Introduction
Usher syndrome is a disease affecting both vision
and hearing, occurring in about 1 in 23,000 individuals worldwide
(1–3). Usher syndrome has three clinical
types. Type I (USH1) is manifested as congenital profound deafness
as well as vestibular dysfunction; USH2 presents as congenital
moderate hearing loss and normal vestibular function; and USH3 is
characterized by progressive hearing impairment and occasional
vestibular dysfunction. USH2 is the most common form accounting for
approximately 70% of all cases. Genetic defects in three genes
[USH2A (4), USH2C
(5), and USH2D (6)] are known to underlie this type of
Usher syndrome. There are three identified proteins (usherin, GPR98
and whirlin) that co-localize and form a complex (USH2 complex)
in vivo (7–10). Whirlin is the key protein in the
USH2 complex, which recruits other USH2 causative proteins at the
periciliary membrane in photoreceptors and the ankle link of the
stereocilia in hair cells. It has been reported that defects in any
of the three proteins may cause mislocalization of the other two
proteins and defects in the USH2 complex, which are the primary
cause for USH2 pathogenesis (8–13).
However, the biological function of the USH2 complex is largely
unknown.
Studies suggest that whirlin is a scaffold protein
and may be essential for the assembly of the USH2 complex (6,14).
Therefore, it is critical to identify proteins that interact with
whirlin and that are part of the USH2 complex (15–18).
Reports indicate that whirlin interacts with several proteins other
than usherin and GPR98, such as myosin XVa, Eps8 and SANS (19–23).
However, evidence to support that any of these proteins are a
component of the USH2 complex is still lacking. There are three PDZ
(postsynaptic density-95/discs large/zona occludens-1) domains and
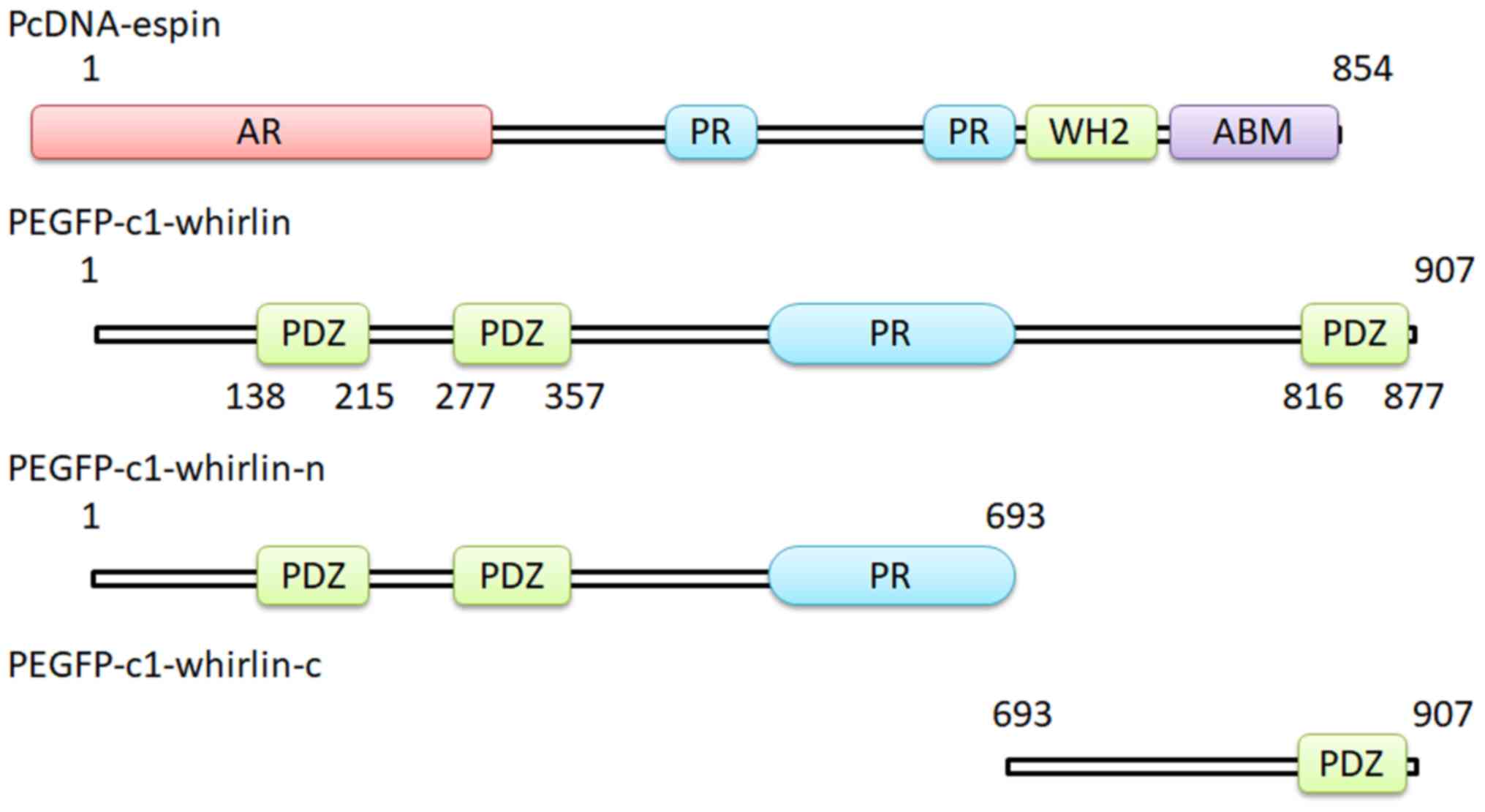
a proline-rich (PR) region in whirlin (Fig. 1). PDZ domains are distributed
throughout the protein from the N-terminal to C-terminal. The USH2
complex proteins are known to bind to each other in vitro
through PDZ domain-mediated interactions (7,8,10).
Espin is a component protein of the USH2 complex and
is a candidate gene for Usher syndrome (24–28).
Mutations in espin have been shown to cause deafness in humans
(24–26). Espin is expressed in vivo in
four isoforms resulting from alternative transcription start site
and gene splicing (29). Wang
et al previously demonstrated that espin is a protein that
interacts with whirlin and that espin expression in photoreceptors
is altered in whirlin-knockout mice (28). However, which domain of whirlin
interacts with espin remains unclear.
