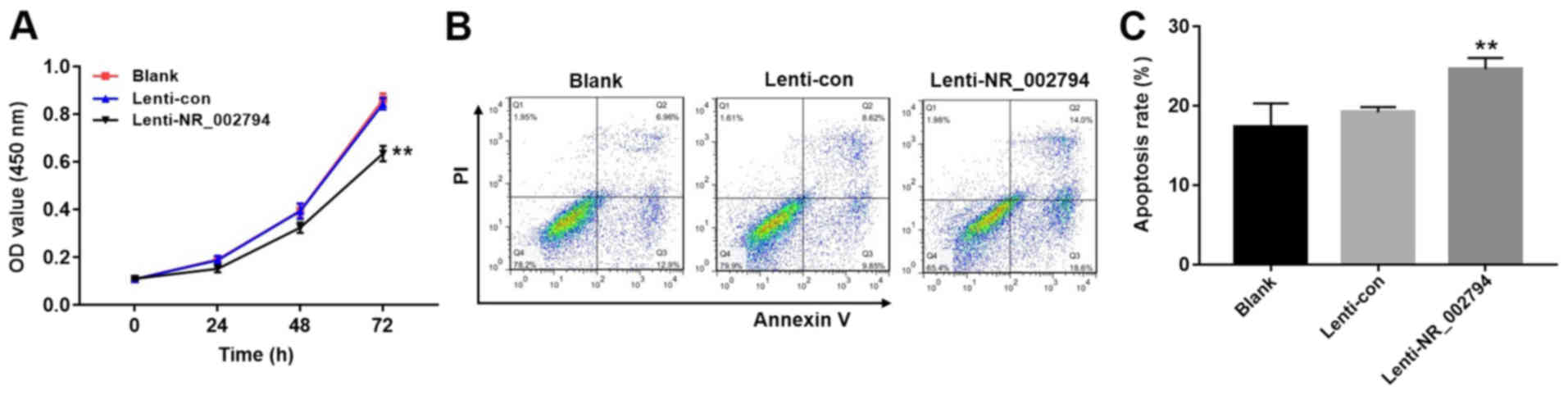
Overexpression of NR_002794 inhibits
the proliferation of trophoblast cells
The significant increase in the expression of
NR_002794 in the placental tissues of patients with pre-eclampsia
prompted an investigation into the possible biological functions of
NR_002794 in pre-eclampsia. To investigate the effects of NR_002794
on the proliferation of trophoblast cells, the CCK-8 assay was used
following the infection of SWAN71 cells with lenti-control or
lenti-NR_002794 vectors. Infection with lenti-NR_002794
significantly decreased cell proliferation compared with the blank
and lenti-control groups (Fig. 3A;
P<0.01). These results suggested that the increased expression
of NR_002794 inhibited the proliferation of SWAN71 cells.
Overexpression of NR_002794 promotes
the apoptosis of trophoblast cells
Flow cytometry was performed to detect the effects
of NR_002794 expression on the level of apoptosis in trophoblast
cells. The infection of SWAN71 cells with lenti-NR_002794 caused a
significant increase in the induction of apoptosis compared with
the lenti-control and blank groups (Fig. 3B and C; P<0.01). These results
indicated that NR_002794 promoted the induction of apoptosis.
