Introduction
Depression is a highly prevalent mood disorder
characterized by depressed mood, declined interest and so-called
autonomic function changes, including alterations in appetite,
sleep, and energy levels (1).
Depression affects many aspects of patients' lives, including
social interaction, relationships with family, and working
efficiency, and >350 million people worldwide battle with the
disorder every day (2). Depression
is one of the leading causes of disabling medical conditions
worldwide, and >60% of those who commit suicide suffer from the
disease (3). Despite its
tremendous burden, high prevalence, the extensive research over the
last half-century, and the increasing number of antidepressant
treatment options available, an efficient cure for depression is
lacking.
The endoplasmic reticulum (ER) is an important
subcellular organelle involved in the synthesis, post-translational
modifications, and proper folding of secretory proteins, and
calcium homeostasis (4). A variety
of physiological conditions, including hypoxia, stress,
hypoglycemia, calcium depletion, oxidative stress, and a high-fat
diet, can disrupt the protein folding process and consequently
result in the accumulation of unfolded and misfolded proteins in
the ER, a condition termed ER stress (5). Under ER stress, the unfolded protein
response (UPR) is activated to minimize the overloading caused by
the unfolded proteins. However, if ER functions are severely
damaged, apoptosis signals are triggered (6).
Maintaining the normal function of the ER requires
the help of ER chaperones, of which the 78-kDa glucose-regulated
protein (GRP78) and 94-kDa glucose-regulated protein (GRP94) are
important members (7). Many
cellular processes require ER chaperone involvement, including the
transfer of newly synthesized peptides on the ER membrane, the
folding and assembly of proteins, the degradation of misfolded
proteins by the ubiquitin-proteasome system, the
autophagy-lysosomal system, and the regulation of calcium
homeostasis, which act as stress sensors for the ER (8–11).
Multiple studies have demonstrated that ER stress is associated
with psychiatric disorders. Bown et al (12) revealed that levels of the ER stress
proteins GRP78, GRP94 and calreticulin are elevated in the
postmortem temporal cortex of patients with major depressive
disorder (MDD), with Nevell et al (13) similarly demonstrating that systemic
and persistent activation of ER stress is associated with MDD.
Another study reported that stressful life events across the
lifespan play an important role in disease etiology (14). Stress is one of the common causes
of depressive episodes; sustained stress can also disrupt
hippocampal nerve regeneration in adults, indirectly inhibit
dopamine function, and exaggerate the amygdala's memory of negative
stimuli (15). Therefore, it is
hypothesized that ER stress may be a major culprit in the
progression of depression. This review aimed to summarize the
current knowledge of the role of ER stress in depression.
ER stress
ER stress refers to the molecular and biochemical
changes in the homeostatic morphology and function of the ER upon
the stimulation of cells by internal or external factors, which
block the processing and transport of proteins, and lead to the
accumulation of large amounts of unfolded or misfolded proteins in
the ER (16). As a result, cells
take corresponding measures to relieve ER stress and promote the
recovery of normal ER function. Cellular survival is only achieved
when the ER stress response resolves these stimuli and produces
normal proteins (17). If residual
unfolded proteins are released as mutants, specific apoptotic
responses occur (18). To relieve
ER stress and restore homeostatic functions within the ER on a
cellular level, the UPR triggers three types of protective cellular
responses depending on the cause of induction: The upregulation of
genes encoding ER chaperones, the attenuation of translation, or
the promotion of ER-associated degradation (ERAD) of aggregated
proteins (19). These are adaptive
responses to protein accumulation in the ER and relieve ER stress
