Introduction
Cataracts are generally treated surgically; however,
excess proliferation and differentiation of the remaining human
lens epithelial cells (HLECs) may result in vision disturbance
following surgery (1–3). Epithelial-to-mesenchymal transition
(EMT) has been implicated in the transition of HLECs to
myofibroblasts (4,5).
EMT cell characteristics include the acquisition of
a spindle-shaped morphology that is accompanied by an accumulation
of α-smooth muscle actin (α-SMA), a redistribution of actin stress
fibers, a loss of cell polarity and epithelial markers such as
cytokeratin, zonula occludens-1 and epithelial cadherin
(E-cadherin), and upregulation of transcription factors including
snail family transcriptional repressor 1 and 2 and twist family
bHLH transcription factor 1 (6–11).
Previous studies have revealed that cataract surgery
may result in cellular stress (12,13).
The endoplasmic reticulum (ER) serves an important role in
detecting cellular stress, and subsequently triggers the ER stress
response (ER stress) to restore cellular homeostasis. Additionally,
the unfolded protein response (UPR) is triggered alongside ER
stress to additionally decrease cellular stress (14).
Evidence indicates that the UPR participates in
crosstalk with EMT in several types of cells: The UPR potentiates
EMT in gastric cancer cells under conditions of severe hypoxia
(15) or prolonged ER stress, and
results in irreversible EMT in human peritoneal mesothelial cells
(16). However, whether ER stress
affects EMT in the human lens epithelium remains unclear.
Therefore, the present study evaluated the role of ER stress in
inducing EMT in HLECs. ER stress resulted in morphological changes,
increased cell migration and altered expression of EMT-associated
proteins in a human lens epithelial cell line in vitro.
Together, these results suggested that ER stress serves an
important role in regulating EMT in HLECs.
Materials and methods
Reagents and antibodies
The ER stress activators thapsigargin (TG) and
tunicamycin (TM) were purchased from Sigma-Aldrich; Merck KGaA and
Beijing Solarbio Science & Technology Co., Ltd., respectively.
The ER stress inhibitors 4-phenylbutyric acid (PBA) and sodium
tauroursodeoxycholate (TUDCA) were purchased from Sigma-Aldrich;
Merck KGaA. TM, TG, PBA and TUDCA were dissolved in dimethyl
sulfoxide (DMSO; Leagene). Anti-glucose-regulated protein 78 kDa
(GRP78; ab12223), anti-activating transcription factor (ATF)6
(ab11909), anti-phosphorylated eukaryotic initiation factor 2α
(p-IRE1α; ab48187), anti-E-Cadherin (ab40772), anti-fibronectin
(ab2413) and anti-α-SMA (ab32575) primary antibodies were purchased
from Abcam. Horseradish peroxidase-conjugated anti-p-eIF2α
(119A11), horse anti-mouse and horse anti-rabbit secondary
antibodies, Alexa Fluor 488-conjugated goat anti-rabbit and Alexa
Fluor 488-conjugated goat anti-mouse secondary antibodies were
purchased from Cell Signaling Technology, Inc. Anti-ATF4 primary
antibody (sc-390063) was purchased from Santa Cruz Biotechnology,
Inc. Primary antibodies against vimentin (10366-1-AP), β-actin
(66009-1-Ig) and Neural cadherin (N-cadherin; 22018-1-AP) were
purchased from ProteinTech Group, Inc.
HLEC culture and treatment
The human lens epithelial SRA01/04 cell line
(supplied by Professor Shang, Zhongshan Ophthalmic Center) was
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin at 37°C. In order to evaluate the role of
ER stress, SRA01/04 cells were treated with TM, TG, PBA and TUDCA
for 24 h at 37°C at the concentrations listed below.
HLEC morphological analysis
SRA01/04 cells were treated with 0.01 µM TG or a
combination of 0.01 µM TG and 0.25 mM PBA for 24 h. Untreated
SRA01/04 cells served as the control group. Cell morphology was
analyzed under an inverted phase-contrast microscope (Axiovert 200;
Carl Zeiss AG), and images were captured using a digital camera
(AxioCam HRC; Carl Zeiss AG; magnification ×20). A minimum of 9
images per group were analyzed using ImageJ software 1.8.0
(National Institutes of Health) and the length of the long axis of
the cells and the aspect ratio, defined as the ratio of the long
axis (width) to the short axis (length) of the cells, were
determined. The experiment was performed in triplicate.
Western blot analysis
SRA01/04 cells were treated with 0.01 µM TG, 0.01 µM
TG and 0.25 mM PBA, 0.01 µM TG and 2 mM TUDCA, 0.1 µM TM, 0.1 µM TM
and 0.25 mM PBA or 0.1 µM TM and 2 mM TUDCA for 24 h. Untreated and
DMSO-treated SRA01/04 cells served as the control groups. Total
protein was subsequently extracted from the cells using
radioimmunoprecipitation assay buffer. Protein samples (30 µg
protein/lane) were separated via SDS-PAGE on a 12% gel. The
separated proteins were subsequently transferred onto a
polyvinylidene fluoride membrane and blocked for 90 min with 5%
skim milk in TBST (0.05% Tween) at room temperature. The membrane
was incubated with primary antibodies against GRP78, ATF6, ATF4,
P-eIF2α, P-IRE1α, fibronectin, vimentin, α-SMA, N-cadherin,
E-cadherin and β-actin (all 1:1,000) overnight at 4°C. Following
the primary incubation, membranes were incubated with horseradish
peroxidase-conjugated horse anti-mouse or horse anti-rabbit
secondary antibodies at room temperature for 90 min. The membrane
was then incubated with enhanced chemiluminescence substrates
(ECL-WBKLS0100; EMD Millipore) at room temperature for 2 min and
the protein bands were visualized using a ChemiDoc XRS+ imager.
