Introduction
Promoting bone defect repair following trauma,
resection, or systemic inflammation is a great challenge for
physicians. Both autografts and allografts for large-scale bone
repair require invasive surgeries, and the recovery varies from one
patient to another. With the rapid development of tissue
engineering, adult stem cells have been revealed to accelerate bone
repair and improve outcomes (1–5).
Adipose-derived stem cells (ADSCs) possess the potential to
differentiate into multiple cell lineages including osteoblasts
(6,7). Notably, since ADSCs can be easily
accessed from fatty tissue and the procedure causes minimal
discomfort compared to procedures used to obtain other types of
adult stem cells, ADSCs are emerging as a competitive adult stem
cell source for bone defect repair (7–9).
Furthermore, surface markers of ADSCs have been well-characterized,
providing standardized protocols for their isolation and
characterization (10). More
importantly, ADSCs are capable of secreting trophic factors that
facilitate bone repair in situ (11,12).
However, it is critical to induce ADSCs with external cues to
initiate osteogenic differentiation and to simultaneously inhibit
non-osteogenic routes, such as adipogenic differentiation.
Osteogenic differentiation of ADSCs is elaborately
regulated by genetic networks and external stimuli. Bone
morphogenetic protein (BMP) signaling, extracellular
signal-regulated kinase (ERK) signaling, Wnt signaling, and Notch
signaling have all been revealed to be involved in the regulatory
network of ADSC differentiation (13–16).
Recently, several bio-active molecules were revealed to play roles
in regulating ADSC differentiation. A histone deacetylase inhibitor
was revealed to have a pro-osteogenic effect on rat ADSCs by
inducing histone hyper-acetylation at the promoter region of Runx2
(17), a well-characterized factor
that controls the osteogenic differentiation of ADSCs (18–20).
Notably, melatonin and vitamin D were demonstrated to inhibit
adipogenic differentiation of human ADSCs (21), further revealing that the
differentiation of ADSCs during clinical bone repair or other
clinical events can potentially be modulated by bio-active
molecules.
Exendin-4 is a biologically active peptide with a
length of 39 amino acids originally isolated from the venom of
Heloderma suspectum, the Gila monster lizard. It was
initially revealed to promote amylase release from the pancreatic
acini of both guinea pigs and rats. Exendin-4 belongs to the
glucagon superfamily of peptide hormones whose function can be
antagonized by the exendin receptor inhibitor exendin (9–39)
(22–24). The synthetic version of exendin-4,
exenatide, was approved in 2005 for treatment of type 2 diabetes by
improving glycemic control (25,26).
Notably, the murine receptor for exendin-4 is essential for the
control of bone resorption, as evidenced by GLP-1 receptor knockout
mice (27). Moreover, exendin-4
also exhibited a protective role in osteopenia by promoting bone
formation and inhibiting bone resorption (28,29).
Recently, accumulating evidence has demonstrated different roles of
exendin-4 in osteogenic differentiation. In MC3T3-E1 cells,
treatment with exendin-4 promoted proliferation and differentiation
by upregulating the phosphorylation level of the MAPK
(mitogen-activated protein kinase) signaling kinases ERK1/2, p38,
and JNK (30), suggesting a role
of exendin-4 in regulating osteogenic differentiation. Furthermore,
exendin-4 treatment could not only protect bone marrow stromal stem
cells (BMSCs) from mitogen-deprivation-induced apoptosis (31) but also promoted osteogenic
differentiation and inhibited adipogenic differentiation of BMSCs
by regulating PKA (Protein Kinase A)/β-catenin signaling (32). Notably, a recent study revealed
that exendin-4 inhibited lipopolysaccharide-induced osteoclast
formation and bone resorption via inhibition of TNF-α expression in
macrophages (33). Collectively,
the data indicate that exendin-4 holds great promise for promoting
osteogenic differentiation in adult stem cells and facilitating
bone repair.
In the present study, the role that exendin-4 plays
in ADSC-mediated bone defect repair in vivo was examined by
establishing a corresponding mouse bone defect model. In addition,
osteogenic and adipogenic differentiation of ADSCs both in
vivo and in vitro under exendin-4 supplementation were
investigated and it was revealed that exendin-4 promoted the
osteogenic differentiation of ADSCs. Moreover, the present results
also indicated that exendin-4 treatment increased the mRNA and
protein levels of genes related to osteogenic differentiation. The
present data further indicated the clinical potential of exendin-4
in improving the osteogenic differentiation of ADSCs, which holds
great promise for bone defect repair based on tissue
engineering.
Materials and methods
ADSC isolation
All animal experiments were performed in accordance
with the guidelines of the NIH (Publication no. 85e23 Rev. 1985)
and were approved by the Animal Care and Use Committee of The
Fourth Military Medical University. C57 mice were purchased from
the Animal Center of The Fourth Military Medical University, and
housed in an environmentally controlled room (20–25°C) with a 12 h
light/dark cycle and free access to food and water. Inguinal fat
pads from C57 black/DBA male mice (3 months) were finely minced and
digested with 0.2% collagenase type I in a 37°C shaking incubator
for 45 min. The digested tissue was filtered through a sterile
100-µm nylon mesh, centrifuged (300 × g at 37°C for 8 min),
resuspended, and cultured in regular growth medium, consisting of
α-minimum essential medium (α-MEM; HyClone; GE Healthcare Life
Sciences), 10% fetal bovine serum (FBS; Gibco BRL; Thermo Fisher
Scientific, Inc.), and penicillin/streptomycin (Sigma-Aldrich;
Merck KGaA). Cell cultures were maintained at 37°C in a humidified
incubator with 5% CO2. Passage 3 cells were used for
identifying ADSC phenotypes and for the following experiments.
Bone defect model
The mouse model of metaphyseal defect of the femur
was established as previously described (34). Briefly, C57 black/DBA male mice (3
months, weight 23–27 g, n=24) were used to establish the bone
defect model under anesthesia via an intraperitoneal injection of
300 mg/kg avertin (Sigma-Aldrich; Merck KGaA) (35,36).
Anesthetic depth was confirmed by dilated pupils, loss of pain,
loss of palpebral reflex and corneal reflexes present. The side
effect of avertin, intestinal ileus, was not observed in these
experiments. After making a 10-mm incision, a blunt 0.9 mm drill
was used to drill through the anterolateral cortical bone into the
metaphyseal cancellous bone to generate a round defect at the
supracondylar region of the right femur. The right hind limbs of
three-month-old wild-type animals were used as the control group.
Hydrogels combined with 3×105 ADSCs were injected into
the defective site after the operation. Exendin-4 (Sigma-Aldrich;
Merck KGaA) was administered intraperitoneally at 4.2 µg/kg/day, as
previously described (37). The
mice were sacrificed by cervical dislocation at day 60 following
the surgery.
Bone imaging
Femurs were removed from sacrificed mice for
micro-computed tomography (µCT) analysis and biomechanical testing.
The samples were scanned using the Explore Locus SPPre-clinical
Specimen Micro-CT (GE Healthcare Life Sciences), and the images
were reconstructed to an isotropic voxel size of 12 µm. All
three-dimensional (3D) image manipulations and analyses were
performed by the system software (MicroView, v.2.1; GE Healthcare
Life Sciences).
Histomorphometry and histology
Mice were injected intraperitoneally with 25 mg/kg
tetracycline and 5 mg/kg calcein (both from Sigma-Aldrich; Merck
KGaA) 10 days later for histomorphometric analysis. For hematoxylin
and eosin (H&E) staining, paraffin sections were prepared.
Four-micrometer-thick sections were stained with H&E (Sigma;
Merck KGaA; 37°C, 30 min) to count the adipocytes.
