Introduction
Insulin resistance (IR) is an impaired insulin
response, which can cause damage to target organs. IR causes
impaired glucose tolerance, as in diabetes mellitus and
cardiovascular disease (1–3). A number of conventional
pharmacological strategies are used to treat IR. However,
limitations, such as costs, patients' compliance and drug side
effects (including weight gain, hypoglycemia and congestive heart
failure) are involved (4,5). Thus, methods to enhance insulin
sensitivity have become a topic of interest.
Multiple studies have demonstrated that electrical
stimulation (ES) can influence the absorption, distribution and
excretion of substances, and even induce the secretion of
endogenous opioid peptides from multiple sites (6,7).
Therefore, plasma glucose levels are decreased. In addition to its
hypoglycemic action, ES also improves insulin sensitivity by
lowering free fatty acid (FFA) levels and increasing plasma
insulin-like growth factor 1 levels, thereby regulating insulin
secretion (8). Although ES has a
hypoglycemic effect, ES treatment combined with other methods are
required to improve therapeutic outcomes in clinical setting. The
exact mechanism of ES on IR is not completely understood because of
the complex pathological factors in IR (4).
PI3K, Akt and mammalian target of rapamycin (mTOR)
signaling are the main pathways that transduce insulin signaling
(9,10). Under normal conditions, insulin
receptor substrate-1 (IRS-1) is phosphorylated and recruited by the
tyrosine kinase, an insulin receptor (11). Then, the downstream PI3K is
triggered, which generates phosphatidylinositol (3–5)-triphosphate and recruits Akt to the
plasma membrane (11). Sufficient
Akt, which phosphorylates the downstream target glycogen synthase
kinase-3 (GSK-3), is recruited to stimulate the insulin response
for glycogen synthesis (12). Akt
also serves as a highly-regulated point of divergence for glucose
transport by accelerating glucose transporter 4 (GLUT4) (13). If excessive calories are consumed,
then the levels of toxic lipids and proinflammatory cytokines in
the blood will be enhanced. Hyperglycemia upregulates the activity
of mTOR/p70 ribosomal S6 protein kinase (p70S6K) (14), which stimulates IRS-1 Ser636/639
phosphorylation and suppresses IRS-1-associated PI3K/Akt signaling,
and GSK-3 is activated (14).
Glycogen synthesis and glucose transport are impaired.
These previous studies have demonstrated that mTOR
signaling is an important effector mediating the action of insulin.
In theory and in clinic application, ES has an effect on IR, but
only a few studies have examined this possibility (6,7).
Additional studies are necessary to investigate the molecular and
intracellular mechanisms by which ES regulates and improves IR. The
present study identified that ES can increase insulin sensitivity
and improve IR by regulating PI3K, Akt and mTOR signaling. In the
current study, IR models were established by a high fat and high
carbohydrate diet (HFHCD). Then, the IR rats were treated by ES and
diet therapy for 2 weeks. Hepatic and pancreas islet pathological
changes and insulin-related signaling pathways were examined to
investigate whether a combination of ES and diet therapy can
improve insulin activity in rats and to explore the mechanism
underlying the function of ES and diet therapy. The results
suggested a theoretical basis for the clinical application of ES to
treat patients with obesity or diabetes having IR.
Materials and methods
Animals
A total of 70 Sprague-Dawley male 4-week-old rats
weighing 90–100 g were provided by the Experimental Animal Center
of Chongqing Medical University (medical animal certification no.
SCXK-2012-0002). The approval for the present study was provided by
Chongqing Medical University's Animal Care and Ethics Committee and
formulated by the Ministry of Science and Technology of China. The
rats were housed in a room under controlled temperature and
humidity (24°C, 50%) with a 12 h light/dark cycle. The rats were
provided with food and water ad libitum. After 1 week, the
rats were randomly divided into normal (n=10) and IR (n=60) groups.
The HFHCD composition and preparation described in a previous study
was used with a few modifications (15). The HFHCD consisted of 25% fructose,
15% lard oil, 60% powdered rat chow and 50 g water per kg of diet.
The IR rats were fed with HFHCD for 5 weeks. On the 6th week, the
IR state was confirmed by measuring fasting plasma glucose (FPG)
insulin (≥100 mg/dl), insulin sensitivity index (ISI) and IR index.
All IR rats that satisfied the IR criteria were randomly divided
into 4 groups, as follows: IR control group (n=15), in which the
rats were fed HFHCD continuously; diet group (n=15), in which the
rats were only fed a low-fat and low-carbohydrate diet (10%
fructose, 5% lard oil, 85% powdered rat chow and 50 g water per kg
of diet); ES group (n=15), in which the rats received ES and normal
diet; and ES + diet group (n=15), in which the rats received
low-fat and low carbohydrate diet and ES. All IR rats were fed for
an additional 2 weeks on the experimental diets after the 5 weeks
of the HFHCD and were subsequently sacrificed for the tissue
collection.
ES application
The rats were positioned in a custom-made holder
(patent no. ZL201420163074.2). ES was performed in bilateral
Zusanli (ST36, located at the anterior aspect of the hind limb, 2
mm straight below the knee joint), Sanyinjiao (Spleen 6, located at
the medial side of the hind limb, 2 mm above the tip of the medial
malleolus posterior to the medial border of the tibia) and
Weiwanxiashu (EX-B3, 1 mm lateral to the no. 8 thoracic spinous
process) acupoints after the IR model was established. Acupuncture
needles (13 mm in length and 0.22 mm in diameter) were inserted
into the acupoints muscle layers to a depth of ~5 mm. The pairs of
needles on the ipsilateral ST36 and SP6 acupoints were connected
with the output terminals of an ES apparatus (Model SDZ-II; Suzhou
Huatuo Medical Instruments Co., Ltd.). The alternating strings of
dense-sparse frequencies (20 Hz for 1.05 sec and 4 Hz for 2.85 sec,
alternately) were used. The intensity was adjusted to induce a
slight twitch of the hind limbs (≤2 mA), with intensity lasting for
20 min/day for 14 days.
Reagents
Glucose oxidase kit (Nanjing Jiancheng Taihao
Biotechnology Co., Ltd. cat. no. F006) and INS ELISA kit (Thermo
Fisher Scientific, cat. no. ERINS) were used. The primary
antibodies were used for western blotting and immunohistochemistry
as follows: Polyclonal rabbit anti-P70S6K antibody (Signalway
Antibody LLC, cat. no. 21276), polyclonal rabbit anti-GSK-3 α/β
antibody (Signalway Antibody LLC, cat. no. 29039), polyclonal
rabbit anti-mTOR antibody (Signalway Antibody LLC, cat. no. 21214),
monoclonal rabbit anti-Akt Antibody (Signalway Antibody LLC, cat.
no. 48888), monoclonal rabbit anti-PI3K antibody (Signalway
Antibody LLC, cat. no. 48868) and polyclonal rabbit anti-GLUT4
antibody (Abcam, cat. no. ab654). The secondary antibody for
western blotting and immunohistochemistry: Goat anti-rabbit IgG
secondary antibody (OriGene Technologies, Inc. cat. no.
TA130015).
