Introduction
In vivo, ischemia/reperfusion (I/R) injury
refers to organ damage produced by the restoration of blood flow
after ischemia. Myocardial I/R injury is a common phenomenon that
occurs after ischemic heart diseases such as myocardial infarction
(MI), or is the result of a reduction in blood flow and oxygen
supply insufficiency (1). MI is a
cause of morbidity in cardiovascular diseases (2,3).
Limiting or reducing the damage following I/R has received
extensive research attention. In vitro, an
hypoxia/reoxygenation (H/R) injury model simulates in vivo
I/R injury (2).
Overexpression of microRNA-101 (miR-101) has been
found in cell inflammation injury, and downregulated expression of
miR-101 can attenuate cell injury (4,5).
miR-101a is upregulated during cell differentiation, and
overexpressed miR-101 can inhibit cell proliferation and migration,
and promote cell apoptosis (6–8).
Thus, it was hypothesized that inhibiting miR-101a-3p could
attenuate H/R-induced damage in H9C2 cells.
The differentiation, proliferation and migration of
cells is critical in the early stages of cell healing, and
apoptosis affects the elimination of inflammatory factors during
cell healing (9). A number of
studies have investigated cell apoptosis via the Bax/Bcl-2
signaling pathway (10–12). In addition, elevated interleukin-6
(IL-6) levels may cause tissue damage and inflammation (13). Studies have demonstrated that tumor
necrosis factor-α (TNF-α) is connected with inflammation and injury
(14–17). STAT3, a member of the STAT family
of transcription factor, regulates cell proliferation, cellular
transformation, metastasis and immune responses, whereas Janus
kinase (JAK)2 participates in the immune system and other signaling
transductions (18,19). Previous studies have demonstrated
that inactivation of the JAK/STAT3 signaling pathway relieved cell
injury, suggesting that activation of the JAK/STAT3 signaling
pathway was correlated with cell injury (20,21).
In the present study, H9C2 cells (rat myocardial
cells) were subjected to H/R treatment and established as an I/R
injury model in vitro. The aim of the study was to
investigate the effect and mechanism of the inhibition of
miR-101a-3p on H/R injury. Cell apoptosis in cardiomyocytes, and
critical regulation of target factors by inhibition of miR-101a-3p
and H/R injury were also studied.
Materials and methods
Cell culture and H/R models
The rat embryonic cardiomyoblast cell line H9C2 was
purchased from the American Type Culture Collection. H9C2 cells
were cultured in DMEM (Thermo Fisher Scientific, Inc.) at 37°C in
an incubator (Thermo Fisher Scientific, Inc.) with 5%
CO2. DMEM contained high-glucose basic DMEM (Invitrogen;
Thermo Fisher Scientific, Inc.), 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1% 10,000 U/ml
penicilin-10,000 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Subculture was conducted when the cells reached
~90% confluence in the culture flask.
To induce H/R models, the cells were cultured in
non-glucose basic DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
containing 1% 10,000 U/ml penicilin-10,000 µg/ml streptomycin at
37°C for 24 h under hypoxic conditions (1% O2/95%
N2). Then the cells were placed in a normal chamber (at
37°C in 5% CO2/95% air) for 6 h and divided into 6
groups: Control group (untreated); H/R group [cells were exposed to
hypoxia/reoxygenation (24/6 h) environment]; miR-101a-3p inhibitor
(I) + H/R (cells were transfected with I for 48 h followed by
exposure to H/R); miR-101a-3p inhibitor control (IC) + H/R (cells
were transfected with IC for 48 h followed by exposure to H/R);
Tyrphostin AG490 (AG490; Selleck Chemicals) + H/R (cells were
pretreated with AG490 for 30 min followed by exposure to H/R);
AG490 + I + H/R (cells were pretreated with AG490 for 30 min,
transfected with I for 48 h followed by exposure to H/R).
miR-101a-3p mimic (M), miR-101a-3p mimic control (MC), I and IC
were purchased from Shanghai GenePharma Co., Ltd.
Cell transfection
H9C2 cells were plated in 35-mm culture dish at
1.5×105 cells/dish for 12 h. The cells were transfected
with I, IC, M, MC (50 nM; Shanghai GenePharma Co., Ltd.) using
riboFECT™ CP Reagent and buffer (Guangzhou RiboBio Co., Ltd.).
RiboFECT™ CP-I/IC mixture and DMEM were added to the cells at 37°C
and incubated for 48 h. Reverse transcription-quantitative PCR was
applied to detect the transfection efficiency of cells. The
sequences of I, IC, M, MC were as follows: M (sense,
5′-UACAGUACUGUGAUAACUGAA-3′ and antisense,
5′-CAGUUAUCACAGUACUGUAUU-3′), MC (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′), I (5′-UUCAGUUAUCACAGUACUGUA-3′) and
IC (5′-AUCGUUCGAUCACGUATT-3′).
Dual luciferase assay
Target genes of miR-101a-3p were predicted using
TargetScan7.2 (http://www.targetscan.org/vert_72/). For the dual
luciferase reporter experiments, the mutation type 3′-untranslated
region (3′-UTR) of JAK2 gene was created by the Quick-Change
Site-Directed Mutagenesis kit (Stratagene; Agilent Technologies,
Inc.). The JAK2 wild-type 3′-UTR or mutate type (MUT) JAK2 3′-UTR
were cloned into psi-CHECK-2 (Promega Corporation) and used to
transfect the cells, M and I were co-transfection with JAK2 3′-UTR
or JAK2 3′-UTR mutant plasmids (400 ng) into H9C2 cells using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
for 48 h. Luciferase activity was measured with the Dual Luciferase
Reporter Assay system (Promega Corporation) according to the
manufacturer's protocols. Renilla luciferase activity served
as an internal control.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was detected by CCK-8
(MedChemExpress, LLC). The cells were plated in 96-well plates at
1.5×103 cells/well for 24 h. Following transfection and
H/R treatment, CCK-8 solution was added into 96-well plates and
diluted in phosphate buffered saline (Gibco; Thermo Fisher
Scientific, Inc.) at 9:1, and then incubated in a 37°C, 5%
CO2 atmosphere for 1 h. Then, the OD value at a
wavelength of 450 nm was measured by Multiskan™ FC (Thermo Fisher
Scientific, Inc.).
Colorimetric assays
The supernatants (centrifugation at 10,000 × g for
30 min at 4°C) from the control group, H/R group, IC + H/R group
and I + H/R group were collected into centrifuge tubes. Creatine
kinase (CK) was detected using a CK test kit (cat. no. BC1140;
Beijing Solarbio Science & Technology Co., Ltd.) and lactate
dehydrogenase (LDH) was analyzed using an LDH test kit (cat. no.
