Introduction
Transverse aortic constriction (TAC) causes
compensatory hypertrophy, chronic maladaptive hemodynamic overload,
cardiac dilatation and heart failure over time (1). TAC is often used as an experimental
method to produce pressure overload, cardiac hypertrophy and heart
failure in mice (2). Although it
has been well demonstrated that TAC-induced myocardial dysfunction
is associated with inflammation, oxidative stress and cardiomyocyte
apoptosis (3–6), the molecular signaling pathways
involved in TAC-induced myocardial dysfunction have yet to be
elucidated. Therefore, it is important to investigate possible
target genes and to identify possible treatments for TAC-induced
myocardial dysfunction.
Asymmetric dimethylarginine (ADMA) is reported to be
an endogenous nitric oxide synthase (NOS) inhibitor, and its
accumulation is associated with various cardiovascular diseases,
including hypertension, diabetes and cardiac dysfunction (7–10).
ADMA attenuates endothelial NOS (eNOS) activity to reduce nitric
oxide (NO) production and induce NOS uncoupling to generate
reactive oxygen species (ROS) (8,11).
Dimethylarginine dimethylaminohydrolase 1 (DDAH1) serves an
important role in regulating vascular endothelial injury repair and
angiogenesis, as well as functions in degrading ADMA to maintain NO
signaling (12,13). Overexpression of the DDAH1 gene
reduces inflammatory infiltration through decreasing ADMA
expression (14). Serum ADMA
concentration was slightly higher when left ventricle DDAH1
expression was low, while DDAH1 knock out (KO) exacerbated left
ventricle hypertrophy, fibrosis and dysfunction in mice after TAC
(15). These findings suggest that
DDAH1 may protect cardiac hypertrophy and ventricular remodeling
against stress conditions.
Berry anthocyanins have recently drawn widespread
scientific interest due to their diverse health benefits, including
antioxidant, anti-inflammatory, antihypertensive,
anti-atherosclerotic, antimicrobial, anticancer and neuroprotective
properties (16). According to
National Health and Nutrition Education Survey (NHANES), an average
of 12.5 mg of anthocyanins are consumed per day by people in the
United States (17). In
vitro and in vivo studies have shown that blueberry
anthocyanins activate cellular antioxidant systems and inhibit
inflammatory infiltration, and protect against inflammation and
oxidative stress, through activation of multiple target genes. The
present study investigated the effects and potential mechanisms of
blueberry anthocyanin-enriched extract (BAE) on TAC-induced
myocardial dysfunction.
Materials and methods
Experimental animals
A total of 30 male C57BL/6 mice (age, 6–8 weeks;
weight, 20–25 g) were obtained from the Experimental Animal Center
of the General Hospital of Shenyang Military Command. All animals
were housed at 20±2°C and 55–60% humidity under a 12-h light/dark
cycle, were provided standard laboratory animal feed and water
ad libitum, and were allowed to acclimate for 7 days before
the study. All experimental animal procedures were approved by the
Animal Ethics Committee of the People's Hospital of Weifang
City.
Materials and reagents
BAE was provided by the Food College of Shenyang
Agricultural University (18). The
total anthocyanin content was ~25.7/100 g of extract. The
composition of BAE, as measured by high-performance liquid
chromatography/mass spectrometry, was: Malvidin 3-galactoside
(28.11%), malvidin 3-arabinoside (16.18%), malvidin 3-glucoside
(14.08%), malvidin 3-(6″-acetyl) glucoside (8.49%), malvidin
3-(6″-acetyl) galactoside (5.50%), petunidin 3-galactoside (5.44%),
petunidin 3-glucoside (5.26%), peonidin3-glucoside (5.22%),
cyanidin3-galactoside (2.96%) and delphinidin 3-glucoside
(1.41%).
Experimental protocol
After acclimation, the 30 mice were divided randomly
into control, TAC and BAE groups. The mice in the control group did
not undergo surgery. The mice in the BAE group were modelled for
TAC and, following a recovery period of 24 h after the surgery,
they were administered with 0.5 g/kg BAE daily by oral gavage for 6
consecutive weeks. Mice in the control and TAC groups were given
the same volume of distilled water.
TAC model
TAC models were created as described previously
(19). Briefly, mice were
anesthetized in an induction chamber with 2% isoflurane mixed with
0.5–1.0 l/min 100% O2. Mice were fixed in a supine
position on top of a heating pad to maintain body temperature.
Partial thoracotomy at the second rib was performed under a
surgical microscope and the sternum was retracted using a chest
retractor. Fine tip 45° angled forceps were used to gently separate
the thymus and fat tissue from the aortic arch. Following
identification of the transverse aorta, a small piece of a 6.0 silk
suture was placed between the innominate and left carotid arteries.
Two loose knots were tied around the transverse aorta and a small
piece of a gauge blunt needle was placed parallel to the transverse
aorta. The first knot was quickly tied against the needle, followed
by the second one, and the needle was promptly removed to yield a
constriction of 0.4 mm in diameter. The chest retractor was removed
and the outflow of the ventilator pinched off for 2 sec to
re-inflate the lungs. The rib cage was closed using a 6.0 prolene
suture with an interrupted suture pattern. The skin was closed
using a 6.0 prolene suture with a continuous suture pattern.
Echocardiogram
All animals were anesthetized with 1.6% isoflurane
and were assessed by echocardiogram (ECHO; Vevo 770, a 12 MHz
transducer; VisualSonics Inc.) according to previous literature
(20). Briefly, to ensure that the
mitral and aortic valves and the apex were visualized, parasternal
long axis views were obtained and recorded. Short axis views were
recorded at the mid-papillary muscle level. To calculate the
end-systolic left ventricular (LV) area, endocardial area tracings
were obtained using 2D mode from digital images captured on
cine-loop. All measurements were made by a single observer and were
averaged over 3–5 consecutive cardiac cycles. The reproducibility
of measurements was assessed in two sets of baseline measurements
in 10 randomly selected rats. Repeated measure variability did not
exceed 65%.
Sample collection
Briefly, mice were anesthetized by intraperitoneal
injection of 2% pentobarbital sodium 50 mg/kg of body weight (1.5
ml/kg; cat. no. 57-33-0; year 2016; Shanghai Haohai Biological
Technology Co., Ltd.) and then sacrificed. Tissues and blood
samples were obtained from abdominal aortas and stored for
analysis. Heart and lung tissues were also collected. Following
weighing on an electronic balance, tissues were either fixed in 10%
formaldehyde at room temperature for 3–5 days or immediately frozen
in liquid nitrogen and then transferred to a −80°C freezer.
ELISA
Levels of inflammatory factors, including
interleukin (IL)-1β and tumor necrosis factor (TNF)-α were measured
with ELISA kits (cat. nos. ab197742 and ab208348, respectively;
Abcam). The concentrations of the heart ADMA (cat. no. CEB301Ge,
Cloud-Clone Corp.) and NO (cat. no. BH3702; Shanghai Kaibo Biology
Technology Co., Ltd.) were measured with ELISA kits, according to
the manufacturers' directions. The absorbance was measured at 450
nm using an Enzyme Labeled Instrument (Bio-Rad Laboratories, Inc.),
and the concentration of NO and ADMA in the samples was calculated
by standard curve.