Protein expression was quantified using Image Lab software
(Imagelab 4.0) with β-actin as the loading control.
Immunofluorescence analysis
SRA01/04 cells were treated as aforementioned in the
previous paragraph (western blot analysis) and 4×105
cells were seeded onto cover slips in a 12-well plate and cultured
for 24 h. Cells were subsequently fixed with ice-cold acetone for
15 min, permeabilized with 0.5% Triton X-100 for 5 min and blocked
with 1% bovine serum albumin in PBS for 90 min at room temperature.
The cells were incubated with primary antibodies against
fibronectin, vimentin, α-SMA and N-cadherin (all 1:200) overnight
at 4°C. Subsequently, the cells were washed with PBS containing
0.1% Tween-20 at room temperature and then incubated with Alexa
Fluor 488-conjugated goat anti-rabbit or Alexa Fluor 488-conjugated
goat anti-mouse secondary antibodies (1:5,000) at room temperature
for 90 min. VECTASHIELD Mounting Medium with DAPI (H1200, Vector
Laboratories, Inc.) was used for DAPI staining at room temperature.
Cells were observed under a fluorescence microscope (AX70; Olympus
Corporation; magnification, ×20). The relative fluorescence
intensity of the specific proteins was quantified in 5
randomly-selected fields using ImageJ software 1.8.0 (National
Institutes of Health), and normalized to the total number of cells.
Data from 3 images per group were used for statistical
analysis.
Wound-healing assay
A wound-healing assay was performed to assess the
migration ability of SRA01/04 cells under specific ER stress
conditions. A serum starving method was used for minimizing cell
proliferation in the wound-healing assay. Dulbecco's Modified Eagle
Medium supplemented with 0.5% fetal bovine serum and 1%
penicillin/streptomycin was used for culturing the cells at 37°C.
The cells were treated with 0.01 µM TG, 0.1 µM TM, 0.01 µM TG and
0.25 mM or 0.1 µM TM and 0.25 mM PBA for 24 h. Untreated and
DMSO-treated SRA01/04 cells served as the control groups.
Sterilized pipette tips were used to create a wound across the
confluent cell monolayer, and debris was removed with PBS.
Migration of the cells into the wound was observed after 24 h in 5
randomly-selected fields under an inverted phase-contrast
microscope at magnification, ×40. Cell migration was analyzed using
ImageJ software (National Institutes of Health) and was expressed
as the repair rate of scarification, defined as the percentage of
the gap relative to the total area of the cell-free region
immediately following wounding. Experiments were performed in
triplicate at least 3 times.
Statistical analysis
The results are expressed as the mean ± standard
deviation of at least 3 independent experiments. Statistical
analysis was performed using GraphPad Prism software (v7; GraphPad
Software, Inc.). A one-way analysis of variance followed by Tukey's
post hoc test was used to compare the different groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
HLEC morphology is altered following
prolonged ER stress
In order to investigate the effect of ER stress on
cell morphology and alignment, SRA01/04 cells were treated with
DMSO, TG and a combination of TG and PBA. Representative images of
SRA01/04 cells from each treatment group are presented in Fig. 1A. Compared with the other groups,
TG-treated cells had a spindle-like appearance and an elongated
long axis (Fig. 1B). The results
indicated that activation of ER stress by TG had a significant
effect on SRA01/04 cell elongation, and that this effect was
eliminated by the ER stress inhibitor PBA. The morphology of
TG-treated cells resembled that of fiber cells rather than
epithelial cells.