TE0159; Beijing Leagene Biotech Co. Ltd.), according to the
manufacturers' protocols.
Reverse transcription-quantitative
(RT-q)PCR
RNA was extracted from H9C2 cells (2×104
cells/well in 6-well plates) using TRIzol regent (Invitrogen;
Thermo Fisher Scientific, Inc.). An iScript™ cDNA Synthesis kit
(Bio-Rad Laboratories, Inc.) was used to synthesize cDNA. The
reverse transcription reaction was performed at 42°C for 15 min,
followed by reverse transcriptase inactivation at 85°C for 15 sec.
RT-qPCR analysis was performed on an ABI Step One Plus sequence
detection system (Thermo Fisher Scientific, Inc.) using the
conditions and primer concentrations suggested by the SYBR-Green
PCR master mix (Thermo Fisher Scientific, Inc.) protocol. Sequence
primers used in for qPCR were as follows: miR-101a-3p (forward,
5′-TACAGTACTGTGATAACTGA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′);
Bcl-2 mRNA (forward, 5′-GGTGCCACCTGTGGTCCACCTG-3′ and reverse,
5′-CTTCACTTGTGGCCCAGATAGG-3′); Bax mRNA (forward,
5′-AAATACCCGGAGCTGATGTTTG-3′ and reverse,
5′-TCCTCTGGCTGAGTTTGCG-3′); IL-6 mRNA (forward,
5′-CCTGAAC-CTTCCAAAGATGG-3′ and reverse,
5′-CATTTGCC-GAAGAGCCCTCA-3) and TNF-α mRNA (forward,
5′-TGAVAAGCCTGTAGCCCACG-3′ and reverse,
5′-TTGTCTTTGAGATCCATGCC-3′). qPCR reactions were performed under
the following conditions: 50°C for 35 min, 85°C for 12 min,
followed by 60 cycles of 95°C for 23 sec and 60°C for 1.5 min.
GAPDH (forward, 5′-TCCCTCAAGATTGTCAGCAA-3′ and reverse,
5′-CCAGAGGCATACAGGGACAAC-3′) and U6 (forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′)
were used as internal controls. The 2−ΔΔCq method
(22) was used to calculate
relative expression levels.
Flow cytometry
Cell apoptosis was determined via flow cytometry.
The cells were suspended in PBS after having been treated for 48 h.
Then, 1 µl Annexin V/FITC 5X, 150 µl annexin-binding buffer and 2.5
µl propidium iodide (PI) in a Dead Cell Apoptosis kit (Thermo
Fisher Scientific, Inc.) were added to 300 µl cell suspension for
conducting flow cytometry at room temperature for 15–20 min in the
dark. The fluorescence was detected by a flow cytometer (BD
Biosciences) and the cell apoptosis was calculated using BD
FACSuite software (version 1.0; BD Biosciences). Apoptosis was
calculated as the sum of early apoptosis and late apoptosis.
Western blot analysis
Following treatment for 48 h, the cells were washed
with PBS 3 times. RIPA buffer (Thermo Fisher Scientific, Inc.) was
added to the dish, and the cell were then scraped for 5–6 min using
a cell scraper on ice. The liquid was mixed by syringe 3 times and
transferred onto the ice and held for 15 min. Next, the liquid was
cleared by centrifugation (Cence Medikal) at 12,000 × g for 15 min
at 4°C. A bicinchoninic acid protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.) was used to determine protein
concentration. An equal quantity of total protein (30 µg) was
separated via 10% SDS-PAGE and then transferred onto polyvinylidene
fluoride membranes (PVDF; Thermo Fisher Scientific, Inc.). After
blocking of non-specific binding sites with 5% non-fat milk at room
temperature for 2 h, the membranes were incubated overnight at 4°C
with antibodies specific for Bcl-2 (cat. no. ab196495; 1:1,000;
Abcam), Bax (cat. no. 2772; 1:1,000; Cell Signaling Technology,
Inc.), IL-6 (cat. no. 12153; 1:1,000; Cell Signaling Technology,
Inc.), phosphorylated (p)-STAT3 (cat. no. 9145; 1:2,000; Cell
Signaling Technology, Inc.), STAT3 (cat. no. 12640; 1:1,000; Cell
Signaling Technology, Inc.), JAK2 (cat. no. 3230; 1:1,000; Cell
Signaling Technology, Inc.), p-JAK2 (cat. no. 3776; 1:1,000; Cell
Signaling Technology, Inc.), TNF-α (cat. no. ab6671; Abcam) and
GAPDH (cat. no. ab9485; 1:1000; Abcam). Next, the membranes were
washed 2–3 times with TBS + 0.05% Tween-20 (TBST) at room
temperature and incubated with the horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
ab6721; 1:2,000; Abcam) for 2–3 h. After the membranes had been
washed with TBST, the proteins were detected using ECL reagent
(Beijing Solarbio Science & Technology Co., Ltd.), and the
protein blots were analyzed using IPP 6.0 software (Media
Cybernetics, Inc.).
Statistical analysis
Data were shown as the mean ± standard deviation.
All statistical analyses were performed using SPSS software
(version 15.0; SPSS, Inc.). Comparisons of cell viability,
apoptosis, CK/LDH and western blot were analyzed using one way
ANOVA and Tukey-Kramer multiple comparison tests. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed ≥3 times.
Results
Effects of miR-101a-3p on the
H/R-induced H9C2 cell injury
M significantly increased miR-101a-3p expression,
and I inhibited miR-101a-3p expression in H9C2 cells (Fig. 1A). Although the expression of
miR-101a-3p was higher in H9C2 cells that had been subjected to H/R
injury, its expression was inhibited by I (Fig. 1B).
