Introduction
Silicone mammary implants (SMI) have been widely
used for breast augmentation worldwide, making these materials the
most commonly consumed in aesthetic surgery (1–3). It
has been reported that approximately two million breast
augmentation procedures are performed annually worldwide (4). Systemic and local complications after
SMI breast augmentation surgery are often observed in patients,
among which capsular contracture is the most common complication,
with an incidence of >20% (5,6).
Capsular contracture is a serious complication and
may resulted in distorted breast shape, pain and other symptoms
(5,6). Based on clinical presentation,
capsular contracture after breast augmentation is classified into 4
stages by the Baker grading system (7). Although a number of strategies have
been proposed to prevent capsular contracture, such as different
implant choices and improved surgical technique, the prevalence of
this complication has not yet decreased (8). One strategy has been to
preferentially choose textured materials over smooth breast
implants (9,10). However, a fraction of patients
would eventually undergo re-operation for capsular contraction,
especially those with Baker-IV capsules, despite application of
textured materials (9,10). As capsular contracture is a
consequence of inflammation induced by the implanted silicone
material surface (5,6), it is important to understand the
mechanism of capsular contracture formation and the corresponding
prophylaxis.
Fibroblasts and macrophages are major components in
fibrotic capsules related to breast implants (10). Fibroblasts play a critical role in
the pathogenesis of fibrotic capsules. For example, fibroblasts are
enriched at the ‘contact zone’ of the implant and at the capsule
responsible for initial capsule formation, by producing collagen
fibres (11–14). The abundance of fibroblasts is
positively associated with the severity of capsular contracture
measured by the Baker grade (15).
On the other hand, macrophages are key players in initiating tissue
repair and remodelling (16). As
it has been reported that macrophages enter the wound before
fibroblasts do (17,18), it is possible that macrophages may
have an influence on fibroblast functions. However, the crosstalk
between macrophages and fibroblasts has not been explored.
Histamine was recognized to promote collagen
formation more than four decades ago (19). Although it has been reported that
blocking of histamine receptor (HR) 1 and 2 has inhibitory roles in
collagen formation, these studies primarily focused on mast cells
(15,20). Therefore, whether other cell types
are involved in histamine-mediated collagen formation remains
unknown. The aim of the present study was to assess the hypothesis
that the HR2 inhibitor roxatidine can prevent capsular contracture
after implantation. In this study, the effect of roxatidine on
macrophages and fibroblasts during the pathogenesis of capsule
formation was investigated.
Materials and methods
Ethics consideration
The ethics committee of the Fourth Affiliated
Hospital of Harbin Medical University approved and supervised the
research proposal (approval number, 170023).
Silicone surface particles
Spherical particles with a diameter of 6 mm were
prepared from silicone implant envelopes under sterile conditions.
The two most common materials in clinical practice were utilised,
Mentor® Perthese™ micro-textured (MT) and smooth (SM)
breast implants (21). Both
implant materials were purchased from Johnson & Johnson,
Inc.
Treatment
Roxatidine acetate (hydrochloride) was purchased
from Sihuan Pharmaceutical Co., Ltd. For in vitro studies,
roxatidine was dissolved in 0.05% DMSO (diluted in PBS). For in
vivo studies, roxatidine was mixed with autoclaved tap water in
a bolus of 100 µl.
Cell lines and culture
The murine macrophage cell line RAW 264.7 (ATCC) and
the fibroblast cell line L929 (ATCC) were utilized in the present
study. Both cell lines were cultured in α-Modified Eagle's Medium
(α-MEM; Thermo Fisher Scientific, Inc.) supplemented with
L-glutamine (Thermo Fisher Scientific, Inc.) and 10% fetal bovine
serum (Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. For pre-treatment with roxatidine, roxatidine (25
µM) was added to the culture medium for 1 h at 37°C (22). For controls, vehicle (0.05% DMSO)
was added to the control wells for 1 h at 37°C. Subsequently, RAW
264.7 macrophages (1×104) were co-cultured with
different silicone surface particles for 24 h at 37°C, and then the
conditioned media was collected for future L929 stimulation.
Stimulated RAW 264.7 cells were then cultured in serum-free medium
for another 24 h at 37°C, by the end of which the cells and media
were used for reverse transcription-quantitative PCR (RT-qPCR) and
ELISA analyses, respectively. Cells in the wells without addition
of silicone implant surface materials served as controls. Cells
were collected by straining culture media through a 100 µm cell
strainer. Following centrifugation at 400 × g for 10 min at 4°C,
cell-free culture media were used for ELISA analyses, whereas cells
were lysed for RT-qPCR analyses. Following the 1 h culture in the
presence or absence of roxatidine, L929 fibroblast cells
(1×104) were co-cultured with different silicone surface
particles or the macrophage-conditioned media for 24 h at 37°C. For
culture of L929 cells using macrophage-conditioned media, at the
end of the 24 h culture the media was replaced with serum-free
media for another 24 h at 37°C, to perform ELISA and RT-qPCR
analyses. For L929 proliferation analyses, implant materials were
added to the wells, and fresh complete α-MEM was changed every 24 h
at 37°C. To neutralize effects of TGFβ, TGFβ neutralizing
antibodies (10 µg/ml, R&D Systems, Inc., cat. no. AB-100-NA)
were applied to the culture medium when the breast implant
materials were added or the conditional medium was used for L929
cells; whereas isotype control antibodies (Rabbit IgG, 10 µg/ml,
R&D Systems, Inc., cat. no. AB-105-C) were used as control.
