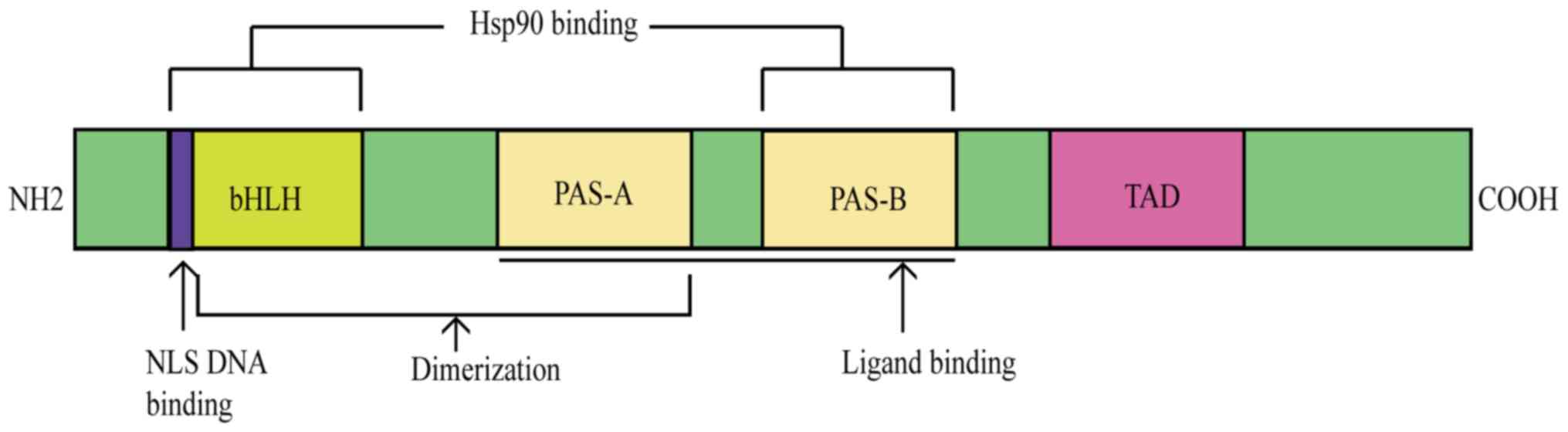
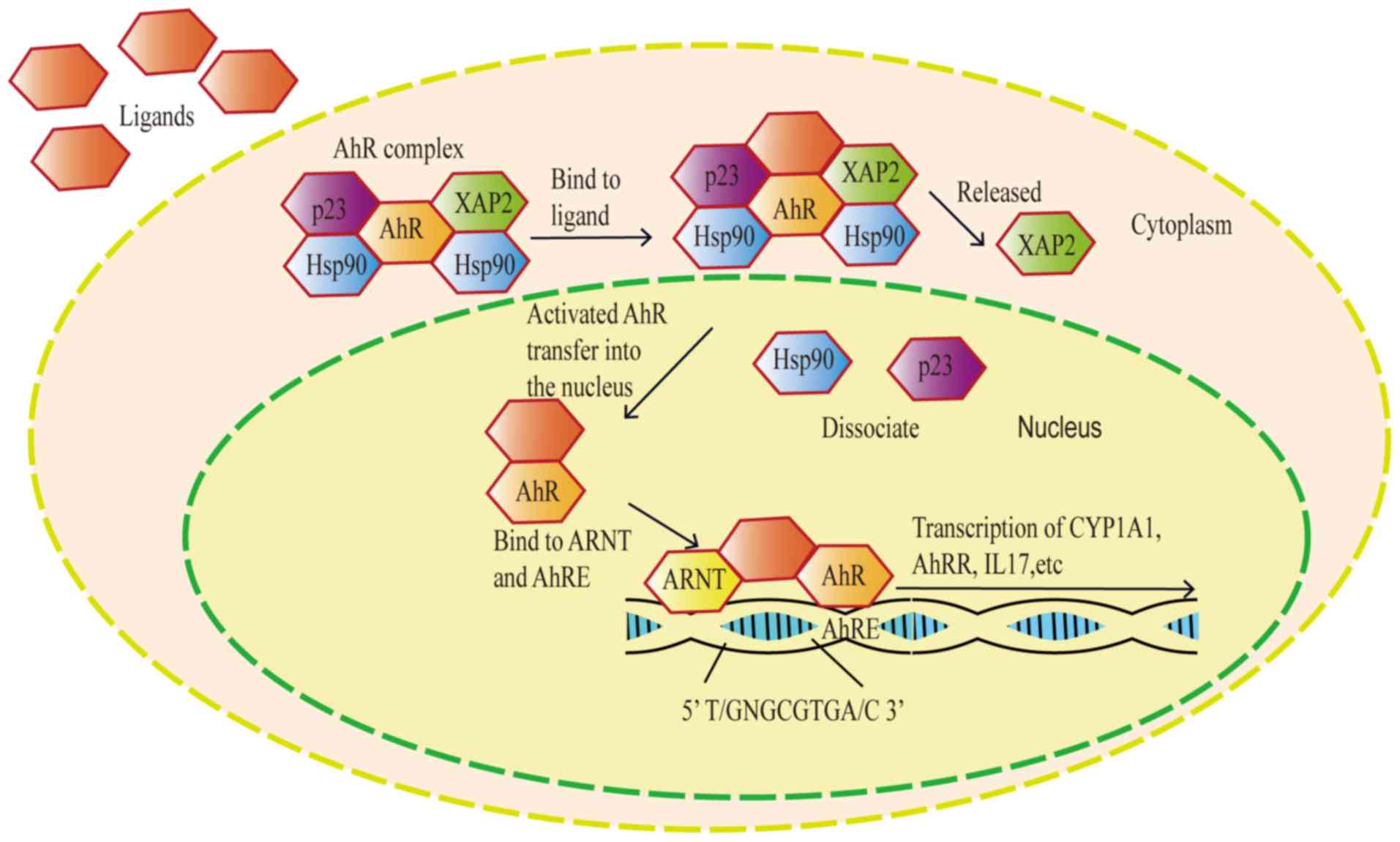
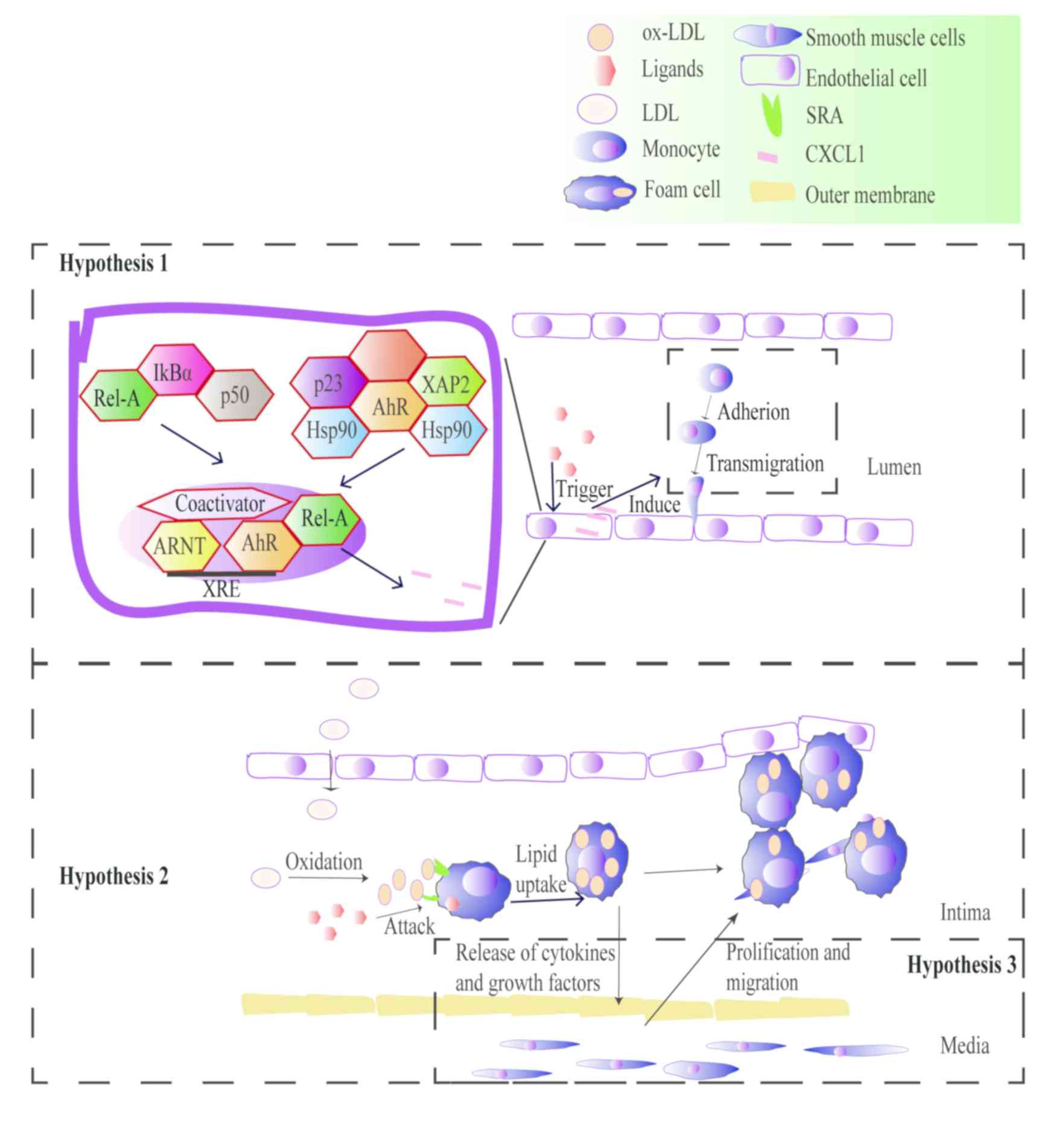
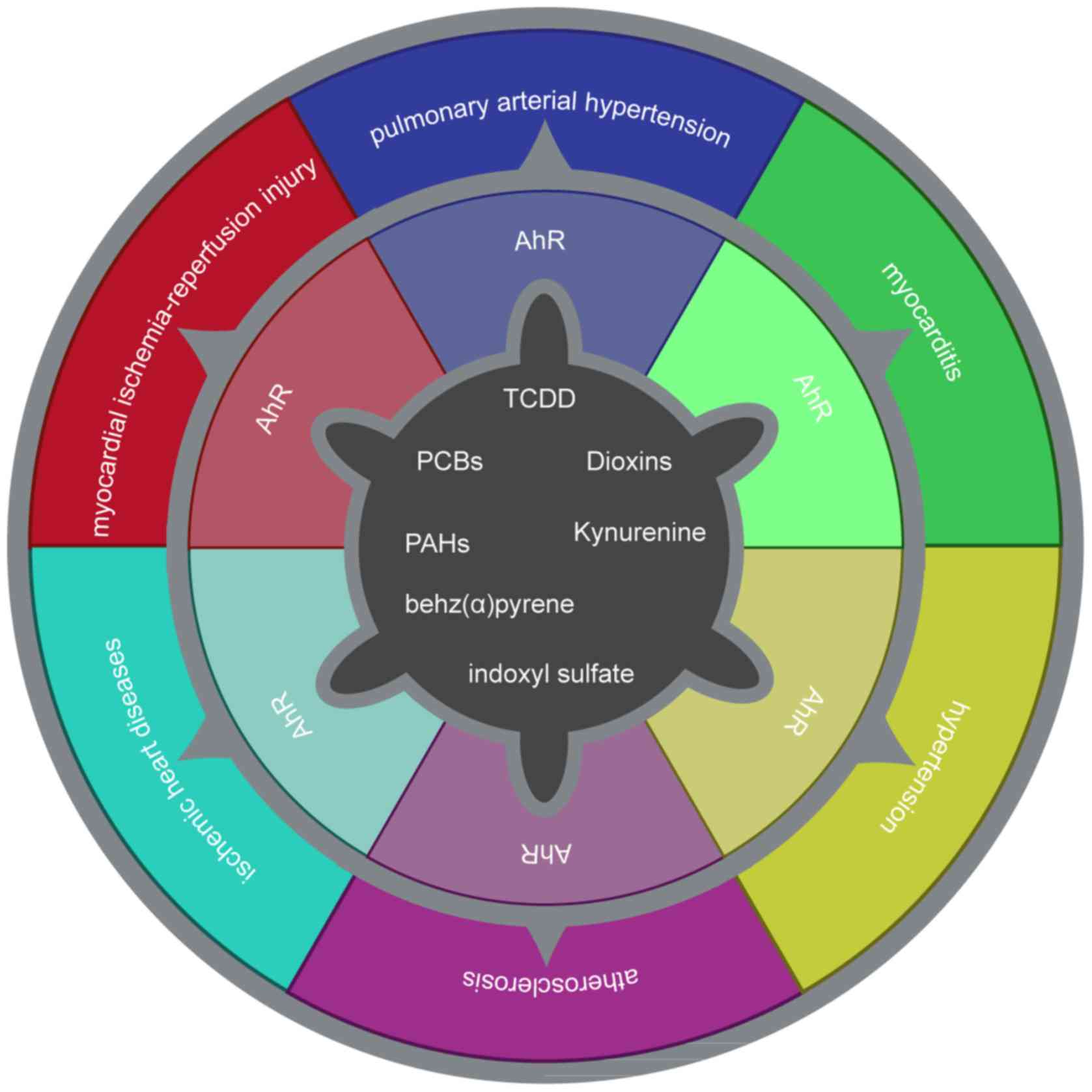
|
1
|
Conney AH, Miller EC and Miller JA: The
metabolism of methylated aminoazo dyes v. evidence for induction of
enzyme synthesis in the rat by 3-methylcholanthre. Cancer Res.
16:450–459. 1956.PubMed/NCBI
|
|
2
|
Hu B, Huang H, Wei Q, Ren M, Mburu DK,
Tian X and Su J: Transcription factors CncC/Maf and AhR/ARNT
coordinately regulate the expression of multiple GSTs conferring
resistance to chlorpyrifos and cypermethrin in spodoptera exigua.
