Introduction
Rheumatoid arthritis is a chronic autoinflammatory
disease characterized by chronic inflammation and bone damage
(1–4). Previous studies have demonstrated
that rheumatoid arthritis is associated with chronic inflammation
of synovial joints, hands and feet (5). Currently, targeted therapy is an
available treatment for patients with rheumatoid arthritis
(6–9). Numerous studies have demonstrated
that targeted therapy for rheumatoid arthritis decreases
inflammation, and many anti-inflammatory drugs have been used to
improve the prognosis of rheumatoid arthritis, such as
non-steroidal anti-inflammatory drugs, methotrexate,
glucocorticoid, infliximab, golimumab and adalimumab (10–14).
However, identifying the molecular signaling pathways underlying
inflammation is required to develop novel treatments for patients
with rheumatoid arthritis.
Although the causes underlying rheumatoid arthritis
are not fully understood, experimental and clinical evidence
suggest that interleukin (IL)-1β may serve an important role in the
pathogenesis of rheumatoid arthritis (15–17).
A previous study has demonstrated that the human anti-IL-1β
monoclonal antibody ACZ885 was effective in blocking inflammatory
responses in a mouse model of joint inflammation and in patients
with rheumatoid arthritis (18).
Theoretically, blocking the IL-1β pathway using specific anti-IL-1β
antibodies would suppress the inflammatory process, limiting joint
damage (19–21). In addition, patients with
rheumatoid arthritis present high circulating levels of
pro-inflammatory IL-1, and clinical trials have revealed that an
IL-1 antagonist presented beneficial effects in patients with
rheumatoid arthritis (22).
Furthermore, a previous study revealed that treatment with an IL-1
receptor antagonist was safe and well-tolerated, and was able to
regulate immune responses, thus providing clinical benefits
(23). ERK and STAT pathways have
been identified as potential molecular targets in the treatment of
rheumatoid arthritis (24–26). Additionally, NF-κB activity is
associated with the severity of rheumatoid arthritis and a
decreased response to infliximab (27). A previous study has reported that
synovial fluid-derived fibroblast-like synoviocytes (sfd-FLSs) can
be used as an in vitro model to evaluate the inflammatory
processes in rheumatoid arthritis (28). Therefore, understanding the role of
IL-1β signaling in sfd-FLSs may be crucial for an improved
understanding of rheumatoid arthritis. Previous studies
demonstrated that blocking NF-κB, ERK and STAT1 expression may be
beneficial for the treatment of human rheumatoid arthritis
(24,29,30).
Therefore, the present study investigated the expression levels of
NF-κB, ERK and STAT1 in sfd-FLSs to explore the role of IL-1β in
rheumatoid arthritis.
In the present study, the expression, the role and
the molecular mechanism underlying IL-1β in sfd-FLSs and in a rat
model of rheumatoid arthritis were investigated. The findings
identified that IL-1β was a pro-inflammatory factor upstream of
NF-κB, which regulated the ERK/STAT1 pathway in sfd-FLSs and in a
rat model of rheumatoid arthritis.
Materials and methods
Establishment of a rat model of
rheumatoid arthritis
A total of 30 8 week-old female Sprague Dawley rats
(200–250 g body weight) were purchased from The Experimental Animal
Center of Jinzhou Medical University (Jinzhou, China). All rats
were housed at 23±1°C, 50±5% humidity with a 12 h light/dark cycle
and free access to food and water. The induction of type II
collagen-induced arthritis was achieved as previously described
(31), by the subcutaneous
injection of 2 mg collagen (ModiQuest Research) per rat (n=10 in
each group). Rats were treated with IL-1β (10 mg/kg, Sigma-Aldrich;
Merck KGaA), PBS (control; equal volume) or anti-IL-1β (10 mg/kg,
ACZ885, Sigma-Aldrich; Merck KGaA) by subcutaneous injection every
4 days for a total of seven times.
Evaluation of arthritis
Rats were examined 28 days after collagen injection,
and an arthritis score was assigned to each rat. The arthritis
scores of experimental rats were evaluated using a scale of 0–2 for
each paw, with a maximum total score of 8, as previously described
(32). A score for each paw was
assigned as follows: 0, normal paw; 0.25, 1–2 swollen toes; 0.5,
3–4 swollen toes; 0.75, slightly swollen footpad or ankle; 1,
swollen footpad or ankle; 1.25, 1–2 swollen toes and swollen
footpad or ankle; and 2.0, swollen toes and swollen footpad and
ankle.
H&E staining
The tibias in experimental rats (n=5 per group) were
fixed in 4% paraformaldehyde for 24 h, decalcified in 10% EDTA (pH
= 7.4) for 5 days and embedded in paraffin. The tibias were cut
into 4 µm tissue sections and then stained with 1% haematoxylin and
eosin (H&E) for 15 min at room temperature. The tissue sections
were imaged using a light microscope (TE2000S; Nikon
Corporation).
ELISA
Blood samples were collected from all rats 28 days
after collagen injection. Samples were centrifuged at 4,000 × g for
15 min at 4°C. The circulating levels of TNF-α (cat. no. RTA00,
R&D Systems, Inc.) and IL-17 (cat. no. HS170, R&D Systems,
Inc.) were analyzed using ELISA kits according to the
manufacturer's protocol.
Immunohistochemical staining
Synovial membranes were collected from rats 28 days
after collagen injection. Tissues were fixed with 4%
paraformaldehyde at room temperature for 12 h. Paraffin-embedded
tissue samples of synovial membranes were obtained and cut into 4
µm sections, deparaffinized and rehydrated using a descending
alcohol series. Sections were prepared and epitope retrieval was
performed using Tris-HCl buffer (cat. no. AP-9005-050; Thermo
Fisher Scientific, Inc.) for 30 min at 37°C. Tissue sections were
stained H&E (Sigma-Aldrich) for 15 min at room temperature.
