Introduction
Bone cancer pain (BCP) is a severe complication of
metastatic or advanced malignancy and is characterized by allodynia
and hyperalgesia (1). In total,
75–90% of patients with metastatic or advanced stage cancer have
chronic severe bone pain (2). BCP
negatively affects the quality of life of those suffering from it
and represents a substantial burden to society (3,4).
Although much effort has been devoted to the study of peripheral
and central nervous system (CNS) sensitizations, the mechanism of
BCP has yet to be elucidated (5),
and pharmacological agents with higher analgesic efficacy and fewer
side effects need to be developed.
The endocannabinoid system comprises the cannabinoid
receptors, corresponding ligands and enzymes that catalyze the
synthesis and degradation of cannabinoids (6). Cannabinoid receptor type 1 (CB1) is
highly expressed in the CNS (7),
whereas the CB2 is mainly distributed in the immune system
(8). Previous studies have
revealed the effects of the cannabinoid signaling system on acute
and chronic pain relief; for example, pharmacological studies have
demonstrated that non-selective cannabinoid agonists and selective
CB1 and CB2 agonists induced antinociceptive effects (9–12).
However, because of the side effects, such as sedation, dependence,
cognitive impairment and psychotic-like behavior, caused by CB1
agonists (13), the pain relief
mechanism of CB2 agonists in cancer pain has become a key
focus.
The analgesic effects of CB2 agonists have been
demonstrated in a number of pain models. (14–16).
Various analgesic mechanisms of CB2 agonists have been proposed
but, to date, there is no definitive explanation. There is evidence
that suggests a fatal role of autophagy in neuropathic pain
(17,18). For example, suberanilohydroxamic
acid (SAHA) was reported to attenuate neuropathic pain through the
autophagy flux mediated by mTOR signaling in spinal astrocytes and
neurons (19). It has also been
reported that antinociception caused by caloric restriction
increased autophagy in diabetic neuropathic pain (20). Previous studies have revealed that
activation of CB2 by JWH015 alleviated autoimmune disease (21), protected against alcoholic liver
disease (22) and exerted an
antitumor effect through the activation of autophagy (23,24).
Therefore, the present study aimed to determine whether the
analgesic effect of the CB2 agonist JWH015 was mediated by the
increased autophagy flux.
The existence of crosstalk between autophagy and
inflammation has been proposed in various diseases, such as
rheumatoid arthritis, systemic lupus erythematosus and cancer
(25,26). Interleukin (IL)-1β and IL-6 are
inflammatory mediators, and their upregulation often occurs with
impaired autophagy (27,28). Based on our previous study that
demonstrated that the activation of CB2 by intrathecal injection of
JWH015 inhibited the activation of glial cells and downregulated
the expression of IL-1β and IL-6 in rat BCP (29), it was hypothesized that autophagy
flux was impaired by the increases in inflammatory mediators in
BCP. However, whether the inflammatory mediator-induced impairment
of autophagy flux is involved in the analgesic effects of JWH015
remains unclear.
Therefore, the present study proposed that the
activation of CB2 by JWH015 may activate autophagy, which was
impaired by glia-derived inflammatory mediators. The present study
investigated the specific mechanism of CB2 in BCP, with a focus on
its modulation of inflammation-mediated autophagy.
Materials and methods
Animals
All experiments were performed in strict accordance
with the appropriate guidelines (30) and were approved by the Animal Care
and Use Committee of the Medical School of Nanjing University
(Nanjing, China).
For in vivo experiments, male C3H/HeN mice
(weight, 20–25 g; age 4–6 weeks, n=141) were purchased from Vital
River Experimental Animal Corporation of Beijing. In total, 6 mice
were housed in one cage under a 12 h light/dark cycle at 20°C, with
a relative humidity of 55% and with free access to water and
food.
For in vitro experiments, 14-day pregnant
Sprague-Dawley rats were used to obtain the fetuses and collected
the primary neuronal cells from the fetuses. Sprague-Dawley rats in
the 14th day of pregnancy (weight, 300–350 g; age 6 weeks, n=3)
were purchased from Qing Long Shan Dong Wu Fan Zhi Chang (Jiangsu,
China, http://www.njqlsdwc.com). Rats were
housed one cage per rat under a 12 h light/dark cycle at 20°C, with
a relative humidity of 55% and with free access to water and
food.
Experimental design
Experiment 1
A total of 64 mice were randomly divided into eight
groups (n=8): Control group, sham group, and tumor group, pain
behavioral tests were performed on the day before (baseline) and on
day 4, 7,10, 14, 21 and 28 after operation. sham + vehicle group,
tumor + vehicle group, tumor + JWH015 (1 µg) group, tumor + JWH015
(2 µg) group, and tumor + JWH015 (1 µg) + AM630 (2 µg) group, pain
behavioral tests were performed on the day before (baseline) and at
4, 8,12, 24, 48 and 72 h after injection.
Experiment 2
A total of 20 mice were randomly divided into two
groups: Sham group (n=6), and tumor group (n=24). 14 days after
operation, mice in sham group were sacrificed and the lumbar spinal
cord was collected for western blotting (n=3) and
immunofluorescence labeling (n=3). Mice in tumor group were
sacrificed on day 0 (n=3), 4 (n=3), 7 (n=3), 10 (n=3), 14 (n=3), 21
(n=3), and 28 (n=3) for western blotting. On day 14 after
operation, the mice in tumor group were sacrificed for
immunofluorescence labeling (n=3).
Experiment 3
A total of 36 mice were randomly divided into eight
groups: Sham + vehicle group (n=6), tumor + vehicle group (n=6),
sham + Baf-A1 group (n=3), tumor + Baf-A1 group, tumor + JWH015 (1
µg) group (n=3), tumor + JWH015 (2 µg) group (n=6), tumor + JWH015
(1 µg) + AM630 (2 µg) group (n=6), and tumor + JWH015 + Baf-A1
group (n=3). Mice were sacrificed at 12 h after injection in each
group for western blotting (n=3). In sham + vehicle group, tumor +
vehicle group, tumor + JWH015 (2 µg) group, tumor + JWH015 (1 µg) +
AM630 (2 µg) group, another 3 mice were sacrificed at 12 h after
injection for immunofluorescence labeling (n=3).
Experiment 4
A total of 21 mice in tumor + JWH015 (2 µg) group
were randomly sacrificed at 0 (n=3), 4 (n=3), 8 (n=3), 12 (n=3), 24
(n=3), 48 (n=3), and 72 (n=3) h after injection for western
blotting.
NCTC 2472 cell culture
NCTC 2472 osteolytic sarcoma cells (cat. no.
2087787; American Type Culture Collection) were cultured in NCTC
135 medium (Sigma-Aldrich; Merck KGaA) containing 10% horse serum
(Gibco; Thermo Fisher Scientific, Inc.) and maintained in a 5% CO2
atmosphere at 37°C (Thermo Forma; Thermo Fisher Scientific,
Inc.).
Primary neuronal cells culture
Sprague-Dawley rats in the 14th day of pregnancy
were deeply anesthetized with 2–3% isofluorane, sacrificed by
cervical dislocation and the fetuses were quickly removed on
embryonic day 14. The meninges and blood vessels were removed from
the fetal cerebral cortices under a dissecting microscope. After
cutting into 1 mm3 pieces, cortical tissues were
digested in 0.25% trypsin at 37°C for 10 min. Supernatants were
passed through a 70 µm cell strainer (Falcon; Thermo Fisher
Scientific, Inc.) and centrifuged for 5 min at 168 × g at 37°C.
Cells were diluted in Neurobasal medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 1% B27 (Gibco; Thermo Fisher
Scientific, Inc.), 2 mM glutamine and 10 µl/ml
penicillin/streptomycin and plated onto poly-L-lysine
(Sigma-Aldrich; Merck KGaA) coated 6-well plates at a density of
1×106 cells/cm2. Cells were cultured at 37°C
in a humidified incubator containing 5% CO2. The culture
medium was changed 3 days after plating and cells were allowed to
grow for 7 days before using in subsequent experiments.
BCP model
The BCP model was constructed as described by Schwei
et al (31). Mice were
anesthetized with sodium pentobarbital (50 mg/kg; i.p.) and an
incision was made in the skin on the right leg and the right joint
was exposed. Then a hole was drilled in the femur-plateau.
Subsequently, 20 µl α-minimum essential medium (Thermo Fisher
Scientific, Inc.) containing 2×105 NCTC 2472 osteolytic
sarcoma cells was injected into the intramedullary space of the
right femur. Mice in the Sham group were injected with the isodose
medium without any cells. The drilled hole was sealed with bone
wax, and the wound was closed with 4-0 silk sutures (Ethicon,
Inc.). Mice recovered from anesthesia on a heated blanket.
Drug treatments
For in vivo experiments, drugs were prepared
and administered as previously described (29,32,33).