Immunohistochemistry
Briefly, serial paraffin sections (5 µm) were
prepared, deparaffinized, and incubated in 3%
H2O2 to block native peroxidases, followed by
incubation at room temperature for 30 min with non-immune animal
serum. Immune-histochemical reactions using antibodies reactive
against COL-1 (1:500, cat. no. ab109025; Abcam), CD29 (1:1,000,
cat. no. 102225), or Sca-1 (1:1,000, cat. no. 122504; both from
BioLegend, Inc.) were conducted at 4°C overnight, followed by
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:1,000, cat. no. ab6721; Abcam) treatment for 30 min, and washing
with phosphate-buffered saline. Antibody binding was detected using
an SP immunohistochemistry kit (Abcam) and DAB Horseradish
Peroxidase Color Development kit (Thermo Fisher Scientific,
Inc.).
Reverse transcription-quantitative
(RT-q) PCR
For each sample, 500 ng of total RNA was
reverse-transcribed using the QuantiTect reverse transcription kit
(Qiagen GmbH). The resulting complementary DNA was diluted 40 times
and RT-qPCR was performed using an ABI StepOne plus Real-Time PCR
System, with 96-well optical reaction plates (both from Applied
Biosystems; Thermo Fisher Scientific, Inc.). Amplification was
performed at 95°C for 10 sec, followed by 40 cycles at 95°C for 5
sec, and 60°C for 20 sec. Gene expression levels were calculated
with the 2−ΔΔCq method (38). The following primer sequences were
used: GAPDH, forward 5′-GCTGAGTATGTCGTGGAGT-3′ and reverse
5′-GTTCACACCCATCACAAAC-3′; Runx-2, forward
5′-CCCAGCCACCTTTACCTACA-3′ and reverse 5′-TGGGAACTGATAGGATGCTG-3′;
ALP, forward 5′-CCGCCTGATCAAGTTCTCCT-3′ and reverse
5′-TTCAGATGATCCATGCGGGG-3′; COL-1A1, forward
5′-CGTCAGCTCGTGTCCTGTGA-3′ and reverse 5′-AGCTTGAGTAGCCATTGTCCA-3′;
OPG, forward 5′-GTCCCTTGCCCTGACCACTCTT-3′ and reverse
5′-AACGCCCTTCCTCACACTCACA-3′; Osterix, forward
5′-CTGCAACTGGCTTTTCTGC-3′ and reverse 5′-CAGCTCCTTAGGGCCACTT-3′;
PPARγ, forward 5′-CATCGAGGACATCCAAGACA-3′ and reverse
5′-TCTGTGACGATCTGCCTGAG-3′.
Western blot analysis
Proteins were obtained with the
radioimmunoprecipitation assay lysis buffer (RIPA; Sigma-Aldrich;
Merck KGaA) and subsequent centrifugation at 13,000 × g for 20 min
at 4°C. Protein concentration was determined by the BCA method.
Proteins (20 µg protein per lane) were separated by sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel
and transferred onto a nitrocellulose membrane (EMD Millipore). The
membrane was blocked with 5% skim milk in TBST (20 mM Tris-HCl, 150
mM NaCl, and 0.1% Tween 20, pH 7.5) for at least 1 h and then
incubated with each primary antibody overnight at 4°C. The
membranes were washed with TBST buffer at least five times for 5
min each, and incubated with the individual horseradish peroxidase
(HRP)-conjugated secondary antibody (1:1,000, cat. no. ab6721;
Abcam) for 1 h at room temperature. The protein bands were
visualized using an enhanced chemiluminescence (ECL) system
(Amersham; GE Healthcare). Equal loading of samples was verified by
immunoblotting of β-actin (Abcam) or GAPDH (Abcam) for total
fraction, and TFIIB for nuclear fraction as previously described
(39). The primary antibodies
against OPG (1:1,000, cat. no. ab124820), COL-1 (1:500, cat. no.
ab109025), ALP (1:1,000, ab83259), Osterix (1:500, cat. no.
ab22552), Runx-2 (1:1,000, ab23981), PPARγ (1:1,000, cat. no.
ab59256), p-GSK3β (1:500, cat. no. ab68476), GSK-3β (1:1,000, cat.
no. ab32391), p-β-catenin (1:500, cat. no. ab27798), β-catenin
(1:1,000, ab223075), p-p38 MAPK (1:1,000, cat. no. ab4822), p38
MAPK (1:1,000, cat. no. ab170099), and Wnt3a (1:500, cat. no.
ab219412) were purchased from Abcam. The expressions of targeted
proteins were quantified by densitometry using ImageJ software
(version 1.49; National Institutes of Health).
ADSC differentiation
ADSCs were induced to differentiate to osteogenic
and adipogenic lineages as previously described (40). For osteogenic differentiation,
confluent cells were incubated in Dulbecco's modified Eagle's
medium (DMEM) with 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.), 0.1 µM dexamethasone (MCE, USA), 100 µg/ml
ascorbate (MCE), and 10 mM β-glycerophosphate (Sigma-Aldrich; Merck
KGaA). After 3 weeks, the cells were fixed in ice-cold 70% ethanol
for 1 h, washed with deionized water, and stained with 1% alizarin
red (Sigma; Merck KGaA) for 5 min at 37°C.
For adipogenic differentiation, ADSCs were cultured
to confluence for 3 days and then incubated in DMEM with 10% FBS,
10 ng/ml insulin (Sigma; Merck KGaA), 500 mM
3-isobutyl-1-methylxanthine (Sigma; Merck KGaA), 1 mM dexamethasone
(MCE), and 1 mM rosiglitazone (Sigma; Merck KGaA). After 3 weeks,
the cells were fixed with 4% paraformaldehyde (PFA), washed with
60% isopropanol for 5 min, and stained with oil red O (0.25%
wt/vol; Sigma; Merck KGaA) for 10 min. After staining, cells were
washed several times with deionized water.
Statistical analysis
Data are presented as the means ± SD from at least
three separate experiments. To assess the significance of
differences between two groups, Student's t-test was performed.
Results for more than two groups were evaluated by one-way ANOVA
followed by Bonferroni's post hoc test. Statistical calculations
were performed using SPSS 20.0 (IBM Corp., USA). A P-value of
<0.05 indicated a statistically significant difference
(*P<0.05, **P<0.01, and ***P<0.001, as indicated in the
figures).
Results
Exendin-4 facilitates ADSC-mediated
bone repair
In order to elucidate the effect of exendin-4 on
bone defect repair, we previously established a bone-defect model
in the distal metaphysis of the femur (34). Compared to the control group, the
bone-defect group exhibited significantly decreased bone/tissue
volume, trabecular bone thickness, and trabecular bone number, as
well as a significant increase in trabecular bone space, indicating
that the bone defect model was successfully established (Fig. 1A and B). When ADSCs were introduced
into the wound, a significant increase in bone volume/tissue volume
and trabecular bone score was observed compared to the non-treated
bone-defect group (Fig. 1A and B),
suggesting a role of ADSCs in repairing the defective bones.
Notably, defective bones that received both ADSCs and exendin-4
markedly recovered, as evidenced by the bone volume/tissue volume,
trabecular bone thickness, and trabecular bone space that were
statistically similar to those of the non-injured control group
(Fig. 1A and B). In addition, the
mineral apposition status was visualized by calcein and
tetracycline labeling, and the results revealed that defective
bones that received both ADSCs and exendin-4 had an identical
mineral apposition status to that of the control group (Fig. 1C).