Sections were treated with 3% hydrogen peroxide for 15 min at 37°C
and subsequently blocked with 5% BSA (Sigma-Aldrich; Merck KGaA)
for 2 h at 37°C. Sections were washed with PBS and incubated with
rabbit anti-rat IL-17 (1:1,000; ab193955; Abcam), TNF-α (1:1,000;
ab109332; Abcam), ERK (1:1,000; ab32537; Abcam), phosphorylated ERK
(pERK; 1:1,000; ab201015; Abcam) and STAT1 (1:1,000; ab2071; Abcam)
at 4°C overnight. Sections were washed three times and incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin G (IgG; 1:2,000; cat. no. 1706515; Bio-Rad
Laboratories, Inc.) for 1 h at 37°C. Diaminobenzidine was used as
substrate for the immunohistochemical reaction. Tissue sections
were visualized at ×200 magnification using a confocal microscope
(LSM780; Carl Zeiss AG).
sfd-FLSs culture
The sfd-FLS line HIG-82 (American Type Culture
Collection cat. no. 1832) was purchased from BeNa Culture
Collection. sfd-FLSs were grown in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2. Cells were
treated with IL-1β (1 mg/ml; cat no. SRP6551; Sigma-Aldrich; Merck
KGaA), anti-IL-1β (1 mg/ml; cat no. PRS4877; Sigma-Aldrich; Merck
KGaA) and/or NF-κB inhibitor (1 mg/ml; cat no. 481412;
Sigma-Aldrich; Merck KGaA) for 12 h at 37°C for further
analysis.
Cells transfection
sfd-FLSs were seeded in 6-well plates at a density
of 1×104 cells/well in 2 ml RPMI-1640 supplemented with
10% FBS. Cells were cultured for 12 h and washed with PBS three
times. NF-κB cDNA was cloned into a pcDNA3.1 plasmid
(pcDNA3.1-NF-κB; Thermo Fisher Scientific, Inc.), and the empty
plasmid pcDNA3.1 (Thermo Fisher Scientific, Inc.) served as
control. sfd-FLSs were transfected with pcDNA3.1-NF-κB (5 µg) or
empty pcDNA3.1 (5 µg) using Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Cells were harvested after 72 h for further analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from sfd-FLSs using the
RNAeasy mini kit (Qiagen GmbH) according to manufacturer's
protocol. RNA was reverse transcribed into cDNA using the
QuantiTect Reverse Transcription Kit (Qiagen GmbH) at 42°C for 2 h
according to on the manufacturer's instrument. All forward and
reverse primers were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.) and are listed in Table I. qPCR was performed as follows:
Initial denaturation at 95°C for 2 min, followed by 45 cycles of
95°C for 30 sec, 59°C for 30 sec and 72°C for 30 sec. The total
volume of each reaction was 25 µl and contained 50 ng of cDNA, 200
µM dNTP, 2.5 units of Taq DNA polymerase (Takara Biotechnology,
Co., Ltd.) and 200 µM primers using the SYBR® Premix Ex
Taq™ kit (Takara Biotechnology, Co., Ltd.). Relative mRNA
expression levels were calculated using the 2−ΔΔCq
method (33). The results are
presented as fold-change relative to the expression level of
β-actin, used as internal control.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Gene symbol | Primer sequence
(5′-3′) |
|---|
| TNF-α | F:
CCCTCACACTCAGATCATCTTCT |
|
| R:
GCTACGACGTGGGCTACAG |
| IL-17 | F:
GGGCCTGGCTTCTGTCTGA |
|
| R:
AAGTTCGTTCTGCCCCATCA |
| NF-κB | F:
CACCCTCACCCTCCAACAAA |
|
| R:
TTCTCTTTCGTTCCCGGTGG |
| ERK | F:
TGGTCCAGGGGTCTTACTCC |
|
| R:
TAAAGCCATGCCAATCTC |
| STAT1 | F:
GCAGGTTCACCAGCTTTATGA |
|
| R:
TGAAGATTACGCTTGCTTTTCCT |
| β-actin | F:
CGGAGTCAACGGATTTGGTC |
|
| R:
AGCCTTCTCCATGGTCGTGA |
Western blotting
sfd-FLSs were homogenized using RIPA lysis buffer
(Thermo Fisher Scientific, Inc.). Protein concentration was
measured using a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.). Subsequently, protein samples (20 µg in each
lane) were separated by 12.5% SDS-PAGE. Protein were blotted on a
nitrocellulose membrane and the membranes were incubated with
primary antibodies anti-IL-17 (1:1,000; ab193955; Abcam), TNF-α
(1:1,000; ab109332; Abcam), ERK (1:1,000; ab32537; Abcam), pERK
(1:1,000; ab201015; Abcam), STAT1 (1:1,000; ab2071; Abcam), pSTAT1
(1:1,000; ab30645; Abcam) and β-actin (1:1,000; ab8226; Abcam) for
12 h at 4°C, after blocking with 5% BSA (Sigma-Aldrich; Merck KGaA)
for 1 h at 37°C. Subsequently, the membranes were incubated with
HRP-conjugated goat anti-rabbit IgG (1:5,000; cat. no. PV-6001;
OriGene Technologies, Inc.) for 24 h at 4°C. The blots were
visualized using an enhanced chemiluminescence detection system
(cat. no. 32209; Pierce; Thermo Fisher Scientific, Inc.).