Histopathological analysis
Samples for histological analysis were immersed in
10% formalin buffer at room temperature for 3–5 days, embedded in
paraffin using a Leica Microsystem tissue processor (ASP 300S), and
sliced into 3–4 µm sections using a Leica Microsystem microtome
(model RM 226; Leica Microsystems GmbH). These sections were
stained with hematoxylin and eosin (H&E) at room temperature
for 10 min. LV fibrosis was estimated by staining with modified
Masson's Trichrome Stain kit (Sigma-Aldrich; Merck KGaA).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from heart tissue with
TRIzol (Takara Bio, Inc.) and reverse-transcribed using the
SuperScript™ Double-Stranded cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.), following the suppliers' protocols.
Reactions were performed in an RT-qPCR thermocycler (Bio-Rad
Laboratories, Inc.) using SYBR Green PCR Master Mix (Takara Bio
Inc.). The thermocycling conditions were: 94°C for 5 min, followed
by 35 cycles of 94°C for 35 sec (denaturation), 57.3°C for 35 sec
(annealing), and 72°C for 50 sec (extension). Primer sequences
were: Atrial natriuretic peptide (ANP), forward
5′-AGCGAGCAGACCGATGAAG-3′ and reverse 5′-AGCCCTCAGTTTGCTTTTCA-3′;
brain natriuretic peptide (BNP), forward 5′-TGATTCTGCTCCTGCTTTC-3′
and reverse 5′-GTGGATTGTTCTGGAGACTG-3′; myosin heavy chain β
(β-MHC), forward 5′-AGAGCAAAAGCAAAGGGTTTC-3′ and reverse
5′-GTGATGGTACGAGATGGGCTA-3′; IL-1β, forward
5′-CCTGTTCTTTGAAGTTGACGG-3′ and reverse 5′-AGCTTCTCCACAGCCACAAT-3′;
TNF-α, forward 5′-CCACCACGCTCTTCTGTCTA-3′ and reverse
5′-GAGAGGGAGGCCATTTGGGA-3′; DDAH1, forward
5′-AGGTGCTGAAATCTTGGCTG-3′ and reverse 5′-GCAGATTCGCTGGACCCTAT-3′;
melanoma differentiation-associated protein 5 (MDA5), forward
5′-AGTGTCTCCACTTGCTGACC-3′ and reverse 5′-CAGCAGCTCTCTTACACCTGA-3′;
inositol-requiring enzyme α (IREα), forward
5′-AGCACAGTTACACTGCCTGAG-3′ and reverse
5′-CTTCCACGTGTGTTGGGACCT-3′; superoxide dismutase-1 (SOD-1),
forward 5′-GAGCATTCCATCATTGGCCG-3′ and reverse
5′-GGCAATCCCAATCACACCAC-3′; Bax, forward 5′-TCCACCAAGAAGCTGAGCGA-3′
and reverse 5′-TTGAAGTTGCCATCAGCAAACA-3′; Caspase-3, forward
5′-ATGGGAGCAAGTCAGTGGAC-3′ and reverse 5′-GTCCACATCCGTACCAGAGC-3′;
Bcl-2, forward 5′-TGAGTACCTGAACCGGCATC-3′ and reverse
5′-AAGCCCAGACTCATTCAACCA-3′; Caspase-8, forward
5′-CCTAGACTGCAACCGAGAGG-3′ and reverse
5′-TCCAACTCGCTCACTTCTTCTG-3′; and GAPDH, forward
5′-CGGATTTGGTCGTATTGGG-3′ and reverse 5′-CTGGAAGATGGTGATGGGATT-3′.
The mRNA expression of the target genes was normalized to GAPDH as
an internal control, and relative fold changes in mRNA expression
were calculated using the formula 2−ΔΔCq (21).
Western blot analysis
Western blotting was performed as previously
described (22). Briefly, heart
tissues were lysed in radioimmunoprecipitation assay (RIPA) buffer
(10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP40, 0.1% sodium dodecyl
sulfate, 1 mM phenylmethylsulfonyl fluoride and 1X protease
inhibitor cocktail; Roche Diagnostics) and homogenized by Sonic
Dismembrator 100 (Thermo Fisher Scientific, Inc.). The protein
concentration of tissue homogenates was measured using a Bio-Rad
Protein Assay (Bio-Rad Laboratories, Inc.). A total of 30 µg
soluble protein was separated on 10% polyacrylamide gels and
transferred into nitrocellulose membrane. The membrane was blocked
with 5% skimmed milk powder at room temperature for 1 h. The
following primary antibodies were used: TNF-α (1:2,000; ab8348;
Abcam), IL-6 (1:1,000; ab83053; Abcam), IREα (1:2,000; 3294;
Abcam), MDA5 (1:200; ab69983; Abcam), ANP (1:500; ab251006; Abcam),
BNP (1:1,000; ab239510; Abcam), β-MHC (1:200; ab23990; Abcam),
SOD-1 (1:1,000; sc-11407; Santa Cruz Biotechnology, Inc.), Bax
(1:2,000; sc-526; Santa Cruz Biotechnology, Inc.), Caspase 3
(1:500; sc-7148; Santa Cruz Biotechnology, Inc.), Bcl-2 (1:1,000;
sc-7382; Santa Cruz Biotechnology, Inc.), Caspase 8 (1:100;
sc-5263; Santa Cruz Biotechnology, Inc.), DDAH1 (1:500; sc-5268;
Santa Cruz Biotechnology, Inc.) and GAPDH (1:5,000; sc-32233; Santa
Cruz Biotechnology, Inc.). The secondary antibodies were:
Horseradish peroxidase (HRP)-labeled goat anti-mouse secondary
antibody (ab6789; 1:4,000; Abcam), goat anti-rabbit HRP-labeled
secondary antibody (ab6721; 1:4,000; Abcam) and goat anti-rat
HRP-labeled secondary antibody (ab7097; 1:2,000; Abcam). Secondary
antibodies were incubated for 1.5 h at room temperature. Proteins
were visualized using a ClarityTM Western ECL Substrate (cat. no.
170-5061; Bio-Rad Laboratories, Inc.) and a Tanon 5200 full
automatic chemiluminescence image analysis system (Tanon Science
and Technology Co., Ltd.).