In the present study, it was determined that the
interaction between whirlin and espin locates at the N-terminal of
whirlin. It was shown that a whirlin fragment with the first two
PDZ domains and the PR region is sufficient for its interaction
with espin. Our findings suggest that the PDZ domain alone is not
sufficient for USH2 complex proteins to interact with each other
and the PR region might be required for protein stability.
Materials and methods
DNA plasmids
Whirlin N- and C-terminal fragments (3–693 amino
acids and 693–907 amino acids, NP_082916) in the pEGFP-C2 vectors
were constructed as described previously (30,31).
All DNA plasmids constructed in this study were confirmed by DNA
sequencing. Whirlin full-length cDNA (3–907 amino acids,
NP_082916), which was originally cloned from the mouse retina into
pEGFP-C2 vectors, was obtained from Dr Jun Yang (University of
Utah, Salt Lake City, UT, USA).
Antibodies
A polyclonal rabbit-espin antibody (K-14; sc-133324)
was purchased from Santa Cruz Biotechnology, Inc. A rabbit
polyclonal-whirlin antibody (25881-1-AP) was obtained from
Invitrogen/Thermo Fisher Scientific, Inc. and was biotin-labeled
prepared according to the manufacturer's instructions (Invitrogen™
FluoReporter™ Mini-Biotin-XX protein Labeling kit; Thermo Fisher
Scientific, Inc.). The rabbit and mouse antibodies against GFP
(ab6556 and ab1218) were from Abcam. Hoechst dye 33342 (4082) and
secondary antibodies (8889) were obtained from Cell Signaling
Technology.
Cell culture and cytochalasin D
treatment
Cell culture preparation and treatment were
performed as described previously by Wang et al with minor
modification as follows (28).
COS-7 and 293 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 5% fetal bovine serum (FBS;
Invitrogen/Thermo Fisher Scientific, Inc.). Transient transfection
was carried out using TurboFect in vitro Transfection
Reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, cells were cultured to ~70%
confluency. One microgram of plasmids was diluted in 100 µl of
serum-free DMEM. Then 2 µl of transfection reagent was added to the
diluted DNA and mixed by vortexing. One hundred milliliters of
transfection reagent/DNA mixture was added to each well (24-well
plate) drop-wise. Cells were collected after incubating at 37°C in
a CO2 incubator for 36 h.
Before being fixed for confocal microscopy analysis,
the cells were treated with cytochalasin D. Cytochalasin D
treatment was performed by incubating cells at room temperature for
2 h in 4 µM cytochalasin D/DMEM/5% FBS, which was freshly prepared
from 2 mM cytochalasin D/DMSO (dimethyl sulfoxide) stock solution.