Densitometric quantification was performed using Quantity-One
software (version 1.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± SD. Differences
were evaluated for significance using one-way ANOVA followed by
Tukey's post hoc test. Data were analyzed using GraphPad Prism
(version 6.0; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of anti-IL-1β on inflammation
and NF-κB-mediated ERK-STAT1 signaling =in a rat model of
rheumatoid arthritis
The effects of IL-1β and of an IL-1β inhibitory
antibody (anti-IL-1β) on inflammation were investigated in a rat
model of rheumatoid arthritis. The results suggested that treatment
with anti-IL-1β decreased the rheumatoid arthritis score, whereas
treatment with IL-1β exacerbated rheumatoid arthritis in
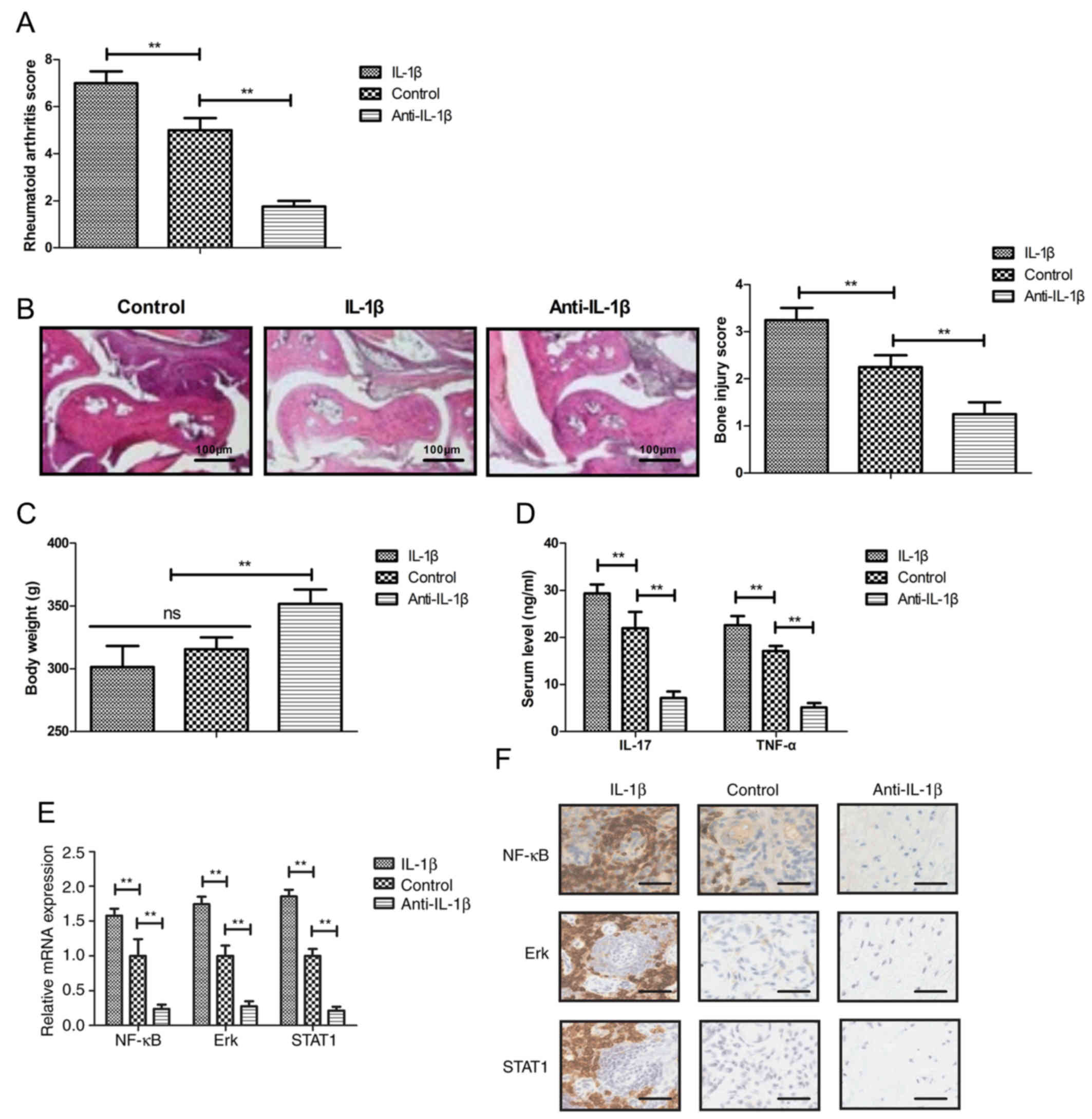
vivo (Fig. 1A).
Histopathological analysis demonstrated decreased synovial
hyperplasia and bone erosion in the anti-IL-1β group compared with
the control and IL-1β-treated groups. Treatment with anti-IL-1β
decreased the bone injury score, whereas IL-1β increased the bone
injury score compared with the control group (Fig. 1B). Treatment with anti-IL-1β
increased the total body weight compared with the control group
(Fig. 1C). Anti-IL-1β treatment
decreased the serum levels of IL-17 and TNF-α in the rheumatoid
arthritis rats, whereas IL-1β treatment increased the serum levels
of IL-17 and TNF-α (Fig. 1D).
Furthermore, the present results indicated that treatment with
anti-IL-1β downregulated the gene and protein expression levels of
NF-κB, ERK and STAT1, whereas treatment with IL-1β exhibited the
opposite effects (Fig. 1E and
F).
Anti-IL-1β downregulates the
expression levels of the inflammatory factors IL-17 and TNF-α in
sfd-FLSs
The effects of anti-IL-1β on the expression levels
of various inflammatory factors were analyzed in sfd-FLSs in
vitro. The results suggested that treatment with anti-IL-1β
decreased the mRNA and protein expression levels of the
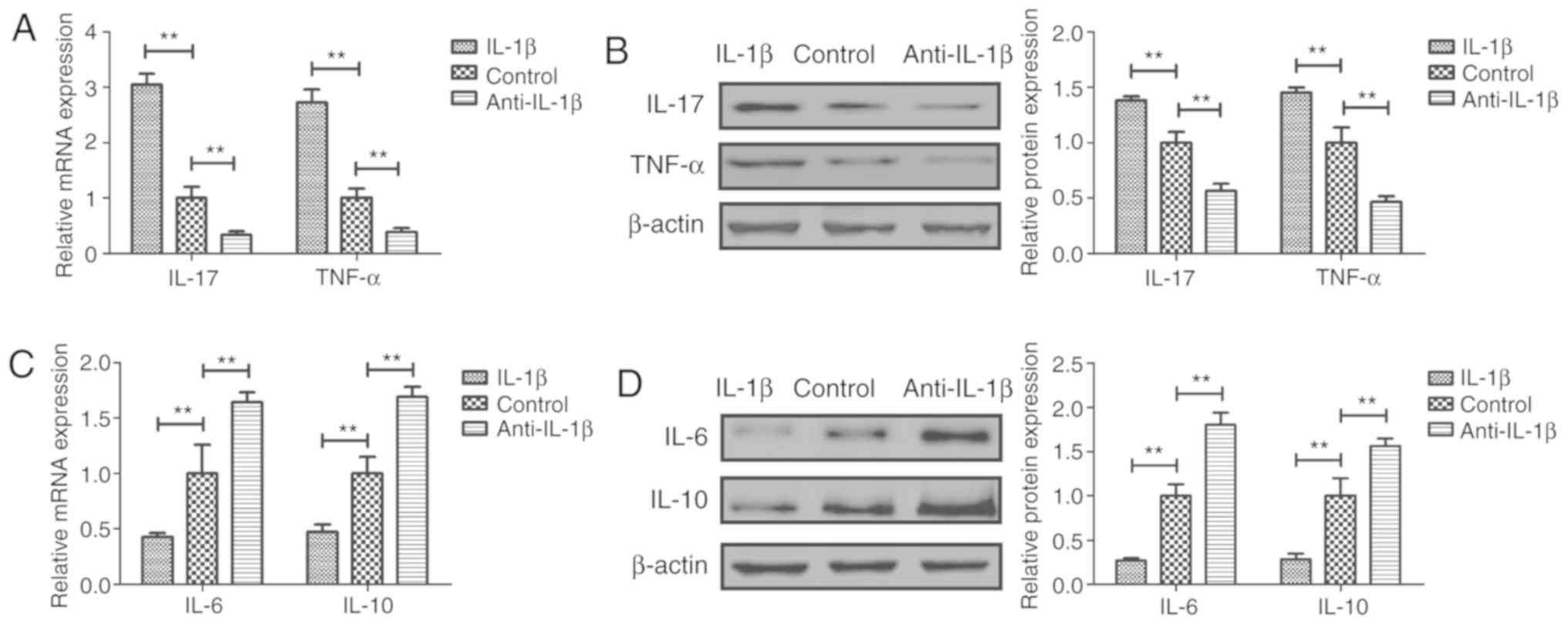
pro-inflammatory factors IL-17 and TNF-α in sfd-FLSs (Fig. 2A and B). By contrast, treatment
with anti-IL-1β increased the expression levels of the
anti-inflammatory factors IL-6 and IL-10 in sfd-FLSs (Fig. 2C and D). Treatment with IL-1β
exhibited the opposite effects (Fig.
