Introduction
Classical histological change of tooth movement
during orthodontic treatment is that the external force generates
two different strains in the periodontal ligament; compression and
tension (1). At the compression
site, the periodontal ligament space narrows, the blood flow slows,
collagen fibers and matrix degrade and absorb, osteoclastogenesis
occurs and alveolar bone tissue is lost. At the tension site, the
periodontal ligament space widens, the blood flow increases,
collagen fibers and matrix proliferate, osteogenesis occurs and
novel alveolar bone tissue is formed (2). Mechanical stress serves a vital
function in bone metabolism during orthodontic tooth movement via
the osteoblast and osteoclast processes. This type of tooth
movement is considered a biological response to the physiologic
equilibrium, when the dentofacial complex is interfered with by an
externally applied force (3).
Osteoblast activity serves a central function in the regulation of
the bone remodeling process and orthodontic tooth movement
(4). Notably, the formation of
alveolar bone includes two aspects; first, that primitive bone
mesenchymal stem cells (BMSCs) differentiate into osteoblast
precursor cells, followed by the maturation of osteoblasts,
including matrix formation and mineralization (5). However, the function of orthodontic
force in the osteogenic differentiation of BMSCs still requires
further study. In order to elucidate the association between
orthodontic tooth movement and orthodontic-periodontal-bone tissue
reconstruction, it is necessary to investigate the mechanism and
regulation of osteogenic differentiation in BMSCs.
It is well recognized that the cellular
microenvironment is necessary for regulating the phenotypic
differentiation of BMSCs. Previous research has suggested that the
differentiation of cells is regulated by complex interactions
between genetic and biochemical factors (6,7).
Subsequent studies have focused on biomechanical signals, which may
serve a complementary or coordinating function to cytokines
(8). Cells may develop
corresponding biological changes in response to multifarious
physiological stresses. Unfortunately, due to the complexity of the
environment in vivo, revealing the impact of mechanical
factors separately is often challenging. It is difficult to
distinguish the effect of a single factor from the combined effect,
particularly when studying the biological behavior of cells.
Therefore, the effect on the cell stimulation system mainly depends
on the in vitro methods (9). Among the various mechanical
stimulations, tensile stretch is a major factor in determining the
strain and function of a cell (10). Under different tensile stress, the
overall framework of the cytoskeleton is rearranged, resulting in
the deformation of cells. Changing of the cell shape may initiate a
signal, which in turn may be transmitted into the cell nucleus and
produce various effects (11).
Notably, the tension that is applied to in vitro culture
cells may provide a greater physiological stimulation that better
recapitulates the growth state of cells in vivo (12). In previous years, more studies have
begun to focus on the osteogenic differentiation induced by tensile
stress, as accumulating evidence suggests that tensile stress
serves an important function in bone remodeling during orthodontic
tooth movement (13,14).
A hypoxic microenvironment may be caused by
orthodontic mechanical forces between the alveolar bone and the
root, and a local hypoxic microenvironment is one of the main
factors initiating bone remodeling (15). Interestingly, bone marrow was also
demonstrated to have a hypoxic environment in nature (1–7%
O2), from which BMSCs are isolated (16). Hypoxia-inducible factor 1α (HIF-1α)
is a specific regulator of hypoxia responses in all cells, and is
also the common pathway of information transmission under
hypoxia-inducible conditions (17), which has a vital influence in the
adaption to hypoxia. However, it is unknown how HIF-1α regulates
osteogenic differentiation induced by cycling stretch. One existing
study demonstrates that regarding HIF-1α in osteogenic
differentiation, being subjected to cycling stretch is still far
from adequate, but it is helpful for clinical orthodontists using
different methods to induce or regulate bone remodeling (18). As a consequence, elucidating the
mechanism of osteogenic differentiation, particularly when
mechanical stretch-induced, is essential to further understand the
mechanism of bone remodeling during orthodontic tooth movement.
To investigate whether HIF-1α affects the osteogenic
differentiation of BMSCs, a model of cyclic tensile stress loading
on cells in vitro was established. Furthermore, different
cycling stretch forces were used to determine the ideal condition
and to investigate the association amongst force, HIF-1α expression
and BMSC osteogenic differentiation. The present study demonstrated
the necessity of mechanical forces in the osteogenic
differentiation of BMSCs and investigated the potential intrinsic
molecular mechanism of the expression of HIF-1α. Furthermore, the
present study provided theoretical guidance for orthodontists
applying different means and methods to induce or regulate bone
remodeling during orthodontic tooth movement.
Materials and methods
Isolating and culture of BMSCs
BMSC primary cell culturing was performed with minor
modifications, as previously described (19). In brief, the tibias and femurs were
isolated from 10 4-week old male Sprague-Dawley rats (140±10 g).
Rats were housed at 18–26°C with ~55% humidity under a 12:12-h
light:dark cycle with access to food and water ad libitum.
The study design was submitted to and ethically approved by the
Animal Ethical Committee of Shandong University (approval no.
ECAESDUSM2013066; Shandong, China). Under aseptic circumstances,
the bone marrow was flushed out and mixed with complete α-minimal
essential medium (α-MEM; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 15% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin. The harvests were plated
into a culture flask and incubated for 3 days to allow for the
attachment of adherent cells, in a humidified atmosphere containing
5% CO2 at 37°C. The culturing medium was changed every 3
days to remove the non-adherent cells and to provide the nutrition
that the cells required.
MTT assay
BMSCs at third passage were cultured in α-MEM
supplement with 10% FBS. Once 80% confluence had been reached,
cells were seeded in 96-well plates at a density of
1×103 cells/well and the culture medium was added up to
100 µl/well. The proliferation of cells was evaluated using a MTT
assay. Cells were cultured for 1 week, and 6 wells/day were
measured. Briefly, the culture medium was replaced with 200 µl MTT
(5 mg/ml; Amresco, LLC, Solon, OH, USA) and the plates were
incubated for 4 h at 37°C. The supernatant was then removed and 150
µl of DMSO was added to each well. The absorbance at 490 nm was
measured in a multiwall spectrophotometer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Osteogenic differentiation and
alkaline phosphatase (ALP) assay
Cells on the 3rd passage were cultured in the growth
medium for 24 h prior to testing the characteristics of osteogenic
differentiation. The osteogenic differentiation medium included
α-MEM containing 10% FBS, 10 mM β-glycerophosphate, 10 nM
dexamethasone and 100 µM Vitamin C (all from Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Osteogenesis analysis was performed at
1-week (ALP) and 4-week (ARS and calcium concentrations) time
points. The extent of mineralization of in vitro-cultured
BMSCs on different substrates was evaluated by using Alizarin red S
(ARS) staining and an ALP activity assay.
For ARS, the samples were fixed in 70% ice-cold
ethanol for 1 h at 4°C, washed, and then were stained with 2%
Alizarin red solution (pH 4.2; Sigma-Aldrich; Merck KGaA) for 10
min at room temperature and finally rewashed with distilled
water.
After staining with Alizarin red, calcium was
dissolved in 1 ml of 10% w/v cetylpyridinium chloride by gentle
rocking for 30 min. The calcium concentration in the eluate (the
solution was diluted at 1:10) was spectrophotometrically determined
at 562 nm at 1-, 2-, 3- and 4-week time points. All values were
normalized against the cultivation area.