by reducing protein synthesis, promoting protein degradation, and
increasing molecular chaperones that help protein folding. Protein
kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α
(IRE1α), and activating transcription factor 6 (ATF6) are important
transmembrane proteins that initiate UPR (20). In unstressed cells, these ER
transmembrane signaling molecules are maintained in an inactive
state by binding to GRP78 (21).
Upon detecting ER stress, the UPR signaling pathway is triggered,
and GRP78 is rapidly released from these three molecules and binds
to unfolded proteins (Fig. 1)
(22).
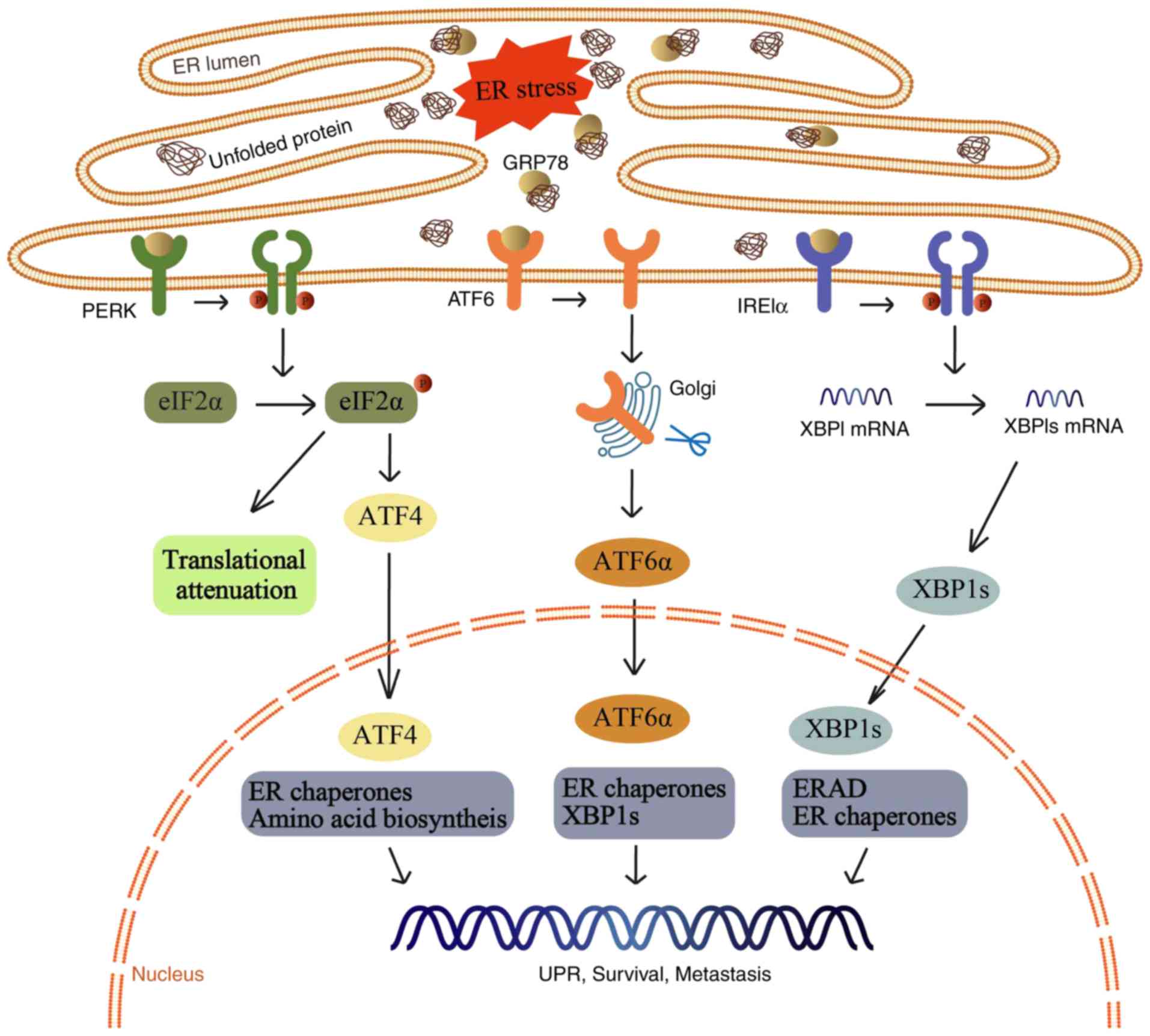 | Figure 1.A variety of physiological conditions
can disrupt the protein folding process and consequently result in
the accumulation of unfolded and misfolded proteins in the ER, a
condition termed ER stress. Under ER stress, the UPR is activated
to minimize overloading. If the UPR is successful in reducing the
number of unfolded and misfolded proteins, UPR signaling is
attenuated and the cell survives. ER, endoplasmic reticulum; GRP78,
78-kDa glucose-regulated protein; PERK, protein kinase R-like ER
kinase; eIF2α, eukaryotic translation initiation factor 2α; ATF4,
activating transcription factor 4; ATF6, activating transcription
factor 6; IRE1α, inositol-requiring enzyme 1α; XBP1, X-box binding
protein 1; ERAD, endoplasmic reticulum associated degradation; UPR,
unfolded protein response. |
Following dissociation from GRP78, PERK
homodimerizes to promote its autophosphorylation and activation
(23). PERK activation then
triggers the phosphorylation of eukaryotic translation initiation
factor 2α (eIF2α), which activates the expression of multiple
transcription factors, such as activating transcription factor 4
(ATF4) (23). Phosphorylated eIF2α
activity is inhibited during the early stages of stress, which
slows down the synthesis and translation of most proteins in the
cell, and reduces the load of protein folding in the ER. In
addition, ATF4 serves a protective role by regulating amino acid
metabolism, redox reactions and protein secretion (24). However, as the time and intensity
of the stress response increases, activated ATF4 induces the
expression of the pro-apoptotic CCAAT/enhancer-binding
protein-homologous protein (CHOP) (25).
IRE1α is a transmembrane protein containing
serine/threonine kinase and endonuclease domain (26). Following the dissociation of IRE1α
from GRP78, IRE1α is self-activated by homodimerization and
phosphorylation of the intracytoplasmic domain, resulting in the
allosteric activation of its surrounding endonuclease domain
(24). Activated IRE1α splices the
26-base intron in the mRNA precursor of X-box binding protein 1
(XBP1), which is induced by ATF6, and the spliced mRNA undergoes
translational frame shifting to encode the steady-state
transcription factor XBP1 (27).
The transcription factor XBP1s not only induces the transcription
of the GRP78 and CHOP genes but also activates the ERAD program
through ER degradation enhancing α mannosidase-like 1 (EDEM1) in an
attempt to restore ER homeostasis (28,29).
Therefore, XBP1s is crucial to restoring ER homeostasis.
In the unstressed state, ATF6 is mainly present in
the ER as a zymogen, but under ER stress, it is induced to
dissociate from GRP78 and transport into Golgi bodies. The ATF6α
transcription factor is released following cleavage by the Golgi
site-1 and −2 proteases (30).
Together with XBP1, ATF6α activates the transcription of targets
such as GRP78, thereby counteract ER stress and exert protective
effects to promote cell survival (31). Collectively, these transduction
pathways coordinate together to counteract ER stress through
negative feedback, reducing the number of unfolded proteins and
facilitating cell survival (17).
Nonetheless, when the mechanism fails, a large amount of calcium is
released into the cytoplasm to induce apoptosis, whilst persistent
UPR signals induce chronic ER stress (18). The UPR actively promotes cell death
through apoptosis following the activation of CHOP, JNKs,
proapoptotic Bcl-2-like protein 11 (Bim; a member of the Bcl-2
family), and caspases (Fig. 2)
(32).