The selective CB2 agonist JWH015 (Sigma-Aldrich; Merck KGaA) was
dissolved in 5% DMSO corresponding to a dose of 1 µg/5 µl (50
µg/kg) or 2 µg/5 µl (100 µg/kg). The CB2-selective antagonist AM630
(Sigma-Aldrich; Merck KGaA) was dissolved in 5% DMSO corresponding
to a dose of 2 µg/5 µl (100 µg/kg). Bafilomycin A1 (Baf-A1), an
inhibitor of autophagosome and lysosome fusion, was dissolved in 5%
DMSO corresponding to a dose of 10 nM. To avoid systemic effects on
tumor cells, intrathecal administration was selected (34). Drugs were intrathecally
administered at a volume of 5 µl at day 14 after the inoculation
with tumor cells. To antagonize the activation of the CB2 receptor,
AM630 was injected 30 min before JWH015.
For in vitro experiments, JWH015 and AM630
were dissolved in culture medium corresponding to a dose of 1 µM.
Lipopolysaccharide (LPS) was dissolved in culture medium
corresponding to a dose of 100 nM.
Pain behavioral tests
Mechanical allodynia and spontaneous pain in mice
were tested prior to operation (day 0) as well as 4, 7, 10, 14, 21
and 28 days after operation in each group, and 0 (baseline), 4, 8,
12, 24, 48 and 72 h after the administration of JWH015, AM630 and
vehicles. The experimenters who performed all behavioral tests were
blinded to the groups.
Paw withdrawal mechanical threshold
(PWMT)
PWMT in the right hind paw was measured using von
Frey filaments (0.16, 0.4, 0.6, 1.0, 1.4 and 2.0 g; Stoelting, USA)
and the ‘up-down’ method as previously described (33). Mice were placed in transparent
plexiglass compartments with a wire mesh bottom and allowed to
acclimate for 30 min. von Frey filaments were stuck to the plantar
surface, and the lowest filament stimulus strength that resulted in
the paw flinching or withdrawing was regarded as the PWMT.
Number of spontaneous flinches
(NSF)
Mice were placed in transparent plexiglass
compartments with a wire mesh bottom and allowed to acclimatize for
30 min. Subsequently, the NSF of the right hind paw in 2 min was
counted; each mouse was tested five times.
Western blotting
Mice were anesthetized with pentobarbital (50 mg/kg,
i.p.) and sacrificed by cervical dislocation on day 0, 4, 7, 10,
14, 21 and 28 after operation and 0, 4, 8, 12, 24, 48 and 72 h
after intrathecal administration. The L3-L5 segments of the spinal
cord were removed and stored at −80°C for further study. In
addition, the primary neuronal cells were collected at 0, 3, 6, 9,
12 and 24 h after LPS-stimulation, and 12 h after JWH015-treatment.
Samples were homogenized in RIPA Lysis Buffer (10 µl/mg for tissue;
Beyotime Institute of Biotechnology) with phenylmethyl sulfonyl
fluoride and incubated on ice for 30 min, followed by
centrifugation at 241 × g at 4°C for 20 min. The supernatant of
each sample was collected. Protein concentrations were determined
using a BCA Protein Assay kit. Each sample of 50 µg protein was
subjected to 10% SDS-PAGE and then transferred onto a PVDF
membrane. The membrane was blocked with 5% BSA (Gibco; Thermo
Fisher Scientific, Inc.) at room temperature for 1 h. The membranes
were incubated with primary antibodies against IL-1β (1:1,000;
Abcam; cat. no. ab2105), IL-6 (1:1,000; Abcam; cat. no. ab6672),
LC3B (1:1,000; Cell Signaling Technology, Inc.; cat. no. 3868), p62
(1:1,000; Abcam; cat. no. ab91526) and β-actin (1:4,000; Abcam;
cat. no. ab8227). The blots were subsequently incubated with a
horseradish peroxidase conjugated goat anti-rabbit secondary
antibody (1:10,000; EMD Millipore; cat. no. AP132P) and developed
in ECL solution (Tanon Science and Technology Co., Ltd.). Images
were captured using a cooled CCD system (Tanon Science and
Technology Co., Ltd.) and quantified using ImageJ v1.8.0 (National
Institutes of Health).
Immunofluorescence labeling
Following the administration of general anesthesia
(pentobarbital, 50 mg/kg; i.p.), mice were transcardially perfused
with normal saline and 4% paraformaldehyde at day 14 after
operation and 12 h after JWH015 (2 µg) administration. Lumbosacral
enlargements were removed and fixed in 4% paraformaldehyde for 6 h,
and then dehydrated in 30% sucrose for 48–72 h at 4°C. After they
were frozen in optimal cutting temperature compound, tissues were
cut into 20 µm sections with a freezing microtome. Sections were
placed in PBS and sequentially blocked with 10% goat serum (Gibco;
Thermo Fisher Scientific, Inc.) containing 0.3% Triton (Tanon
Science and Technology Co., Ltd.) for 2 h at room temperature.
Sections were then incubated with primary antibodies for glial
fibrillary acidic protein (GFAP; mouse, 1:100, Cell Signaling
Technology, Inc. #80788), ionized calcium-binding adaptor molecule
1 (Iba1; rabbit, 1:300, Wako, Japan), LC3B (rabbit; 1:100; Cell
Signaling Technology, Inc.; cat. no. 3868), and neuronal nuclei
antigen (NeuN; mouse; 1:1,000; Abcam; cat. no. ab104224) separately
overnight at 4°C. After washing with PBS, sections were incubated
with Alexa 488-conjugated goat anti-rabbit (1:3,000, Thermo Fisher
Scientific, Inc.; cat. no. R37116) or Alexa 594-conjugated goat
anti-mouse (1:3,000; Thermo Fisher Scientific, Inc.; cat. no.
R37121) secondary antibodies. The sections were mounted on glass
slides, air-dried and incubated with DAPI (Abcam) for 5 min at room
temperature for nuclear staining. Images were captured using a
laser-scanning confocal microscope (Olympus Corporation) and the
staining density was analyzed using ImageJ (National Institutes of
Health).
Statistical analyses
Data are presented as the mean ± standard deviation.
Results from the behavioral study were analyzed using repeated
measurements ANOVA followed by Bonferroni test post-hoc test to
assess differences at each time point among and with. Western
blotting results were analyzed using one-way ANOVA followed by
Bonferroni test for between-group comparisons. Statistical analyses
were performed using SPSS 22.0 (IBM Corporation); GraphPad Prism
Version 7 (GraphPad Software, Inc.) was used to plot graphs.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Intrathecal injection of JWH015
attenuates BCP in mice
PWMT and NSF of the right hind paw of mice were
tested to monitor the progression of BCP generated by injecting
NCTC 2472 osteolytic sarcoma cells into the femur. No significant
differences in PWMT and NSF were observed between groups at
baseline. PWMT decreased slightly (1.15±0.207 g in the Sham group;
1.30±0.185 g in the tumor group; Fig.
1A) and NSF increased (3.500±0.756 in the Sham group;
2.875±0.641 in the tumor group; Fig.
1B) on day 4 in the Sham and Tumor groups compared with the
values at baseline (all P<0.05). The PWMT and NSF values
recovered to near baseline level in the Sham group from day 7. In
the Tumor group, PWMT recovered on day 7 and decreased starting on
day 10 (0.9±0.28 g on day 10; 0.52±0.21 g on day 14; 0.34±0.11 g on
days 21 and 28; P<0.05; Fig.
1A) compared with the baseline. NSF in the Tumor group remained
significantly higher compared with the baseline value at days 10–28
(5.875±1.12599 on day 10; 9.5±0.92582 on day 14; 11±1.06904 on day
21 and 12.5±0.92582 on day 28; P<0.05; Fig. 1B). These results confirmed the
successful establishment of the bone cancer pain model.