ALP activity of each sample (n=3) was assayed using
an ALP assay kit (Sigma-Aldrich; Merck KGaA). After incubation for
24 h, the medium was changed to mineralization medium for 7 days.
Then, the BMSCs were rinsed twice with PBS, fixed with 10%
formaldehyde for 30 min at room temperature, stained with 1 ml
sodium nitrate, 1 ml FRV Alkaline and 1 ml Napthol As-BI ALK in 45
ml deuterium-depleted water (ddw) for 30 min at room temperature in
the dark, and then washed with ddw and imaged using an optical
microscope (magnification, ×10). Red staining indicated an ALP
activity positive rate of 80–90%.
Colony-forming ability of BMSCs
Cells that had been passaged 3 times were seeded in
6-well culture plate at a gradient density of 0.01–1×105
cells/ml, in a humidified atmosphere containing 5% CO2
at 37°C. Culturing medium was changed every 3 days as described
above. When formed, the colony was fixed using alcohol for 10 min
at room temperature and washed using phosphate buffered saline.
Then, 0.1% crystal violet dye was added into the plate for 5 min at
room temperature and then washed away. Subsequent to being
air-dried, the plate was observed using an optical microscope
(magnifications, ×10 and ×100), and the colony forming
units-fibroblastic (CFU-F) enrichment index was counted (one
colony-forming unit was counted as >50 cells).
Surface antigen analysis of BMSCs
Flow cytometry was used to analyze the surface
antigens of BMSCs. Following digestion for three times at room
temperature and centrifugation at 150 × g for 3 min at room
temperature, the density of the cells in the 3rd generation were
adjusted to 1×106 ml. Cell-surface antigens were
detected using FITC-conjugated [cluster of differentiation CD44
(1:100; cat. no. 203906), CD90 (1:100; cat. no. 206106)] or
phycoerythrin-conjugated [CD45 (1:100; cat. no. 202207)] antibodies
(Biolegend, Inc.), and the results were analyzed using FlowJo 7.6
(FlowJo LLC).
Application of cyclic tensile
strain
Cells were plated at a density of 1×105
cells/ml in 2 ml serum-free medium on 6-well flexible silicone
rubber BioFlex™ plates which were coated with rat tail collagen
type I (Flexcell International Corp., Hillsborough, NC, USA). When
the cells reached 80–90% confluence, the growth medium was replaced
and mechanical strain was applied. The cyclic mechanical strain
with a 1 HZ and sinusoidal curve set at 1, 5 and 15% elongation
were applied for each treatment group, using a FX-5000T™ Flexcell
Tension Plus™ unit (Flexcell International Corp.). The culture
environment was the same as aforementioned. The BMSCs were
collected after 0.5, 2, 6, 8 and 12 h of stretch stimulation.
Alkaline phosphatase (ALP) activity
assay
The ALP activity of each sample (n=3) was assayed
using an ALP assay kit (Sigma-Aldrich; Merck KGaA). Once the
absorbance was measured at 405 nm, the optical density values were
normalized to the amount of total proteins in each sample
lysate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using RNAiso Plus (Takara
Bio, Inc., Otsu, Japan). The concentration of the isolated RNA was
determined at 260 nm using Gene Quant pro (GE Healthcare, Chicago,
IL, USA) and reverse transcription was performed with the Prime
Script® RT reagent kit with gDNA Eraser (Takara Bio,
Inc.). RT-qPCR was performed using SYBR® Premix Ex TaqTM
(Takara Bio, Inc.). The thermocycling conditions of the qPCR were
as follows: Denaturation at 95°C for 30 sec; 40 cycles at 95°C for
50 sec and 60°C for 20 sec; and a final dissociation stage (65°C
for 15 sec) was added at the end of the amplification procedure.
GAPDH was used as an internal control. Each experiment for each
sample was performed three times. The data were analyzed using the
comparative quantitation cycle (2−∆∆Cq) method and
expressed as a fold change respective to the control (20). The primer sequences are listed in
Table I.
 | Table I.Oligodeoxynucleotide primers used for
the reverse transcription-quantitative polymerase chain
reaction. |
Table I.
Oligodeoxynucleotide primers used for
the reverse transcription-quantitative polymerase chain
reaction.
| Gene | GenBank accession
number | Primer
sequence | Annealing (°C) | Cycle (sec) | Length (base
pairs) |
|---|
| GAPDH | NM 017008 | F:
5′-AGGTCGGTGTGAACGGATTTG-3′ | 60 | 40 | 123 |
|
| 8 | R:
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
|
|
|
|
Hypoxia-inducible | NM 024359 | F:
5′AAGTCAGCAACGTGGAAGGT-3′ |
|
|
|
| factor-1α |
| R:
5′-ATCAGCACCAAGCACGTCAT-3 | 55 | 35 | 116 |
Western blotting
Cell-aggregate lysates were extracted by lysing the
cells in a western & immunoprecipitation protein extraction
reagent (Beyotime Institute of Biotechnology, Haimen, China) with a
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) for 5 min
on ice, and then the concentration of the total protein was
determined using a BCA protein assay (Nanjing Keygen Biotech Co.,
Ltd., Nanjing, China). Subsequent to being heated for 5 min at
100°C in a loading buffer, 20 µg of each detected protein sample
was separated by 10% SDS-PAGE and then was electro-transferred into
polyvinylidenedifluoride membranes (PVDF; EMD Millipore, Billerica,
MA, USA). Then, the PVDF membranes were blocked for 0.5 h at room
temperature with Tris-buffered saline (TBS) containing 5% nonfat
dry milk powder and 0.05% Tween 20, and then incubated overnight at
4°C with primary antibodies against HIF-1α (1:1,000; cat. no.
ab216842) and GAPDH (1:5,000; cat. no. ab9485; both Abcam,
Cambridge, MA, USA). The membranes were washed 3 times for 10 min
each with TBS containing 0.05% Tween 20. Bound primary antibodies
were detected by incubating for 1 h with horseradish
peroxidase-conjugated goat anti-mouse (1:5,000; cat. no CW0102S) or
anti-rabbit immunoglobulin G secondary antibodies (1:5,000; cat. no
CW0103S; both CoWin Biosciences Co., Ltd., Beijing, China). The
membranes were washed and developed using an ECL Reagent (EMD
Millipore) according to the manufacturer's protocol, and were
quantified using ImageQuant software (TL 8.1; GE Healthcare Life
Sciences).
Statistical analysis
All data were presented as the mean ± the standard
deviation of at least three independent experiments performed in
duplicate. Statistical significance was evaluated using a one-way
analysis of variance followed by multiple comparisons performed
using a post-hoc Bonferroni's test using SPSS22.0 software (IBM
Corp., Armonk, NY, USA). P≤0.05 was considered to indicate a
statistically significant difference.