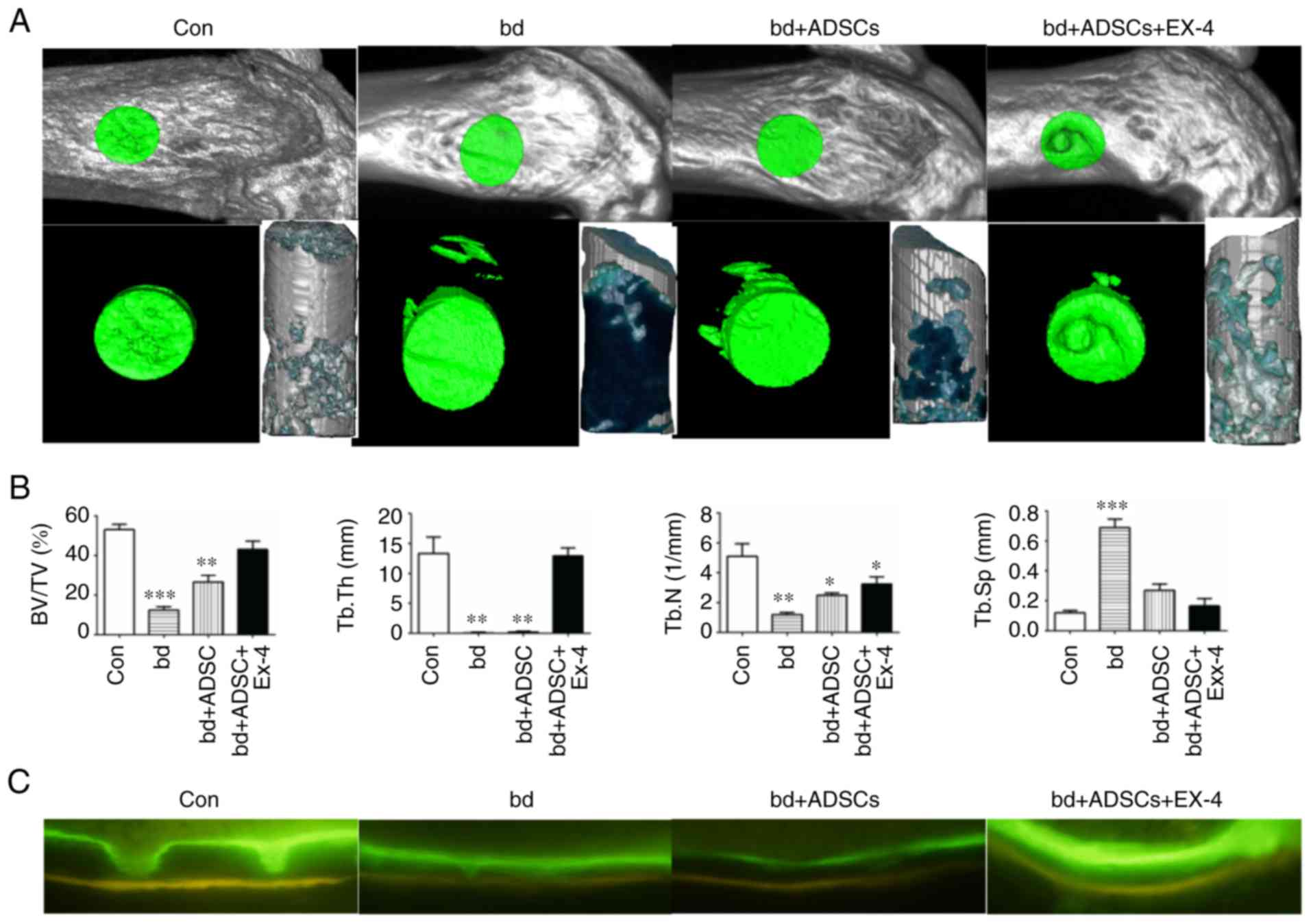 | Figure 1.Exendin-4 facilitates bone defect
repair. (A) Representative micro-computed tomography (µCT) scanning
results of control mice bone (Con; n=6), defective bone (bd; n=6),
defective bone receiving ADSCs (bd+ADSCs; n=6), and defective bone
receiving ADSCs and exendin-4 (bd+ADSCs+Ex-4; n=6). (B)
Quantitative presentation of microarchitectural parameters of
control mice bone (Con; n=6), defective bone (bd; n=6), defective
bone receiving ADSCs (bd+ADSCs; n=6), and defective bone receiving
ADSCs and exendin-4 (bd+ADSCs+Ex-4; n=6). Error bars represent the
mean ± SD. *P<0.05, **P<0.01 and ***P<0.001. (C) Calcein
and tetracycline double staining of control mice bone (Con; n=6),
defective bone (bd; n=6), defective bone receiving ADSCs (bd+ADSCs;
n=6), and defective bone receiving ADSCs and exendin-4
(bd+ADSCs+Ex-4; n=6). ADSCs, adipose-derived stem cells. |
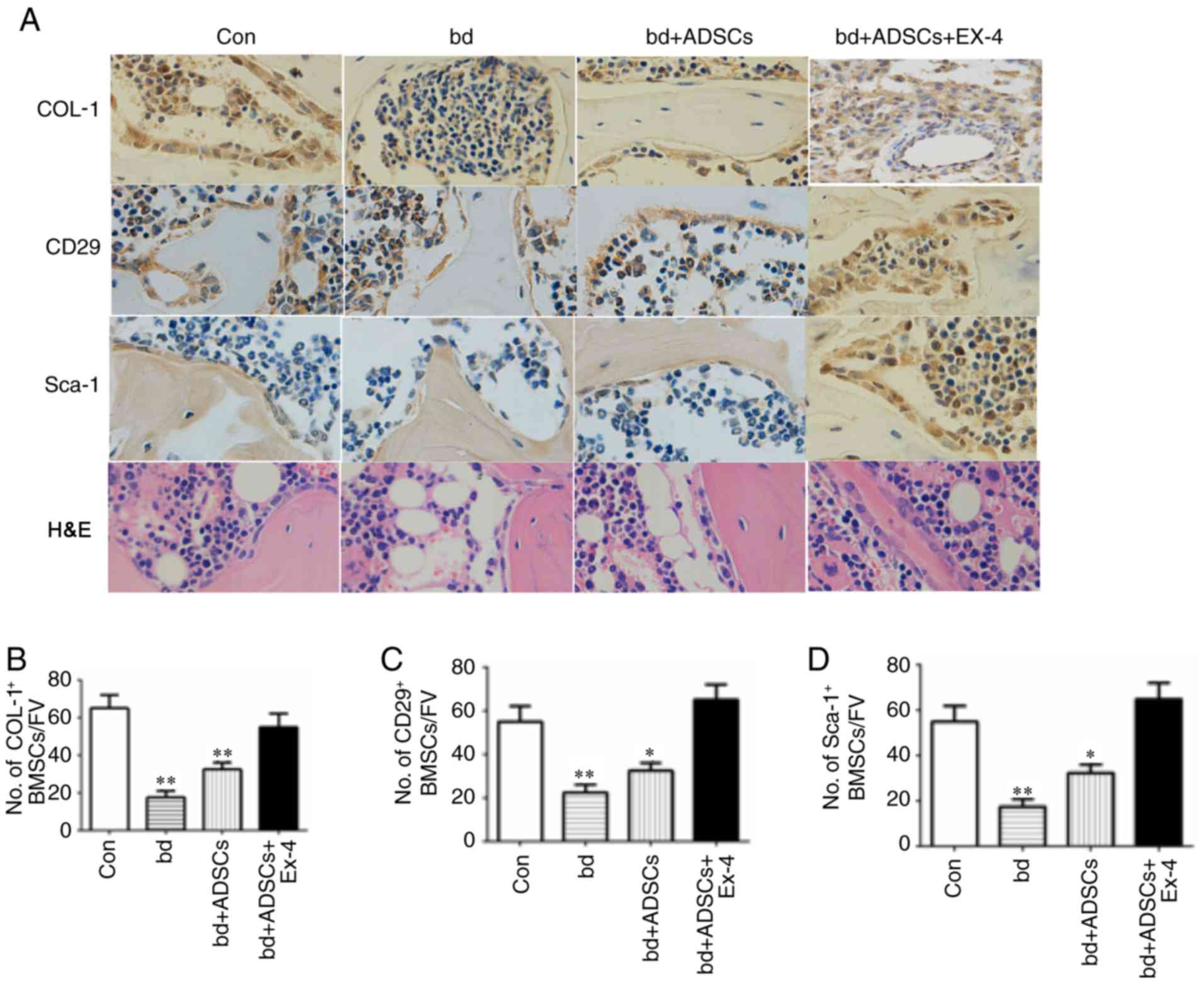
Exendin-4 promotes the generation of
osteoblasts during bone defect repair
To reveal the cellular mechanism through which
exendin-4 facilitates bone repair, the osteogenic and adipogenic
differentiation in defective bones subjected to ADSC and exendin-4
treatments were analyzed using immunohistochemical staining.