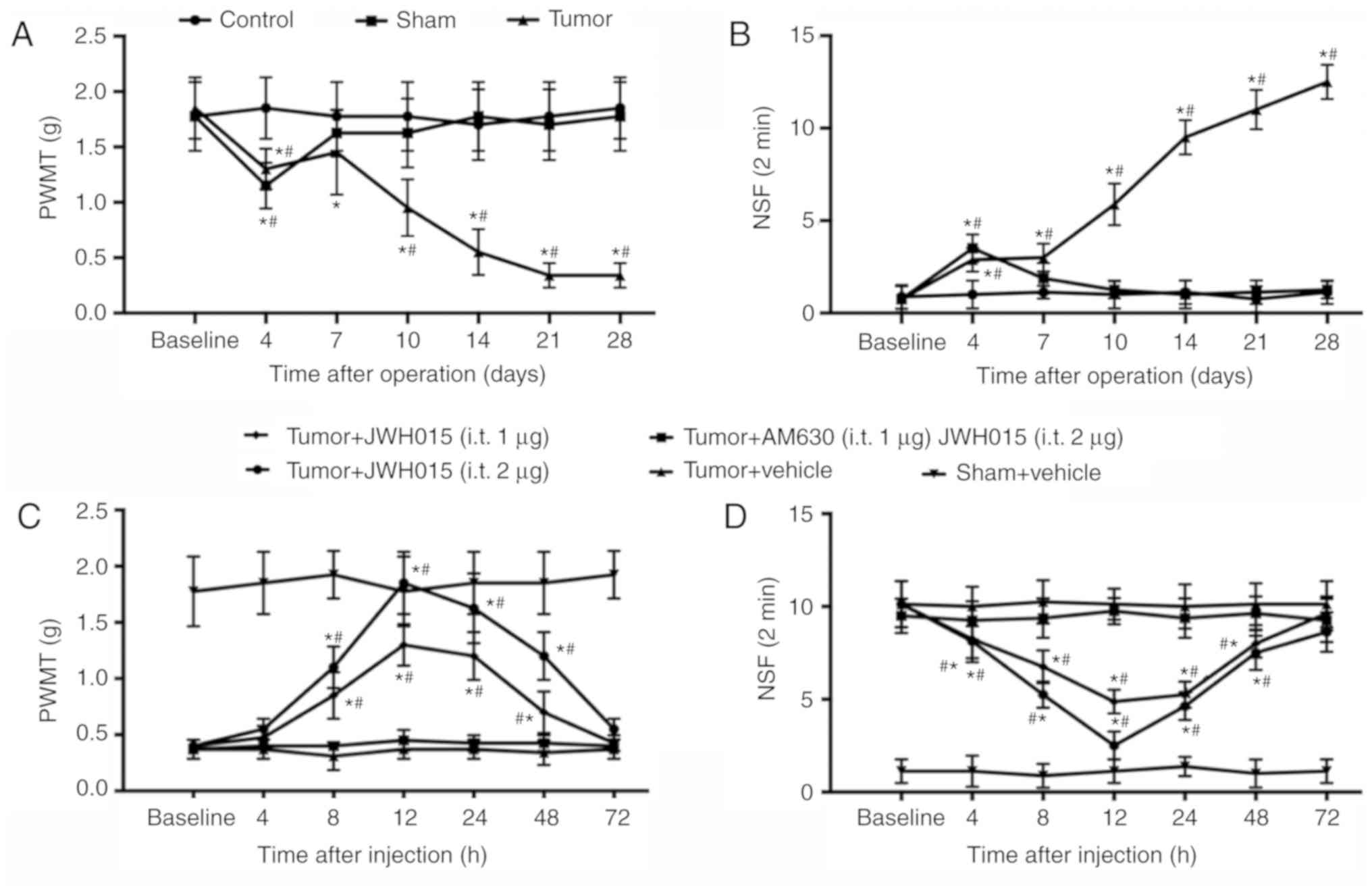 | Figure 1.Intrathecal administration of JWH015
attenuates bone cancer pain. Intra-femur implantation of NCTC 2476
cells induced mechanical hypersensitivity in the ipsilateral hind
paw. The (A) PWMT and (B) NSF were measured on days 0, 4, 7, 10,
14, 21 and 28 after operation in Control, Sham and Tumor group
mice. JWH015 and AM630 were intrathecally injected on day 14 after
the operation. (C) PWMT and (D) NSF were tested before
administration (baseline, 0 h) and at 4, 8, 12, 24, 48 and 72 h
after administration of JWH015. Data are expressed as the mean ±
SD; n=8; *P<0.05 vs. Baseline; #P<0.05 vs. Sham at
each time point. PWMT, paw withdrawal mechanical threshold; NSF,
Number of spontaneous flinches. |
Activation of CB2 by intrathecal administration of
JWH015 on day 14 significantly improved the pain behaviors. There
were no significant differences were identified in PWMT and NSF in
the Sham group following vehicle treatment. JWH015-treated mice
exhibited a large increase in PWMT and a decrease in NSF in a
dose-dependent manner (Fig. 1C and
D, respectively). PWMT increased and NSF decreased from 8 to 48
h after administration in the Tumor + JWH015 (1 µg) group
(P<0.05) and the Tumor + JWH015 (2 µg) group (P<0.05)
compared with the respective baseline values. The analgesic effect
of JWH015 peaked at 12 h after injection, with an increase in PWMT
to 1.85±0.278 g in the Tumor + JWH015 (2 µg) group and 1.3±0.185 g
in the Tumor+JWH015 (1 µg) group. However, the pain-relief effect
of JWH015 was completely prevented by pretreatment with the CB2
antagonist AM630 (Fig. 1C and
D).
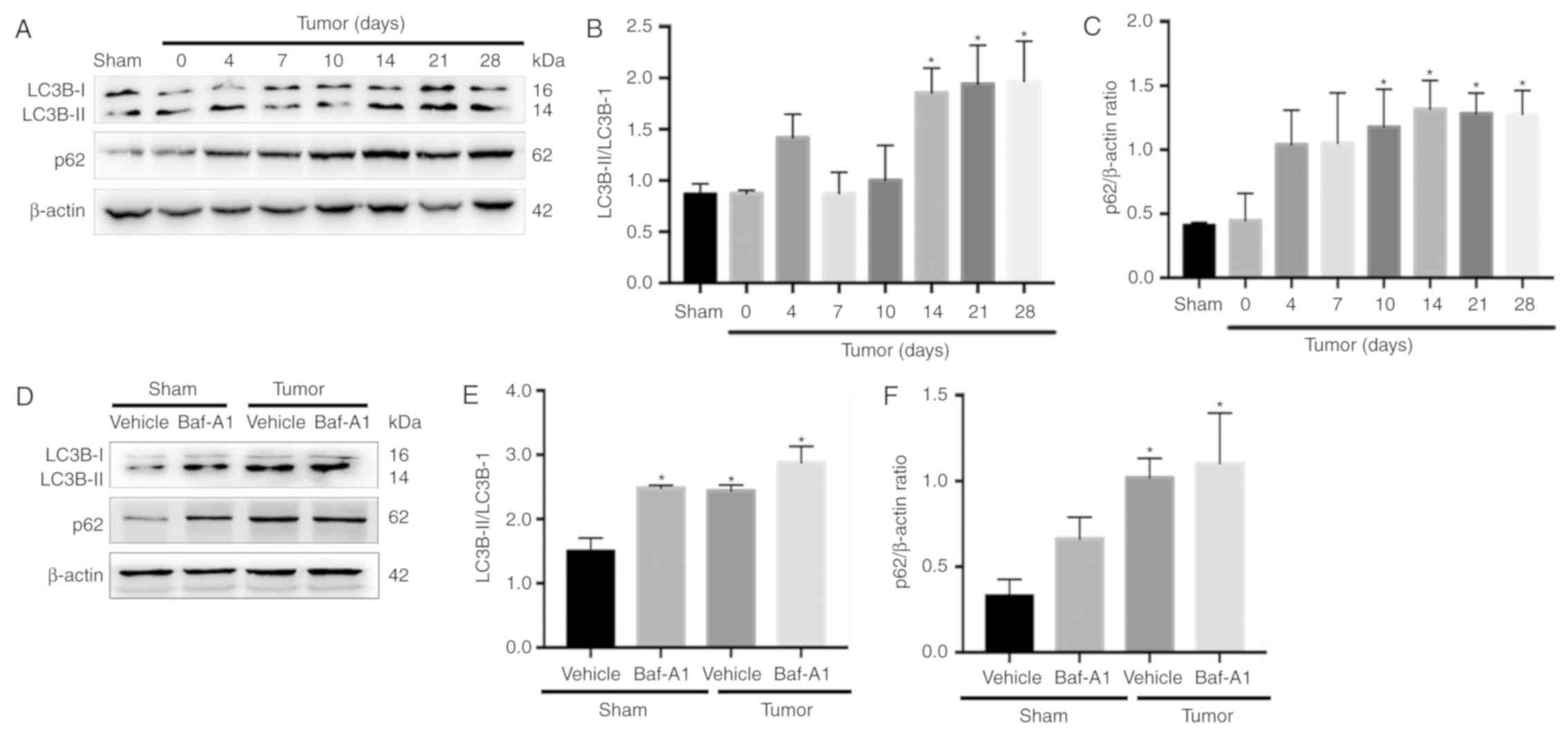
Autophagy flux is impaired in BCP
mice
There are two major autophagic marker proteins, LC3B
and p62. LC3B-I is converted to LC3B-II during the formation of
autophagosomes (35), whereas p62
is degraded by the autophagosome-lysosome pathway and represents
the degradation level of autophagosomes (36). To determine whether autophagy flux
was impaired in the spinal cord during BCP, western blotting was
performed. The results indicated that LC3B-II the ratio of
LC3B-II/LC3B-I were significantly increased in BCP mice at 14, 21
and 28 days after operation compared with Sham group (Fig. 2A and B; P<0.05). In addition,
the expression of p62 was significantly increased from day 10 to 28
(Fig. 2C; P<0.05).
Baf-A1 suppresses autophagosome-lysosome fusion and
results in the accumulation of autophagosomes. As shown in Fig. 2D-F, intrathecal injection of 10 nM
Baf-A1 increased the ratio of LC3B-II/LC3B-I and the expression of
p62 in the Sham mice compared with the values in the Sham + vehicle
mice (P<0.05). These results indicated that autophagy flux was
impaired in BCP model mice.