Tissue processing for ultrastructure
observation and immunohistochemistry
The rats from the various groups were anesthetized
by intraperitoneally injecting 2% pentobarbital sodium (40 mg/kg).
Physiological saline (250 ml, 4°C) and 4% paraformaldehyde in 500
ml of 0.1 mmol/l phosphate buffered saline (PBS; 4°C, pH of 7.4)
were perfused into the left ventricle. The liver tissues, pancreas
and the quadriceps femoris muscle tissues in left leg were
harvested and assigned into two segments. First segments were fixed
with 4% paraformaldehyde for 24–48 h (4°C) and dehydrated with 30%
sucrose overnight (4°C). Second segments were used for
ultrastructure observation.
Hematoxylin-eosin (H&E)
staining
To evaluate the pathologic changes and effectiveness
of ES, H&E staining was performed. Liver and pancreas tissues
were fixed in formalin for >24 h and then moved to 70% ethanol.
Then, the tissues were dehydrated in graded alcohols, immersed in
xylol and embedded in paraffin wax. The tissues were sliced into
serial sections with a thickness of 6 µm each using a paraffin
slicing machine and stained with H&E (0.5–1% eosin; 3.3%
hematoxylin) under room temperature for 80 min. Digital images were
captured using an Olympus light microscope with a ×10 objective and
an Olympus camera (Olympus-45; Olympus Corporation) with a total
magnification of ×100. The images were further analyzed by a
blinded investigator.
Immunohistochemical analysis
For the immunohistochemical evaluation, 10 µm thick
sections of the liver, pancreas and muscle tissues from each group
were prepared. Briefly, endogenous peroxidase was blocked by 3%
H2O2 for 15 min at room temperature. The
sections were further washed with 0.01 mmol/l PBS and blocked with
10% horse serum (Thermo Fisher Scientific, Inc., cat. no. 31874)
for 20 min at room temperature. Next, the sections were incubated
with primary antibodies (PI3K, Akt, GLUT 4, GSK-3α, GSK-3β, mTOR,
P70S6K; all 1:200) at 4°C overnight. The sections were rinsed with
0.01 mmol/l PBS and reacted with secondary antibodies (1:400) for
20 min at 37°C. The slides were rewashed with 0.01 mmol/l PBS. DAB
horseradish peroxidase color development kit (Beyotime Institute of
Biotechnology, cat. no. P0202) was used for developing at room
temperature for 5–10 min. The slides were counterstained by 3.3%
hematoxylin at room temperature for 2 min after the slides were
rewashed with 0.01 mmol/l PBS. Lastly, the slides were mounted in
50% glycerol dissolved in 0.01 mmol/l PBS.
Ultrastructure observation
A portion of each specimen from the sections
perfused with 4% paraformaldehyde was immediately fixed in 2.5%
glutaraldehyde for 2 days at room temperature after washing with
PBS. The glutaraldehyde-fixed specimens were treated in 2% osmic
acid for 1 h, dehydrated in serial alcohol (50% ethanol, 75%
ethanol, 95% ethanol and absolute alcohol), infiltrated with
propylene oxide and embedded in araldite for morphometric analyses
on semithin sections (70–90 nm). The sections were mounted on Cu
grids and stained with 3% uranyl acetate for 30 min and then 2%
lead citrate for 15 min at room temperature. Images were obtained
by transmission electron microscopy (Hitachi-7500; Hitachi,
Ltd.).
Measurement of metabolic parameters
FPG, insulin, FFA, total cholesterol (TC) and triglycerides
(TG)
Blood was collected from the left cardiac cavity and
centrifuged at 3,000 × g for 10 min at 4°C to isolate the
supernatant/plasma. FPG levels were determined using a glucose
oxidase kit according to the manufacturer's protocol. The plasma
levels of the fasting insulin were measured by INS ELISA kit
(Thermo Fisher Scientific, Inc., cat. no. ERINS). Non-esterified
free fatty acids assay kit (Nanjing Jiancheng Bioengineering
Institute, cat. no. A042-2-1), total cholesterol assay kit (Nanjing
Jiancheng Bioengineering Institute, cat. no. F002-1-1) and
triglyceride assay kit (Nanjing Jiancheng Bioengineering Institute,
cat. no. A110-1-1) were used to measure FFA, TC and TG,
respectively. The ISI was defined as follows: ISI=l g (1/FPG ×
serum insulin). The IR index was defined as follows: IR index=FPG
level (mg/dl) × fasting serum insulin level (ng/ml)/22.5 (16).
Western blot analysis
Protein extraction was performed according to
standard procedures. Briefly, samples were homogenized in 0.3 ml of
disrupting RIPA lysis buffer (Beyotime Institute of Biotechnology,
cat. no. P0013B) and 1% protease inhibitor (Beyotime Institute of
Biotechnology, cat. no. ST506). The homogenate was centrifuged at
12,000 × g for 15 min at 4°C. The supernatant was diluted with 5X
protein loading buffer (Beyotime Institute of Biotechnology) and
then heated at 95°C for 5 min. Samples containing 40 µg protein
were separated by 12% SDS-PAGE and then transferred onto a
polyvinylidene difluoride membrane. Blotted membranes were blocked
with 5% skimmed milk at room temperature for 4 h. For
immunoblotting, the following primary antibodies were used:
Monoclonal rabbit anti-PI3 kinase antibody (1:1,000), monoclonal
rabbit anti-Akt antibody (1:1,000), polyclonal rabbit anti-GLUT4
antibody (1:1,000), polyclonal rabbit anti-GSK-3 α/β antibody
(1:1,000), polyclonal rabbit anti-P70S6K antibody (1:1,000) and
polyclonal rabbit anti-mTOR antibody (1:1,000). All the primary
antibodies were incubated at 4°C overnight. The secondary antibody
used was goat anti-rabbit IgG (1:500) at room temperature for 2 h.
Western bands were quantified by gel densitometry (GS-900™
calibrated densitometry system, cat. no. 1707991, Bio-Rad
Laboratories, Inc.). The ratio between protein and GAPDH expression
for each sample was obtained (each point was repeated in
triplicate).
Statistical analysis
The statistical analyses were performed using SPSS
11.0 (SPSS, Inc.). The results were expressed as the mean ±
standard error of the mean. Data were compared using the Duncan
Multiple Range test following one-way ANOVA. P<0.05 was
considered to indicate a statistically significant difference.
Results
Metabolic parameters and weight
After being fed with HFHCD for 5 weeks, the rats
demonstrated signs of IR; however, no significant changes in
overall body weight were observed in the IR rats compared with the
normal rats (Table I). Following
the administration of specific diets, the weight of rats in the
diet, ES and ES + diet group were significantly decreased compared
with those in the IR control group (P<0.05; Table I). The weight of rats in the ES +
diet group significantly decreased compared with in the diet and ES
groups (P<0.05; Table I). This
demonstrated that ES combined with low-carbohydrate, low-fat diet
can reduce weight significantly. The HFHCD caused IR, as indicated
by a significant increase in FPG, insulin, IR index, TG, FFA and
TC, and a decrease in ISI compared with the normal group (Table I). After 14 days, the rats in the
ES + diet group demonstrated significantly lower serum levels of
FPG, insulin, IR index, TG, FFA and TC compared with those in the
IR control, diet and ES groups (P<0.05, n=15; Table I).
 | Table I.Biochemical characteristics of
metabolic parameters in groups. |
Table I.