Experiments (n=6 wells/group) were conducted in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from RAW 264.7 and L929
cells using a commercial kit (RNeasy Mini kit; Qiagen GmbH)
following the manufacturer's instructions. All RNA samples were
stored at −80°C until analysis. Total RNA (2 µg) was
reverse-transcribed to cDNA using an RT kit (High-Capacity cDNA
Reverse Transcription kit, Thermo Fisher Scientific, Inc.),
according to the manufacturer's manual. qPCR for target genes and
the housekeeping gene GAPDH were performed using the
SYBR® Green Master mix (Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Relative mRNA expression
values were normalized according to levels of GAPDH. The fold
change of mRNA expression was calculated using the formula:
2−ΔΔCq (23). The
sequences of primers used are listed in Table I.
 | Table I.Primers used for RT-qPCR. |
Table I.
Primers used for RT-qPCR.
|
| Primers
(5′→3′) |
|---|
|
|
|
|---|
| Target gene | Forward | Reverse |
|---|
| IL1β |
AACCTGCTGGTGTGTGACGTTC |
CAGCACGAGGCTTTTTTGTTGT |
| IL6 |
GACAAAGCCAGAGTCCTTCAGAGAG |
CTAGGTTTGCCGAGTAGATCTC |
| TNFα |
GCCTCTTCTCATTCCTGCTTG |
CTGATGAGAGGGAGGCCATT |
| TGFβ |
GCTAATGGTGGACCGCAACAACG |
CTTGCTGTACTGTGTGTCCAGGC |
| GAPDH |
TTCTTGTGCAGTGCCAGCCTCGTC |
TAGGAACACGGAAGGCCATGCCAG |
ELISA
ELISA was performed using R&D Systems
DuoSet® kits equipped with corresponding mouse
antibodies (R&D Systems, Inc., cat. nos.: IL1β, DY407; IL6,
DY406; TNFα, DY470; and TGFβ, DY1679), according to the
manufacturer's instructions. Briefly, a 96-well ELISA plate was
incubated with the capture antibody (based on the target protein)
overnight at room temperature. Following thorough washes using the
Wash Buffer (R&D Systems, Inc., cat. no. WA126) the plate was
blocked using 10% FBS at room temperature for 1 h. Cell culture
media and standard samples were then added to the plate, followed
by incubation at room temperature for 2 h. Biotinylated detection
antibodies (provided with the DuoSet®kits) were then
applied to the plate. Subsequently, the streptavidin-horseradish
peroxidase solution was added and incubated for 20 min at room
temperature, followed by the substrate solution for 20 min at room
temperature. After adding the stop solution, the optical density of
each well was detected by a microtiter plate reader at a wavelength
of 450 nm.
Flow cytometry
At the end of culture, cells were collected from
cell culture plates using 0.5 mM EDTA (Thermo Fisher Scientific,
Inc.). Cells originating from tissue surrounding breast implant
materials in mice were obtained using mechanical dissociation and
enzymatic digestion, according to a previous paper (24). Cells were subsequently stained with
antibodies listed in Table II for
30 min at 4°C, at the designated dilutions. To analyze
proliferation, cells were stained with anti-Ki67 antibodies. To
analyze signalling, RAW 264.7 macrophages were collected. Briefly,
RAW 264.7 macrophages were cultured in serum-free media at 37°C for
24 h. Roxatidine (25 µM) was subsequently added to the media 1 h
prior to stimulation with silicone surface materials. Macrophages
were collected for analysis of phosphorylation of NF-κB subunit p65
and p38 MAPK by flow cytometry 15 minutes after adding silicone
surface materials to culture media. The primary gate of cells was
set for viable cells according to the plots of forward scatter side
scatter. Corresponding isotype control antibodies were used for
setting the negative population. Cells were analyzed using an
Attune NxT flow cytometer (Thermo Fisher Scientific, Inc.) and
Kaluza software (version 2.1; Beckman Coulter, Inc.).
 | Table II.Antibodies used for flow
cytometry. |
Table II.
