Introduction
Liver fibrosis, the typical response to chronic
liver disease and the main factor contributing to the development
of liver failure, is regarded as a mixture of fibrogenesis and
fibrosis resolution (1–3). The degree of liver fibrosis is
largely dependent on the balance between fiber generation and
degradation (4,5). Matrix metalloproteinases (MMPs) have
been demonstrated to serve a critical role in liver fibrosis by
promoting fibrogenesis and fibrosis resolution (6–8).
MMPs are a family of zinc metallo-endopeptidases and
their role in promoting liver fibrosis has been investigated
extensively (9). The general
principle is that MMPs promote the expression of cytokines and
chemokines, particularly transforming growth factor β (TGFβ), by
which MMPs activate hepatic stellate cells (HSCs) and promote
fibrogenesis (10–12). Previous studies have demonstrated
that the depletion of MMP9 or MMP13 suppressed the activation of
TGFβ and the generation of fibrosis in response to acute liver
injury (13,14). During liver fibrosis, the
generation and degradation of collagen occur simultaneously
(2–4). Until now, although numerous MMPs,
including MMP1, MMP2, MMP12, MMP13 and MMP14, have been
demonstrated to be involved in the regression of liver fibrosis
(4,10,15),
the underlying molecular mechanism by which MMPs are involved in
the degradation of extracellular matrix (ECM) remains unknown.
Using a transgene of MMP9, a previous study observed that MMP9
accelerated the resolution of liver fibrosis by neutralizing tissue
inhibitor of metalloproteinase-1 (TIMP1) (16). Other studies have indicated that
MMPs have a critical role in the apoptosis of activated HSCs, or
facilitate the generation of restorative macrophages, which has
been proven to promote fibrosis resolution (17,18).
As one of the most important members of the MMP
family, MMP9 promotes collagen deposition and degradation. This
two-edged effect of MMP9 makes it difficult to understand the
precise role of MMP9 in liver fibrosis. The present study aimed to
investigate the dynamic features of liver fibrogenesis and fibrosis
resolution in the absence of matrix metalloproteinase-9. It was
revealed that the absence of MMP9 attenuated liver fibrosis in the
early stage of disease, but collagens accumulated in the liver
tissues with time and reached the same levels as those in the
control by 8 weeks. The regression of fibrosis was induced by
stopping TAA treatment, and collagen levels were higher in
MMP9−/− mice. It has also been suggested that
fibronectin peptides generated by limited digestion by MMP9 during
liver injury act as local regulatory signals that induce the
apoptosis of HSCs, and so prevent chronic fibrogenesis and fibrosis
(19).
Materials and methods
Animal experiments
MMP9−/− (FVB) mice from the Model Animal
Research Center of Nanjing University (Nanjing, China) were crossed
into the B6/C57 background for six generations, and homozygous
wild-type (WT) mice were used as controls (13,14).
A total of 100 8-weeks old, male mice (20–25 g) were maintained
under controlled temperature (22–24°C) and humidity (50%) with a
12-h light/dark cycle and were fed standard laboratory chow and
water (supplied ad libitum). Animal experimental protocols
were approved by the Institutional Animal Care and Research
Advisory Committee of Nanjing Drum Tower Hospital (Nanjing, China).
The murine model of liver fibrogenesis and fibrosis resolution was
prepared as previously described (4) with some modification. Briefly, liver
fibrogenesis was induced by repeated intraperitoneal administration
of thioacetamide (TAA, 0.1 mg/g body weight; Sigma-Aldrich; Merck
KGaA) every 2 days for 8 weeks. Following all challenges,
spontaneous fibrosis resolution was observed for 9 days. Control
mice were injected with the same volume of saline.
Serum transaminase and ELISA
Serum was collected by ocular blood extraction at
the indicated time points (0, 12, 24, 36 and 48 h). Liver injury
was estimated according to the increased activity of serum alanine
aminotransferase (ALT), which was measured in a clinical
biochemical laboratory of Nanjing Drum Tower Hospital. The levels
of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-10
and TGFβ in the liver homogenate were determined using commercial
ELISA kits (R&D Systems, Inc.) according to the manufacturer's
protocol. All samples and standards were measured in duplicate.
Western blot analysis
The liver samples were prepared as previously
described (20). Equal quantities
(30 µg) of protein extracted from liver tissues were run on 10%
SDS-PAGE gels, followed by electrotransfer onto a polyvinylidene
fluoride membrane. The membrane was cut into three for and blocked
with 5% skimmed milk for 2 h at room temperature and then incubated
overnight with primary antibodies at 4°C. Primary antibodies
targeting collagen-I (ab34710, 1:1,000), collagen-III (ab7778,
1:1,000), collagen-IV (ab6586, 1:1,000) and GAPDH (ab181602,
1:2,000) were purchased from Abcam. Horseradish
peroxidase-conjugated secondary antibodies (sc-2004, 1:500, Santa
Cruz Biotechnology, Inc.) were used 1 h at room temperature prior
to detection with Super Signal West Femto Chemiluminescent
substrate (Pierce; Thermo Fisher Scientific, Inc.). The protein
band intensities in the western blot analysis were quantified using
Image Quant software (version 5.2; GE Healthcare Life
Sciences.).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from liver tissue using
TRIzol® (Life Technologies; Thermo Fisher Scientific,
Inc.). The analysis was performed as described previously (20). Briefly, reverse transcription was
performed with random primers and the RNAPCR kit (Takara
Biotechnology Co., Ltd.). RT-qPCR was conducted according to the
manufacturer's instructions using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.). Amplifications were performed in a final
volume of 20 ml containing 2 ml of cDNA. The reactions were run on
the StepOnePlus Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using the following program: 95°C for 10
min for the holding stage and 40 cycles of 95°C for 15 sec and 60°C
for 1 min. The expression levels of the target genes were
normalized to the housekeeping gene GAPDH. The final result of gene
transcription was calculated as 2(Ct GAPDH-2Ct Gene)
(21). Analyses were performed
using the StepOne Software 2.0 (Applied Biosystems).
The primer sequences used for PCR amplification of
the mouse genes were as follows: MMP9, forward
5′-CGTGTCTGGAGATTCGACTTGA-3′ and reverse
5′-TGGAAGATGTCGTGTGAGTTCC-3′; MMP13, forward
5′-CCTTCTGGTCTTCTGGCACAC-3′, reverse,
5′-GGCTGGGTCGTCACACTTCTCTGG-3′; MMP2, forward,
5′-CAACGGTCGGGAATACAGCAG-3′ and reverse
5′-CCAGGAAAGTGAAGGGGAAGA-3′; MMP14, forward
5′-ATCTCACAGCTCGGTGTGTGTTCA-3′ and reverse
5′-AAGGTCAGAGGGTCTTGCCTTCAA-3′; TIMP1, forward
5′-GCATGGACATTTATTCTCCACTGT-3′ and reverse
5′-TCTCTAGGAGCCCGATCTG-3′; TIMP2, forward
5′-GCCAAAGCAGTGAGCGAGAAG-3′ and reverse
5′-GGGGAGGAGATGTAGCAAGGG-3′; GAPDH, forward
5′-AACTTTGGCATTGTGGAAGG-3′ and reverse
5′-ACACATTGGGGGTAGGAACA-3′.