Biochemical characteristics of
metabolic parameters in groups.
|
|
Group
(n=15) |
|---|
|
|
|
|---|
|
| Normal | IR group | Diet group | ES group | ES + diet
group |
|---|
|
|
|
|
|
|
|
|---|
| Parameter | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | Baseline | Treatment |
|---|
| Weight (g) | 349.67±1.52 | 388.33±6.65 | 388.67±7.07 | 391.64±6.80 | 388.33±5.85 |
343.33±5.85b–d | 389.67±4.16 |
341.67±13.31b–d | 389.33±2.51 |
326.57±5.13c,d |
| Fasting glucose
(mg/dl) | 112.33±2.28 | 112.33±2.28 |
123.53±0.87a | 123.89±3.30 | 122.96±5.87 |
119.15±4.57b–d | 124.57±4.77 |
117.71±3.94b–d | 123.61±8.02 |
115.28±3.71c,d |
| Fasting insulin
(ng/ml) | 0.46±0.07 | 0.46±0.07 |
1.54±0.17a | 1.57±0.25 | 1.59±0.03 |
1.18±0.11b–d | 1.58±0.26 |
1.13±0.12b–d | 1.57±0.10 |
0.81±0.15c,d |
| ISI | 1.71±0.08 | 1.70±0.08 |
2.28±0.06a | 2.29±0.05 | 2.29±0.01 |
2.14±0.05b–d | 2.29±0.08 |
2.12±0.06b–d | 2.28±0.03 |
1.96±0.07c,d |
| IR index | 2.28±0.41 | 2.28±0.41 |
8.49±1.16a | 8.64±1.11 | 8.68±0.26 |
6.59±0.99b–d | 8.74±1.63 |
5.94±0.76b–d | 8.83±0.17 |
4.11±0.66c,d |
| TC (mmol/l) | 2.19±0.25 | 2.16±0.17 |
10.56±1.26a | 10.81±0.85 | 9.95±1.51 |
8.11±0.41b–d | 9.84±1.99 |
7.33±0.72b–d | 10.31±2.07 |
5.25±0.96c,d |
| TG (mmol/l) | 1.29±0.35 | 1.05±0.075 |
2.32±0.34a | 2.23±0.097 | 2.26±0.32 |
1.59±0.095b–d | 2.17±0.26 |
1.76±0.14b–d | 2.21±0.28 |
1.36±0.08c,d |
| FFA (µmol/l) | 392.22±9.07 | 387.33±8.51 |
594.33±5.50a | 593.33±4.04 | 582.01±11.13 |
475.32±13.86b–d | 583.12±16.37 |
478.67±9.50b–d | 585.10±7.21 |
437.67±8.51c,d |
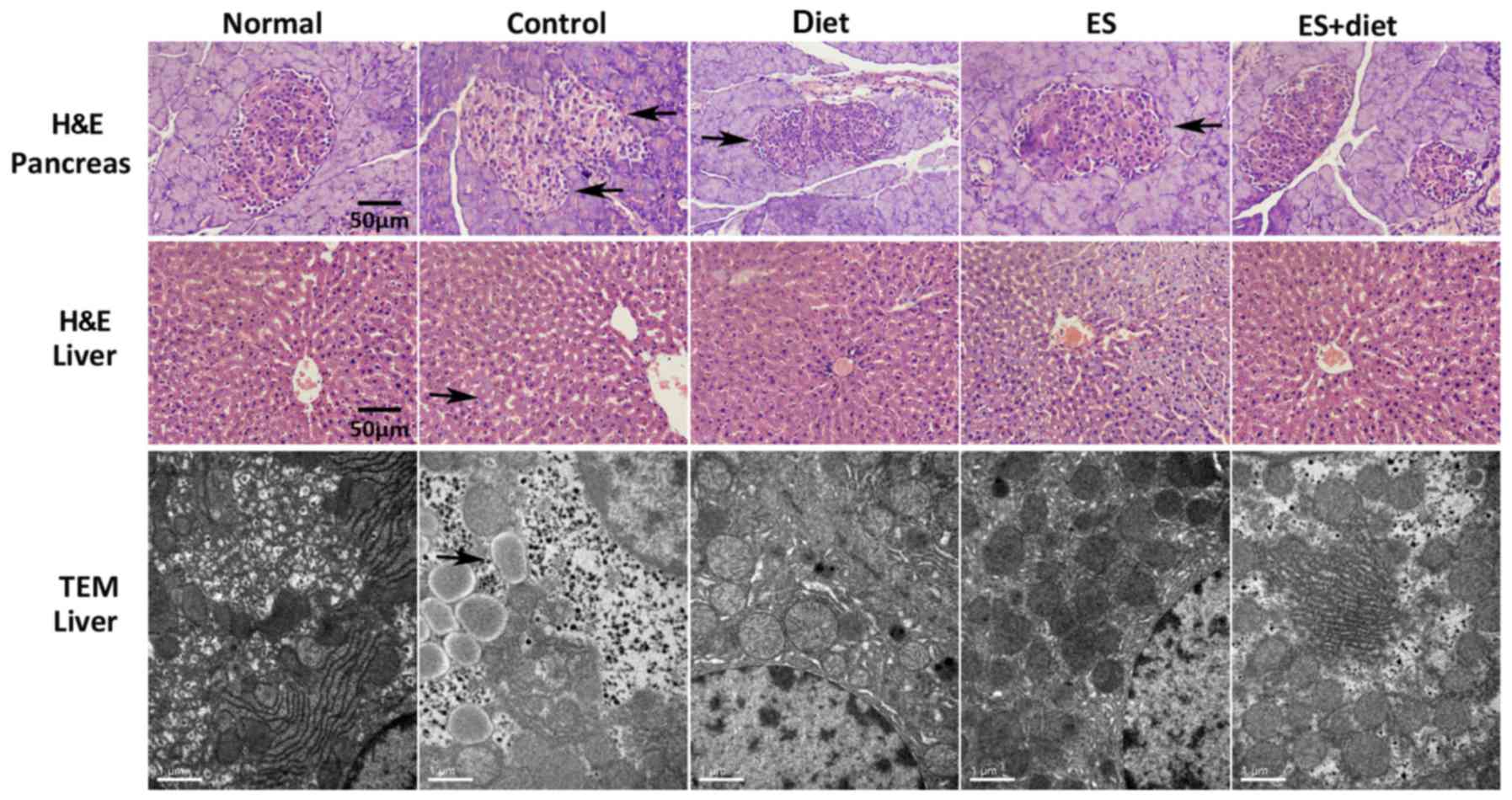
Pathologic changes in liver tissue and
pancreatic islet
As demonstrated in Fig.
1, hepatocytes in the normal group were distributed around the
central veins radially and presented cord-like arrangement.
Hepatocellular nuclei were clear without inflammation and necrosis.
HFHCD induced considerable hepatic damage with evident necrotic
foci and inflammatory cell infiltration. The hepatic cord structure
in the IR control group was destroyed and the hepatocyte
arrangement became disorderly (Fig.