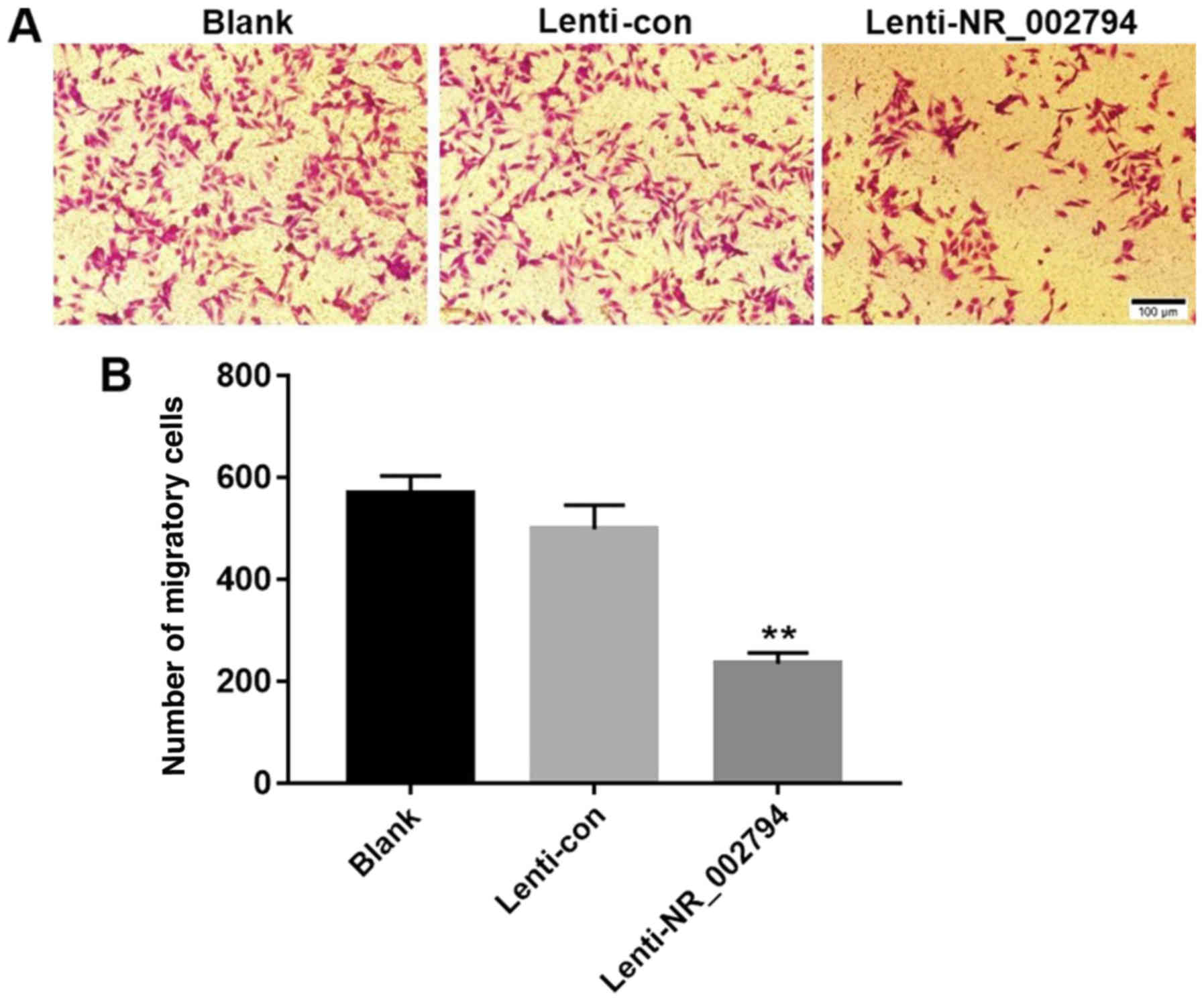
Overexpression of NR_002794 inhibits
the migration of trophoblast cells
The transwell assay was used to investigate the
effects of NR_002794 expression on the migration of SWAN71 cells.
The results indicated that expression of NR_002794 significantly
decreased the number of migrating cells compared with the blank and
lenti-control groups (Fig. 4),
indicating that NR_002794 inhibited the migration of trophoblast
cells. Cells expressing lenti-NR_002794 exhibited no significant
difference in proliferation compared with the blank and
lenti-control groups at 24 h. Thus, the inhibitory effect of
lenti-NR_002794 on migration was not a result of cell death.
