Introduction
Endometriosis is a disorder characterized by
implantation and growth of endometrial tissue outside the uterine
cavity. It has been estimated that approximately 10–15% of women of
reproductive age are affected by endometriosis (1). To date, the etiology of endometriosis
has yet to be elucidated; many theories have been proposed, but
none of them have been substantiated (2–4).
Recently, substantial evidence indicates that oxidative stress,
which is defined as an imbalance between reactive oxygen species
(ROS) and antioxidants, may be involved in the pathophysiology of
endometriosis by triggering a general inflammatory response in the
peritoneal cavity (5).
Consequently, ROS are likely to be associated with different
pregnancy-related disorders, such as spontaneous abortions, fetal
growth restriction and preeclampsia. Therefore, targeting oxidative
imbalance may be applicable as an effective therapeutic strategy
against endometriosis.
MicroRNAs (miRNAs) are a group of small non-coding
RNAs that regulate the expression of genes at the
post-transcriptional level via inhibition of protein translation
and decay of mRNAs (6). It is well
documented that miRNAs are involved in various biological processes
including the response to oxidative stress. For instance, a recent
study showed that miR-182-5p inhibits oxidative stress via
targeting Toll-like receptor 4 (TLR4) in macrophages (7). Forced expression of miR-210 was found
to lead to the reduction of BNIP3 and protected rat myocardial
cells from oxidative stress induced by H2O2
(8). miR-29b was found to regulate
oxidative stress via targeting SIRT1 in ovarian cancer cells
(9). miR-455 has been found to be
involved in a variety of cancers, and it was shown that miR-455
could protect osteoblasts from oxidative stress via activation of
the Nrf2 signaling pathway (10,11).
However, whether miR-455 can alleviate oxidative stress in
endometrial stromal cells remains unknown.
Fatty acid binding protein 4 (FABP4), also known as
adipocyte FABP (aP2), is a small 15-kDa lipid chaperone that
participates in various biological processes such as glucose and
lipid homeostasis, inflammation and intracellular fatty acid
trafficking (12). A recent study
has shown that FABP4−/− macrophages exhibit diminished
ROS production (13). In contrast,
it was also found that FABP4 plays a cytoprotective role against
oxidative and ER stress in adipocytes and that the knockdown of
FABP4 causes an increase in cellular ROS levels (14). These findings indicate that FABP4
plays an essential role in the regulation of oxidative stress, and
thus, further investigation is warranted in terms of its role in
endometriosis.
In the present study, we identified miR-455 as a
putative FABP4-targeting miRNA. Forced expression of miR-455
protected endometrial stromal cells from cytotoxicity induced by
H2O2. Moreover, silencing of FABP4 generated
protective effects similar to those of miR-455, which were
abrogated by the ectopic expression of FABP4 in endometrial stromal
cells.
Materials and methods
Cell culture and transfection
Immortalized human endometrial stromal cells (HESCs)
were purchased from Applied Biological Materials Ltd.. Cells were
cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium
(DMEM) (Gibco™; Thermo Fisher Scientific, Inc.) and Ham's F-12
medium (Gibco™; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal calf serum (FCS) (HyClone; GE Healthcare), 100 U/ml
penicillin and streptomycin (HyClone; GE Healthcare). Cells were
cultured in a humidified atmosphere with 5% CO2 at 37°C.
miR-455 mimics (miR-455), negative control mimics (miR-NC), siRNA
against FABP4 (si-FABP4), negative control siRNA (si-NC) and
pcDNA3.1-FABP4 (pcFABP4) were purchased from GenePharm.
Transfection was performed using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Unless indicated, the dose of H2O2 used in
this study was 20 µM.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, 1 µg of total RNA was used to
synthesize cDNA using a TIANScript II cDNA First Chain Synthesis
kit (Tiangen Biotech Co., Ltd). Then, qPCR was performed with a
miScript SYBR-Green PCR kit (Qiagen, Inc.) on an ABI 7500 system
(PE Applied Biosystems™; Thermo Fisher Scientific, Inc.). GAPDH and
U6 were used as internal controls. The thermocycling conditions
were as follows: 95°C for 1 min, then 40 cycles of 95°C for 15 sec,
55°C for 30 sec and 70°C for 30 sec. The expression levels in
tissues and cells were calculated using the 2−ΔΔCq
method (15).
Dual-luciferase reporter assay
Two oligonucleotide pairs including FABP4 3′UTR
sequences of both the mutant and wild-type sequences were
synthesized by Genepharm. After annealing, the oligonucleotides
were inserted into the pmirGLO vector (Fig. S1) (Promega, Madison, WI, USA). For
luciferase assays, the cells were co-transfected with the
corresponding vectors and miR-455a mimics using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Then, 24 h after transfection, the firefly and
Renilla luciferase activities were detected consecutively
using a Dual-Luciferase Kit (Promega Corp.). The relative
luciferase activity was normalized to that of Renilla
luciferase.
Cell viability assay
A CCK-8 assay was performed to measure cell
viability after treatment. Briefly, cells (5,000 cells/well) were
seeded in a 96-well plate. After treatment, 10 µl of Cell Counting
Kit-8 solution (CCK-8; cat. no. c0038, Beyotime Institute of
Biotechnology) was added, and the optical density (OD) value of
each well was measured at a wavelength of 595 nm using an ELISA
microplate reader. Wells without cells served as blanks. The
experiments were performed in triplicate and were repeated at least
three times.
Apoptosis assay
For apoptosis detection by flow cytometry, the cells
were stained with propidium iodide (PI) and Annexin V-FITC (cat.
no. v13242; Invitrogen; Thermo Fisher Scientific, Inc.); the
fluorescence was then determined by a BD FACSVia™ flow cytometry
system (BD Biosciences).
Caspase-3/7 activity assay
The activity of caspase-3/7 was measured using a
Caspase-Glo 3/7 Assay kit (cat. no. g8090, Promega) according to
the manufacturer's protocol. Briefly, 100 µl of caspase-3/7 reagent
was added to each well followed by incubation for 1 h at room
temperature. Luminescence was measured as the absorbance at 405 nm.