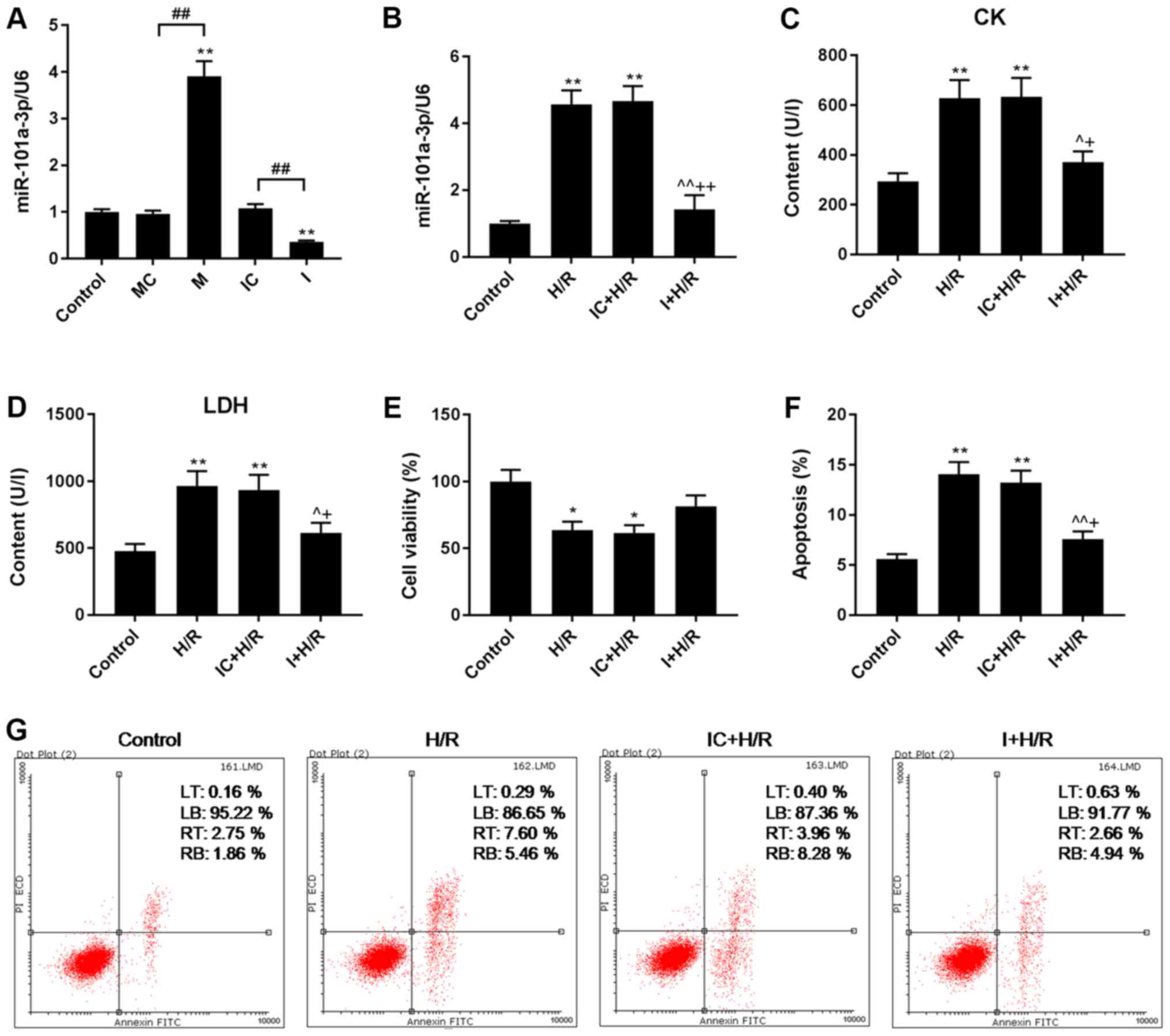 | Figure 1.Effects of I on apoptosis, viability,
and CK, LDH and miR-101a-3p levels in H9C2 cells during H/R injury.
I was transfected into H9C2 cells for 48 h in the H/R model. (A and
B) miR-101a-3p expression levels in H9C2 cells were determined via
reverse transcription-quantitative PCR analysis. (C) CK and (D) LDH
contents in H9C2 cells were analyzed by colorimetric assays. (E)
H9C2 cell viability was detected using a Cell Counting Kit-8 assay.
(F and G) H9C2 cell apoptosis was determined via flow cytometry
using a Dead Cell Apoptosis kit. Data are presented as the mean ±
standard deviation and were analyzed by ANOVA with Tukey-Kramer
multiple comparison test. *P<0.05, **P<0.01 vs. Control;
^P<0.05, ^^P<0.01 vs. H/R;
+P<0.05, ++P<0.01 vs. IC + H/R;
##P<0.01. miR, microRNA; CK, creatine kinase; LDH,
lactate dehydrogenase; H/R, hypoxia/reoxygenation; I, miR-101a-3p
inhibitor; IC, miR-101a-3p inhibitor control; M, miR-101a-3p mimic;
MC, miR-101a-3p mimic control; LT, upper left quadrant; LB, lower
left quadrant; RT, upper right quadrant; RB, lower right quadrant;
PI, propidium iodide. |
Effects of I on viability, and the
contents of CK and LDH in H/R model H9C2 cells
CK has been reported to be linked to diastolic
dysfunction in rats (23), and
cardiomyocyte damage can be assessed by detecting the content of
LDH (24). The expression levels
of CK and LDH increased when the cells were subjected to H/R
injury, and such expression was decreased by I transfection during
H/R (Fig. 1C and D). The data also
demonstrated that H/R decreased H9C2 cell viability, whereas I
improved cell viability following H/R (Fig. 1E).
Effects of I on H/R-induced H9C2 cell
apoptosis
The effects of I and AG490 on H9C2 cell apoptosis
were determined via flow cytometry using a cell apoptosis kit with
Annexin V-FITC and PI. It was determined that the apoptosis rate of
H/R group was significantly higher than that of control group,
while the apoptosis rate of I + H/R group was significantly lower
than that of H/R group, suggesting that H/R induced H9C2 cell
apoptosis, and that I can decrease H9C2 cell apoptosis during H/R
injury (P<0.01; Fig. 1F and
G).
Effects of I on the expression levels
of Bcl-2, Bax, IL-6 and TNF-α in H/R-treated H9C2 cells
The expression levels of Bcl-2, Bax, IL-6 and TNF-α
were detected via RT-qPCR and western blot analyses. Compared with
the H/R group, the I + H/R group had a lower level of Bax but a
higher level of Bcl-2 expression (Fig.
2A, B, E and F), suggesting that I inhibited the Bax/Bcl-2
signaling pathway during cell H/R injury. The I + H/R group
exhibited significantly lower expression levels of IL-6 and TNF-α
in comparison with the H/R group (Fig.
2C-F). Of note, the expression levels of Bcl-2, Bax, IL-6 and
TNF-α in the H/R group were similar to those in the IC + H/R group,
and the significant differences between groups in the RT-qPCR
assays were similar to those identified via western blot
analysis.