Statistical analysis
Statistics were performed using SPSS 20.0
statistical software (IBM Corp.). Data were expressed as means ±
standard error of the mean and were analyzed using unpaired t-test
and one-way ANOVA followed by a Bonferroni correction post hoc test
for differences among >2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
BAE ameliorates TAC-induced cardiac
hypertrophy in mice
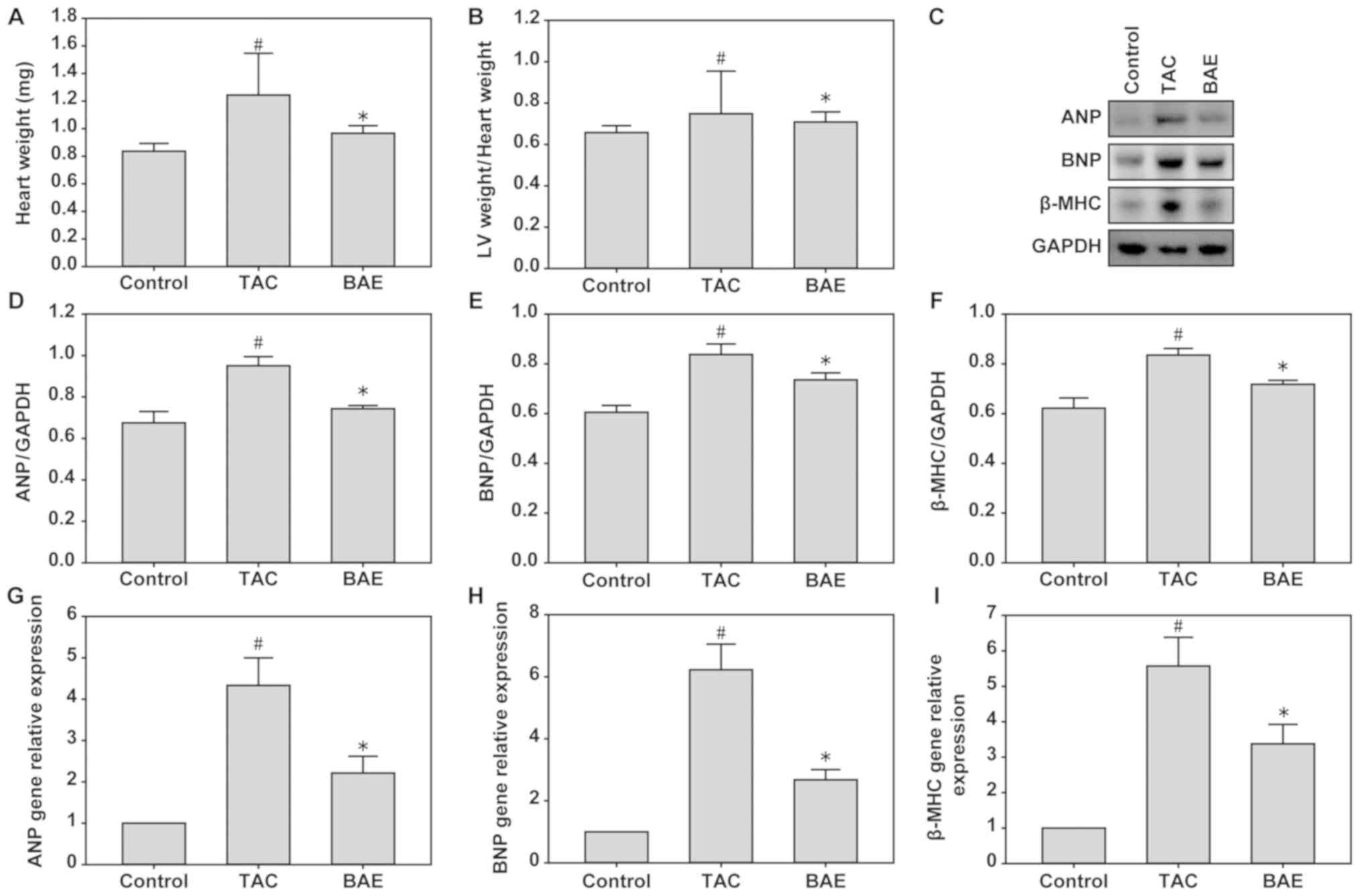
Compared with the control group, the TAC group had
significantly higher heart weight and LV weight/heart weight ratio,
but these effects were significantly attenuated by BAE treatment
(Fig. 1A and B; P<0.05).
Additionally, compared with the control group, the TAC group had
significantly increased protein and mRNA expression levels of ANP,
BNP and β-MHC in their heart LV tissues, and these were
significantly ameliorated following BAE treatment (Fig. 1C-I; P<0.05).
BAE ameliorates TAC-induced LV
dysfunction in mice
Compared with the control group, the TAC group
demonstrated reduced LV fractional shorting and LV ejection
fraction, and increased LV end-systolic diameter, LV end-diastolic
diameter, and LV wall thickness. BAE treatment markedly elevated
the LV ejection fraction and LV fractional shorting and decreased
the LV end-diastolic and end-systolic diameters compared with the
TAC group (Table I;
P<0.05).
 | Table I.Cardiac function parameters in the
experimental groups. |
Table I.
Cardiac function parameters in the
experimental groups.
| Parameters | Control (n=10) | TAC (n=10) | BAE (n=10) |