Histological examination
The liver tissues were collected at definite times
(1, 5 and 9 days after TAA withdrawal) and fixed in 4% formalin and
subsequently embedded in paraffin. The sections were cut into 5-mm
slices and were deparaffinized and rehydrated. The slides were
incubated with hematoxylin (5 min, room temperature) followed by
rinsing with water and quick dips into acid ethanol to destain. For
eosin staining, the slides were incubated with eosin (1 min, room
temperature) followed by 95, 100% ethanol and xylene. For Sirius
Red staining, the sections were deparaffinized and then stained by
Sirius Red (10–15 min, room temperature). The slides were evaluated
using light microscopy, as previously described (4).
Statistical analysis
All data are expressed as the mean ± standard
deviation. The differences between two groups were analyzed using
two-tailed Student's t-test. Differences between multiple groups
were tested with analysis of variance. Statistics and graphs were
generated using GraphPad Prism 5.0 (GraphPad Software Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Attenuation of acute liver injury and
liver fibrogenesis in the absence of MMP9
Inflammation has been revealed to induce the
progression of liver fibrosis (2).
In the present study, the role of MMP9 in acute liver injury was
investigated. All animals were subjected to the challenge course
presented in Fig. 1A. Certain
animals were sacrificed just prior to the second challenge with TAA
in order to analyze the degree of acute liver injury. The serum
level of ALT was increased markedly in the WT animals 24 h after
TAA injection, whereas the elevation was less marked in the
MMP9−/− mice (Fig. 1B).
The same variation was observed in the levels of TNF-α and IL-1β
(Fig. 1C and D). The level of
IL-10 was increased by 98% at 12 h and decreased by 48% at 24 h in
the WT mice compared with that in the saline-treated animals.
However, no significant change was observed in the
MMP9−/− animals (Fig.
1E). The level of TGFβ was increased 5-fold in the WT animals
2-fold in the MMP9−/− animals 24 h after TAA injection,
compared with that in the controls (Fig. 1F).
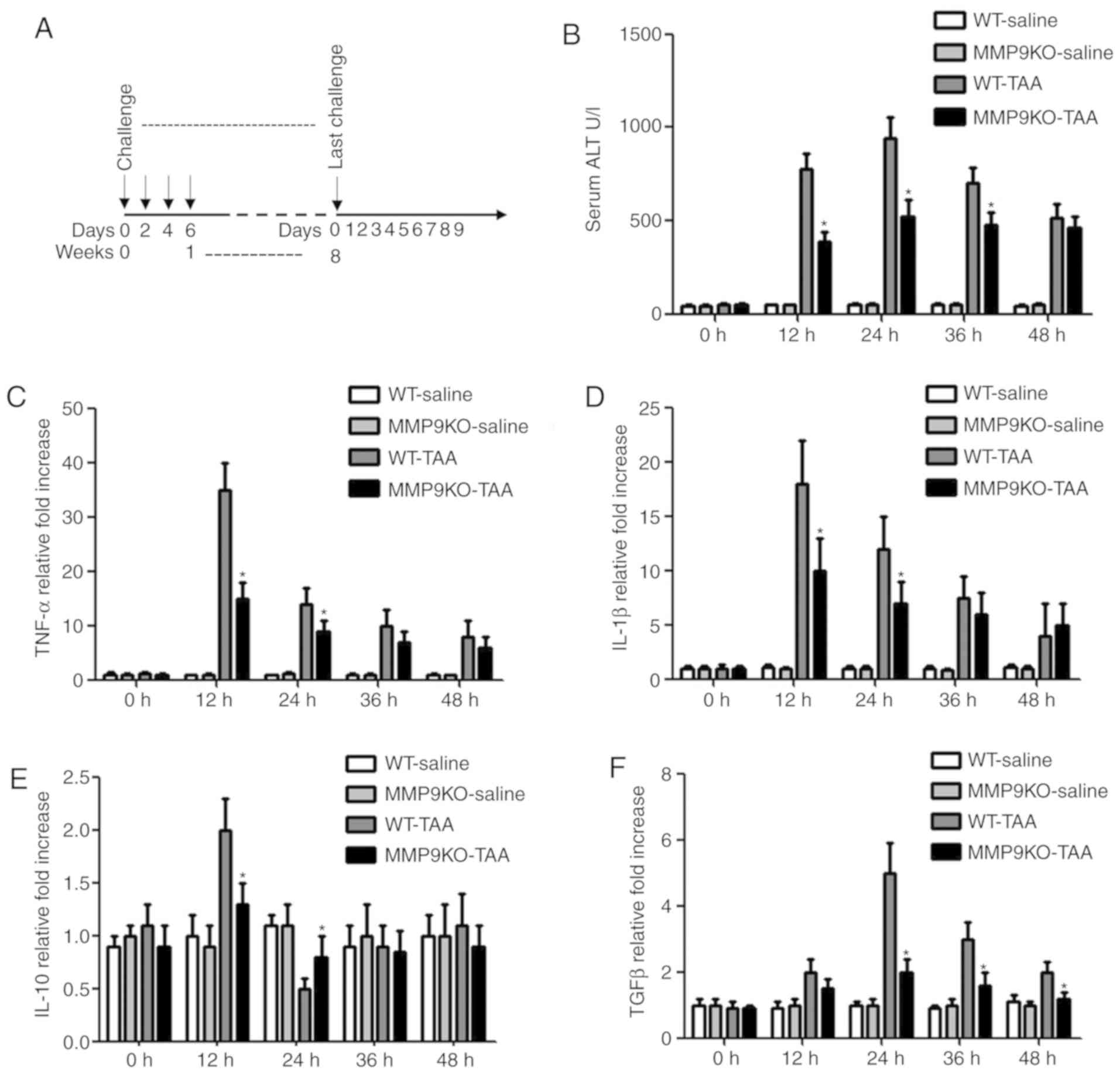 | Figure 1.Attenuation of liver injury by
depleting MMP9. (A) WT and MMP9−/− mice were treated
with TAA (0.1 mg/g body weight; intraperitoneally) every 2 days for
8 weeks, following which the TAA challenge was withdrawn and