1). Considerable lipid droplets and cavitations were observed
in hepatocytes by H&E staining. The diet treatment resulted in
insignificant changes compared with the IR control group. The
ES-treated group demonstrated fewer fat droplets, whereas the
steatosis in the livers of the rats in the ES + diet group was
improved (Fig. 1). These
observations were confirmed by electron microscopy (Fig. 1), which revealed hepatocytes with
abundant lipid droplets in the IR control group and few fat
droplets in the diet group. In the ES and ES + diet group,
inflammatory cell infiltration was improved. Lipid droplets and
cavitations in the hepatocyte decreased. The hepatocyte arrangement
in the ES + diet group was notably enhanced compared with that in
the IR control group. These results suggested that treatment with
ES and diet could ameliorate the effects of HFHCD.
The H&E-stained sections revealed that the
pancreatic islet in the normal group was oval and large compared
with the IR control group, thereby indicating that the pancreatic
islet was injured. The pancreatic islet was improved in the ES +
diet group compared with the other two treatment groups. This
result suggested that ES facilitated the maintenance of the
pancreatic islet cell morphology.
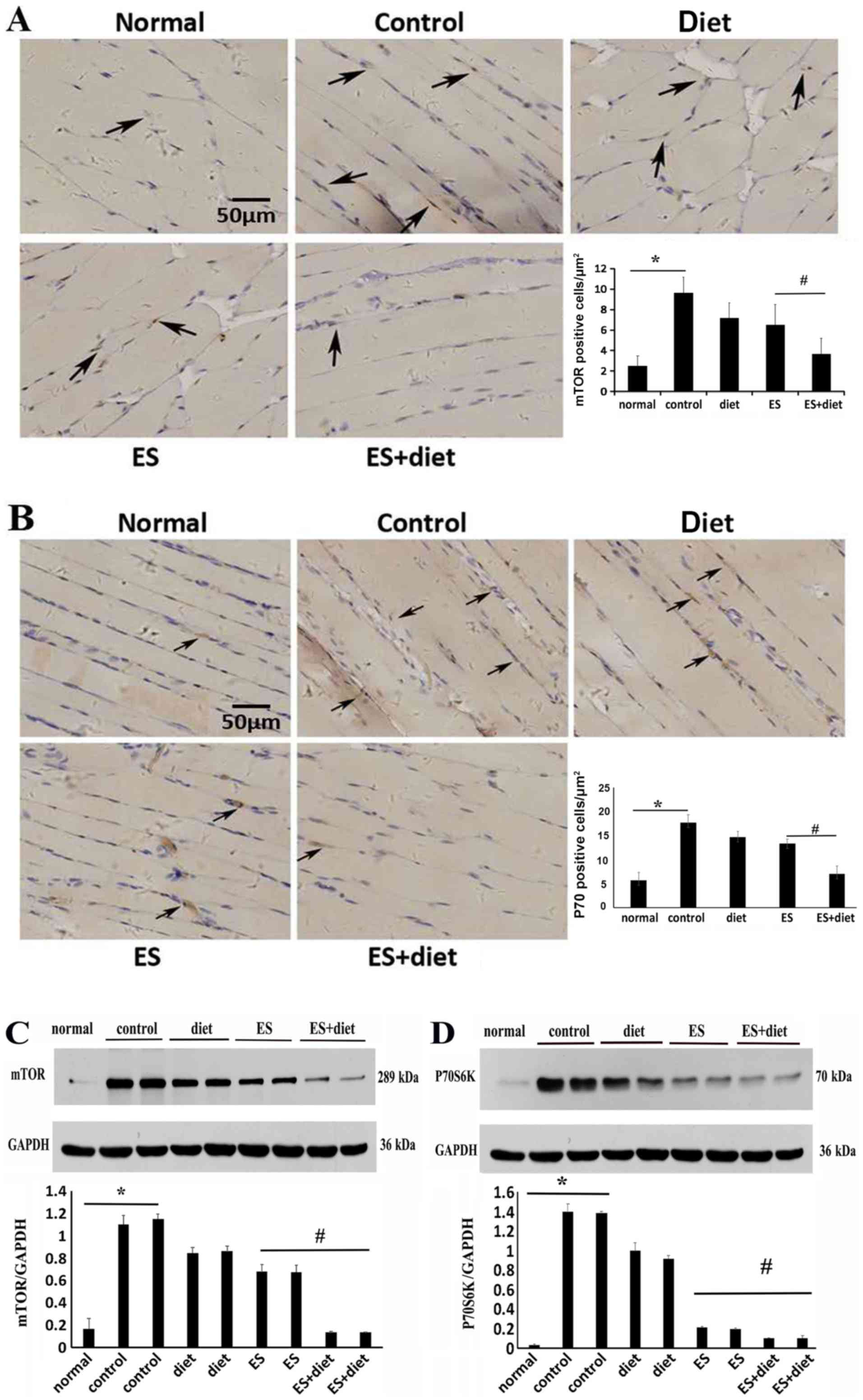
PI3K/Akt/mTOR signaling in muscle or
hepatic tissues is regulated by ES and diet therapy
To investigate whether PI3K/Akt/mTOR signaling was
involved with the mechanism of ES and diet therapy in insulin
activity in rats, mTOR, p70S6K, PI3K and Akt expression levels were
examined at the lesion sites in the different groups by
immunohistochemistry and western blot analysis. As demonstrated in
Fig. 2, the protein expression
levels of mTOR and its downstream substrate, p70S6K, were
significantly increased in the muscle tissue of IR rats fed with
HFHCD (P<0.05; Fig. 2).
Following treatment, the protein expression levels of mTOR and
p70S6K significantly decreased in the ES + diet group compared with
the other groups (P<0.05; Fig.
2).