2).
Anti-IL-1β downregulates the
NF-κB-mediated ERK/STAT1 pathway in sfd-FLSs
The effects of anti-IL-1β on the NF-κB-mediated
ERK/STAT1 signaling pathway were analyzed in sfd-FLSs in
vitro. The results indicated that treatment of sfd-FLSs with
anti-IL-1β decreased the mRNA expression levels and the protein
phosphorylation of NF-κB, ERK and STAT1 (Fig. 3A and B). Conversely, treatment with
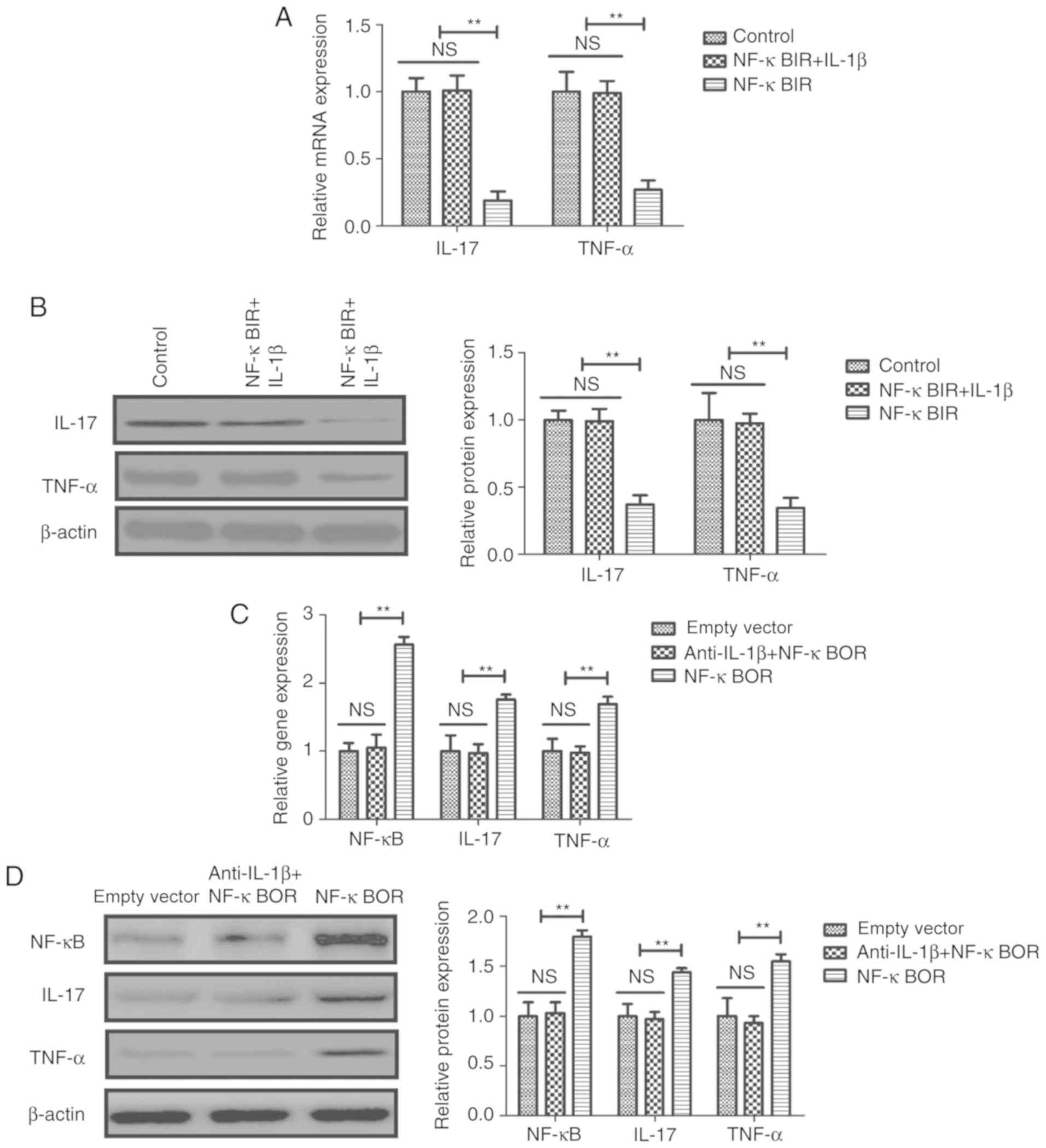
IL-1β exhibited the opposite effects (Fig. 3A and B). Treatment with an NF-κB
inhibitor (NF-κBIR) suppressed the IL-1β-mediated increase in the
mRNA expression levels of NF-κB, ERK and STAT1 in sfd-FLSs
(Fig. 3). Additionally, NF-κBIR
inhibited the IL-1β-mediated increase in pNF-κB/NF-κB, p-ERK/ERK
and pSTAT1/STAT1 protein expression ratios in sfd-FLSs (Fig. 3D). Conversely, NF-κB overexpression
(NF-κBOR) suppressed the anti-IL-1β-mediated decrease in the mRNA
expression and protein phosphorylation levels of NF-κB, ERK and
STAT1 (Fig. 3E and F).