|---|
| LVPW (mm) | 2.39±0.36 |
12.01±0.27a |
5.71±0.38b |
| LVEDd (mm) |
3.25±0.53 |
4.91±0.47a |
3.91±0.32b |
| LVESd (mm) |
1.72±0.47 |
3.75±0.71a |
2.28±0.53b |
| LVEDV (µl) |
47.25±6.04 |
89.54±9.53a |
68.45±4.53b |
| LVESV (µl) |
18.05±7.31 |
55.34±6.93a |
31.13±2.05b |
| LVEF (%) |
72.32±5.95 |
31.42±3.18a |
51.34±0.34b |
| LVFS (%) |
42.64±4.42 |
23.65±3.08a |
32.32±6.38b |
BAE ameliorates TAC-induced LV
inflammation and fibrosis in mice
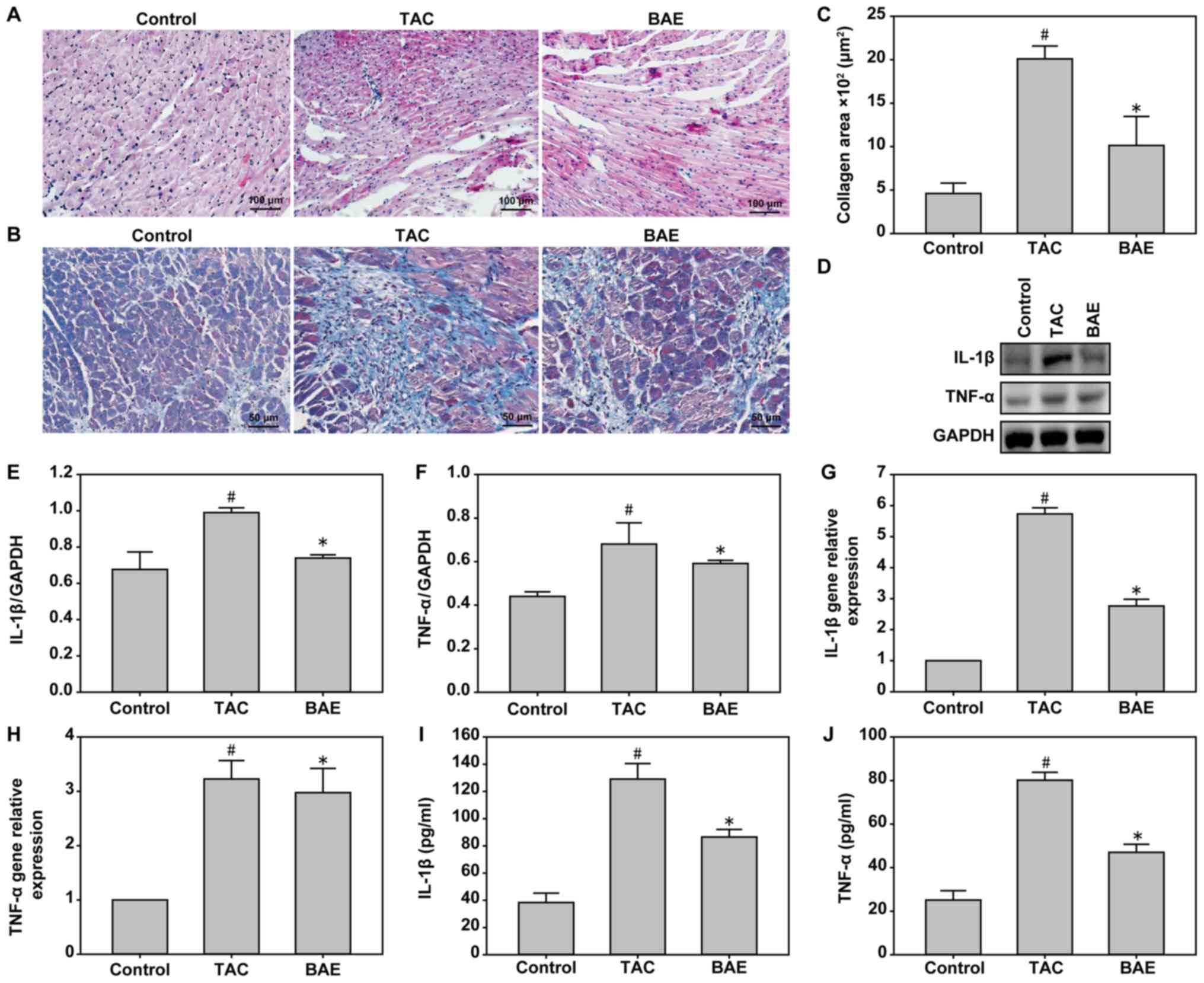
Compared with the control group, the TAC group
experienced heart hypertrophy, edema, inflammatory cell
infiltration, as well as a significant increase in the levels of
pro-inflammatory cytokines IL-1β and TNF-α (Fig. 2). BAE treatment significantly
reversed the TAC-induced pro-inflammatory factor secretion
(Fig. 2; P<0.05). In addition,
TAC induced significant LV fibrosis compared with the control
group, and this effect was ameliorated following BAE treatment
(Fig. 2; P<0.05).
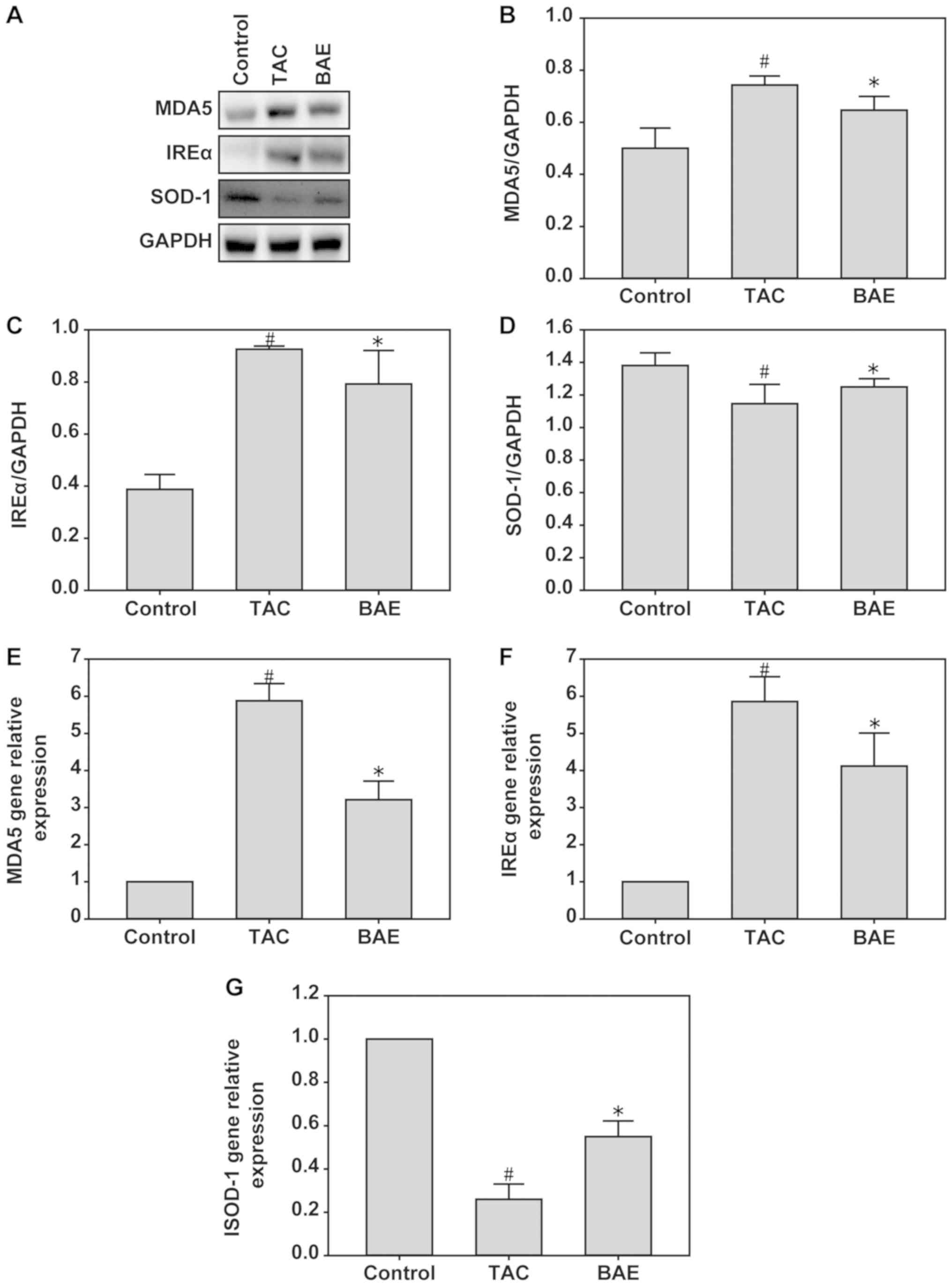
BAE ameliorates TAC-induced oxidative
stress in mice
To assess the effect of BAE on TAC-induced oxidative
stress, the protein and mRNA expression levels of MDA5 and IREα
(markers of oxidative stress), and of SOD-1 (antioxidant status
marker), were assessed in the heart tissues of the experimental
mice. The data demonstrated that TAC significantly increased MDA5
and IREα expression, but decreased SOD-1 expression. By contrast,
BAE treatment enhanced the expression of MDA5 and IREα and reduced
the expression of SOD-1 (Fig. 3;
P<0.05).