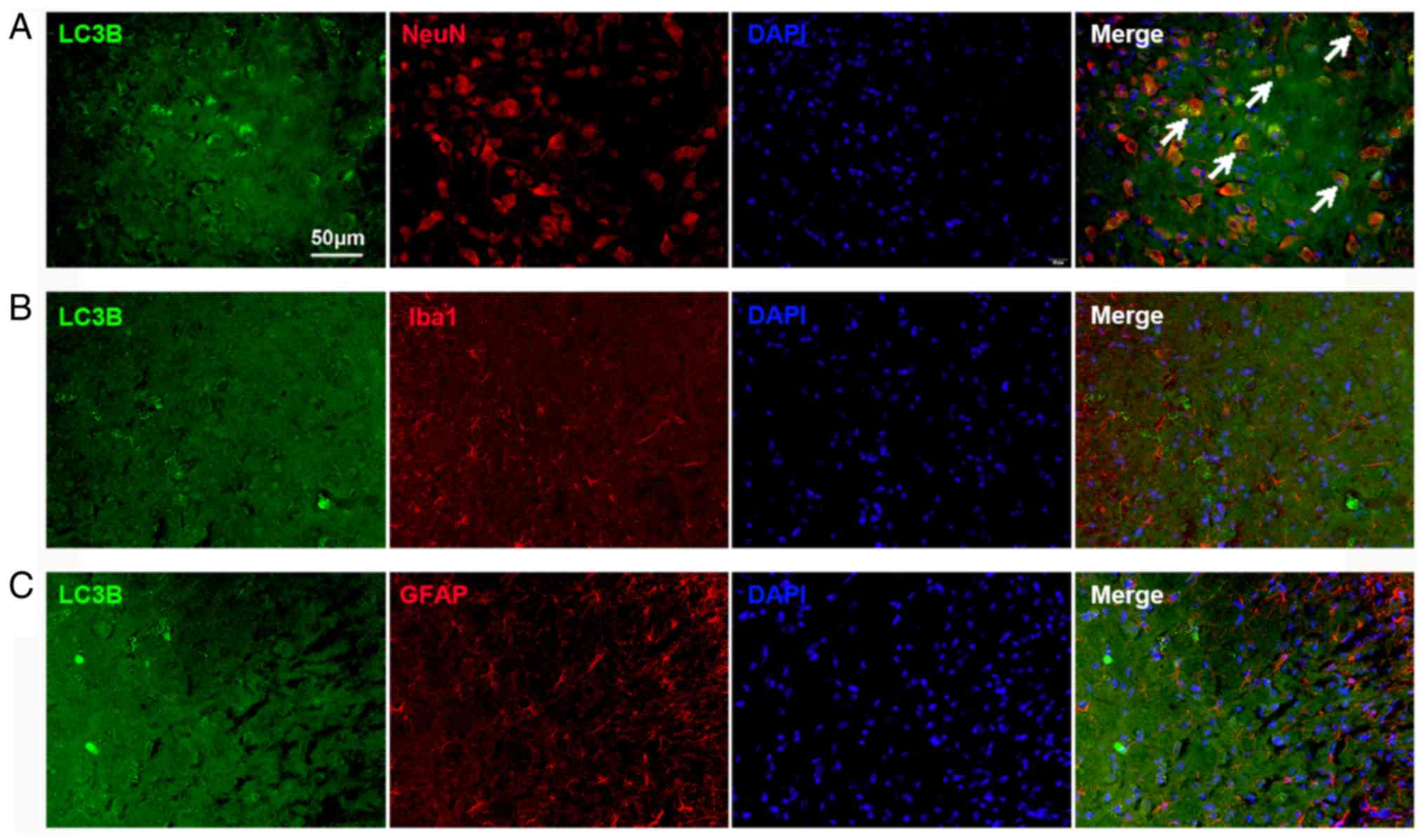
Impaired autophagy flux is located in
neurons in the spinal cord
The cellular localization of LC3B expression was
determined using double immunofluorescence staining. The spinal
tissues were collected on day 14 from the Tumor groups. The results
demonstrated substantial LC3B expression in the spinal dorsal horn,
which was mostly colocalized with NeuN, a neuronal marker (Fig. 3A). The expression of LC3B in
astrocytes and microglia was also examined. No colocalization with
GFAP (an astrocyte marker; Fig.
3B) and Iba1 (a microglial marker; Fig. 3C) in the spinal cord was found.
These results indicated that the autophagy flux in the dorsal horn
neurons was impaired in BCP.
Intrathecal administration of JWH015
ameliorates impaired autophagy flux in BCP
Based on the protective role of autophagy in pain
and the relationship between autophagy and the CB2 in several
diseases (19–24), the relationship between the CB2 and
autophagy was involved in BCP was investigated. There were
significant increase of the ratio of LC3B-II/LC3B-I and the
expression of p62 in the Tumor + vehicle group compared with the
Sham + vehicle group (P<0.05). Intrathecal administration of
JWH015 decreased the ratio of LC3B-II/LC3B-I in the Tumor + JWH015
(1 µg) and Tumor + JWH015 (2 µg) groups, as well as decreased the
expression of p62 in the tumor + JWH015 (2 µg) group, compared with
the values in the Tumor + vehicle group (Fig. 4A-C; P<0.05). Pretreatment with
the CB2 antagonist AM630 reversed the increased autophagy flux in
the JWH015-treated mice. The protein expression levels of LC3B and
p62 were also examined at different time points following JWH015 (2
µg) injection; the ratio of LC3B-II/LC3B-I and the expression of
p62 decreased from 8 to 72 h after injection (Fig. 4D-F; P<0.05). In addition, the
ratio of LC3B-II/LC3B-I and the expression of p62 increased in the
Tumor + JWH015 (2 µg) + Baf-A1 (10 nM) group compared with the
values in the Tumor + JWH015 (2 µg) group (Fig. 4G-I; P<0.05). Collectively, these
results suggested that JWH015 ameliorated the impaired autophagy
flux in BCP.
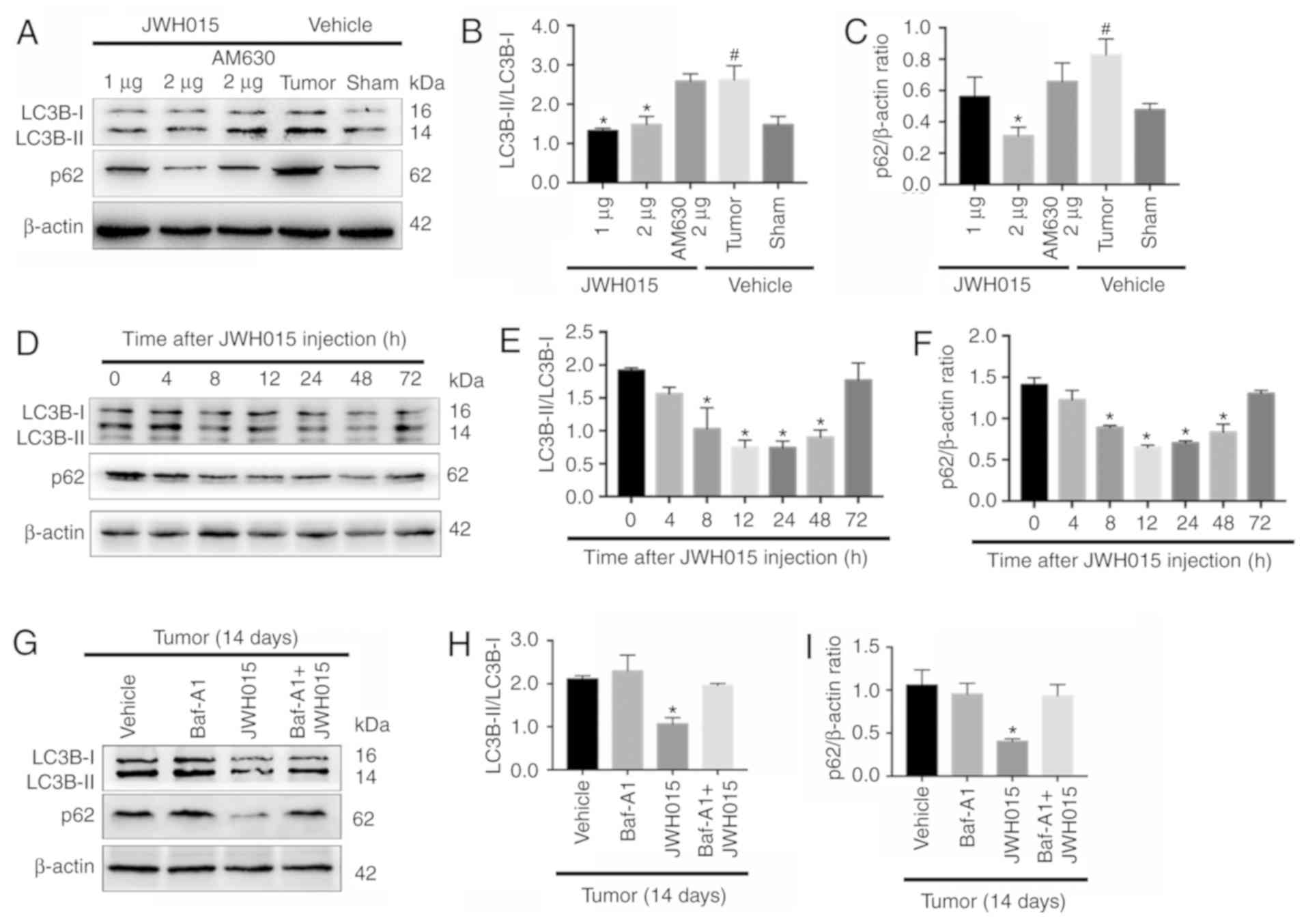 | Figure 4.Intrathecal administration of JWH015
ameliorates impaired autophagy flux in bone cancer pain. (A)
Representative western blotting images of LC3B and p62 in the
spinal cord 12 h after treatment with JWH015. (B) Ratio of
LC3B-II/LC3B-I and (C) quantification of p62 protein expression
levels. #P<0.05 vs. Sham + vehicle group; *P<0.05
vs. Tumor + vehicle group. (D) Representative western blots of LC3B
and p62 protein expression in the spinal cord at 0, 4, 8, 12, 24,
48 and 72 h after JWH015 (2 µg) injection. (E) Ratio of
LC3B-II/LC3B-I and (F) quantification of p62. *P<0.05 vs. 0 h.