Pest Manag Sci. 75:2009–2019. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higgins LG and Hayes JD: Mechanisms of
induction of cytosolic and microsomal glutathione transferase (GST)
genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev.
43:92–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Anna JS, Leggieri LR, Arias Darraz L,
Cárcamo JG, Venturino A and Luquet CM: Effects of sequential
exposure to water accommodated fraction of crude oil and
chlorpyrifos on molecular and biochemical biomarkers in rainbow
trout. Comp Biochem Physiol C Toxicol Pharmacol. 212:47–55. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davisson MT: Rules and guidelines for
genetic nomenclature in mice: Excerpted version. Transgenic Res.
6:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baricza E, Tamási V, Marton N, Buzás EI
and Nagy G: The emerging role of aryl hydrocarbon receptor in the
activation and differentiation of Th17 cells. Cell Mol Life Sci.
73:95–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt JV and Bradfield CA: Ah receptor
signaling pathways. Annu Rev Cell Dev Biol. 12:55–89. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Go RE, Hwang KA and Choi KC: Cytochrome
P450 1 family and cancers. J Steroid Biochem Mol Biol. 147:24–30.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang N: The role of endogenous aryl
hydrocarbon receptor signaling in cardiovascular physiology. J
Cardiovasc Dis Res. 2:91–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Curran CP, Altenhofen E, Ashworth A, Brown
A, Kamau-Cheggeh C, Curran M, Evans A, Floyd R, Fowler J, Garber H,
et al: AhrdCyp1a2(−/−) mice show increased
susceptibility to PCB-induced developmental neurotoxicity.
Neurotoxicology. 33:1436–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bock KW: From TCDD-mediated toxicity to
searches of physiologic AHR functions. Biochem Pharmacol.
155:419–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian Y, Rabson AB and Gallo MA: Ah
receptor and NF-κB interactions: Mechanisms and physiological
implications. Chem Biol Interact. 141:97–115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stejskalova L, Dvorak Z and Pavek P:
Endogenous and exogenous ligands of aryl hydrocarbon receptor:
Current state of art. Curr Drug Metab. 12:198–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamas B, Natividad JM and Sokol H: Aryl
hydrocarbon receptor and intestinal immunity. Mucosal Immunol.
11:1024–1038. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wakamatsu T, Yamamoto S, Ito T, Sato Y,
Matsuo K, Takahashi Y, Kaneko Y, Goto S, Kazama JJ, Gejyo F and
Narita I: Indoxyl sulfate promotes macrophage IL-1β production by
activating aryl hydrocarbon receptor/NF-κ/MAPK cascades, but the
NLRP3 inflammasome was not activated. Toxins (Basel). 10:E1242018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishiumi S, Yamamoto N, Kodoi R, Fukuda I,
Yoshida K and Ashida H: Antagonistic and agonistic effects of
indigoids on the transformation of an aryl hydrocarbon receptor.
Arch Biochem Biophys. 470:187–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peter Guengerich F, Martin MV, McCormick
WA, Nguyen LP, Glover E and Bradfield CA: Aryl hydrocarbon receptor
response to indigoids in vitro and in vivo. Arch Biochem Biophys.
423:309–316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Li Y, Sun R, Zhou S, Li M, Feng M
and Xie Y: Dual character of flavonoids in attenuating and
aggravating ischemia-reperfusion-induced myocardial injury. Exp
Ther Med. 14:1307–1314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hubbard TD, Murray IA and Perdew GH:
Indole and tryptophan metabolism: Endogenous and dietary routes to
Ah Receptor activation. Drug Metab Dispos. 43:1522–1535. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kewley RJ, Whitelaw ML and Chapman-Smith
A: The mammalian basic helix-loop-helix/PAS family of
transcriptional regulators. Int J Biochem Cell Biol. 36:189–204.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bock KW: Human AHR functions in vascular
tissue: Pro- and anti-inflammatory responses of AHR agonists in
atherosclerosis. Biochem Pharmacol. 159:116–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidt JV, Su GH, Reddy JK, Simon MC and
Bradfield CA: Characterization of a murine ahR null allele:
Involvement of the Ah receptor in hepatic growth and development.
Proc Natl Acad Sci USA. 93:6731–6736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu CX, Wang C, Zhang ZM, Jaeger CD, Krager
SL, Bottum KM, Liu J, Liao DF and Tischkau SA: Aryl hydrocarbon
receptor deficiency protects mice from diet-induced adiposity and
metabolic disorders through increased energy expenditure. Int J
Obes (Lond). 39:1300–1309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gutiérrez-Vázquez C and Quintana FJ:
Regulation of the immune response by the aryl hydrocarbon receptor.