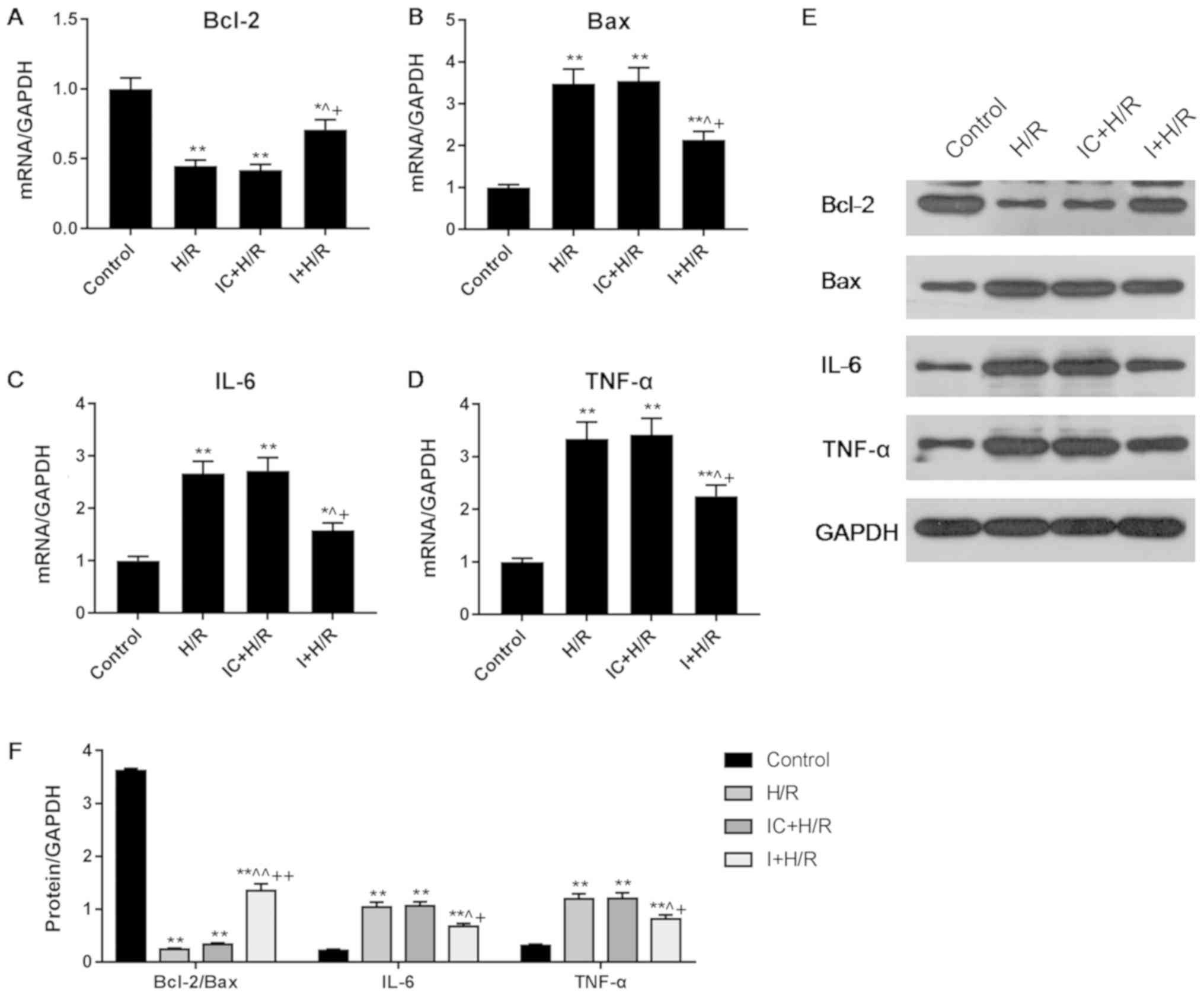 | Figure 2.Effects of I on the expression levels
of Bax, Bcl-2, IL-6 and TNF-α in H9C2 cells during H/R injury. H9C2
cells were treated with I for 48 h in an H/R environment. mRNA
levels of (A) Bcl-2, (B) Bax, (C) IL-6 and (D) TNF-α were
determined via reverse transcription-quantitative PCR analysis. (E
and F) Protein levels of Bax, Bcl-2, IL-6 and TNF-α were detected
via western blot analysis. Data are presented as the mean ±
standard deviation, and were analyzed by ANOVA and Tukey-Kramer
multiple comparison test. *P<0.05, **P<0.01 vs. Control;
^P<0.05, ^^P<0.01 vs. H/R;
+P<0.05, ++P<0.01 vs. IC + H/R. miR,
microRNA; IL, interleukin; TNF, tumor necrosis factor; H/R,
hypoxia/reoxygenation; I, miR-101a-3p inhibitor; IC, miR-101a-3p
inhibitor control. |
Effects of I, M and AG490 on the
expression levels of JAK2, p-JAK2, STAT3, and p-STAT3 in
H/R-treated H9C2 cells
AG490 can inhibit the activation of STAT3 and JAK2
(25). A dual luciferase assay
identified that I promoted luciferase activity, which was inhibited
by M, supporting the hypothesis that I promoted JAK2 expression and
M was able to inhibit expression (Fig.
3A,B). The H/R, IC + H/R and MC + H/R groups exhibited lower
levels of JAK2 compared with control group (Fig. 3C-E). M inhibited the expression of
JAK2, which was found to be upregulated by I in the H/R model.
AG490 + M + H/R group was found to have the lowest expression
levels of JAK2. In addition, M inhibited the phosphorylation of
JAK2, which was significantly promoted by I in comparison (Fig. 3C-E). It was also found that I
increased the level of STAT3 in the H/R group, while M decreased
the level of STAT3 in the H/R group, and that the AG490 + M + H/R
group had the lowest levels of STAT3 (Fig. 3F and G).