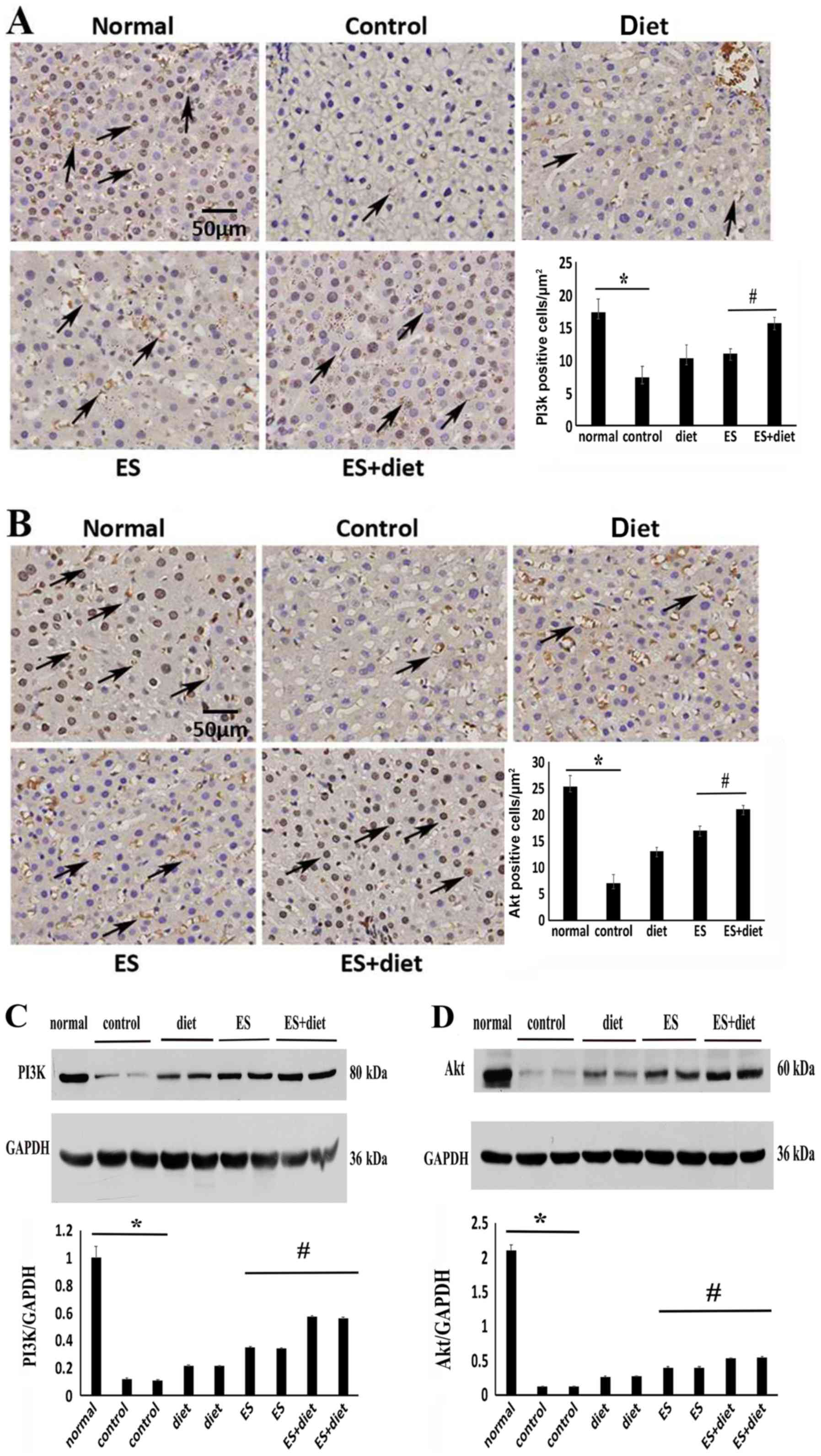
PI3K and Akt protein expression levels were analyzed
in the hepatic tissues. Western blot analysis demonstrated that the
PI3K and Akt expression levels significantly decreased in the IR
rats compared with the normal rats (P<0.05) (Fig. 3C and D). This result was consistent
with the immunohistochemistry results (Fig. 3A and B). Following treatment, the
PI3K and Akt expression levels were upregulated in the ES + diet
group compared with rats in other groups (P<0.05; Fig. 3). These results suggested that the
excessive nutrients hyperactivated the mTOR pathway and may have
led to IR via suppression of PI3K/Akt signaling. This result was
consistent with previous findings (14). ES and diet treatment may have
improved IR by mTOR inhibition and PI3K/Akt signaling
activation.
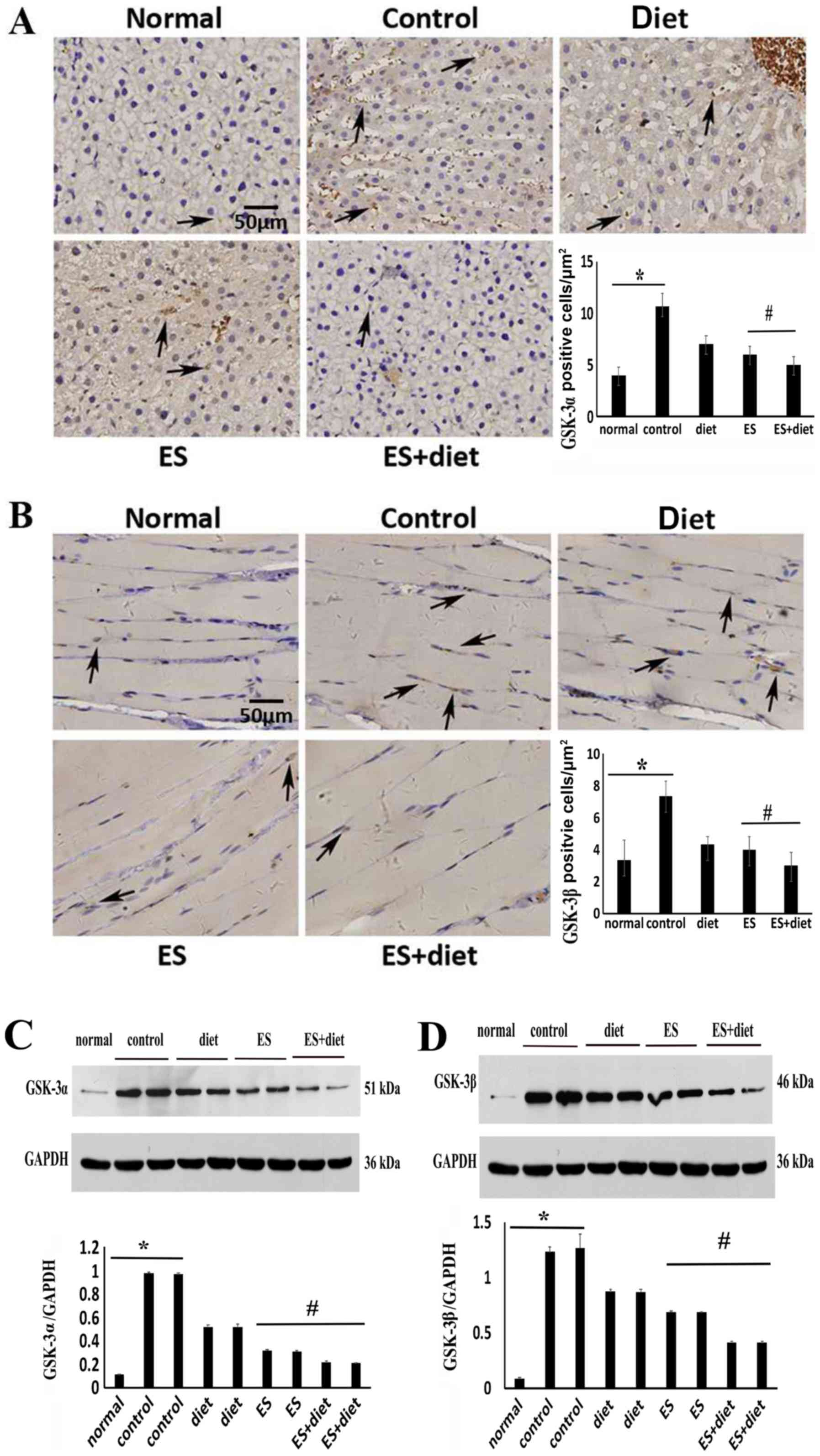
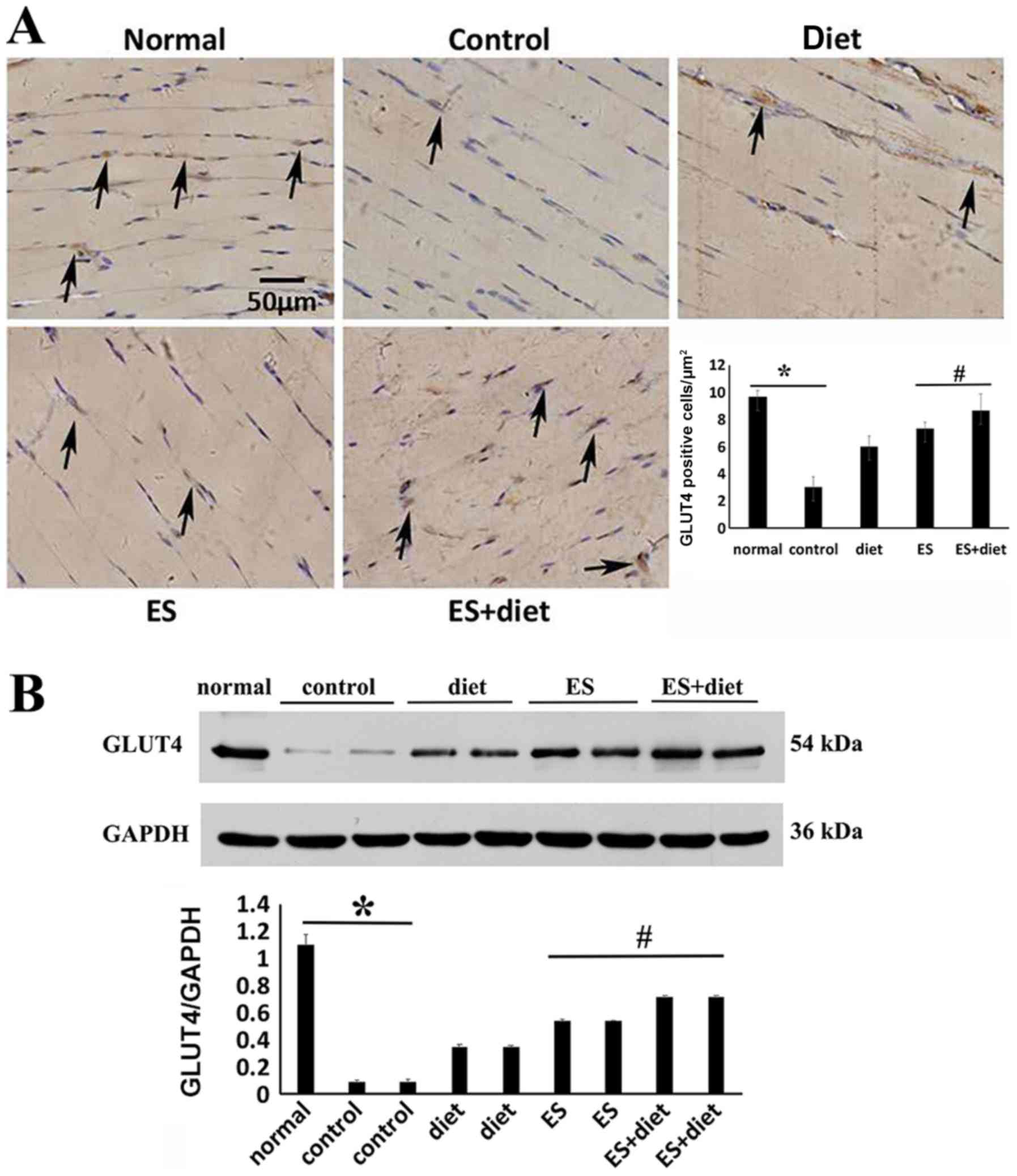
ES downregulates GSK-3α/β and
upregulates GLUT4 expression
The protein expression levels of GSK-3α in liver
tissue and GSK-3β in muscle tissue, which are serine kinases and
identified as key negative regulators of glycogen synthesis
(17), significantly increased in
the IR control group compared with the normal groups (Fig. 4). Following treatment, the GSK-3α/β
expression levels in the ES + diet group were significantly
downregulated compared with the other groups (Fig. 4; P<0.05). Thus, ES may improve
glycogen synthesis and IR by inhibiting GSK-3α/β expression
(18). To detect the effect of ES
and diet therapy on glycogen synthesis, the protein expression
levels of GLUT4, which maintains glucose homeostasis in striated
muscle (19), were also
investigated. The results demonstrated that the GLUT4 protein
expression levels increased significantly in the quadriceps femoris
muscle tissues of the ES + diet group compared with those of the
other groups (Fig. 5; P<05).
This result suggested that combination treatment may improve
glucose transportation by enhancing GLUT4 expression.
Discussion
Dietary excesses, including high fat and
hyperglycemia, increase the toxic lipid and proinflammatory
cytokine levels in the blood, which are major risk factors for
chronic inflammation and IR development in the skeletal muscle