Positive staining for COL-1 was significantly decreased in the
defective bones, while ADSC administration improved the level of
COL-1-positive staining. Notably, COL-1-positive signals in the
defective bones treated with ADSCs and exendin-4 were comparable to
those of the control group (Fig. 2A
and B). The number of ADSCs were also examined in the defective
bones after various treatments by immunohistochemical staining for
CD29 and Sca-1, two molecular markers of ADSCs. The present results
revealed a reduced number of ADSCs in defective bones, while
exendin-4 treatment significantly attenuated the loss of ADSCs
resulting from bone defects (Fig. 2C
and D). Concurrently, H&E staining revealed that the number
of adipocytes was reduced in the defective bones treated with ADSCs
and exendin-4 (Fig. 2A).
Collectively, the present results revealed that exendin-4 promoted
the generation of osteoblasts and inhibited the generation of
adipocytes.
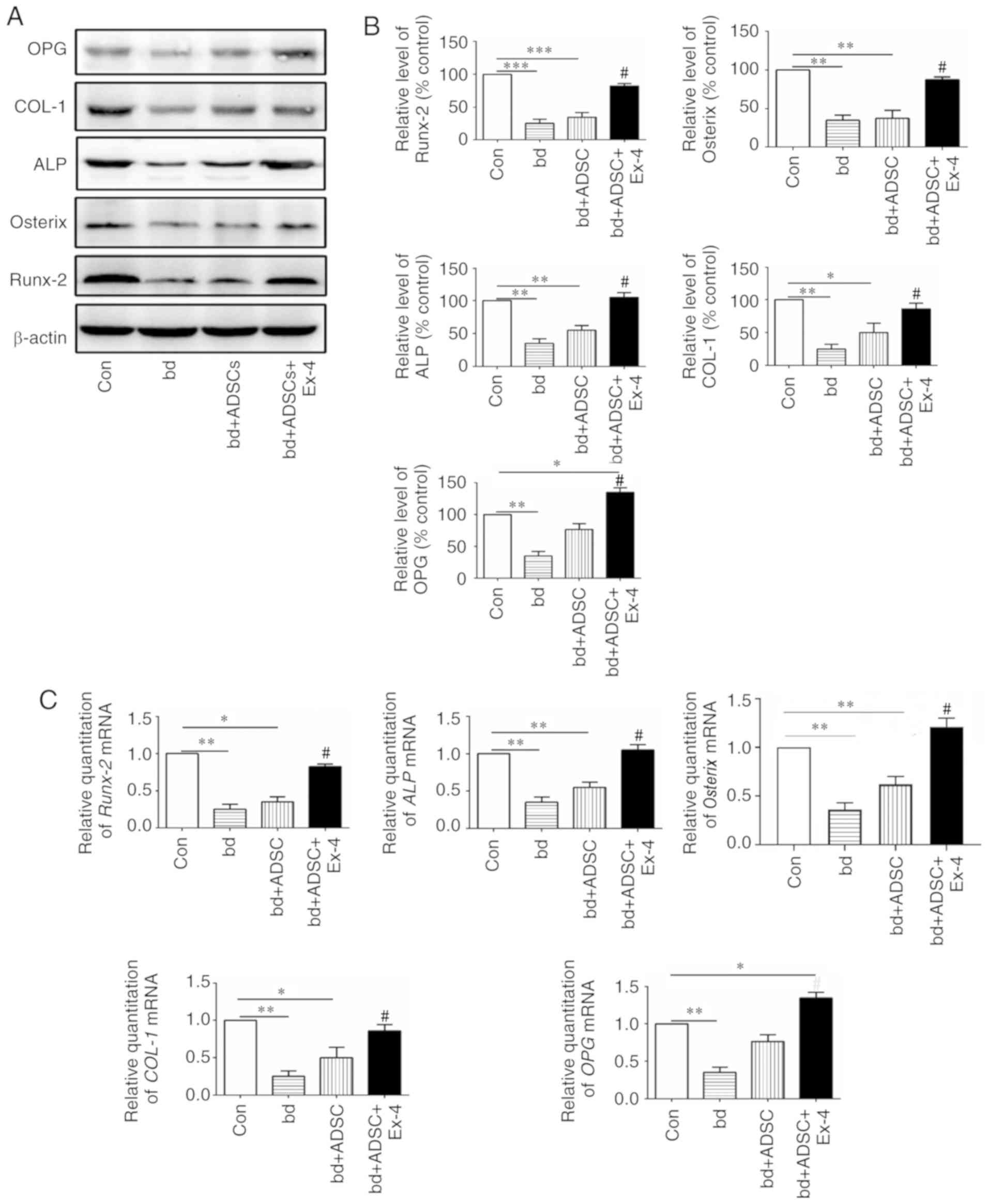
Exendin-4 induces the expression of
genes related to osteogenic differentiation
Since exendin-4 treatment significantly promoted
osteogenic differentiation, the expression of genes related to
osteogenic differentiation at both the protein and mRNA levels were
next examined. The protein levels of osteoprotegerin (OPG), COL-1,
alkaline phosphatase (ALP), Osterix, and Runx-2, all of which are
closely related to bone metabolism, were first assessed (41–45).
The present results revealed that the protein levels of OPG, COL-1,
ALP, Osterix, and Runx-2 were significantly decreased in the
defective bones, and ADSC treatment had little effect on the
expression of these proteins in the defective bones. However, when
the defective bones received both ADSCs and exendin-4 treatment,
the decrease in protein levels of OPG, COL-1, ALP, Osterix, and
Runx-2 was significantly abolished (Fig. 3A and B, P<0.05 vs. the bd
group). Notably, it was observed that ADSC supplementation alone
rescued the expression of OPG, and administration of exendin-4
further increased the protein levels of OPG (Fig. 3A and B). These data indicated that
administration of exendin-4 increased the protein levels of genes
related to osteogenic differentiation. The mRNA levels of OPG,
COL-1, ALP, Osterix, OPG, and Runx-2 were also examined, and it was
revealed that they were in agreement with the protein levels of the
respective genes (Fig. 3C).