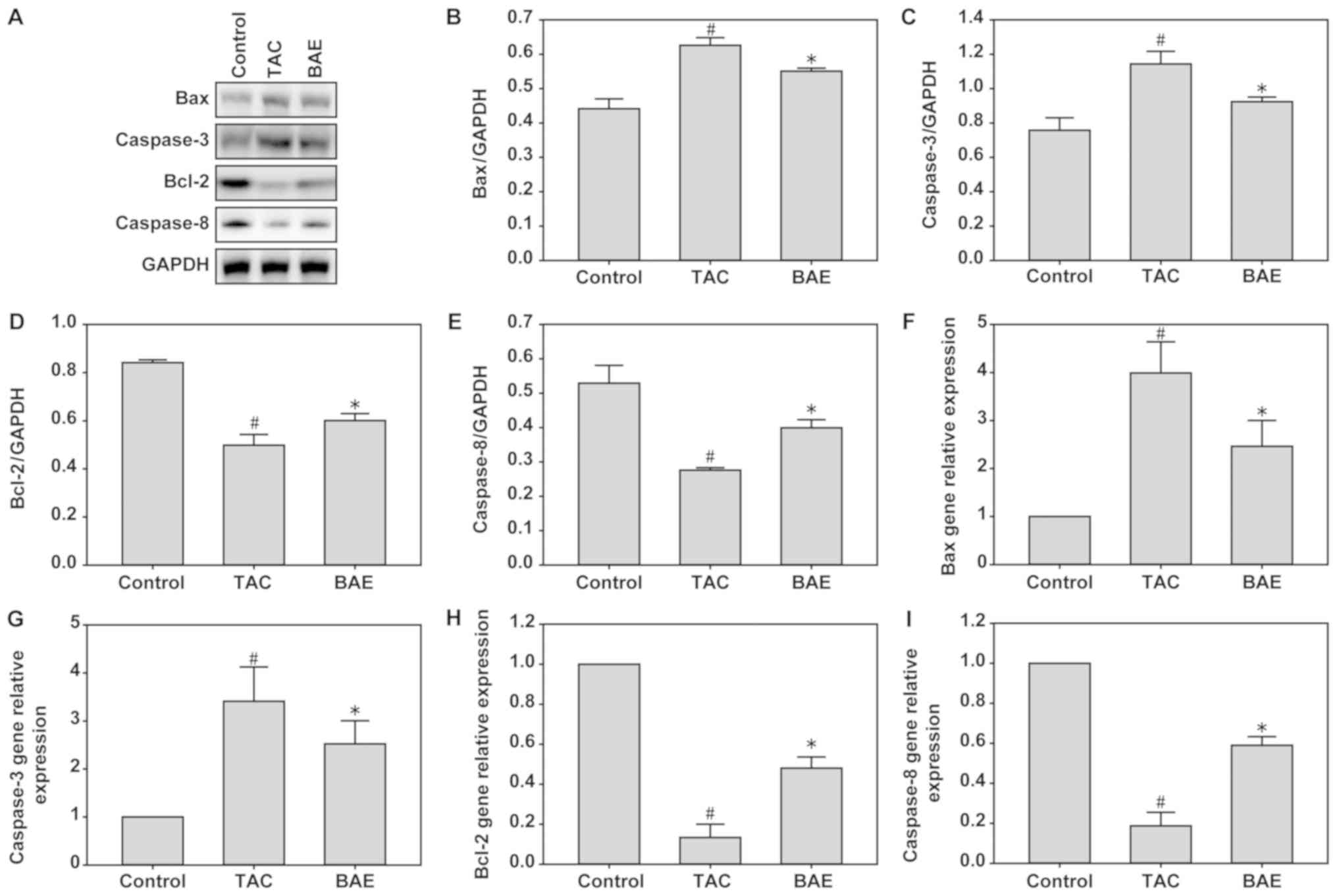
BAE ameliorates TAC-induced heart
tissue apoptosis in mice
The present data demonstrated that TAC significantly
increased Bax and Caspase-3 expression, but decreased Bcl-2 and
Caspase-8 expression, compared with the control group, at both the
mRNA and protein levels. By contrast, BAE treatment significantly
reversed the upregulation of Bax and Caspase-3 and the
downregulation of Bcl-2 and Caspase-8 induced by TAC (Fig. 4; P<0.05).
BAE ameliorates TAC-induced myocardial
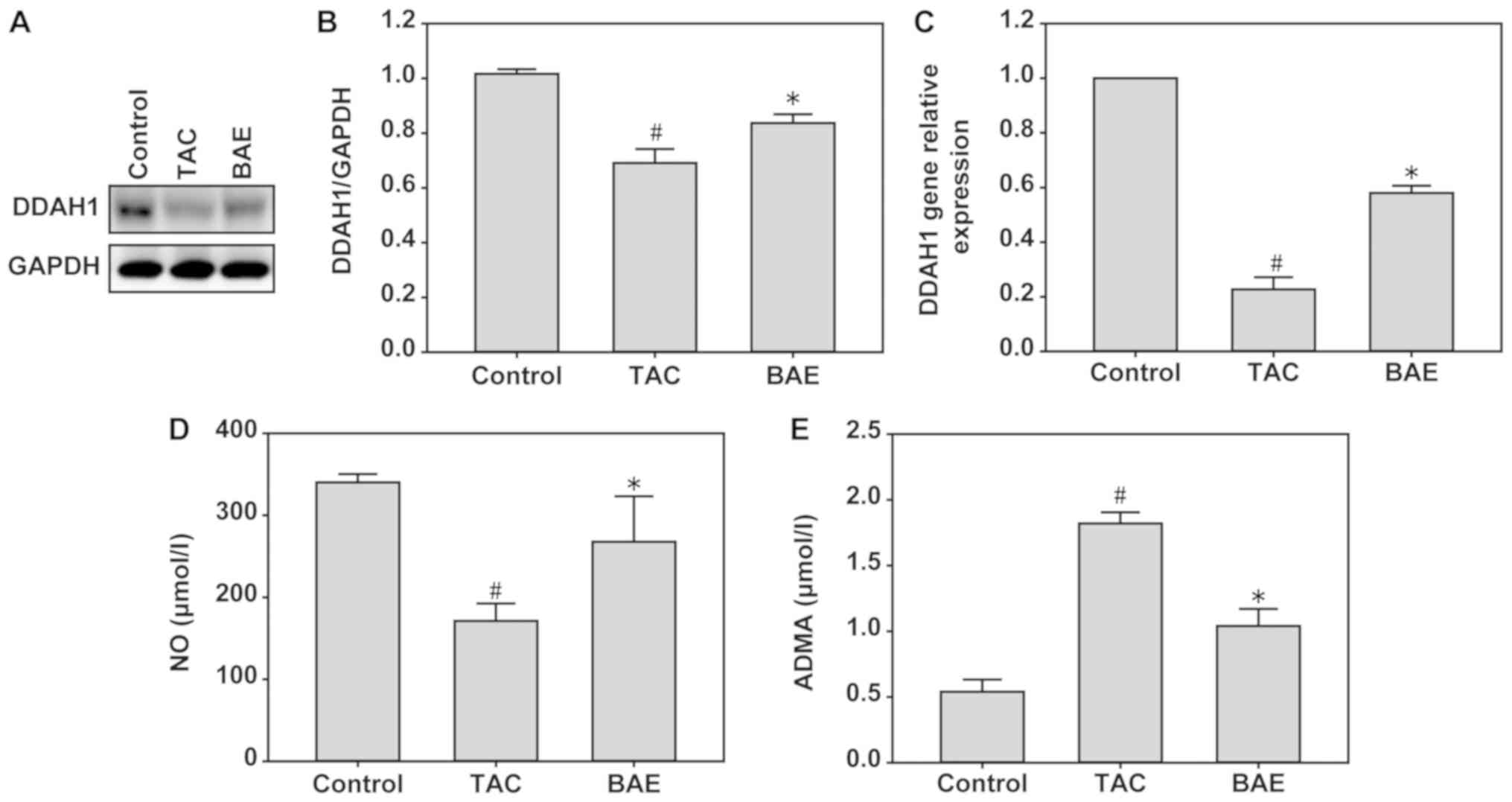
dysfunction via DDAH1/ADMA/NO signaling in mice
To investigate whether DDAH1/ADMA/NO signaling was
involved in TAC-induced myocardial dysfunction, the protein
expression levels of DDAH1 and the concentrations of ADMA and NO
were measured in heart tissues by western blot analysis, RT-qPCR
and ELISA. Compared with the control group, TAC significantly
increased ADMA concentration (Fig.