Immunity. 48:19–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brinchmann BC, Skuland T Rambøl MH, Szoke
K, Brinchmann JE, Gutleb AC, Moschini E, Kubátová A, Kukowski K, Le
Ferrec E, et al: Lipophilic components of diesel exhaust particles
induce Pro-inflammatory responses in human endothelial cells
through AhR dependent pathway(s). Part Fibre Toxicol. 15:212018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Liu Q, Guo S, Zhang Q, Tang J, Liu
G, Kong D, Li J, Yan S, Wang R, et al:
2,3,7,8-Tetrachlorodibenzo-p-dioxin promotes endothelial cell
apoptosis through activation of EP3/p38MAPK/Bcl-2 pathway. J Cell
Mol Med. 21:3540–3551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan ZQ and Hansson GK: Innate immunity,
macrophage activation, and atherosclerosis. Immunol Rev.
219:187–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vogel CF, Khan EM, Leung PS, Gershwin ME,
Chang WL, Wu D, Haarmann-Stemmann T, Hoffmann A and Denison MS:
Cross-talk between aryl hydrocarbon receptor and the inflammatory
response: A role for nuclear factor-κB. J Biol Chem. 289:1866–1875.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vogel CF, Sciullo E, Wong P, Kuzmicky P,
Kado N and Matsumura F: Induction of proinflammatory cytokines and
C-reactive protein in human macrophage cell line U937 exposed to
air pollution particulates. Environ Health Perspect. 113:1536–1541.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ledda A, González M, Gulfo J, Díaz
Ludovico I, Ramella N, Toledo J, Garda H, Grasa M and Esteve M:
Decreased OxLDL uptake and cholesterol efflux in THP1 cells
elicited by cortisol and by cortisone through 11β-hydroxysteroid
dehydrogenase type 1. Atherosclerosis. 250:84–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Y, Du KL, Guo PY, Zhao RM, Wang B,
Zhao XL and Zhang CQ: IL-16 regulates macrophage polarization as a
target gene of mir-145-3p. Mol Immunol. 107:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sieve I, Ricke-Hoch M, Kasten M, Battmer
K, Stapel B, Falk CS, Leisegang MS, Haverich A Scherr M and
Hilfiker-Kleiner D: A positive feedback loop between IL-1β, LPS and
NEU1 may promote atherosclerosis by enhancing a pro-inflammatory
state in monocytes and macrophages. Vascul Pharmacol. 103:16–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdolmaleki F, Gheibi Hayat SM, Bianconi
V, Johnston TP and Sahebkar A: Atherosclerosis and immunity: A
perspective. Trends Cardiovasc Med. 28:S1050–1738. 2018.(Epub ahead
of print).
|
|
35
|
Ohtsuka M, Miyashita Y and Shirai K:
Lipids deposited in human atheromatous lesions induce apoptosisof
human vascular smooth muscle cells. J Atheroscler Thromb.
13:256–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baumer Y, McCurdy S, Weatherby TM, Mehta
NN, Halbherr S, Halbherr P, Yamazaki N and Boisvert WA:
Hyperlipidemia-induced cholesterol crystal production by
endothelial cells promotes atherogenesis. Nat Commun. 8:11292017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barnes MJ and Farndale RW: Collagens and
atherosclerosis. Exp Gerontol. 34:513–525. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chitra P, Saiprasad G, Manikandan R and
Sudhandiran G: Berberine inhibits smad and non-smad signaling
cascades and enhances autophagy against pulmonary fibrosis. J Mol
Med (Berl). 93:1015–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ke S, Rabson AB, Germino JF, Gallo MA and
Tian Y: Mechanism of suppression of cytochrome P-4501A1 expression
by tumor necrosis Factor-α and lipopolysaccharide. J Biol Chem.
276:39638–39644. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aung HH, Lame MW, Gohil K, He G, Denison
MS, Rutledge JC and Wilson DW: Comparative gene responses to
collected ambient particles in vitro: Endothelial responses.
