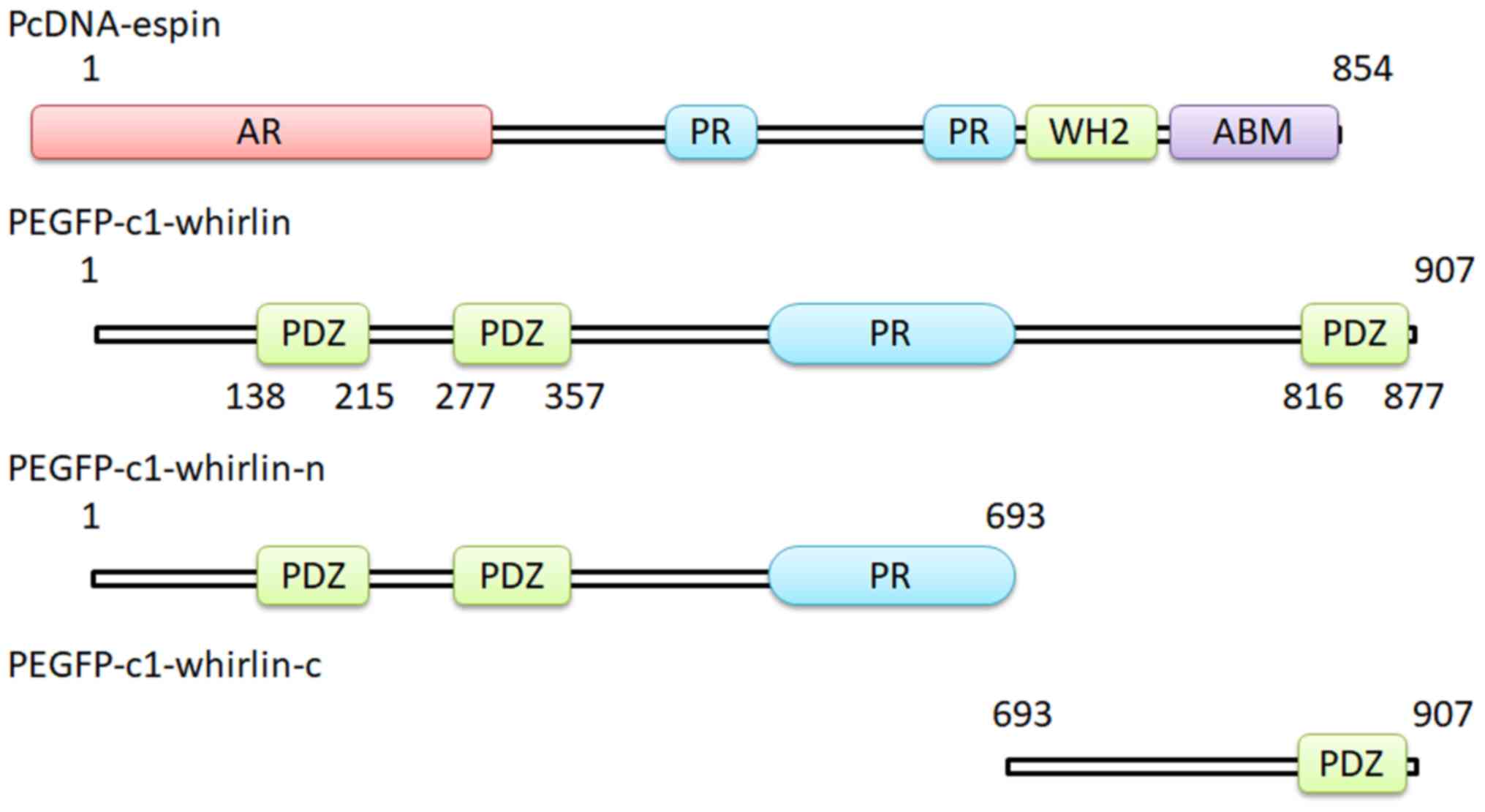
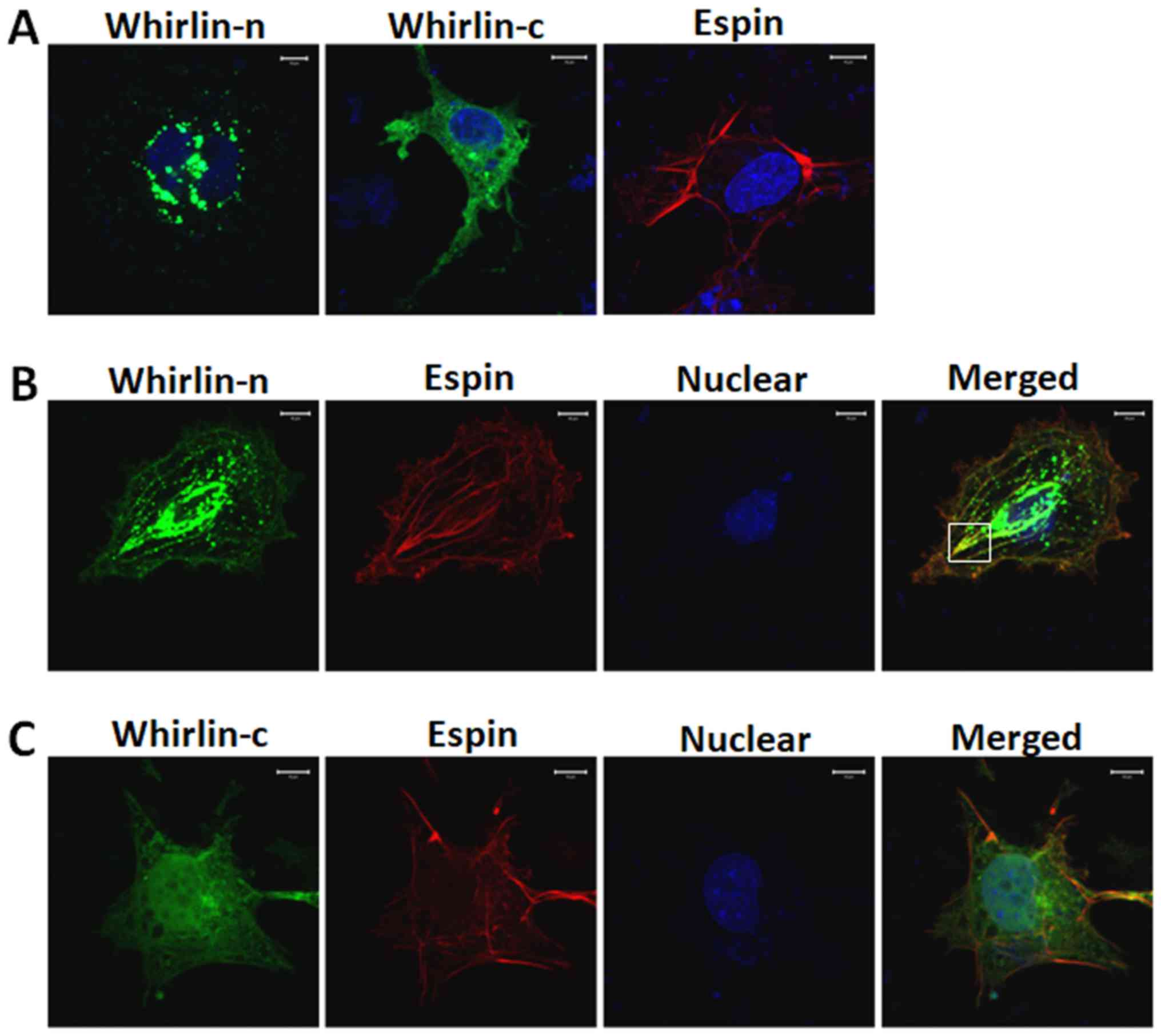
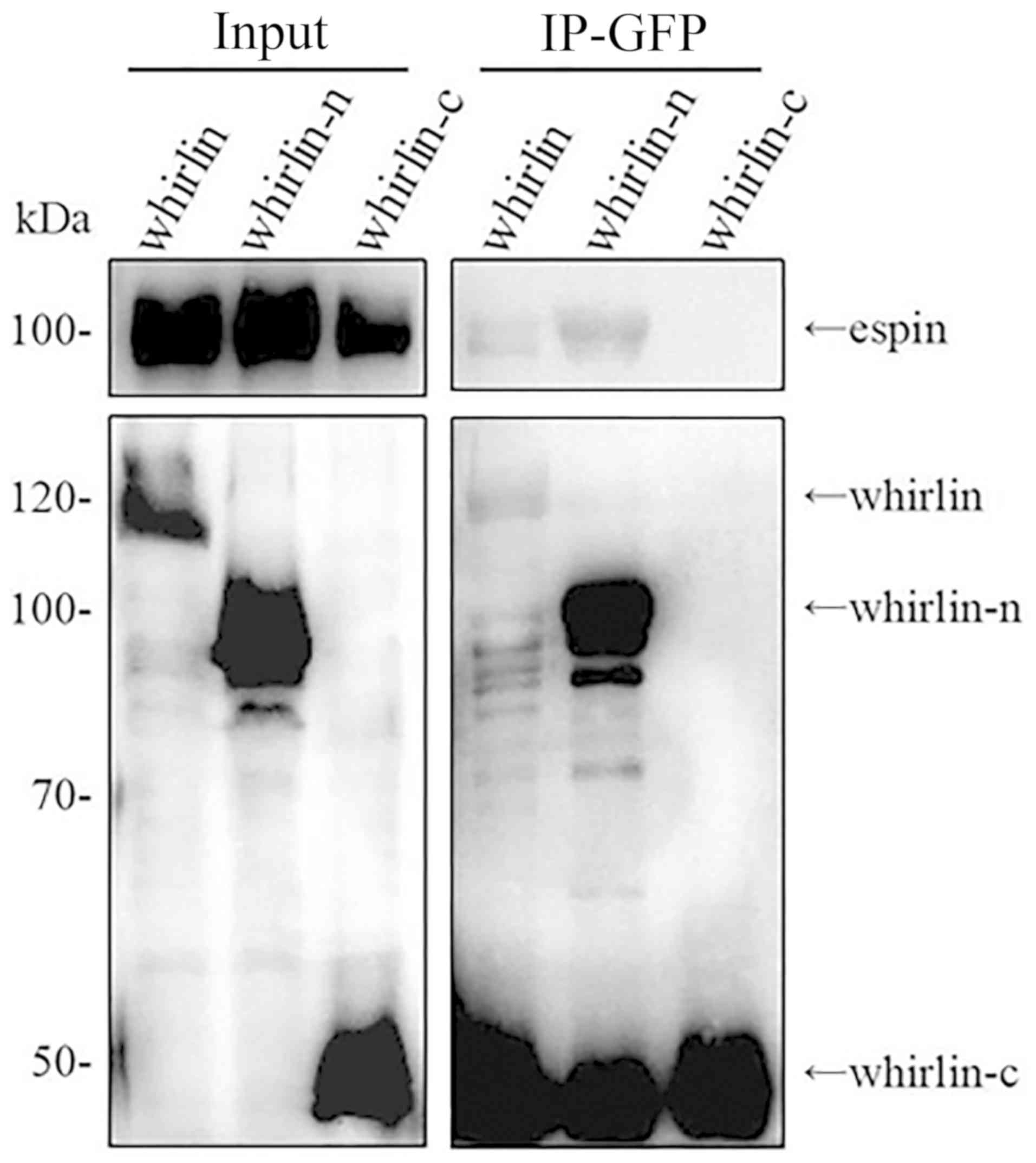
|
1
|
Boughman JA, Vernon M and Shaver KA: Usher
syndrome: Definition and estimate of prevalence from two high-risk
populations. J Chronic Dis. 36:595–603. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartong DT, Berson EL and Dryja TP:
Retinitis pigmentosa. Lancet. 368:1795–1809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keats BJ and Corey DP: The usher
syndromes. Am J Med Genet. 89:158–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eudy JD, Weston MD, Yao S, Hoover DM, Rehm
HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, et al:
Mutation of a gene encoding a protein with extracellular matrix
motifs in Usher syndrome type IIa. Science. 280:1753–1757. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weston MD, Luijendijk MW, Humphrey KD,
Möller C and Kimberling WJ: Mutations in the VLGR1 gene implicate
G-protein signaling in the pathogenesis of Usher syndrome type II.