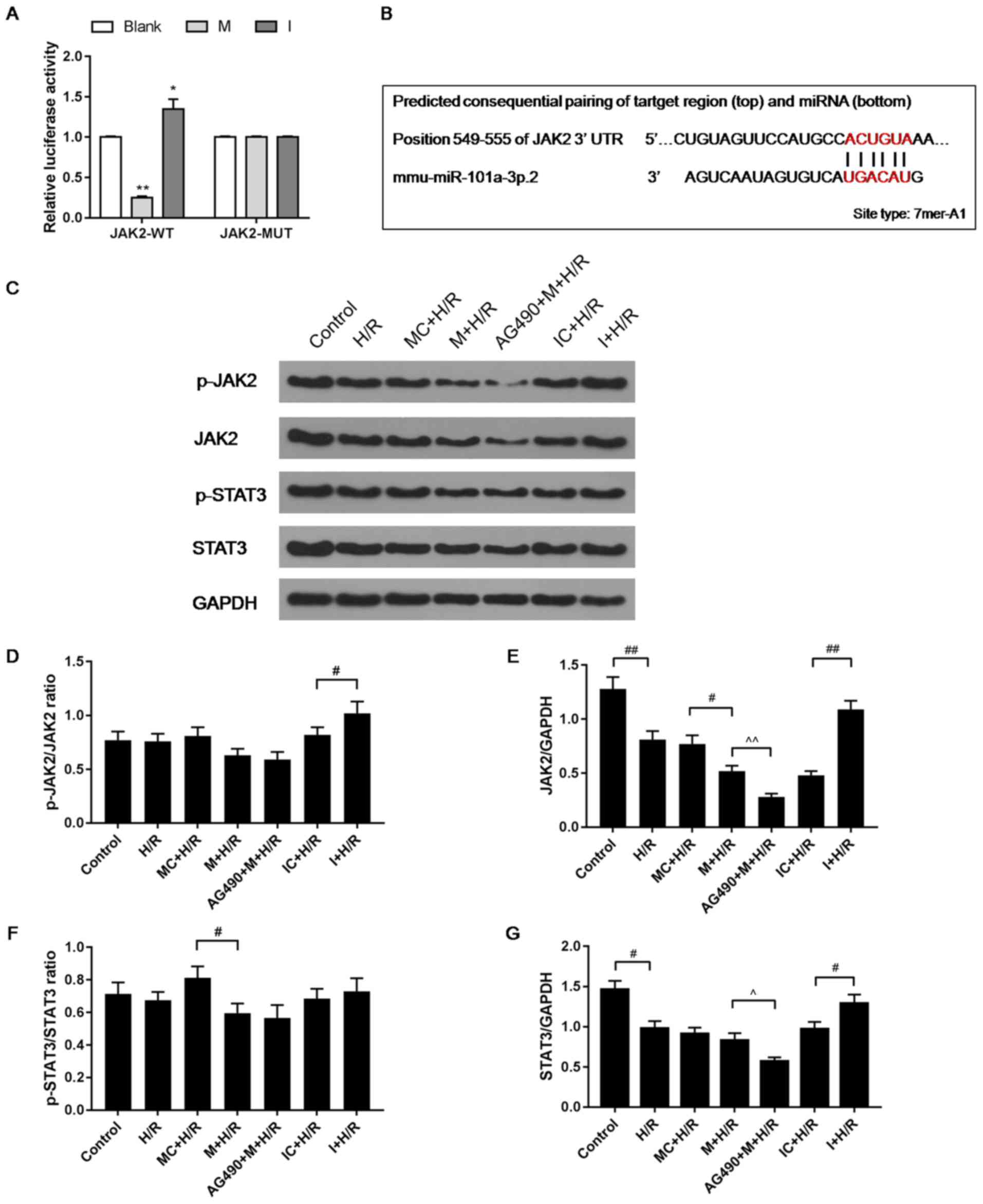 | Figure 3.Effects of I and AG490 on the
expression levels of JAK2, p-JAK2, STAT3, and p-STAT3 in H9C2 cells
during H/R injury. (A) psi-CHECK-2 containing the JAK2 3′UTR, and M
or I were used to transfect cells, and a dual luciferase assay kit
was used to detect luciferase activity. (B) TargetScan7.2 was used
to predict target genes of miR-101a-3p. (C) Protein levels of JAK2,
p-JAK2, STAT3 and p-STAT3 were detected via western blot analysis.
Ratios of (D) p-JAK2/JAK2 and (E) total JAK2 were used to assess
the activation of JAK2. (F) p-STAT3/STAT3 and (G) total STAT3 were
used to assess the activation of STAT3. Data are presented as the
mean ± standard deviation and were analyzed by ANOVA with
Tukey-Kramer multiple comparison. *P<0.05, **P<0.01 vs.
Blank; #P<0.05, ##P<0.01;
^P<0.05, ^^P<0.01. miR, microRNA; JAK,
Janus kinase; H/R, hypoxia/reoxygenation; p-, phosphorylated; UTR,
untranslated region; WT, wild-type; MUT, mutant; I, miR-101a-3p
inhibitor; IC, miR-101a-3p inhibitor control; M, miR-101a-3p mimic;
MC, miR-101a-3p mimic control. |
Effects of AG490 on H/R-induced H9C2
cell apoptosis
H/R treatment significantly induced apoptosis of
H9C2 cells, while I inhibited the promoting effect of H/R on
apoptosis of H9C2 cells (Fig. 4);
AG490, a JAK2-specific inhibitor (26), was found to induce H9C2 cell
apoptosis during H/R injury (Fig.
4); the results also showed that the apoptosis rate of AG490 +
I + H/R group were higher than that of I + H/R group (Fig. 4), indicating that AG490 reversed
the inhibitory effect of I on H/R-induced cell apoptosis.