Physiol Genomics. 43:917–929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vogel CF and Matsumura F: A new cross-talk
between the aryl hydrocarbon receptor and RelB, a member of the
NF-ĸB family. Biochem Pharmacol. 77:734–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vogel CF, Sciullo E, Li W, Wong P,
Lazennec G and Matsumura F: RelB, a new partner of aryl hydrocarbon
receptor-mediated transcription. Mol Endocrinol. 21:2941–2955.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang C, Petriello MC, Zhu B and Hennig B:
PCB 126 induces monocyte/macrophage polarization and inflammation
through ahR and NF-κB pathways. Toxicol Appl Pharmacol. 367:71–81.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hennig B, Meerarani P, Slim R, Toborek M,
Daugherty A, Silverstone AE and Robertson LW: Proinflammatory
properties of coplanar PCBs: In vitro and in vivo evidence. Toxicol
Appl Pharmacol. 181:174–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yisireyili M, Saito S, Abudureyimu S,
Adelibieke Y, Ng HY, Nishijima F, Takeshita K, Murohara T and Niwa
T: Indoxyl sulfate-induced activation of (Pro)renin receptor
promotes cell proliferation and tissue factor expression in
vascular smooth muscle cells. PLoS One. 9:e1092682014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hennig B, Hammock BD, Slim R, Toborek M,
Saraswathi V and Robertson LW: PCB-induced oxidative stress in
endothelial cells: Modulation by nutritients. Int J Hyg Environ
Health. 205:95–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Watanabe I, Tatebe J, Namba S, Koizumi M,
Yamazaki J and Morita T: Activation of aryl hydrocarbon receptor
mediates indoxyl sulfate-induced monocyte chemoattractant Protein-1
expression in human umbilical vein endothelial Cells. Circ J.
77:224–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Puga A, Barnes SJ, Chang C, Zhu H, Nephew
KP, Khan SA and Shertzer HG: Activation of transcription factors
activator protein-1 and nuclear factor-κB by
2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Pharmacol.
59:997–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chistiakov DA, Melnichenko AA, Myasoedova
VA, Grechko AV and Orekhov AN: Mechanisms of foam cell formation in
atherosclerosis. J Mol Med. 95:1153–1165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chan DC, Watts F, Ooi EM, Ji J, Johnson AG
and Barrett PH: Atorvastatin and fenofibrate have comparable
effects on VLDL-Apolipoprotein C-III kinetics in men with the
metabolic syndrome. Arterioscler Thromb Vasc Biol. 28:1831–1837.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mesnage R, Biserni M, Balu S, Frainay C,
Poupin N, Jourdan F, Wozniak E, Xenakis T, Mein CA and Antoniou MN:
Integrated transcriptomics and metabolomics reveal signatures of
lipid metabolism dysregulation in hepaRG liver cells exposed to PCB
126. Arch Toxicol. 92:2533–2547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Podechard N, Le Ferrec E, Rebillard A,
Fardel O and Lecureur V: NPC1 repression contributes to lipid
accumulation in human macrophages exposed to environmental aryl
hydrocarbons. Cardiovasc Res. 82:361–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ambolet-Camoit A, Ottolenghi C, Leblanc A,
Kim MJ, Letourneur F, Jacques S, Cagnard N, Guguen-Guillouzo C,
Barouki R and Aggerbeck M: Two persistent organic pollutants which
act through different xenosensors (alpha-endosulfan and 2,3,7,8
tetrachlorodibenzo-p-dioxin) interact in a mixture and downregulate
multiple genes involved in human hepatocyte lipid and glucose.
Biochimie. 116:79–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yao L, Wang C, Zhang X, Peng L, Liu W,
Zhang X, Liu Y, He J, Jiang C, Ai D and Zhu Y: Hyperhomocysteinemia
activates the aryl hydrocarbon receptor/CD36 pathway to promote
hepatic steatosis in mice. Hepatology. 64:92–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Van Breda SGJ, Claessen SMH, van Herwijnen
M, Theunissen DHJ, Jennen DGJ, de Kok TMCM and Kleinjans JCS:
Integrative omics data analyses of repeated dose toxicity of
valproic acid in vitro reveal new mechanisms of steatosis
induction. Toxicology. 393:160–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang L, Hatzakis E, Nichols RG, Hao R,
Correll J, Smith PB, Chiaro CR, Perdew GH and Patterson AD:
Metabolomics reveals that aryl hydrocarbon receptor activation by
environmental chemicals induces systemic metabolic dysfunction in
mice. Environ Sci Technol. 49:8067–8077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lu P, Yan J, Liu K, Garbacz WG, Wang P, Xu
M, Ma X and Xie W: Activation of aryl hydrocarbon receptor
dissociates fatty liver from insulin resistance by inducing FGF21.