Cells treated with DMSO (1:1,000) in DMEM/5% FBS under the same
condition were used as a negative control for cytochalasin D
treatment.
Immunoprecipitation and western
blotting
Transfected 293 cells were grown as described above
and collected from the culture medium. Cells were lysed by
homogenization in lysis buffer [50 mM Tris–HCl pH 8.0, 150 mM NaCl,
0.5% Triton X-100, 5 mM ethylene diamine triacetic acid (EDTA), 0.5
mM phenylmethanesulfonyl fluoride, 1X protease inhibitor and 1 mM
DTT] and sonication on ice briefly. The cells were centrifuged at
18,000 × g for 10 min, and the supernatant (Input) was precleared
by incubation with protein G sepharose (Amersham Biosciences) for 1
h. The precleared supernatant was then incubated with a primary
antibody for 3.5 h and centrifuged at 18,000 × g for 10 min. The
resulting supernatant was incubated with protein G sepharose for 1
h. After centrifugation at 2,000 × g for 10 min, the pellet was
washed with lysis buffer for three times and then boiled in Laemmli
sample buffer. All the procedures were performed at 4°C. A
non-immune rabbit immunoglobulin served as a negative control.
Cells without the immunoprecipitation were included as a second
negative control.
The various cell lysates or the above
immunoprecipitate was separated on a 10% SDS polyacrylamide gel and
transferred to a polyvinylidene fluoride (PVDF) membrane. The
resulting PVDF membrane was sequentially subjected to blocking with
Tris-buffered saline and Tween 20 (TBST, pH 7.5, 20 mM Tris, 150 mM
NaCl, 0.1% Tween 20) containing 1% BSA (A7906, Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature, and then incubated with the
anti-GFP antibody at a 1:1,000 dilution (ab6556, Abcam) overnight
at 4°C and the anti-rabbit IgG at a 1:5,000 dilution (#8889, Cell
Signaling Technology) for 2 h at 37°C. The protein bands were
detected using the chemiluminescent substrate with Alpha Innotech
Alpha View software on a FluorChem Q machine (Cell Biosciences,
Inc.). The intensities of the protein bands were determined by
Image J Software (National Institutes of Health, Bethesda, MD, USA)
and normalized using sample loading control bands.
Immunofluorescence staining and
co-localization analysis
Cultured cells were fixed in a mixture of methanol
and acetone (1:1) at −20°C for 10 min and rinsed with PBS for 5 min
three times. The fixed cells were then blocked in 5% goat serum/PBS
for 1 h, incubated with a rabbit polyclonal-whirlin antibody
(25881-1-AP, Invitrogen/Thermo Fisher Scientific) in 5% goat
serum/PBS at 1:100 dilution at 4°C overnight, washed three times
with PBS and then incubated with the anti-rabbit IgG (8889, Cell
Signaling Technology) at a 1:5,000 dilution and Hoechst 33342
(4082, Cell Signaling Technology) in 5% goat serum/PBS for 1 h. For
staining of espin in cultured cells, A polyclonal rabbit-espin
antibody with a 1:100 dilution (K-14; sc-133324, Santa Cruz
Biotechnology) was used. The stained cells were viewed and
photographed on a confocal laser scanning microscope (Model FV1000;
Olympus, Tokyo, Japan).
Ethics statement
This is a basic research study. There were no
animals or patients involved in this study. The cell lines used in
the in vitro experiments are commercial products, obtained
from American Type Culture Collection (ATCC). Moreover, other
materials used were normal reagents. There were no ethical issues
involved in the study.
Results
Construction of GFP-tagged whirlin
fragment expression plasmids
To identify parts of the whirlin protein required
for interaction with espin, two pEGFP-C2 vector derivative plasmids
expressing the N-terminal fragment and the C-terminal fragment of
the whirlin protein, respectively, were constructed (Fig. 1). The N-terminal fragment contains
the first two PDZ domains and the PR region. The C-terminal
fragment includes the third PDZ domain. Both plasmids stably
expressed whirlin fragments.