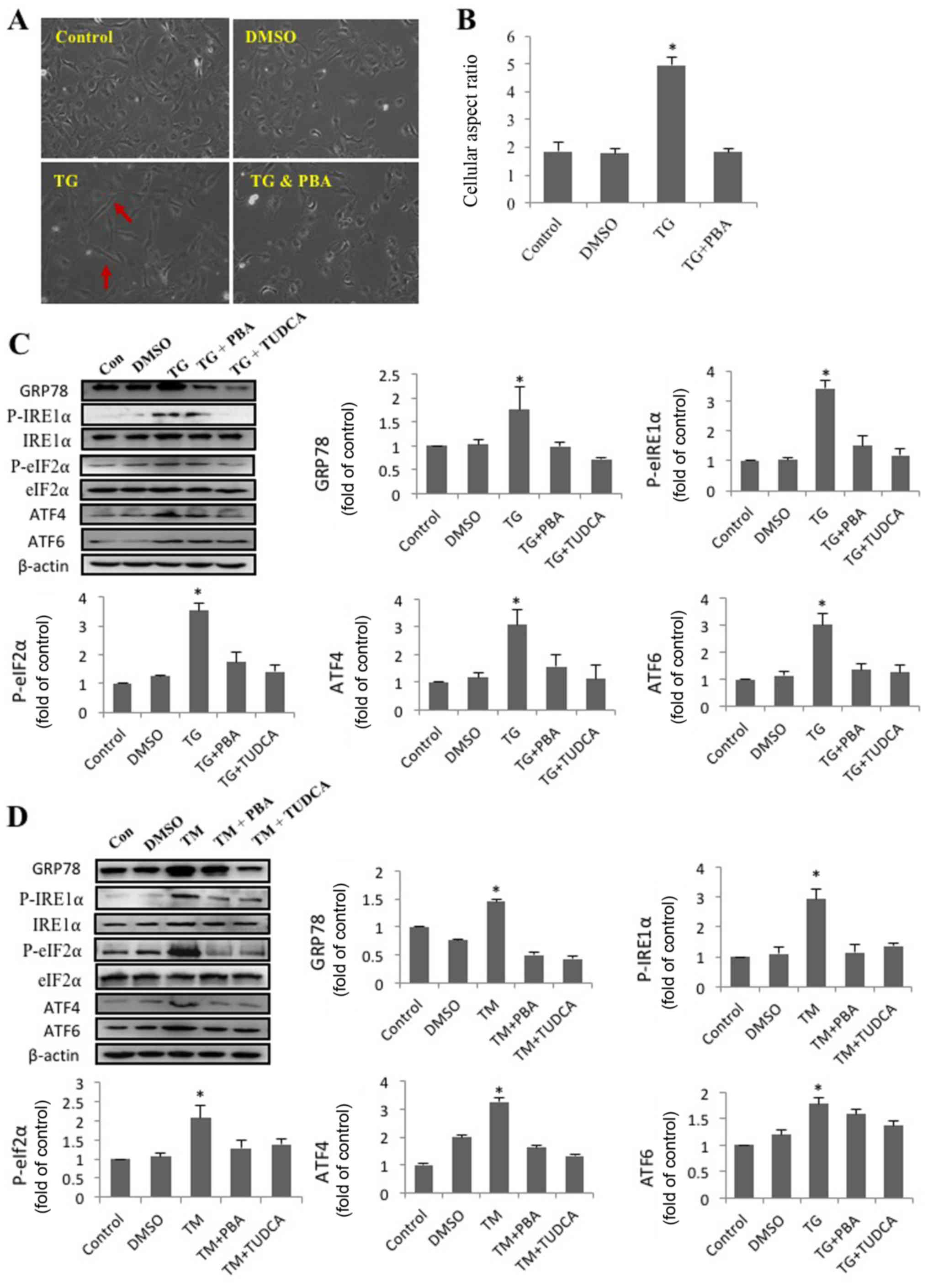 | Figure 1.SRA01/04 cells treated with
endoplasmic reticulum stress inducers exhibit a morphological
change, from an epithelial- to a fibroblast-like morphology.
SRA01/04 cells were treated with 0.01 µM TG or a combination of
0.01 µM TG and 0.25 mM PBA for 24 h. Untreated SRA01/04 cells
served as the control group. (A) Micrographs were obtained under an
inverted phase-contrast microscope (magnification, ×20). The red
arrows indicate that TG-treated cells had a spindle-like appearance
and an elongated long axis. (B) The cellular aspect ratio was
analyzed with ImageJ software (n=3). *P<0.05 vs. control.
SRA01/04 cells were treated with 0.01 µM TG, 0.01 µM TG and 0.25 mM
PBA, 0.01 µM TG and 2 mM TUDCA, 0.1 µM TM, 0.1 µM TM and 0.25 mM
PBA or 0.1 µM TM and 2 mM TUDCA for 24 h. (C) ER stress markers
were determined by western blot analysis. (D) The expression levels
of GRP78, p-IRE1α, p-eIF2α, ATF4 and ATF6 were quantified by
densitometry (n=3). *P<0.05 vs. control. TG, thapsigargin; TM,
tunicamycin; PBA, 4-phenylbutyric acid; TUDCA,
tauroursodeoxycholate; GRP78, glucose-regulated protein 78 kDa;
P-IRE1α, phosphorylated inositol-requiring protein 1α; P-eIf2α,
phosphorylated eukaryotic initiation factor 2α; ATF6, activating
transcription factor 6; ATF4, activating transcription factor 4;
DMSO, dimethyl sulfoxide; Con, control. |
TM and TG trigger ER stress in
HLECs
The present study revealed that TG altered the
morphology of SRA01/04 cells and that this effect was inhibited by
PBA. ER stress following treatment with TM, TG, PBA or TUDCA was
subsequently investigated. Western blot analysis was performed to
determine the levels of proteins associated with 3 pathways of the
UPR. The expression levels of GRP78, p-eIf2α, ATF6, ATF4 and
P-IRE1α expression in SRA01/04 cells were significantly increased
following treatment with TM or TG and significantly decreased
following treatment PBA or TUDCA (Fig.
1C and D). The results suggested that ER stress was triggered
by TM or TG and inhibited by PBA or TUDCA.