Results
Culture of BMSCs
In the primary culture, the suspension of monocytes
were round in shape. The suspension also contained other types of
cells, including blood cells and other cells with the
characteristics of adherent growth. BMSCs were isolated following
adherence for 24–36 h. On the 3rd day, BMSCs grew at a rapid rate
and other suspended cells were gradually discarded by exchanging
the medium. Elongated spindle and polygon-shaped cells were
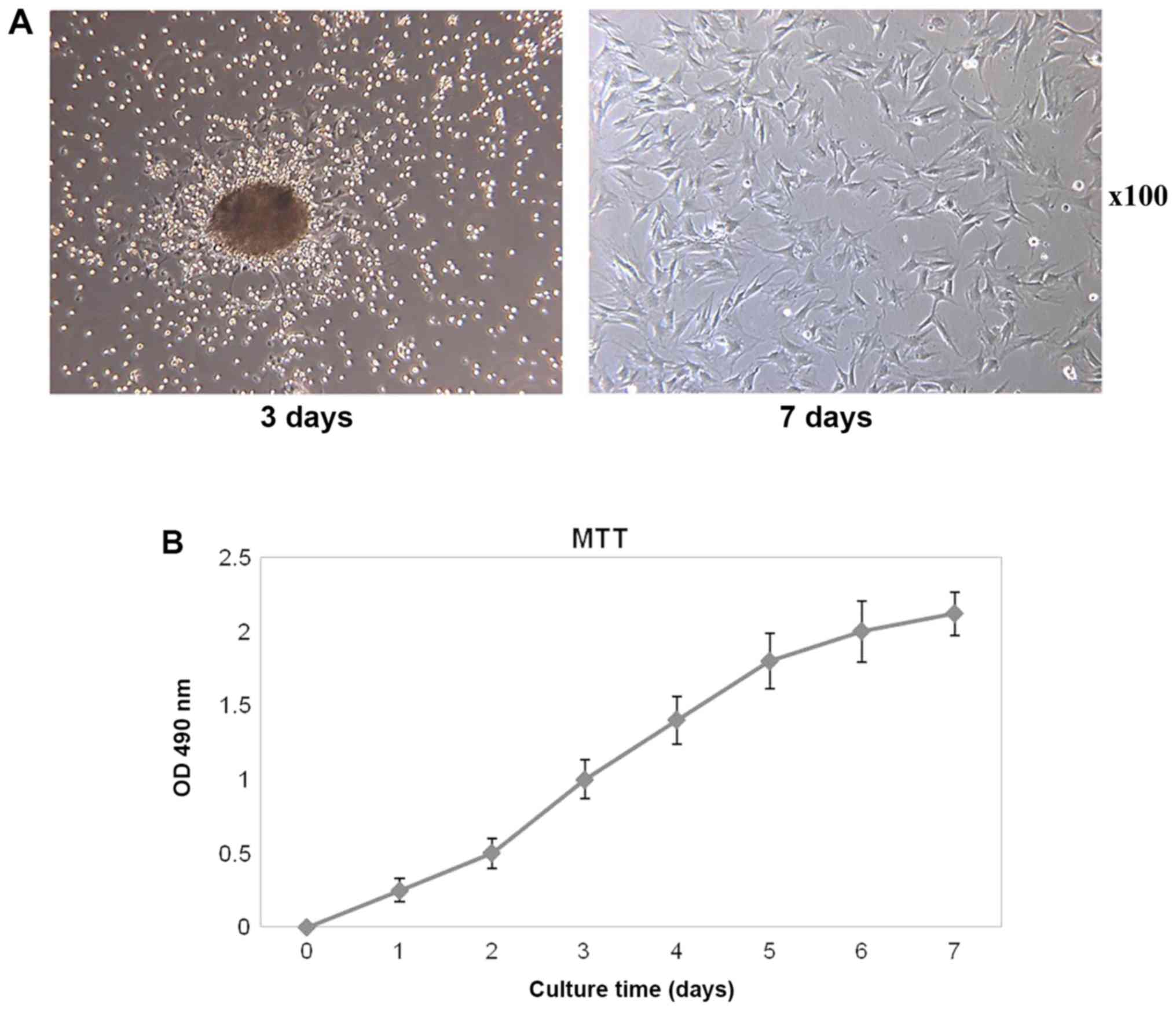
observed via light microscopy, and cell clusters appeared (Fig. 1A). On the 7th day, cells were in
typical spindle-shape and cell cluster was fused. Subsequent to the
primary culture, the cells were passaged at a 1:2 ratio at 7 days
when the 90% of the area of the bottom was covered by cells,
suggesting the cells were in a good condition (Fig. 1A). As the cells in the 3rd
generation still had decent cell viability, it suggested that the
BMSCs possessed the capability to passage stably and may be used
for further experimental study.
Proliferation capability of BMSCs
To assess the cell proliferation capability, an MTT
test kit was used to detect the proliferative rates. The cell
proliferation curve presented a slower rate initially (within the
first 2 days), and followed by a logarithmic growth during days 3
to 6, which was similar to an S-shape curve. Subsequent to 7 days,
the proliferation of the BMSCs entered a relative stable phase
(Fig. 1B).
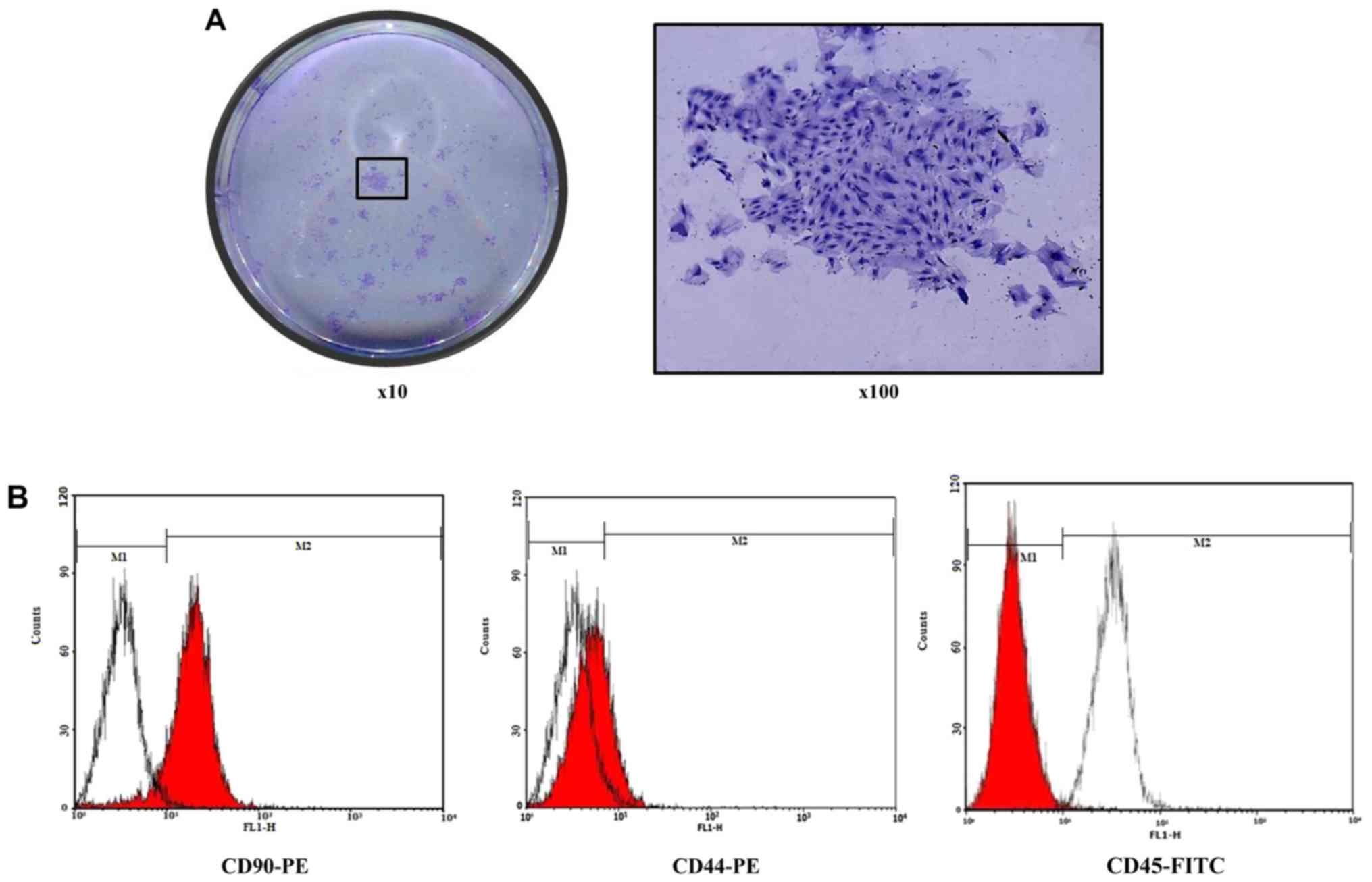
Clonal characterization of BMSCs
BMSCs were originally isolated from other cells via
their ability to adhere to culture plastic and then form
density-independent colonies termed CFU-F. To assess the clone
formation characteristics of BMSCs, a limited dilution method was
used by adding crystal violet dye into the plate. Following one
week, it was observed through a light microscope that there was
substantial purple colony formation, and a CFU-F was formed
(Fig. 2A).
