Introduction
Tuberculosis is a chronic infectious disease caused
by various strains of Mycobacterium tuberculosis (Mtb) that
causes ~200 million mortalities annually worldwide (1). Vitamin D deficiency is closely
associated with tuberculosis (2,3). Mtb
affects cell morphology, immunogenicity and drug resistance.
Mutations lead to the emergence of multi-drug resistance (4).
Prior to the identification of specific
anti-tuberculosis drugs, vitamin D was used for the clinical
treatment of tuberculosis. Following the development of
anti-tuberculosis drugs in the mid-1950s, the use of vitamin D
treatment for tuberculosis decreased (5). The most important members of the
vitamin D family are vitamin D2 and vitamin D3. Vitamin D3 is
metabolized to 25 hydroxyvitamin D3 [25(OH)D3] in hepatocytes.
Subsequently, 25(OH)D3 is bound with α-globulin and transferred to
the kidney, where it is hydroxylated by 25-hydroxyvitamin D-1 α
hydroxylase, mitochondrial (CYP27B1) and converted into the
biologically active 1,25(OH)2D3, which exerts biological functions
(6,7).
The biological effects of 1,25(OH)2D3 depend on
intracellular specific vitamin D receptor (VDR)-mediated signaling
(8,9). Vitamin D regulates the innate immune
response and the release of cytokines and chemokines against
pathogenic microorganisms (10).
Vitamin D has been demonstrated to have significant
anti-inflammatory effects against a variety of bacterial infections
(11). As early as the 1980s, Rook
et al (12) demonstrated
that vitamin D enhanced the bactericidal activity of macrophages
in vivo, in order to combat treatment resistance of Mtb.
Vitamin D serves an important role in anti-tuberculosis immunity
(13).
With the progression of studies examining the role
of 25(OH)D3 in immunity, the mechanism of vitamin D in the
prevention and treatment of tuberculosis has been gradually
elucidated. Liu et al (2)
confirmed that vitamin D deficiency may inhibit natural immune
function, resulting in susceptibility to tuberculosis infection,
and suggested that vitamin D enhanced the ability of macrophages to
kill bacteria by increasing the antimicrobial peptide secreted by
macrophages. Following the activation of the macrophagic toll-like
receptor 2/1 signaling pathway by the Mtb 19-kDa lipoprotein, the
expression levels of VDR and CYP27B1 are upregulated (2). The majority of the biological effects
of 1,25(OH)2D3 are mediated by its interaction with VDR. VDRs are
present in numerous types of cells, and serve important roles as an
agonist-activated transcription factor in the immune system
(8). VDR is expressed in T
lymphocytes, B lymphocytes and monocytes that differentiate into
dendritic cells (DCs) (14).
Mature DCs express sterol 26-hydroxylase, mitochondrial (CYP27A1)
and CYP27B1 (14). Cultures of DCs
are able to convert vitamin D3 to 1,25(OH)2D3 (14).
In the present study, the effects of Mtb infection
on DCs derived from vitamin D-deficient mice or normal mice were
investigated.
Materials and methods
Construction of vitamin D-deficient
mouse model
A total of 30 male SPF C57BL/6 mice, aged 6–8 weeks
old and weighing 20–25 g, were purchased from Changzhou Cavens
Laboratory Animal, Co., Ltd. The animals were treated in accordance
with animal ethics standards (15)
during the experiment and the study was approved by the Committee
for Experiments with the Use of Laboratory Animals in Shenyang
Military Region General Hospital (Shenyang, China; approval no.
AE20160823).
The mice were randomly divided into the following
two groups, which were housed separately: Vitamin D-deficient mice
model group; and normal control group. The vitamin D-deficient mice
model group was given vitamin D-deficient feed, and the normal
control group was provided with normal feed. The mice in the two
groups were placed on an ultra-clean bench and protected from
light. The mice were then exposed to air for 12 h and irradiated
with a yellow fluorescent lamp (18W/41/4P, Philips Healthcare)
containing no UV light for 12 h. Following exposure, the mice were
kept in the dark for an additional 12 h. After 12 weeks, the mice
were sacrificed by exsanguination under 2% isoflurane anesthesia
(16) in order to minimize animal
suffering, and were continuously monitored for respiration and
temperature. Complete anesthetization was defined as a loss of
consciousness, loss of awareness of sensation and suppression of
the autonomous reflex activity. Death was confirmed by either
cervical dislocation or cessation of circulation.
Animals were checked twice daily. Moribund animals
were sacrificed during the experiments using humane endpoints,
using following criteria (17): i)
Major organ failure or medical conditions unresponsive to treatment
such as severe respiratory distress, icterus, uremia, intractable
diarrhea or self-mutilation; ii) clinical or behavioral signs
unresponsive to appropriate intervention persisting for 24 h
including significant inactivity, labored breathing, sunken eyes,
hunched posture, piloerection/matted fur, one or more unresolving
skin ulcers and abnormal vocalization when handled. The NC3Rs
Animal Research: Reporting of in vivo experiments guidelines
(18) were followed. After 12
weeks, there were 10 mice in the vitamin D-deficient mice model
group and 12 mice in the normal control group. The tail of each
mouse was clamped under isoflurane anesthesia and 100 µl blood was
collected. The levels of 25(OH)D3 (cat. no. LS-F5643-1; LifeSpan
BioSciences) and 1,25(OH)2D3 (cat. no. MBS263019; MyBioSource,
Inc.) in the serums were determined by ELISA.