(20,21). The prevalence of IR disorders is
mainly associated with obesity, type 2 diabetes mellitus,
dyslipidemia and hypertension in western/westernized populations
(22). IR includes impaired fatty
acid oxidation, lipotoxicity and ectopic fat deposition (23), whereas lipotoxicity and ectopic fat
are the common features of the diverse IR states (24).
Feeding rats with HFHCD induces IR (25). The duration of HFHCD feeding in the
literature varies predominantly from 4–12 weeks (25,26).
In the present study, the IR rat models were established by HFHCD
for 5 weeks. The increased FPG, insulin and IR index and decreased
ISI in rats indicated the successful establishment of the IR model.
The FFA, TG and TC concentrations in the control IR group
increased. These findings were consistent with those of previous
studies, which demonstrated that hyperglycemia induces IR, vascular
dysfunction and hepatic steatosis in rats (25,27).
Hepatic steatosis caused by TG accumulation is a
major contributor to IR (28).
This phenomenon was confirmed in the rat liver specimens of the
present study by histopathological examination (Fig. 1). mTOR is one of the intermediates
produced during TG synthesis and acts as an inhibitor for insulin
signaling (29). Hyperglycemia
upregulates the activity of the mTOR/p70S6K axis (14). Increased mTOR/p70S6K1 affects
insulin signaling by the phosphorylation of insulin receptor
substrate-1, thereby inhibiting PI3K and Akt activation (30,31).
The serine/threonine protein kinase Akt, which is also known as
protein kinase B and is a downstream effector of PI3K, is a
critical mediator of mTOR activity (32,33).
In the present study, HFHCD-induced IR was associated with
increased mTOR and decreased PI3K and Akt protein levels (Figs. 2 and 3).
Skeletal muscle is the main target tissue of insulin
action (13). At the cellular
level, IR in the skeletal muscle is characterized by impaired
insulin stimulation of glucose uptake and glycogen synthesis
(34,35). The promotion of glucose transport
and uptake and glycogen synthesis are the key biological actions of
insulin in skeletal muscle (13).
A number of studies have proved the important role
of ES in hypoglycemic action in IR (8,36–38).