5E; P<0.05), but decreased DDAH1 expression (Fig. 5A-C; P<0.05) and NO production
(Fig. 5D; P<0.05). Notably, BAE
treatment significantly reversed these effects (Fig. 5; P<0.05).
Discussion
Cardiovascular risk factors, including diabetes,
smoking, hypertension, dyslipidemia, aging, and obesity, are
associated with ROS, oxidative stress, inflammatory response and
apoptosis (23). TAC increases
free oxygen radicals, inflammation and apoptosis in heart tissues.
The present study demonstrated that BAE ameliorated cardiac
dysfunction, LV hypertrophy and fibrosis induced by TAC. The
present data also demonstrated that BAE treatment attenuated
TAC-induced LV leukocyte infiltration, inflammatory cytokine
expression, oxidative stress and apoptosis. Furthermore, BAE
significantly increased DDAH1 expression and NO production, and
decreased ADMA concentration. These results suggested that
TAC-induced myocardial dysfunction may be ameliorated by BAE
treatment via the DDAH1/ADMA/NO signaling pathway.
A previous study demonstrated that 20 and 80 mg/kg
BAE treatment attenuated cyclophosphamide-induced cardiac
dysfunction, left ventricular hypertrophy and fibrosis (24). In addition, BAE significantly
reduced systolic blood pressure and increased aortic vessel
relaxation in response to acetylcholine in high fat/high
cholesterol diet-fed rats (25,26).
Oxidative stress activates several intracellular signaling pathways
and upregulates the expression of a large number of
pro-inflammatory cytokines (27).
A previous study demonstrated that a long-term blueberry-enriched
diet decreases blood pressure and attenuates oxidative status in
the kidneys of spontaneously hypertensive rats (28). BAE significantly inhibits hydrogen
peroxide-induced human retinal pigment epithelial cell apoptosis,
decreased vascular endothelial growth factor levels and activates
protein kinase B (Act)-signal pathways (29). Song et al (30) demonstrated that BAE protects
retinal cells against diabetes-induced oxidative stress and
inflammation. In addition to the antioxidant effects, a previous
study demonstrated that blueberry supplementation decreases serum
inflammatory markers, including TNF-α, IL-6 and C-reactive protein
(31). Freeze-dried blueberries
markedly reduce pro-inflammatory cytokines TNF-α and IL-6
production in macrophages of apolipoprotein E KO mice by inhibiting
nuclear factor (NF)-κB activation and the mitogen-activated protein
kinase pathway (32). BAE
attenuates C-C motif chemokine ligand 4-induced liver fibrosis,
associated with reducing sources of ROS generation and associated
oxidative damage, decreasing the influence of pro-inflammatory
cytokines (33). Blackberry and
blueberry anthocyanin supplementation significantly reduces serum
and hepatic lipid levels, markedly elevates hepatic SOD and
glutathione peroxidase activities, and reduces the expression of
TNF-α, IL-6 and NF-κB genes in high-fat diet fed C57BL/6 mice
(34). Additionally, blueberry
suppresses cell cycle progression and induces
mitochondrial-mediated cell apoptosis by abolishing the Janus
kinase/STAT3 pathway (35). In
brief, the present data, in agreement with the previous literature,
suggested that BAE may have a protective effect against TAC-induced
LV inflammation, oxidative stress and apoptosis.
In vivo and in vitro experiments have
demonstrated that the DDAH1/ADMA/NO signaling pathway is closely
associated with cardiovascular disease (10,12),
cancer (13,36), liver diseases (37), preeclampsia (38,39)
and ischemic and reperfusion injury (40). The present study demonstrated that
TAC-induced a significant increase in ADMA concentration and a
decrease in DDAH1 expression and NO production, which were
ameliorated by BAE treatment. Previous studies have demonstrated
that dietary blueberries have vascular beneficial effects by
activating multiple targets including eNOS/NO/cyclic guanosine
monophosphate (cGMP), redox and inflammatory signaling pathways
(41–43). The production of NO by eNOS
involves multiple steps and can be activated in different ways
(44). In addition to
vasodilation, NO exerts many beneficial vascular effects through
anti-inflammation, antiplatelet, antiproliferation and
antimigration activity (45). A
randomized, double-blinded, placebo-controlled clinical trial study
found that daily blueberry supplement reduces blood pressure and
arterial stiffness, which may be related to increase NO production
(46). In addition, pterostilbene,
an active constituent of blueberries, causes eNOS phosphorylation
and subsequent NO production through activation of the PI3K/Akt
pathway (47); this blueberry
supplement may change the intestinal microbiota and the
anti-hypertensive effect of blueberries may be regulated by the
NO-dependent pathway (48).
Collectively, these studies suggest that the vascular effects of
blueberries could occur via eNOS/NO/cGMP signaling, but the direct
effect of blueberry anthocyanins on eNOS activation remains to be
elucidated.