Am J Hum Genet. 74:357–366. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebermann I, Scholl HP, Charbel Issa P,
Becirovic E, Lamprecht J, Jurklies B, Millán JM, Aller E, Mitter D
and Bolz H: A novel gene for Usher syndrome type 2: Mutations in
the long isoform of whirlin are associated with retinitis
pigmentosa and sensorineural hearing loss. Hum Genet. 121:203–211.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adato A, Lefèvre G, Delprat B, Michel V,
Michalski N, Chardenoux S, Weil D, El-Amraoui A and Petit C:
Usherin, the defective protein in Usher syndrome type IIA, is
likely to be a component of interstereocilia ankle links in the
inner ear sensory cells. Hum Mol Genet. 14:3921–3932. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Liu X, Zhao Y, Adamian M, Pawlyk
B, Sun X, McMillan DR, Liberman MC and Li T: Ablation of whirlin
long isoform disrupts the USH2 protein complex and causes vision
and hearing loss. PLoS Genet. 6:e10009552010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou J, Chen Q, Almishaal A, Mathur PD,
Zheng T, Tian C, Zheng QY and Yang J: The roles of USH1 proteins
and PDZ domain-containing USH proteins in USH2 complex integrity in
cochlear hair cells. Hum Mol Genet. 26:624–636. 2017.PubMed/NCBI
|
|
10
|
van Wijk E, van der Zwaag B, Peters T,
Zimmermann U, Te Brinke H, Kersten FF, Märker T, Aller E, Hoefsloot
LH, Cremers CW, et al: The DFNB31 gene product whirlin connects to
the Usher protein network in the cochlea and retina by direct
association with USH2A and VLGR1. Hum Mol Genet. 15:751–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McGee J, Goodyear RJ, McMillan DR,
Stauffer EA, Holt JR, Locke KG, Birch DG, Legan PK, White PC, Walsh
EJ and Richardson GP: The very large G-protein-coupled receptor
VLGR1: A component of the ankle link complex required for the
normal development of auditory hair bundles. J Neurosci.
26:6543–6553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michalski N, Michel V, Bahloul A, Lefèvre
G, Barral J, Yagi H, Chardenoux S, Weil D, Martin P, Hardelin JP,
et al: Molecular characterization of the ankle-link complex in
cochlear hair cells and its role in the hair bundle functioning. J
Neurosci. 27:6478–6488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mathur P and Yang J: Usher syndrome:
Hearing loss, retinal degeneration and associated abnormalities.
Biochim Biophys Acta. 1852:406–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciardo MG, Andrés-Bordería A, Cuesta N,
Valente P, Camprubí-Robles M, Yang J, Planells-Cases R and
Ferrer-Montiel A: Whirlin increases TRPV1 channel expression and
cellular stability. Biochim Biophys Acta. 1863:115–127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou J, Luo L, Shen Z, Chiodo VA, Ambati
BK, Hauswirth WW and Yang J: Whirlin replacement restores the
formation of the USH2 protein complex in whirlin knockout
photoreceptors. Invest Ophthalmol Vis Sci. 52:2343–2351. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Green JA, Yang J, Grati M, Kachar B and
Bhat MA: Whirlin, a cytoskeletal scaffolding protein, stabilizes
the paranodal region and axonal cytoskeleton in myelinated axons.