Flow cytometry
The 3rd passage of the BMSCs was used to investigate
its phenotypes. The result revealed that 99.7% of the cells
expressed CD44 and CD90. In contrast, expression of CD45, a
pan-hematopoietic marker, was observed in a distinct population of
0.3% of the cells (Fig. 2B). The
results indicated that the cells obtained through isolated culture
were bone marrow stromal cells.
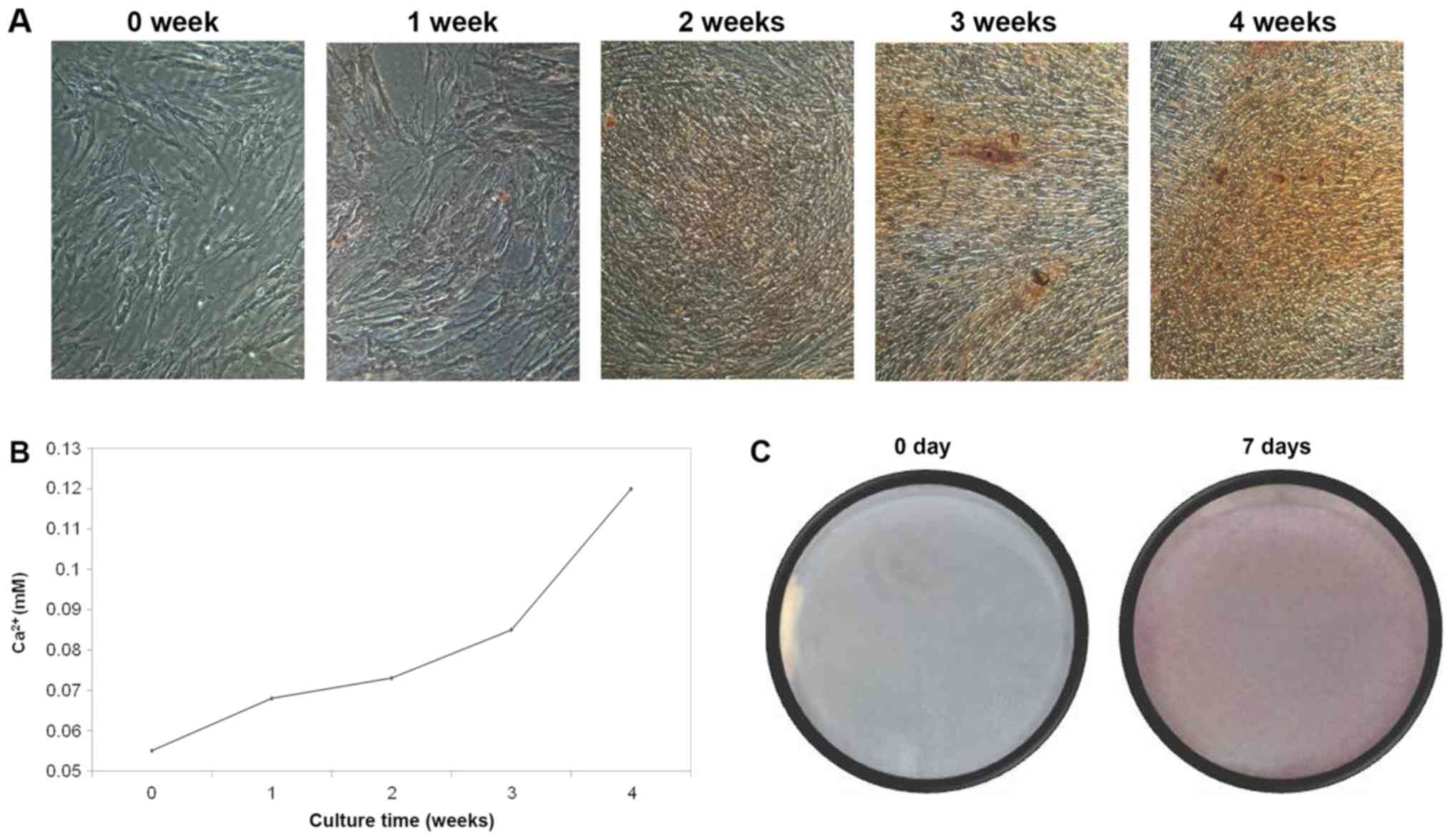
Osteogenic differentiation
Four weeks subsequent to the BMSCs being placed in
osteogenic medium, mineralization nodules were observed following
ARS staining. As presented in Fig.
3A, with increased culture time, the proportion of mineralized
nodules was increased. Following staining, the concentration of
calcium ions was increased, which also exhibited a similar trend to
ARS staining, reaching a peak at 4 weeks (Fig. 3B). In addition, ALP staining was
used to detect the activity of ALP. The positive rate was as high
as 80–90%, which indicates that the activity of ALP was elevated
notably (Fig. 3C). The
aforementioned results demonstrate that when in osteogenic medium,
BMSCs may be induced to differentiate into osteoblasts in
vitro.