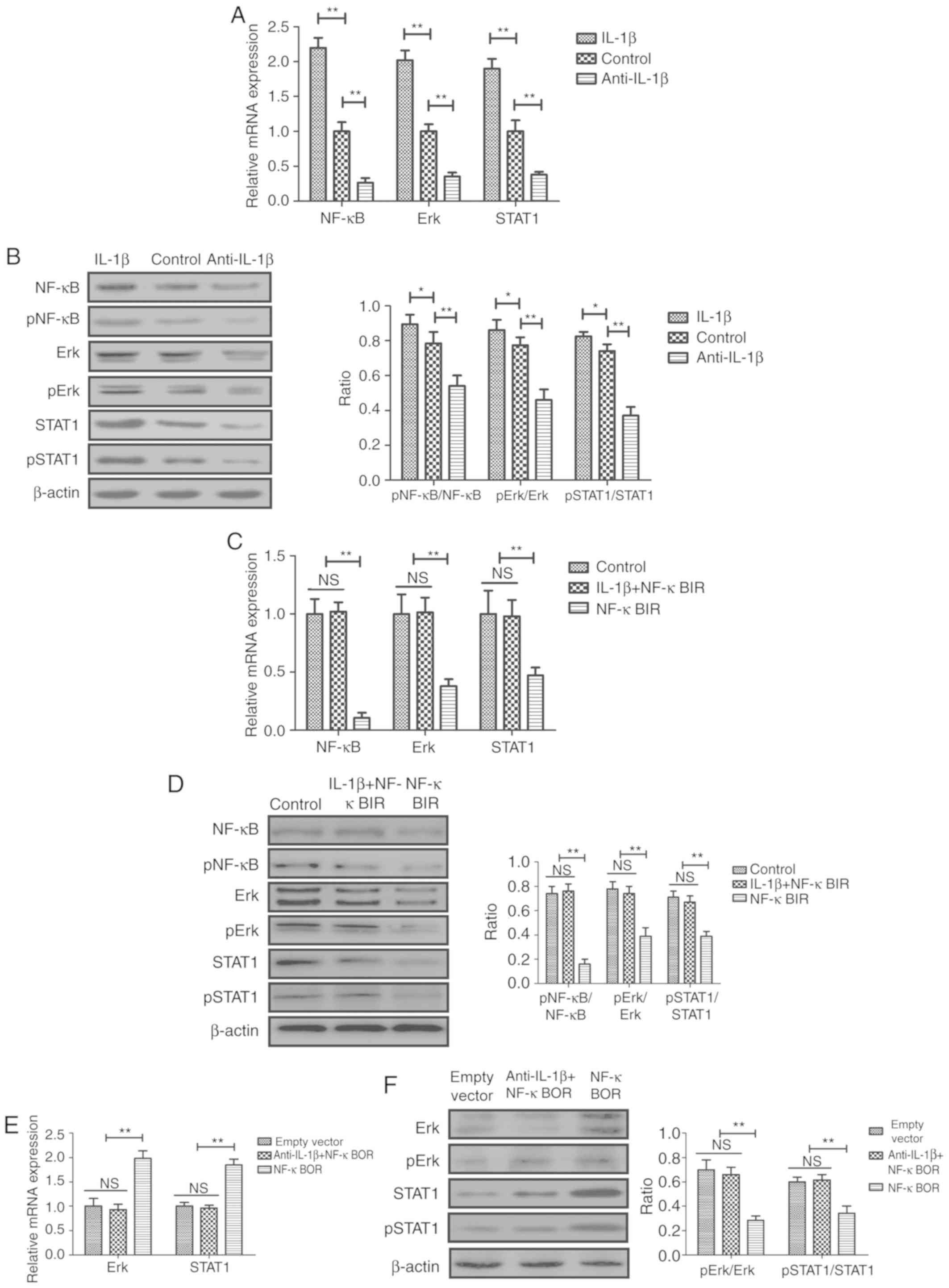 | Figure 3.Effects of IL-1β and anti-IL-1β on
the activity of the NF-κB-mediated ERK/STAT1 signaling pathway in
sfd-FLSs in vitro. (A) mRNA expression levels of NF-κB, ERK
and STAT1 in the different cell groups. (B) Protein expression
levels of phosphorylated and total NF-κB, ERK and STAT1 in the
different cell groups. (C) Effects of NF-κBIR on IL-1β-mediated
increase in the mRNA expression and the (D) protein phosphorylation
levels of NF-κB, ERK and STAT1 in sfd-FLSs. (E) Effects of NF-κBOR
on anti-IL-1β-mediated decrease in the mRNA expression and (F)
protein phosphorylation levels of ERK and STAT1 in sfd-FLSs.
*P<0.05 and **P<0.01, with comparisons indicated by brackets.
sfd-FLSs, synovial fluid-derived fibroblast-like synoviocytes;
NF-κBIR, NF-κB inhibitor; NF-κBOR, NF-κB overexpression; IL,
interleukin; p, phosphorylated; NS, not significant. |
IL-1β increases the expression levels
of inflammatory factors in sfd-FLSs via the NF-κB-mediated
ERK/STAT1 signaling pathway
The mechanism underlying IL-1β-mediated inflammation
was further investigated in sfd-FLSs. The results suggested that
NF-κB inhibition suppressed the IL-1β-mediated increase in the mRNA
and protein expression levels of IL-17 and TNF-α in sfd-FLSs
(Fig. 4A and B). Similarly, NF-κB
overexpression inhibited the anti-IL-1β-mediated decrease in the
mRNA and protein expression levels of NF-κB, IL-17 and TNF-α in
sfd-FLSs (Fig. 4C and D).
Discussion
Rheumatoid arthritis affects the function of joints
and tissues, which may lead to various pathological symptoms,
including fatigue, general discomfort and body weight loss
(34). A previous study has
demonstrated that NF-κB and various pro-inflammatory cytokines are
involved in the inflammation of the joints through multiple
signaling pathways both in vivo and in vitro
(35). In the present study, the
role of the pro-inflammatory cytokine IL-1β was investigated in
vitro, using sfd-FLSs, and in vivo, using a rat model of
rheumatoid arthritis. The present study suggested the importance of
the NF-κB-mediated ERK/STAT signaling pathway in rheumatoid
arthritis and revealed a novel mechanism by which IL-1β inhibition
ameliorated inflammatory factor expression through inhibition of
NF-κB in sfd-FLSs. The decrease in the activity of the ERK/STAT
pathway induced by anti-IL-1β was identified to protect rheumatoid
arthritis rat against arthritic inflammation, possibly by
inhibiting the IL-1β-mediated activation of the NF-κB signaling
pathway.