Antibodies used for flow
cytometry.
| Antibody | Manufacturer | Cat. no. | Clone | Dilution |
|---|
| Ki67-PE | Abcam | Ab16667
(self-conjugated) | Polyclonal | 1:100 |
| p-p65-PE | eBioscience; Thermo
Fisher Scientific, Inc. | MA5-15160
(self-conjugated) | T.849.2 | 1:100 |
| p-p38-PE | eBioscience; Thermo
Fisher Scientific, Inc. | MA5-15218
(self-conjugated) | C.7.8 | 1:100 |
| αSMA-PE | Abcam | Ab7817
(self-conjugated) | 1A4 | 1:100 |
Mouse breast implant studies
Prepared breast implant surface materials were
seeded into 8-week old Balb/c female mice (weight ~20 g, Jackson
Laboratory), according to a published paper (25). Mice were maintained in the
specific-pathogen-free environment (control at constant 23°C and
40–60% humidity) with a 12-h light/dark cycle. Normal chow and
water were allowed ad libitum. General anesthesia was
administered by intraperitoneal injection of one dose of ketamine
hydrochloride (100 mg/kg) and xylazine hydrochloride (10 mg/kg;
Sihuan Pharmaceutical Co., Ltd.). Animals were maintained on a
heated blanket at 37°C. Each mouse received one piece of implant
material (up to 6 mm in diameter) in a subcutaneous pocket
surgically created in the left flank and the incision was closed
using 6-0 Vicryl sutures (Ethicon, Inc.). No animals died during
the experimental period. Starting the day after surgery for 14
days, implant-bearing mice were treated daily by oral gavage with
roxatidine (15.62 mg/kg) or PBS (control) (26). At day 90 post-surgery, mice were
sacrificed under anesthesia and the fat tissue surrounding the
implant material (5 mm distance from the implant surface) was
collected for flow cytometry analysis of fibroblast abundance.
Experiments (n=9 mice/group) were performed in duplicate.
Statistical analysis
Data are presented as the mean ± SD. Data were
compared using the Student's t-test for two groups or one-way ANOVA
followed by a Bonferroni post hoc test for more than two groups.
Data with >2 groups at different time points were analyzed using