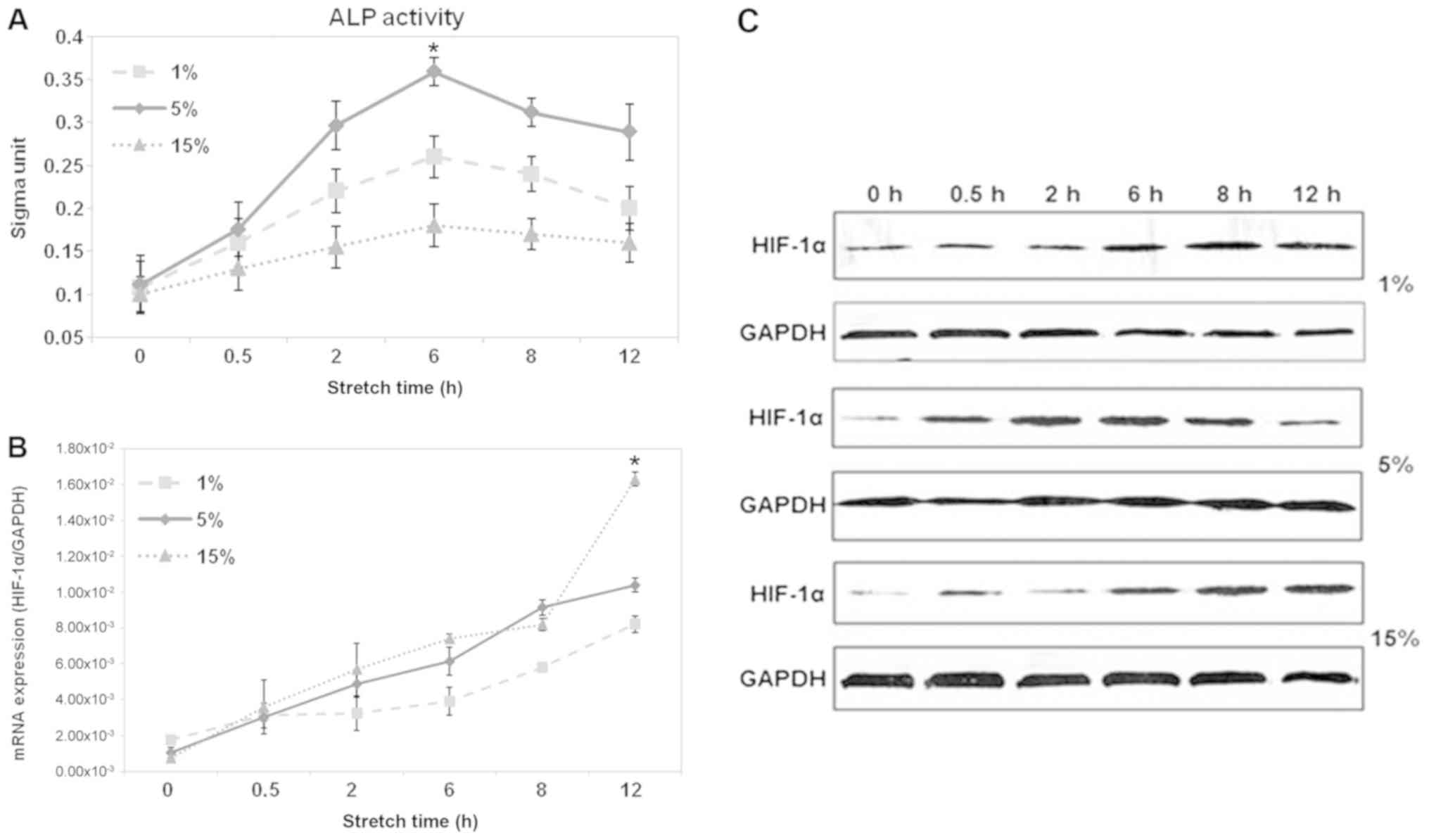
Optimal force by ALP activity
detection
In the present study, cycling stretch stimulation at
1 HZ was applied to BMSCs and whether the process of osteogenesis
was switched on was assessed. The progression of osteogenesis was
assessed using the presence of the osteoblast differentiation
marker ALP. The results indicated that ALP activity increased with
increasing culture time for all assessed groups (Fig. 4A). Additionally, the ALP activity
of the medium force group (5%) was substantially higher compared
with that of the weak force group (1%) and the strong force group
(15%). Furthermore, at 6 h, the ALP activity of cells was
substantially higher compared with any other time, which indicated
that 6 h at 5% 1 HZ stretch is the ideal condition to stimulate
osteogenic differentiation of BMSCs.
Effect of different cyclic stretch
strengths on the expression of HIF-1α in BMSCs
To investigate whether HIF-1α participates in the
stretch-induced osteogenic differentiation of BMSCs, the expression
of HIF-1α at the mRNA and protein levels was determined. In the
present study, the cells were exposed to different strengths of
loading mechanical stimulation: A weak force group (1%), a medium
force group (5%) and a strong force group (15%). Each group was
stretched for 0, 0.5, 2, 6, 8 and 12 h, at 1 HZ. According to
RT-qPCR analysis, it was revealed that there was a time-dependent
increase in the expression of HIF-1α at the mRNA level, when BMSCs
were exposed to 1 and 15% cycling stretch. Notably in 5% group, the
HIF-1α mRNA level was slightly increased during the first 8 h, and
peaked at 12 h, but then decreased a little (Fig. 4B). In accordance with the changes
in mRNA levels, the protein expression of HIF-1α increased with
increased stretch time in BMSCs, and reached a maximum at 12 h
(Fig. 4C). The above results
suggest that mechanical stretch energized the activation of HIF-1α.
Their changes were consistent with BMSC osteogenic differentiation.
All results suggested that HIF-1α participated in stretch-induced
BMSC osteogenic differentiation.
Discussion
The bone remodeling cycle may be altered by a number
of different types of stimulation, including mechanobiological
factors. Previous research has demonstrated that mechanical stress
may participate in osteogenic differentiation (21). To date, numerous studies have
focused on the mechanism of HIF-1α in different types of tumor
(22,23). However, only a few reports so far
have been able to shed light on the osteogenic differentiation of
BMSCs (24). A clear molecular
biological mechanism will help the application and generalization
of HIF-1α in a clinical setting. Therefore, it is necessary to
elucidate its mechanism in the osteogenic differentiation of BMSCs.
In this context, the present study examined this topic, and
concluded that a dynamic overexpression of osteogenic genes and
HIF-1α occurs in response to biomechanical cycling stretch. In the
process of osteogenesis induced by mechanical stress, osteogenic
differentiation is continuously enhanced under appropriate
mechanical stretch, and HIF-1α is involved in this process.
BMSCs reside in bone marrow aspirated from tibias
and femurs, which easily produce large quantities of BMSCs and
which are available for transplantation (25). Furthermore, BMSCs are multipotent
cells that favor the bone remodeling cycle and other mesenchymal
tissues (25). Isolated expanded
BMSC cultures differentiate, in a controlled manner, to multiple
lineages (26). Therefore they are
suitable ‘volunteers’ for studying osteogenic differentiation and
bone formation, which was confirmed by the results of the present
study. In the present study, Alizarin Red S staining was used to
confirm that the isolated bone marrow monocytes exhibited
multipotential differentiation. One previous study demonstrated
that BMSCs emerged from marrow cell suspensions by selectively
attaching to a culture dish and dividing to form colonies (25). In the present study, it was
revealed that a large number of colonies were formed once the BMSCs
grew against the wall of flask, whereas other cell types, such as
blood cells, would not have formed colonies until several days of
culture. This difference in the rate of colony formation is well
documented for BMSCs in cultures derived from clonal and mass cell
origin (27). Furthermore, to
confirm the purity of the BMSCs, flow cytometry was performed. The
positive expression of CD90 and CD44 markers indicated the presence
of BMSCs in the pool of bone marrow cells. CD45, on the other hand,
was negative, as a marker for hematopoietic cells (28). One previous study revealed that the
third and fifth passage of the cells exhibited a uniform fiber-like
shape similar to fibroblasts and the optimal cell purity was
obtained (29). Due to this, cells
in these passages are often used for experimental research. The
results of the present study were consistent with the
characteristics of BMSCs. Altogether, these results suggested that
the cells that were isolated from rats were identified as BMSCs,
which lay the foundation of the subsequent experiment.
Orthodontic tooth movement induced by mechanical
stimuli depends on the remodeling capacity of local alveolar bone.