Elevated serum levels of IL-1β have been reported in
patients with rheumatoid arthritis (36). Decreasing the expression levels of
IL-1β could decrease inflammation and facilitate the treatment of
rheumatoid arthritis (37). The
present results suggested that inhibition of IL-1β using a IL-1β
blocking antibody decreased the mRNA and protein expression levels
of IL-17 and TNF-α in sfd-FLSs and in rat models of rheumatoid
arthritis. In vivo experiments suggested that blocking IL-1β
decreased the rheumatoid arthritis score, bone injury and increased
the body weight in rheumatoid arthritis rat. Although treatment
with IL-1β affected the serum levels of various cytokines and the
pathology of rheumatoid arthritis, it did not affect the body
weight of the animals. Notably, further experiments are required to
determine the cellular specificity of the protective effects of
anti-IL1β treatment by generating transgenic rodents presenting
cell-specific IL-1β inhibition.
In the present study it was hypothesized that the
inflammatory response induced by IL-1β was able to promote a
positive feedback loop leading to the upregulation of IL-17 and
TNF-α, which may be potential targets in the treatment of
rheumatoid arthritis. Previous studies have reported that the
expression levels of IL-6 and IL-10 are downregulated in patients
with rheumatoid arthritis (38–40).
The present data suggested that IL-1β decreased IL-6 and IL-10
expression, whereas anti-IL-1β increased IL-6 and IL-10 expression
in sfd-FLSs, which further indicated the therapeutic potential of
anti-IL-1β in treating rheumatoid arthritis. Inhibition of IL-6
modulated type III collagen and C-reactive protein degradation in
patients with rheumatoid arthritis exhibiting an inadequate
response to anti-TNF therapy (41). IL-6 is an independent predictive
factor of drug survival after dose escalation of infliximab in
patients with rheumatoid arthritis (38). Additionally, STAT3 increases the
expression level of IL-10 in a subset of regulatory B cells in
patients with rheumatoid arthritis (42), suggesting that IL-10 may promote
the occurrence and progression of rheumatoid arthritis (43). The present results suggested that
anti-IL-1β markedly upregulated IL-6 and IL-10 in sfd-FLSs,
suggesting that blocking IL-1β may have anti-inflammatory effects
that may be beneficial for the treatment of rheumatoid arthritis.
Notably, our results demonstrated that anti-IL-1β treatment
increased the total body weight compared with the control group,
which may suggest contributed to body weight loss of patients with
rheumatoid arthritis. The increased total body weight of
experimental animals in anti-IL-1B treatment may due to the
reduction of inflammation.
NF-κB signaling is essential for the development and
progression of rheumatoid arthritis (44). A previous study found that the ERK
signaling pathway served a central role in the initiation and
progression of rheumatoid arthritis and ERK inhibitors were
described as novel potential treatments for rheumatoid arthritis
(24). STAT1 expression is
increased in inflammatory arthritis, suggesting that its
pro-apoptotic and anti-inflammatory effects are not able to
effectively counteract inflammation (45–47).
In the present study, the mRNA and protein expression levels of
NF-κB, ERK and STAT1 were analyzed and the results suggested that
anti-IL-1β treatment downregulated NF-κB, ERK and STAT1 expression
in sfd-FLSs and in a rat model of rheumatoid arthritis. NF-κB
inhibitor suppressed IL-1β-mediated upregulation of IL-17 and TNF-α
in sfd-FLSs, whereas NF-κB overexpression suppressed
anti-IL-1β-mediated downregulation of IL-17 and TNF-α in sfd-FLSs.
In addition, NF-κB overexpression suppressed the
anti-IL-1β-mediated decrease in the mRNA expression and protein
phosphorylation levels of NF-κB, ERK and STAT1, indicating that
anti-IL-1β may regulate the ERK/STAT1 pathway by targeting NF-κB.
Therefore, the present results suggested that NF-κB may be involved
in the pathogenesis of IL-1β-induced rheumatoid arthritis mediated
by the ERK/STAT1 signal pathway, and that anti-IL-1β improved the
symptoms associated with rheumatoid arthritis by inhibiting the
NF-κB signaling pathway.
Collectively, systemic administration of anti-IL-1β
decreased arthritis severity and tissue inflammation in a rat model
of rheumatoid arthritis. In addition, IL-1β increased the
expression levels of inflammatory factors via the upregulation of
the NF-κB-mediated ERK/STAT1 signaling pathway. The present results
suggested that IL-1β may be a crucial inflammatory factor involved
in rheumatoid arthritis and that the NF-κB-mediated ERK/STAT1
signaling pathway may represent a potential therapeutic target for
the treatment of rheumatoid arthritis.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Xi'an Health
and Family Planning Commission (grant no. J20161008) and The Study
of Structural Changes of Subchondral Bone in Post-Traumatic
Arthritis In Rabbits (grant no. XA20170502).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY performed all experiments in the present study.
JW, XL, HZ, QM and BJ analyzed the experimental data. FT designed
the present study. JL performed the experiments and wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethic
Committee of Honghui Hospital, Xi'an Jiaotong University (approval
no. JS20160215X).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma J, Bhar S and Devi CS: A review on
interleukins: The Key manipulators in rheumatoid arthritis. Mod
Rheumatol. 27:723–746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lage-Hansen PR, Lindegaard H, Chrysidis S
and Terslev L: The role of ultrasound in diagnosing rheumatoid
arthritis, what do we know? An updated review. Rheumatol Int.
37:179–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tarp S, Furst DE, Dossing A, Østergaard M,
Lorenzen T, Hansen MS, Singh JA, Choy EH, Boers M, Suarez-Almazor
ME, et al: Defining the optimal biological monotherapy in
rheumatoid arthritis: A systematic review and meta-analysis of
randomised trials. Semin Arthritis Rheum. 46:699–708. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang M, Ren F, Zheng Y, Yan R, Huang W,
Xia N, Luo L, Zhou J and Tang L: Efficacy and safety of
down-titration versus continuation strategies of biological
disease-modifying anti-rheumatic drugs in patients with rheumatoid
arthritis with low disease activity or in remission: A systematic
review and meta-analysis. Clin Exp Rheumatol. 35:152–160.