ER stress facilitates EMT in
HLECs
To address the effect of ER stress on EMT, SRA01/04
cells were exposed to the classic ER stress inducers TM or TG alone
or in combination with classic ER stress inhibitors PBA or TUDCA
for 24 h. The expression of fibronectin, vimentin, α-SMA,
N-cadherin, E-cadherin and β-actin was measured by western blot
analysis. Compared with the control groups, N-cadherin, vimentin,
α-SMA and fibronectin protein levels in TM-treated cells were
increased by 1.82-, 1.76-, 2.18- and 2.69-fold, respectively.
Furthermore, the protein level of E-cadherin decreased to 37% of
the control groups (Fig. 2A).
Compared with the control groups, N-cadherin, vimentin, α-SMA and
fibronectin protein levels in TG-treated cells were increased by
2.21-, 1.66-, 2.49- and 1.86-fold. Furthermore, the protein level
of E-cadherin had decreased by 0.61-fold (Fig. 2B). By contrast, SRA01/04 cells
treated with a combination of PBA or TUDCA and TM or TG did not
exhibit significant changes in the expression of the aforementioned
EMT-associated proteins compared with the control groups (Fig. 2A and B). These results indicated
that ER stress triggered EMT in SRA01/04 cells, and
immunofluorescence staining was performed to corroborate these
results. As expected, the average fluorescence intensities
corresponding to fibronectin, vimentin, α-SMA and N-cadherin per
cell were significantly increased in the TM- and TG-treated groups
compared with the control groups (Fig.
3). Furthermore, the increased expression was attenuated in