Hepatology. 61:1908–1919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kurtz CL, Fannin EE, Toth CL, Pearson DS,
Vickers KC and Sethupathy P: Inhibition of miR-29 has a significant
lipid-lowering benefit through suppression of lipogenic programs in
liver. Sci Rep. 5:129112015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wahlang B, Falkner KC, Gregory B, Ansert
D, Young D, Conklin DJ, Bhatnagar A, McClain CJ and Cave M:
Polychlorinated biphenyl 153 is a diet-dependent obesogen that
worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J
Nutr Biochem. 24:1587–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Han SG, Han SS, Toborek M and Hennig B:
EGCG protects endothelial cells against PCB 126-induced
inflammation through inhibition of AhR and induction of
Nrf2-regulated genes. Toxicol Appl Pharmacol. 261:181–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Z, Yang H, Ramesh A, Roberts LJ, Zhou
L, Lin X, Zhao Y and Guo Z: Overexpression of Cu/Zn-superoxide
dismutase and/or catalase accelerates benzo(a)pyrene detoxification
by upregulation of the aryl hydrocarbon receptor in mouse
endothelial cells. Free Radic Biol Med. 47:1221–1229. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim JB, Pjanic M, Nguyen T, Miller CL,
Iyer D, Liu B, Wang T, Sazonova O, Carcamo-Orive I, Matic LP, et
al: TCF21 and the environmental sensor aryl-hydrocarbon receptor
cooperate to activate a pro-inflammatory gene expression program in
coronary artery smooth muscle cells. PLoS Genet. 13:e10067502017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li X, Liu N, Wang Y, Liu J, Shi H, Qu Z,
Du T, Guo B and Gu B: Brain and muscle aryl hydrocarbon receptor
nuclear translocator-like protein-1 cooperates with glycogen
synthase kinase-3β to regulate osteogenesis of bone-marrow
mesenchymal stem cells in type 2 diabetes. Mol Cell Endocrinol.
440:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wnuk A and Kajta M: Steroid and xenobiotic
receptor signalling in apoptosis and autophagy of the nervous
system. Int J Mol Sci. 18:E23942017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: American heart association council on
epidemiology and prevention statistics committee and stroke
statistics subcommittee: Heart disease and stroke statistics-2018
update: A report from the american heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zou JG, Ma YT, Xie X, Yang YN, Pan S, Adi
D, Liu F and Chen BD: The association between CYP1A1 genetic
polymorphisms and coronary artery disease in the uygur and han of
China. Lipids Health Dis. 13:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Taspinar M, Aydos S, Sakiragaoglu O, Duzen
IV, Yalcinkaya A, Oztuna D, Bardakci H, Tutar E and Sunguroglu A:
Impact of genetic variations of the CYP1A1, GSTT1, and GSTM1 genes
on the risk of coronary artery disease. DNA Cell Biol. 31:211–218.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Manfredi S, Federici C, Picano E, Botto N,
Rizza A and Andreassi MG: GSTM1, GSTT1 and CYP1A1 detoxification
gene polymorphisms and susceptibility to smoking-related coronary
artery disease: A case-only study. Mutat Res. 621:106–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huang S, Shui X, He Y, Xue Y, Li J, Li G,
Lei W and Chen C: AhR expression and polymorphisms are associated
with risk of coronary arterial disease in Chinese population. Sci
Rep. 5:80222015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Peng DD, Xie W and Yu ZX: Impact of
interaction between CYP1A1 genetic polymorphisms and smoking on
coronary artery disease in the han of China. Clin Exp Hypertens.
39:339–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang X, Lv S, Guo C, Shi C, Chi Y, Zhao
L, Wang G and Wang Z: Gene-gene interaction between PPARG and
CYP1A1 gene on coronary artery disease in the Chinese han
population. Oncotarget. 8:34398–34404. 2017.PubMed/NCBI
|
|
72
|
Zhang ZX and Zhang Y: Glutathione
S-transferase M1 (GSTM1) null genotype and coronary artery disease
risk: A meta-analysis. Int J Clin Exp Med. 7:3378–3384.
2014.PubMed/NCBI
|
|
73
|
Pašalić D and Marinković N: Genetic
polymorphisms of the CYP1A1, GSTM1, and GSTT1 enzymes and their
influence on cardiovascular risk and lipid profile in people who
live near a natural gas plant. Arh Hig Rada Toksikol. 68:46–52.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mir R, Bhat MA, Javaid J, Shah N, Kumar P,
Sharma E, Jhu C, Basak S, Amle D, Ray PC, et al: Glutathione
S-transferase M1 and T1 (rs4025935 and rs71748309) null genotypes
are associated with increased susceptibility to coronary artery
disease in Indian Populations. Acta Cardiol. 71:678–684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bhat MA and Gandhi G: Association of GSTT1
and GSTM1 gene polymorphisms with coronary artery disease in north
Indian punjabi population: A case-control study. Postgrad Med J.
92:701–706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ramprasath T, Senthil Murugan P,
Prabakaran AD, Gomathi P, Rathinavel A and Selvam GS: Potential
risk modifications of GSTT1, GSTM1 and GSTP1
(glutathione-S-transferases) variants and their association to CAD
in patients with type-2 diabetes. Biochem Biophys Res Commun.