2017.PubMed/NCBI
|
|
5
|
Haus E, Sackett-Lundeen L and Smolensky
MH: Rheumatoid arthritis: Circadian rhythms in disease activity,
signs and symptoms, and rationale for chronotherapy with
corticosteroids and other medications. Bull NYU Hosp Jt Dis. 70
(Suppl 1):S3–S10. 2012.
|
|
6
|
Steunebrink LM, Versteeg GA, Vonkeman HE,
Ten Klooster PM, Kuper HH, Zijlstra TR, van Riel PL and van de Laar
MA: Initial combination therapy versus step-up therapy in treatment
to the target of remission in daily clinical practice in early
rheumatoid arthritis patients: Results from the dream registry.
Arthritis Res Ther. 18:602016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernard NJ: Rheumatoid arthritis: Choline
kinase-more than a cancer therapy target? Nat Rev Rheumatol.
10:6992014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwata S, Nakayamada S, Fukuyo S, Kubo S,
Yunoue N, Wang SP, Yoshikawa M, Saito K and Tanaka Y: Activation of
syk in peripheral blood B cells in patients with rheumatoid
arthritis: A potential target for abatacept therapy. Arthritis
Rheumatol. 67:63–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernard NJ: Rheumatoid arthritis: Are
FcRL4+ B cells the next target for RA biologic therapy? Nat Rev
Rheumatol. 10:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pincus T and Castrejon I: Evidence that
the strategy is more important than the agent to treat rheumatoid
arthritis. Data from clinical trials of combinations of
non-biologic DMARDs, with protocol-driven intensification of
therapy for tight control or treat-to-target. Bull Hosp Jt Dis. 71
(Suppl 1):S33–S40. 2013.
|
|
11
|
Rabquer BJ and Koch AE: NK4 therapy: A new
approach to target angiogenesis and inflammation in rheumatoid
arthritis. Arthritis Res Ther. 15:1192013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakai R, Tanaka M, Nanki T, Watanabe K,
Yamazaki H, Koike R, Nagasawa H, Amano K, Saito K, Tanaka Y, et al:
Drug retention rates and relevant risk factors for drug
discontinuation due to adverse events in rheumatoid arthritis
patients receiving anticytokine therapy with different target
molecules. Ann Rheum Dis. 71:1820–1826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svanstrom H, Lund M, Melbye M and
Pasternak B: Concomitant use of low-dose methotrexate and NSAIDs
and the risk of serious adverse events among patients with
rheumatoid arthritis. Pharmacoepidemiol Drug Saf. 27:885–893. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Best JH, Kong AM, Lenhart GM, Sarsour K,
Stott-Miller M and Hwang Y: Association between glucocorticoid
exposure and healthcare expenditures for potential
glucocorticoid-related adverse events in patients with rheumatoid
arthritis. J Rheumatol. 45:320–328. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pasi S, Kant R, Gupta S and Surolia A:
Novel multimeric IL-1 receptor antagonist for the treatment of
rheumatoid arthritis. Biomaterials. 42:121–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang HQ, Liu XG, Yang X, Chen T and Yu SG:
Effect of different types of moxibustion intervention on expression
of inflammatory cytokines IL-1 and TNF-alpha in rabbits with
rheumatoid arthritis. Zhen Ci Yan Jiu. 38:134–139. 2013.(In
Chinese). PubMed/NCBI
|
|
17
|
Adachi M, Okamoto S, Chujyo S, Arakawa T,
Yokoyama M, Yamada K, Hayashi A, Akita K, Takeno M, Itoh S, et al:
Cigarette smoke condensate extracts induce IL-1-beta production
from rheumatoid arthritis patient-derived synoviocytes, but not
osteoarthritis patient-derived synoviocytes, through aryl
hydrocarbon receptor-dependent NF-kappa-B activation and novel
NF-kappa-B sites. J Interferon Cytokine Res. 33:297–307. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alten R, Gram H, Joosten LA, van den Berg
WB, Sieper J, Wassenberg S, Burmester G, van Riel P, Diaz-Lorente
M, Bruin GJ, et al: The human anti-IL-1 beta monoclonal antibody
ACZ885 is effective in joint inflammation models in mice and in a
proof-of-concept study in patients with rheumatoid arthritis.
Arthritis Res Ther. 10:R672008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalliolias GD and Liossis SN: The future
of the IL-1 receptor antagonist anakinra: From rheumatoid arthritis
to adult-onset Still's disease and systemic-onset juvenile
idiopathic arthritis. Expert Opin Investig Drugs. 17:349–359. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Settas LD, Tsimirikas G, Vosvotekas G,
Triantafyllidou E and Nicolaides P: Reactivation of pulmonary
tuberculosis in a patient with rheumatoid arthritis during
treatment with IL-1 receptor antagonists (anakinra). J Clin
Rheumatol. 13:219–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Botsios C, Sfriso P, Ostuni PA, Todesco S
and Punzi L: Efficacy of the IL-1 receptor antagonist, anakinra,
for the treatment of diffuse anterior scleritis in rheumatoid
arthritis. Report of two cases. Rheumatology (Oxford).
46:1042–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nikfar S, Saiyarsarai P, Tigabu BM and
Abdollahi M: Efficacy and safety of interleukin-1 antagonists in
rheumatoid arthritis: A systematic review and meta-analysis.