Caspase-3/7 activity was indicated as a percentage of the untreated
control. Three independent experiments were performed.
Western blot analysis
After treatment, cells were collected and lysed in
RIPA buffer (Beyotime Institute of Biotechnology). Equal amounts of
protein extracts (20 µg) were subjected to 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a polyvinylidene difluoride (PVDF) membrane
(Millipore). The membrane was blocked with 5% skim milk for 1 h at
room temperature. Then, the membrane was incubated with the primary
antibody overnight at 4°C. The following primary antibodies were
used: FABP4 (cat. no. ab66682; Abcam), actin (cat. no. ab179467;
Abcam), and caspase-3 (cat. no. 9662; Cell Signaling Technology).
The primary antibodies were diluted at the ratio of 1:1,000 in
TBST. Following three washes in TBST for 15 min each, the membranes
were incubated with a goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (cat. no. 7074; Cell
Signaling Technology) for 1 h at room temperature. The secondary
antibody was diluted at the ratio of 1:10,000 in TBST. The results
were visualized using the Super Signal Chemiluminescent Substrate
(Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Protein bands were quantified by
densitometric analysis using Quantity One software v4.6.6 (Bio-Rad
Laboratories).
Determination of ROS, LDH, CAT,
GSH-Px, MDA, and SOD activity
For the assessment of reactive oxygen species (ROS),
DCF-DA (Thermo Scientific) was used as an ROS probe, as previously
described (16). After different
treatments, the cells were incubated with 5 µM DCF-DA for 30 min at
37°C. The stained cells were then analyzed by flow cytometry (FACS
Caliber, BD Biosciences). Lactate dehydrogenase (LDH) activity was
measured using an LDH ELISA kit (cat. no. MAK066, Sigma-Aldrich;
Merck KGaA) according to the manufacturer's instructions. The
activities of catalase (CAT), glutathione peroxidase (GSH-Px),
malondialdehyde (MDA) and superoxide dismutase (SOD) were
determined using commercially available colorimetric assay kits
(cat. nos. ab83464, ab239727, ab118970, ab211096, respectively;
Abcam) according to the manufacturer's protocols.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) and were analyzed using SPSS 18.0 (SPSS, Inc.). Statistical
comparisons between different groups were measured using Student's
t-test or a one-way analysis of variance (ANOVA) with post-hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant result.
Results
Hydrogen peroxide induces apoptosis
and decreases the expression of miR-455 in human endometrial
stromal cells
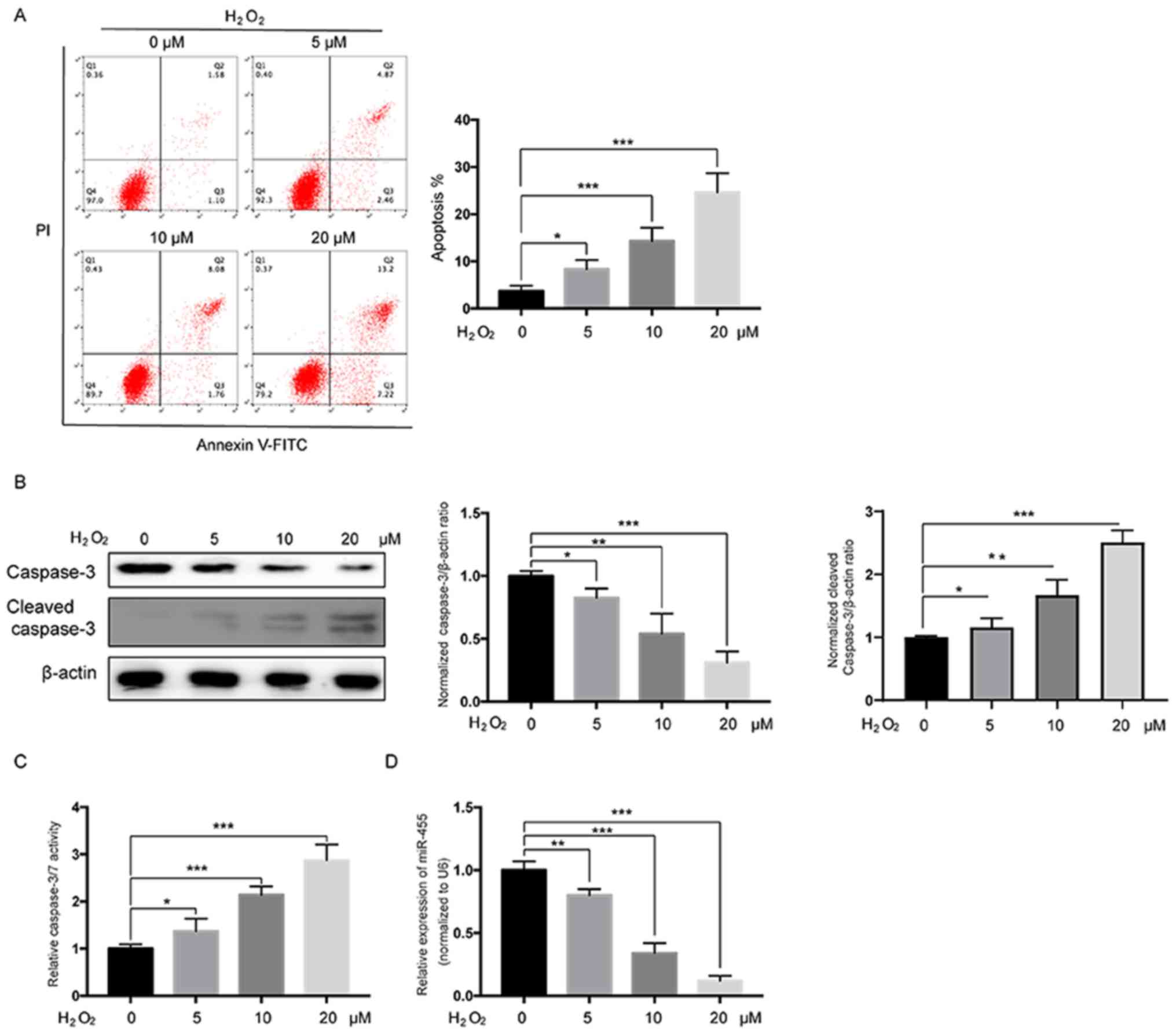
First, HESCs were treated with various doses of
H2O2 for 24 h after which the apoptosis rates
were measured. As shown in Fig.