(G) Representative blots of LC3B and p62 in the spinal cord with
treatment of vehicle, Baf-A1 (10 nM), JWH015 (2 µg), and Baf-A1 (10
nM) + JWH015 (2 µg) in BCP mice on day 14. (H) Ratio of
LC3B-II/LC3B-I. (I) Quantification of p62. *P<0.05 vs. tumor +
vehicle group. For all western blots, β-actin was used as a loading
control; data are expressed as the mean ± SD n=3 per group. LC3B,
microtubule-associated protein 1 light chain 3β. |
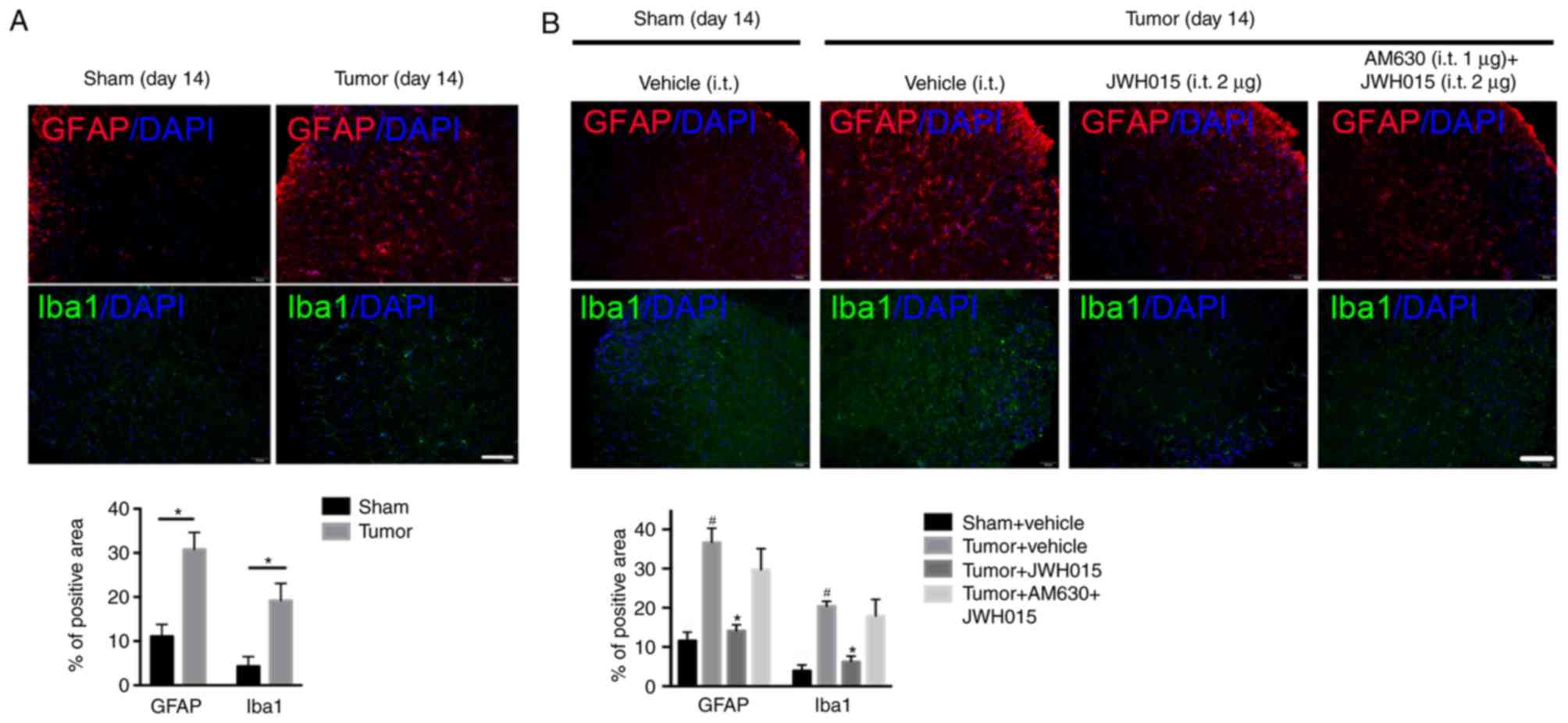
Intrathecal injection of JWH015
inhibits the activation of astrocytes and microglia, and
downregulates IL-1β and IL-6 in BCP mice
Although the results aforementioned demonstrated the
involvement of CB2-mediated autophagy flux in BCP, the mechanism by
which CB2 affected autophagy was not clarified. Accumulating
evidence has indicated the role of inflammation as the bridge
between the CB2 and autophagy (21,22).
Thus, whether the amelioration of autophagy flux by JWH015 was
mediated by the modulation of inflammation was determined.
Glial cell-derived pro-inflammatory mediators, such
as IL-1β and IL-6, have been reported to play key roles in the
pathophysiology of pain (29).
Increases in the staining density of GFAP (P<0.05) and Iba1
(P<0.05) were observed on day 14 after operation in BCP mice
compared with the levels in the Sham mice (Fig. 5A). Western blotting revealed the
significantly increased expression levels of IL-1β on days 4, 14,
21 and 28 (Fig. 6A and B;
P<0.05) and of IL-6 on day 14 (Fig.
6A and B; P<0.05) in the spinal cord in BCP mice compared
with the levels in the Sham mice.