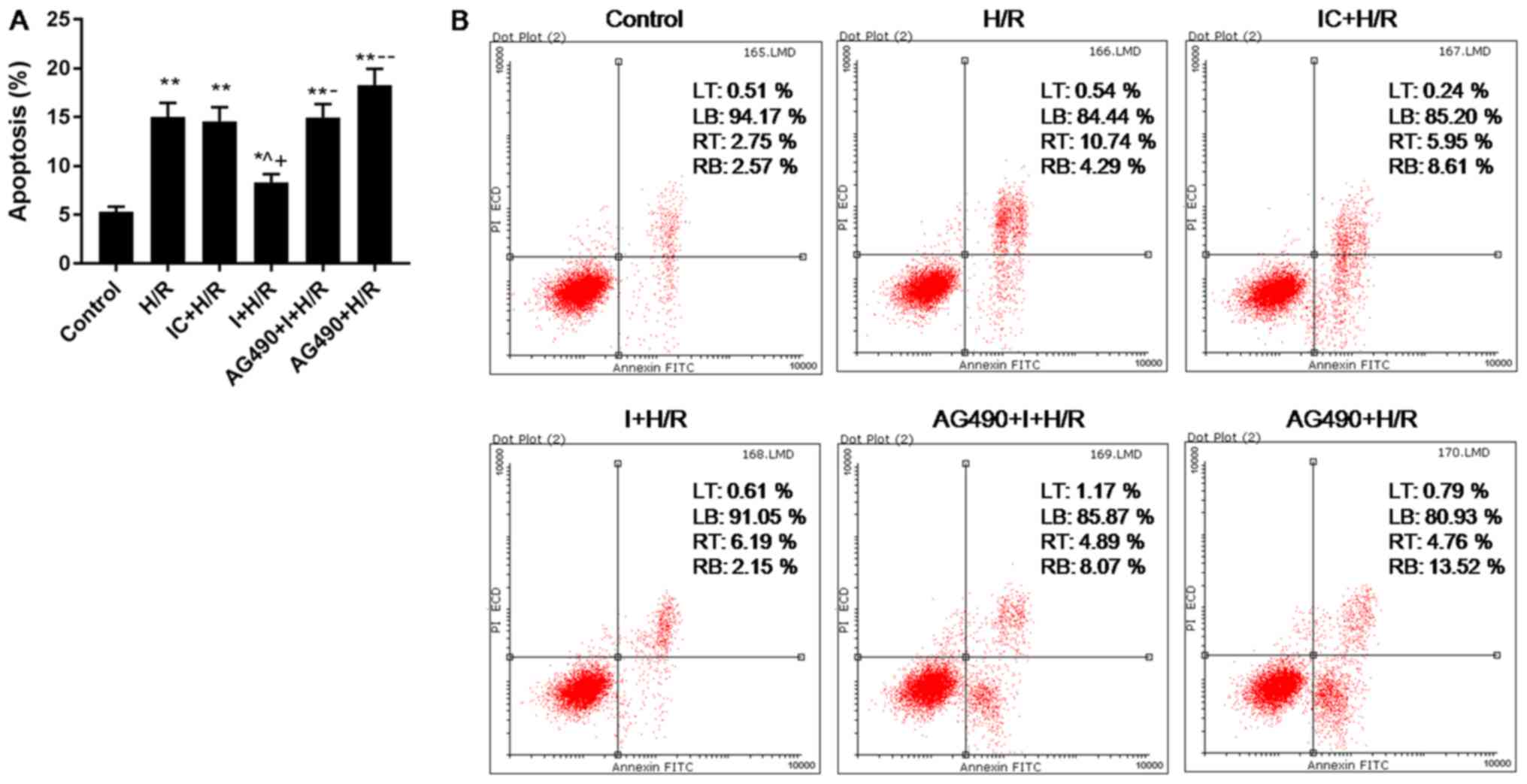 | Figure 4.Effects of I and AG490 on H9C2 cell
apoptosis during H/R injury. (A and B) H9C2 cell apoptosis was
analyzed via flow cytometry. Data are presented as the mean ±
standard deviation, and were analyzed by ANOVA and Tukey-Kramer
multiple comparison test. *P<0.05, **P<0.01 vs. Control;
^P<0.05 vs. H/R; +P<0.05 vs. IC + H/R;
−P<0.05, −−P<0.01 vs. I + H/R. miR,
microRNA; H/R, hypoxia/reoxygenation; I, miR-101a-3p inhibitor; IC,
miR-101a-3p inhibitor control; LT, upper left quadrant; LB, lower
left quadrant; RT, upper right quadrant; RB, lower right quadrant;
PI, propidium iodide. |
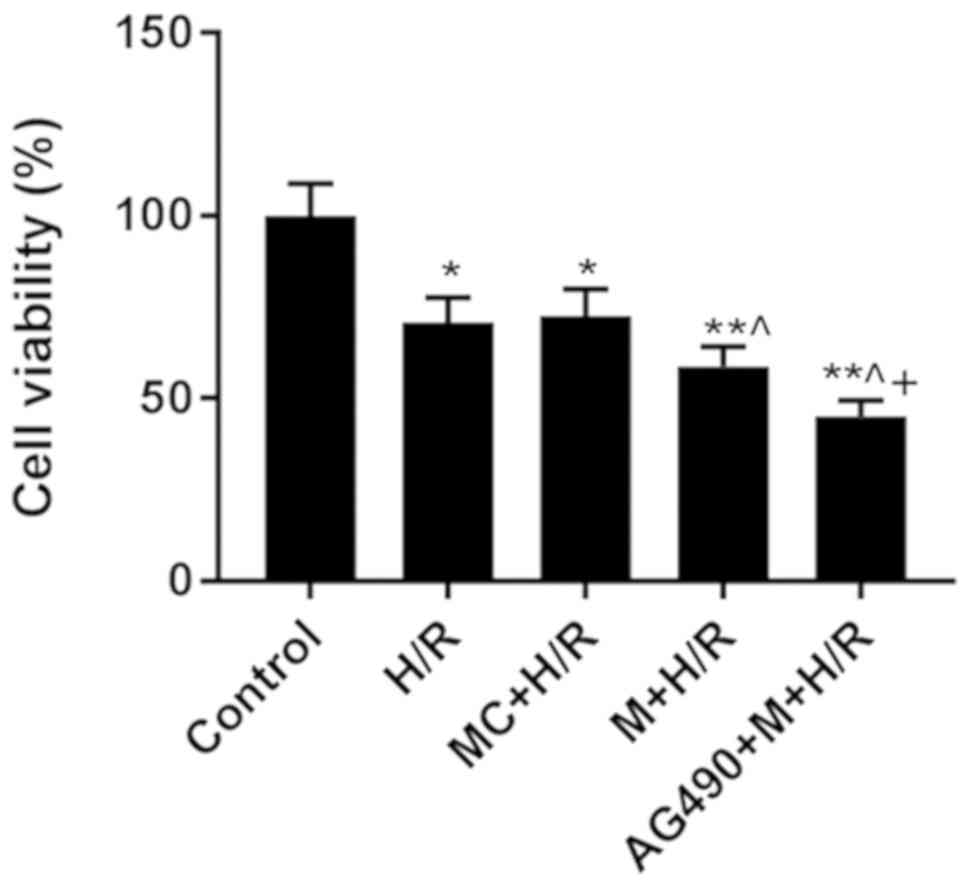
Effects of M on H9C2 cell
viability
The M + H/R group exhibited significantly reduced
cell viability compared with the MC + H/R group (Fig. 5), suggesting that miR-101a-3p
upregulation aggravated H/R-induced damage in H9C2 cells. In
addition, the AG490 + M + H/R group exhibited the lowest cell
viability (Fig. 5), indicating
that AG490 enhanced the inhibitory effect of M on H/R-induced cell
viability.
Discussion
The results of the present study demonstrated that I
can improve H9C2 cell viability and decrease cell apoptosis during
cell H/R injury. As damage of cardiomyocytes gradually intensifies,
LDH and CK will be increasingly released from cardiomyocytes
(27,28). In the H9C2 cells, H/R caused
cardiomyocyte damage, which was attenuated by I.
The Bcl-2 family contains Bcl-2 and Bax, and
downregulating the level of Bax and upregulating the level of Bcl-2
can reduce cell apoptosis (29).
The Bax/Bcl-2 ratio is regarded as a main factor in determining
cell apoptosis, and the majority of studies investigate apoptosis
via the Bax/Bcl signaling pathway (30–33).