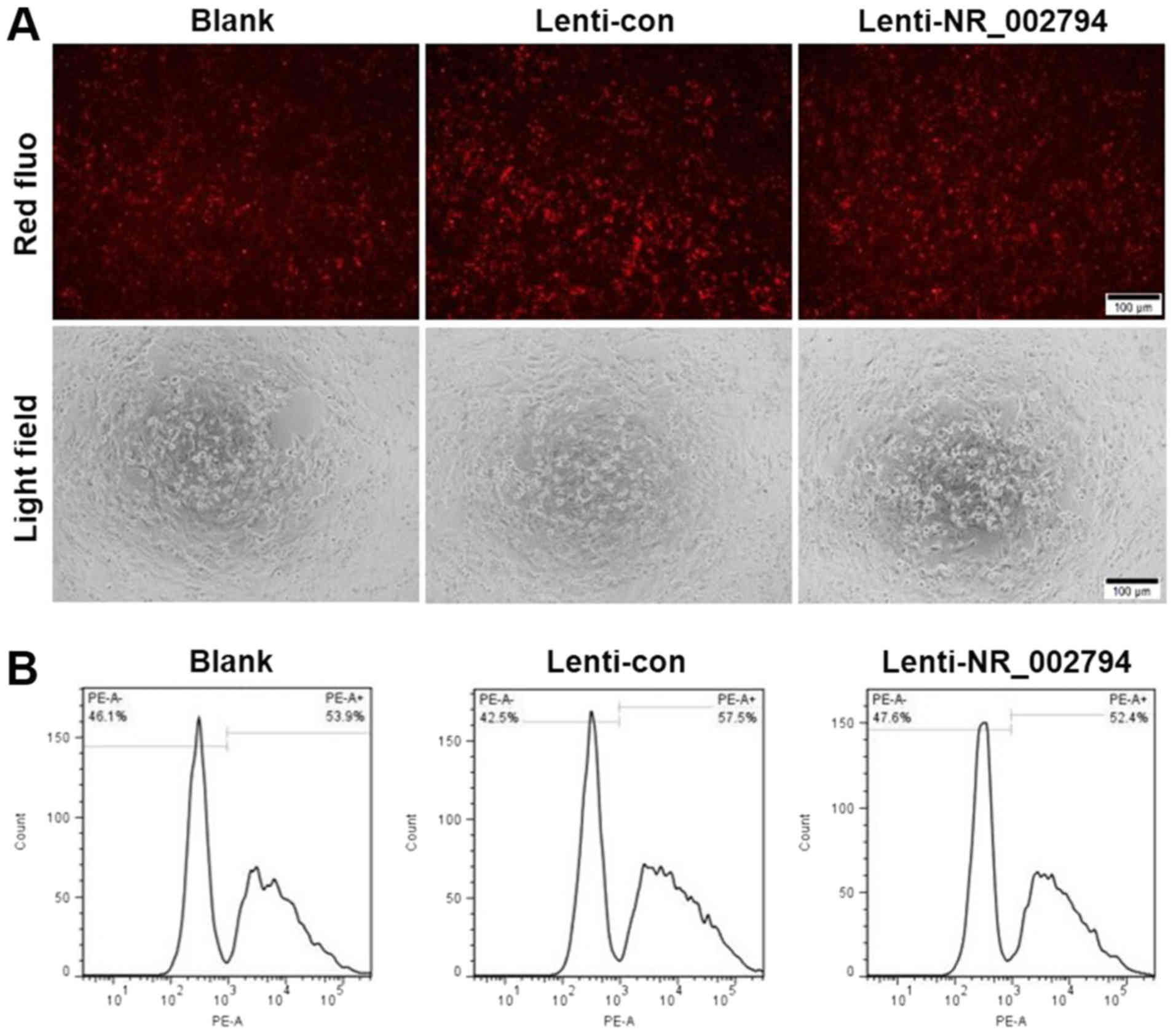
Overexpression of NR_002794 does not
affect the phagocytic activity of trophoblast cells
SWAN71 cells that overexpressed NR_002794 exhibited
no significant difference in their phagocytic activity compared
with the blank and lenti-control groups (Fig. 5). This was demonstrated by
fluorescence and flow cytometry analysis. These data suggested that
NR_002794 did not affect the phagocytic activity of trophoblast
cells.
Discussion
Pre-eclampsia is characterized by hypertension and
proteinuria. It is a common and severe complication encountered
during pregnancy (22,23). Patients with pre-eclampsia often
suffer from increased uric acid levels and serum transaminase
concentrations (24). Currently,
the only effective treatment for pre-eclampsia is delivery or
interruption of pregnancy (25).