fibrosis resolution was observed for 9 days. (B) Serum was
collected at the indicated time points. ALT level was measured in a
clinical biochemical laboratory. The levels of (C) TNF-α, (D)
IL-1β, (E) IL-10 and (F) TGFβ in the liver homogenate were
determined using commercial ELISA kits. All samples and standards
were measured in duplicate. Data are expressed as fold-change
compared with the control. *P<0.05 vs. WT animals. MMP9, matrix
metalloproteinase-9; WT, wild-type mice; MMP9KO, MMP9-deficient
mice; ALT, alanine aminotransferase; TAA, thioacetamide; TNF-α,
tumor necrosis factor α; IL, interleukin; TGFβ, transforming growth
factor β. |
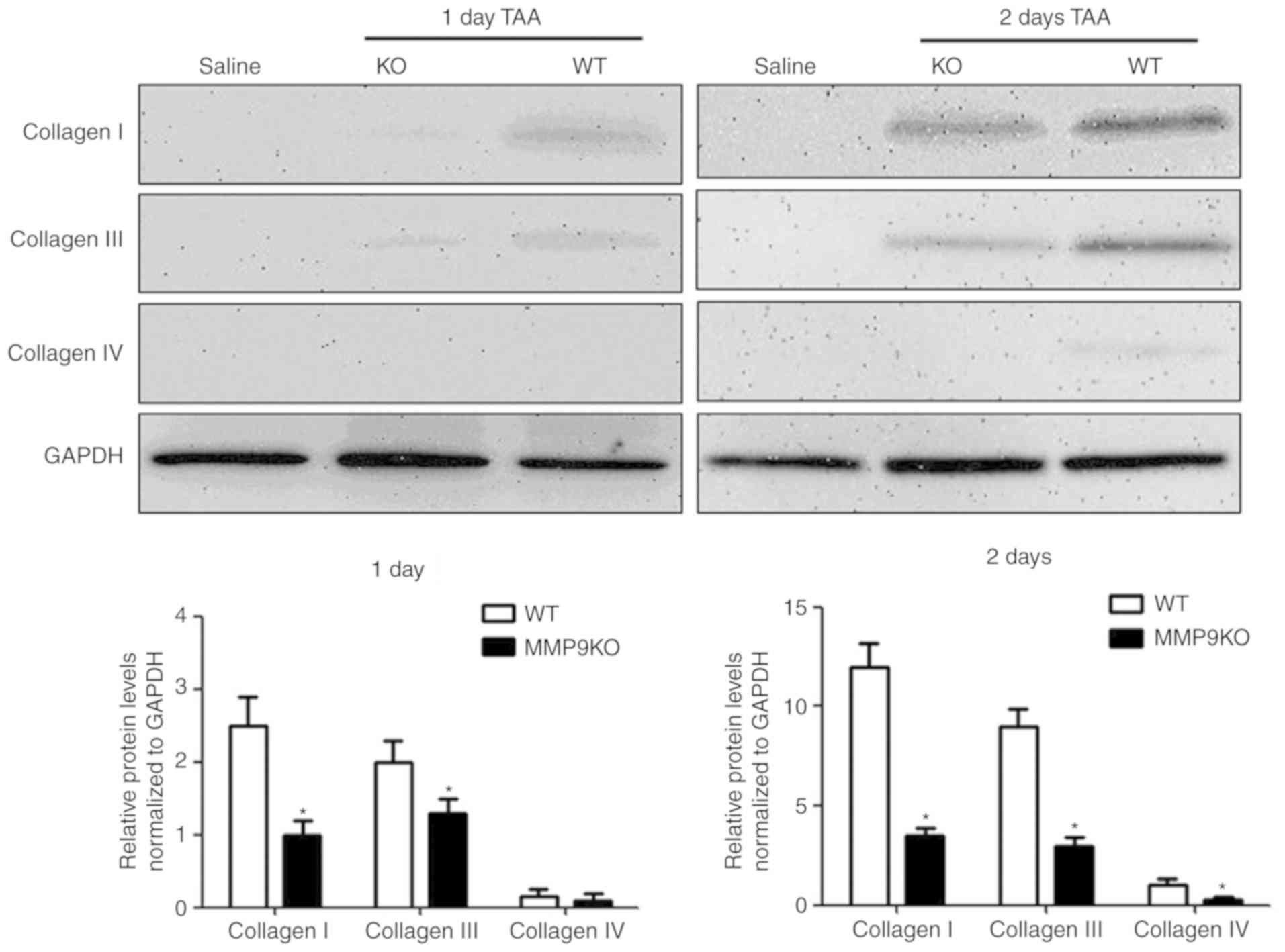
Liver fibrogenesis occurs alongside the development
of liver injury (2). Increasing
deposition of collagen types I and III is the paramount feature of
liver fibrogenesis (22,23). In the present study, enhanced
expression levels of collagens I and III were observed in both
genotypic mice following TAA treatment (Fig. 2). However, the quantified data
revealed that the depletion of MMP9 decreased the level of collagen
I by 55% at day 1, and by 72% at day 2, compared with that in the
WT mice. Collagen III exhibited the same variation in the absence
of MMP9 (Fig. 2). Collagen IV is a
substrate of MMP9 and also contributes to liver fibrogenesis,
although it only presents along vessels or around myofibroblasts
(23–25). In the present study, the expression
of collagen IV was significantly decreased in the absence of MMP9 2
days after TAA treatment, compared with that in the control
(Fig. 2).
Dynamic features of collagen
deposition and degradation in the absence of MMP9
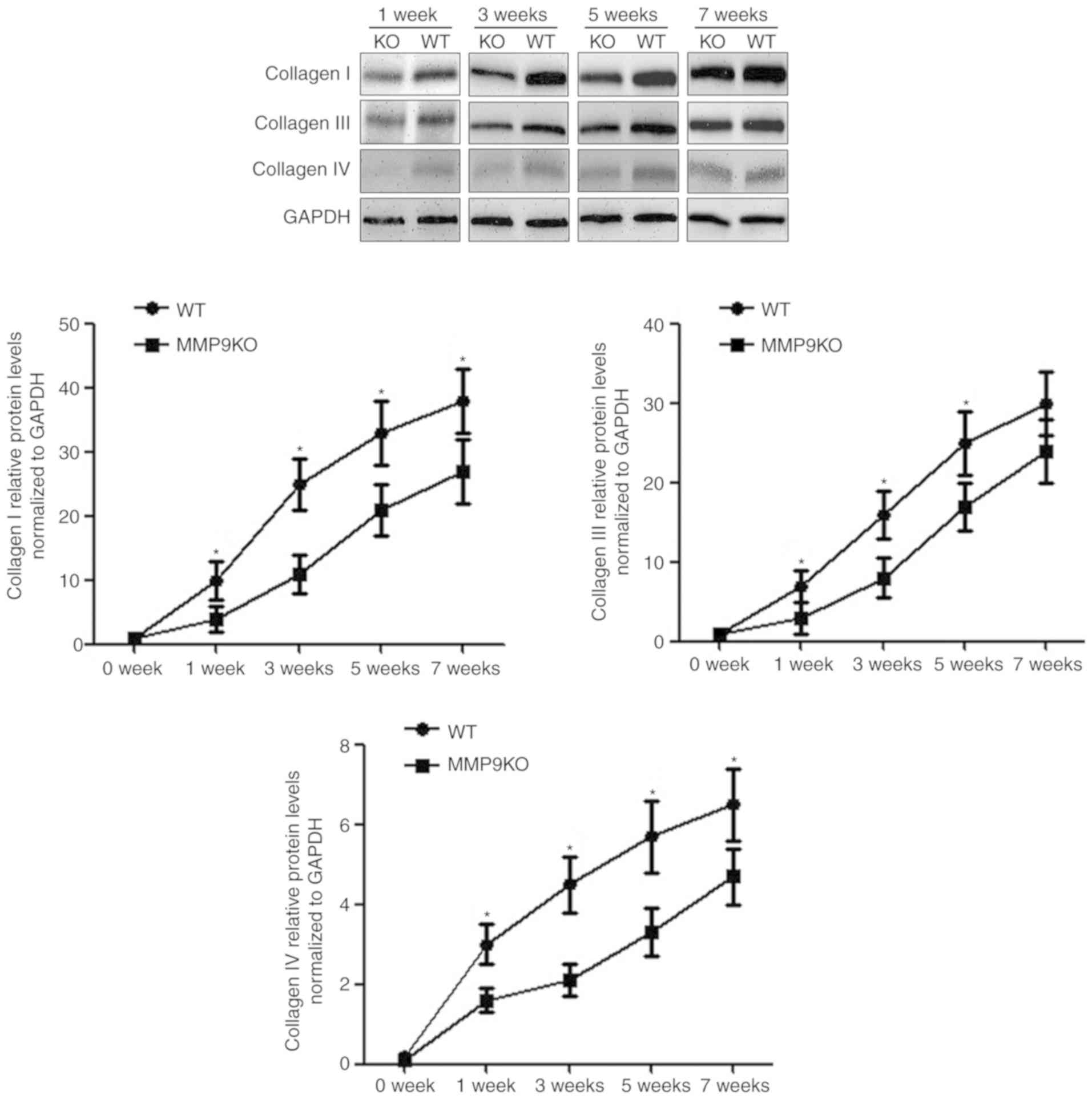
With continual TAA challenge, the levels of
collagens increased with time in both genotypic animals. Compared
with that in the WT animals, collagen deposition was slower in the
MMP9−/− mice at the early stage of liver fibrosis,
particularly during the first 3 weeks. The quantified data
demonstrated that the absence of MMP9 decreased collagen I by 46%
at week 1, 57% at week 3, 49% at week 5 and 32% at week 7 (Fig. 3). The expression of collagen III
decreased by 33% at week 1, 51% at week 3 and 37% at week 5, but
there was no significant change at week 7. The expression of
collagen IV decreased by 46% at week 1, 57% at week 3, 49% at week
5 and 32% at week 7 (Fig. 3).