BMC Neurosci. 14:962013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Q, Zou J, Shen Z, Zhang W and Yang J:
Whirlin and PDZ domain-containing 7 (PDZD7) proteins are both
required to form the quaternary protein complex associated with
Usher syndrome type 2. J Biol Chem. 289:36070–36088. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mathur PD, Zou J, Zheng T, Almishaal A,
Wang Y, Chen Q, Wang L, Vashist D, Brown S, Park A and Yang J:
Distinct expression and function of whirlin isoforms in the inner
ear and retina: An insight into pathogenesis of USH2D and DFNB31.
Hum Mol Genet. 24:6213–6228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Belyantseva IA, Boger ET, Naz S, Frolenkov
GI, Sellers JR, Ahmed ZM, Griffith AJ and Friedman TB: Myosin-XVa
is required for tip localization of whirlin and differential
elongation of hair-cell stereocilia. Nat Cell Biol. 7:148–156.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delprat B, Michel V, Goodyear R, Yamasaki
Y, Michalski N, El-Amraoui A, Perfettini I, Legrain P, Richardson
G, Hardelin JP and Petit C: Myosin XVa and whirlin, two deafness
gene products required for hair bundle growth, are located at the
stereocilia tips and interact directly. Hum Mol Genet. 14:401–410.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kikkawa Y, Mburu P, Morse S, Kominami R,
Townsend S and Brown SD: Mutant analysis reveals whirlin as a
dynamic organizer in the growing hair cell stereocilium. Hum Mol
Genet. 14:391–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manor U, Disanza A, Grati M, Andrade L,
Lin H, Di Fiore PP, Scita G and Kachar B: Regulation of stereocilia
length by myosin XVa and whirlin depends on the actin-regulatory
protein Eps8. Curr Biol. 21:167–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mburu P, Kikkawa Y, Townsend S, Romero R,
Yonekawa H and Brown SD: Whirlin complexes with p55 at the
stereocilia tip during hair cell development. Proc Natl Acad Sci
USA. 103:10973–10978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng L, Sekerková G, Vranich K, Tilney
LG, Mugnaini E and Bartles JR: The deaf jerker mouse has a mutation
in the gene encoding the espin actin-bundling proteins of hair cell
stereocilia and lacks espins. Cell. 102:377–385. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Naz S, Griffith AJ, Riazuddin S, Hampton
LL, Battey JF Jr, Khan SN, Riazuddin S, Wilcox ER and Friedman TB:
Mutations of ESPN cause autosomal recessive deafness and vestibular
dysfunction. J Med Genet. 41:591–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donaudy F, Zheng L, Ficarella R, Ballana
E, Carella M, Melchionda S, Estivill X, Bartles JR and Gasparini P:
Espin gene (ESPN) mutations associated with autosomal dominant
hearing loss cause defects in microvillar elongation or
organisation. J Med Genet. 43:157–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Wang L, Song H and Sokolov M:
Current understanding of usher syndrome type II. Front Biosci.
17:1165–1183. 2012. View
Article : Google Scholar :
|
|
28
|
Wang L, Zou J, Shen Z, Song E and Yang J:
Whirlin interacts with espin and modulates its actin-regulatory
function: An insight into the mechanism of Usher syndrome type II.
Hum Mol Genet. 21:692–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sekerková G, Zheng L, Loomis PA, Mugnaini
E and Bartles JR: Espins and the actin cytoskeleton of hair cell
stereocilia and sensory cell microvilli. Cell Mol Life Sci.
63:2329–2341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Liu X, Yue G, Adamian M, Bulgakov
O and Li T: Rootletin, a novel coiled-coil protein, is a structural
component of the ciliary rootlet. J Cell Biol. 159:431–440. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen B, Li A, Wang D, Wang M, Zheng L and
Bartles JR: Espin contains an additional actin-binding site in its
N terminus and is a major actin-bundling protein of the Sertoli
cell-spermatid ectoplasmic specialization junctional plaque. Mol
Biol Cell. 10:4327–4339. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Williamson MP: The structure and function
of proline-rich regions in proteins. Biochem J. 297:249–260. 1994.
View Article : Google Scholar : PubMed/NCBI
|