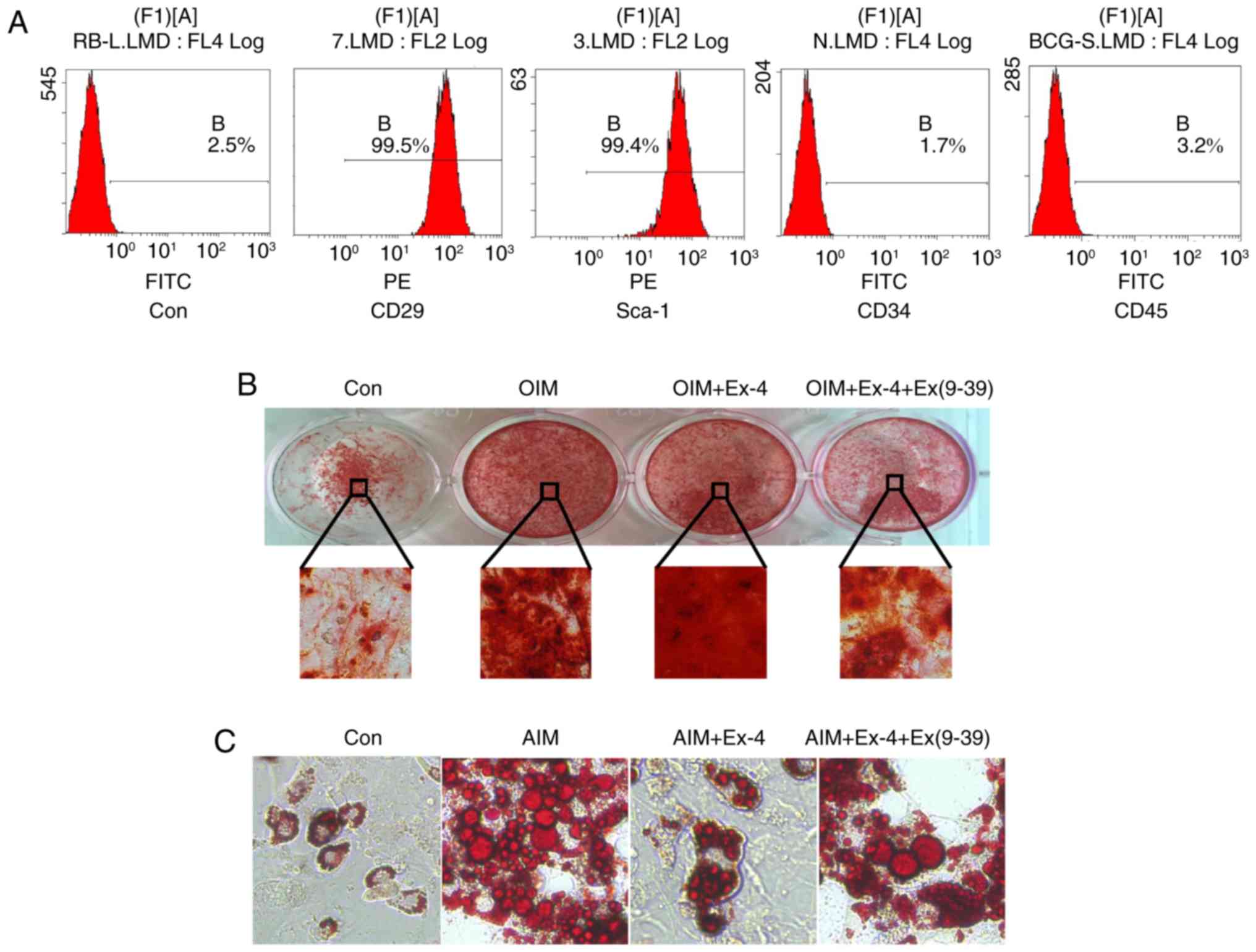
Exendin-4 promotes osteogenic
differentiation in vitro
To further validate the pro-osteogenic role of
exendin-4, ADSCs were isolated and their differentiation in
vitro was examined under exendin-4 treatment. First, the
characteristics of cultured ADSCs were verified using flow
cytometry, and the results revealed that the cells were positive
for the mesenchymal stem cell marker CD29 and the ADSC marker
Sca-1. Concurrently, the in vitro-cultured cells were
negative for CD34 and CD45, indicating that the isolated cells
maintained ADSC characteristics (Fig.
4A). Then in vitro osteoblast differentiation was
induced with osteoblast induction medium (OIM). The results
revealed that administration of exendin-4 increased the osteogenic
differentiation in vitro and notably, when exendin-4 was
antagonized by its specific antagonist Ex (9–39),
the osteogenic differentiation of ADSCs in vitro was
significantly impaired (Fig. 4B).
In addition, the adipogenic differentiation was also induced with
adipose induction medium (AIM) in vitro. The results
revealed that adipogenic differentiation was markedly inhibited by
exendin-4. However, when exendin-4 was antagonized by Ex (9–39),
adipogenic differentiation increased (Fig. 4C).
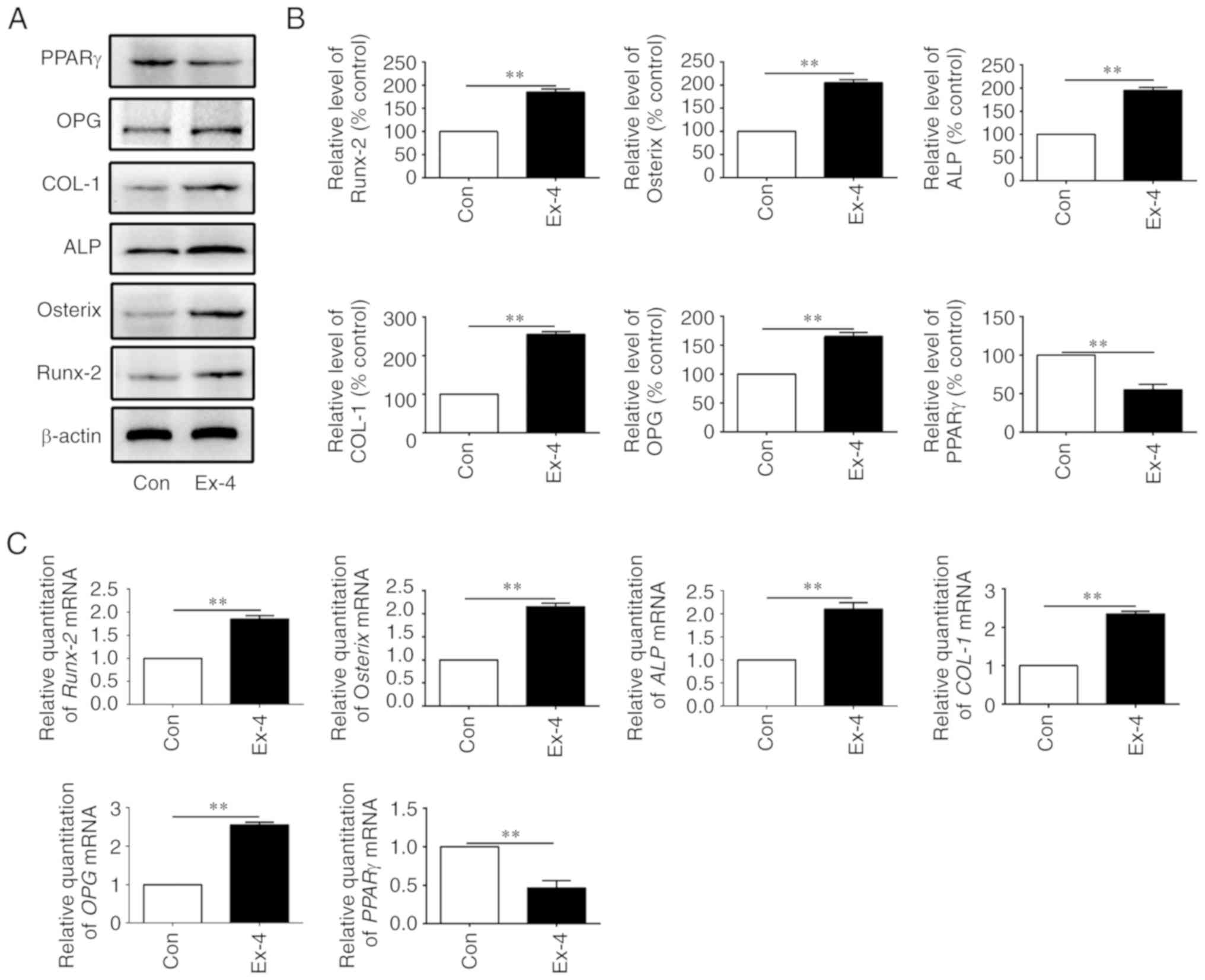
The expression levels of genes related to osteogenic
differentiation were next examined in the in vitro-cultured
ADSCs. As anticipated, the expression levels of the relevant genes,
OPG, COL-1, ALP, Osterix, and Runx-2, were all increased at both
the protein and the mRNA levels when the cells were treated with
exendin-4 (Fig. 5A-C).
Concurrently, peroxisome proliferator-activated receptor-g (PPARγ),
a molecular marker that indicates adipogenic differentiation, was
significantly downregulated at both the mRNA and protein level when
ADSCs were treated with exendin-4 (Fig. 5A-C). Collectively, the present data
indicated that exendin-4 promoted osteogenic differentiation and
inhibited adipogenic differentiation in vitro.