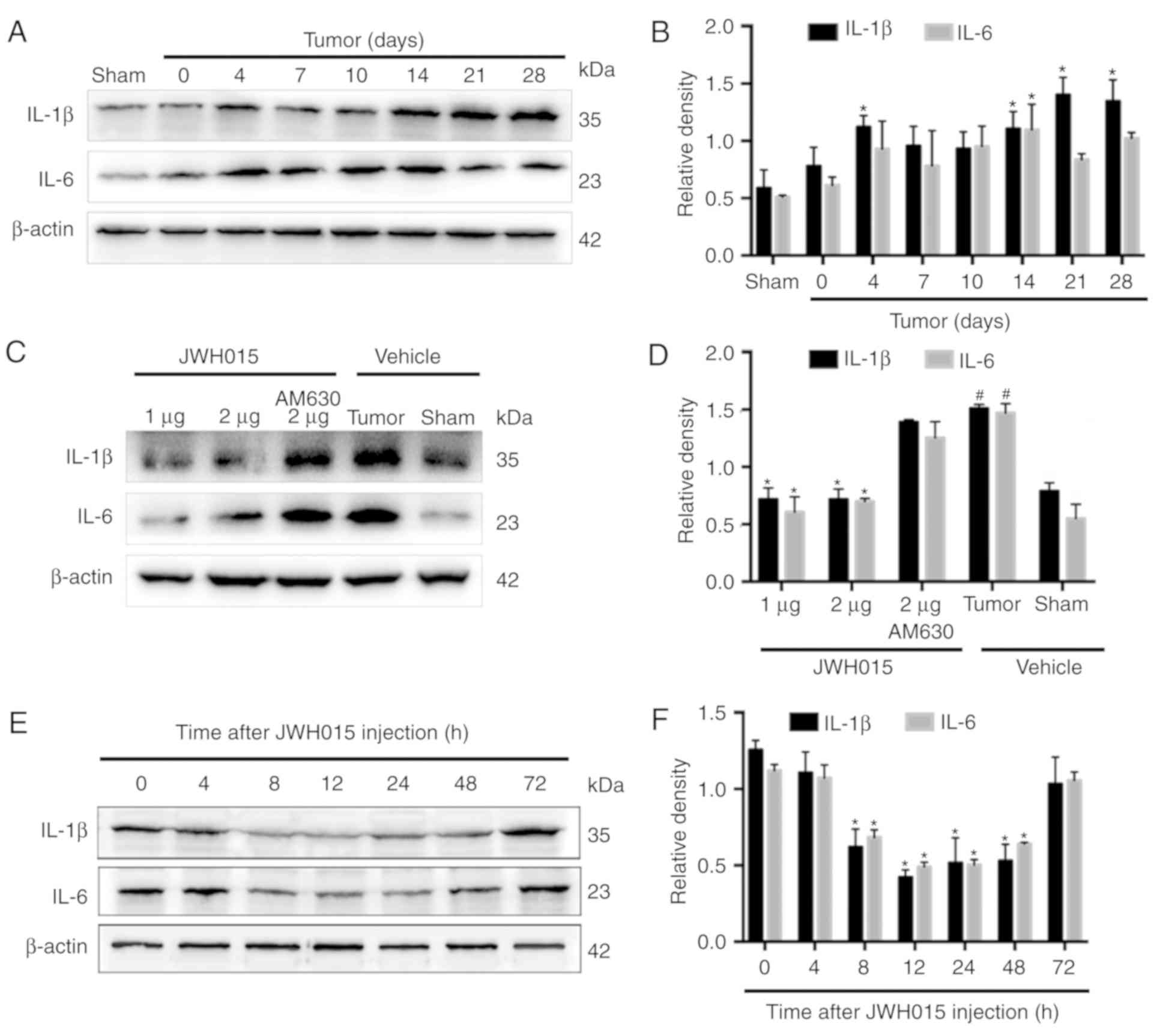 | Figure 6.Intrathecal administration of JWH015
downregulated the expression of pro-inflammatory mediators IL-1β
and IL-6. (A) Representative western blotting images and (B)
quantification of IL-1β and IL-6 protein expression in the spinal
cord in Sham and Tumor mice on day 0, 4, 7, 10, 14, 21 and 28 after
the operation. *P<0.05 vs. Sham. (C) Representative western
blots and (D) quantification of IL-1β and IL-6 expression in the
spinal cord 12 h after the treatment of JWH015.
#P<0.05 vs. Sham + vehicle group; *P<0.05 vs.
Tumor + vehicle. (E) Representative blots and (F) quantification of
IL-1β and IL-6 in the spinal cord at 0, 4, 8, 12, 24, 48 and 72 h
after JWH015 (2 µg) injection. *P<0.05 vs. 0 h. For all blots,
β-actin was used as a loading control; data are expressed as the
mean ± SD; n=3. IL, interleukin. |
Intrathecal administration of JWH015 at a dosage of
2 µg decreased the staining density of GFAP and Iba1 at 12 h after
treatment (Fig. 5B; P<0.05)
compared with the values in the Tumor + vehicle group. Compared
with the values in the Tumor + vehicle group, intrathecal injection
of 1 and 2 µg JWH015 significantly lowered the protein expression
levels of IL-1β and IL-6 (Fig. 6C and
D; P<0.05). The expression of IL-1β and IL-6 decreased
between 8 and 72 h after JWH015 (2 µg) injection (Fig. 6E and F, P<0.05). However, the
suppressive effect of JWH015 was completely prevented by
pretreatment with AM630 (Fig. 6C and
D; P>0.05).
In summary, the activation of CB2 by the intrathecal
administration of JWH015 inhibited the activation of glial cells,
and downregulated glial cell-derived IL-1β and IL-6, and this may
be the underlying mechanism by which autophagy flux was
ameliorated.
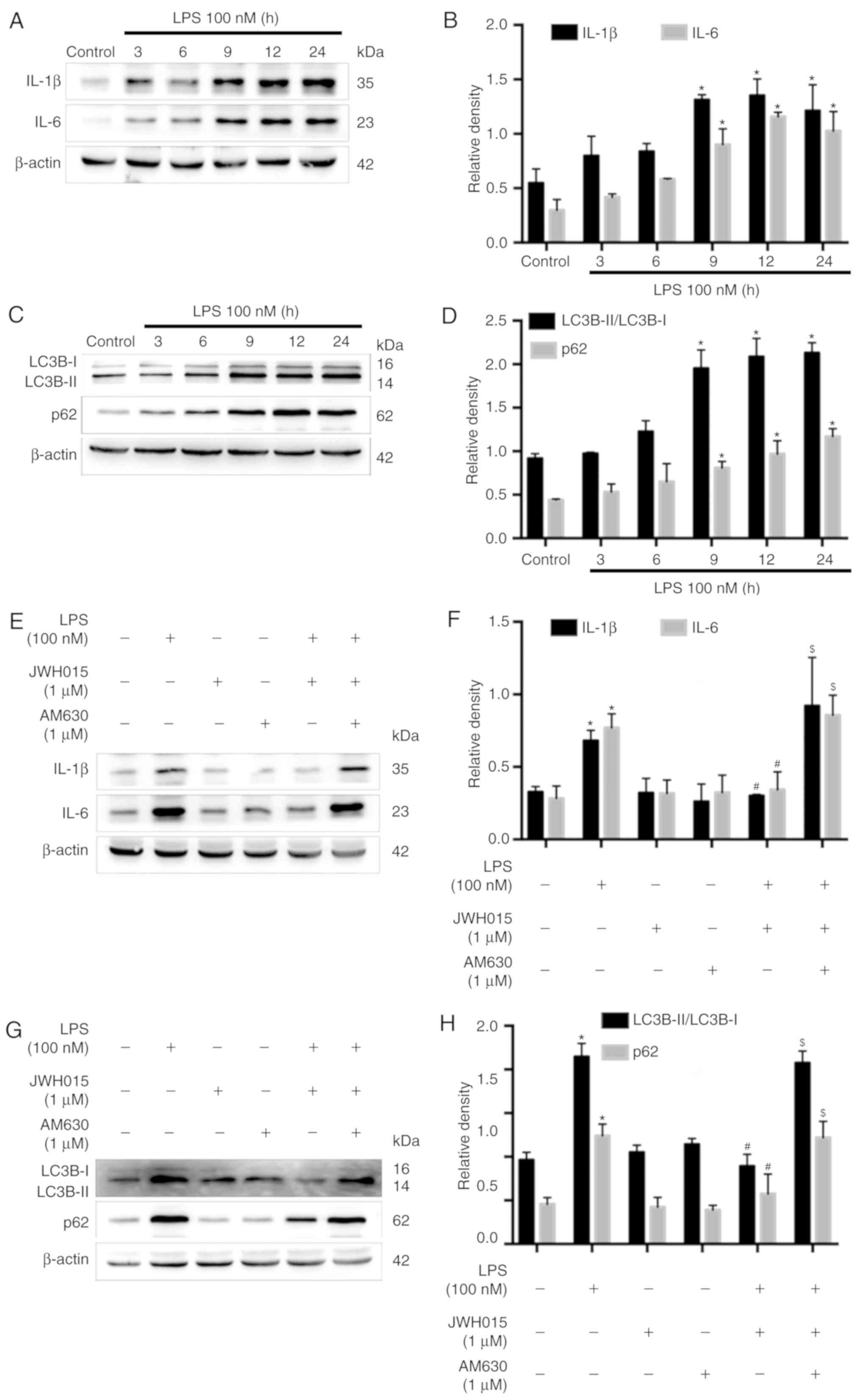
Amelioration of impaired autophagy
flux in LPS-stimulated primary neurons by JWH015 is mediated by the
downregulation of IL-1β and IL-6
Previous studies have reported the increased
expression of IL-1β and IL-6 following LPS-stimulation in various
cells (37,38). Therefore, LPS-stimulated primary
neurons were used as an in vitro model to further explore
the role of IL-1β and IL-6 in the activation of autophagy by
JWH015. Primary neurons were stimulated with LPS (100 nM) for 0, 3,
6, 9, 12 or 24 h. Western blotting revealed significantly increased
protein expression levels of IL-1β and IL-6 in primary neurons at
9, 12 and 24 h after LPS stimulation, compared with untreated
Control cells (Fig. 7A and B;
P<0.05), which indicated an inflammatory environment induced by
LPS. IL-1β and IL-6 were upregulated over time following LPS
stimulation, along with increases in the ratio of LC3B-II/LC3B-I
and expression levels of p62 at 9, 12 and 24 h after stimulation
(Fig. 7C and D; P<0.05), which
indicated the impairment of autophagy flux induced by the
LPS-mediated production of IL-1β and IL-6 in primary neurons.