The present study demonstrated that I attenuated H/R-induced
apoptosis by decreasing Bax and increasing Bcl-2. IL-6, a
pro-inflammatory factor, is rapidly produced in response to tissue
injury (34,35). A reduction of IL-6 has beneficial
functions in tissues and prevents destructive effects (36), as it can reduce inflammation and
prevent further injury (37).
Injury causes significant immune dysfunction, and TNF-α is a potent
proinflammatory cytokine (38,39).
It was revealed in the present study that I could reduce immune
dysfunction caused by H/R by decreasing expression of the
inflammatory cytokines IL-6 and TNF-α.
Previous studies have demonstrated that the
downregulation of p-JAK2 and p-STAT3 can increase cell apoptosis
and decrease cell viability (40–42).
It has also been reported that proapoptotic factors such as Bax are
upregulated in H/R injury-treated cells (3,43).
The present study revealed that M could not only inhibit p-JAK2 and
p-STAT3, but also inhibit cell viability during H/R, whereas I
promoted JAK2 and STAT3 during H/R and increased cell viability. In
order to investigate whether the JAK2/STAT3 signaling pathway
contributed to the effects of I on H/R damage, AG490 (which
inhibits JAK2 and STAT3 during H/R) was used to investigate cell
viability during H/R. Notably, AG490 inhibited cell viability
during H/R, and AG490 inhibited the p-JAK2/IAK2 and p-STAT3/STAT3
ratios in M-transfected and H/R-treated cells. However, I promoted
cell viability, and the p-JAK2/JAK and p-STAT3/STAT3 ratios during
H/R. Therefore, it was hypothesized that the levels of JAK2, p-JAK2
and p-STAT3 were important indicators to regulate the effects of
miR-101a-3p on H9C2 cells during H/R. However, the present study
did not investigate in depth whether the levels of these molecules
were of clear importance for H9C2 cells during H/R. In future
studies, the effects of the levels of JAK2, p-JAK2 and p-STAT3 on
H9C2 cells during H/R should be studied via quantitative analysis.
It has been demonstrated that the activation of JAK2 results in
phosphorylation of downstream STAT3 signaling pathways (44). However, the role of STAT3 in
I-treated cardiomyocytes during H/R, and the association between
JAK2 activation and STAT activation during H/R remains unclear and
should be determined in future studies.
In conclusion, the present study indicated that
downregulation of miR-101a-3p prevented not only H/R-induced damage
to cardiomyocytes by decreasing inflammatory responses, but also
reduced cell apoptosis during H/R injury via the Bax/Bcl-2
signaling pathway. The present study provided evidence that the
JAK2/STAT3 signaling pathway served an important role in decreasing
H/R injury in cardiomyocytes following inhibition of
miR-101a-3p.
Acknowledgements
Not applicable.
Funding
This work was supported by Inner Mongolia Health and
Family Planning Commission Class A Item (grant no. 201701072).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JL made substantial contributions to the conception
and design of the study. JW, FC and YN contributed to acquisition,
analysis and interpretation of data. JL drafted the article and
critically revised it for important intellectual content. All
authors approved the final version of the manuscript to be
published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Shi X, Li J, Zhang M and Yu B:
Knockdown of KLF11 attenuates hypoxia/reoxygenation injury via
JAK2/STAT3 signaling in H9c2. Apoptosis. 22:510–518. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Chen G, Zhong S, Zheng F, Gao F,
Chen Y, Huang Z, Cai W, Li W, Liu X, et al: N-n-butyl haloperidol
iodide ameliorates cardiomyocytes hypoxia/reoxygenation injury by
extracellular calcium-dependent and -independent mechanisms. Oxid
Med Cell Longev. 2013:9123102013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ouyang F, Huang H, Zhang M, Chen M, Huang
H, Huang F and Zhou S: HMGB1 induces apoptosis and EMT in
association with increased autophagy following H/R injury in
cardiomyocytes. Int J Mol Med. 37:679–689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S, Man Y and Zhao L: Sinomenine
inhibits lipopolysaccharide-induced inflammatory injury by
regulation of miR-101/MKP-1/JNK pathway in keratinocyte cells.
Biomed Pharmacother. 101:422–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Hua R, Gong Z, Shang B, Huang Y,
Guo L, Liu T and Xue J: Human amniotic epithelial cells inhibit
CD4+ T cell activation in acute kidney injury patients
by influencing the miR-101-c-Rel-IL-2 pathway. Mol Immunol.
81:76–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin C, Huang F, Li QZ and Zhang YJ:
miR-101 suppresses tumor proliferation and migration, and induces
apoptosis by targeting EZH2 in esophageal cancer cells. Int J Clin
Exp Pathol. 7:6543–6550. 2014.PubMed/NCBI
|
|
7
|
Li D, Zhan S, Wang Y, Wang L, Zhong T, Li
L, Fan J, Xiong C, Wang Y and Zhang H: Role of microRNA-101a in the
regulation of goat skeletal muscle satellite cell proliferation and
differentiation. Gene. 572:198–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao K, Li J, Zhao Y, Wang Q, Zeng Q, He S,
Yu L, Zhou J and Cao P: miR-101 inhibiting cell proliferation,
migration and invasion in hepatocellular carcinoma through
downregulating Girdin. Mol Cells. 39:96–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon JY, Park BS, Kim YH, Kim YD, Kim CH,
Yoon JY and Yoon JU: Remifentanil protects human keratinocytes
against hypoxia-reoxygenation injury through activation of
autophagy. PLoS One. 10:e01169822015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu LS, Bai XQ, Gao Y, Wu Q, Ren Z, Li Q,
Pan LH, He NY, Peng J and Tang ZH: PCSK9 promotes oxLDL-induced
PC12 cell apoptosis through the Bcl-2/Bax-Caspase 9/3 signaling
pathway. J Alzheimers Dis. 57:723–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yating Q, Yuan Y, Wei Z, Qing G, Xingwei
W, Qiu Q and Lili Y: Oxidized LDL induces apoptosis of human
retinal pigment epithelium through activation of ERK-Bax/Bcl-2
signaling pathways. Curr Eye Res. 40:415–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanda T and Takahashi T: Interleukin-6 and
cardiovascular diseases. Jpn Heart J. 45:183–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adefolaju GA, Theron KE and Hosie MJ:
Effects of HIV protease, nucleoside/non-nucleoside reverse
transcriptase inhibitors on Bax, Bcl-2 and apoptosis in two
cervical cell lines. Biomed Pharmacother. 68:241–251. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang S, Sun K, Wang Y, Dong S, Wang C,
Liu L and Wu Y: Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS
death receptor pathway in apoptosis induced by zinc oxide
nanoparticles in human aortic endothelial cells and the protective
effect by alpha-lipoic acid. Chem Biol Interact. 258:40–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zahs A, Bird MD, Ramirez L, Choudhry MA
and Kovacs EJ: Anti-IL-6 antibody treatment but not IL-6 knockout
improves intestinal barrier function and reduces inflammation after
binge ethanol exposure and burn injury. Shock. 39:373–379. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esposito E and Cuzzocrea S: TNF-alpha as a
therapeutic target in inflammatory diseases, ischemia-reperfusion
injury and trauma. Curr Med Chem. 16:3152–3167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji K, Zhang M, Chu Q, Gan Y, Ren H, Zhang
L, Wang L, Li X and Wang W: The role of p-STAT3 as a prognostic and
clinicopathological marker in colorectal cancer: A systematic
review and meta-analysis. PLoS One. 11:e01601252016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu R, Xu N, Yi W, Huang K and Su M:
Electroacupuncture effect on neurological behavior and tyrosine
kinase-JAK 2 in rats with focal cerebral ischemia. J Tradit Chin
Med. 32:465–470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv X, Zhang Y, Cui Y, Ren Y, Li R and Rong
Q: Inhibition of microRNA155 relieves sepsisinduced liver injury
through inactivating the JAK/STAT pathway. Mol Med Rep.