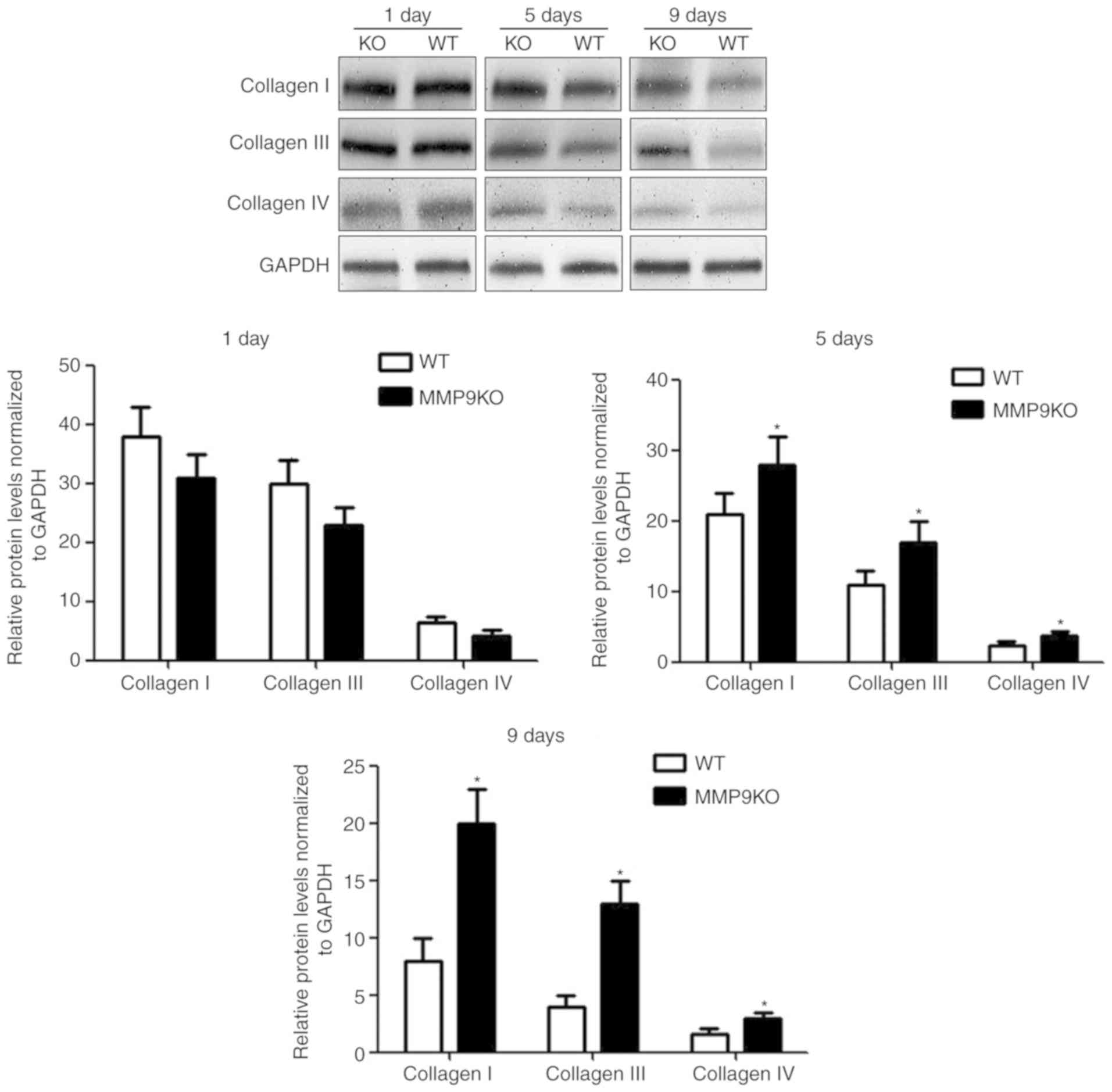
The resolution of reversible fibrosis is initiated
through the cessation of insults causing liver injury (6). The effect of MMP9 on liver fibrosis
resolution was observed for 9 days following the withdrawal of TAA.
By quantifying the data from the western blotting, it was revealed
that there was no significant difference in the expression of
collagen between the two genotypic animals after 8 weeks of TAA
treatment when measured on the first day of TAA withdrawal. The
levels of collagens decreased faster in the WT animals (Fig. 4). The quantified data revealed that
the expression of collagen I decreased by 29% at day 5 and 58% at
day 9; collagen III decreased by 33% at day 5 and 69% at day 9;
collagen IV decreased by 21% at day 5; and 48% at day 9, compared
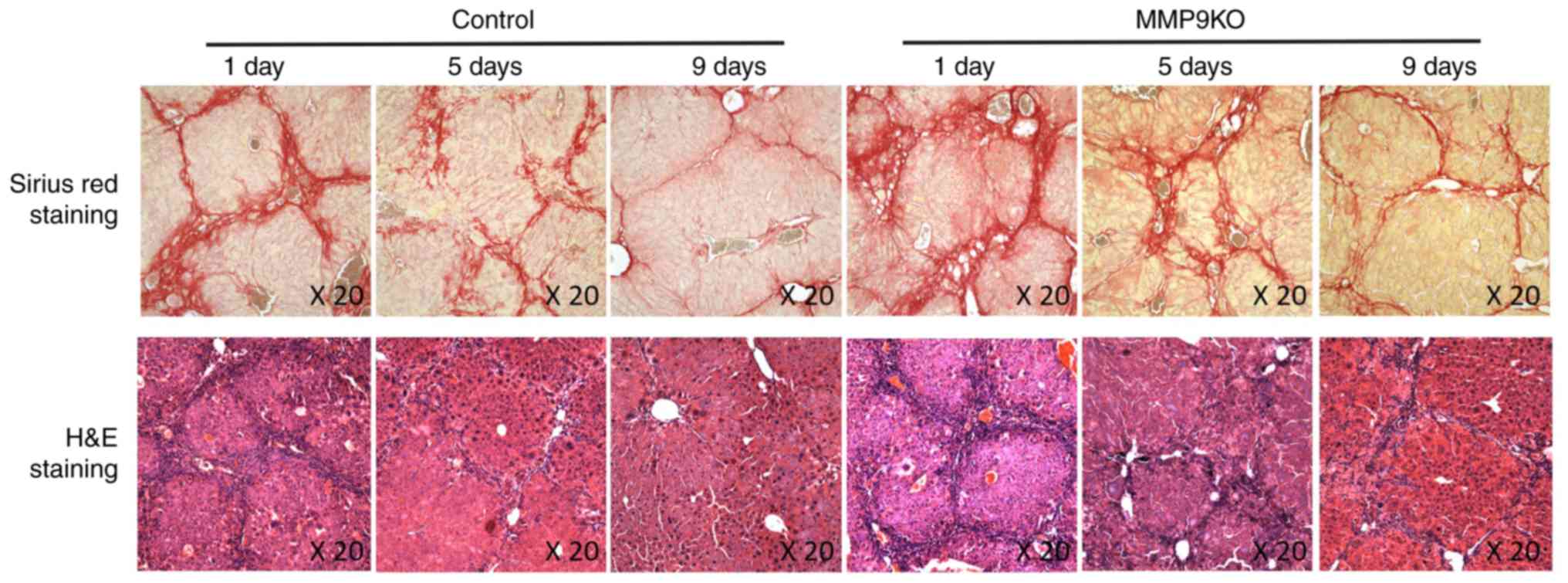
with levels in the MMP9-depleted controls (Fig. 4). The histological examination
indicated that the resolution of fibrosis was suppressed by the
depletion of MMP9 (Fig. 5).