two-way ANOVA with Tukey post hoc tests. P<0.05 was considered
to indicate a statistically significant difference. Statistical
analyses were performed using GraphPad Prism software (version 7;
GraphPad Software, Inc.).
Results
Responses of macrophages cultured with
silicone implant surface materials in the presence or absence of
roxatidine
RAW 264.7 murine macrophages (1×104
cells/well) were maintained in 6-well plates containing each of the
aforementioned materials for 24 h at 37 °C. Proinflammatory
cytokines, interleukin (IL) 1β, IL6, tumor necrosis factor α (TNFα)
and transforming growth factor β (TGFβ), have been reported to have
critical roles in fibrosis generation after breast implant surgery
(4,27). Therefore, the levels of these
cytokines were assessed. As shown in Fig. 1A, culture of RAW 264.7 cells with
different silicone implant surface materials increased mRNA levels
of the proinflammatory cytokines assessed. Similar results were
also observed at the protein level (Fig. 1B).
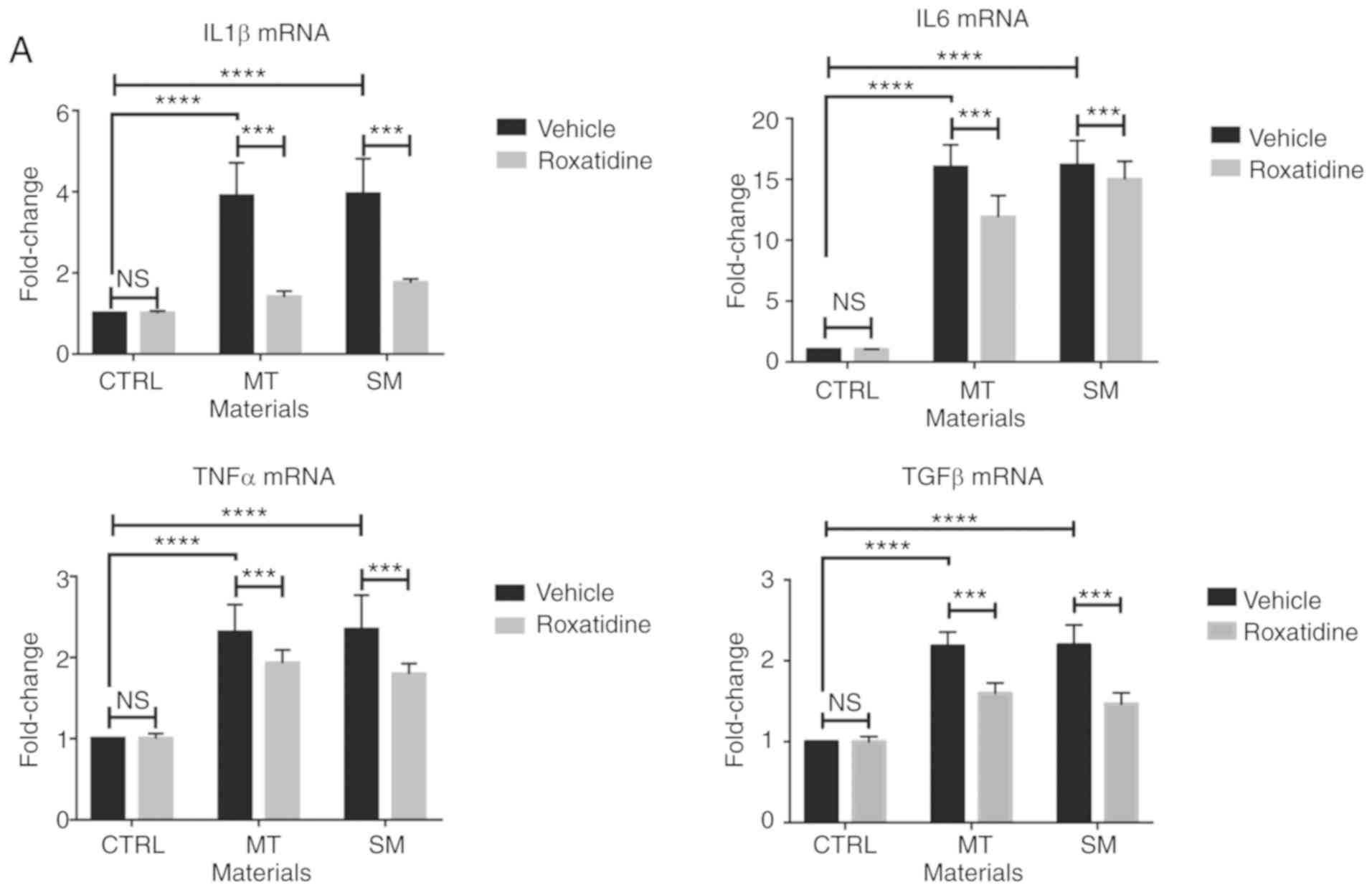 | Figure 1.Pretreatment of roxatidine in the
culture medium of macrophages stimulated with silicone surface
materials reduced the production of proinflammatory cytokines.
Cells were pretreated with roxatidine (25 µM) or vehicle for 1 h at
37°C before adding implant materials. At the end of culture (24 h
after co-culture with implant materials at 37°C), cells were
collected for another 24 h culture in serum-free medium at 37°C for
subsequent RT-qPCR and ELISA. (A) Administration of roxatidine
reduced proinflammatory cytokine production by macrophages
stimulated with breast implant materials at the mRNA level. (B)
Roxatidine decreased secretion of proinflammatory cytokines by
stimulated macrophages. n=6 wells/group, from one of triplicated
experiments. n.s., not significant, **P<0.01, ***P<0.001 and
****P<0.0001, as indicated. Data were analyzed using one-way
ANOVA followed by a Bonferroni post hoc test. RT-qPCR, reverse