cells treated with a combination of TM and TUDCA or TG and
TUDCA.
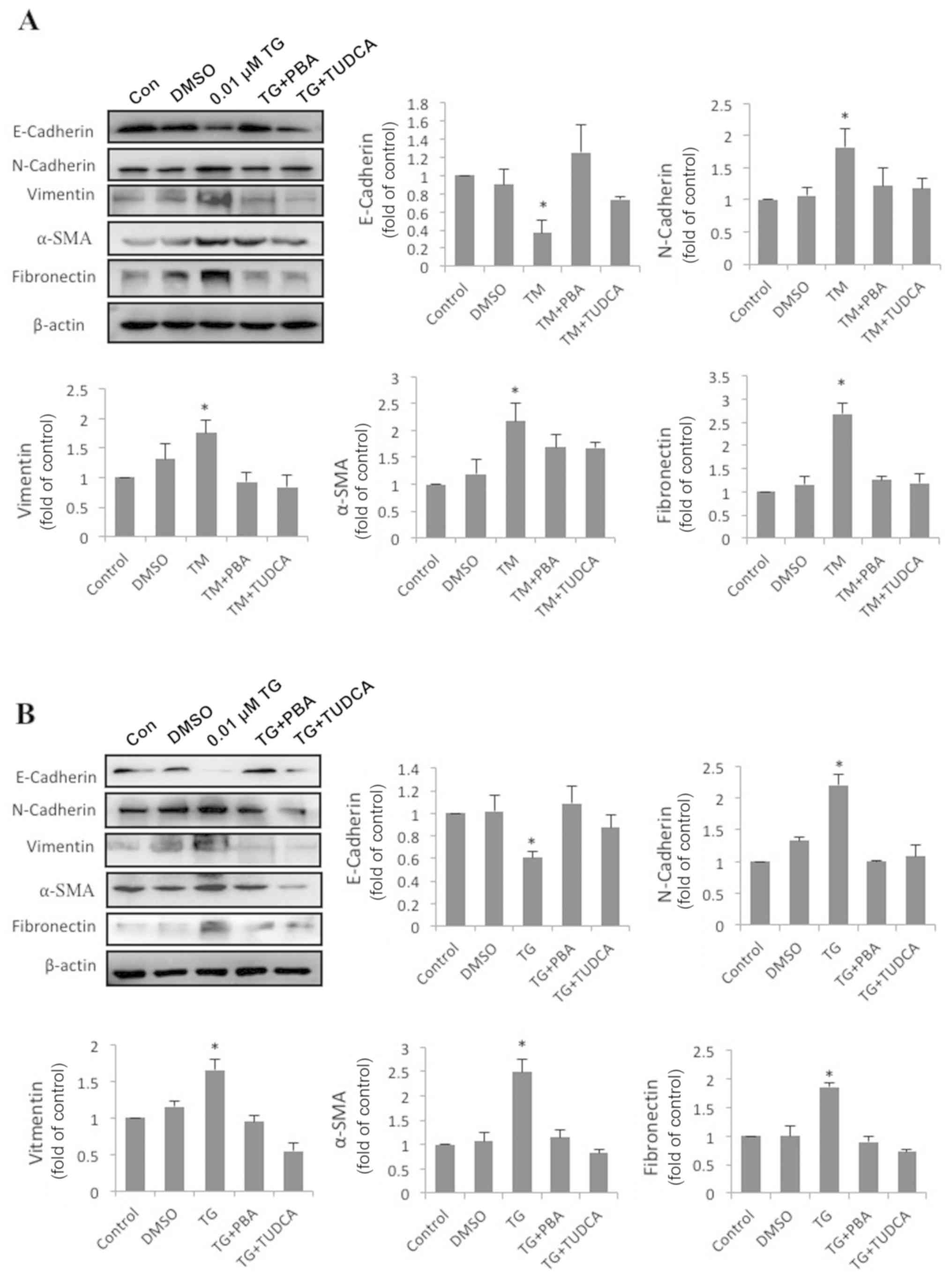 | Figure 2.Enhanced endoplasmic reticulum stress
upregulates EMT-associated protein expression and downregulates
epithelial marker expression. (A) SRA01/04 cells were treated with
0.1 µM TM, 0.1 µM TM and 0.25 mM PBA or 0.1 µM TM and 2 mM TUDCA
for 24 h. (B) SRA01/04 cells were treated with 0.01 µM TG, 0.01 µM
TG and 0.25 mM PBA or 0.01 µM TG and 2 mM TUDCA for 24 h. The
expression levels of the EMT-associated markers fibronectin,
vimentin, α-SMA, N-cadherin and E-cadherin were measured by western
blot analysis (n=3). *P<0.05 vs. control. EMT,
epithelial-to-mesenchymal transition; TG, thapsigargin; TM,
tunicamycin; PBA, 4-phenylbutyric acid; TUDCA,
tauroursodeoxycholate; α-SMA, α-smooth muscle actin. N-cadherin,
neural cadherin; E-cadherin, epithelial cadherin; DMSO, dimethyl
sulfoxide; Con, control. |
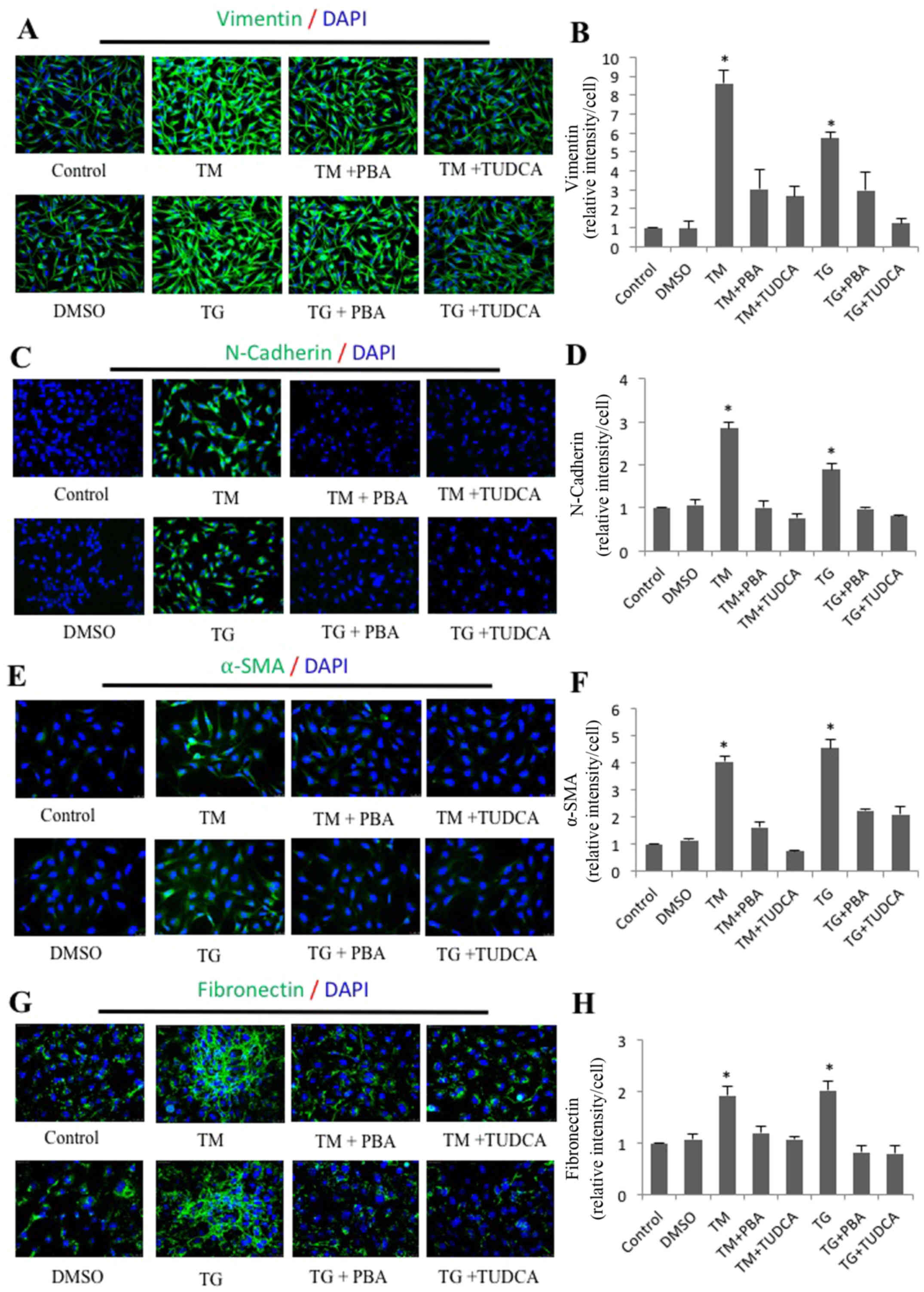 | Figure 3.Immunofluorescence analysis of
endoplasmic reticulum stress-induced expression of vimentin,
N-cadherin, α-SMA and fibronectin in SRA01/04 cells. SRA01/04 cells
were treated with 0.01 µM TG, 0.01 µM TG and 0.25 mM PBA, 0.01 µM
TG and 2 mM TUDCA, 0.1 µM TM, 0.1 µM TM and 0.25 mM PBA or 0.1 µM
TM and 2 mM TUDCA for 24 h. The expression of (B) vimentin, (D)
N-cadherin, (F) α-SMA and (H) fibronectin was determined by
immunofluorescence analysis. The average fluorescence of (A)
vimentin, (C) N-cadherin, (E) α-SMA and (G) fibronectin was
quantified (n=3). *P<0.05 vs. control, magnification, ×20.