Mechanical strain is known to be a fundamental physiological factor
regulating bone formation and renewal (30). Numerous studies have examined the
mechanism of mechanical stimuli. One focus in this area of research
is regulating the osteogenic differentiation of stem cells
(31). However, less attention has
been dedicated to the consequences of cyclic equiaxial stretch
(32). One prior study has
suggested that equibiaxial cyclic strain stimulates human
mesenchymal stem cells increase in matrix mineralization (33). Furthermore, another previous study
demonstrated that cyclical stretching was a differentiation factor
which was stronger compared with dexamethasone in the short term
(34). However, another study
reported that cyclic tensile stretch inhibits the osteogenic
differentiation of human dental pulp stem cells (21). Therefore, the effect of cyclic
tensile stress on stem cells remains controversial. In lieu of
this, the present study established an external mechanical
stimulation model using rat BMSCs and a mechanical loading device.
Additionally, an ALP assay kit was used to examine the ALP activity
of the BMSCs, as ALP is an important early stage osteogenic marker
of stem cells (24). The results
revealed that levels of the enzyme were low in the first half hour
but increased substantially within 2 h, reaching maximal levels
around 6 h and decreasing following that point (Fig. 3C), which indicated that ALP
activity increased substantially and that cyclic tensile strain
promotes the osteogenic differentiation of BMSCs under a certain
conditions in vitro. One potential reason for the different
results is that cells in vivo did not receive the same
mechanical signal in the cell stretching loading system since the
bone matrix filters the physical load. Furthermore, external
biomechanical conditions are too complex to be simulated. It was
reported that 20% of strain-activated apoptotic signaling pathways,
and 25% of cyclic deformation, induced cell death directly
(35). Therefore, the present
study selected a 15% cyclic deformation at 1 HZ (60 cycles/min)
stretch as a physiological limit of stress to induce the osteogenic
differentiation of BMSCs. Three different loading mechanical
stimulation groups were created: A weak force group (1%), a medium
force group (5%) and a strong force group (15%), which mimicked the
physiological conditions of occlusal force in vivo (36). In the present experiments, it was
also revealed that the 5% elongation group exhibited the highest
ALP activity, followed by 1% elongation. It was substantially
increased at 6 h following loading and then decreased (Fig. 4A), which suggested that 6 h at 5% 1
HZ stretch is the ideal condition to stimulate the osteogenic
differentiation of BMSCs. Similar optimal force was also identified
in photothermal stress-induced osteogenesis (37). These results will form the
theoretical guidance for application during orthodontic tooth
movement.
Bone formation is initiated following the expansion
of BMSCs, and ends with osteoblastic cells originating from the
BMSCs (38). Numerous factors are
involved in ensuring that BMSCs differentiate along osteoblastic
cell lines, including growth factors, cytokines and the surrounding
microenvironmental conditions (39). Interestingly, one previous study
has demonstrated that a hypoxic microenvironment is a necessary
condition during the bone regeneration process (40), whereas little is known on the
mechanism underlying hypoxia in osteoblast differentiation and bone
formation. Research has demonstrated that the transcription factor
HIF-1α constitutes the principal mediator of cellular adaptation to
hypoxia (41). HIF-1α is a
transcriptional activator and participates in numerous
pathophysiological processes under hypoxia (42). Previously, the classical theory was
that HIF-1α activates the process of the epithelial-mesenchymal
transition, which is fundamental for embryonic development and of
particular importance to organ formation and differentiation
(43). Additionally, De Luna et
al (44) reported that hypoxia
mediated HIF-1α accumulation results in reduced proinflammatory
gene expression and ameliorates inflammation. These results
indicate that hypoxia followed by HIF-1α production is closely
associated with the proliferation, differentiation, invasion,
metastasis and even prognosis of a tumor. Since stem cells and
cancer cells share a lot of similarities in gene expression,
cellular processes and signal transductions, HIF-1α may be involved
in the BMSC osteogenesis process. In fact, others have reported the
presence of a hypoxic micro-environment in the bone remodeling
process and orthodontic tooth movement (15). Furthermore BMSCs originated from
hypoxic stem cell niches, and survived under hypoxia, particularly
in the bone marrow (45). Thus, it
is reasonable to investigate whether HIF-1α was involved in the
cyclic stretch-induced osteogenic differentiation of BMSCs. In the
present study, RT-qPCR and western blot analysis revealed that the
mRNA and protein expression of HIF-1α increased uniformly under
various conditions during osteogenic differentiation. The present
results demonstrate that the in vitro mechanical stimulation
model that was established contained a hypoxic microenvironment.
Meanwhile extrinsic cyclic stretch may be used to realistically
simulate the microenvironment of the cells in vivo (36). All these results indicate that the
activation of HIF-1α was required for the cyclic stretch-induced
osteogenic differentiation of BMSCs, which were in agreement with a
previous report (46). These
results reveal the mechanism of HIF-1α involvement in
hypoxia-induced osteogenic differentiation.
The mechanism by which hypoxia regulates osteogenic
differentiation has been investigated. Hsu et al (47) demonstrated that osteoblasts
differentiated under hypoxia rely mainly on glycolysis for energy.
They also confirmed that glycolysis was enhanced under hypoxia, and
the metabolic switch to mitochondrial respiration during the
osteogenic differentiation of human MSCs (hMSCs) was strongly
compromised by hypoxia (47).
Additionally, research suggests that vascular endothelial growth
factor, a transcripional target of HIF-1α, serves an important
function in the angiogenesis (48). It was indicated that osteogenesis
is tightly coupled with angiogenesis during bone development and
regeneration (40). These results
are consistent with previous reports (49–51),
which revealed HIF-1α is involved in hypoxia-induced osteogenic
differentiation. However, the controversy remains. One previous
study demonstrated that the overexpression of HIF-1α will decrease
the expression of RUNX family transcription factor 2 (Runx2; a
critical transcriptional regulator of osteoblast differentiation)
and bone morphogenetic protein 2 (BMP2; another important
osteogenic factor) in MSCs (52).
Furthermore, a previous study reported that HIF-1α inhibits
osteogenesis in hMSCs and suggested that hypoxia inhibits the
expression of type 1 Runx2 and its downstream targets (53). Nevertheless, a number of studies
have differing views on this issue. One study has suggested that
hypoxia promotes the osteogenesis of hMSCs in a HIF-1α-dependent
manner, which directly enhances the expression of Runx2 (54). Interestingly, Huang et al
(40) hypothesized that hypoxia
increases ALP activity and the production of type I/III collagen,
which are all hallmarkers of MSC differentiation into osteogenic
lineage cells. This discrepancy, however, may reflect the fact that
different cells respond differently to hypoxia. Additionally, there
is evidence confirming that at an early stage of osteogenesis, the
hypoxic-mediated HIF-1α-Twist1 pathway promotes osteoblast
differentiation, whereas at a late stage hypoxia substantially
diminishes bone formation in vivo (55). This has provided a novel viewpoint
defining the association between hypoxia and osteoblast
differentiation. In other words, it may be stage-dependent. In
brief, it was assumed that the potential reasons for these
different results include a different cell species (mouse, rat and
human), different stage of osteoblast differentiation (early,
middle and late), differences in the experimental design (hypoxic
state or mechanical stimuli) and different oxygen concentration
(from 0.02 to 5% oxygen). One previous study has implicated Twist,
a downstream target of HIF-1α, functioning as a transcription
repressor of Runx2 through binding to the E-box located on the
promoter of type 1 Runx2, resulting in the suppression of type 1
Runx2, followed by the suppression of BMP2, type 2 Runx2 and
downstream targets of Runx2 in MSCs undergoing osteogenic
differentiation (49). This has
provided a neoteric molecular mechanism to explain the effect of
hypoxia. Thus, the HIF-1α-Twist1 axis is involved in the MSC
osteogenesis process, which may provide novel ideas to elucidate
the association between HIF-1α and stretch-induced osteogenic
differentiation.