transcription-quantitative PCR; IL, interleukin; TNF, tumor
necrosis factor; TGF, transforming growth factor; CTRL, control;
MT, micro-textured; SM, smooth. |
As it has been reported that administration of
roxatidine inhibits macrophage activation upon stimulation
(22), the subsequent
investigation assessed whether roxatidine suppressed secretion of
these proinflammatory cytokines by macrophages in a co-culture
system with silicone implant surface materials. As shown in
Fig. 1A and B, addition of
roxatidine significantly reduced both the mRNA and protein levels
of proinflammatory cytokines (P<0.01), indicating that
roxatidine inhibited silicone implant material induced activation
of macrophages.
Roxatidine has no effect on cytokine
production or proliferation of cultured L929 cells in the presence
of silicone surface materials
Subsequently, the effects of roxatidine on cultured
L929 fibroblast cells in the presence or absence of silicone
surface materials were investigated. As shown in Fig. 2A and B, addition of silicone
surface materials did not affect the production of TGFβ at either
the mRNA or protein level. Moreover, roxatidine treatment did not
influence production of TGFβ by L929 fibroblast cells. Similarly,
the levels of other proinflammatory cytokines did not change when
L929 fibroblast were stimulated with or without the presence of
roxatidine. The influence of roxatidine on L929 proliferation was
then investigated, as excessive fibrosis caused by fibroblast
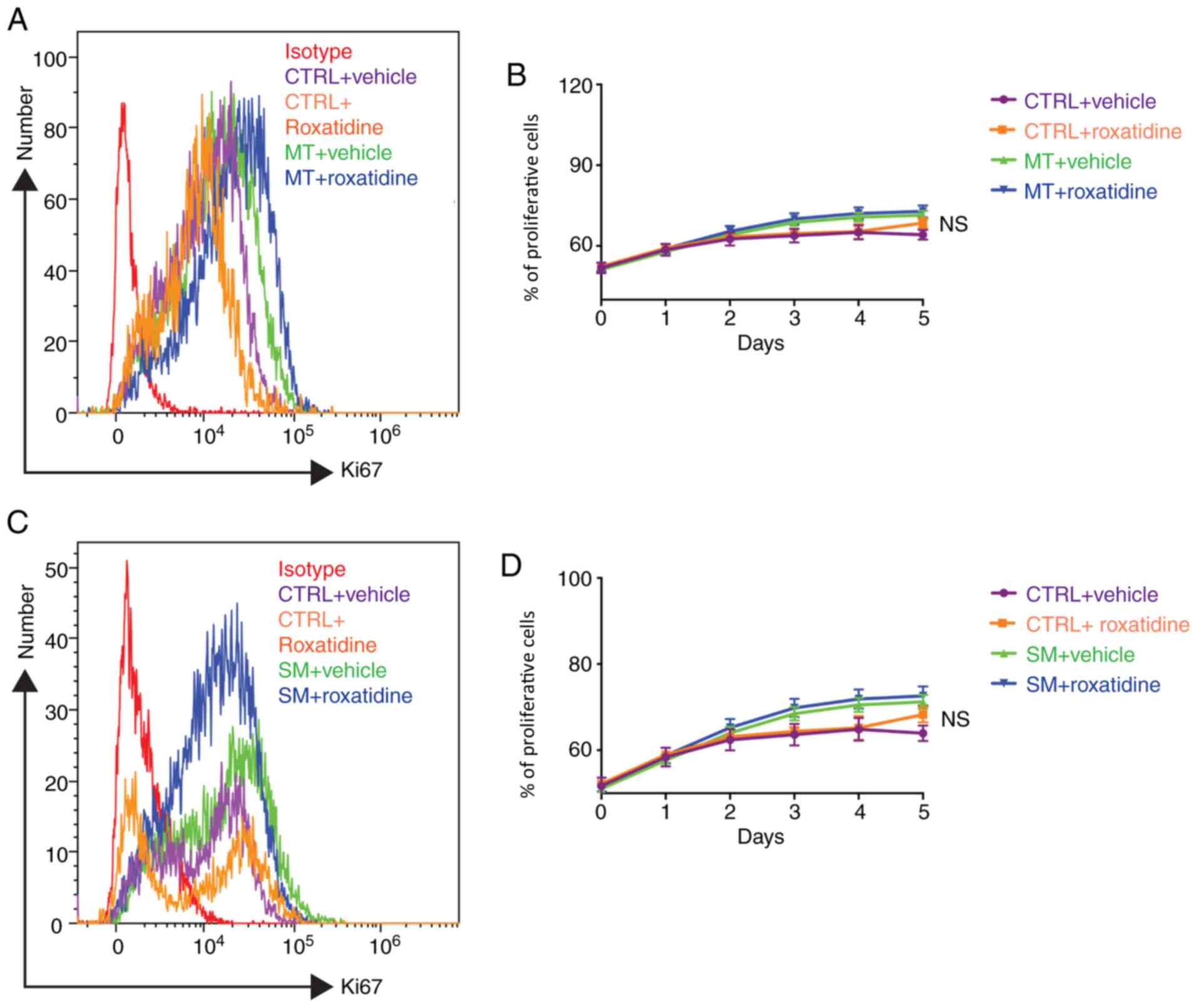
proliferation is the main cause of implant failure (28). As shown in Fig. 3, silicone surface materials had no
effect on L929 proliferation over time, measured by Ki67
expression, and neither did roxatidine.
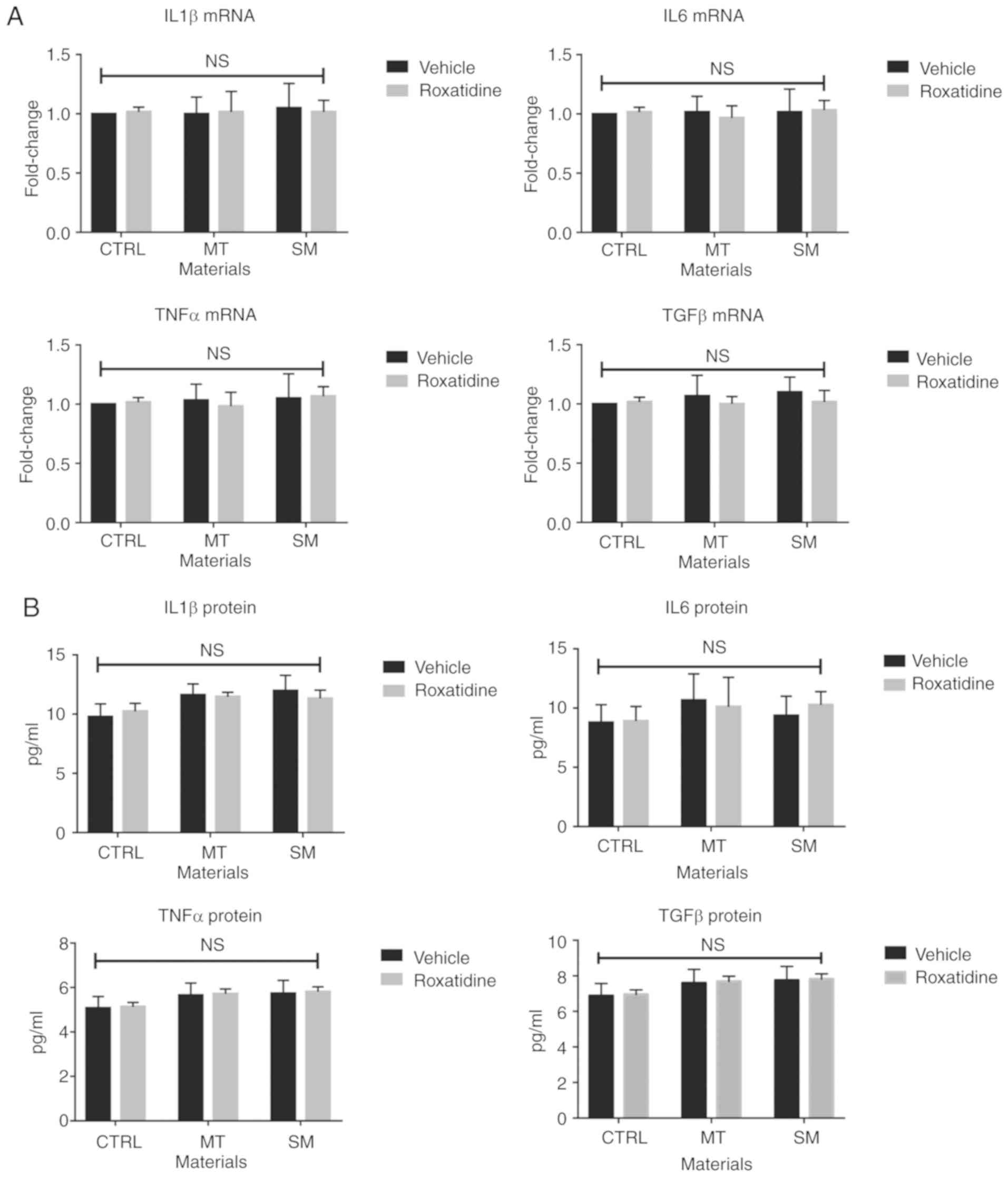 | Figure 2.Pretreatment of roxatidine in the
culture medium of fibroblasts stimulated with silicone surface
materials did not affect production of proinflammatory cytokines.
Cells were pretreated with roxatidine (25 µM) or vehicle for 1 h at