α-SMA, α-smooth muscle actin; N-cadherin, neural cadherin;
E-cadherin; epithelial cadherin; TG, thapsigargin; TM, tunicamycin;
PBA, 4-phenylbutyric acid; TUDCA, tauroursodeoxycholate. |
ER stress enhances migration of
HLECs
The wound-healing assay revealed that ER stress
promoted the migration of SRA01/04 cells (Fig. 4A and B). The migration of SRA01/04
cells was significantly increased in TM- or TG-treated cells
compared with the untreated control group. The repair rate of
scarification of the TM- and TG-treated cells after 24 h was 60 and
65%, respectively. However, cells treated with a combination of TM
and PBA or TG and PBA did not exhibit a significant difference in
the repair rate of scarification compared with the control group.
The DMSO-treated cells served as the negative control. No
significant differences were observed between the DMSO-treated
cells and the untreated cells.
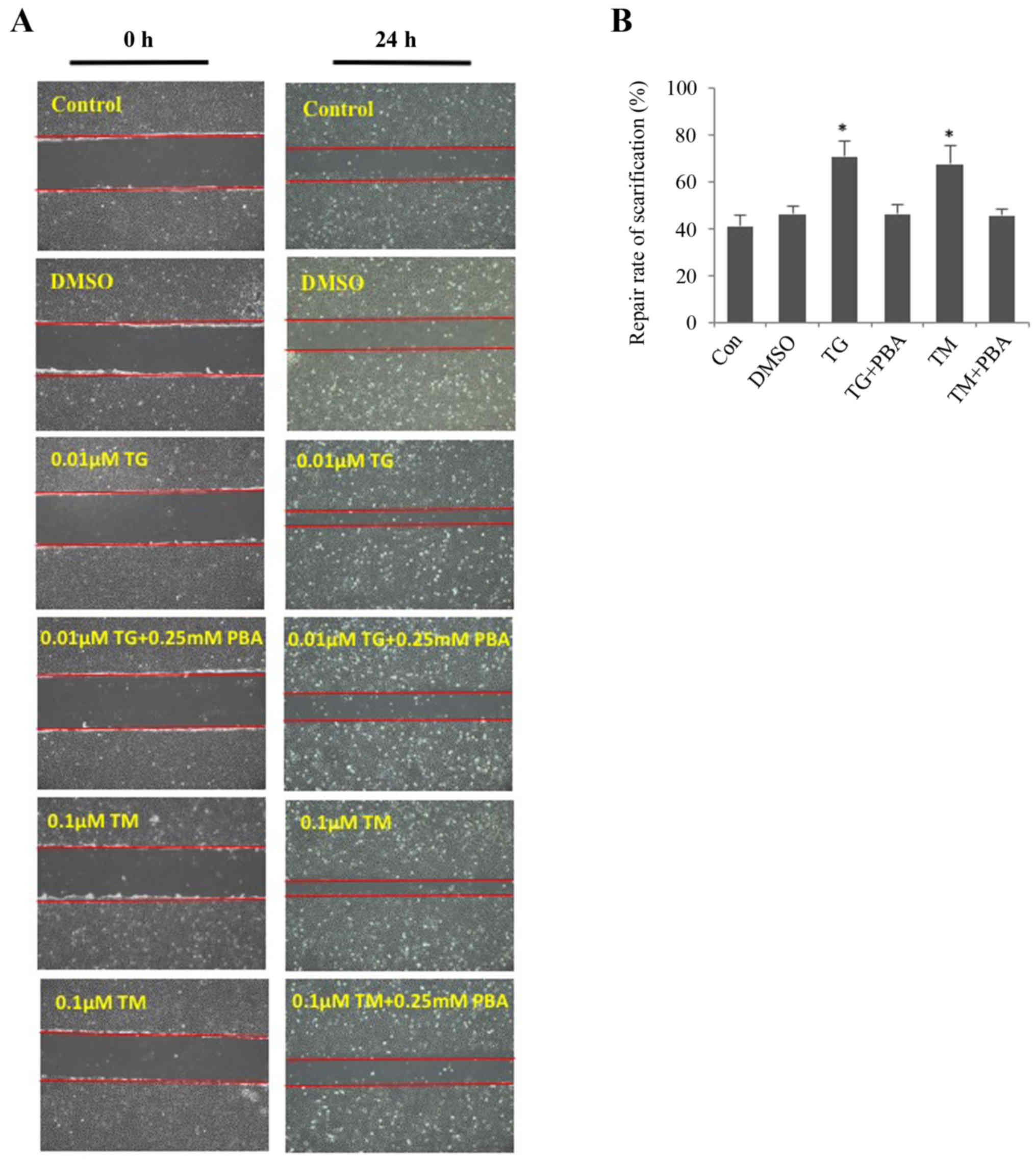 | Figure 4.Endoplasmic reticulum stress
facilitates the cell migration of SRA01/04 cells. SRA01/04 cells
were treated with 0.01 µM TG, 0.1 µM TM, 0.01 µM TG and 0.25 mM or
0.1 µM TM and 0.25 mM PBA for 24 h and were subsequently subjected
to a wound healing assay. Untreated and dimethyl sulfoxide-treated
SRA01/04 cells served as the control groups. (A) Cells that
migrated into the wounded area from the border of the wound after
24 h were visualized and images were captured under an inverted
phase-contrast microscope (magnification, ×10). (B) Cell migration
was used to calculate the repair rate of scarification, expressed
as the percentage of the gap relative to the total area of the
cell-free region, using ImageJ software (n=3). *P<0.05 vs.