1A, flow cytometric analysis revealed that treatment with
H2O2 significantly induced apoptosis of HESCs
in a dose-dependent manner. Western blot analysis also demonstrated
that H2O2 treatment led to a decrease in
pro-caspase-3 and an increase in cleaved caspase-3 in a
dose-dependent manner in HESCs (Fig.
1B). Moreover, a caspase-3/7 activity assay further confirmed
that treatment with H2O2 significantly
increased the activation of caspase-3/7 in a dose-dependent manner
in HESCs (Fig. 1C). Then, miR-455
levels were examined in H2O2-treated HESCs,
and the results showed that miR-455 levels were significantly
reduced in HESCs in a dose-dependent manner following
H2O2 treatment. Taken together, these data
suggest that miR-455 is likely negatively correlated with apoptosis
induced by H2O2 in HESCs.
Overexpression of miR-455 protects
HESCs from H2O2-induced apoptosis
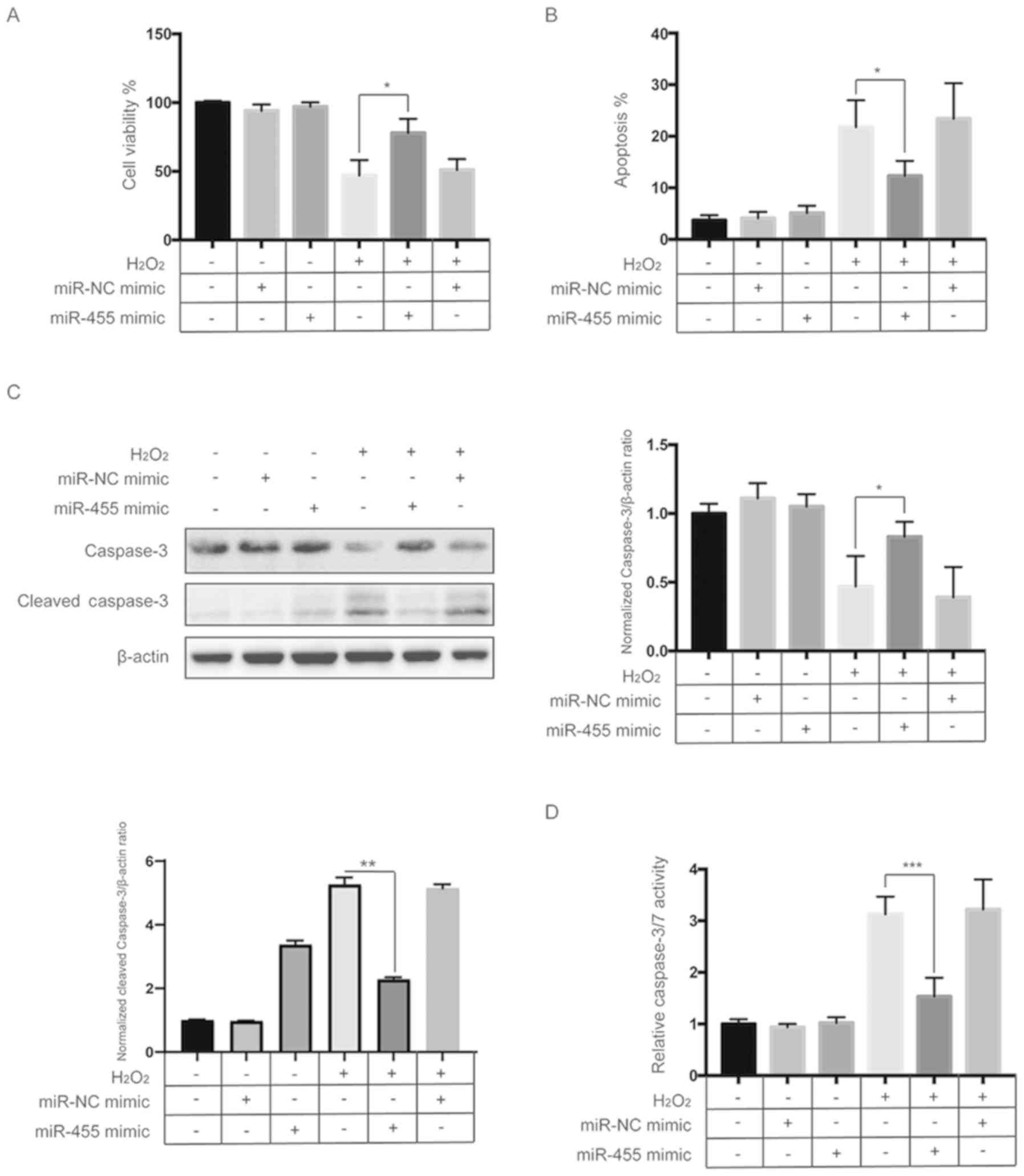
Next, we explored whether miR-455 plays a role in
H2O2-induced apoptosis of HESCs. A CCK-8
assay showed that the H2O2-induced reduction
in cell viability was significantly reversed by overexpression of
miR-455 mimics in HESCs (Fig. 2A).
Flow cytometric analysis also indicated that upregulation of
miR-455 significantly reduced the apoptosis induced by
H2O2 in HESCs (Fig. 2B and Fig. S2). Western blot analysis found
that cleavage of caspase-3 induced by H2O2
was diminished by overexpression of miR-455 in HESCs (Fig. 2C). Furthermore, the caspase-3/7
activity assay also revealed that activation of caspase-3/7 induced
by H2O2 was repressed by upregulation of
miR-455 (Fig. 2D). These results
indicate that miR-455 plays a protective role in
H2O2-induced apoptosis of HESCs.
miR-455 alleviates the oxidative
stress induced by H2O2 in HESCs
We then investigated the effects of miR-455 on
oxidative stress induced by H2O2 in HESCs.