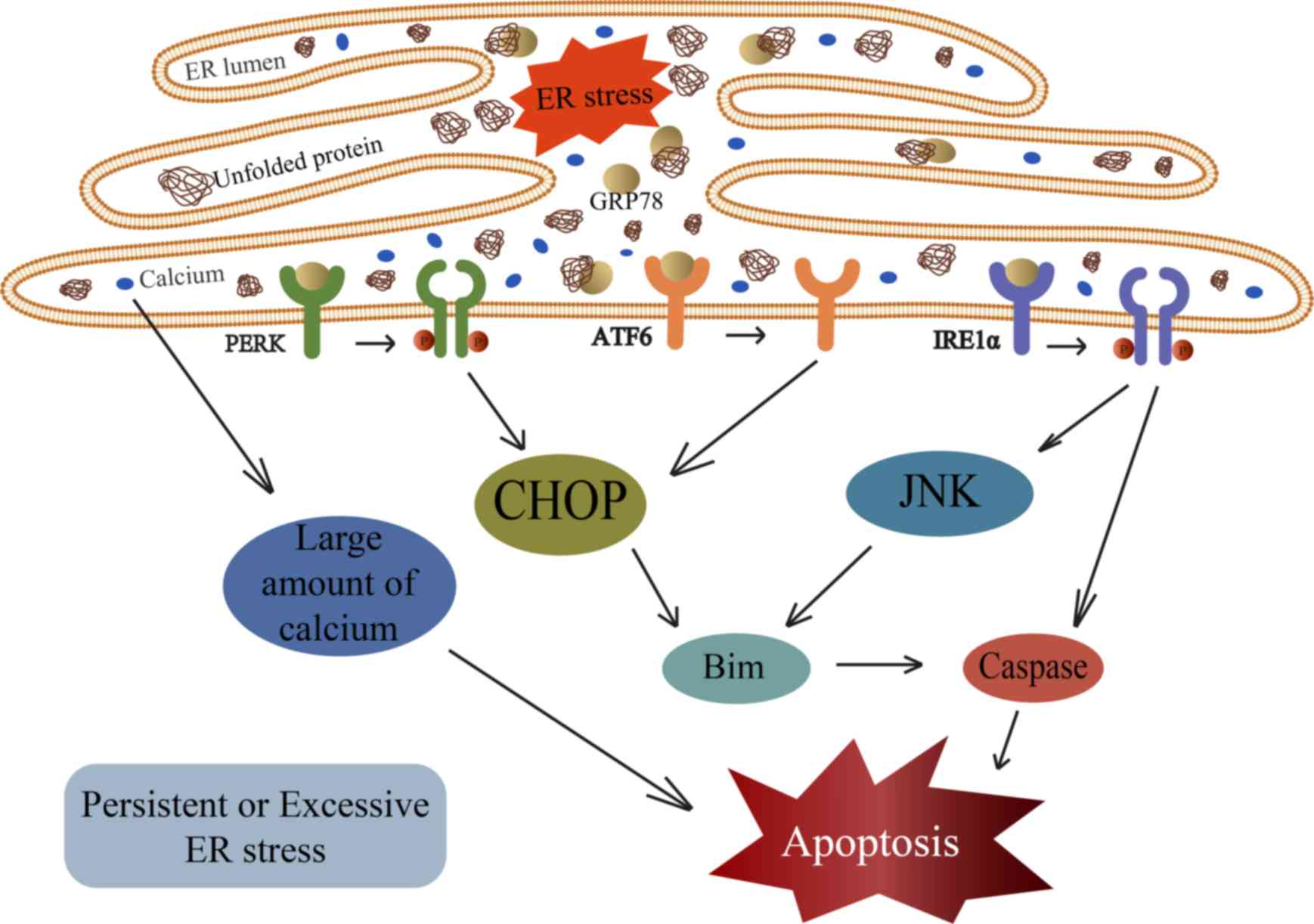 | Figure 2.When the function of ER is severely
impaired, a large amount of calcium is released into the cytoplasm
to induce apoptosis. UPR also triggers apoptotic signals through
CHOP, JNKs, Bim, and caspases. ER, endoplasmic reticulum; GRP78,
78-kDa glucose-regulated protein; PERK, protein kinase R-like ER
kinase; ATF6, activating transcription factor 6; IRE1α,
inositol-requiring enzyme 1α; CHOP, CCAAT/enhancer-binding
protein-homologous protein; Bim, Bcl-2-like protein 11. |
ER stress and depression
Animal studies
Rats or mice exposed to chronic unpredictable mild
stress (CUMS) are widely used as depression models (33). Previous results have suggested that
the depressive behavior of CUMS mice is related to ER stress, and
that the underlying mechanism is the insufficient synthesis of ATP,
and the overactivated oxidative and ER stress which leads to
neuronal apoptosis or death (34).
Another previous study reported that CUMS-induced depression-like
behavior in rats is due to the disturbance of hippocampal hydrogen
sulfide (H2S) generation, which in turn promotes
hippocampal ER stress responses including the upregulation of
GRP78, CHOP, and cleaved caspase-12 expression (35). H2S is the third most
prevalent endogenous signaling gasotransmitter and serves a key
role in both neuromodulation and neuroprotection (36). Moreover, H2S has been
suggested to inhibit hippocampal ER stress by upregulating
hippocampal silence signal regulator 1, an essential metabolically
regulated transcription factor, thereby attenuating depression-like
behaviors in rats (37). These
findings suggest that H2S may provide a novel target for
the treatment of depression.
Recent evidence has demonstrated that chronic
restraint stress (CRS) induces cognitive impairment, and anxiety-
and depression-like behavior in rodents (38). Previous findings by Jangra et
al suggest that honokiol (a traditional Chinese medicine
isolated from the bark of Magnolia officinalis tree) eliminates
CRS-induced cognitive impairment and depressive-like behavior by
preventing the elevation of GRP78 and CHOP in the hippocampus of
mice (39). Furthermore, in CRS
mice, the expression levels of GRP78 and CHOP in the hippocampus,
and of CHOP in the prefrontal cortex, are significantly upregulated
compared with those in the control group, while sodium
phenylbutyrate (an ER stress inhibitor) and edaravone (a free
radical scavenger) not only reverse the high expression of these
genes, but also improve the cognitive deficits and depression-like
behavior observed in the model mice (40). The expression levels of GRP78,
ATF6, XBP1, CHOP, and growth arrest and DNA damage-inducible
protein (a regulatory subunit of an eIF2α-specific phosphatase
complex) are also increased in the CRS rat striatum, indicating
that ER stress is involved in CRS-induced depression-like abnormal
behaviors (41).