control. TG, thapsigargin; TM, tunicamycin; PBA, 4-phenylbutyric
acid; TUDCA, tauroursodeoxycholate; α-SMA, α-smooth muscle actin;
DMSO, dimethyl sulfoxide; Con, control. |
Discussion
The present study investigated the role of ER stress
on EMT in HLECs. ER stress triggers a response termed the UPR,
which serves to decrease cell stress (17). The UPR is activated following
cellular stress in several disorders, including cataracts (18). A previous study revealed that ER
stress and the UPR participate in the formation of several types of
cataracts (19). In the present
study, TM and TG were used to activate the UPR, and PBA and TUDCA
were used to inhibit its activation. TM- or TG-treated SRA01/04
cells exhibited increased GRP78, p-eIf2α, ATF6, ATF4 and P-IRE1α
expression levels compared with the control groups, indicating that
3 UPR pathways were activated. Conversely, treatment with PBA or
TUDCA decreased the expression of these proteins. The results
obtained in the present study are in concordance with the
well-known effects of TM, TG, PBA and TUDCA on HLECs.
During EMT, epithelial cancer cells lose polarity
and cell-cell adhesion while gaining a more mesenchymal and
invasive phenotype. Additionally, the cells appear to adopt a
fibroblastic spindle-like morphology (7,20).
The present study revealed that SRA01/04 cells exhibited a more
spindle-like morphology following treatment with TG. Additionally,
the long axis of the cells was significantly elongated compared
with the control group.
The present study subsequently investigated whether
chemically-induced ER stress may have an effect on EMT in SRA01/04
cells. Typical markers for EMT include the downregulation of
E-cadherin and the upregulation of N-cadherin, vimentin,
fibronectin and α-SMA (21).
Previous studies revealed that ER stress and EMT markers are
commonly observed under stress conditions in multiple tissues,
including the lungs, liver and kidneys (22–26).
The western blot analysis and immunofluorescence staining performed
in the present study revealed a decreased expression level of
E-cadherin and increased expression levels of N-cadherin, vimentin,
fibronectin and α-SMA in TM- or TG-treated SRA01/04 cells compared
with the controls. However, these changes in expression were not
significant when the disruption of ER stress was inhibited by TUDCA
or PBA, suggesting that ER stress may serve regulatory role in
EMT.
Cataract surgery is an effective treatment strategy;
however, it is not possible to completely remove the lens
epithelial cells attached to an intact lens capsule. A number of
previously published studies suggested that EMT is implicated in
the pathogenesis of fibrotic posterior capsular opacification (PCO)
(27,28). Therefore, an increased
understanding of how cataract surgery initiates EMT and how to
modulate EMT following cataract surgery is required. The present
study investigated the migration of SRA01/04 cells under conditions
that regulate ER stress. The results revealed that TM- or
TG-treated cells exhibited enhanced migration compared with the
control groups, an effect that was abrogated in the presence of
TUDCA or PBA.
In summary, the results from the present study
suggested that ER stress is an important regulator of EMT in HLECs.
Therefore, suppressing ER stress and regulating the EMT may serve
as a novel therapeutic strategy to reduce PCO following cataract
surgery.
Acknowledgements
The authors would like to thank Professor Fu Shang
(Zhongshan Ophthalmic Center) who provided the human lens
epithelial cell line SRA01/04 for the present study.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500742) awarded to
JY, the Science and Technology Foundation of Guangdong Province of
China (grant no. 2017A020215187) awarded to JY and the Natural
Science Research Foundation of Guangdong Province of China (grant
nos. 2017A030313614 and 2018A030313117) awarded to SZ.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ performed the western blot analysis, statistical
analysis and participated in drafting the manuscript. JY performed
the molecular experiments, participated in the design of the study,
revised the statistical analysis and drafted the manuscript. MW
performed the immunofluorescence staining and statistical analysis.