Bone marrow-derived DC (BMDC)
preparation and induction of DCs in vitro
The C57BL/6 mice were sacrificed by cervical
dislocation and then were immersed in 75% alcohol for 5 min. The
tibia and femur were removed under aseptic conditions and immersed
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
containing 1% fetal bovine serum (Hyclone; GE Healthcare Life
Sciences). The ends of the bones were removed with scissors and a 1
ml syringe was used to repeatedly rinse the marrow cavity with
RPMI-1640 medium. The bone marrow was washed into a sterile culture
dish. The cell suspension in the culture dish was collected, and
the supernatant and red blood cells were discarded using
centrifugation at 300 × g for 5 min at 4°C. The cells were
resuspended with RPMI-1640 medium + 10% FBS and inoculated in
24-well culture plates (1×106 cells/ml per well) with
the cytokines granulocyte-macrophage colony-stimulating factor (20
ng/ml; cat. no. G5035; Sigma-Aldrich; Merck KGaA) and interleukin
(IL)-4 (10 ng/ml; SRP3211; Sigma-Aldrich; Merck KGaA) for 6 days in
a cell culture incubator at 37°C and 5% CO2. The cells
were cultured for an additional 24 h in the presence of 0, 1 and 2
mg/ml Bacillus Calmette-Guérin antigen (BCG; Sinopharm Chemical
Reagent Co., Ltd.), and then harvested as DCs. Cell morphology
during BMDC induction from days 1 to 6 was observed by inverted
light microscopy (magnification, ×40; Leica Microsystems GbmH).
Identification of DC phenotype
The DCs were digested with 0.25% trypsin for 3 min,
centrifuged at 300 × g for 5 min at 4°C and then the supernatant
was discarded. The cells were resuspended in 1 ml of 1% BSA/PBS
buffer. The cell concentration was adjusted to 1×106
cells/ml and the cells were fixed with 0.1 ml polyformaldehyde for
30 min at 25°C Subsequently, fluorescein isothiocyanate-labeled
integrin alpha-X (CD11c; 1:50; 130-110-700; Miltenyi Biotech),
T-lymphocyte activation antigen CD86 (CD86; 1:50; 130-102-506;
Miltenyi Biotech), T-lymphocyte activation antigen CD80 (CD80;
1:50; 130-116-459; Miltenyi Biotech), major histocompatibility
complex class I (MHC-I; 1:50; ab95572; Abcam) and MHC-II (1:50;
130-112-386; Miltenyi Biotech) were added, respectively. Following
incubation for 1 h at 4°C in the dark, the cells were washed with
1% BSA/PBS buffer and measured by a MACSQuant analyzer 10 (Miltenyi
Biotech).
Detection of cytokines in the cell
supernatant by ELISA
There are 2 types of helper T (Th) lymphocytes: Th1
and Th2 cells. On day 7, the supernatants of the BMDC cultures were
collected and an ELISA was used to detect Th1 cytokines, including
IL-2 (cat. no. E-EL-M0042c; Elabscience), IL-12 (cat. no.
E-EL-M0726c; Elabscience), interferon-γ (IFN-γ; cat. no.
E-EL-M0048c; Elabscience) and tumor necrosis factor-α (TNF-α; cat.
no. E-EL-M0049c; Elabscience), and Th2 cytokines, including IL-4
(cat. no. E-EL-M0043c; Elabscience) and IL-5 (cat. no. E-EL-M0722c;
Elabscience), according to the manufacturer's protocol. The
expression levels of IL-6 (cat. no. E-EL-M0044c; Elabscience) and
IL-10 (cat. no. E-EL-M0046c; Elabscience) were also detected in the
DCs.
Western blot analysis
Western blot analysis was used to detect the
expression levels of VDR and CYP27B1 in the induced DCs following
treatment with BCG. Proteins were extracted with RIPA buffer
(Beyotime Institute of Biotechnology) and quantified using a BCA
Protein Assay Kit (Beyotime Institute of Biotechnology). In total,
25–50 µg protein per lane was separated on an 10% SDS-PAGE gel and
transferred to a polyvinylidene difluoride (PVDF) membrane
(19). The PVDF membrane was cut
and incubated with 5% skimmed milk blocking buffer for 1 h, and
then with corresponding primary antibodies against VDR (1:250;
sc-1008; Santa Cruz Biotechnology, Inc.) and CYP27B1 (1:400;
ab206655; Abcam) at 4°C overnight. β-actin (1:500; cat. no BM0627;
Wuhan Boster Biological Technology, Ltd.) was used as loading
control. Following 3 washes with PBS plus 0.1% Tween-20, the
membrane was incubated with a horseradish peroxidase-conjugated
anti-rabbit secondary antibody (1:5,000; ab7097; Abcam) for 45 min
at 25°C. Protein expression was detected using a Bio-Rad ChemiDoc™
MP imaging system (Bio-Rad Laboratories, Inc.) and performed the
densitometric analysis using ImageJ v1.4 (National Institute of
Health) (20).
T lymphocyte separation
Blood vessels were collected from the abdominal
aorta and placed in lithium heparin anticoagulation vacuum tubes
for isolation and culture of peripheral blood mononuclear cells.
Lithium heparin anticoagulant (Wuhan DeSheng Biochemical Technology
Co., Ltd.) and RPMI-1640 were thoroughly mixed (1:1), and then 2 ml
Ficoll lymphocyte separation solution (Sigma-Aldrich; Merck KGaA)
was added to the mixture. Following centrifugation for 15 min at
500 × g at 4°C, the mononuclear cell layer was aspirated and the
cell suspension concentration was adjusted to 106
cells/ml with RPMI-1640. Subsequently, the CD4+ T cells
were isolated.