Regulatory effect on the ratio of
MMPs/TIMPs by MMP9
Liver injury, wound healing and the activation of
MMPs occur in the process of fibrogenesis or fibrosis resolution
(2,13). In the present study, the dynamic
features of these factors were also demonstrated, which may
influence the role of MMP9 in liver fibrosis, however, the
underlying molecular mechanism by which MMP9 promotes fibrogenesis
or fibrosis resolution requires further investigation. It was
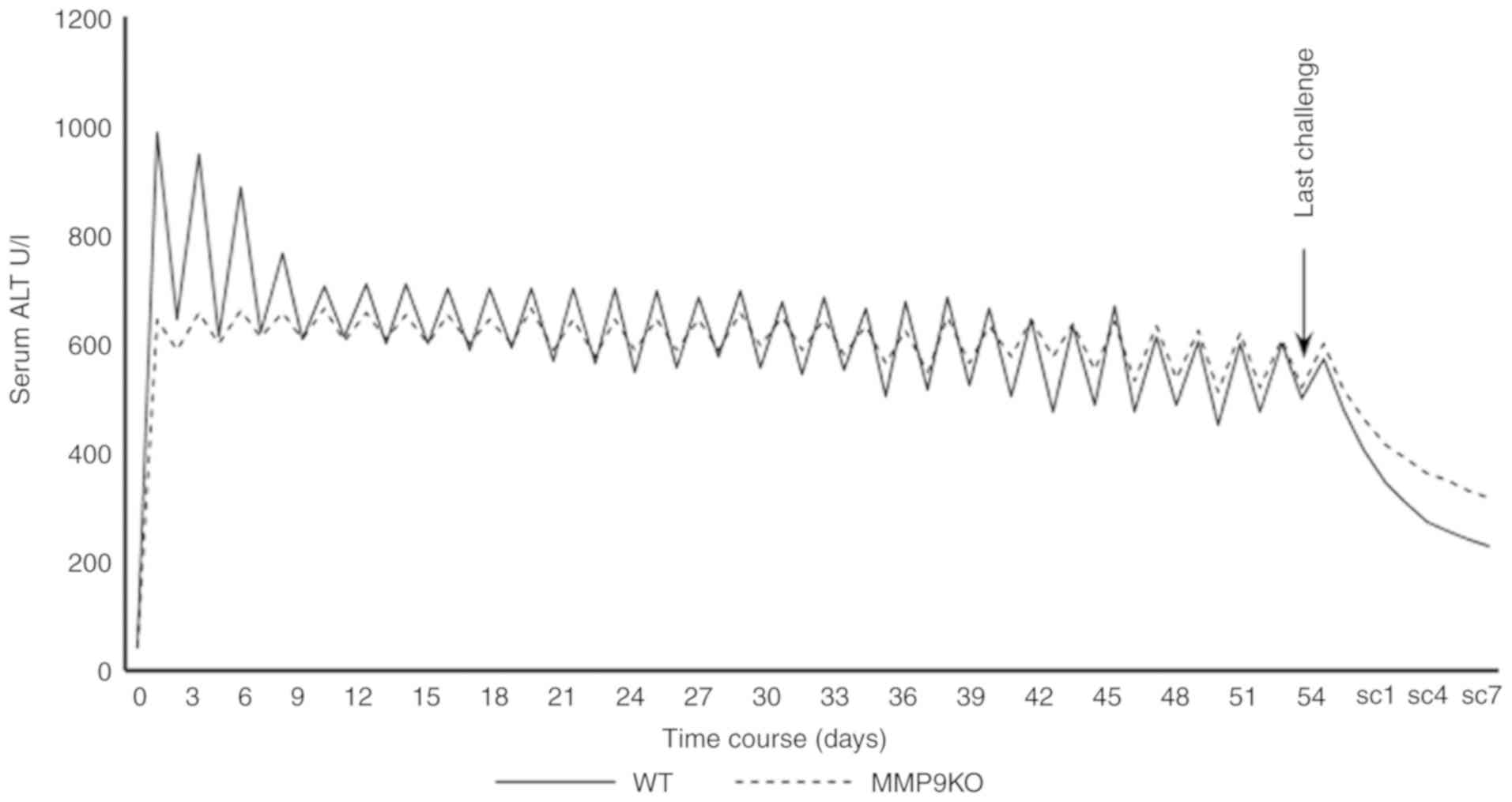
observed that the absence of MMP9 decreased the serum level of ALT
at the acute injury phase, and modulated the fluctuation of serum
ALT at the chronic injury period, although there was no significant
difference in the average level of serum ALT compared with that in
the controls (Fig. 6).
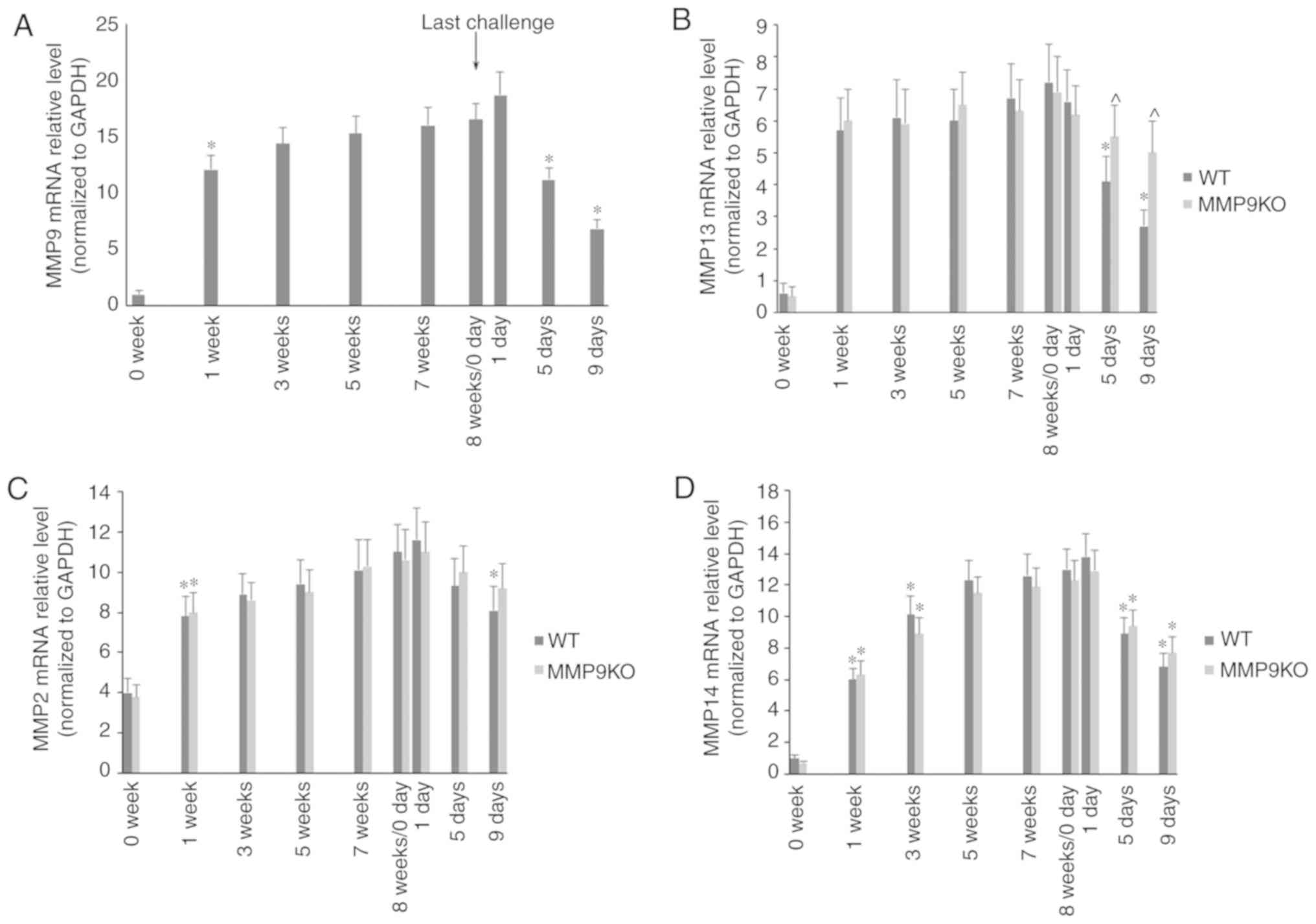
During the development of fibrosis, the mRNA levels
of MMP9 (Fig. 7A), MMP13 (Fig. 7B), MMP2 (Fig. 7C) and MMP14 (Fig. 7D) were marginally increased, but
there was no significant difference between the two genotypic
animals. During the resolution of fibrosis, the mRNA levels of MMPs
were significantly decreased. The absence of MMP9 significantly
attenuated the decrease in the mRNA level of MMP13 (Fig. 7B).