First, we examined the effect of miR-455 on the intracellular ROS
levels induced by H2O2. As shown in Fig. 3A, exposure to
H2O2 significantly increased the ROS levels,
which were significantly reduced by overexpression of miR-455. MDA
is a degradation product of membrane lipid oxidation that can serve
as an indicator of oxidative damage (17). To further investigate the
antioxidant function of miR-455, the levels of MDA were measured
after treatment. As indicated in Fig.
3B, H2O2 treatment led to a significant
upregulation of intracellular MDA levels, while overexpression of
miR-455 significantly diminished this effect. Furthermore, the
activities of endogenous antioxidative enzymes such as SOD, CAT and
GSH-Px were also determined. As shown in Fig. 3C-E, H2O2
significantly decreased the SOD, CAT and GSH-Px activities, which
were partly reversed by upregulation of miR-455. Taken together,
these findings confirmed that miR-455 alleviates oxidative stress
in HESCs.
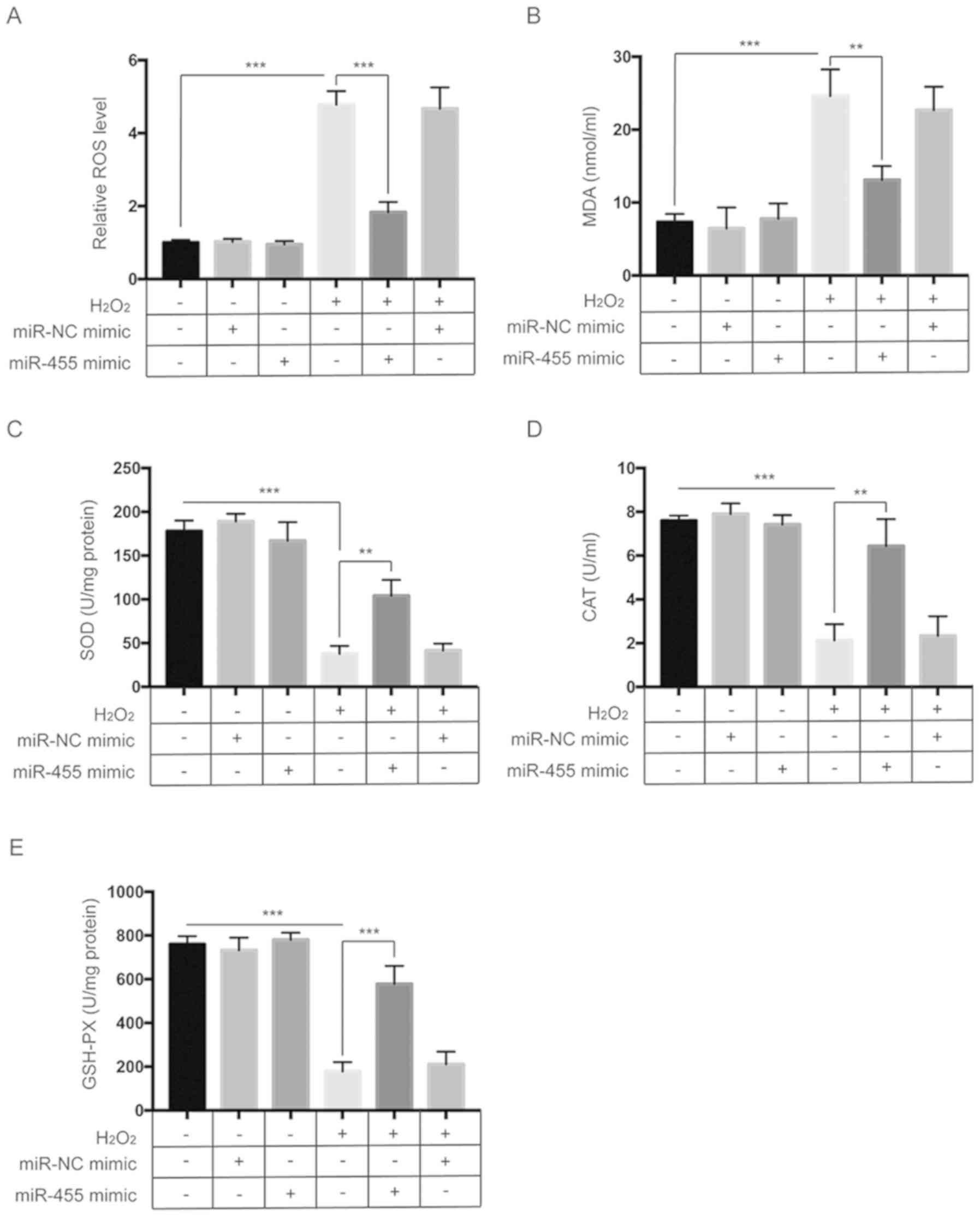 | Figure 3.miR-455 alleviates oxidative stress
in HESCs. (A) HESCs were treated as indicated, and then
intracellular ROS levels were measured by flow cytometry. (B) HESCs
were treated as indicated, after which the MDA activity was
assessed. (C) HESCs were treated as indicated, after which the SOD
activity was assessed. (D) HESCs were treated as indicated, after
which CAT activity was assessed. (E) HESCs were treated as
indicated, after which assessment of GSH-Px activity was
determined. All data are shown as the mean ± SD of three