In summary, the results of the present study suggest
that cyclic tensile stretch promotes the osteogenic differentiation
of BMSCs in vitro, as indicated by the upregulation of ALP
activity. The present experimental results also revealed that the
expression of HIF-1α increased specifically in response to certain
mechanical tension with defined intensity and duration. Therefore,
future research will investigate HIF-1α as a biological target of
osteogenic differentiation induced by tensile stress. The function
and mechanism of HIF-1α in bone remodeling are not well studied,
nor is it clear whether HIF-1α functions as a promoter or an
inhibitor for osteogenic differentiation.
Acknowledgements
The authors would like to thank Dr Qingyuan Guo
(Department of Stomatology, The Affiliated Qingdao Municipal
Hospital, Qingdao University) who provided guidance, and the
technical support from the Director of Shandong Provincial Key
Laboratory of Oral Tissue Regeneration.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 11602122) and the
China Postdoctoral Science Foundation (grant no. 2017m623396).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HY and QG designed the study and edited the
manuscript. WY and YL performed the experiments. XY and RY analyzed
the data. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Provincial Key Laboratory of Oral Tissue
Regeneration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dibart S, Yee C, Surmenian J, Sebaoun JD,
Baloul S, Goguet-Surmenian E and Kantarci A: Tissue response during
Piezocision-assisted tooth movement: A histological study in rats.
Eur J Orthod. 36:457–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoun JS, Mei L, Gibbs K and Farella M:
Effect of orthodontic treatment on the periodontal tissues.
Periodontol. 74:140–157. 2017. View Article : Google Scholar
|
|
3
|
Vlaikou AM, Kouroupis D, Sgourou A,
Markopoulos GS, Bagli E, Markou M, Papadopoulou Z, Fotsis T, Nakos
G, Lekka ME and Syrrou M: Mechanical stress affects methylation
pattern of GNAS isoforms and osteogenic differentiation of
hAT-MSCs. Biochim Biophys Acta Mol Cell Res. 1864:1371–1381. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barbieri G, Solano P, Alarcón JA, Vernal
R, Rios-Lugo J, Sanz M and Martín C: Biochemical markers of bone
metabolism in gingival crevicular fluid during early orthodontic
tooth movement. Angle Orthod. 83:63–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohgushi H: Osteogenically differentiated
mesenchymal stem cells and ceramics for bone tissue engineering.
Expert Opin Biol Ther. 14:197–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connelly JT, García AJ and Levenston ME:
Interactions between integrin ligand density and cytoskeletal
integrity regulate BMSC chondrogenesis. J Cell Physiol.
217:145–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moreau JE, Chen J, Horan RL, Kaplan DL and
Altman GH: Sequential growth factor application in bone marrow
stromal cell ligament engineering. Tissue Eng. 11:1887–1897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreau JE, Bramono DS, Horan RL, Kaplan DL
and Altman GH: Sequential biochemical and mechanical stimulation in
the development of tissue-engineered ligaments. Tissue Eng Part A.
14:1161–1172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaffer JL, Rizen M, L'Italien GJ,
Benbrahim A, Megerman J, Gerstenfeld LC and Gray ML: Device for the
application of a dynamic biaxially uniform and Isotropic strain to
a flexible cell culture membrane. J Orthop Res. 12:709–719. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Susilo ME, Bell BJ, Roeder BA,
Voytik-Harbin SL, Kokini K and Nauman EA: Prediction of equibiaxial
loading stress in collagen-based extracellular matrix using a
three-dimensional unit cell model. Acta Biomater. 9:5544–5553.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi YX, Yao QP, Huang K, Shi Q, Zhang P,
Wang GL, Han Y, Bao H, Wang L, Li HP, et al: Nuclear envelope
proteins modulate proliferation of vascular smooth muscle cells
during cyclic stretch application. Proc Natl Acad Sci USA.
113:5293–5298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ursekar CP, Teo SK, Hirata H, Harada I,
Chiam KH and Sawada Y: Design and construction of an equibiaxial
cell stretching system that is improved for biochemical analysis.
PLoS One. 9:e906652014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lohberger B, Kaltenegger H, Stuendl N,
Payer M, Rinner B and Leithner A: Effect of cyclic mechanical
stimulation on the expression of osteogenesis genes in human
intraoral mesenchymal stromal and progenitor cells. Biomed Res Int.
2014:1895162014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Huang X, Yu H, Yang J, Li Y, Yuan X
and Guo Q: HIF-1α-TWIST pathway restrains cyclic mechanical
stretch-induced osteogenic differentiation of bone marrow
mesenchymal stem cells. Connect Tissue Res. 60:544–554. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niklas A, Proff P, Gosau M and Römer P:
The role of hypoxia in orthodontic tooth movement. Int J Dent.
2013:8418402013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Padmanabha D, Gentile LB, Dumur CI,
Beckstead RB and Baker KD: HIF- and non-HIF-regulated hypoxic
responses require the estrogen-related receptor in Drosophila
melanogaster. PLoS Genet. 9:e10032302013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou N, Hu N, Liao JY, Lin LB, Zhao C, Si
WK, Yang Z, Yi SX, Fan TX, Bao W, et al: HIF-1a as a regulator of
BMP2-induced chondrogenic differentiation, osteogenic
differentiation, and endochondral ossification in stem cells. Cell
Physiol Biochem. 36:44–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nemeth K, Mayer B, Sworder BJ, Kuznetsov
SA and Mezey E: A practical guide to culturing mouse and human bone
marrow stromal cells. Curr Protoc Immunol. 102:Unit 22F.12.
2013.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu HC, Wu TC, Chen MR, Liu SW, Chen JH and
Lin KM: Mechanical stretching induces osteoprotegerin in
differentiating C2C12 precursor cells through noncanonical Wnt
pathways. J Bone Miner Res. 25:1128–1137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang Y, Han J, Kim SJ, Kim J, Lee MJ,
Jeong S, Ryu MJ, Seo KS, Choi SY, Shong M, et al: Suppression of
mitochondrial respiration with auraptene inhibits the progression
of renal cell carcinoma: Involvement of HIF-1a degradation.
Oncotarget. 6:38127–38138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung WW, Chu YC, Chen PR, Liao MH and Lee
JW: Positive regulation of HIF-1A expression by EBV oncoprotein
LMP1 in nasopharyngeal carcinoma cells. Cancer Lett. 382:21–31.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sha Y, Lv Y, Xu Z, Yang L, Hao X and
Afandi R: MGF E peptide pretreatment improves the proliferation and
osteogenic differentiation of BMSCs via MEK-ERK1/2 and PI3K-Akt
pathway under severe hypoxia. Life Sci. 189:52–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bruder SP, Jaiswal N and Haynesworth SE:
Growth kinetics, selfrenewal, and the osteogenic potential of
purified human mesenchymal stem cells during extensive
subcultivation and following cryopreservation. J Cell Biochem.