37°C before adding implant materials. At the end of culture (24 h
after co-culture with implant materials at 37°C), cells were
collected for another 24 h culture in serum-free medium at 37°C for
RT-qPCR and ELISA. (A) mRNA levels of proinflammatory cytokines.
(B) Protein levels of proinflammatory cytokines. n=6 wells/group,
from one of triplicated experiments. n.s., not significant, by
one-way ANOVA among all groups. Data were analyzed by one-way
ANOVA. RT-qPCR, reverse transcription-quantitative PCR; IL,
interleukin; TNF, tumor necrosis factor; TGF, transforming growth
factor; CTRL, control; MT, micro-textured; SM, smooth. |
Macrophage-conditioned media
stimulated with silicone implant materials increases L929 cell
proliferation and TGFβ production
The following investigation assessed whether
conditioned media collected from macrophages co-cultured with
silicone implant materials would have an effect on L929 functions
in terms of proinflammatory cytokine production. As illustrated in
Fig. 4A, although silicone implant
materials had no effect on L929 proliferation, media collected from
macrophages stimulated with MT or SM increased proliferation of
L929, measured by flow cytometry. Moreover, production of TGFβ, a
key player in fibrosis (28), was
also increased in L929 cultured in MT or SM stimulated
macrophage-conditioned media (Fig.
4B). However, when roxatidine was added to the culture media of
macrophages in the presence of silicone implant materials, the
levels of L929 proliferation and TGFβ production decreased to
baseline (Fig. 4A and B). This
result indicated that roxatidine-induced macrophage hypoactivation
inhibited L929 proliferation and TGFβ secretion. Furthermore, when
TGFβ neutralizing antibodies were added to the MT or SM stimulated
macrophage-conditioned media for L929 culture, increased
proliferation and TGFβ secretion were not observed (Fig. 4C). This indicated that the
TGFβ-induced TGFβ production (a forward feedback loop) was
essential for L929 proliferation.