independent experiments. **P<0.01 and ***P<0.001. HESCs,
human endometrial stromal cells; ROS, reactive oxygen species; MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px,
glutathione peroxidase. |
FABP4 is negatively regulated by
miR-455
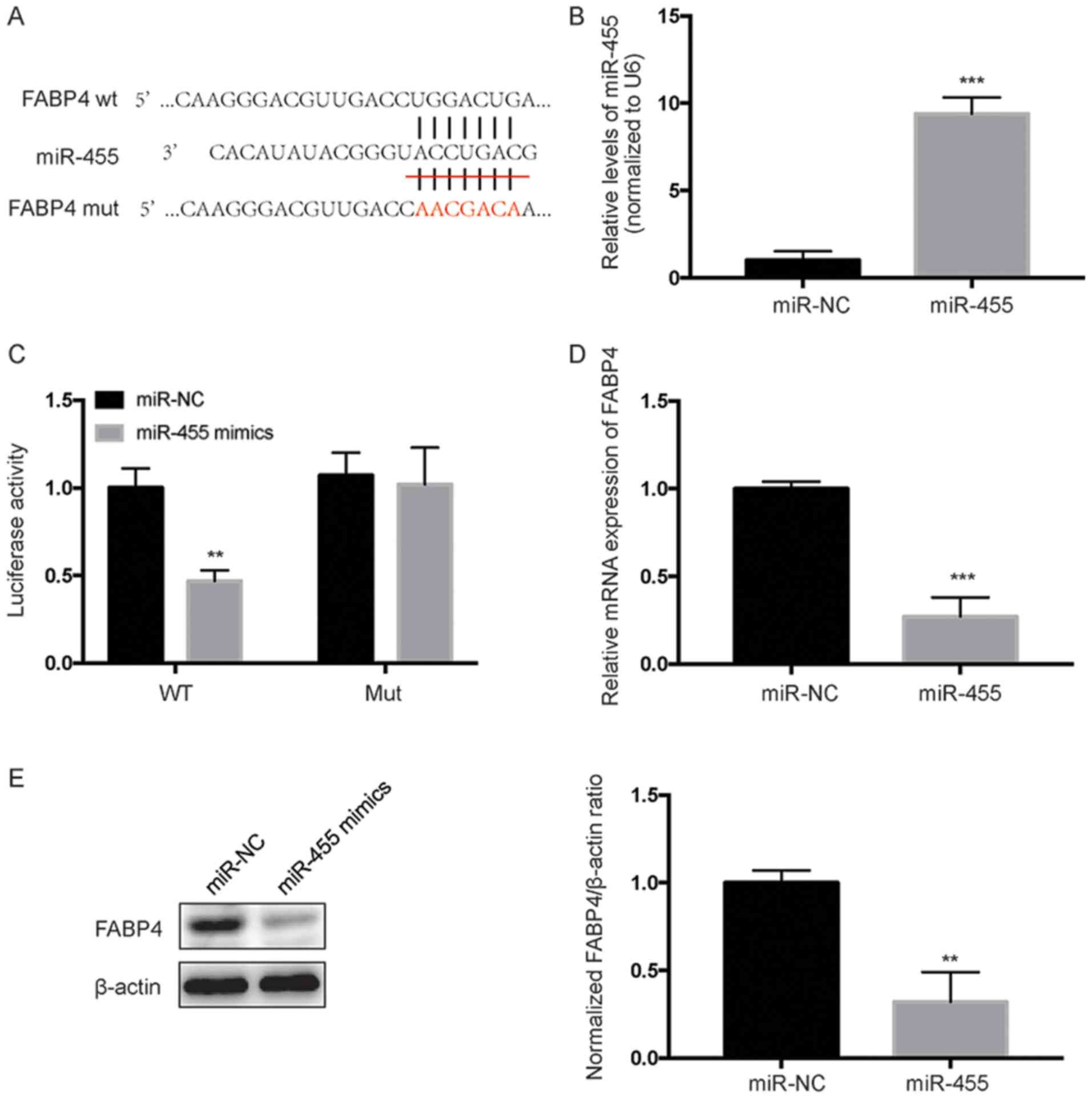
To identify potential targets of miR-455, the
bioinformatic tools TargetScan (www.targetscan.org) and StarBase V2.0 (starbase.sysu.edu.cn) were used. It was shown that
miR-455 putatively targets the 3′-UTR of FABP4 mRNA (Fig. 4A). Furthermore, we performed a
luciferase reporter assay to further confirm whether miR-455 can
directly target the 3′-UTR region of FABP4. Both the FABP4
wild-type (WT) 3′UTR containing the miR-455 binding site and a
mutated FABP4 3′-UTR sequence were cloned into luciferase reporter
vectors, which were co-transfected with miR-NC or a miR-455 mimic.
qPCR analysis revealed that transfection of cells with miR-455
mimics successfully increased the endogenous level of miR-455
compared with the negative control (Fig. 4B). As indicated in Fig. 2C, overexpression of miR-455
significantly (P<0.01) decreased the activity of luciferase
encoded by a gene containing FABP4 3′-UTR WT, while it did not
affect the activity of luciferase encoded by a gene containing
FABP4 3′UTR Mut. Further investigation revealed that miR-455
overexpression significantly decreased the mRNA and protein levels
of FABP4 in HESCs (Fig. 4D and E).
Collectively, these data indicate that FABP4 is a direct target of
miR-455 in HESCs.