In conclusion, the data form the present study
suggested that BAE exerted potential beneficial vascular effects
against TAC-induced LV inflammation, oxidative stress and
apoptosis, by decreasing ADMA concentration and by elevating DDAH1
expression and NO production. BAE treatment ameliorated the
TAC-induced myocardial dysfunction, oxidative stress and
inflammatory response and apoptosis, potentially via the
DDAH1/ADMA/NO signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
WW, QM, TL and JFZ performed the experiments,
analyzed and interpreted data. WH and JCZ made substantial
contributions to the conception and design of the present study,
and wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in compliance
with the Guide for the Care and Use of Laboratory Animals published
by the National Institutes of Health, and were approved by the
Animal Ethics Committee of the People' Hospital of Weifang City
(Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Muller OJ, Heckmann MB, Ding L, Rapti K,
Rangrez AY, Gerken T, Christiansen N, Rennefahrt UEE, Witt H,
González Maldonado S, et al: Comprehensive plasma and tissue
profiling reveals systemic metabolic alterations in cardiac
hypertrophy and failure. Cardiovasc Res. 115:1296–1305. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quast C, Alter C, Ding Z, Borg N and
Schrader J: Adenosine formed by CD73 on T cells inhibits cardiac
inflammation and fibrosis and preserves contractile function in
transverse aortic constriction-induced heart failure. Circ Heart
Fail. 10(pii): e0033462017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shang L, Weng X, Wang D, Yue W, Mernaugh
R, Amarnath V, Weir EK, Dudley SC, Xu Y, Hou M and Chen Y:
Isolevuglandin scavenger attenuates pressure overload-induced
cardiac oxidative stress, cardiac hypertrophy, heart failure and
lung remodeling. Free Radic Biol Med. 141:291–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Luo HQ, Sun LL, Xu MT, Yu J, Liu
LL, Zhang JY, Wang YQ, Wang HX, Bao XF and Meng GL:
Dihydromyricetin attenuates myocardial hypertrophy induced by
transverse aortic constriction via oxidative stress inhibition and
SIRT3 pathway enhancement. Int J Mol Sci. 19(pii): E25922018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng J, Li S and Chen H: Tanshinone IIA
inhibits myocardial remodeling induced by pressure overload via
suppressing oxidative stress and inflammation: Possible role of
silent information regulator 1. Eur J Pharmacol. 791:632–639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gröschel C, Sasse A, Monecke S, Röhrborn
C, Elsner L, Didié M, Reupke V, Bunt G, Lichtman AH, Toischer K, et
al: CD8+-T cells with specificity for a model antigen in
cardiomyocytes can become activated after transverse aortic
constriction but do not accelerate progression to heart failure.
Front Immunol. 9:26652018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wieczór AM, Wieczór R, Kulwas A and Rość
D: Asymmetric dimethylarginine and angiogenesis: Biological
significance. Int Angiol. 37:431–436. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dovinová I, Hrabárová E, Jansen E,
Kvandová M, Majzúnová M, Berenyiová A and Barančík M: ADMA,
homocysteine and redox status improvement affected by
7-nitroindazole in spontaneously hypertensive rats. Biomed
Pharmacother. 106:1478–1483. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zarezadeh M, Saedisomeolia A, Khorshidi M,
Kord Varkane H, Makhdoomi Arzati M, Abdollahi M, Yekaninejad MS,
Hashemi R, Effatpanah M and Mohammadzadeh Honarvar N: Asymmetric
dimethylarginine and soluble inter-cellular adhesion molecule-1
serum levels alteration following ginger supplementation in
patients with type 2 diabetes: A randomized double-blind,
placebo-controlled clinical trial. J Complement Integr Med.
16:2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Xu X, Shang R and Chen Y:
Asymmetric dimethylarginine (ADMA) as an important risk factor for
the increased cardiovascular diseases and heart failure in chronic
kidney disease. Nitric Oxide. 78:113–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirkby NS, Tesfai A, Ahmetaj-Shala B,
Gashaw HH, Sampaio W, Etelvino G, Leão NM, Santos RA and Mitchell
JA: Ibuprofen arginate retains eNOS substrate activity and reverses
endothelial dysfunction: Implications for the COX-2/ADMA axis.
FASEB J. 30:4172–4179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amir M, Hassanein SI, Abdel Rahman MF and
Gad MZ: AGXT2 and DDAH-1 genetic variants are highly correlated
with serum ADMA and SDMA levels and with incidence of coronary
artery disease in Egyptians. Mol Biol Rep. 45:2411–2419. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reddy KRK, Dasari C, Duscharla D, Supriya
B, Ram NS, Surekha MV, Kumar JM and Ummanni R: Dimethylarginine
dimethylaminohydrolase-1 (DDAH1) is frequently upregulated in
prostate cancer, and its overexpression conveys tumor growth and
angiogenesis by metabolizing asymmetric dimethylarginine (ADMA).
Angiogenesis. 21:79–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YE, Tong CC, Zhang YB, Cong PF, Shi
XY, Liu Y, Shi L, Tong Z, Jin HX and Hou MX: Chitosan
oligosaccharide ameliorates acute lung injury induced by blast
injury through the DDAH1/ADMA pathway. PLoS One. 13:e01921352018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu X, Zhang P, Kwak D, Fassett J, Yue W,
Atzler D, Hu X, Liu X, Wang H, Lu Z, et al: Cardiomyocyte
dimethylarginine dimethylaminohydrolase-1 (DDAH1) plays an
important role in attenuating ventricular hypertrophy and
dysfunction. Basic Res Cardiol. 112:552017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Wang P, Luo Y, Zhao M and Chen F:
Health benefits of anthocyanins and molecular mechanisms: Update
from recent decade. Crit Rev Food Sci Nutr. 57:1729–1741. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Beecher GR, Holden JM, Haytowitz DB,
Gebhardt SE and Prior RL: Concentrations of anthocyanins in common
foods in the United States and estimation of normal consumption. J
Agric Food Chem. 54:4069–4075. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Lin J, Tian J, Si X, Jiao X, Zhang
W, Gong E and Li B: Blueberry malvidin-3-galactoside suppresses
hepatocellular carcinoma by regulating apoptosis, proliferation,
and metastasis pathways in vivo and in vitro. J Agric Food Chem.
67:625–636. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Almeida AC, van Oort RJ and Wehrens XH:
Transverse aortic constriction in mice. J Vis Exp:. (pii):
17292010.PubMed/NCBI
|
|
20
|
Hampton C, Rosa R, Campbell B, Kennan R,
Gichuru L, Ping X, Shen X, Small K, Madwed J and Lynch JJ: Early
echocardiographic predictors of outcomes in the mouse transverse
aortic constriction heart failure model. J Pharmacol Toxicol
Methods. 84:93–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong C, Liu Y, Zhang Y, Cong P, Shi X, Liu
Y, Shi Hongxu Jin L and Hou M: Shock waves increase pulmonary
vascular leakage, inflammation, oxidative stress, and apoptosis in
a mouse model. Exp Biol Med (Maywood). 243:934–944. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park KH and Park WJ: Endothelial
dysfunction: Clinical implications in cardiovascular disease and
therapeutic approaches. J Korean Med Sci. 30:1213–1225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Tan D, Shi L, Liu X, Zhang Y, Tong
C, Song D and Hou M: Blueberry anthocyanins-enriched extracts
attenuate cyclophosphamide-induced cardiac injury. PLoS One.