Lipopolysaccharide is an endotoxin that causes
anxiety- and depression-like behavior in rodents after central or
peripheral administration (42).
Its administration is reported to significantly upregulate GRP78
mRNA expression in the hippocampus, suggesting that UPR is involved
in lipopolysaccharide-evoked behavioral anomalies (43). Social defeat stress-vulnerable mice
also demonstrate depression-like behavior, which is associated with
a significant increase in the expression of GRP78, CHOP and choline
acetyltransferase in the amygdala (44). Liu et al found that the
expression of GRP78 and XBP1 were significantly increased in the
hippocampus of mice with social defeat stress (45). Furthermore, chronic social defeat
stress can activate the PERK-eIF2α signaling pathway in the
hippocampus, leading to the downregulation of brain-derived
neurotrophic factor expression levels, and eventually
depression-like behavior and memory impairment in mice (46). Sharma et al also
demonstrated that inhibition of PERK expression in the hippocampus
can enhance hippocampal-dependent memory and reverse memory
deterioration in mice (47),
suggesting that cognitive function can be improved by regulating
the expression levels of PERK at the Cornu Ammon 1 region of the
hippocampus. In addition, plasma levels of corticosterone and the
expression of genes encoding GRP78, GRP94, ATF6, XBP1, ATF4, and
CHOP increased in the hippocampus of rats with learned
helplessness, suggesting that depression-like behavior may be
associated with excessive persistence of ER stress (48). Spinal cord injury in rodent models
causes depressive-like behavior and impairment of spatial memory
retention (49–51). Experimental studies have reported
that chronically activated ER stress in the brain and newly formed
immature neurons in the subgranular area of the hippocampus may be
detrimental to the survival and regeneration of impaired cognitive
and depressive neurons following spinal cord injury (52). Finally, the introduction of an
Alzheimer's patient-associated mutation in the gene encoding
carboxypeptidase E/NF-α1 into transgenic mice leads to increased ER
stress and decreased expression of a pro-survival protein, Blc-2,
ultimately resulting in symptoms resembling dementia and depression
(53). In summary, the above
animal studies have demonstrated a link between ER stress and
depression.
Clinical studies
To explore the hypothesis that ER stress has an
important influence on the pathophysiology of depression in human
studies, Behnke et al (8)
measured the expression levels of GRP78, GRP94, and calreticulin in
postmortem samples of psychiatric patients. Higher expression
levels of GRP78, GRP94, and calreticulin were found in the temporal
cortex of patients with MDD who died by suicide compared with MMD
patients with non-suicide deaths or other groups (8). Although some of the patients with
bipolar disorder or schizophrenia died by suicide, no such
differences in the expression levels of these proteins were found
in these patients (12).
Furthermore, xanthine oxidase activity, which produces reactive
oxygen species and is a central mechanism of oxidative stress, is
increased in temporal lobe tissue of patients with recurrent
depression (54,55). In addition, upon analyzing the
expression levels of GRP78, EDEM1, CHOP, and XBP1, the major
indicators of the ER stress response, with reverse
transcription-quantitative PCR using leukocyte-derived RNA samples
from 86 participants in the Detroit Neighborhood Health Study,
patients with MDD had significantly higher levels of GRP78, EDEM1,
CHOP, and XBP1 compared to control patients (13). These data suggest that the ER
stress response pathways in those MDD patients are indicative of
sustained activation. Altogether, these studies have suggested that
the ER stress response pathway is continuously activated in MDD
patients and warrants further investigation.
ER stress and medicine
Herbal medicine
Previous studies have found that numerous compounds
exert antidepressive properties that may be linked to their ability
to affect ER stress. Chronic luteolin treatment results in an
antidepressant-like effect by inhibiting apoptosis induced by
hippocampal ER stress, leading to the increased expression of the
genes encoding GRP78 and GRP94 and reduced cleavage of caspase-3
(56). By contrast, longistyline C
exerts antidepressive properties by inhibiting the levels of GRP78,
CHOP and XBP1, thus, relieving ER stress (57). Another previous study reported that
the water extraction product of Panax ginseng possesses
antidepressant-like activity in both acute and chronic stress
models of depression, with the underlying molecular mechanism
depending on the downregulation of ER stress-related proteins, such
as CHOP, GRP78, XBP1, and caspase-12 (58). Saikosaponins may also exert
antidepressant-like effects through their protective effects on
neuronal cells, including their ability to downregulate GRP78, CHOP
and XBP1 to stabilize ER stress and restore mitochondrial membrane
potential activity to recover mitochondrial function (59). Finally, a recent study demonstrated
that gastrodin can improve depression-like behavior in mice by
inhibiting ER stress (60), and
the mechanism by which Aralia elata treats depression after
illness also involves ER stress (61).
Antidepressants
Fluvoxamine is a selective serotonin reuptake
inhibitor (SSRI) with high affinity for the σ-1 receptor (Sig-1R)
(62). In a leptin resistance
study, Hosoi et al revealed that fluvoxamine attenuated ER
stress through the Sig-1R (63).
Sig-1R forms a complex with another molecular chaperone, such as
GRP78, at the ER-mitochondrial interface to regulate calcium ion
signaling and cell survival (64).
Similarly, a previous study by Omi et al demonstrated that
fluvoxamine-mediated upregulation of Sig-1R expression promoted
neuroprotection by inhibiting ER stress-mediated apoptosis and
increasing ATF4 translation (65).