DZ participated in designing the study and culturing the cells, and
was responsible for revising the statistical analysis. YL was
involved in designing the study and revising the manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HLECs
|
human lens epithelial cells
|
|
ER stress
|
endoplasmic reticulum stress
response
|
|
ER
|
endoplasmic reticulum
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
PCO
|
posterior capsular opacification
|
|
UPR
|
unfolded protein response
|
|
α-SMA
|
α-smooth muscle actin
|
|
GRP78
|
glucose-regulated protein 78
|
|
IRE1α
|
phosphorylated inositol-requiring
protein 1α
|
|
eIf2α
|
phosphorylated eukaryotic initiation
factor 2α
|
|
p-
|
phosphorylated
|
|
ATF6
|
activating transcription factor 6
|
|
ATF4
|
activating transcription factor 4
|
|
TG
|
thapsigargin
|
|
TM
|
tunicamycin
|
|
PBA
|
4-phenylbutyric acid
|
|
TUDCA
|
tauroursodeoxycholate
|
References
|
1
|
Spalton D: Posterior capsule
opacification: Have we made a difference? Br J Ophthalmol. 97:1–2.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaumberg DA, Dana MR, Christen WG and
Glynn RJ: A systematic overview of the incidence of posterior
capsule opacification. Ophthalmology. 105:1213–1221. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Apple DJ, Escobar-Gomez M, Zaugg B,
Kleinmann G and Borkenstein AF: Modern cataract surgery: Unfinished
business and unanswered questions. Surv Ophthalmol. 56 (6
Suppl):S3–S53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mamuya FA, Wang Y, Roop VH, Scheiblin DA,
Zajac JC and Duncan MK: The roles of alphaV integrins in lens EMT
and posterior capsular opacification. J Cell Mol Med. 18:656–670.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wormstone IM and Eldred JA: Experimental
models for posterior capsule opacification research. Exp Eye Res.
142:2–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeichi M: Cadherin cell adhesion
receptors as a morphogenetic regulator. Science. 251:1451–1455.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lovicu FJ, Shin EH and McAvoy JW: Fibrosis
in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT
leading to cataract. Exp Eye Res. 142:92–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erler P and Monaghan JR: The link between
injury-induced stress and regenerative phenomena: A cellular and
genetic synopsis. Biochim Biophys Acta. 1849:454–461. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Hussuna A, Qvist N, Zangenberg MS,
Langkilde A, Siersma V, Hjort S and Gögenur I: No effect of
anti-TNF-a agents on the surgical stress response in patients with
inflammatory bowel disease undergoing bowel resections: A
prospective multi-center pilot study. BMC Surg. 18:912018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakka VP, Prakash-Babu P and Vemuganti R:
Crosstalk between endoplasmic reticulum stress, oxidative stress,
and autophagy: Potential therapeutic targets for acute CNS
injuries. Mol Neurobiol. 53:532–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen X, Xue Y, Si Y, Wang Q, Wang Z, Yuan
J and Zhang X: The unfolded protein response potentiates
epithelial-to-mesenchymal transition (EMT) of gastric cancer cells
under severe hypoxic conditions. Med Oncol. 32:4472015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin HS, Ryu ES, Oh ES and Kang DH:
Endoplasmic reticulum stress as a novel target to ameliorate
epithelial-to-mesenchymal transition and apoptosis of human
peritoneal mesothelial cells. Lab Invest. 95:1157–1173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lindholm D, Korhonen L, Eriksson O and
Kõks S: Recent Insights into the role of unfolded protein response
in ER stress in health and disease. Front Cell Dev Biol. 5:482017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Zhou S, Gu J, Wang Y, Guo M and
Liu Y: Differences in unfolded protein response pathway activation
in the lenses of three types of cataracts. PLoS One.
10:e01307052015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Puls TJ, Tan X, Whittington CF and
Voytik-Harbin SL: 3D collagen fibrillar microstructure guides
pancreatic cancer cell phenotype and serves as a critical design
parameter for phenotypic models of EMT. PLoS One. 12:e01888702017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y,
Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, et al:
Role of endoplasmic reticulum stress in epithelial-mesenchymal
transition of alveolar epithelial cells: Effects of misfolded
surfactant protein. Am J Respir Cell Mol Biol. 45:498–509. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pallet N: New insights on stress-induced
epithelial phenotypic changes. Nephrol Dial Transplant. 27:483–485.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanjore H, Cheng DS, Degryse AL, Zoz DF,
Abdolrasulnia R, Lawson WE and Blackwell TS: Alveolar epithelial
cells undergo epithelial-to-mesenchymal transition in response to
endoplasmic reticulum stress. J Biol Chem. 286:30972–30980. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawson WE, Crossno PF, Polosukhin VV,
Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB,
et al: Endoplasmic reticulum stress in alveolar epithelial cells is
prominent in IPF: Association with altered surfactant protein
processing and herpesvirus infection. Am J Physiol Lung Cell Mol
Physiol. 294:L1119–L1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shah PP, Dupre TV, Siskind LJ and Beverly
LJ: Common cytotoxic chemotherapeutics induce
epithelial-mesenchymal transition (EMT) downstream of ER stress.
Oncotarget. 8:22625–22639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Iongh RU, Wederell E, Lovicu FJ and
McAvoy JW: Transforming growth factor-beta-induced
epithelial-mesenchymal transition in the lens: A model for cataract
formation. Cells Tissues Organs. 179:43–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hales AM, Schulz MW, Chamberlain CG and
McAvoy JW: TGF-beta 1 induces lens cells to accumulate alpha-smooth
muscle actin, a marker for subcapsular cataracts. Curr Eye Res.
13:885–890. 1994. View Article : Google Scholar : PubMed/NCBI
|


