To further understand how exendin-4 regulates ADSCs,
the levels of GSK3β, phosphorylated (p)-GSK3β, p38 MAPK, p-p38
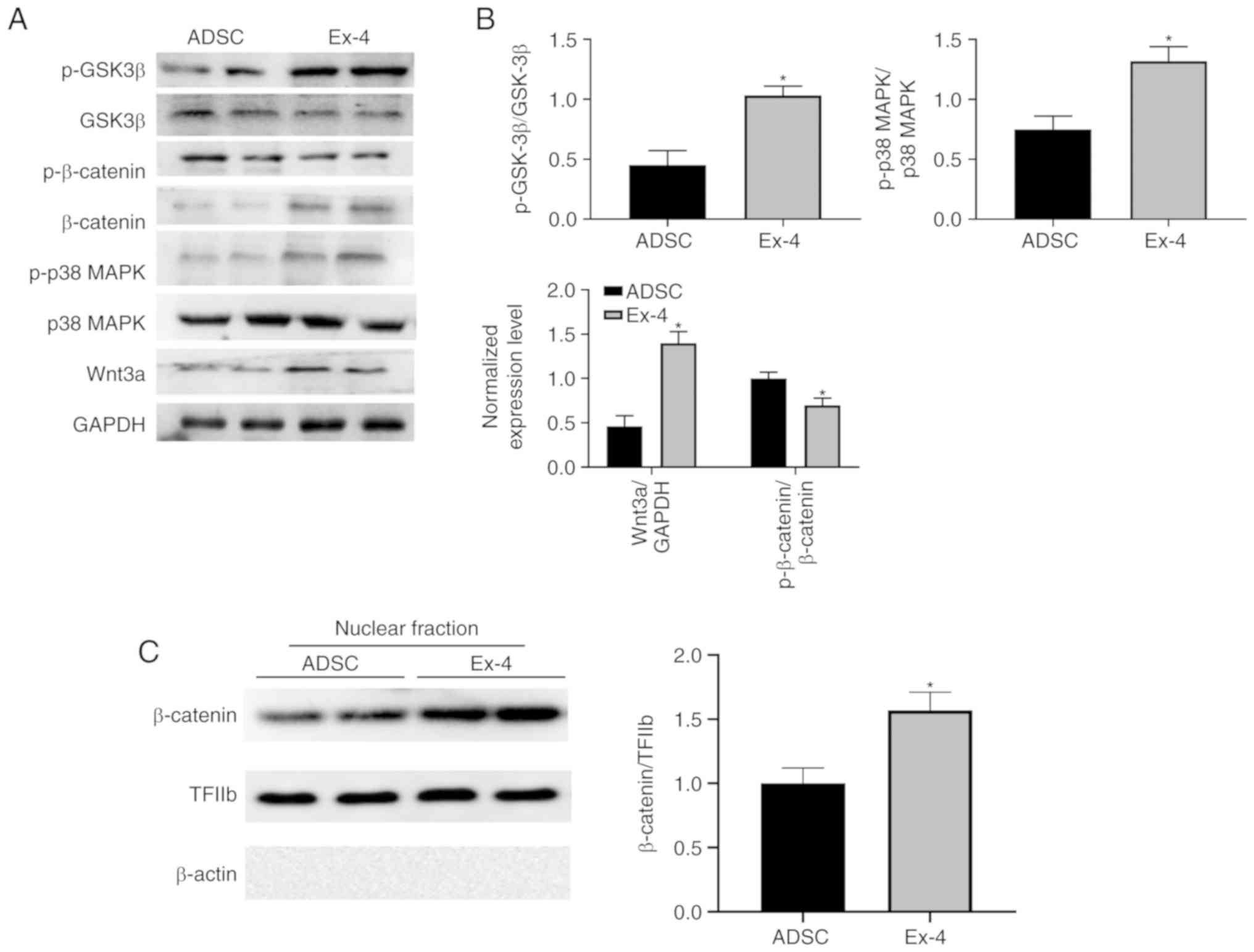
MAPK, β-catenin, p-β-catenin, and Wnt3a were examined. Notably,
administration of exendin-4 significantly increased the levels of
Wnt3a and β-catenin, as well as nuclear localization of β-catenin
(Fig. 6A-C), which corresponds to
previous study (46). Moreover,
the present results revealed that p-p38 MAPK and p-GSK3β were
significantly upregulated when ADSCs were treated with exendin-4
(Fig. 6A and B) indicating that
exendin-4 exhibited identical function as in the neuronal system
(47,48). These alterations in critical
signaling pathways may induce indirect regulation of the expression
of genes related to osteogenic differentiation. Hence, the present
data demonstrated that multiple signaling pathways were involved in
exendin-4-mediated ADSC differentiation.
Discussion
A key to the successful application of ADSCs in bone
tissue regeneration is to accurately adjust the cell
differentiation after effective delivery. In the present study, the
glucagon-like peptide 1 receptor agonist exendin-4, which promoted
osteogenic differentiation of ADSCs both in vitro and in
vivo was characterized and the transcription of genes related
to osteogenic differentiation was induced. The present results
revealed that exendin-4 facilitated bone repair in mice injected
with ADSCs after a large-scale bone defect in vivo,
indicating that exendin-4 is a promising candidate for assisting in
the repair of bone defects using adult stem cells.
Several types of adult stem cells have demonstrated
potential in bone tissue engineering (5,7),
however, ADSCs display multiple advantages among the various types
of adult stem cells. The abundance of ADSCs is higher than that of
other adult stem cells and it is easier to obtain these cells
(49,50). The role of ADSCs in promoting bone
regeneration has been revealed for a long time (51,52),
and several supplements that were used to induce osteogenic
differentiation of BMSCs also promoted osteogenic differentiation
of ADSCs in vitro (53–55).
In the present study, it was demonstrated that exendin-4, a
clinically approved drug for type 2 diabetes treatment, facilitated
the osteogenic differentiation of ADSCs both in vivo and
in vitro. In a rat bone loss model caused by mechanical
unloading, exendin-4 was revealed to improve bone mass and bone
strength during the natural bone healing process (32). In exendin-4-induced bone
reconstruction, a significant increase in osteoblasts generated by
BMSC was observed (32). The
present results further revealed the pro-osteogenic role of
exendin-4 in ADSC-based bone repair, which not only agreed with
previous research but further demonstrated a clinical application
prospect for exendin-4 in tissue engineering-based bone repair.
Exendin-4 promotes osteogenic differentiation
through various molecular mechanisms. The present results revealed
that both mRNA and protein levels of Runx-2, Osterix, APL, COL-1,
and OPG were significantly increased in the regenerating bones
in situ (Fig. 3). However,
the addition of ADSCs alone did not result in a marked increase in
Runx-2 and Osterix at the protein level, suggesting that exendin-4
is partially required to trigger a complete osteogenic
differentiation. Although, evidence to illustrate the detailed
mechanism of how these genes were transcriptionally activated was
not obtained, the present results revealed that multiple critical
signaling pathways were altered when exendin-4 was administered,
including GSK3β, MAPK, and Wnt/β-catenin signaling. These signals
have been revealed to promote osteogenic differentiation by
increasing the expression of genes including Runx-2, Osterix, APL,
COL-1, and OPG (56). These
alterations may indirectly contribute to the expression changes of
genes related to osteogenic differentiation. Notably, the present
data also indicated that the induction of osteogenic
differentiation by exendin-4 depends on transcriptional activation
of genes related to osteogenic differentiation (Fig. 3). Thus, it is crucial to
characterize the downstream signaling activated by exendin-4 in
ADSCs to further understand the detailed molecular mechanism and
guide clinical applications. Meng et al suggested that
exendin-4 treatment induced the expression and nuclear localization
of β-catenin in BMSCs, which in turn further directed the
differentiation of BMSCs (32). In
addition, the present data also revealed that exendin-4
significantly promoted osteogenic differentiation and inhibited
adipogenic differentiation in vitro, providing a unified
cellular model to study the underlying molecular mechanisms.