MTT detection
Following the stimulation of the DCs with different
concentrations of BCG, the cells were collected and adjusted to a
cell density of 1×105 cells/ml at 37°C in a 5%
CO2 cell incubator for 30 min. Lymphocytes at a cell
density of 1×106 cells/ml were mixed with equal amounts
of DCs. Following incubation of the cells at 37°C for 3 days, 10 µl
MTT (Beyotime Institute of Biotechnology) was added into each well
and then the cells were incubated at 37°C for 3–4 h in the dark.
Subsequently, 200 µl dimethyl sulfoxide was added for 10 min. The
optical density value was measured at a wavelength of 492 nm in
order to analyze cell viability.
Statistical analysis
SPSS version 13.0 (SPSS, Inc.) was used for
statistical analysis. The data are presented as the mean ± standard
deviation. The inter-group differences were analyzed using one-way
analysis of variance followed by the Least Significant Difference
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of a vitamin D-deficient
mouse model
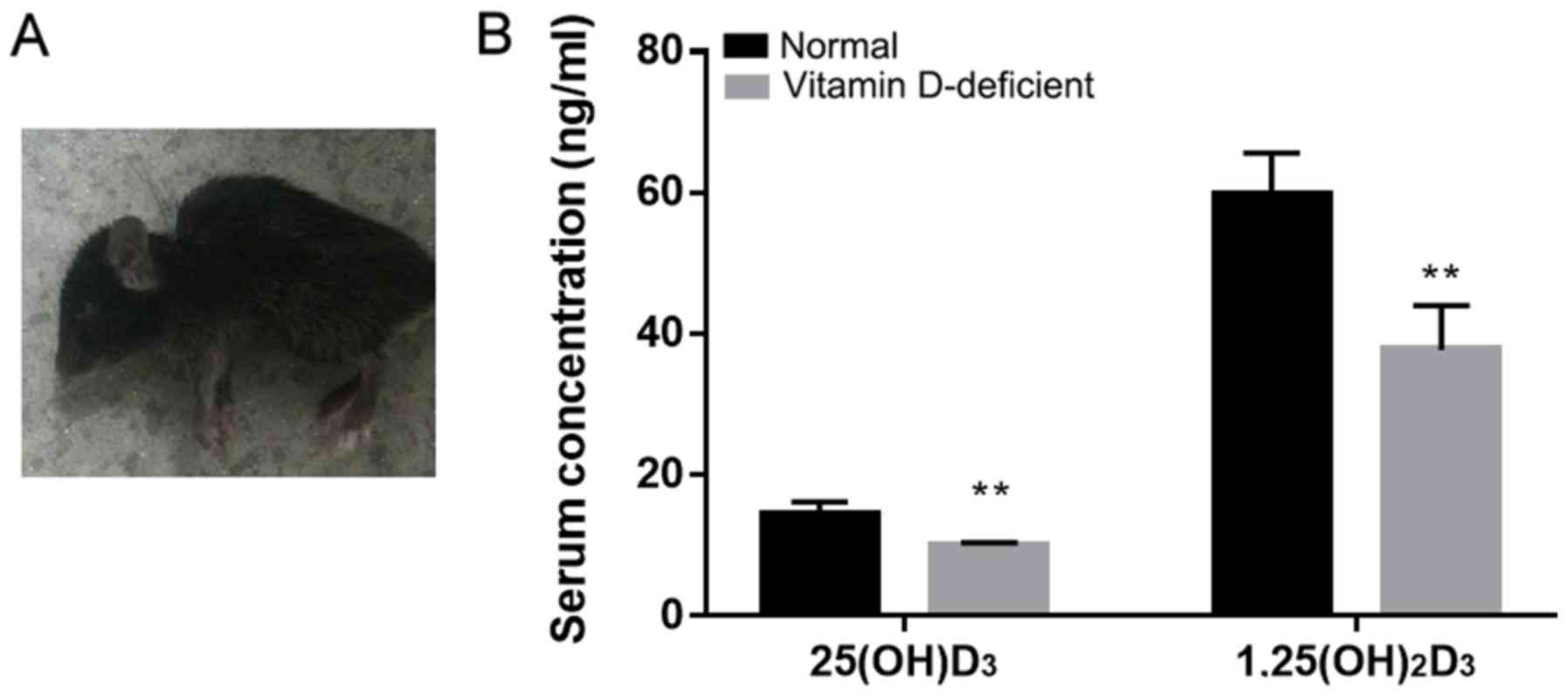
The vitamin D-deficient mice exhibited a significant
decrease in walking activity from the 10th week of birth compared
with the normal mice. The vitamin D-deficient mice presented with
slow movement, darkening of the coat color and back arching
(Fig. 1A). The serum levels of
25(OH)D3 and 1,25(OH)2D3 were significantly different between the
vitamin D-deficient mice and the normal mice (Fig. 1B). The serum 25(OH)D3 concentration
in the normal mice was 14.43±1.74 ng/ml, and it was 9.99±0.35 ng/ml
in the vitamin D-deficient mice. The concentration of serum
1,25(OH)2D3 in the normal mice was 59.73±5.91 and 37.72±6.28 ng/ml
in the vitamin D-deficient mice. The results suggest that the
vitamin D-deficient mouse model was established successfully.
Morphological observation and
identification of BMDCs
Optical microscope observation of
BMDCs
On day 1 of the DC culture, a large number of bone
marrow cells were floating in the medium, and a number of cells had
adhered to the bottom of the culture plate, which were identified
as mononuclear macrophages (Fig.
2A). On the second day, the number of adherent macrophages had
increased, and on the third day, the adherent cell mass was
increased further. On the fourth day, cells with a small number of
short ‘needles’ were observed floating in the medium and a number
of the cell clusters were semi-adherent, which were classified as
DC clusters. On the sixth day of culture, numerous DC clusters were
suspended in the medium. There was a large number of needle-like
protrusions on the surface of the cell membranes, which is a
typical feature of DC morphology (Fig.