Whirlin N-terminal fragment
co-localizes with espin
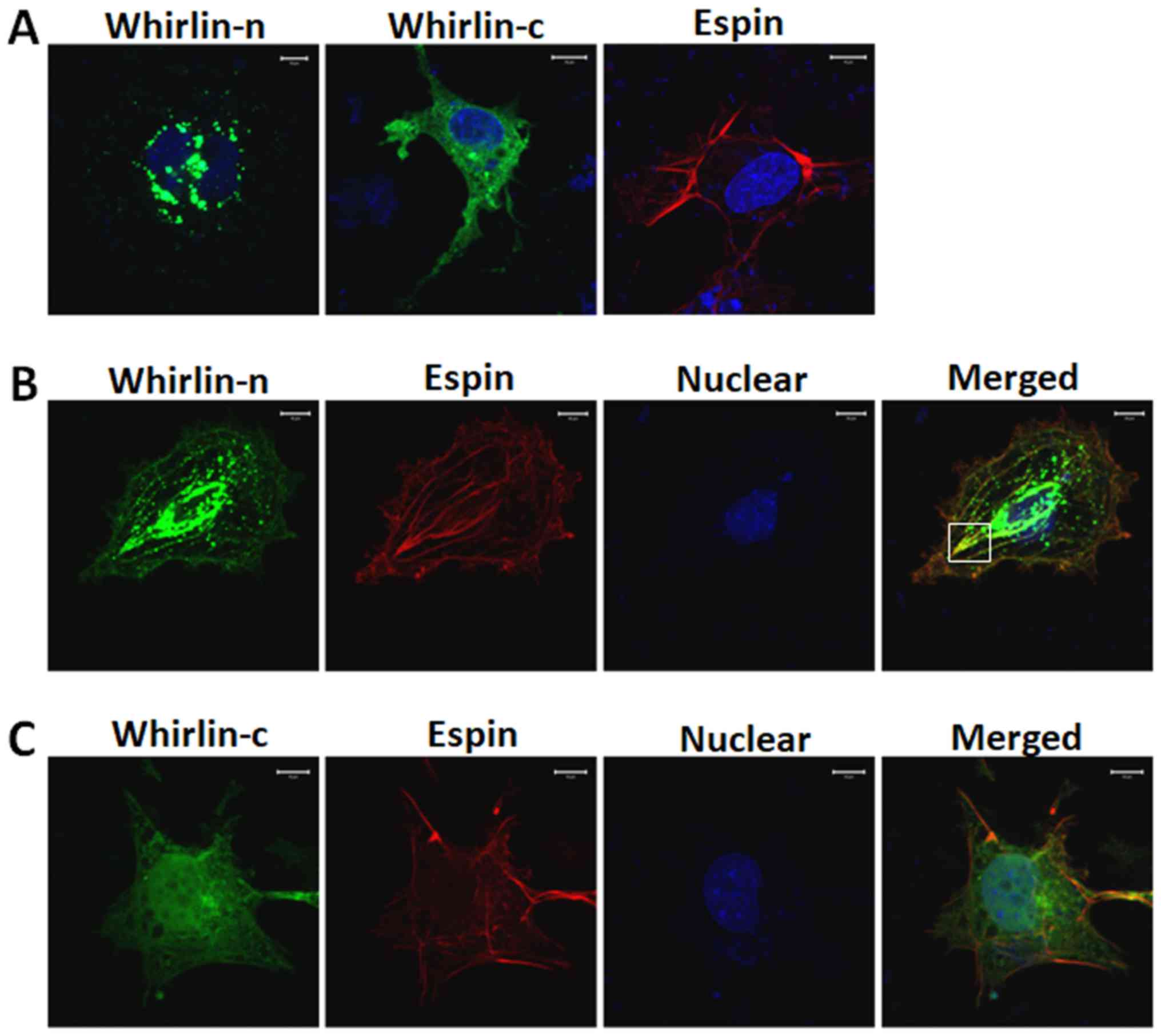
Yang et al and Wang et al reported
partial co-localization of whirlin and espin in the retina and the
inner ear (27,28). To further elucidate the interaction
between whirlin and espin, two whirlin fragments were tested for
their abilities to colocalize with full-length espin (Fig. 2). Plasmids expressing GFP-tagged
whirlin fragments were cotransfected with mCherry-tagged espin in
COS-7 cells. The reason why we used COS-7 cells to study the
interaction between whirlin and espin is that the size of COS-7
cells is larger than the majority of other cells, including
photoreceptors; thereby, it is more convenient and precise to
observe the colocalization of the proteins of interest by confocal
laser scanning microscopy. As known to date, whirlin is highly
expressed in photoreceptors, while there is little whirlin in the
COS-7 cell line. These are the reasons why we chose COS-7 cells as
a material to carry out immunofluorescent analysis in vitro
and used photoreceptors to perform other functional studies in
vivo. In the present study, the whirlin N-terminal fragment was
shown to partially co-localize with espin in COS-7 cells. In
comparison, the whirlin C-terminal fragment did not appear to
colocalize with espin. This suggests that PDZ domains in the
whirlin N-terminal fragment may be responsible for whirlin-espin
interaction.