407:49–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kim SJ, Kim MG, Kim KS, Song JS, Yim SV
and Chung JH: Impact of glutathione s-transferase M1 and T1 gene
polymorphisms on the smoking-related coronary artery disease. J
Korean Med Sci. 23:365–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Manfredi S, Calvi D, del Fiandra M, Botto
N, Biagini A and Andreassi MG: Glutathione S-transferase T1- and
M1-null genotypes and coronary artery disease risk in patients with
type 2 diabetes mellitus. Pharmacogenomics. 10:29–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang LS, Tang JJ, Tang NP, Wang MW, Yan
JJ, Wang QM, Yang ZJ and Wang B: Association of GSTM1 and GSTT1
gene polymorphisms with coronary artery disease in relation to
tobacco smoking. Clin Chem Lab Med. 46:1720–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Masetti S, Botto N, Manfredi S, Colombo
MG, Rizza A, Vassalle C, Clerico A, Biagini A and Andreassi MG:
Interactive effect of the glutathione S-transferase genes and
cigarette smoking on occurrence and severity of coronary artery
risk. J Mol Med (Berl). 81:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kalpana C, Rajasekharan KN and Menon VP:
Modulatory effects of curcumin and curcumin analog on circulatory
lipid profiles during nicotine-induced toxicity in wistar rats. J
Med Food. 8:246–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li ZZ, Guo ZZ, Zhang Z, Cao QA, Zhu YJ,
Yao HL, Wu LL and Dai QY: Nicotine-induced upregulation of VCAM-1,
MMP-2, and MMP-9 through the α7-nAChR-JNK pathway in RAW264.7 and
MOVAS cells. Mol Cell Biochem. 399:49–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Abdel Fattah S, Rizk AAE, Motawie AG, Abd
El-Galil TI and El Sebaie M: Effects of nicotine on rat adrenal
gland: Crosstalk between oxidative and inflammatory markers, and
amelioration by melatonin. Biotech Histochem. 19:1–10. 2018.
|
|
84
|
O'Donnel MJ, Chin SL, Rangarajan S, Xavier
D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapary S, et
al: Global and regional effects of potentially modifiable risk
factors associated with acute stroke in 32 countries (INTERSTROKE):
A case control study. Lancet. 388:761–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhu H, Li Z, Lv J and Zhao R: Effects of
cerebral small vessel disease on the outcome of patients with
ischemic stroke caused by large artery atherosclerosis. Neurol Res.
40:381–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Moon KS, Lee HJ, Hong SH, Kim HM and Um
JY: CYP1A1 and GSTM1/T1 genetic variation in predicting risk for
cerebral infarction. J Mol Neurosci. 32:155–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Demirdöğen BC, Adali AÇ, Bek S, Demirkaya
Ş and Adali O: Cytochrome P4501A1 genotypes and smoking- and
hypertension-related ischemic stroke risk. Hum Exp Toxicol.
32:483–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sultana S, Kolla VK, Peddireddy V,
Jeedigunta Y, Penagaluru PK, Joshi S, Penagaluru UR and Penagaluru
PR: Association of cyp1a1 gene polymorphism with ischemic stroke in
south Indian population. Transl Stroke Res. 2:26–32. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang M, Wu JM, Zhang QS, Yan DW, Ren LJ
and Li WP: The association of CYP1A1 genetic polymorphisms and
additional gene-gene interaction with ischemic stroke in the
eastern han of China. Neurol Sci. 37:1679–1684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sindhu S, Akhter N, Kochumon S, Thomas R,
Wilson A, Shenouda S, Tuomilehto J and Ahmad R: Increased
expression of the innate immune receptor TLR10 in obesity and
Type-2 diabetes: Association with ROS-mediated oxidative stress.
Cell Physiol Biochem. 45:575–590. 2018. View Article : Google Scholar
|
|
91
|
Raza ST, Abbas S, Ahmad A, Ahmed F, Zaidi
Zh and Mahdi F: Association of glutathione-s-transferase (GSTM1 and
GSTT1) and FTO gene polymorphisms with type 2 diabetes mellitus
cases in northern India. Balkan J Med Genet. 17:47–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Etemad A, Vasudevan R, Aziz AF, Yusof AK,
Khazaei S, Fawzi N, Jamalpour S, Arkani M, Mohammad NA and Ismail
P: Analysis of selected glutathione S-transferase gene
polymorphisms in malaysian type 2 diabetes mellitus patients with
and without cardiovascular disease. Genet Mol Res. 15:2016.
View Article : Google Scholar :
|
|
93
|
Porojan MD, Bala C, Ilies R, Catana A,
Popp RA and Dumitrascu DL: Combined glutathione S transferase M1/T1
null genotypes is associated with type 2 diabetes mellitus. Clujul
Med. 88:159–163. 2015.PubMed/NCBI
|
|
94
|
Hori M, Oniki K, Ueda K, Goto S, Mihara S,
Marubayashi T and Nakagawa K: Combined glutathione S-transferase T1
and M1 positive genotypes afford protection against type 2 diabetes
in japanese. Pharmacogenomics. 8:1307–1314. 2007. View Article : Google Scholar : PubMed/NCBI
|