2A).
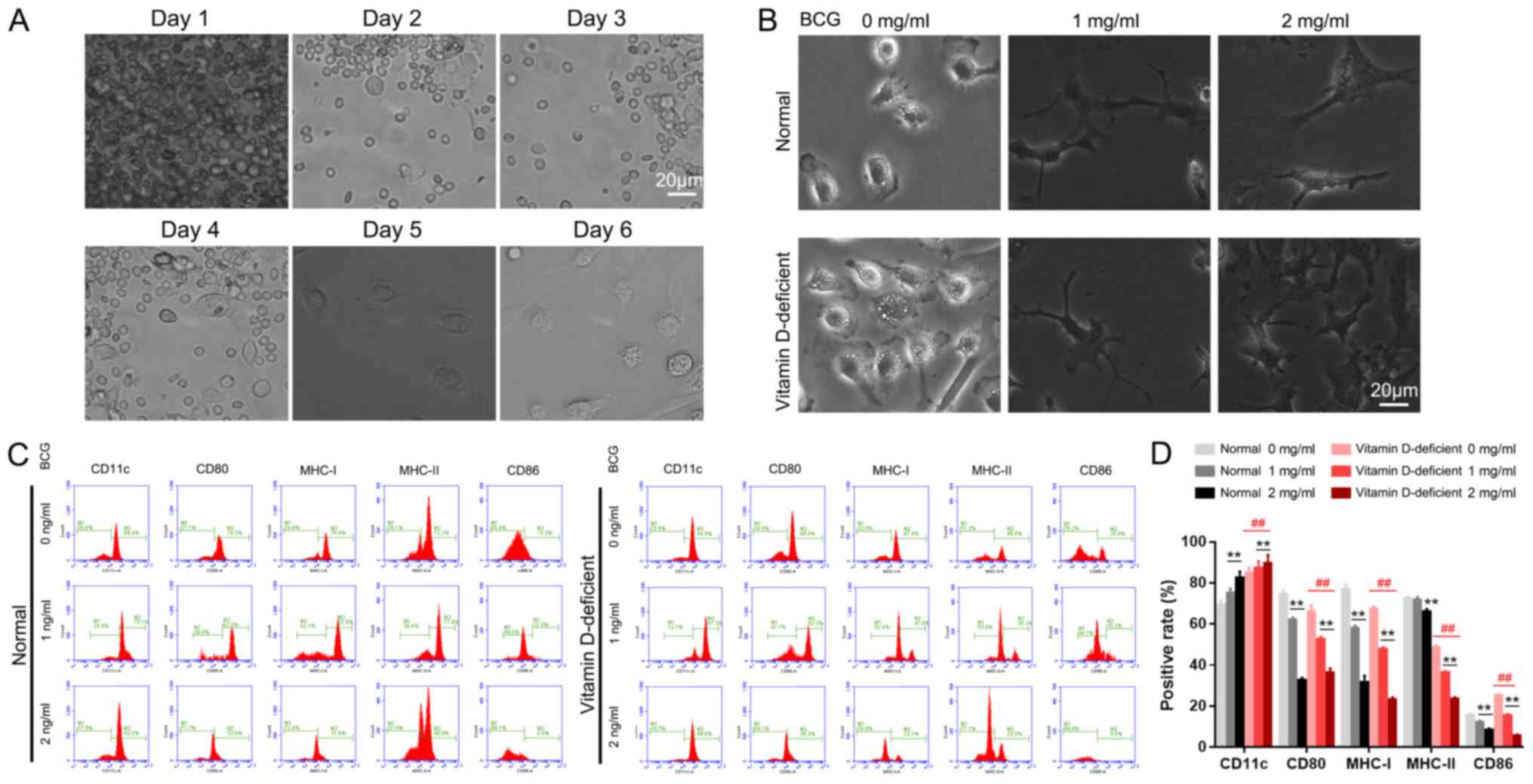 | Figure 2.Effect of BCG on BMDC differentiation
and maturation. (A) Cell morphology during BMDC induction from days
1 to 6, as observed by light microscopy. (B) BCG stimulated BMDC
maturation. (C) Phenotypic changes of the BMDCs induced by BCG
stimulation and (D) the levels of the surface molecules of DCs. All
images were captured under identical magnification and microscopy
conditions. **P<0.01 vs. 0 mg/ml in the normal control or
vitamin D-deficiency groups. ##P<0.01, vitamin
D-deficiency group vs. normal control group. Scale bar, 20 µm. BCG,
Bacillus Calmette-Guérin; BMDC, bone marrow-derived dendritic cell;
CD11c, integrin alpha-X; CD80, T-lymphocyte activation antigen
CD80; MHC-I, major histocompatibility complex class I; MHC-II,
major histocompatibility complex class II; CD86, T-lymphocyte
activation antigen CD86. |
BCG stimulates the maturity of
BMDCs
The blood cell count plate revealed that the
proportions of DCs in each of the normal groups were as follows:
Normal group, ~10%; normal + 1 mg/ml BCG stimulation group, ~50%;
and normal + 2 mg/ml BCG stimulation group, ~85%. The DC content in
the vitamin D-deficient groups were as follows: Vitamin D-deficient
mice model group, ~15%; model + 1 mg/ml BCG stimulation group,
~78%; and model + 2 mg/ml BCG stimulation group, ~95% (Fig. 2B).