Since exendin-4 has been approved as an orally
administered medication, its use in conjunction with genetically
modified stem cells and other bio-active molecules provides new
perspectives for bone healing. Jin et al reported that the
ERK signaling pathway balanced the osteogenesis and adipogenesis of
ADSCs during bone defect repair (14). Moreover, epigenetic modifications
and basic fibroblast growth factor (bFGF) signaling were also
revealed to be critical to improve bone defect repair using BMSCs
(57,58). The combined use of inhibitors or
agonists of these signaling pathways may provide fine tuning of
ADSC proliferation and differentiation, which may finally satisfy
the needs of various bone repair technologies. Recent studies also
suggested that miR-146a-regulated osteogenesis of ADSCs can be
adjusted through the BMP signaling pathway during bone regeneration
(59). Furthermore, miR-342-3p was
reported to increase osteogenic differentiation of umbilical cord
mesenchymal stem cells (60).
Based on these findings, it will be of great significance to
combine exendin-4 and miRNAs to develop practical therapies for
bone regeneration.
In summary, the present results demonstrated that
exendin-4 promoted the osteogenic differentiation of transplanted
ADSCs in situ, which in turn repaired the defective bones.
In addition, exendin-4 inhibited the adipogenic differentiation of
transplanted ADSCs to guarantee eventual bone formation. The
present data further revealed the clinical potential of ADSCs in
tissue engineering-based bone defect repair and characterized the
role of exendin-4 in facilitating the osteogenic differentiation of
transplanted ADSCs.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant nos. 81771107
and 81470775).
Availability of data and materials
The datasets used and/or analyzed for the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BLD and WZZ performed the statistical analyses,
evaluated the results, and drafted the study. YLS, BLD and WZZ
participated in the conception and design of the study. YSD, YQH
and XFC contributed to data collection and interpretation, and
manuscript revision. SS and ZY contributed to analyzing data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the guidelines of the NIH (Publication no. 85e23 Rev. 1985)
and were approved by the Animal Care and Use Committee of The
Fourth Military Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADSC
|
adipose-derived stem cells
|
|
BMSCs
|
bone marrow stromal stem cells
|
|
PKA
|
protein kinase A
|
|
GLP-1
|
glucagon-like peptide 1
|
|
bFGF
|
basic fibroblast growth factor
|
|
OPG
|
osteoprotegerin
|
|
ALP
|
alkaline phosphatase
|
References
|
1
|
Dufrane D: Impact of age on human adipose
stem cells for bone tissue engineering. Cell Transplant.
26:1496–1504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weinand C, Neville CM, Weinberg E, Tabata
Y and Vacanti JP: Optimizing biomaterials for tissue engineering
human bone using mesenchymal stem cells. Plast Reconstr Surg.
137:854–863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bertolo A, Mehr M, Janner-Jametti T,
Graumann U, Aebli N, Baur M, Ferguson SJ and Stoyanov JV: An in
vitro expansion score for tissue-engineering applications with
human bone marrow-derived mesenchymal stem cells. J Tissue Eng
Regen Med. 10:149–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alizadeh A, Moztarzadeh F, Ostad SN, Azami
M, Geramizadeh B, Hatam G, Bizari D, Tavangar SM, Vasei M and Ai J:
Synthesis of calcium phosphate-zirconia scaffold and human
endometrial adult stem cells for bone tissue engineering. Artif
Cells Nanomed Biotechnol. 44:66–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang P, Liu X, Zhao L, Weir MD, Sun J,
Chen W, Man Y and Xu HH: Bone tissue engineering via human induced
pluripotent, umbilical cord and bone marrow mesenchymal stem cells
in rat cranium. Acta Biomater. 18:236–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amini AR, Laurencin CT and Nukavarapu SP:
Bone tissue engineering: Recent advances and challenges. Crit Rev
Biomed Eng. 40:363–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oryan A, Alidadi S, Moshiri A and Maffulli
N: Bone regenerative medicine: Classic options, novel strategies,
and future directions. J Orthop Surg Res. 9:182014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oryan A, Kamali A, Moshiri A and Baghaban
Eslaminejad M: Role of mesenchymal stem cells in bone regenerative
medicine: What is the evidence? Cells Tissues Organs. 204:59–83.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tapp H, Hanley EN Jr, Patt JC and Gruber
HE: Adipose-derived stem cells: Characterization and current
application in orthopaedic tissue repair. Exp Biol Med (Maywood).
234:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orbay H, Busse B, Leach JK and Sahar DE:
The effects of Adipose-derived stem cells differentiated into
endothelial cells and osteoblasts on healing of critical size
calvarial defects. J Craniofac Surg. 28:1874–1879. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knippenberg M, Helder MN, Zandieh Doulabi
B, Wuisman PI and Klein-Nulend J: Osteogenesis versus
chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem
Biophys Res Commun. 342:902–908. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin Y, Zhang W, Liu Y, Zhang M, Xu L, Wu
Q, Zhang X, Zhu Z, Huang Q and Jiang X: rhPDGF-BB via ERK pathway
osteogenesis and adipogenesis balancing in ADSCs for critical-sized
calvarial defect repair. Tissue Eng Part A. 20:3303–3313. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lough DM, Chambers C, Germann G, Bueno R,
Reichensperger J, Swanson E, Dyer M, Cox L, Harrison C and
Neumeister MW: Regulation of ADSC Osteoinductive potential using
notch pathway inhibition and gene rescue: A Potential On/Off Switch
for clinical applications in bone formation and reconstructive
efforts. Plast Reconstr Surg. 138:642e–652e. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Hu C, Li J, Liu L, Jing W, Tang W,
Tian W and Long J: Effect of miR-26a-5p on the Wnt/Ca(2+) pathway
and osteogenic differentiation of mouse Adipose-derived mesenchymal
stem cells. Calcif Tissue Int. 99:174–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu X, Fu Y, Zhang X, Dai L, Zhu J, Bi Z,
Ao Y and Zhou C: Histone deacetylase inhibitor sodium butyrate
promotes the osteogenic differentiation of rat adipose-derived stem
cells. Dev Growth Differ. 56:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gersbach CA, Byers BA, Pavlath GK and
Garcia AJ: Runx2/Cbfa1 stimulates transdifferentiation of primary
skeletal myoblasts into a mineralizing osteoblastic phenotype. Exp
Cell Res. 300:406–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee B, Thirunavukkarasu K, Zhou L, Pastore
L, Baldini A, Hecht J, Geoffroy V, Ducy P and Karsenty G: Missense
mutations abolishing DNA binding of the osteoblast-specific
transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat
Genet. 16:307–310. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Yang M, Lin L, Chen P, Ma KT,
Zhou CY and Ao YF: Runx2 overexpression enhances osteoblastic
differentiation and mineralization in adipose-derived stem cells in
vitro and in vivo. Calcif Tissue Int. 79:169–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Basoli V, Santaniello S, Cruciani S,
Ginesu GC, Cossu ML, Delitala AP, Serra PA, Ventura C and Maioli M:
Melatonin and Vitamin D interfere with the adipogenic fate of
Adipose-derived stem cells. Int J Mol Sci. 18:E9812017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malhotra R, Singh L, Eng J and Raufman JP:
Exendin-4, a new peptide from Heloderma suspectum venom,
potentiates cholecystokinin-induced amylase release from rat
pancreatic acini. Regul Pept. 41:149–156. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eng J, Kleinman WA, Singh L, Singh G and
Raufman JP: Isolation and characterization of exendin-4, an
exendin-3 analogue, from Heloderma suspectum venom. Further
evidence for an exendin receptor on dispersed acini from guinea pig
pancreas. J Biol Chem. 267:7402–7405. 1992.PubMed/NCBI
|
|
24
|
Eng J: Exendin peptides. Mt Sinai J Med.