Silencing of FABP4 is a phenocopy of
the effect of miR-455
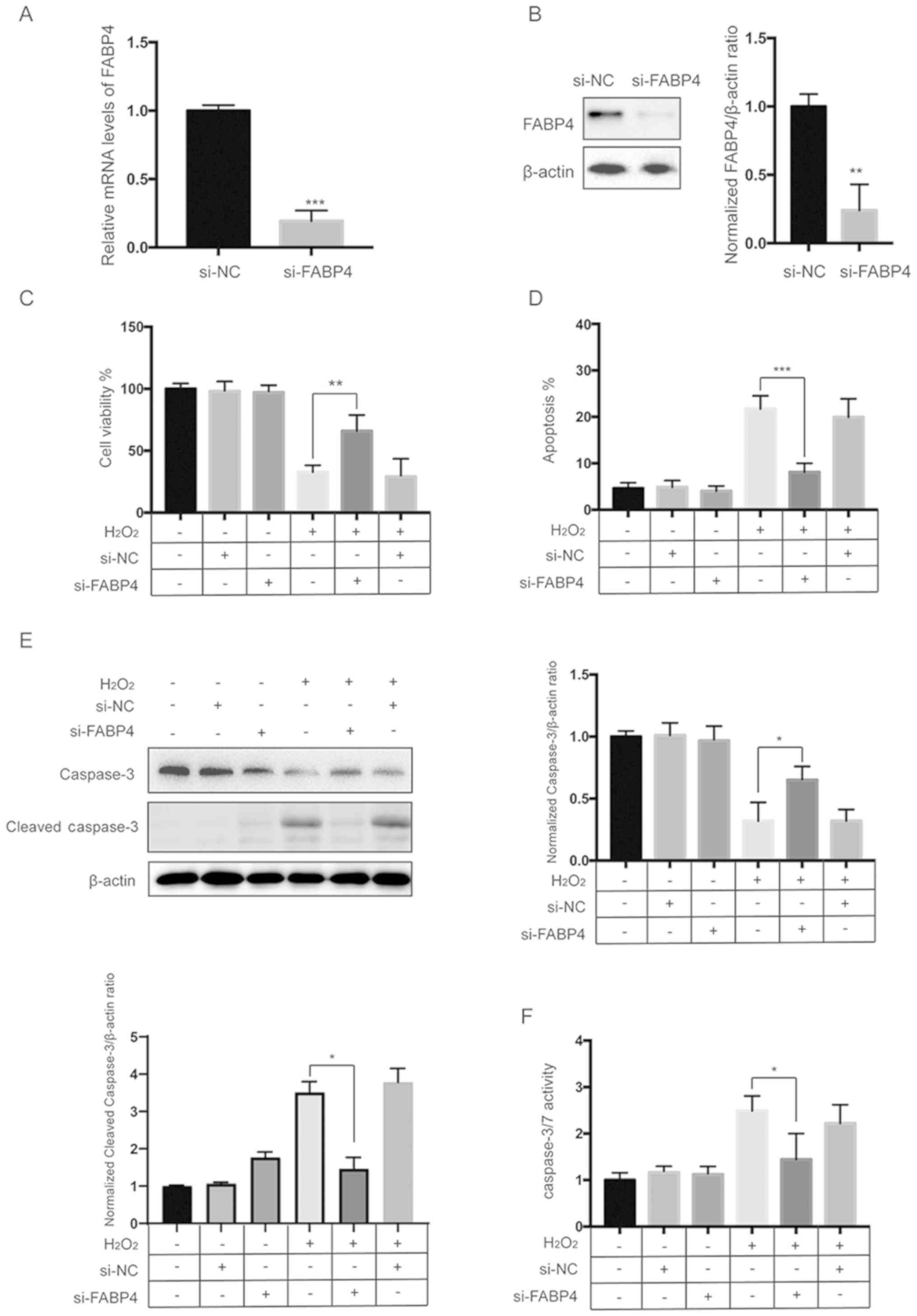
Next, to investigate the role of FABP4 in response
to oxidative stress in HESCs, we used siRNA to specifically knock
down the expression of FABP4. Twenty-four hours after transfection,
the expression of FABP4 was measured by qPCR and western blot
analysis. The results showed that transfection of cells with
si-FABP4 successfully repressed the expression of FABP4 (Fig. 5A and B). CCK-8 assays showed that
siFABP4 protects HESCs from the H2O2-induced
decrease in cell viability (Fig.
5C). Flow cytometric analysis of apoptosis showed that
H2O2-induced apoptosis was reversed by
siFABP4 (Fig. 5D and Fig. S3). Furthermore, western blot
analysis and caspase-3/7 activity assay revealed that the
H2O2-induced activation of caspase-3 was
diminished by siFABP4 (Fig. 5E and
F).
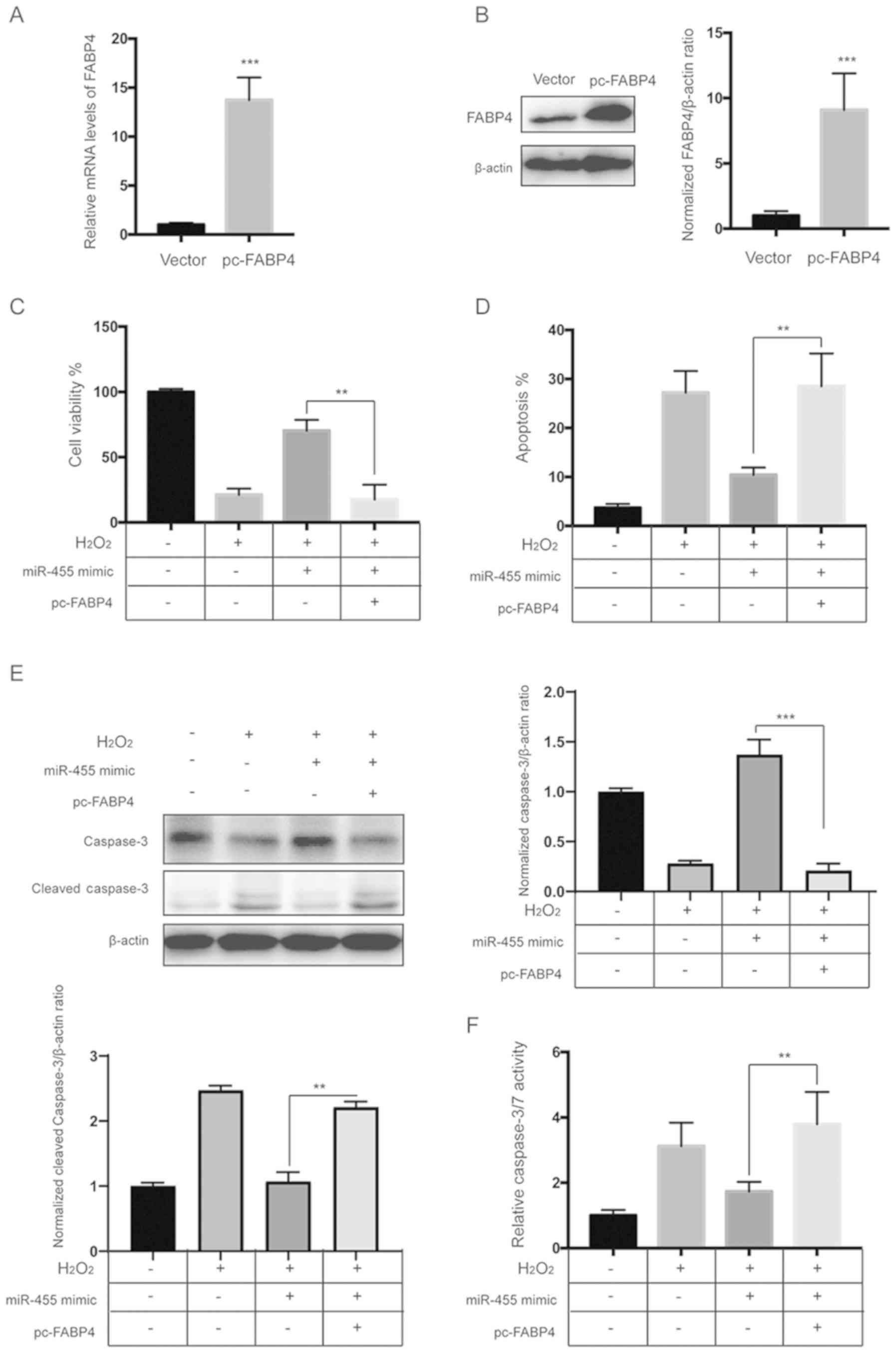
Upregulation of FABP4 diminishes the
protective effects of miR-455 against
H2O2
To further investigate the role of FABP4, HESCs were
transfected with miR-455 mimics and the FABP4 overexpression vector
alone or in combination. qPCR and western blot analysis revealed
that the FABP4 vector significantly upregulated the level of FABP4
(Fig. 6A and B). A CCK-8 assay
showed that overexpression of FABP4 significantly diminished the
protective effects of miR-455 against H2O2
(Fig. 6C). In addition, flow
cytometric analysis showed that upregulation of FABP4 abrogated the
protective role of miR-455 against apoptosis induced by
H2O2 (Fig.