Phenotypic analysis of the DCs
Flow cytometry was used to examine the expression
levels of the surface molecules of DCs. The average positive rates
for CD11c, CD80, major histocompatibility complex class I (MHC-1),
MHC-II and CD86 were as follows: In the control group (0 mg/ml
BCG), 69.4, 74.5, 76.8, 72.2 and 15.3%, respectively; in the 1
mg/ml BCG group, 75.1, 62, 57.9, 71.6 and 12%, respectively; and in
the 2 mg/ml BCG group, 82.5, 32.6, 31.6, 66.0 and 8.4%,
respectively (Fig. 2C). In the
vitamin D-deficient mice model groups, the average positive rates
of CD11c, CD80, MHC-1, MHC-II and CD86 were as follows: In the 0
mg/ml BCG group, 84.9, 66.0, 67.4, 48.5 and 25.0%, respectively; in
the 1 mg/ml BCG group, 87.3, 52.3, 47.6, 36.2 and 15.3%,
respectively; and in the 2 mg/ml BCG group, 89.6, 36.2, 23.1, 23.5
and 5.5%, respectively (Fig. 2C).
The levels of these surface molecules in the vitamin D-deficient
mice model group following BCG treatment were significantly
different compared with the control groups (P<0.01; Fig. 2D).
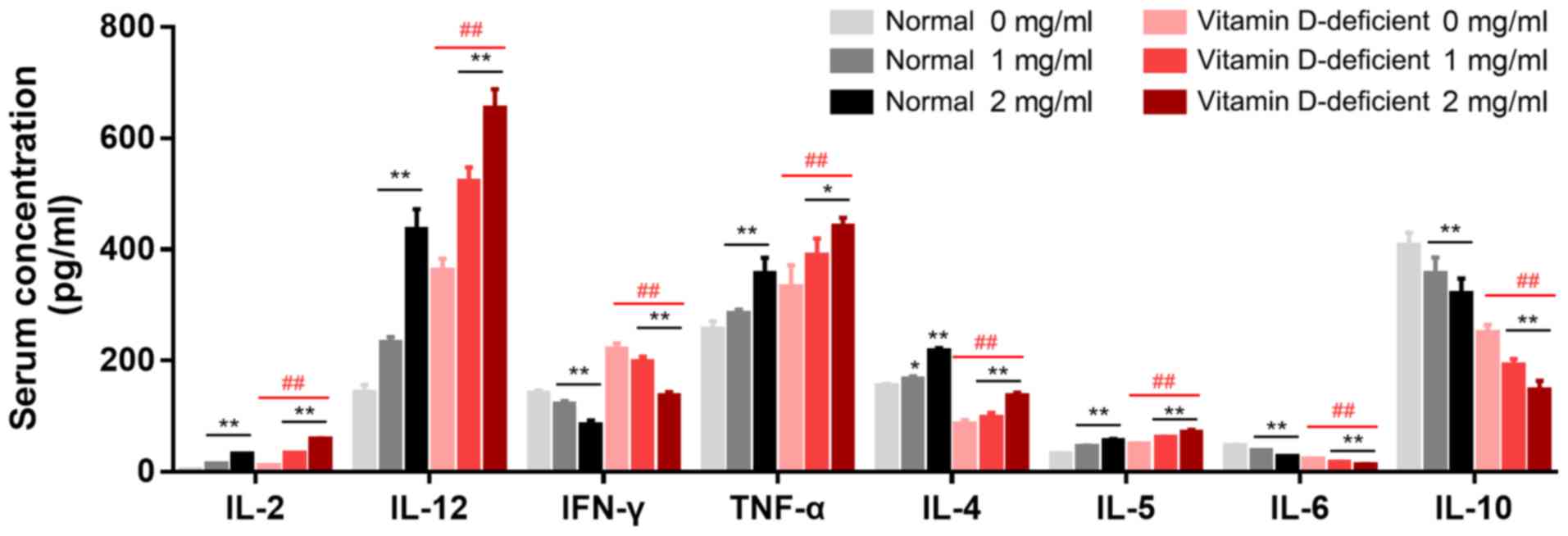
Production of cytokines in BMDCs
The levels of IL-2, IL-12, IFN-γ, TNF-α and IL-5
were significantly increased, and those of IL-4, IL-6 and IL-10
were decreased in the media of the BMDCs from the vitamin
D-deficient mice when compared with the normal mice (Fig. 3). In the presence of 1 mg/ml BCG,
the levels of IL-2 (P<0.01), IL-12 (P<0.01) and IL-5
(P<0.01) were significantly increased in the BMDCs from the
vitamin D-deficient mice compared with the control mice, while
those of IFN-γ (P<0.05) and IL-6 (P<0.05) were significantly
decreased, and those of TNF-α, IL-4 and IL-10 were not
significantly different. In the presence of BCG, the levels of
IL-2, IL-12, IFN-γ, TNF-α and IL-5 were significantly increased in
the BMDCs from the vitamin D-deficient mice in a
concentration-dependent manner, while those of IFN-γ (P<0.01)
and IL-10 (P<0.05) were significantly reduced. The differences
among the cytokines in the BMDCs from the normal and vitamin
D-deficient mice were significant in the presence of BCG (Fig. 3).