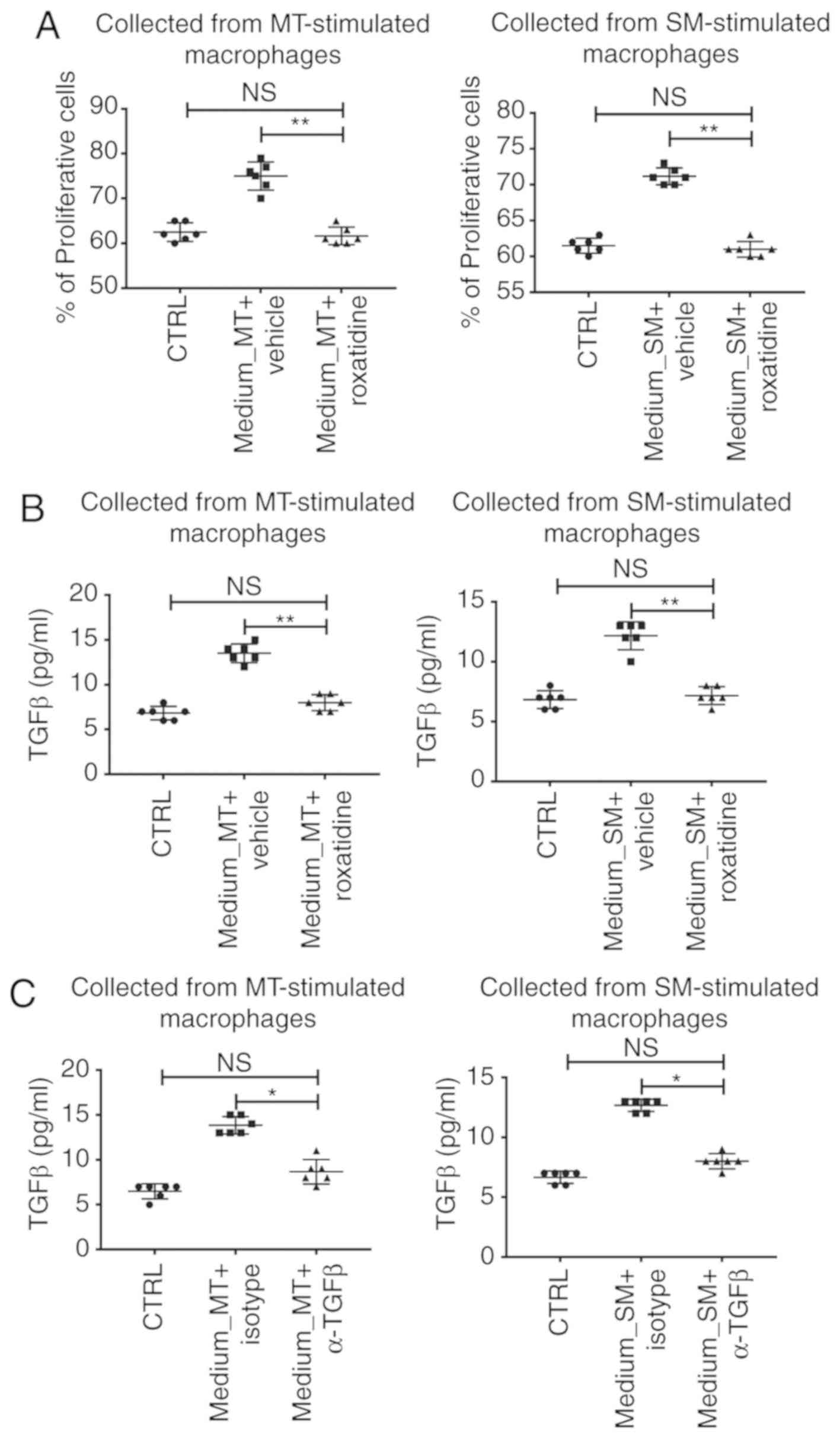 | Figure 4.Conditioned media collected from
stimulated macrophages increased L929 cell proliferation and TGFβ
production. Cell-free conditioned media, collected from the
co-culture system of macrophages and silicone material implants
with or without pretreatment with roxatidine, were added to L929
culture for 24 h at 37°C, and then the medium was switched to the
serum-free medium for another 24 h at 37°C. By the end of L929
culture, cells and media were harvested for analyses. (A) Presence
of roxatidine during macrophage culture inhibited
macrophage-conditioned media-induced enhanced proliferation in L929
cells. (B) The presence of roxatidine during macrophage culture
decreased TGFβ production by L929 cultured in
macrophage-conditioned media. (C) Presence of TGFβ neutralizing
antibodies during fibroblast culture suppressed production of TGFβ
by L929 cultured in macrophage-conditioned media. Left panels,
media collected from MT-stimulated macrophages. Right panels, media
collected from SM-stimulated macrophages. n=6 wells/group, from one
of triplicated experiments. n.s., no significance; *P<0.05 and
**P<0.01, as indicated. Data were analyzed by one-way ANOVA
followed by the Bonferroni post hoc test. TGF, transforming growth
factor; MT, micro-textured; SM, smooth; CTRL, control. |
Roxatidine inhibits macrophage
activation by suppressing NF-κB and p38/MAPK signaling
It has been reported that roxatidine displays
anti-inflammatory roles in other cell types by inhibiting
activation of the NF-κB and p38/MAPK signaling pathways (22,29).
Based on this, it was asked whether this would be the same case in
macrophages stimulated with silicone implant materials. As shown in
Fig. 5, administration of
roxatidine to macrophages stimulated with silicone implanted
materials inhibited activation of both signaling pathways. This
suggested the possibility that inhibition of certain
proinflammatory pathways might be the mechanism of
roxatidine-mediated antifibrosis effects.