Finally, Terada et al reported that fluvoxamine improved
depression-like behavior in mice following chronic dexamethasone
infusions via the recovery of brain-derived neurotrophic
factor/XBP1/Sig-1R/5-hydroxytryptamine 2A receptor signaling
cascades (66).
Fluoxetine is a widely used SSRI in clinical
practice that can prolong and increase the action of serotonin
(67). Ma et al revealed
that fluoxetine induced apoptosis through CHOP-dependent ER
stress-related apoptotic pathways in glioma cells, such as the
PERK/eIF2α/ATF4/CHOP and ATF6/CHOP signaling pathways (68). In addition, desipramine promotes
antitumor activity in glioma by inducing autophagy through the
PERK/eIF2α and ATF6 signaling pathways (69) and the rapid antidepressant effects
of ketamine may also be related to ER stress (70). Notably, previous experimental
studies suggest that liver damage caused by some drugs is also
associated with excessive activation of ER stress. For example, the
commonly used SSRI antidepressant sertraline leads to an increase
in PERK, IRE1α, and CHOP expression levels (71) and nefazodone significantly
increases CHOP, ATF4, and phosphorylated eIF2α levels (72). In addition, a recent study
demonstrated that the SSRI escitalopram significantly reduces the
expression level of ER stress marker proteins, thereby exerting its
antidepressant effect (73). To
sum up, most studies suggest that antidepressants work by reducing
ER stress.
Other
The Bax inhibitor 1 (BI-1) is an evolutionarily
conserved ER protein that has an antiapoptotic effect on ER
stress-induced cell death (74).
Therefore, in the short term, BI-1 may serve a protective role in
the depression-like behaviors induced by chronic mild stress
(75). In addition, previous
findings by Hunsberger et al indicated that the expression
of BI-1 was protective against anhedonia (76). Alternatively, evidence suggests
that an estrogen receptor β agonist could ameliorate ER
stress-induced anxiety- and depression-like behavior through the
IRE1α/XBP1 pathway (77). Finally,
in rats, a soluble epoxide hydrolase inhibitor mitigated the
development of depression-like behaviors by alleviating ER stress
(78).
Conclusions
Current research suggests that ER stress serves a
key role in the pathophysiology of depression. ER stress-related
proteins are significantly increased in animal models of
depression, including within the hippocampus, prefrontal cortex,
amygdala, and striatum, indicating that ER stress is involved in
the pathogenesis of depression. Clinical studies are relatively
limited, but evidence has revealed that patients with depression
exhibit persistent activation of the ER stress system. This
suggests that the treatment and prevention of depression should
focus on ER stress-related signaling pathways. Future research
should elucidate further the signaling pathways involved in
depression-related ER stress. Furthermore, studies with
antidepressant drugs are contradictory; herbal medicine and
fluvoxamine inhibit the excessive activation of the ER stress
system, whereas fluoxetine, desipramine, sertraline, and nefazodone
potentiate ER stress. Although the latter can activate ER stress to
promote cell apoptosis, no studies have definitely demonstrated
that they cause neuronal apoptosis. Thus, the effects of these
antidepressant drugs on different types of cellular ER stress
systems should be explored in depth. Concurrently, the role of ER
stress in depression should be further clarified from the
perspective of gene transcription and translation. In addition, the
activation of ER stress in animal models of depression is often
accompanied by the occurrence of oxidative stress and inflammation.
Hence, the mechanism of the interactions among oxidative stress,
inflammation, and ER stress in the development of depression
requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Medical and
Health Science and Technology Plan Project of Zhejiang Province
(grant nos. 2018KY671 and 2019KY564), The Ningbo Health Branding
Subject Fund (grant no. PPXK2018-01), The Major Social Development
Special Foundation of Ningbo (grant no. 2017C510010), The Natural
Science Foundation of Zhejiang province (grant no. LY14H090003) and
The Natural Science Foundation of Ningbo (grant no.
2016A610156).
Availability of data and materials
Not applicable.
Authors' contributions
JXM, YRH and ZZL drafted the manuscript. JXM, YXJ
and ZZL conceived and designed the framework of this article, LMR,
YRH, YXJ and ZZL collected and analyzed the literature. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
endoplasmic reticulum
|
|
UPR
|
unfolded protein response
|
|
GRP78
|
78-kDa glucose-regulated protein
|
|
GRP94
|
94-kDa glucose-regulated protein
|
|
ERAD
|
ER-associated degradation
|
|
MDD
|
major depressive disorder
|
|
PERK
|
protein kinase R-like ER kinase
|
|
IRE1α
|
inositol-requiring enzyme 1α
|
|
ATF6
|
activating transcription factor 6
|
|
eIF2α
|
eukaryotic translation initiation
factor 2α
|
|
ATF4
|
activating transcription factor 4
|
|
CHOP
|
CCAAT/enhancer-binding
protein-homologous protein
|
|
XBP1
|
X-box binding protein 1
|
|
EDEM1
|
ER degradation-enhancing-α-
mannosidase-like 1
|
|
CUMS
|
chronic unpredictable mild stress
|
|
H2S
|
hydrogen sulfide
|
|
CRS
|
chronic restraint stress
|
|
SSRI
|
selective serotonin reuptake
inhibitor
|
|
Sig-1R
|
σ-1 receptor
|
References
|
1
|
Malhi GS and Mann JJ: DepressionLancet.
London, England: 392. pp. 2299–2312. 2018, View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The burden of depression. Nature.
515:1632014. View
Article : Google Scholar
|
|
3
|
Ledford H: Medical research: If depression
were cancer. Nature. 515:182–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCaffrey K and Braakman I: Protein
quality control at the endoplasmic reticulum. Essays Biochem.
60:227–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hetz C and Papa FR: The unfolded protein
response and cell fate control. Mol Cell. 69:169–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hetz C, Chevet E and Oakes SA:
Proteostasis control by the unfolded protein response. Nat Cell
Biol. 17:829–838. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Lee J, Liem D and Ping P: HSPA5
Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum.