10:e01278132015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basu A, Du M, Leyva MJ, Sanchez K, Betts
NM, Wu M, Aston CE and Lyons TJ: Blueberries decrease
cardiovascular risk factors in obese men and women with metabolic
syndrome. J Nutr. 140:1582–1587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodriguez-Mateos A, Ishisaka A, Mawatari
K, Vidal-Diez A, Spencer JP and Terao J: Blueberry intervention
improves vascular reactivity and lowers blood pressure in
high-fat-, high-cholesterol-fed rats. Br J Nutr. 109:1746–1754.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paneni F, Beckman JA, Creager MA and
Cosentino F: Diabetes and vascular disease: Pathophysiology,
clinical consequences, and medical therapy: Part I. Eur Heart J.
34:2436–2443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elks CM, Reed SD, Mariappan N,
Shukitt-Hale B, Joseph JA, Ingram DK and Francis J: A
blueberry-enriched diet attenuates nephropathy in a rat model of
hypertension via reduction in oxidative stress. PLoS One.
6:e240282011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang WY, Wu H, Li DJ, Song JF, Xiao YD,
Liu CQ, Zhou JZ and Sui ZQ: Protective effects of blueberry
anthocyanins against H2O2-induced oxidative injuries in human
retinal pigment epithelial cells. J Agric Food Chem. 66:1638–1648.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song Y, Huang L and Yu J: Effects of
blueberry anthocyanins on retinal oxidative stress and inflammation
in diabetes through Nrf2/HO-1 signaling. J Neuroimmunol. 301:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vendrame S, Daugherty A, Kristo AS, Riso P
and Klimis-Zacas D: Wild blueberry (Vaccinium angustifolium)
consumption improves inflammatory status in the obese Zucker rat
model of the metabolic syndrome. J Nutr Biochem. 24:1508–1512.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie C, Kang J, Ferguson ME, Nagarajan S,
Badger TM and Wu X: Blueberries reduce pro-inflammatory cytokine
TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB
activation and the MAPK pathway. Mol Nutr Food Res. 55:1587–1591.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun J, Wu Y, Long C, He P, Gu J, Yang L,
Liang Y and Wang Y: Anthocyanins isolated from blueberry
ameliorates CCl4 induced liver fibrosis by modulation of
oxidative stress, inflammation and stellate cell activation in
mice. Food Chem Toxicol. 120:491–499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu T, Gao Y, Guo X, Zhang M and Gong L:
Blackberry and blueberry anthocyanin supplementation counteract
high-fat-diet-induced obesity by alleviating oxidative stress and
inflammation and accelerating energy expenditure. Oxid Med Cell
Longev. 2018:40512322018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baba AB, Nivetha R, Chattopadhyay I and
Nagini S: Blueberry and malvidin inhibit cell cycle progression and
induce mitochondrial-mediated apoptosis by abrogating the
JAK/STAT-3 signalling pathway. Food Chem Toxicol. 109:534–543.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye J, Xu J, Li Y, Huang Q, Huang J, Wang
J, Zhong W and Lin X, Chen W and Lin X: DDAH1 mediates gastric
cancer cell invasion and metastasis via Wnt/β-catenin signaling
pathway. Mol Oncol. 11:1208–1224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li T, Feng R, Zhao C, Wang Y, Wang J, Liu
S, Cao J, Wang H, Wang T, Guo Y and Lu Z: Dimethylarginine
dimethylaminohydrolase 1 protects against high-fat diet-induced
hepatic steatosis and insulin resistance in mice. Antioxid Redox
Signal. 26:598–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akbar F, Heinonen S, Pirskanen M, Uimari
P, Tuomainen TP and Salonen JT: Haplotypic association of DDAH1
with susceptibility to pre-eclampsia. Mol Hum Reprod. 11:73–77.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Powers R and Gefter J: OS075.
Endothelial-dependent vascular function is significantly impaired
in obesity and restored by overexpression of DDAH1: Evidence for
the role of ADMA. Pregnancy Hypertens. 2:2182012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Trocha M, Nowak B, Merwid-Lad A, Szuba A,
Dzięgiel P, Pieśniewska M, Gomułkiewicz A, Wiśniewski J, Piasecki
T, Gziut M, et al: The impact of sitagliptin, inhibitor of
dipeptidyl peptidase-4 (DPP-4), on the ADMA-DDAH-NO pathway in
ischemic and reperfused rat livers. Adv Clin Exp Med. 27:1483–1490.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gielis JF, Lin JY, Wingler K, Van Schil
PE, Schmidt HH and Moens AL: Pathogenetic role of eNOS uncoupling
in cardiopulmonary disorders. Free Radic Biol Med. 50:765–776.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bharat D, Cavalcanti RRM, Petersen C,
Begaye N, Cutler BR, Costa MMA, Ramos RKLG, Ferreira MR, Li Y,
Bharath LP, et al: Blueberry metabolites attenuate
lipotoxicity-induced endothelial dysfunction. Mol Nutr Food Res.
62:2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi D, Xu M, Ren M, Pan E, Luo C, Zhang W
and Tang Q: Immunomodulatory effect of flavonoids of blueberry
(Vaccinium corymbosum L.) leaves via the NF-κB signal pathway in
LPS-stimulated RAW 264.7 cells. J Immunol Res. 2017:54769032017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Quillon A, Fromy B and Debret R:
Endothelium microenvironment sensing leading to nitric oxide
mediated vasodilation: A review of nervous and biomechanical
signals. Nitric Oxide. 45:20–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Su JB: Vascular endothelial dysfunction
and pharmacological treatment. World J Cardiol. 7:719–741. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Johnson SA, Figueroa A, Navaei N, Wong A,
Kalfon R, Ormsbee LT, Feresin RG, Elam ML, Hooshmand S, Payton ME
and Arjmandi BH: Daily blueberry consumption improves blood
pressure and arterial stiffness in postmenopausal women with pre-
and stage 1-hypertension: A randomized, double-blind,
placebo-controlled clinical trial. J Acad Nutr Diet. 115:369–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park SH, Jeong SO, Chung HT and Pae HO:
Pterostilbene, an active constituent of blueberries, stimulates
nitric oxide production via activation of endothelial nitric oxide
synthase in human umbilical vein endothelial cells. Plant Foods Hum
Nutr. 70:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ahrén IL, Xu J, Önning G, Olsson C, Ahrné
S and Molin G: Antihypertensive activity of blueberries fermented
by Lactobacillus plantarum DSM 15313 and effects on the gut
microbiota in healthy rats. Clin Nutr. 34:719–726. 2015. View Article : Google Scholar : PubMed/NCBI
|