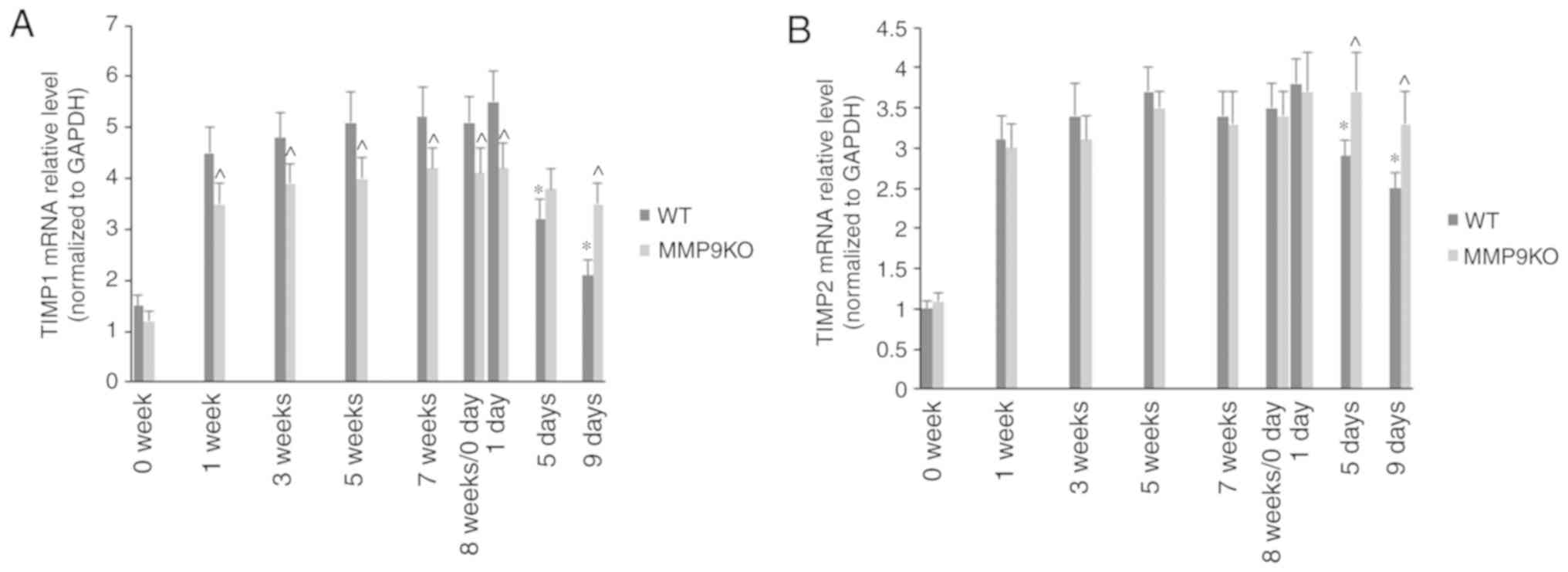
It was observed that the mRNA levels of TIMP1
(Fig. 8A) and TIMP2 (Fig. 8B) also increased slightly during
the first 5 weeks of TAA treatment. The absence of MMP9 decreased
the mRNA level of TIMP1, rather than that of TIMP2. During the
resolution of fibrosis, the mRNA levels of TIMP1 and TIMP2 were
markedly decreased in the WT animals, but only marginally in the
MMP9−/− mice (Fig. 8A and
B).