Gene. 618:14–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Behnke J, Mann MJ, Scruggs FL, Feige MJ
and Hendershot LM: Members of the Hsp70 family recognize distinct
types of sequences to execute ER quality control. Mol Cell.
63:739–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otero JH, Lizák B and Hendershot LM: Life
and death of a BiP substrate. Semin Cell Dev Biol. 21:472–478.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Ni M, Lee B, Barron E, Hinton DR and
Lee AS: The unfolded protein response regulator GRP78/BiP is
required for endoplasmic reticulum integrity and stress-induced
autophagy in mammalian cells. Cell Death Differ. 15:1460–1471.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura K, Bossy-Wetzel E, Burns K, Fadel
MP, Lozyk M, Goping IS, Opas M, Bleackley RC, Green DR and Michalak
M: Changes in endoplasmic reticulum luminal environment affect cell
sensitivity to apoptosis. J Cell Biol. 150:731–740. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bown C, Wang JF, MacQueen G and Young LT:
Increased temporal cortex ER stress proteins in depressed subjects
who died by suicide. Neuropsychopharmacology. 22:327–332. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nevell L, Zhang K, Aiello AE, Koenen K,
Galea S, Soliven R, Zhang C, Wildman DE and Uddin M: Elevated
systemic expression of ER stress related genes is associated with
stress-related mental disorders in the Detroit Neighborhood Health
Study. Psychoneuroendocrinology. 43:62–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koenig JI, Walker CD, Romeo RD and Lupien
SJ: Effects of stress across the lifespan. Stress. 14:475–480.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dillon DG and Pizzagalli DA: Mechanisms of
memory disruption in depression. Trends Neurosci. 41:137–149. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kadowaki H, Satrimafitrah P, Takami Y and
Nishitoh H: Molecular mechanism of ER stress-induced pre-emptive
quality control involving association of the translocon, Derlin-1,
and HRD1. Sci Rep. 8:73172018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shore GC, Papa FR and Oakes SA: Signaling
cell death from the endoplasmic reticulum stress response. Curr
Opin Cell Biol. 23:143–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scheper W and Hoozemans JJ: The unfolded
protein response in neurodegenerative diseases: A neuropathological
perspective. Acta Neuropathol. 130:315–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bettigole SE and Glimcher LH: Endoplasmic
reticulum stress in immunity. Annu Rev Immunol. 33:107–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korennykh A and Walter P: Structural basis
of the unfolded protein response. Annu Rev Cell Dev Biol.
28:251–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Casas C: GRP78 at the centre of the stage
in cancer and neuroprotection. Front Neurosci. 11:1772017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harding HP, Zhang Y, Bertolotti A, Zeng H
and Ron D: Perk is essential for translational regulation and cell
survival during the unfolded protein response. Mol Cell. 5:897–904.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carrara M, Prischi F, Nowak PR, Kopp MC
and Ali MM: Noncanonical binding of BiP ATPase domain to Ire1 and
Perk is dissociated by unfolded protein CH1 to initiate ER stress
signaling. Elife. 4:2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han J, Back SH, Hur J, Lin YH,
Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M,
et al: ER-stress-induced transcriptional regulation increases
protein synthesis leading to cell death. Nat Cell Biol. 15:481–490.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, Liu H, Li L, Liu H, Shi W, Yuan X
and Wu L: Structural insights into IRE1 functions in the unfolded
protein response. Curr Med Chem. 23:4706–4716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee AH, Iwakoshi NN and Glimcher LH: XBP-1
regulates a subset of endoplasmic reticulum resident chaperone
genes in the unfolded protein response. Mol Cell Biol.
23:7448–7459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu R, Zhang QH, Lu YJ, Ren K and Yi GH:
Involvement of the IRE1α-XBP1 pathway and XBP1s-dependent
transcriptional reprogramming in metabolic diseases. DNA Cell Biol.
34:6–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Papaioannou A, Higa A, Jégou G, Jouan F,
Pineau R, Saas L, Avril T, Pluquet O and Chevet E: Alterations of
EDEM1 functions enhance ATF6 pro-survival signaling. FEBS J.
285:4146–4164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hillary RF and FitzGerald U: A lifetime of
stress: ATF6 in development and homeostasis. J Biomed Sci.
25:482018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shoulders MD, Ryno LM, Genereux JC,
Moresco JJ, Tu PG, Wu C, Yates JR III, Su AI, Kelly JW and Wiseman
RL: Stress-independent activation of XBP1s and/or ATF6 reveals
three functionally diverse ER proteostasis environments. Cell Rep.
3:1279–1292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hiramatsu N, Chiang WC, Kurt TD, Sigurdson
CJ and Lin JH: Multiple mechanisms of unfolded protein
response-induced cell death. Am J Pathol. 185:1800–1808. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hill MN, Hellemans KG, Verma P, Gorzalka
BB and Weinberg J: Neurobiology of chronic mild stress: Parallels
to major depression. Neurosci Biobehav Rev. 36:2085–2117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Y, Yang N, Hao W, Zhao Q, Ying T, Liu
S, Li Q, Liang Y, Wang T, Dong Y, et al: Dynamic proteomic analysis
of protein expression profiles in whole brain of Balb/C mice
subjected to unpredictable chronic mild stress: Implications for
depressive disorders and future therapies. Neurochem Int.
58:904–913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan H, Zou W, Jiang J, Tian Y, Xiao Z, Bi
L, Zeng H and Tang X: Disturbance of hippocampal H2S generation
contributes to CUMS-induced depression-like behavior: Involvement
in endoplasmic reticulum stress of hippocampus. Acta Biochim
Biophys Sin (Shanghai). 47:285–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X and Bian JS: Hydrogen sulfide: A
neuromodulator and neuroprotectant in the central nervous system.