The pathogenesis of pre-eclampsia remains elusive. The failure of
cells to infiltrate into the trophoblast and the abnormal
development of the placenta are considered to be the main factors
in the development of this disease (26). EVTs differentiate and invade the
maternal endometrium for intravascular transformation, forming
normal placental vessels. However, trophoblastic cellular
dysfunction or the failure of invasion can lead to maternal spiral
artery-remodeling failure (11,12).
The differentiation of trophoblast cells is regulated by various
factors and the regulatory role of lncRNAs has received attention
(8).
It has been shown that >90% of the human genome
does not encode protein products: The transcripts of these genes
are called non-protein-encoding RNAs (ncRNAs) (27). ncRNAs are broadly divided into two
major classes: microRNAs and lncRNAs (28). With the advancement of
transcriptomics, the role of lncRNAs in various cellular activities
and ontogeny is being recognized (29). lncRNAs are capable of regulating
the development and metabolism of several cellular activities at
the epigenetic, transcriptional and post-transcriptional levels
(30). A number of studies have
shown that the abnormal expression of lncRNAs in placental tissues
during pregnancy is closely related to the onset of pre-eclampsia
(31,32). He et al (16) reported that 738 lncRNAs were
differentially expressed in the placental tissues of patients with
pre-eclampsia, including 479 that were upregulated and 259 that
were downregulated. These differentially expressed lncRNAs were
identified following the microarray analysis of tissues derived
from 6 patients with pre-eclampsia and 5 normal pregnancies
(16). Zou et al (11) examined the expression levels of
specific lncRNAs in the placental tissues of 25 patients with
pre-eclampsia and found a 2.8-fold increase in the expression level
of the lncRNA SPRY4-IT1 compared with placental tissues from normal
pregnancies. Moreover, the increased expression of SPRY4-IT1 was
found to reduce the rate of invasion, metastasis and proliferation
of trophoblast cells and increase the rate of apoptosis. Zhang
et al (12) demonstrated
that the expression level of the lncRNA MEG3 in the placental
tissues from patients with pre-eclampsia were significantly lower
than those from normal pregnancies. The low expression of MEG3
promoted the induction of trophoblast cell apoptosis and inhibited
the invasive and metastatic abilities of the cells. Chen et
al (17) further showed that
MALAT-1, which is considered to be a significant tumor marker,
played an important role in trophoblast cell invasion and
metastasis.
In the present study, tissues from 3 cases of
pre-eclampsia and 3 cases of normal pregnancy were investigated. A
total of 26 lncRNAs with differential expression levels were
identified, this included 9 upregulated lncRNAs and 17
downregulated lncRNAs. In addition, 208 differentially expressed
mRNAs were identified that were classified as upregulated (87) or
downregulated (121). GO and KEGG pathway analyses were performed
and indicated that these genes were involved in invasion,
metastasis and phagocytosis. In the present study, NR_002794, a
lncRNA that was found to be highly expressed in pre-eclampsia, was
selected as a target to investigate the effects of lncRNAs on the
function of the trophoblast cell line SWAN71. It was observed that
the upregulation of NR_002794 suppressed the proliferation and
migration of SWAN71 cells, and increased the induction of
apoptosis. As such, it is hypothesized that NR_002794 may play an
important role in the proliferation, migration and apoptosis of
trophoblast cells in pre-eclampsia. NR_002794 may play an important
role in trophoblast cell activity, in the remodeling of the uterine
spiral artery and in the normal development of the placenta.
Abnormal expression of NR_002794 may inhibit the invasion of
trophoblast cells into the maternal endometrium, resulting in
insufficient remodeling of the spiral arteries, incomplete
development of the placenta and insufficient perfusion. These
processes eventually lead to the development of pre-eclampsia.
However, the molecular mechanism underlying the role of NR_002794
with regards to trophoblast invasion and apoptosis remains unclear
and requires further investigation.