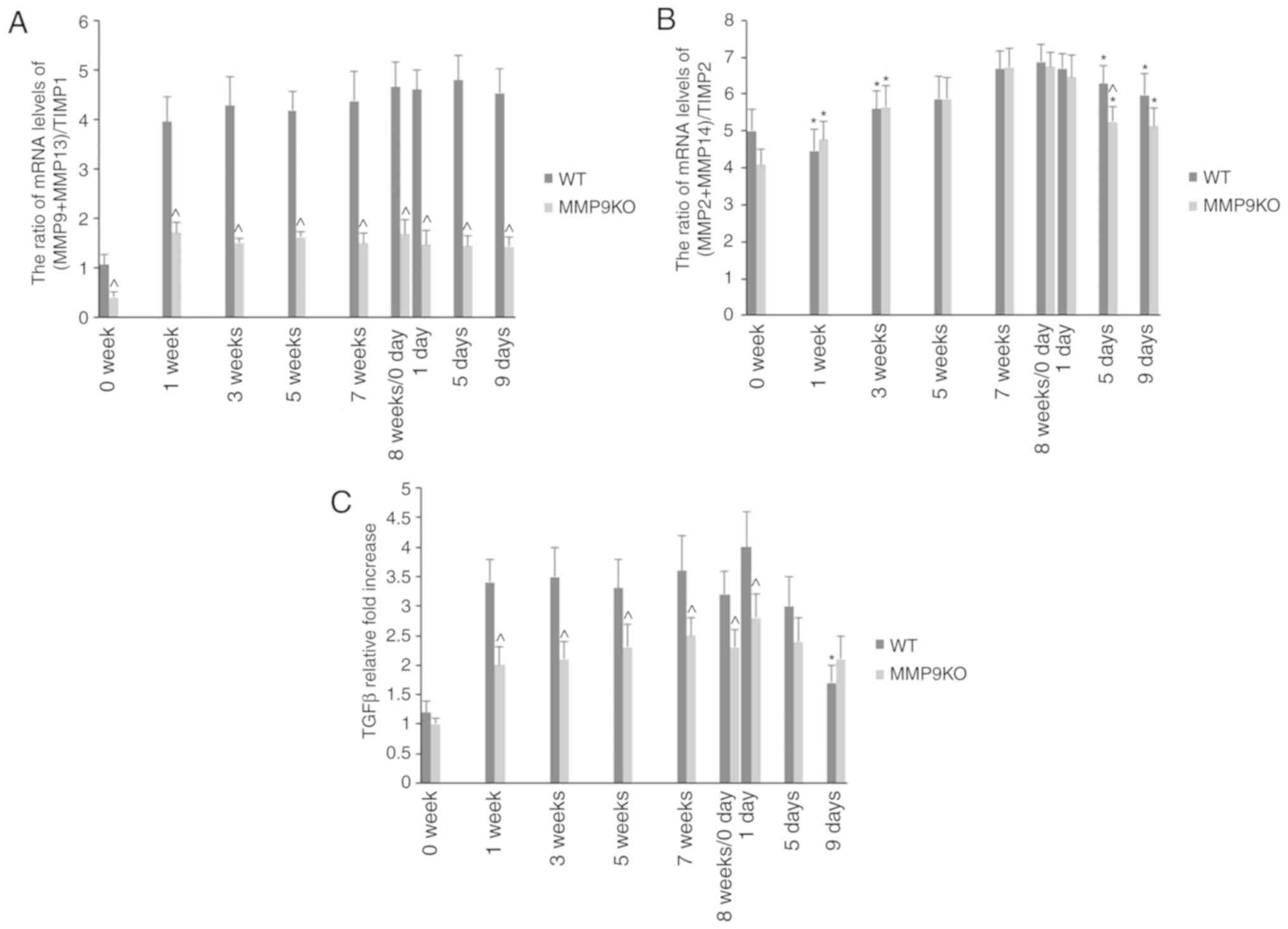
The actual proteolytic activation of MMPs is
dependent on the ratio of MMPs with their corresponding TIMPs. In
the present study, it was observed that the absence of MMP9
decreased the (MMP9 + MMP13)/TIMP1 ratio (Fig. 9A). The (MMP2 + MMP14)/TIMP2 ratio
was slightly increased during the development of fibrosis and
decreased on fibrosis resolution (Fig.
9B). It was also observed that the absence of MMP9 decreased
the hepatic level of TGFβ in the development of fibrosis, whereas
its expression was enhanced during fibrosis resolution,
particularly notable IX days following TAA withdrawal (Fig. 9C).
Discussion
Despite MMP9 being important in liver fibrogenesis
and fibrosis resolution, the dynamic features of liver fibrosis
induced by the two-edged effect of MMP9 remain unclear. The present
study used the previously established liver fibrotic model of
TAA-induced liver injury and investigated the dynamic features of
fibrosis development and regression in the absence of MMP9.
Liver fibrogenesis is driven by inflammation,
therefore, inflammatory cytokines in acute liver injury were first
determined during the present study. In line with previous studies
on ischemia and reperfusion and hepatic toxin-induced liver injury,
the depletion of MMP9 attenuated acute liver injury and decreased
liver fibrogenesis in the present study (14). The continual TAA challenge utilized
in the present study revealed for the first time, to the best of
our knowledge, that the depletion of MMP9 attenuated collagen
deposition at the early stage of liver fibrosis, although the
deposition remained increased with time. At the end of the
challenge course, the expression of these collagens reached the
same levels as those in the control. α-smooth muscle actin (α-SMA)
is a marker for active HSCs. The depletion of MMP9 suppresses the
activation of HSCs and decreases the expression of α-SMA (14). Why the long-term effect of MMP9 is
not obvious requires further investigation in the future. However,
to the best of our knowledge, MMP9 is only one of the MMPs
regulating liver fibrogenesis and fibrosis resolution. In the
absence of MMP9, other MMPs may serve an important role (10–14).
The resolution of fibrosis is initiated when the
cause of cirrhosis is removed. The liver adapts to a near-normal
structure (26). In the present
study, the absence of MMP9 increased collagen levels. However, the
underlying mechanism by which MMP9 regulates the resolution of
liver fibrosis remains to be fully elucidated. Our previous study
demonstrated that the adoptive transfer of MMP9-expressing kupffer
cells promoted fibrosis resolution (20).
Increasing evidence has demonstrated that MMP9
promotes fibrogenesis by activating latent TGFβ, which stimulates
the activation of HSCs (10–12).
However, the underlying molecular mechanism by which MMP9
facilitates the regression of fibrosis requires further
investigation. In general, the process of fibrosis resolution
consists of three steps: The transformation of immunoreaction
phenotypes following insult withdrawal, particularly the generation
of restorative macrophages; the decline in activated HSCs; and the
degradation of ECM (6). MMP8 has
been demonstrated to promote transformation of the macrophage
phenotype (18), whereas MMP9 has
been demonstrated to induce the apoptosis of activated HSCs
(16). Kupffer-derived MMP9, MMP12
or MMP13 serve critical roles in ECM degradation (6,27).
Previous studies have revealed that MMP9 indirectly promoted the
resolution of fibrosis by promoting the expression of vascular
endothelial growth factor to facilitate transformation to the
macrophage phenotype (3,28) and decreasing collagen I to promote
the apoptosis of activated HSCs (16,29).
In the present study, the dynamic features of liver
injury, wound healing and MMP activation were demonstrated, which
are associated with liver fibrosis and its regression (3). It was observed that the absence of
MMP9 attenuated acute, rather than chronic, liver injury. Previous
studies have demonstrated that MMP9 caused collapse of the hepatic
sinus and worsened acute liver injury (30), however, why the absence of MMP9 has
no effect on chronic injury remains to be elucidated.
The actual activation of MMPs is dependent on their
expression levels and the levels of TIMPs (4,31).
In the present study, it was observed that the mRNA levels of MMPs
(MMP2, MMP9, MMP13 and MMP14) and TIMPs (TIMP1 and TIMP2) were
increased with TAA challenge and were decreased following TAA
withdrawal. The (MMP9 + MMP13)/TIMP1 and (MMP2 + MMP14)/TIMP2
ratios were calculated and it was revealed that both ratios were
lower in the absence of MMP9. In addition, it was revealed that the
absence of MMP9 increased the expression of TGFβ during the late
stage of fibrosis resolution compared with that in the control. The
effect of the loss of MMP9 on liver fibrosis can be partially
explained by the features of these promotive factors for
fibrogenesis or fibrosis resolution, although the precise
underlying molecular mechanism requires further investigation.
In conclusion, the present study demonstrated the
dynamic features of liver fibrogenesis and fibrosis resolution in
the absence of MMP9. These results indicate that the two-edged
effect of MMP9 is regulated by the time and state of liver injury.
An improved understanding of the role of MMP9 in liver fibrosis
will provide a stepping stone for novel clinical applications by
targeting MMP9 in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81670561 and
81300336), the Nature Science Foundation of Jiangsu Province (grant
nos. QNRC2016022 and 2014-WSW-046), the Nature Science Foundation
of Nanjing (grant no. JQX14003) and the Fundamental Research Funds
for the Central Universities (grant no. 021414380445).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QW and LL contributed to the conception and design.
MF and JW made important modifications to conception and design.