Rheumatol Int. 38:1363–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu X, He D, Deng S, Li W, Xi Y, Xie C,
Jiang T, Zhang JZ, Dong C and Chen G: Regulatory immune responses
induced by IL-1 receptor antagonist in rheumatoid arthritis. Mol
Immunol. 49:290–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohori M: ERK inhibitors as a potential new
therapy for rheumatoid arthritis. Drug News Perspect. 21:245–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Isomaki P, Junttila I, Vidqvist KL,
Korpela M and Silvennoinen O: The activity of JAK-STAT pathways in
rheumatoid arthritis: Constitutive activation of STAT3 correlates
with interleukin 6 levels. Rheumatology (Oxford). 54:1103–1113.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boyle DL, Soma K, Hodge J, Kavanaugh A,
Mandel D, Mease P, Shurmur R, Singhal AK, Wei N and Rosengren S:
The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT
signalling in rheumatoid arthritis. Ann Rheum Dis. 74:1311–1316.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Torices S, Julia A, Munoz P, Varela I,
Balsa A, Marsal S, Fernández-Nebro A, Blanco F, López-Hoyos M,
Martinez-Taboada V and Fernández-Luna JL: A functional variant of
TLR10 modifies the activity of NFkB and may help predict a worse
prognosis in patients with rheumatoid arthritis. Arthritis Res
Ther. 18:2212016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trzybulska D, Olewicz-Gawlik A, Sikora J,
Frydrychowicz M, Kolecka-Bednarczyk A, Kaczmarek M and Hrycaj P:
The effect of caveolin-1 knockdown on interleukin-1β-induced
chemokine (C-C motif) ligand 2 expression in synovial fluid-derived
fibroblast-like synoviocytes from patients with rheumatoid
arthritis. Adv Clin Exp Med. 27:1491–1497. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin G, Wang Y, Cen XM, Yang M, Liang Y and
Xie QB: Lipid peroxidation-mediated inflammation promotes cell
apoptosis through activation of NF-κB pathway in rheumatoid
arthritis synovial cells. Mediators Inflamm. 2015:4603102015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gordon RA, Grigoriev G, Lee A, Kalliolias
GD and Ivashkiv LB: The interferon signature and STAT1 expression
in rheumatoid arthritis synovial fluid macrophages are induced by
tumor necrosis factor alpha and counter-regulated by the synovial
fluid microenvironment. Arthritis Rheum. 64:3119–3128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakajima H, Takamori H, Hiyama Y and
Tsukada W: The effect of treatment with interferon-gamma on type II
collagen-induced arthritis. Clin Exp Immunol. 81:441–445. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saha S, Qi J, Wang S, Wang M, Li X, Kim
YG, Núñez G, Gupta D and Dziarski R: PGLYRP-2 and Nod2 are both
required for peptidoglycan-induced arthritis and local
inflammation. Cell Host Microbe. 5:137–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murray E, Ellis A, Butylkova Y, Skup M,
Kalabic J and Garg V: Systematic review and network meta-analysis:
Effect of biologics on radiographic progression in rheumatoid
arthritis. J Comp Eff Res. 7:959–974. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang QH, Lv SW, Guo YY, Duan JX, Dong SY,
Wang QS, Yu FM, Su H and Kuang HX: Pharmacological effect of
caulophyllum robustum on collagen-induced arthritis and regulation
of nitric oxide, NF-κB, and proinflammatory cytokines in vivo and
in vitro. Evid Based Complement Alternat Med. 2017:81343212017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruscitti P, Cipriani P, Cantarini L,
Liakouli V, Vitale A, Carubbi F, Berardicurti O, Galeazzi M,
Valenti M and Giacomelli R: Efficacy of inhibition of IL-1 in
patients with rheumatoid arthritis and type 2 diabetes mellitus:
Two case reports and review of the literature. J Med Case Rep.
9:1232015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chabaud M, Page G and Miossec P: Enhancing
effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory
protein-3alpha production in rheumatoid arthritis: Regulation by
soluble receptors and Th2 cytokines. J Immunol. 167:6015–6020.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takasugi K, Nishida K, Natsumeda M,
Yamashita M, Yamamoto W and Ezawa K: IL-6 is an independent
predictive factor of drug survival after dose escalation of
infliximab in patients with rheumatoid arthritis. Mod Rheumatol.
28:452–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamana J, Yamamura M, Okamoto A, Aita T,
Iwahashi M, Sunahori K and Makino H: Resistance to IL-10 inhibition
of interferon gamma production and expression of suppressor of
cytokine signaling 1 in CD4+ T cells from patients with rheumatoid
arthritis. Arthritis Res Ther. 6:R567–R577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sabry D, Elamir A, Mahmoud RH, Abdelaziz
AA and Fathy W: Role of LncRNA-AF085935, IL-10 and IL-17 in
rheumatoid arthritis patients with chronic Hepatitis C. J Clin Med
Res. 9:416–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Juhl P, Thudium CS, Gudmann NS, Karsdal
MA, Bay-Jensen AC and Siebuhr AS: IL-6 receptor inhibition
modulates type III collagen and C-reactive protein degradation in
rheumatoid arthritis patients with an inadequate response to
anti-tumour necrosis factor therapy: Analysis of connective tissue
turnover in the tocilizumab RADIATE study. Clin Exp Rheumatol.
36:568–574. 2018.PubMed/NCBI
|
|
42
|
Banko Z, Pozsgay J, Szili D, Tóth M, Gáti
T, Nagy G, Rojkovich B and Sármay G: Induction and differentiation
of IL-10-Producing regulatory B cells from healthy blood donors and
rheumatoid arthritis patients. J Immunol. 198:1512–1520. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ouyang BS, Che JL, Gao J, Zhang Y, Li J,
Yang HZ, Hu TY, Wu YJ and Yang M: Effects of electroacupuncture and
simple acupuncture on changes of IL-1, IL-4, IL-6 and IL-10 in
peripheral blood and joint fluid in patients with rheumatoid
arthritis. Zhongguo Zhen Jiu. 30:840–844. 2010.(In Chinese).
PubMed/NCBI
|
|
44
|
Li G, Xia Z, Liu Y, Meng F, Wu X, Fang Y,
Zhang C and Liu D: SIRT1 inhibits rheumatoid arthritis
fibroblast-like synoviocyte aggressiveness and inflammatory
response via suppressing NF-κB pathway. Biosci Rep.
38:BSR201805412018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Scheinman RI, Trivedi R, Vermillion S and
Kompella UB: Functionalized STAT1 siRNA nanoparticles regress
rheumatoid arthritis in a mouse model. Nanomedicine (Lond).
6:1669–1682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Walker JG, Ahern MJ, Coleman M, Weedon H,
Papangelis V, Beroukas D, Roberts-Thomson PJ and Smith MD:
Expression of Jak3, STAT1, STAT4, and STAT6 in inflammatory
arthritis: Unique Jak3 and STAT4 expression in dendritic cells in
seropositive rheumatoid arthritis. Ann Rheum Dis. 65:149–156. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kasperkovitz PV, Verbeet NL, Smeets TJ,
van Rietschoten JG, Kraan MC, van der Pouw Kraan TC, Tak PP and
Verweij CL: Activation of the STAT1 pathway in rheumatoid
arthritis. Ann Rheum Dis. 63:233–239. 2004. View Article : Google Scholar : PubMed/NCBI
|