ACS Chem Neurosci. 5:876–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu SY, Li D, Zeng HY, Kan LY, Zou W,
Zhang P, Gu HF and Tang XQ: Hydrogen sulfide inhibits chronic
unpredictable mild stress-induced depressive-like behavior by
upregulation of Sirt-1: Involvement in suppression of hippocampal
endoplasmic reticulum stress. Int J Neuropsychopharmacol.
20:867–876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiba S, Numakawa T, Ninomiya M, Richards
MC, Wakabayashi C and Kunugi H: Chronic restraint stress causes
anxiety- and depression-like behaviors, downregulates
glucocorticoid receptor expression, and attenuates glutamate
release induced by brain-derived neurotrophic factor in the
prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry.
39:112–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jangra A, Dwivedi S, Sriram CS, Gurjar SS,
Kwatra M, Sulakhiya K, Baruah CC and Lahkar M: Honokiol abrogates
chronic restraint stress-induced cognitive impairment and
depressive-like behaviour by blocking endoplasmic reticulum stress
in the hippocampus of mice. Eur J Pharmacol. 770:25–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jangra A, Sriram CS, Dwivedi S, Gurjar SS,
Hussain MI, Borah P and Lahkar M: Sodium phenylbutyrate and
edaravone abrogate chronic restraint stress-induced behavioral
deficits: Implication of oxido-nitrosative, endoplasmic reticulum
stress cascade, and neuroinflammation. Cell Mol Neurobiol.
37:65–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pavlovsky AA, Boehning D, Li D, Zhang Y,
Fan X and Green TA: Psychological stress, cocaine and natural
reward each induce endoplasmic reticulum stress genes in rat brain.
Neuroscience. 246:160–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lawson MA, Parrott JM, McCusker RH,
Dantzer R, Kelley KW and O'Connor JC: Intracerebroventricular
administration of lipopolysaccharide induces
indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J
Neuroinflammation. 10:872013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jangra A, Sriram CS and Lahkar M:
Lipopolysaccharide-induced behavioral alterations are alleviated by
sodium phenylbutyrate via attenuation of oxidative stress and
neuroinflammatory cascade. Inflammation. 39:1441–1452. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang GB, Zhao T, Muna SS, Bagalkot TR,
Jin HM, Chae HJ and Chung YC: Effects of chronic social defeat
stress on behaviour, endoplasmic reticulum proteins and choline
acetyltransferase in adolescent mice. Int J Neuropsychopharmacol.
16:1635–1647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu L, Zhao Z, Lu L, Liu J, Sun J, Wu X
and Dong J: Icariin and icaritin ameliorated hippocampus
neuroinflammation via inhibiting HMGB1-related pro-inflammatory
signals in lipopolysaccharide-induced inflammation model in
C57BL/6J mice. Int Immunopharmacol. 68:95–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li MX, Li Q, Sun XJ, Luo C, Li Y, Wang YN,
Chen J, Gong CZ, Li YJ, Shi LP, et al: Increased Homer1-mGluR5
mediates chronic stress-induced depressive-like behaviors and
glutamatergic dysregulation via activation of PERK-eIF2α. Prog
Neuropsychopharmacol Biol Psychiatry. 95:1096822019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sharma V, Ounallah-Saad H, Chakraborty D,
Hleihil M, Sood R, Barrera I, Edry E, Kolatt Chandran S, Ben Tabou
de Leon S, Kaphzan H and Rosenblum K: Local inhibition of PERK
enhances memory and reverses age-related deterioration of cognitive
and neuronal properties. J Neurosci. 38:648–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Timberlake MA II and Dwivedi Y: Altered
expression of endoplasmic reticulum stress associated genes in
hippocampus of learned helpless rats: Relevance to depression
pathophysiology. Front Pharmacol. 6:3192016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luedtke K, Bouchard SM, Woller SA, Funk
MK, Aceves M and Hook MA: Assessment of depression in a rodent
model of spinal cord injury. J Neurotrauma. 31:1107–1121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maldonado-Bouchard S, Peters K, Woller SA,
Madahian B, Faghihi U, Patel S, Bake S and Hook MA: Inflammation is
increased with anxiety- and depression-like signs in a rat model of
spinal cord injury. Brain Behav Immun. 51:176–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu J, Zhao Z, Sabirzhanov B, Stoica BA,
Kumar A, Luo T, Skovira J and Faden AI: Spinal cord injury causes
brain inflammation associated with cognitive and affective changes:
Role of cell cycle pathways. J Neurosci. 34:10989–11006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu J, Zhao Z, Kumar A, Lipinski MM, Loane
DJ, Stoica BA and Faden AI: Endoplasmic reticulum stress and
disrupted neurogenesis in the brain are associated with cognitive
impairment and depressive-like behavior after spinal cord injury. J
Neurotrauma. 33:1919–1935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheng Y, Cawley NX, Yanik T, Murthy SR,
Liu C, Kasikci F, Abebe D and Loh YP: A human carboxypeptidase
E/NF-α1 gene mutation in an Alzheimer's disease patient leads to
dementia and depression in mice. Transl Psychiatry. 6:e9732016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Michel TM, Camara S, Tatschner T, Frangou
S, Sheldrick AJ, Riederer P and Grünblatt E: Increased xanthine
oxidase in the thalamus and putamen in depression. World J Biol
Psychiatry. 11:314–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Harrison R: Structure and function of
xanthine oxidoreductase: Where are we now? Free Radic Biol Med.
33:774–797. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ishisaka M, Kakefuda K, Yamauchi M,
Tsuruma K, Shimazawa M, Tsuruta A and Hara H: Luteolin shows an
antidepressant-like effect via suppressing endoplasmic reticulum
stress. Biol Pharm Bull. 34:1481–1486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu Y, Zhao N, Li C, Chang Q, Liu X, Liao
Y and Pan R: Longistyline C acts antidepressant in vivo and
neuroprotection in vitro against glutamate-induced cytotoxicity by
regulating NMDAR/NR2B-ERK pathway in PC12 cells. PLoS One.