64:278–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kusuyama J, Kamisono A, ChangHwan S, Amir
MS, Bandow K, Eiraku N, Ohnishi T and Matsuguchi T: Spleen tyrosine
kinase influences the early stages of multilineage differentiation
of bone marrow stromal cell lines by regulating phospholipase C
gamma activities. J Cell Physiol. 233:2549–2559. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Y, Xu Y, Xiao Z, Zhao Y, Li J, Han S,
Chen L, Dai B, Wang L, Chen B and Wang H: The combination of
three-dimensional and rotary cell culture system promotes the
proliferation and maintains the differentiation potential of rat
BMSCs. Sci Rep. 7:1922017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia CS, Zuo AJ, Wang CY and Wang YZ:
Isolation of rabbit bone marrow mesenchymal stem cells using
density gradient centrifugation and adherence screening methods.
Minerva Med. 104:519–525. 2013.PubMed/NCBI
|
|
29
|
Steinert AF, Kunz M, Prager P, Göbel S,
Klein-Hitpass L, Ebert R, Nöth U, Jakob F and Gohlke F:
Characterization of bursa subacromialis-derived mesenchymal stem
cells. Stem Cell Res Ther. 6:1142015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang F, Xia Z, Li S, Eckert G and Chen J:
Mechanical environment change in root, periodontal ligament, and
alveolar bone in response to two canine retraction treatment
strategies. Orthod Craniofac Res. 18 (Suppl 1):S29–S38. 2015.
View Article : Google Scholar
|
|
31
|
Stanley A, Heo SJ, Mauck RL, Mourkioti F
and Shore EM: Elevated BMP and mechanical signaling through
YAP1/RhoA poises FOP mesenchymal progenitors for osteogenesis. J
Bone Miner Res. May 20–2019.doi: 10.1002/jbmr.3760 (Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Estes BT, Gimble JM and Guilak F:
Mechanical signals as regulators of stem cell fate. Curr Top Dev
Biol. 60:91–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simmons CA, Matlis S, Thornton AJ, Chen S,
Wang CY and Mooney DJ: Cyclic strain enhances matrix mineralization
by adult human mesenchymal stem cells via the extracellular
signal-regulated kinase (ERK1/2) signaling pathway. J Biomech.
36:1087–1096. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jagodzinski M, Drescher M, Zeichen J,
Hankemeier S, Krettek C, Bosch U and van Griensven M: Effects of
cyclic longitudinal mechanical strain and dexamethasone on
osteogenic differentiation of human bone marrow stromal cells. Eur
Cell Mater. 7:35–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boccafoschi F, Sabbatini M, Bosetti M and
Cannas M: Overstressed mechanical stretching activates survival and
apoptotic signals in fibroblasts. Cells Tissues Organs.
192:167–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parandakh A, Tafazzoli-Shadpour M and
Khani MM: Stepwise morphological changes and cytoskeletal
reorganization of human mesenchymal stem cells treated by
short-time cyclic uniaxial stretch. In Vitro Cell Dev Biol Anim.
53:547–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kajiya H, Katsumata Y, Sasaki M, Tsutsumi
T, Kawaguchi M and Fukushima T: Photothermal stress triggered by
near-infrared-irradiated carbon nanotubes up-regulates osteogenesis
and mineral deposition in tooth-extracted sockets. Int J
Hyperthermia. 31:635–642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Friedenstein AJ: Precursor cells of
mechanocytes. Int Rev Cytol. 47:327–359. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han I, Kwon BS, Park HK and Kim KS:
Differentiation potential of mesenchymal stem cells is related to
their intrinsic mechanical properties. Int Neurourol J. 21 (Suppl
1):S24–S31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang J, Deng F, Wang L, Xiang XR, Zhou
WW, Hu N and Xu L: Hypoxia induces osteogenesis-related activities
and expression of core binding factor alpha1 in mesenchymal stem
cells. Tohoku J Exp Med. 224:7–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shah YM: The role of hypoxia in intestinal
inflammation. Mol Cell Pediatr. 3:12016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu L and Zhao Q: Hypoxia-inducible factor
1a participates in hypoxia-induced epithelial-mesenchymal
transition via response gene to complement 32. Exp Ther Med.
14:1825–1831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Luna N, Suárez-Calvet X, Lleixà C,
Diaz-Manera J, Olivé M, Illa I and Gallardo E: Hypoxia triggers
IFN-I production in muscle: Implications in dermatomyositis. Sci
Rep. 7:85952017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moirangthem RD, Singh S, Adsul A,
Jalnapurkar S, Limaye L and Kale VP: Hypoxic niche-mediated
regeneration of hematopoiesis in the engraftment window is
dominantly affected by oxygen tension in the milieu. Stem Cells
Dev. 24:2423–2436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang Y, Wang Y and Tang G: Cyclic tensile
strain promotes the osteogenic differentiation of a bone marrow
stromal cell and vascular endothelial cell co-culture system. Arch
Biochem Biophys. 607:37–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hsu SH, Chen CT and Wei YH: Inhibitory
effects of hypoxia on metabolic switch and osteogenic
differentiation of human mesenchymal stem cells. Stem Cells.
31:2779–2788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wei F, Yang S, Xu H, Guo Q, Li Q, Hu L,
Liu D and Wang C: Expression and function of hypoxia inducible
factor-1α and vascular endothelial growth factor in pulp tissue of
teeth under orthodontic movement. Mediators Inflamm.
2015:2157612015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Y, Wan C, Gilbert SR and Clemens TL:
Oxygen sensing and osteogenesis. Ann N Y Acad Sci. 1117:1–11. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wan C, Shao J, Gilbert SR, Riddle RC, Long
F, Johnson RS, Schipani E and Clemens TL: Role of HIF-1alpha in
skeletal development. Ann N Y Acad Sci. 1192:322–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Menon A, Creo P, Piccoli M, Bergante S,
Conforti E, Banfi G, Randelli P and Anastasia L: Chemical
activation of the hypoxia-inducible factor reversibly reduces
tendon stem cell proliferation, inhibits their differentiation, and
maintains cell undifferentiation. Stem Cells Int. 2018:94680852018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lampert FM, Kütscher C, Stark GB and
Finkenzeller G: Overexpression of Hif-1a in mesenchymal stem cells
affects cell-autonomous angiogenic and osteogenic parameters. J
Cell Biochem. 117:760–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang DC, Yang MH, Tsai CC, Huang TF, Chen
YH and Hung SC: Hypoxia inhibits osteogenesis in human mesenchymal
stem cells through direct regulation of RUNX2 by TWIST. PLoS One.
6:e239652011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wagegg M, Gaber T, Lohanatha FL, Hahne M,
Strehl C, Fangradt M, Tran CL, Schönbeck K, Hoff P, Ode A, et al:
Hypoxia promotes osteogenesis but suppresses adipogenesis of human
mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent
manner. PLoS One. 7:e464832012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen X, Gu S, Chen BF, Shen WL, Yin Z, Xu
GW, Hu JJ, Zhu T, Li G, Wan C, et al: Nanoparticle delivery of
stable miR-199a-5p agomir improves the osteogenesis of human
mesenchymal stem cells via the HIF1a pathway. Biomaterials.
53:239–250. 2015. View Article : Google Scholar : PubMed/NCBI
|