Treatment with JWH015 at a dosage of 1 µM for 12 h in LPS
stimulated-primary neurons significantly reduced IL-1β and IL-6
expression levels (Fig. 7E and F;
P<0.05). In addition, the ratio of LC3B-II/LC3B-I and the
expression of p62 decreased in the JWH015-treated group (Fig. 7G and H; P<0.05). These effects
could be prevented by pretreatment with AM630 as the expression of
IL-1β and IL-6, the ratio of LC3B-II/LC3B-I, and the expression of
p62 was increased in the LPS + JWH015 + AM630 group compared with
the LPS + JWH015 group (P<0.05).
In summary, the results of the in vitro
experiment demonstrated that the impairment of autophagy flux was
induced by the production of IL-1β and IL-6 in LPS-stimulated
primary neurons. JWH015 may ameliorate autophagy by the
downregulation of the inflammatory mediators IL-1β and IL-6.
Discussion
BCP is common in patients with advanced cancer, and
the extreme pain is difficult to completely control. The results of
the present study demonstrated that autophagy flux was impaired
during the progression of BCP. Intrathecal administration of the
selective CB2 agonist JWH015 alleviated BCP through the
amelioration of impaired autophagy flux in spinal neurons. The
production of IL-1β and IL-6 in LPS-stimulated primary neurons
impaired autophagy, and treatment with JWH015 reversed the impaired
autophagy by downregulating IL-1β and IL-6 expression levels. The
results indicated a potential mechanism by which the CB2 affects
pain relief. Activation of the CB2 by JWH015 alleviated
hyperalgesia by ameliorating the impaired autophagy induced by
glia-derived inflammatory mediators in spinal neurons.
BCP remains a clinically challenging problem
(39,40). Its etiology and mechanisms are
complex and poorly elucidated. The current therapeutic options for
BCP are not very effective and have many unresolvable side effects
(41). The endocannabinoid system
serves important roles in pain states, and the analgesic properties
of cannabinoid receptor agonists have been extensively described
(9–12). The present study verified the pain
relief effect of the intrathecal administration of the
CB2-selective agonist JWH015 on tumor-evoked mechanical
hyperalgesia, which was similar to results reported in our previous
studies (29,32,33).
CB2 agonists have been shown to exert analgesic effects in various
models of pain, such as inflammatory pain (42), neuropathic pain (15) and BCP. Our previous studies
indicated that the CB2 could attenuate bone cancer pain via the
modification of N-methyl D-aspartate receptor subtype 2B (NR2B; a
subunit of NMDAR) (33), G-protein
coupled receptor kinase 2 (32),
inflammatory cytokines, and glial cells (29). Other studies have reported that CB2
could modulate the NACHT, LRR and PYD domains-containing protein 3
(NLRP3) inflammasome (14) and
microglial phenotype (43) in
different models of pain relief. These results suggested that the
CB2 may induce analgesic effects through various pathways; however,
little attention has been given to autophagy.
Autophagy, a cellular self-digestive process
(44), is associated with several
diseases, such as cancers and neurodegenerative and inflammation
diseases (45,46). Autophagy has been proposed to serve
a role in pain, and the impairment of autophagy has been reported
in neuropathic pain (47).
Metformin was demonstrated to relieve neuropathic pain through
autophagy flux stimulation (48).
Moreover, impaired autophagy in GABAergic interneurons was found in
neuropathic pain (49). In the
present study, the ratio of LC3B-II/LC3B-I and the expression of
p62 increased, which indicated decreased formation of
autophagosomes and degradation of the autophagosome-lysosome
pathway. Baf-A1, an inhibitor of autophagosome and lysosome fusion,
induced an increase in the ratio of LC3B-II/LC3B-I in the Sham mice
and exacerbated the increase in the ratio of LC3B-II/LC3B-I in the
Tumor mice. These results provided further evidence of the impaired
autophagy flux in BCP. A relationship between the CB2 and autophagy
has been proposed in various diseases (21,22).
The CB2-selective agonist JWH015 is capable of inhibiting tumor
cell growth through the stimulation of autophagy (23,24).
Thus, the expression levels of autophagy proteins in BCP mice
treated with JWH015 were investigated. A decrease in the ratio of
LC3B-II/LC3B-I and the downregulation of p62 in neurons were found
in JWH015-treated mice compared with the levels in vehicle-treated
mice. The increase in autophagy flux together with the attenuation
of tumor-evoked mechanical hyperalgesia indicated that autophagy
may serve an important role in the analgesic mechanism of CB2.
Several mechanisms for the modulation of autophagy
by the CB2 have been proposed, mainly focusing on inflammation
(50,21,20).
For example, cannabinoids can inhibit energetic metabolism and
induce AMPK-dependent autophagy in pancreatic cancer cells
(50). Activation of CB2 by JWH133
was reported to inhibit hepatic inflammation and activate autophagy
in macrophages in alcoholic liver disease (21). Inhibition of NLRP3 inflammasomes
and activation of autophagy were found in HU308 (a specific agonist
of CB2R)-stimulated BV2 cells (20). Crosstalk between autophagy and
inflammation is involved in many pathological conditions. For
example, autophagy suppresses inflammation in kidney diseases
(51) and environmental ultrafine
particulate matter (PM)-induced airway epithelial injury (52). Only limited studies have focused on
the effect of inflammation on autophagy (27,28).
The present study explored the possible mechanism by which
glia-derived pro-inflammatory mediators induced autophagy in BCP.
It was demonstrated that the impairment of autophagy in BCP mainly
occurred in spinal neurons. Thus, the mechanism of
inflammation-induced autophagy in primary neurons was investigated.
After stimulation by LPS, the expression of IL-1β and IL-6 was
increased and autophagy was impaired in primary neurons. These
results suggested that stimulation by inflammatory mediators may
impair autophagy in neurons. Treatment with JWH015 increased the
expression of autophagy-related proteins and downregulated IL-1β
and IL-6, which further verified the results of the in vivo
experiments.
In conclusion, the results of the present study
indicated that intrathecal administration of the selective CB2
agonist JWH015 alleviated BCP and that the amelioration of impaired
neuronal autophagy mediated by the downregulation of glia-derived
IL-1β and IL-6 may underlie this pain-relief effect. This was a
preliminary study and further studies are required to elucidate the
function of autophagy in different cell types.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant. nos. 81471129,
81671087, 81500954, 81701102 and 81771142) and a grants from The
Department of Health of Jiangsu Province of China (grant. nos.
XK101140 and RC2011006).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YM, ZM and XG conceived and designed the
experiments. YM, YH, YZ and CW carried out all experiments. YM, YH,
HW, XT, YuL, BH, YiL and HR helped conduct the experiments and
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments conformed with the Regulation
of Animal Care Management of the Ministry of Public Health,
People's Republic of China and were approved by the Ethical
Committee of the Medical School of Nanjing University (Nanjing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mantyh P: Bone cancer pain: Causes,
consequences, and therapeutic opportunities. Pain. 154 (Suppl
1):S54–S62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantyh PW: Cancer pain and its impact on
diagnosis, survival and quality of life. Nat Rev Neurosci.
7:797–809. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honore P, Luger NM, Sabino MA, Schwei MJ,
Rogers SD, Mach DB, O'keefe PF, Ramnaraine ML, Clohisy DR and
Mantyh PW: Osteoprotegerin blocks bone cancer-induced skeletal
destruction, skeletal pain and pain-related neurochemical
reorganization of the spinal cord. Nat Med. 6:521–528. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller K, Steger GG, Niepel D and Luftner
D: Harnessing the potential of therapeutic agents to safeguard bone
health in prostate cancer. Prostate Cancer Prostatic Dis.
21:461–472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji RR, Xu ZZ and Gao YJ: Emerging targets
in neuroinflammation-driven chronic pain. Nat Rev Drug Discov.
13:533–548. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ligresti A, De Petrocellis L and Di Marzo
V: From phytocannabinoids to cannabinoid receptors and
endocannabinoids: Pleiotropic physiological and pathological roles
through complex pharmacology. Physiol Rev. 96:1593–1659. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kendall DA and Yudowski GA: Cannabinoid
receptors in the central nervous system: Their signaling and roles
in disease. Front Cell Neurosci. 10:2942016.PubMed/NCBI
|
|
8
|
Devane WA, Dysarz FA III, Johnson MR,
Melvin LS and Howlett AC: Determination and characterization of a
cannabinoid receptor in rat brain. Mol Pharmacol. 34:605–613.
1988.PubMed/NCBI
|
|
9
|
Pernia-Andrade AJ, Kato A, Witschi R,
Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W,
Schüttler J, et al: Spinal endocannabinoids and CB1 receptors
mediate C-fiber-induced heterosynaptic pain sensitization. Science.
325:760–764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Racz I, Nadal X, Alferink J, Baños JE,
Rehnelt J, Martín M, Pintado B, Gutierrez-Adan A, Sanguino E,
Manzanares J, et al: Crucial role of CB(2) cannabinoid receptor in
the regulation of central immune responses during neuropathic pain.