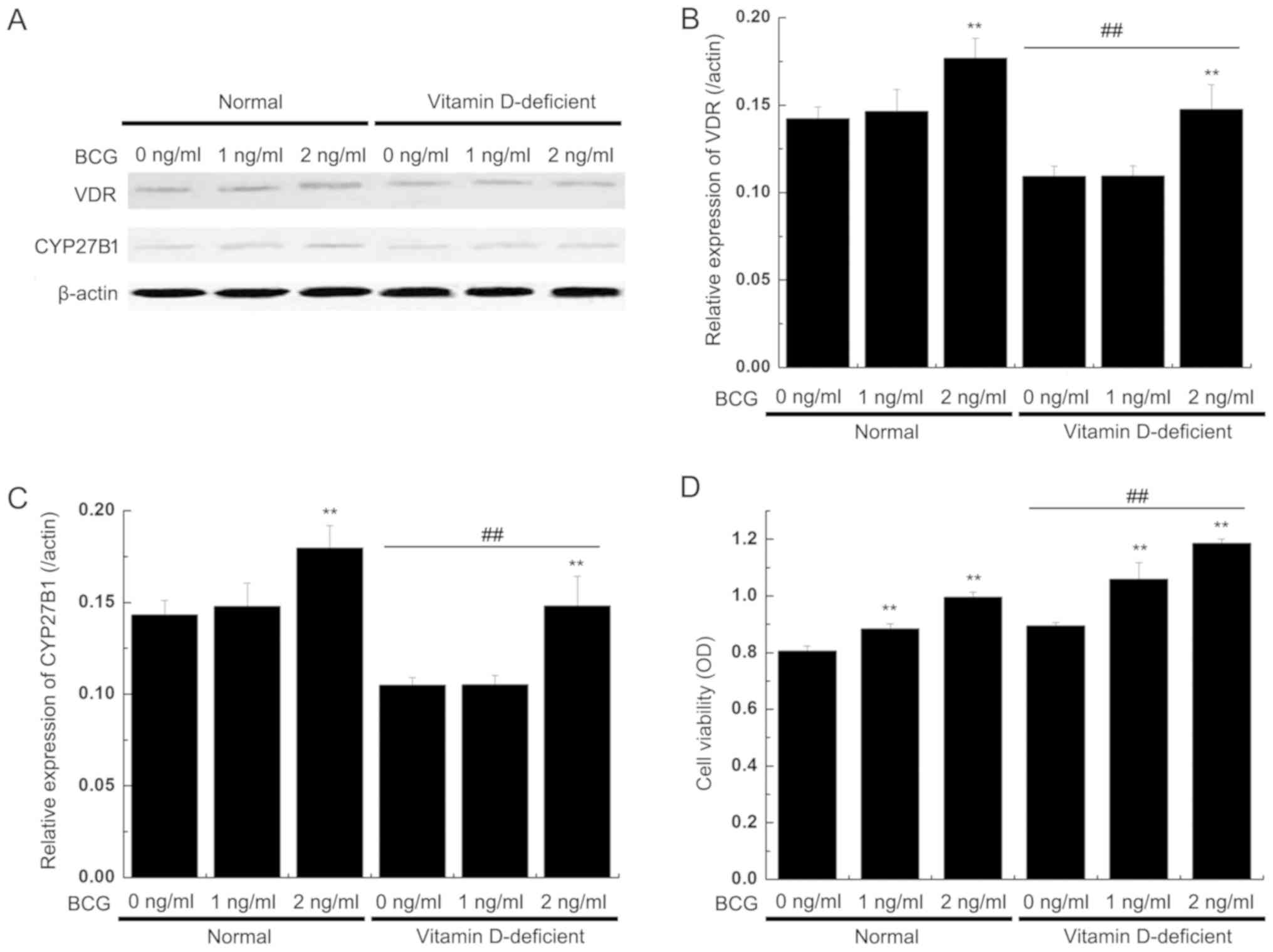
VDR and CYP27B1 protein
expression
The western blot analysis results revealed that the
expression levels of the VDR and CYP27B1 proteins were decreased in
the BMDCs from the vitamin D-deficient mice compared with the
normal mice, but BCG (2 mg/ml) significantly increased the
expression levels of VDR and CYP27B1. In the normal mice group, the
expression levels of VDR were also significantly increased in the
BMDCs following BCG stimulation (2 mg/ml). The two proteins
exhibited similar patterns of expression in each model group
(Fig. 4A-C).
Viability of T lymphocytes is induced
by DCs
The induced DCs were mixed with allogeneic T
lymphocytes on day 6, with different concentrations of
BCG-stimulated maturations for an additional 24 h. The
CD4+ T cells were mixed with equal amounts of DCs and
then incubated for 3 days. Subsequently, the viability of the
CD4+ T cells was observed. The MTT results demonstrated
that T cell viability was markedly increased in the coculture with
BMDCs from the vitamin D-deficient mice, compared to those
cocultured with BMDCs from the normal mice. T cell viability was
additionally increased by BCG treatment (Fig. 4D).
Discussion
Vitamin D deficiency is more likely to be a risk
factor for tuberculosis than a consequence of it (21,22).
Studies on the status of vitamin D deficiency with the response of
the immune system to tuberculosis infection are urgently required,
along with clarification of the mechanisms of vitamin D-based
prevention and treatment of tuberculosis.
Penna and Adorini (23) demonstrated that 1,25(OH)2D3 may
inhibit the differentiation and maturation of DCs and affect their
activity. In the present study, the changes in the phenotypes and
cytokines releases of DCs derived from vitamin D-deficient mice
were observed under treatment with different concentrations of BCG.
Using a BMDC co-culture system, the viability of T cells was
observed under different BCG treatment conditions. The results
revealed that the positive rates of CD86 and CD11c in the DCs from
vitamin D-deficient mice were increased compared with the normal
mice, and the positive rates of MHC-I, MHC II and CD80 were
decreased. The expression of the BMDC differentiation-associated
surface molecules MHC-I, MHC-II, CD80 and CD86 was significantly
inhibited by BCG in a concentration-dependent manner. CD11c is an
important marker for the identification of BMDCs. CD11c expression
was upregulated by BCG in a concentration-dependent manner. These
results suggest that BCG promotes the immune function and
specificity of DCs.