6D and Fig S4). Moreover,
western blot analysis and the caspase-3/7 activity assay showed
that overexpression of FABP4 significantly abrogated the inhibitory
effect of miR-455 on the activation of caspase-3 induced by
H2O2 (Fig. 6E
and F).
Discussion
In the present study, we examined the effects of
miR-455 on oxidative stress in human endometrial stromal cells
(HESCs). It was found that miR-455 was decreased by
H2O2 in a dose-dependent manner. In addition,
ectopic expression of miR-455 alleviated cellular damage induced by
H2O2 in HESCs. It was also demonstrated that
miR-455 inhibited H2O2-induced intracellular
reactive oxygen species (ROS) production and the malondialdehyde
(MDA) level; this effect was accompanied by significantly increased
activities of catalase (CAT), superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px). Moreover, fatty acid binding
protein 4 (FABP4) was identified as a target of miR-455. Since
oxidative stress is implicated in the pathophysiology of
endometriosis as it causes a general inflammatory response in the
peritoneal cavity, our findings suggest that miR-455 may be applied
as part of a more optimized therapy for endometriosis.
H2O2 has been extensively
applied as an inducer of oxidative stress in various in
vitro models. H2O2 readily enters the
cytoplasm and thereafter produces a more toxic hydroxyl radical
that interacts with macromolecules such as lipids, proteins and
DNA, which leads to cellular damage (18). To evaluate
H2O2-related cellular damage, a CCK-8 assay
and flow cytometry, which are widely used to evaluate the effect of
pharmacological agents on cell proliferation and apoptosis, were
performed. The results of our study revealed that
H2O2 decreased the viability and increased
the apoptosis of HESCs in a dose-dependent manner. Another
significant finding was that H2O2 also
decreased the expression of miR-455 in a dose-dependent manner.
Although no previous study to date has reported the protective
effect of miR-455 against oxidative stress in HESCs, Xu et
al demonstrated that miR-455 targets cullin 3 to activate Nrf2
signaling and protects human osteoblasts from cellular damage
induced by H2O2 (11). Moreover, in a recent study, Zhang
et al showed that miR-455 protects osteoblasts from
oxidative stress through activation of Nrf2/ARE signaling (10). Our results further confirmed that
miR-455 overexpression largely relieved oxidative stress in HESCs
and revealed that H2O2 inhibited HESC growth,
at least in part, via downregulation of miR-455.
Many studies have shown that
H2O2 exerts oxidative stress via inducing
apoptosis in vitro (19, 20). Oxidative stress activates
caspases, which are a group of cysteine proteases that act as death
effector molecules, and after activation, they cleave various
substrates in the cytoplasm or nucleus (21,22).
Two pathways lead to apoptosis, namely, the extrinsic and intrinsic
pathways (22). The extrinsic
pathway is initiated by caspase-8, while the intrinsic pathway is
initiated by caspase-9 (22). The
activation of caspase-8 and/or caspase-9 leads to the activation of
caspase-3, which is the executioner caspase that can cleave
different substrates and finally induce apoptosis (21,22).
Our study, which is in agreement with a previous study, indicated
that a relatively low concentration of H2O2
induced apoptosis, which was accompanied by activation of caspase-3
in HESCs (23). In the present
study, ectopic expression of miR-455 obviously restrained the
H2O2-induced apoptosis in HESCs. Mounting
evidence has confirmed that H2O2-induced
accumulation of ROS, which can lead to cell damage and apoptosis,
is implicated in the pathophysiology of endometriosis (24). The results of our study indicated
that 20 µM of H2O2 markedly increased the
level of ROS, which was repressed by overexpression of miR-455.
Moreover, overexpression of miR-455 markedly alleviated oxidative
stress, as evidenced by the decreased MDA level and increased SOD,
CAT and GSH-Px activities. Therefore, we concluded that miR-455
alleviated oxidative stress and thereby provides protection against
H2O2 in HESCs.
FABP4, which has been identified as a direct
downstream target of miR-455, belongs to the FABP family. It has
been reported that FABP1, another member of the FABP family,
functions as an antioxidant protein since it neutralizes free
radicals through its methionine and cysteine amino acids (25). However, the role of FABP4 in the
regulation of oxidative stress is still controversial. Many studies
have shown that inhibition of FABP4 could suppress inflammation and
oxidative stress in various models (26,27).