Whirlin N-terminal fragment interacts
with espin
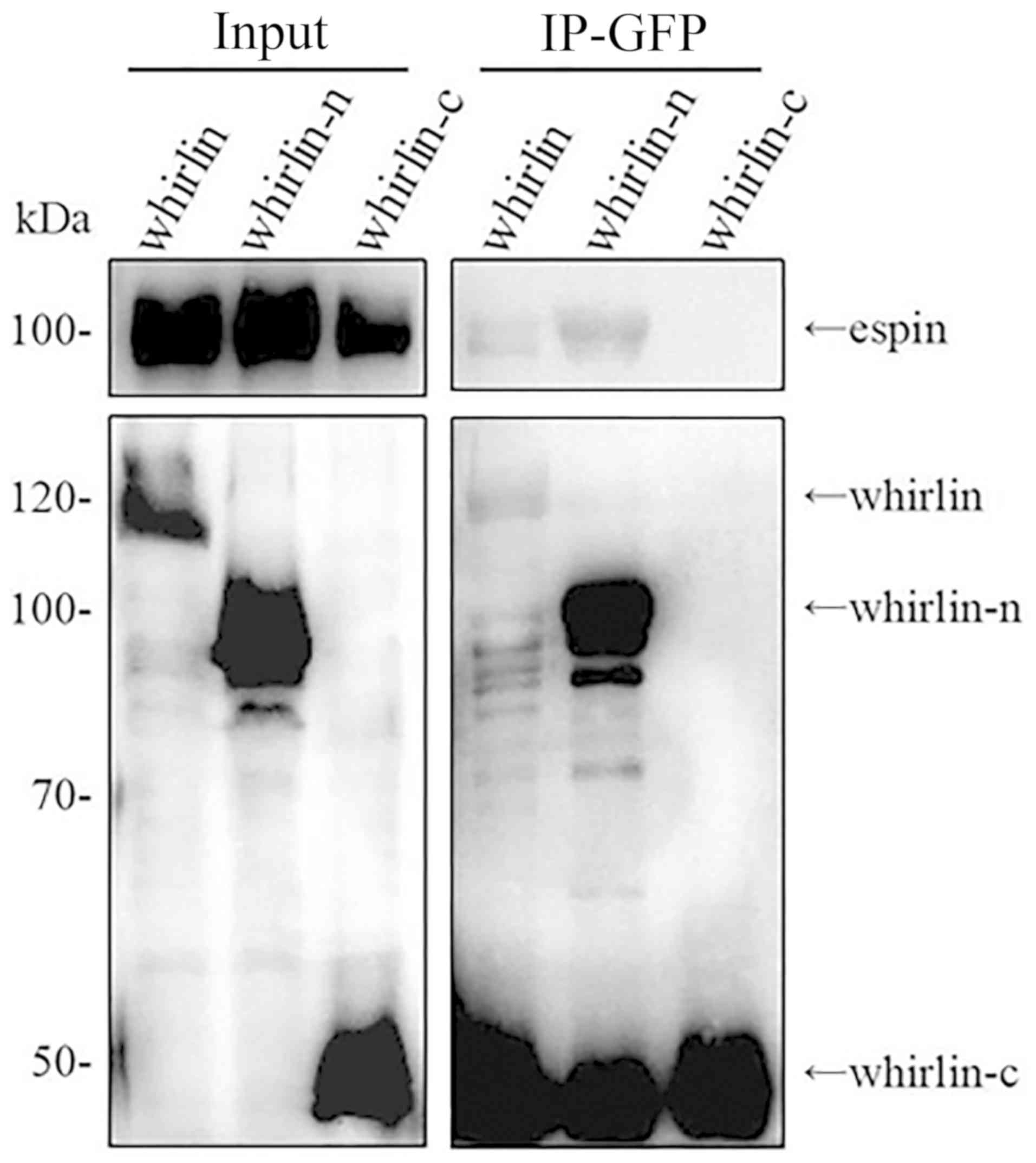
Co-localization does not necessarily mean
interaction between two proteins. In order to confirm that the
whirlin N-terminal fragment indeed interacts with espin, we
examined the two whirlin fragments for their abilities to interact
with full-length espin using a co-immunoprecipitation (CoIP) assay
as described in Materials and methods. In our previous study, we
tested two different whirlin fragments, where the N-terminal
fragment lacked the PR region and the C-terminal fragment contained
the PR region, and could not co-immunoprecipitate either whirlin
fragment with espin (data not shown). In addition to construct two
different fragments, we also modified the CoIP procedure slightly
by adding cytochalasin D treatment after co-transfection of whirlin
and espin expression plasmids. Cytochalasin D inhibits actin
polymerization and induces depolymerization of actin filaments.