12:6013–6018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Z, Zhao X, Gao Y, Liu M, Hou M, Jin H
and Cui Y: Recombinant human brain natriuretic peptide ameliorates
trauma-induced acute lung injury via inhibiting JAK/STAT signaling
pathway in rats. J Trauma Acute Care Surg. 78:980–987. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fowler ED, Benoist D, Drinkhill MJ, Stones
R, Helmes M, Wust RC, Stienen GJ, Steele DS and White E: Decreased
creatine kinase is linked to diastolic dysfunction in rats with
right heart failure induced by pulmonary artery hypertension. J Mol
Cell Cardiol. 86:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Wang K, Jiang Y and Chen J:
Protective effect of heart-fatty acid binding protein on
lipopolysaccharide-induced cardiomyocyte damage. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 40:457–463. 2015.(In Chinese). PubMed/NCBI
|
|
25
|
Chai HT, Yip HK, Sun CK, Hsu SY and Leu S:
AG490 suppresses EPO-mediated activation of JAK2-STAT but enhances
blood flow recovery in rats with critical limb ischemia. J Inflamm
(Lond). 13:182016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu X and Li Z, Wan Q, Cheng X, Zhang J,
Pathak JL and Li Z: Inhibition of JAK2/STAT3 signaling suppresses
bone marrow stromal cells proliferation and osteogenic
differentiation, and impairs bone defect healing. Biol Chem.
399:1313–1323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang J, Wang G, Liu Y, Fu Y, Chi J, Zhu Y,
Zhao Y and Yin X: Cyclosporin A induces cardiomyocyte injury
through calcium-sensing receptor-mediated calcium overload.
Pharmazie. 66:52–57. 2011.PubMed/NCBI
|
|
28
|
Yan H, Zhang Y, Lv SJ, Wang L, Liang GP,
Wan QX and Peng X: Effects of glutamine treatment on myocardial
damage and cardiac function in rats after severe burn injury. Int J
Clin Exp Pathol. 5:651–659. 2012.PubMed/NCBI
|
|
29
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohseni M, Mihandoost E, Shirazi A,
Sepehrizadeh Z, Bazzaz JT and Ghazi-khansari M: Melatonin may play
a role in modulation of bax and bcl-2 expression levels to protect
rat peripheral blood lymphocytes from gamma irradiation-induced
apoptosis. Mutat Res. 738-739:19–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and Bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pepper C, Hoy T and Bentley DP: Bcl-2/Bax
ratios in chronic lymphocytic leukaemia and their correlation with
in vitro apoptosis and clinical resistance. Br J Cancer.
76:935–938. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen D, Zheng X, Kang D, Yan B, Liu X, Gao
Y and Zhang K: Apoptosis and expression of the Bcl-2 family of
proteins and P53 in human pancreatic ductal adenocarcinoma. Med
Princ Pract. 21:68–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rose-John S: IL-6 trans-signaling via the
soluble IL-6 receptor: Importance for the pro-inflammatory
activities of IL-6. Int J Biol Sci. 8:1237–1247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Van Wagoner NJ and Benveniste EN:
Interleukin-6 expression and regulation in astrocytes. J
Neuroimmunol. 100:124–139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu W, Kang J, Hu K, Tang S, Zhou X, Yu S
and Xu L: Angiotensin-(1–7) relieved renal injury induced by
chronic intermittent hypoxia in rats by reducing inflammation,
oxidative stress and fibrosis. Braz J Med Biol Res. 50:e55942017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao G, Yu YM, Kaneki M, Bonab AA,
Tompkins RG and Fischman AJ: Simvastatin reduces burn
injury-induced splenic apoptosis via downregulation of the
TNF-alpha/NF-kappaB pathway. Ann Surg. 261:1006–1012. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheong CU, Chang CP, Chao CM, Cheng BC,
Yang CZ and Chio CC: Etanercept attenuates traumatic brain injury
in rats by reducing brain TNF-α contents and by stimulating newly
formed neurogenesis. Mediators Inflamm. 2013:6208372013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roshan S, Liu YY, Banafa A, Chen HJ, Li
KX, Yang GX, He GY and Chen MJ: Fucoidan induces apoptosis of HepG2
cells by down-regulating p-Stat3. J Huazhong Univ Sci Technolog Med
Sci. 34:330–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Z, Gan L, Zhou Z, Jin W and Sun C:
SOCS3 promotes inflammation and apoptosis via inhibiting JAK2/STAT3
signaling pathway in 3T3-L1 adipocyte. Immunobiology. 220:947–953.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trung LQ, Espinoza JL, An DT, Viet NH,
Shimoda K and Nakao S: Resveratrol selectively induces apoptosis in
malignant cells with the JAK2V617F mutation by inhibiting the JAK2
pathway. Mol Nutr Food Res. 59:2143–2154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen C, Jia KY, Zhang HL and Fu J: MiR-195
enhances cardiomyocyte apoptosis induced by hypoxia/reoxygenation
injury via downregulating c-myb. Eur Rev Med Pharmacol Sci.
20:3410–3416. 2016.PubMed/NCBI
|
|
44
|
Quintás-Cardama A and Verstovsek S:
Molecular pathways: Jak/STAT pathway: Mutations, inhibitors, and
resistance. Clin Cancer Res. 19:1933–1940. 2013. View Article : Google Scholar : PubMed/NCBI
|