1,25(OH)2D3 regulates the immune system by
inhibiting the maturation of DCs, affecting the first stage of the
immune response (23). In the
present study, the viability of T cells cocultured with BMDCs from
normal mice in the presence of BCG was decreased compared with
vitamin D-deficient mice. A previous study demonstrated that
CD4+ T cells produce different types of cytokines based
on cell clones in mice (24). Th1
cells primarily express IL-2 and IFN-γ, enhancing the cytotoxicity
of killer cells and stimulating delayed type hypersensitivity. Th2
cells primarily express IL-4, IL-5 and IL-10, promoting the
production of antibodies and mediating the immune response. An
imbalance of Th1 and Th2 cells induces immune escape of tumor cells
and infection with bacteria and viruses, and results in allergic
and autoimmune diseases, and transplant rejection (25). Th1 and Th2 abnormalities are also
significantly associated with the severity of tuberculosis
(26). IL-4 is an important
cytokine that promotes Th2 cell viability and is positively
associated with tissue fibrosis and necrosis (27). IFN-γ and IL-2 are essential
anti-inflammatory cytokines in tuberculosis (28). IL-6 has been used as a reference
indicator for observing the prognosis of patients with tuberculosis
(29). IL-10 is a key cytokine in
suppressing the Th1 cellular immune responses (30). IL-12 is an important cytokine of
Th1 cells that controls Mtb infection and the survival rate of
BALB/c mice (27,31). The release of cytokines in the
immune response is an important function of DCs. In the present
study, the levels of IL-2, IL-12, IFN-γ and TNF-α in the DCs from
normal mice were decreased compared with those from vitamin
D-deficient mice, while the levels of IL-4, IL-6 and IL-10 were
increased. Treatment with BCG reversed the levels of those
cytokines in a concentration-dependent manner.
In the present study, it was observed that vitamin D
may affect cytokine secretion. The expression levels of CPY27B1 and
VDR protein in the BMDCs from vitamin D-deficient mice were
significantly decreased, and this effect was reversed by BCG
stimulation. Vitamin D is a hormone precursor with low biological
activity that is only converted into active 1,25(OH)2D3, which may
maximize the activation of VDR and exert its important
physiological roles in the body (9). The production of active 1,25(OH)2D3
requires 2 important hydroxylation processes: Hydroxylation at
position 25 catalyzed by CYP27A1 in the liver mitochondria, and 1α
hydroxylation in the kidney catalyzed by CYP27B1 (32). 1,25(OH)2D3 inhibits DC
differentiation and maturation, B cell differentiation, T cell
proliferation and secretion of IL-12 and TNF-α (14,33,34).
1,25(OH)2D3 may also promote the Th-2 T cell response and
production of regulatory T cells (35). The results of the present study
indicated that BCG increased DCs viability and enhanced the
immunofunction via CPY27B1 and VDR. Furthermore, BCG increased the
viability of CD4+ T cells.
In conclusion, BCG increased DCs viability and may
enhance immunofunction, which may assist in preventing the risk of
tuberculosis in patients with vitamin D deficiency.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81301575).
Availability of data and materials
The datasets analyzed during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
LX and JL designed the experiments. HY, HZ and YL
performed the experiments. All authors were involved in the
preparation and revision of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee for
Experiments with the Use of Laboratory Animals in Shenyang Military
Region General Hospital (Shenyang, China; approval no.
AE20160823).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Flynn JL: Immunology of tuberculosis and
implications in vaccine development. Tuberculosis (Edinb).
84:93–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu PT, Stenger S, Li H, Wenzel L, Tan BH,
Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al: Toll-like
receptor triggering of a vitamin D-mediated human antimicrobial
response. Science. 311:1770–1773. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talat N, Perry S, Parsonnet J, Dawood G
and Hussain R: Vitamin D deficiency and tuberculosis progression.
Emerg Infect Dis. 16:853–855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zignol M, van Gemert W, Falzon D,
Sismanidis C, Glaziou P, Floyd K and Raviglione M: Surveillance of
anti-tuberculosis drug resistance in the world: An updated
analysis, 2007–2010. Bull World Health Organ. 90:111–119D. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goswami R, Mishra SK and Kochupillai N:
Prevalence & potential significance of vitamin D deficiency in
Asian Indians. Indian J Med Res. 127:229–238. 2008.PubMed/NCBI
|
|
6
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Querfeld U: Vitamin D and inflammation.
Pediatr Nephrol. 28:605–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dusso AS, Brown AJ and Slatopolsky E:
Vitamin D. Am J Physiol Renal Physiol. 289:F8–F28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pike JW and Meyer MB: The vitamin D
receptor: New paradigms for the regulation of gene expression by
1,25-dihydroxyvitamin D3. Rheum Dis Clin North Am. 38:13–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb
DK, Yoon D, Kong J, Thadhani R and Li YC: 1,25-Dihydroxyvitamin D
promotes negative feedback regulation of TLR signaling via
targeting microRNA-155-SOCS1 in macrophages. J Immunol.
190:3687–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Von Essen MR, Kongsbak M, Schjerling P,
Olgaard K, Ødum N and Geisler C: Vitamin D controls T cell antigen
receptor signaling and activation of human T cells. Nat Immunol.
11:344–349. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rook GA, Steele J, Fraher L, Barker S,
Karmali R, O'Riordan J and Stanford J: Vitamin D3, gamma
interferon, and control of proliferation of Mycobacterium
tuberculosis by human monocytes. Immunology. 57:159–163.