In conclusion, the present study demonstrated that
lncRNA NR_002794 was upregulated in the placental tissues of
patients with pre-eclampsia. The results further showed that the
expression levels of NR_002794 may alter the biological functions
of trophoblast cells in vitro.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
Guangxi Natural Science Foundation (grant nos. 2017GXNSFAA198084
and 2014GXNSFBA118176).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YiM and XL performed experiments, analyzed data and
were major contributors in the development of the manuscript. HW
and CZ collected tissues, interpreted patient data and performed
experiments. YaM interpreted patient data and reviewed the final
version of the manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was received
from the People's Hospital of Guangxi Zhuang Autonomous Region.
Written consent was obtained from all donors.
Patient consent for publication
All patients provided written informed consent for
the publication of all associated information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mol BWJ, Roberts CT, Thangaratinam S,
Magee LA, de Groot CJM and Hofmeyr GJ: Pre-eclampsia. Lancet.
387:999–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn S, Jeong E, Min JW, Kim E, Choi SS,
Kim CJ and Lee DC: Identification of genes dysregulated by
elevation of microRNA-210 levels in human trophoblasts cell line,
Swan 71. Am J Reprod Immunol. 78:e127222017. View Article : Google Scholar
|
|
3
|
Souza JP, Gulmezoglu AM, Vogel J, Carroli
G, Lumbiganon P, Qureshi Z, Costa MJ, Fawole B, Mugerwa Y, Nafiou
I, et al: Moving beyond essential interventions for reduction of
maternal mortality (the WHO Multicountry Survey on Maternal and
Newborn Health): A cross-sectional study. Lancet. 381:1747–1755.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hansen AR, Barnes CM, Folkman J and
McElrath TF: Maternal preeclampsia predicts the development of
bronchopulmonary dysplasia. J Pediatr. 156:532–536. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strand KM, Heimstad R, Iversen AC,
Austgulen R, Lydersen S, Andersen GL, Irgens LM and Vik T:
Mediators of the association between pre-eclampsia and cerebral
palsy: Population based cohort study. BMJ. 347:f40892013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Irgens HU, Reisaeter L, Irgens LM and Lie
RT: Long term mortality of mothers and fathers after pre-eclampsia:
Population based cohort study. BMJ. 323:1213–1217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huppertz B: Placental origins of
preeclampsia: Challenging the current hypothesis. Hypertension.
51:970–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du MR, Wang SC and Li DJ: The integrative
roles of chemokines at the maternal-fetal interface in early
pregnancy. Cell Mol Immunol. 11:438–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Chen H, Kong W, Zhang Y, Cao L, Gao
L and Zhou R: Down-regulated long non-coding RNA-ATB in
preeclampsia and its effect on suppressing migration,
proliferation, and tube formation of trophoblast cells. Placenta.
49:80–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chakraborty C, Gleeson LM, McKinnon T and
Lala PK: Regulation of human trophoblast migration and
invasiveness. Can J Physiol Pharmacol. 80:116–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou Y, Jiang Z, Yu X, Sun M, Zhang Y, Zuo
Q, Zhou J, Yang N, Han P, Ge Z, et al: Upregulation of long
noncoding RNA SPRY4-IT1 modulates proliferation, migration,
apoptosis, and network formation in trophoblast cells HTR-8SV/neo.
PLoS One. 8:e795982013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Zou Y, Wang W, Zuo Q, Jiang Z,
Sun M, De W and Sun L: Down-regulated long non-coding RNA MEG3 and
its effect on promoting apoptosis and suppressing migration of
trophoblast cells. J Cell Biochem. 116:542–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akhade VS, Pal D and Kanduri C: Long
noncoding RNA: Genome organization and mechanism of action. Adv Exp
Med Biol. 1008:47–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo X and Hua Y: CCAT1: An oncogenic long
noncoding RNA in human cancers. J Cancer Res Clin Oncol.
143:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson R: Long non-coding RNAs in
Huntington's disease neurodegeneration. Neurobiol Dis. 46:245–254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He X, He Y, Xi B, Zheng J, Zeng X, Cai Q,
Ouyang Y, Wang C, Zhou X, Huang H, et al: LncRNAs expression in
preeclampsia placenta reveals the potential role of LncRNAs
contributing to preeclampsia pathogenesis. PLoS One. 8:e814372013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen H, Meng T, Liu X, Sun M, Tong C, Liu
J, Wang H and Du J: Long non-coding RNA MALAT-1 is downregulated in
preeclampsia and regulates proliferation, apoptosis, migration and
invasion of JEG-3 trophoblast cells. Int J Clin Exp Pathol.
8:12718–12727. 2015.PubMed/NCBI
|
|
18
|
Zou YF and Sun LZ: Long noncoding RNA
HOTAIR modulates the function of trophoblast cells in
pre-eclampsia. Sichuan Da Xue Xue Bao Yi Xue Ban. 46113–117.
(122)2015.(In Chinese). PubMed/NCBI
|
|
19
|
Gao C, Zhao D, Zhao Q, Dong D, Mu L, Zhao
X, Guo M, Xu A, Fang L, Liu Q and Che J: Microarray profiling and
co-expression network analysis of lncRNAs and mRNAs in ovarian
cancer. Cell Death Discov. 5:932019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mlecnik B, Galon J and Bindea G:
Comprehensive functional analysis of large lists of genes and
proteins. J Proteomics. 171:2–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ray JG, Vermeulen MJ, Schull MJ and
Redelmeier DA: Cardiovascular health after maternal placental
syndromes (CHAMPS): Population-based retrospective cohort study.
Lancet. 366:1797–1803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
ACOG Committee on Practice
Bulletins-Obstetrics, . ACOG practice bulletin. Diagnosis and
management of preeclampsia and eclampsia. Number 33, January 2002.
Obstet Gynecol. 99:159–167. 2002.PubMed/NCBI
|
|
24
|
Álvarez-Cabrera MC, Barrientos-Galeana E,
Barrera-García A, Osorio-Caballero M, Acevedo JF, Flores-Herrera O,
Díaz NF, Molina-Hernández A, García-López G and Flores-Herrera H:
Secretion of heat shock −60, −70 kD protein, IL-1β and TNFα levels
in serum of a term normal pregnancy and patients with pre-eclampsia
development. J Cell Mol Med. 22:5748–5752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schlichting LE, Insaf TZ, Zaidi AN, Lui GK
and Van Zutphen AR: Maternal comorbidities and complications of
delivery in pregnant women with congenital heart disease. J Am Coll
Cardiol. 73:2181–2191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heazell AE, Buttle HR, Baker PN and
Crocker IP: Altered expression of regulators of caspase activity
within trophoblast of normal pregnancies and pregnancies
complicated by preeclampsia. Reprod Sci. 15:1034–1043. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gloss BS and Dinger ME: The specificity of
long noncoding RNA expression. Biochim Biophys Acta. 1859:16–22.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi Q and Yang X: Circulating MicroRNA and
long noncoding RNA as biomarkers of cardiovascular diseases. J Cell
Physiol. 231:751–755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Tang J, Di R, Liu Q, Wang X, Gan
S, Zhang X, Zhang J, Hu W and Chu M: Comparative transcriptomics
reveal key sheep (Ovis aries) hypothalamus LncRNAs that affect
reproduction. Animals (Basel). 9(pii): E1522019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun W, Yang Y, Xu C and Guo J: Regulatory
mechanisms of long noncoding RNAs on gene expression in cancers.
Cancer Genet. 216-217:105–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pengjie Z, Xionghui C, Yueming Z, Ting X,
Na L, Jianying T and Zhice X: LncRNA uc003fir promotes CCL5
expression and negatively affects proliferation and migration of
trophoblast cells in preeclampsia. Pregnancy Hypertens. 14:90–96.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Xi L, Ma Y, Zhu X, Chen R, Luan L,
Yan J and An R: The lncRNA small nucleolar RNA host gene 5
regulates trophoblast cell proliferation, invasion, and migration
via modulating miR-26a-5p/N-cadherin axis. J Cell Biochem.
120:3173–3184. 2019. View Article : Google Scholar : PubMed/NCBI
|