Although Wang et al (28)
found that whirlin exhibited effects on actin bundle network in
photoreceptors and hair cells, cytochalasin D treatment here
promoted whirlin N-terminal fragment CoIP with espin, but not the
whirlin C-terminal fragment (Fig.
3). It appears that with the actin network disrupted, the
whirlin N-terminus is able to interact with espin. The results also
showed two strong bands with an approximate molecular weight 50
kilodalton (KDa) in both lanes of IP-GFP whirlin and
IP-GFP-whirlin-n. In fact, those strong bands represent the heavy
chain of the antibody. The molecular weight of whirlin-c is a
little higher than that of the heavy chain. Therefore, whirlin-c
only exists in the sample of IP-GFP-whirlin-c.
Discussion
Whirlin mutations cause retinal degeneration and
hearing loss in Usher syndrome type II (USH2) and nonsyndromic
deafness (1–3,6,27).
It has been shown that whirlin is critical for recruiting other
USH2 causative proteins to form a complex at the periciliary
membrane complex in photoreceptors and the ankle link of the
stereocilia in hair cells (6,8–12,28).
However, the biological function and mechanism of the USH2 protein
complex are largely unknown. Espin is a component of the USH2
protein complex and is a candidate gene for Usher syndrome. Espin
is an actin-binding/bundling protein and may cause human deafness
when it is defective (24–26). Wang et al identified that
espin interacts with whirlin and crosslinks actin filaments
(28). It induces the formation of
actin bundles, which are presumed to be stabilized and elongated
through prevention from disassembly of these bundles and from
depolymerization of actin filaments. The interaction of whirlin
with a pool of espin does not bind to actin monomers or filaments,
but activates the exchange rate of espin between the pools of
actin-free and actin-bound, with the destabilization and shortening
of the espin cross-linked actin bundles. In the present study, it
was demonstrated that only the whirlin N-terminal fragment was able
to interact with espin and that the PR regions in both whirlin and
espin proteins are important for their interaction. This finding
lays a foundation for further research on the dynamic equilibrium
between espin and actin.
The PR regions of whirlin are involved in its
interaction with espin. To learn more about the interaction between
whirlin and espin, two whirlin fragments were tested for their
abilities to interact with full-length espin (Figs. 2 and 3). Previously, we tested two whirlin
fragments and did not observe any interaction with espin. We
constructed a new set of whirlin fragments to identify their
interaction with espin. One distinct difference in the construction
is that we moved the PR region into the N-terminal fragment and
left the C-terminal fragment with only the third PDZ domain. The
interaction between the whirlin N-terminal and espin suggests that
the PR region is required for the PDZ domains to interact with
espin. PR regions of proteins occur widely in both prokaryotes and
eukaryotes. Their functions have been found to be important for
protein conformation and sometimes involved in direct binding
(32). It is not clear which
function the PR regions in whirlin and espin play in whirlin-espin
interaction. However, studies have shown that the USH2 complex
proteins can interact with each other through PDZ domains, at least
in vitro (7,8,10).
The requirement of the PR region demonstrated here suggests that
the PR region may be important to enable the truncated whirlin
protein to form a conformation that is accessible to espin.
In conclusion, whirlin-espin interaction is
important for the architecture of the USH2 complex and actin
bundles cross-linked by espin. Our demonstration of whirlin
N-terminal fragment interaction with espin, is significantly novel,
providing insight into how these two proteins interact to form the
USH2 complex. Our findings suggest that although whirlin and espin
probably interact through PDZ domains, the PR regions are important
for the folding of these proteins into functional conformations. It
is crucial to understand the USH2 complex and USH2 pathogenesis.
However, the present study did not investigate the interaction
between whirlin and espin without the PR domain which warrants
further research by our group.
Acknowledgements
The authors would like to thank Dr Jun Yang (Utah
University, Salt Lake City, UT, USA) for providing the cDNA
plasmids.
Funding
This study was supported by the National Natural
Science Foundation of China (81400404 and 31670795); The Project of
the Foundation for Changbai Mountain Scholars; Jilin Province
Science and Technology Youth Fund (20140520034JH); Young Scholars
Program of Norman Bethune Health Science Center of Jilin University
(2013205030).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
JH, XF and SX designed the study and analyzed data.