J Neurosci. 28:12125–12135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pertwee RG: Targeting the endocannabinoid
system with cannabinoid receptor agonists: Pharmacological
strategies and therapeutic possibilities. Philos Trans R Soc Lond B
Biol Sci. 367:3353–3363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernandez-Ruiz J, Romero J, Velasco G,
Tolon RM, Ramos JA and Guzman M: Cannabinoid CB2 receptor: A new
target for controlling neural cell survival? Trends Pharmacol Sci.
28:39–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalant H: Adverse effects of cannabis on
health: An update of the literature since 1996. Prog
Neuropsychopharmacol Biol Psychiatry. 28:849–863. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao F, Xiang HC, Li HP, Jia M, Pan XL, Pan
HL and Li M: Electroacupuncture inhibits NLRP3 inflammasome
activation through CB2 receptors in inflammatory pain. Brain Behav
Immun. 67:91–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niu J, Huang D, Zhou R, Yue M, Xu T, Yang
J, He L, Tian H, Liu X and Zeng J: Activation of dorsal horn
cannabinoid CB2 receptor suppresses the expression of P2Y12 and
P2Y13 receptors in neuropathic pain rats. J Neuroinflammation.
14:1852017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
La Porta C, Bura SA, Aracil-Fernandez A,
Manzanares J and Maldonado R: Role of CB1 and CB2 cannabinoid
receptors in the development of joint pain induced by monosodium
iodoacetate. Pain. 154:160–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li AL, Lin X, Dhopeshwarkar AS, Thomaz AC,
Carey LM, Liu Y, Nikas SP, Makriyannis A, Mackie K and Hohmann AG:
Cannabinoid CB2 Agonist AM1710 differentially suppresses distinct
pathological pain states and attenuates morphine tolerance and
withdrawal. Mol Pharmacol. 95:155–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu J, Hocevar M, Bie B, Foss JF and Naguib
M: Cannabinoid Type 2 receptor system modulates Paclitaxel-induced
microglial dysregulation and central sensitization in rats. J Pain.
20:501–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng XL, Deng HB, Wang ZG, Wu Y, Ke JJ and
Feng XB: Suberoylanilide hydroxamic acid triggers autophagy by
influencing the mTOR pathway in the spinal dorsal horn in a rat
neuropathic pain model. Neurochem Res. 44:450–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coccurello R, Nazio F, Rossi C, De Angelis
F, Vacca V, Giacovazzo G, Procacci P, Magnaghi V, Ciavardelli D and
Marinelli S: Effects of caloric restriction on neuropathic pain,
peripheral nerve degeneration and inflammation in normometabolic
and autophagy defective prediabetic Ambra1 mice. PLoS One.
13:e02085962018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao BZ, Wei W, Ke P, Xu ZQ, Zhou JX and
Liu C: Activating cannabinoid receptor 2 alleviates pathogenesis of
experimental autoimmune encephalomyelitis via activation of
autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther.
20:1021–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Denaes T, Lodder J, Chobert MN, Ruiz I,
Pawlotsky JM, Lotersztajn S and Teixeira-Clerc F: The Cannabinoid
Receptor 2 protects against alcoholic liver disease via a
macrophage autophagy-dependent pathway. Sci Rep. 6:288062016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vara D, Salazar M, Olea-Herrero N, Guzman
M, Velasco G and Diaz-Laviada I: Anti-tumoral action of
cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent
activation of autophagy. Cell Death Differ. 18:1099–1111. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salazar M, Carracedo A, Salanueva IJ,
Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C,
Torres S, García S, et al: Cannabinoid action induces
autophagy-mediated cell death through stimulation of ER stress in
human glioma cells. J Clin Invest. 119:1359–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Netea-Maier RT, Plantinga TS, van de
Veerdonk FL, Smit JW and Netea MG: Modulation of inflammation by
autophagy: Consequences for human disease. Autophagy. 12:245–260.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian R, Li Y and Yao X: PGRN suppresses
inflammation and promotes autophagy in keratinocytes through the
Wnt/β-Catenin signaling pathway. Inflammation. 39:1387–1394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sil S, Niu F, Tom E, Liao K, Periyasamy P
and Buch S: Cocaine mediated neuroinflammation: Role of
dysregulated autophagy in pericytes. Mol Neurobiol. 56:3576–3590.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piippo N, Korhonen E, Hytti M, Kinnunen K,
Kaarniranta K and Kauppinen A: Oxidative stress is the principal
contributor to inflammasome activation in retinal pigment
epithelium cells with defunct proteasomes and autophagy. Cell
Physiol Biochem. 49:359–367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu C, Liu Y, Sun B, Sun Y, Hou B, Zhang Y,
Ma Z and Gu X: Intrathecal injection of JWH-015 attenuates bone
cancer pain via time-dependent modification of pro-inflammatory
cytokines expression and astrocytes activity in spinal cord.
Inflammation. 38:1880–1890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Demers G, Griffin G, De Vroey G, Haywood
JR, Zurlo J and Bédard M: Animal research. Harmonization of animal
care and use guidance. Science. 312:700–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schwei MJ, Honore P, Rogers SD,
Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR and Mantyh
PW: Neurochemical and cellular reorganization of the spinal cord in
a murine model of bone cancer pain. J Neurosci. 19:10886–10897.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu C, Shi L, Sun B, Zhang Y, Hou B, Sun Y,
Ma Z and Gu X: A single intrathecal or intraperitoneal injection of
CB2 receptor agonist attenuates bone cancer pain and induces a
time-dependent modification of GRK2. Cell Mol Neurobiol.
37:101–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu X, Mei F, Liu Y, Zhang R, Zhang J and
Ma Z: Intrathecal administration of the cannabinoid 2 receptor
agonist JWH015 can attenuate cancer pain and decrease mRNA
expression of the 2B subunit of N-methyl-D-aspartic acid. Anesth
Analg. 113:405–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hylden JL and Wilcox GL: Intrathecal
morphine in mice: A new technique. Eur J Pharmacol. 67:313–316.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mizushima N and Klionsky DJ: Protein
turnover via autophagy: Implications for metabolism. Annu Rev Nutr.
27:19–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bjorkoy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: p62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu Y, Li G, Zhang Y, Liu N, Zhang P, Pan
C, Nie H, Li Q and Tang Z: Upregulated TSG-6 expression in ADSCs
inhibits the BV2 Microglia-mediated inflammatory response. Biomed
Res Int. 2018:72391812018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye J, Guan M, Lu Y, Zhang D, Li C and Zhou
C: Arbutin attenuates LPS-induced lung injury via
Sirt1/Nrf2/NF-kappaBp65 pathway. Pulm Pharmacol Ther. 54:53–59.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rubens RD: Bone metastases-the clinical
problem. Eur J Cancer. 34:210–213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sturge J, Caley MP and Waxman J: Bone
metastasis in prostate cancer: Emerging therapeutic strategies. Nat
Rev Clin Oncol. 8:357–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li MH, Suchland KL and Ingram SL:
Compensatory Activation of cannabinoid CB2 receptor inhibition of
GABA release in the rostral ventromedial medulla in inflammatory
pain. J Neurosci. 37:626–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luongo L, Palazzo E, Tambaro S, Giordano
C, Gatta L, Scafuro MA, Rossi FS, Lazzari P, Pani L, de Novellis V,
et al:
1-(2′,4′-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide,
a novel CB2 agonist, alleviates neuropathic pain through functional
microglial changes in mice. Neurobiol Dis. 37:177–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin-induced autophagy leads to the apoptosis in breast cancer
stem cells: Molecular mechanisms. Mol Cancer. 12:1712013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Deretic V, Saitoh T and Akira S: Autophagy
in infection, inflammation and immunity. Nat Rev Immunol.
13:722–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Piao Y, Gwon DH, Kang DW, Hwang TW, Shin
N, Kwon HH, Shin HJ, Yin Y, Kim JJ, Hong J, et al: TLR4-mediated
autophagic impairment contributes to neuropathic pain in chronic
constriction injury mice. Mol Brain. 11:112018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weng W, Yao C, Poonit K, Zhou X, Sun C,
Zhang F and Yan H: Metformin relieves neuropathic pain after spinal
nerve ligation via autophagy flux stimulation. J Cell Mol Med.
23:1313–1324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yin Y, Yi MH and Kim DW: Impaired
autophagy of GABAergic interneurons in neuropathic pain. Pain Res
Manag. 2018:91853682018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dando I, Donadelli M, Costanzo C, Dalla
Pozza E, D'Alessandro A, Zolla L and Palmieri M: Cannabinoids
inhibit energetic metabolism and induce AMPK-dependent autophagy in
pancreatic cancer cells. Cell Death Dis. 4:e6642013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kimura T, Isaka Y and Yoshimori T:
Autophagy and kidney inflammation. Autophagy. 13:997–1003. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen ZH, Wu YF, Wang PL, Wu YP, Li ZY,
Zhao Y, Zhou JS, Zhu C, Cao C, Mao YY, et al: Autophagy is
essential for ultrafine particle-induced inflammation and mucus
hyperproduction in airway epithelium. Autophagy. 12:297–311. 2016.
View Article : Google Scholar : PubMed/NCBI
|