However, the true effect and mechanism of ES on IR is unclear and
remains controversial. The results of the present study
demonstrated that ES can accelerate glucose translocation and
glycogen synthesis to improve IR by regulating the PI3K/Akt/mTOR
signaling pathway. This model may provide a mechanistic explanation
for the findings. Following the combination of ES and diet
treatment, FFA, TG and TC concentrations were significantly
decreased and inflammatory cell infiltration in the liver and the
pancreatic islet were ameliorated compared with the other groups.
mTOR, which acts as an inhibitor for insulin signaling, was
increased by HFHCD (29) and
attenuated by ES and diet treatment. In addition to the skeletal
muscle, hepatic tissue is also a main target tissue of insulin
action (13). The levels of PI3K
and Akt proteins in the liver were regulated by ES and diet
treatment.
In the further downstream signaling from Akt, the
insulin-mediated translocation of GLUT4 starts from its
intracellular localization to the plasma membrane for glucose
transport (13). GLUT4 in the
striated muscle and adipose cells is the major glucose transporter
in maintaining glucose homeostasis (24,25).
Insulin stimulates glucose transport by promoting GLUT4 exocytosis
(13). The impaired glucose
transport activity and/or defective intracellular metabolism of the
transported glucose can decrease glucose uptake (19). This result is consistent with the
present study, showing that the decreased protein levels for GLUT4
in IR rats induced the increased fasting glucose. In rodents and
humans, the skeletal muscle is responsible for >80% of
insulin-mediated glucose uptake in vivo through muscle
contraction (26), which induces
GLUT4 translocation from the intracellular depots to the plasma
membrane to allow large transport of glucose (23). Only a moderate increase in the
protein levels of GLUT4 is required to enhance insulin sensitivity
after exercise in skeletal muscle (27). In the present study, alternating
strings of low and dense-sparse frequencies used in the ES + diet
group may have stimulated skeletal muscle contraction and induced
glucose transportation by increasing PI3K, Akt and GLUT4 protein
levels. ES combined with diet also increased GLUT4 protein levels,
which may improve glucose transport and uptake.
In addition to glucose transport, ES and diet
therapy may improve glycogen synthesis by inhibiting GSK3, which
includes GSK-3 α/β and is involved in IR (17). GSK-3 is a key negative regulator of
glycogen synthesis, which is a major form of glucose storage
(28,29). GSK-3α functions as the hepatic
glycogen synthesis kinase regulating glycogen synthesis and
deposition primarily in the liver. GSK-3β serves an important role
in the skeletal muscle tissue and β-islet cells where its knockout
leads to enhanced glycogen synthesis activity and glycogen
accumulation or insulin responsiveness, respectively (39). Excessive calorie intake enhances
IRS-1 Ser636/639 phosphorylation, suppresses IRS-1-associated
PI3K/Akt signaling and activates GSK-3α and GSK-3β (14). Thus, insulin signaling is impaired.
The current study demonstrated that Akt decreased, whereas GSK-3α/β
protein levels in the liver and skeletal muscle tissue increased in
the IR control groups, respectively. Following combination
treatment, Akt was activated by ES, and GSK-3α and GSK-3β were
inhibited. These findings suggested that the effects of ES on
GSK-3α and GSK-3β may contribute to improving glucose
homeostasis.
The present study proposed a mechanism for the
effect of ES on IR. The results indicated that ES and diet therapy
improved insulin sensitivity in the IR rats via mTOR signaling. ES
increased GLUT4 expression and inhibited GSK-3 expression, which
may result in regulating glucose transport and glycogen synthesis
in the skeletal muscle and liver tissue, respectively. The present
in vivo data supplied the theoretical basis for the clinical
use of ES to treat patients with obesity or diabetes having IR.
However, these mechanisms have not been fully elucidated. Thus, the
molecular consequences of insulin-mediated changes in mTOR
signaling in vitro should be established in future
studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81403466 and
81273870), the Natural Science Foundation Project of CQ CSTC (grant
nos. cstc2017jcyjAX0363 and cstc2018jcyjAX0036), the Joint Project
of CQ CSTC and Health Commission of Chongqing (grant no.
ZY201802026) and the Venture & Innovation Support Program for
Chongqing Overseas Returnees (grant no. cx2018106).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH was involved in data acquisition, analysis and
interpretation, as well as manuscript drafting and revision. NT and
HZ were involved in data acquisition and analysis and manuscript
drafting. CT was involved in the conception and design of the study
and gave final approval of the manuscript to be submitted. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The approval for the present study was provided by
Chongqing Medical University's Animal Care and Ethics Committee and
formulated by the Ministry of Science and Technology of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IR
|
insulin resistance
|
|
ES
|
electrical stimulation
|
|
mTOR
|
mammalian target of rapamycin
|
|
FPG
|
fasting plasma glucose
|
|
ISI
|
insulin sensitivity index
|
|
TG
|
triglycerides
|
|
FFA
|
free fatty acids
|
|
TC
|
total cholesterol
|
References
|
1
|
Crescenzo R, Bianco F, Mazzoli A, Giacco
A, Liverini G and Iossa S: Mitochondrial efficiency and insulin
resistance. Front Physiol. 5:5122015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anusree SS, Nisha VM, Priyanka A and Raghu
KG: Insulin resistance by TNF-α is associated with mitochondrial
dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic
acid, a PPARγ agonist. Mol Cell Endocrinol. 413:120–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawaguchi T, Nakano D, Oriishi T and
Torimura T: Effects of isomaltulose on insulin resistance and
metabolites in patients with non-alcoholic fatty liver disease: A
metabolomic analysis. Mol Med Rep. 18:2033–2042. 2018.PubMed/NCBI
|
|
4
|
Lin RT, Tzeng CY, Lee YC, Chen YI, Hsu TH,
Lin JG and Chang SL: Acupoint-specific, frequency-dependent, and
improved insulin sensitivity hypoglycemic effect of
electroacupuncture applied to drug-combined therapy studied by a
randomized control clinical trial. Evid Based Complement Alternat
Med. 2014:3714752014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee TI, Bai KJ, Chen YC, Lee TW, Chung CC,
Tsai WC, Tsao SY and Kao YH: Histone deacetylase inhibition of
cardiac autophagy in rats on a high-fat diet with low-dose
streptozotocin-induced type 2 diabetes mellitus. Mol Med Rep.
17:594–601. 2018.PubMed/NCBI
|
|
6
|
Lin JG, Chen WC, Hsieh C, Tsai CC, Cheng
YW, Cheng JT and Chang SL: Multiple sources of endogenous opioid
peptide involved in the hypoglycemic response to 15 Hz
electroacupuncture at the Zhongwan acupoint in rats. Neurosci Lett.
366:39–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Senna-Fernandes V, Franca D, Moreno SF,
Santos-Filho S, Rogers PA, Bernardo-Filho M and Guimarães MA: The
effect of ‘Zusanli’ (ST. 36) acupuncture on the bio-availability of
sodium pertechnetate in Wistar rats. Acupunct Electrother Res.