LW, BW, YW and YS performed the research. JM and XG provided
assistance in designing the study and analyzing data. LW, JH, XF
and SX wrote and revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This is a basic research study. There were no
animals or patients involved in this study. The cell lines used in
the in vitro experiments are commercial products, obtained
from American Type Culture Collection (ATCC). There were no ethical
issues involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
USH2
|
Usher syndrome type II
|
|
PDZ
|
postsynaptic density-95/discs
large/zona occludens-1
|
|
PR
|
proline-rich
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
CoIP
|
co-immunoprecipitation
|
|
EDTA
|
ethylene diamine triacetic acid
|
|
PVDF
|
polyvinylidene fluoride
|
References
|
1
|
Boughman JA, Vernon M and Shaver KA: Usher
syndrome: Definition and estimate of prevalence from two high-risk
populations. J Chronic Dis. 36:595–603. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartong DT, Berson EL and Dryja TP:
Retinitis pigmentosa. Lancet. 368:1795–1809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keats BJ and Corey DP: The usher
syndromes. Am J Med Genet. 89:158–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eudy JD, Weston MD, Yao S, Hoover DM, Rehm
HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, et al:
Mutation of a gene encoding a protein with extracellular matrix
motifs in Usher syndrome type IIa. Science. 280:1753–1757. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weston MD, Luijendijk MW, Humphrey KD,
Möller C and Kimberling WJ: Mutations in the VLGR1 gene implicate
G-protein signaling in the pathogenesis of Usher syndrome type II.
Am J Hum Genet. 74:357–366. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebermann I, Scholl HP, Charbel Issa P,
Becirovic E, Lamprecht J, Jurklies B, Millán JM, Aller E, Mitter D
and Bolz H: A novel gene for Usher syndrome type 2: Mutations in
the long isoform of whirlin are associated with retinitis
pigmentosa and sensorineural hearing loss. Hum Genet. 121:203–211.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adato A, Lefèvre G, Delprat B, Michel V,
Michalski N, Chardenoux S, Weil D, El-Amraoui A and Petit C:
Usherin, the defective protein in Usher syndrome type IIA, is
likely to be a component of interstereocilia ankle links in the
inner ear sensory cells. Hum Mol Genet. 14:3921–3932. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Liu X, Zhao Y, Adamian M, Pawlyk
B, Sun X, McMillan DR, Liberman MC and Li T: Ablation of whirlin
long isoform disrupts the USH2 protein complex and causes vision
and hearing loss. PLoS Genet. 6:e10009552010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou J, Chen Q, Almishaal A, Mathur PD,
Zheng T, Tian C, Zheng QY and Yang J: The roles of USH1 proteins
and PDZ domain-containing USH proteins in USH2 complex integrity in
cochlear hair cells. Hum Mol Genet. 26:624–636. 2017.PubMed/NCBI
|
|
10
|
van Wijk E, van der Zwaag B, Peters T,
Zimmermann U, Te Brinke H, Kersten FF, Märker T, Aller E, Hoefsloot
LH, Cremers CW, et al: The DFNB31 gene product whirlin connects to
the Usher protein network in the cochlea and retina by direct
association with USH2A and VLGR1. Hum Mol Genet. 15:751–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McGee J, Goodyear RJ, McMillan DR,
Stauffer EA, Holt JR, Locke KG, Birch DG, Legan PK, White PC, Walsh
EJ and Richardson GP: The very large G-protein-coupled receptor
VLGR1: A component of the ankle link complex required for the
normal development of auditory hair bundles. J Neurosci.
26:6543–6553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michalski N, Michel V, Bahloul A, Lefèvre
G, Barral J, Yagi H, Chardenoux S, Weil D, Martin P, Hardelin JP,
et al: Molecular characterization of the ankle-link complex in
cochlear hair cells and its role in the hair bundle functioning. J
Neurosci. 27:6478–6488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mathur P and Yang J: Usher syndrome:
Hearing loss, retinal degeneration and associated abnormalities.
Biochim Biophys Acta. 1852:406–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciardo MG, Andrés-Bordería A, Cuesta N,
Valente P, Camprubí-Robles M, Yang J, Planells-Cases R and
Ferrer-Montiel A: Whirlin increases TRPV1 channel expression and
cellular stability. Biochim Biophys Acta. 1863:115–127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou J, Luo L, Shen Z, Chiodo VA, Ambati
BK, Hauswirth WW and Yang J: Whirlin replacement restores the
formation of the USH2 protein complex in whirlin knockout
photoreceptors. Invest Ophthalmol Vis Sci. 52:2343–2351. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Green JA, Yang J, Grati M, Kachar B and
Bhat MA: Whirlin, a cytoskeletal scaffolding protein, stabilizes
the paranodal region and axonal cytoskeleton in myelinated axons.