QW, XL and JZ made substantial contributions to acquisition and
analysis of data. QW interpreted data with help from MF and JW. QW,
MF and JW were involved in drafting the manuscript and/or its
critical revision for important intellectual content. MF and JW
provided final approval of the version to be published. All authors
agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Institutional Animal Care and Research Advisory Committee of
Nanjing Drum Tower Hospital (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Campana L and Iredale JP: Regression of
liver fibrosis. Semin Liver Dis. 37:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo RC and Kim H: Histopathological
evaluation of liver fibrosis and cirrhosis regression. Clin Mol
Hepatol. 23:302–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramachandran P, Iredale JP and Fallowfield
JA: Resolution of liver fibrosis: Basic mechanisms and clinical
relevance. Semin Liver Dis. 35:119–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Feng M, Liu X, Bai L, Kong M,
Chen Y, Zheng S, Liu S, Wan YJ, Duan Z and Han YP: Persistence of
cirrhosis is maintained by intrahepatic regulatory T cells that
inhibit fibrosis resolution by regulating the balance of tissue
inhibitors of metalloproteinases and matrix metalloproteinases.
Transl Res. 169:67–79.e1-e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Altamirano-Barrera A, Barranco-Fragoso B
and Méndez-Sánchez N: Management strategies for liver fibrosis. Ann
Hepatol. 16:48–56. 2017. View Article : Google Scholar
|
|
6
|
Tacke F and Trautwein C: Mechanisms of
liver fibrosis resolution. J Hepatol. 63:1038–1039. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
George J, Tsutsumi M and Tsuchishima M:
MMP-13 deletion decreases profibrogenic molecules and attenuates
N-nitrosodimethylamine-induced liver injury and fibrosis in mice. J
Cell Mol Med. 21:3821–3835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koyama Y and Brenner DA: Liver
inflammation and fibrosis. J Clin Invest. 127:55–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han YP: Matrix metalloproteinases, the
pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 21
(Suppl 3):S88–S91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krantz SB, Shields MA, Dangi-Garimella S,
Cheon EC, Barron MR, Hwang RF, Rao MS, Grippo PJ, Bentrem DJ and
Munshi HG: MT1-MMP cooperates with Kras(G12D) to promote pancreatic
fibrosis through increased TGF-β signaling. Mol Cancer Res.
9:1294–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi T, Kim H, Liu X, Sugiura H,
Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, et al:
Matrix metalloproteinase-9 activates TGF-β and stimulates
fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol
Physiol. 306:L1006–L1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dayer C and Stamenkovic I: Recruitment of
matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface
by lysyl hydroxylase 3 (LH3) triggers transforming growth factor-β
(TGF-β) activation and fibroblast differentiation. J Biol Chem.
290:13763–13778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng M, Wang H, Wang Q and Guan W: Matrix
metalloprotease 9 promotes liver recovery from ischemia and
reperfusion injury. J Surg Res. 180:156–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu L, Feng M, Gu J, Xia Z, Zhang H, Zheng
S, Duan Z, Hu R, Wang J, Shi W, et al: Restoration of intrahepatic
regulatory T cells through MMP-9/13-dependent activation of TGF-β
is critical for immune homeostasis following acute liver injury. J
Mol Cell Biol. 5:369–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Hovell CJ, Pawley S, Hutchings MI,
Arthur MJ, Iredale JP and Benyon RC: Expression of matrix
metalloproteinase-2 and −14 persists during early resolution of
experimental liver fibrosis and might contribute to fibrolysis.
Liver Int. 24:492–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Atta H, El-Rehany M, Hammam O, Abdel-Ghany
H, Ramzy M, Roderfeld M, Roeb E, Al-Hendy A, Raheim SA, Allam H and
Marey H: Mutant MMP-9 and HGF gene transfer enhance resolution of
CCl4-induced liver fibrosis in rats: Role of ASH1 and EZH2
methyltransferases repression. PLoS One. 9:e1123842014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murphy FR, Issa R, Zhou X, Ratnarajah S,
Nagase H, Arthur MJ, Benyon C and Iredale JP: Inhibition of
apoptosis of activated hepatic stellate cells by tissue inhibitor
of metalloproteinase-1 is mediated via effects on matrix
metalloproteinase inhibition: Implications for reversibility of
liver fibrosis. J Biol Chem. 277:11069–11076. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen G, Zhang C, Chen Q, Luong le A,
Mustafa A, Ye S and Xiao Q: A novel role of matrix
metalloproteinase-8 in macrophage differentiation and polarization.
J Biol Chem. 290:19158–19172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Modol T, Brice N, Ruiz de Galarreta M,
García Garzón A, Iraburu MJ, Martínez-Irujo JJ and López-Zabalza
MJ: Fibronectin peptides as potential regulators of hepatic
fibrosis through apoptosis of hepatic stellate cells. J Cell
Physiol. 230:546–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng M, Ding J, Wang M, Zhang J, Zhu X and
Guan W: Kupffer-derived matrix metalloproteinase-9 contributes to
liver fibrosis resolution. Int J Biol Sci. 14:1033–1040. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karsdal MA, Henriksen K, Nielsen MJ,
Byrjalsen I, Leeming DJ, Gardner S, Goodman Z, Patel K, Krag A,
Christiansen C and Schuppan D: Fibrogenesis assessed by serological
type III collagen formation identifies patients with progressive
liver fibrosis and responders to a potential antifibrotic therapy.
Am J Physiol Gastrointest Liver Physiol. 311:G1009–G1017. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nieto N: A systems biology approach for
understanding the collagen regulatory network in alcoholic liver
disease. Liver Int. 32:189–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mak KM, Chu E, Lau KH and Kwong AJ: Liver
fibrosis in elderly cadavers: Localization of collagen types I,
III, and IV, α-smooth muscle actin, and elastic fibers. Anat Rec
(Hoboken). 295:1159–1167. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu SB, Ikenaga N, Peng ZW, Sverdlov DY,
Greenstein A, Smith V, Schuppan D and Popov Y: Lysyl oxidase
activity contributes to collagen stabilization during liver
fibrosis progression and limits spontaneous fibrosis reversal in
mice. FASEB J. 30:1599–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Vinicius I, Baptista AP, Barbosa AA and
Andrade ZA: Morphological signs of cirrhosis regression.
Experimental observations on carbon tetrachloride-induced liver
cirrhosis of rats. Pathol Res Pract. 201:449–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramachandran P, Pellicoro A, Vernon MA,
Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A,
Gordon-Walker TT, et al: Differential Ly-6C expression identifies
the recruited macrophage phenotype, which orchestrates the
regression of murine liver fibrosis. Proc Natl Acad Sci USA.
109:E3186–E3195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamamoto N, Otsuka T, Kondo A,
Matsushima-Nishiwaki R, Kuroyanagi G, Kozawa O and Tokuda H: Rac
limits TGF-β-induced VEGF synthesis in osteoblasts. Mol Cell
Endocrinol. 405:35–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu L, Ni B, Liu J, Yang J, Guo Q and Zhou
W: Hydroxycamptothecin liposomes inhibit collagen secretion and
induce fibroblast apoptosis in a postlaminectomy rabbit model. Eur
J Orthop Surg Traumatol. 23 (Suppl 1):S85–S91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Syed I, Rathod J, Parmar M, Corcoran GB
and Ray SD: Matrix metalloproteinase-9, −10, and −12, MDM2 and p53
expression in mouse liver during dimethylnitrosamine-induced
oxidative stress and genomic injury. Mol Cell Biochem. 365:351–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang JC: Importance of plasma matrix
metalloproteinases (MMP) and tissue inhibitors of metalloproteinase
(TIMP) in development of fibrosis in agnogenic myeloid metaplasia.
Leuk Lymphoma. 46:1261–1268. 2005. View Article : Google Scholar : PubMed/NCBI
|