59:147–149. 1992.PubMed/NCBI
|
|
25
|
Edwards CM, Stanley SA, Davis R, Brynes
AE, Frost GS, Seal LJ, Ghatei MA and Bloom SR: Exendin-4 reduces
fasting and postprandial glucose and decreases energy intake in
healthy volunteers. Am J Physiol Endocrinol Metab. 281:E155–E161.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nielsen LL, Young AA and Parkes DG:
Pharmacology of exenatide (synthetic exendin-4): A potential
therapeutic for improved glycemic control of type 2 diabetes. Regul
Pept. 117:77–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamada C, Yamada Y, Tsukiyama K, Yamada K,
Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y and Inagaki
N: The murine glucagon-like peptide-1 receptor is essential for
control of bone resorption. Endocrinology. 149:574–579. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nuche-Berenguer B, Lozano D,
Gutierrez-Rojas I, Moreno P, Mariñoso ML, Esbrit P and
Villanueva-Peñacarrillo ML: GLP-1 and exendin-4 can reverse
hyperlipidic-related osteopenia. J Endocrinol. 209:203–210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma X, Meng J, Jia M, Bi L, Zhou Y, Wang Y,
Hu J, He G and Luo X: Exendin-4, a glucagon-like peptide-1 receptor
agonist, prevents osteopenia by promoting bone formation and
suppressing bone resorption in aged ovariectomized rats. J Bone
Miner Res. 28:1641–1652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng Y, Su L, Zhong X, Guohong W, Xiao H,
Li Y and Xiu L: Exendin-4 promotes proliferation and
differentiation of MC3T3-E1 osteoblasts by MAPKs activation. J Mol
Endocrinol. 56:189–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He J, Wang C, Sun Y, Lu B, Cui J, Dong N,
Zhang M, Liu Y and Yu B: Exendin-4 protects bone marrow-derived
mesenchymal stem cells against oxygen/glucose and serum
deprivation-induced apoptosis through the activation of the
cAMP/PKA signaling pathway and the attenuation of ER stress. Int J
Mol Med. 37:889–900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng J, Ma X, Wang N, Jia M, Bi L, Wang Y,
Li M, Zhang H, Xue X, Hou Z, et al: Activation of GLP-1 receptor
promotes bone marrow stromal cell osteogenic differentiation
through β-Catenin. Stem Cell Reports. 6:579–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen WR, Kimura K, Ishida M, Sugisawa H,
Kishikawa A, Shima K, Ogawa S, Qi J and Kitaura H: The
glucagon-like Peptide-1 receptor agonist Exendin-4 inhibits
lipopolysaccharide-induced osteoclast formation and bone resorption
via inhibition of TNF-α expression in macrophages. J Immunol Res.
2018:57836392018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Uusitalo H, Rantakokko J, Ahonen M, Jämsä
T, Tuukkanen J, KäHäri V, Vuorio E and Aro HT: A metaphyseal defect
model of the femur for studies of murine bone healing. Bone.
28:423–429. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin M, Liu R, Gozal D, Wead WB, Chapleau
MW, Wurster R and Cheng ZJ: Chronic intermittent hypoxia impairs
baroreflex control of heart rate but enhances heart rate responses
to vagal efferent stimulation in anesthetized mice. Am J Physiol
Heart Circ Physiol. 293:H997–H1006. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soranno DE, Rodell CB, Altmann C,
Duplantis J, Andres-Hernando A, Burdick JA and Faubel S: Delivery
of interleukin-10 via injectable hydrogels improves renal outcomes
and reduces systemic inflammation following ischemic acute kidney
injury in mice. Am J Physiol Renal Physiol. 311:F362–F372. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang N, Gao J, Jia M, Ma X, Lei Z, Da F,
Yan F, Zhang H, Zhou Y, Li M, et al: Exendin-4 induces bone marrow
stromal cells migration through bone marrow-derived macrophages
polarization via PKA-STAT3 signaling pathway. Cell Physiol Biochem.
44:1696–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, et al: FoxM1 promotes
β-catenin nuclear localization and controls Wnt target-gene
expression and glioma tumorigenesis. Cancer Cell. 20:427–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roxburgh J, Metcalfe AD and Martin YH: The
effect of medium selection on adipose-derived stem cell expansion
and differentiation: Implications for application in regenerative
medicine. Cytotechnology. 68:957–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gerstenfeld LC and Shapiro FD: Expression
of bone-specific genes by hypertrophic chondrocytes: Implication of
the complex functions of the hypertrophic chondrocyte during
endochondral bone development. J Cell Biochem. 62:1–9. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Greep RO, Fischer CJ and Morse A: Alkaline
phosphatase in odontogenesis and osteogenesis and its histochemical
demonstration after demineralization. J Am Dent Assoc. 36:427–442.
1948. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCarthy TL, Ji C, Chen Y, Kim KK, Imagawa
M, Ito Y and Centrella M: Runt domain factor (Runx)-dependent
effects on CCAAT/enhancer-binding protein delta expression and
activity in osteoblasts. J Biol Chem. 275:21746–21753. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Seo MH, Lee J, Hong SW, Rhee EJ, Park SE,
Park CY, Oh KW, Park SW and Lee WY: Exendin-4 inhibits hepatic
lipogenesis by increasing beta-catenin signaling. PLoS One.
11:e01669132016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu W, Yang Y, Yuan G, Zhu W, Ma D and Hu
S: Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces
Alzheimer disease-associated tau hyperphosphorylation in the
hippocampus of rats with type 2 diabetes. J Investig Med.
63:267–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hayes MR: Neuronal and intracellular
signaling pathways mediating GLP-1 energy balance and glycemic
effects. Physiol Behav. 106:413–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mainil-Varlet P, Aigner T, Brittberg M,
Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker
K, Roberts S, et al: Histological assessment of cartilage repair: A
report by the Histology endpoint committee of the international
cartilage repair society (ICRS). J Bone Joint Surg Am. 85-A (Suppl
2):S45–S57. 2003. View Article : Google Scholar
|
|
50
|
Veronesi F, Maglio M, Tschon M, Aldini NN
and Fini M: Adipose-derived mesenchymal stem cells for cartilage
tissue engineering: State-of-the-art in in vivo studies. J Biomed
Mater Res A. 102:2448–2466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Di Bella C, Farlie P and Penington AJ:
Bone regeneration in a rabbit critical-sized skull defect using
autologous adipose-derived cells. Tissue Eng Part A. 14:483–490.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS
and Lee SH: In vivo bone formation following transplantation of
human adipose-derived stromal cells that are not differentiated
osteogenically. Tissue Eng Part A. 14:1285–1294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jurgens WJ, Oedayrajsingh-Varma MJ, Helder
MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ and van
Milligen FJ: Effect of tissue-harvesting site on yield of stem
cells derived from adipose tissue: Implications for cell-based
therapies. Cell Tissue Res. 332:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Al-Salleeh F, Beatty MW, Reinhardt RA,
Petro TM and Crouch L: Human osteogenic protein-1 induces
osteogenic differentiation of adipose-derived stem cells harvested
from mice. Arch Oral Biol. 53:928–936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ahn HH, Kim KS, Lee JH, Lee JY, Kim BS,
Lee IW, Chun HJ, Kim JH, Lee HB and Kim MS: In vivo osteogenic
differentiation of human adipose-derived stem cells in an
injectable in situ-forming gel scaffold. Tissue Eng Part A.
15:1821–1832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Asserson DB, Orbay H and Sahar DE: Review
of the pathways involved in the osteogenic differentiation of
Adipose-derived stem cells. J Craniofac Surg. 30:703–708. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang H, Kot A, Lay YE, Fierro FA, Chen H,
Lane NE and Yao W: Acceleration of fracture healing by
overexpression of basic fibroblast growth factor in the mesenchymal
stromal cells. Stem Cells Transl Med. 6:1880–1893. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Deng Y, Guo T, Li J, Guo L, Gu P and Fan
X: Repair of calvarial bone defect using Jarid1a-knockdown bone
mesenchymal stem cells in rats. Tissue Eng Part A. 24:711–718.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xie Q, Wei W, Ruan J, Ding Y, Zhuang A, Bi
X, Sun H, Gu P, Wang Z and Fan X: Effects of miR-146a on the
osteogenesis of adipose-derived mesenchymal stem cells and bone
regeneration. Sci Rep. 7:428402017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huang M, Qing Y, Shi Q, Cao Y and Song K:
miR-342-3p elevates osteogenic differentiation of umbilical cord
mesenchymal stem cells via inhibiting Sufu in vitro. Biochem
Biophys Res Commun. 491:571–577. 2017. View Article : Google Scholar : PubMed/NCBI
|