BMC Neurosci. 14:962013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Q, Zou J, Shen Z, Zhang W and Yang J:
Whirlin and PDZ domain-containing 7 (PDZD7) proteins are both
required to form the quaternary protein complex associated with
Usher syndrome type 2. J Biol Chem. 289:36070–36088. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mathur PD, Zou J, Zheng T, Almishaal A,
Wang Y, Chen Q, Wang L, Vashist D, Brown S, Park A and Yang J:
Distinct expression and function of whirlin isoforms in the inner
ear and retina: An insight into pathogenesis of USH2D and DFNB31.
Hum Mol Genet. 24:6213–6228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Belyantseva IA, Boger ET, Naz S, Frolenkov
GI, Sellers JR, Ahmed ZM, Griffith AJ and Friedman TB: Myosin-XVa
is required for tip localization of whirlin and differential
elongation of hair-cell stereocilia. Nat Cell Biol. 7:148–156.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delprat B, Michel V, Goodyear R, Yamasaki
Y, Michalski N, El-Amraoui A, Perfettini I, Legrain P, Richardson
G, Hardelin JP and Petit C: Myosin XVa and whirlin, two deafness
gene products required for hair bundle growth, are located at the
stereocilia tips and interact directly. Hum Mol Genet. 14:401–410.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kikkawa Y, Mburu P, Morse S, Kominami R,
Townsend S and Brown SD: Mutant analysis reveals whirlin as a
dynamic organizer in the growing hair cell stereocilium. Hum Mol
Genet. 14:391–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manor U, Disanza A, Grati M, Andrade L,
Lin H, Di Fiore PP, Scita G and Kachar B: Regulation of stereocilia
length by myosin XVa and whirlin depends on the actin-regulatory
protein Eps8. Curr Biol. 21:167–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mburu P, Kikkawa Y, Townsend S, Romero R,
Yonekawa H and Brown SD: Whirlin complexes with p55 at the
stereocilia tip during hair cell development. Proc Natl Acad Sci
USA. 103:10973–10978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng L, Sekerková G, Vranich K, Tilney
LG, Mugnaini E and Bartles JR: The deaf jerker mouse has a mutation
in the gene encoding the espin actin-bundling proteins of hair cell
stereocilia and lacks espins. Cell. 102:377–385. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Naz S, Griffith AJ, Riazuddin S, Hampton
LL, Battey JF Jr, Khan SN, Riazuddin S, Wilcox ER and Friedman TB:
Mutations of ESPN cause autosomal recessive deafness and vestibular
dysfunction. J Med Genet. 41:591–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donaudy F, Zheng L, Ficarella R, Ballana
E, Carella M, Melchionda S, Estivill X, Bartles JR and Gasparini P:
Espin gene (ESPN) mutations associated with autosomal dominant
hearing loss cause defects in microvillar elongation or
organisation. J Med Genet. 43:157–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Wang L, Song H and Sokolov M:
Current understanding of usher syndrome type II. Front Biosci.
17:1165–1183. 2012. View
Article : Google Scholar :
|
|
28
|
Wang L, Zou J, Shen Z, Song E and Yang J:
Whirlin interacts with espin and modulates its actin-regulatory
function: An insight into the mechanism of Usher syndrome type II.
Hum Mol Genet. 21:692–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sekerková G, Zheng L, Loomis PA, Mugnaini
E and Bartles JR: Espins and the actin cytoskeleton of hair cell
stereocilia and sensory cell microvilli. Cell Mol Life Sci.
63:2329–2341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Liu X, Yue G, Adamian M, Bulgakov
O and Li T: Rootletin, a novel coiled-coil protein, is a structural
component of the ciliary rootlet. J Cell Biol. 159:431–440. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen B, Li A, Wang D, Wang M, Zheng L and
Bartles JR: Espin contains an additional actin-binding site in its
N terminus and is a major actin-bundling protein of the Sertoli
cell-spermatid ectoplasmic specialization junctional plaque. Mol
Biol Cell. 10:4327–4339. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Williamson MP: The structure and function
of proline-rich regions in proteins. Biochem J. 297:249–260. 1994.
View Article : Google Scholar : PubMed/NCBI
|