31:33–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YC, Li TM, Tzeng CY, Cheng YW, Chen
YI, Ho WJ, Lin JG and Chang SL: Electroacupuncture-induced
cholinergic nerve activation enhances the hypoglycemic effect of
exogenous insulin in a rat model of streptozotocin-induced
diabetes. Exp Diabetes Res. 2011:9471382011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Könner AC and Brüning JC: Selective
insulin and leptin resistance in metabolic disorders. Cell Metab.
16:144–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng L, Song J, Li G, Liu Y, Wang Y, Meng
X, Sun G and Sun X: Effects of the Tangningtongluo formula as an
alternative strategy for diabetics via upregulation of insulin
receptor substrate-1. Mol Med Rep. 16:703–709. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo S, Copps KD, Dong X, Park S, Cheng Z,
Pocai A, Rossetti L, Sajan M, Farese RV and White MF: The Irs1
branch of the insulin signaling cascade plays a dominant role in
hepatic nutrient homeostasis. Mol Cell Biol. 29:5070–5083. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tzatsos A and Kandror KV: Nutrients
suppress phosphatidylinositol 3-kinase/Akt signaling via
raptor-dependent mTOR-mediated insulin receptor substrate 1
phosphorylation. Mol Cell Biol. 26:63–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panchal SK, Poudyal H, Iyer A, Nazer R,
Alam A, Diwan V, Kauter K, Sernia C, Campbell F, Ward L, et al:
High-carbohydrate high-fat diet-induced metabolic syndrome and
cardiovascular remodeling in rats. J Cardiovasc Pharmacol.
57:611–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nozaki Y, Fujita K, Wada K, Yoneda M,
Shinohara Y, Imajo K, Ogawa Y, Kessoku T, Nakamuta M, Saito S, et
al: Deficiency of eNOS exacerbates early-stage NAFLD pathogenesis
by changing the fat distribution. BMC Gastroenterol. 15:1772015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leng S, Zhang W, Zheng Y, Liberman Z,
Rhodes CJ, Eldar-Finkelman H and Sun XJ: Glycogen synthase kinase 3
beta mediates high glucose-induced ubiquitination and proteasome
degradation of insulin receptor substrate 1. J Endocrinol.
206:171–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nikoulina SE, Ciaraldi TP, Mudaliar S,
Carter L, Johnson K and Henry RR: Inhibition of glycogen synthase
kinase 3 improves insulin action and glucose metabolism in human
skeletal muscle. Diabetes. 51:2190–2198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kahn BB, Rossetti L, Lodish HF and Charron
MJ: Decreased in vivo glucose uptake but normal expression of GLUT1
and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest.
87:2197–2206. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Keung W, Samokhvalov V, Wang W
and Lopaschuk GD: Role of fatty acid uptake and fatty acid
beta-oxidation in mediating insulin resistance in heart and
skeletal muscle. Biochim Biophys Acta. 1801:1–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeyda M and Stulnig TM: Obesity,
inflammation, and insulin resistance-a mini-review. Gerontology.
55:379–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sparks LM, Xie H, Koza RA, Mynatt R,
Hulver MW, Bray GA and Smith SR: A high-fat diet coordinately
downregulates genes required for mitochondrial oxidative
phosphorylation in skeletal muscle. Diabetes. 54:1926–1933. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McGarry JD: Banting lecture 2001:
Dysregulation of fatty acid metabolism in the etiology of type 2
diabetes. Diabetes. 51:7–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lund S, Holman GD, Schmitz O and Pedersen
O: Contraction stimulates translocation of glucose transporter
GLUT4 in skeletal muscle through a mechanism distinct from that of
insulin. Proc Natl Acad Sci USA. 92:5817–5821. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
James DE, Strube M and Mueckler M:
Molecular cloning and characterization of an insulin-regulatable
glucose transporter. Nature. 338:83–87. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zorzano A, Wilkinson W, Kotliar N, Thoidis
G, Wadzinkski BE, Ruoho AE and Pilch PF: Insulin-regulated glucose
uptake in rat adipocytes is mediated by two transporter isoforms
present in at least two vesicle populations. J Biol Chem.
264:12358–12363. 1989.PubMed/NCBI
|
|
26
|
DeFronzo RA, Jacot E, Jequier E, Maeder E,
Wahren J and Felber JP: The effect of insulin on the disposal of
intravenous glucose. Results from indirect calorimetry and hepatic
and femoral venous catheterization. Diabetes. 30:1000–1007. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ivy JL and Kuo CH: Regulation of GLUT4
protein and glycogen synthase during muscle glycogen synthesis
after exercise. Acta Physiol Scand. 162:295–304. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grimes CA and Jope RS: The multifaceted
roles of glycogen synthase kinase 3beta in cellular signaling. Prog
Neurobiol. 65:391–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaidanovich-Beilin O and Eldar-Finkelman
H: Long-term treatment with novel glycogen synthase kinase-3
inhibitor improves glucose homeostasis in ob/ob mice: Molecular
characterization in liver and muscle. J Pharmacol Exp Ther.
316:17–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pearce NJ, Arch JR, Clapham JC, Coghlan
MP, Corcoran SL, Lister CA, Llano A, Moore GB, Murphy GJ, Smith SA,
et al: Development of glucose intolerance in male transgenic mice
overexpressing human glycogen synthase kinase-3beta on a
muscle-specific promoter. Metabolism. 53:1322–1330. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun XJ and Liu F: Phosphorylation of IRS
proteins Yin-Yang regulation of insulin signaling. Vitam Horm.
80:351–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signalling pathways: Insights into insulin
action. Nat Rev Mol Cell Biol. 7:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Højlund K and Beck-Nielsen H: Impaired
glycogen synthase activity and mitochondrial dysfunction in
skeletal muscle: Markers or mediators of insulin resistance in type
2 diabetes? Curr Diabetes Rev. 2:375–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shulman GI, Rothman DL, Jue T, Stein P,
DeFronzo RA and Shulman RG: Quantitation of muscle glycogen
synthesis in normal subjects and subjects with
non-insulin-dependent diabetes by 13C nuclear magnetic resonance
spectroscopy. N Engl J Med. 322:223–228. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang SL, Lin KJ, Lin RT, Hung PH, Lin JG
and Cheng JT: Enhanced insulin sensitivity using electroacupuncture
on bilateral Zusanli acupoints (ST 36) in rats. Life Sci.
79:967–971. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pai HC, Tzeng CY, Lee YC, Chang CH, Lin
JG, Cheng JT and Chang SL: Increase in plasma glucose lowering
action of rosiglitazone by electroacupuncture at bilateral Zusanli
acupoints (ST.36) in rats. J Acupunct Meridian Stud. 2:147–151.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan H, Huang H, Zhang L, Ma S, Yang H and
Wang H: ‘Adjusting internal organs and dredging channel’
electroacupuncture treatment prevents the development of diabetic
peripheral neuropathy by downregulating glucose-related protein 78
(GRP78) and caspase-12 in streptozotocin-diabetic rats. J Diabetes.
Mar 8–2019.(Epub ahead of print). View Article : Google Scholar :
|
|
39
|
MacAulay K and Woodgett JR: Targeting
glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2
diabetes. Expert Opin Ther Targets. 12:1265–1274. 2008. View Article : Google Scholar : PubMed/NCBI
|