In contrast, another study showed that the knockdown of FABP4
upregulates the intracellular ROS levels in adipocytes (14). The discrepancy may be due to
different cell models, and therefore, further investigation is
needed. In the present study, it was found that the antioxidant
effects of miR-455 could be mimicked by downregulation of FABP4 and
abrogated by overexpression of FABP4. Therefore, our study implies
that targeting of FABP4 may be an effective strategy against
oxidative stress in the treatment of endometriosis.
Taken together, our study provides new insight into
the mechanisms by which miR-455 alleviates oxidative stress in
HESCs. Additionally, we identified FABP4 as a direct target of
miR-455. Hence, these results provide strong evidence that miR-455
may be an effective target for the treatment of endometriosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Natural
Foundation of Ningbo Science and Technology Bureau, China (grant
no. 2018A610323).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WT and OC performed experiments; FY performed the
statistical analysis of the data; LC designed the study and drafted
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Kennedy S, Bergqvist A, Chapron C,
D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A and
Saridogan E; ESHRE Special Interest Group for Endometriosis and
Endometrium Guideline Development Group, : ESHRE guideline for the
diagnosis and treatment of endometriosis. Hum Reprod. 20:2698–2704.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sampson JA: Metastatic or embolic
endometriosis, due to the menstrual dissemination of endometrial
tissue into the venous circulation. Am J Pathol. 3:93–110.43.
1927.PubMed/NCBI
|
|
3
|
Sourial S, Tempest N and Hapangama DK:
Theories on the pathogenesis of endometriosis. Int J Reprod Med.
2014:1795152014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vinatier D, Orazi G, Cosson M and Dufour
P: Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol.
96:21–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christodoulakos G, Augoulea A,
Lambrinoudaki I, Sioulas V and Creatsas G: Pathogenesis of
endometriosis: The role of defective ‘immunosurveillance’. Eur J
Contracept Reprod Health Care. 12:194–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin SB, Peng DY, Lu JM and Ke ZP:
miR-182-5p inhibited oxidative stress and apoptosis triggered by
oxidized low-density lipoprotein via targeting toll-like receptor
4. J Cell Physiol. 233:6630–6637. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diao H, Liu B, Shi Y, Song C, Guo Z, Liu
N, Song X, Lu Y, Lin X and Li Z: MicroRNA-210 alleviates oxidative
stress-associated cardiomyocyte apoptosis by regulating BNIP3.
Biosci Biotechnol Biochem. 81:1712–1720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou M, Zuo X, Li C, Zhang Y and Teng Y:
Mir-29b regulates oxidative stress by targeting SIRT1 in ovarian
cancer cells. Cell Physiol Biochem. 43:1767–1776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Wu W, Jiao G, Li C and Liu H:
miR-455-3p activates Nrf2/ARE signaling via HDAC2 and protects
osteoblasts from oxidative stress. Int J Biol Macromol.
107:2094–2101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu D, Zhu H, Wang C, Zhu X, Liu G, Chen C
and Cui Z: microRNA-455 targets cullin 3 to activate Nrf2 signaling
and protect human osteoblasts from hydrogen peroxide. Oncotarget.
8:59225–59234. 2017.PubMed/NCBI
|
|
12
|
Furuhashi M and Hotamisligil GS: Fatty
acid-binding proteins: Role in metabolic diseases and potential as
drug targets. Nat Rev Drug Discov. 7:489–503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steen KA, Xu H and Bernlohr DA: FABP4/aP2
regulates macrophage redox signaling and inflammasome activation
via control of UCP2. Mol Cell Biol. 37(pii): e00282–16.
2017.PubMed/NCBI
|
|
14
|
Kajimoto K, Minami Y and Harashima H:
Cytoprotective role of the fatty acid binding protein 4 against
oxidative and endoplasmic reticulum stress in 3T3-L1 adipocytes.
FEBS Open Bio. 4:602–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Wang W, Jin K, Zhu Q, Lin H, Xie M
and Wang D: Perillyl alcohol protects human renal tubular
epithelial cells from hypoxia/reoxygenation injury via inhibition
of ROS, endoplasmic reticulum stress and activation of
PI3K/Akt/eNOS pathway. Biomed Pharmacother. 95:662–669. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsukahara H: Biomarkers for oxidative
stress: Clinical application in pediatric medicine. Curr Med Chem.
14:339–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halliwell B, Clement MV, Ramalingam J and
Long LH: Hydrogen peroxide. Ubiquitous in cell culture and in vivo?
IUBMB Life. 50:251–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh M, Sharma H and Singh N: Hydrogen
peroxide induces apoptosis in HeLa cells through mitochondrial
pathway. Mitochondrion. 7:367–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clement MV, Ponton A and Pervaiz S:
Apoptosis induced by hydrogen peroxide is mediated by decreased
superoxide anion concentration and reduction of intracellular
milieu. FEBS Lett. 440:13–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thornberry NA: Caspases: A decade of death
research. Cell Death Differ. 6:1023–1027. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zal F, Khademi F, Taheri R and
Mostafavi-Pour Z: Antioxidant ameliorating effects against
H2O2-induced cytotoxicity in primary
endometrial cells. Toxicol Mech Methods. 28:122–129. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scutiero G, Iannone P, Bernardi G,
Bonaccorsi G, Spadaro S, Volta CA, Greco P and Nappi L: Oxidative
stress and endometriosis: A systematic review of the literature.
Oxid Med Cell Longev. 2017:72652382017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan J, Gong Y, She YM, Wang G, Roberts MS
and Burczynski FJ: Molecular mechanism of recombinant liver fatty
acid binding protein's antioxidant activity. J Lipid Res.
50:2445–2454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong Y, Yu Z, Gao Y, Deng L, Wang M, Chen
Y, Li J and Cheng B: FABP4 inhibitors suppress inflammation and
oxidative stress in murine and cell models of acute lung injury.
Biochem Biophys Res Commun. 496:1115–1121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahman N, Jeon M and Kim YS: Methyl
gallate, a potent antioxidant inhibits mouse and human adipocyte
differentiation and oxidative stress in adipocytes through
impairment of mitotic clonal expansion. Biofactors. 42:716–726.
2016. View Article : Google Scholar : PubMed/NCBI
|