1986.PubMed/NCBI
|
|
13
|
Bikle DD: Vitamin D and the immune system:
Role in protection against bacterial infection. Curr Opin Nephrol
Hypertens. 17:348–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Veldman CM, Cantorna MT and DeLuca HF:
Expression of 1,25-dihydroxyvitamin D (3) receptor in the immune
system. Arch Biochem Biophys. 374:334–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miziara ID, Magalhaes AT, Santos M, Gomes
EF and Oliveira RA: Research ethics in animal models. Braz J
Otorhinolaryngol (Article in English, Portuguese). 78:128–131.
2012. View Article : Google Scholar
|
|
16
|
Wu SY, Chen WH, Hsieh CL and Lin YW:
Abundant expression and functional participation of TRPV1 at
Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an
‘acupuncture-responding channel’. BMC Complement Altern Med.
14:962014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang Z, Xie Y, Dominguez JA, Breed ER,
Yoseph BP, Burd EM, Farris AB, Davidson NO and Coopersmith CM:
Intestine-specific deletion of microsomal triglyceride transfer
protein increases mortality in aged mice. PLoS One. 9:e1018282014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
https://www.nc3rs.org.uk/arrive-guidelines
|
|
19
|
Zeng Y and Liu J: Role of glypican-1 in
endothelial NOS activation under various steady shear stress
magnitudes. Exp Cell Res. 348:184–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng Y, Yao X, Liu X, He X, Li L, Liu X,
Yan Z, Wu J and Fu BM: Anti-angiogenesis triggers exosomes release
from endothelial cells to promote tumor vasculogenesis. J
Extracellular Vesicles. 8:16298652019. View Article : Google Scholar
|
|
21
|
Gibney KB, MacGregor L, Leder K, Torresi
J, Marshall C, Ebeling PR and Biggs BA: Vitamin D deficiency is
associated with tuberculosis and latent tuberculosis infection in
immigrants from Sub-Saharan Africa. Clin Infect Dis. 46:443–446.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang SJ, Wang XH, Liu ZD, Cao WL, Han Y,
Ma AG and Xu SF: Vitamin D deficiency and the risk of tuberculosis:
A meta-analysis. Drug Des Devel Ther. 11:91–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Penna G and Adorini L: 1
Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation,
activation, and survival of dendritic cells leading to impaired
alloreactive T cell activation. J Immunol. 164:2405–2411. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mosmann TR, Cherwinski H, Bond MW, Giedlin
MA and Coffman RL: Two types of murine helper T cell clone. I.
Definition according to profiles of lymphokine activities and
secreted proteins. J Immunol. 136:2348–2357. 1986.PubMed/NCBI
|
|
25
|
Lichtner M, Rossi R, Mengoni F, Vignoli S,
Colacchia B, Massetti AP, Kamga I, Hosmalin A, Vullo V and
Mastroianni CM: Circulating dendritic cells and interferon-alpha
production in patients with tuberculosis: Correlation with clinical
outcome and treatment response. Clin Exp Immunol. 143:329–337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dominguez J, De Souza-Galvao M,
Ruiz-Manzano J, Latorre I, Prat C, Lacoma A, Milà C, Jiménez MA,
Blanco S, Maldonado J, et al: T-cell responses to the mycobacterium
tuberculosis-specific antigens in active tuberculosis patients at
the beginning, during, and after antituberculosis treatment. Diagn
Microbiol Infect Dis. 63:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Infante-Duarte C and Kamradt T: Th1/Th2
balance in infection. Springer Semin Immunopathol. 21:317–338.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He XY, Xiao L, Chen HB, Hao J, Li J, Wang
YJ, He K, Gao Y and Shi BY: T regulatory cells and Th1/Th2
cytokines in peripheral blood from tuberculosis patients. Eur J
Clin Microbiol Infect Dis. 29:643–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nemeth J, Winkler HM, Boeck L, Adegnika
AA, Clement E, Mve TM, Kremsner PG and Winkler S: Specific cytokine
patterns of pulmonary tuberculosis in Central Africa. Clin Immunol.
138:50–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Handzel ZT, Barak V, Altman Y, Bibi H,
Lidgi M, Iancovici-Kidon M, Yassky D and Raz M: Increased Th1 and
Th2 type cytokine production in patients with active tuberculosis.
Isr Med Assoc J. 9:479–483. 2007.PubMed/NCBI
|
|
31
|
Flynn JL, Goldstein MM, Triebold KJ, Sypek
J, Wolf S and Bloom BR: IL-12 increases resistance of BALB/c mice
to mycobacterium tuberculosis infection. J Immunol. 155:2515–2524.
1995.PubMed/NCBI
|
|
32
|
Tissandié E, Guéguen Y, Lobaccaro JM,
Aigueperse J and Souidi M: Vitamin D: Metabolism, regulation and
associated diseases. Med Sci (Paris). 22:1095–1100. 2006.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nebbioso M, Buomprisco G, Pascarella A and
Pescosolido N: Modulatory effects of 1,25-dihydroxyvitamin D3 on
eye disorders: A critical review. Crit Rev Food Sci Nutr.
57:559–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boonstra A, Barrat FJ, Crain C, Heath VL,
Savelkoul HF and O'Garra A: 1α,25-Dihydroxyvitamin D3 has a direct
effect on naive CD4+ T Cells to enhance the development
of Th2 cells. J Immunol. 167:4974–4980. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gregori S, Casorati M, Amuchastegui S,
Smiroldo S, Davalli AM and Adorini L: Regulatory T cells induced by
1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment
mediate transplantation tolerance. J Immunol. 167:1945–1953. 2001.
View Article : Google Scholar : PubMed/NCBI
|