12:e01837022017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang Y, Li Z, Liu Y, Liu X, Chang Q, Liao
Y and Pan R: Neuroprotective effect of water extract of Panax
ginseng on corticosterone-induced apoptosis in PC12 cells and its
underlying molecule mechanisms. J Ethnopharmacol. 159:102–112.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li ZY, Guo Z, Liu YM, Liu XM, Chang Q,
Liao YH and Pan RL: Neuroprotective effects of total saikosaponins
of Bupleurum yinchowense on corticosterone-induced apoptosis in
PC12 cells. J Ethnopharmacol. 148:794–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ye T, Meng X, Wang R, Zhang C, He S, Sun G
and Sun X: Gastrodin alleviates cognitive dysfunction and
depressive-like behaviors by inhibiting ER stress and NLRP3
inflammasome activation in db/db Mice. Int J Mol Sci. 19:2018.
View Article : Google Scholar
|
|
61
|
Shikov AN, Pozharitskaya ON and Makarov
VG: Aralia elata var. mandshurica (Rupr. & Maxim.)
J.Wen: An overview of pharmacological studies. Phytomedicine.
23:1409–1421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Narita N, Hashimoto K, Tomitaka S and
Minabe Y: Interactions of selective serotonin reuptake inhibitors
with subtypes of sigma receptors in rat brain. Eur J Pharmacol.
307:117–119. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hosoi T, Miyahara T, Kayano T, Yokoyama S
and Ozawa K: Fluvoxamine attenuated endoplasmic reticulum
stress-induced leptin resistance. Front Endocrinol (Lausanne).
3:122012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hayashi T and Su TP: Sigma-1 receptor
chaperones at the ER-mitochondrion interface regulate Ca(2+)
signaling and cell survival. Cell. 131:596–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Omi T, Tanimukai H, Kanayama D, Sakagami
Y, Tagami S, Okochi M, Morihara T, Sato M, Yanagida K, Kitasyoji A,
et al: Fluvoxamine alleviates ER stress via induction of Sigma-1
receptor. Cell Death Dis. 5:e13322014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Terada K, Izumo N, Suzuki B, Karube Y,
Morikawa T, Ishibashi Y, Kameyama T, Chiba K, Sasaki N, Iwata K, et
al: Fluvoxamine moderates reduced voluntary activity following
chronic dexamethasone infusion in mice via recovery of BDNF signal
cascades. Neurochem Int. 69:9–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Perez-Caballero L, Torres-Sanchez S, Bravo
L, Mico JA and Berrocoso E: Fluoxetine: A case history of its
discovery and preclinical development. Expert Opin Drug Discov.
9:567–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ma J, Yang YR, Chen W, Chen MH, Wang H,
Wang XD, Sun LL, Wang FZ and Wang DC: Fluoxetine synergizes with
temozolomide to induce the CHOP-dependent endoplasmic reticulum
stress-related apoptosis pathway in glioma cells. Oncol Rep.
36:676–684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ma J, Hou LN, Rong ZX, Liang P, Fang C, Li
HF, Qi H and Chen HZ: Antidepressant desipramine leads to C6 glioma
cell autophagy: Implication for the adjuvant therapy of cancer.
Anticancer Agents Med Chem. 13:254–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Abelaira HM, Réus GZ, Ignácio ZM, Dos
Santos MA, de Moura AB, Matos D, Demo JP, da Silva JB, Michels M,
Abatti M, et al: Effects of ketamine administration on mTOR and
reticulum stress signaling pathways in the brain after the infusion
of rapamycin into prefrontal cortex. J Psychiatr Res. 87:81–87.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen S, Xuan J, Couch L, Iyer A, Wu Y, Li
QZ and Guo L: Sertraline induces endoplasmic reticulum stress in
hepatic cells. Toxicology. 322:78–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ren Z, Chen S, Zhang J, Doshi U, Li AP and
Guo L: Endoplasmic reticulum stress induction and ERK1/2 activation
contribute to nefazodone-induced toxicity in hepatic cells. Toxicol
Sci. 154:368–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang L, Zheng L, Wan Y, Chen Z, Li P and
Wang Y: Metoprolol, N-acetylcysteine, and escitalopram prevents
chronic unpredictable mild stress-induced depression by inhibition
of endoplasmic reticulum stress. Front Psychiatry. 9:6962018.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li B, Yadav RK, Jeong GS, Kim HR and Chae
HJ: The characteristics of Bax inhibitor-1 and its related
diseases. Curr Mol Med. 14:603–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sui ZY, Chae HJ, Huang GB, Zhao T,
Shrestha Muna S and Chung YC: Effects of chronic mild stress in
female bax inhibitor-1-gene knockout mice. Clin Psychopharmacol
Neurosci. 10:155–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hunsberger JG, Machado-Vieira R, Austin
DR, Zarate C, Chuang DM, Chen G, Reed JC and Manji HK: Bax
inhibitor 1, a modulator of calcium homeostasis, confers affective
resilience. Brain Res. 1403:19–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Crider A, Nelson T, Davis T, Fagan K,
Vaibhav K, Luo M, Kamalasanan S, Terry AV Jr and Pillai A: Estrogen
receptor β agonist attenuates endoplasmic reticulum stress-induced
changes in social behavior and brain connectivity in mice. Mol
Neurobiol. 55:7606–7618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Swardfager W, Hennebelle M, Yu D, Hammock
BD, Levitt AJ, Hashimoto K and Taha AY:
Metabolic/inflammatory/vascular comorbidity in psychiatric
disorders; soluble epoxide hydrolase (sEH) as a possible new
target. Neurosci Biobehav Rev. 87:56–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
