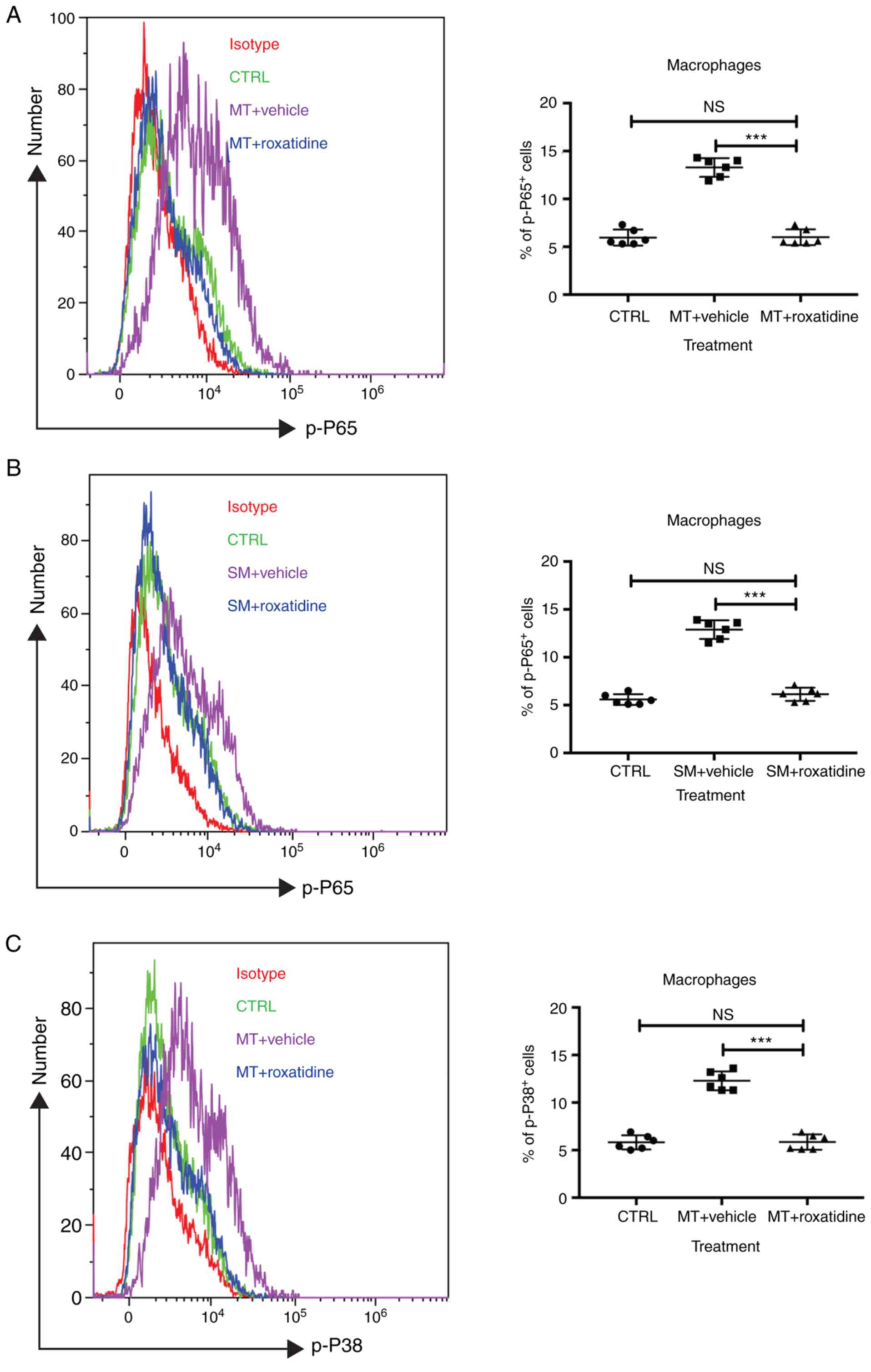 | Figure 5.Addition of roxatidine to the culture
medium of RAW 264.7 macrophages inhibited the activation of the
NF-κB and MAPK signaling pathways. RAW 264.7 macrophages were
cultured in serum-free media at 37°C. Then, 24 h after cells were
seeded, roxatidine (25 µM) was added to the media 1 h prior to
stimulation with silicone surface materials. Cells were collected
for analysis of phosphorylation of NF-κB subunit p65 and p38 MAPK
by flow cytometry 15 minutes after adding silicone surface
materials to culture media. Administration of roxatidine suppressed
p65 activation stimulated by (A) MT and (B) SM, as well as p38
phosphorylation stimulated by (C) MT. p38 phosphorylation
stimulated by (D) SM. n=6 wells/group, from one of triplicated
experiments. n.s., not significant and ***P<0.001, as indicated.
Data were analyzed by one-way ANOVA followed by the Bonferroni post
hoc test. MAPK, mitogen-activated protein kinase; MT,
micro-textured; SM, smooth; CTRL, control. |
Administration of roxatidine in
silicone implant bearing mice reduces fibrosis
Finally, the effects of roxatidine in silicone
implant bearing mice were examined. Silicone implant surface
materials were surgically seeded into the subcutaneous pocket of
mice. At three months post-surgery, the surrounding tissue of the
implants was removed and the amount of fibroblasts present was
assessed by flow cytometry. As shown in Fig. 6A, administration of roxatidine
reduced serum levels of TGFβ in both MT- and SM- bearing mice at
day 90 post-surgery, suggesting that roxatidine decreased systemic
TGFβ levels in mice with silicone implant materials. Furthermore,
at three months post-surgery, there were fewer fibroblasts in the
surrounding tissues of implant materials (Fig. 6B and C), indicating that roxatidine
protected against fibrosis.
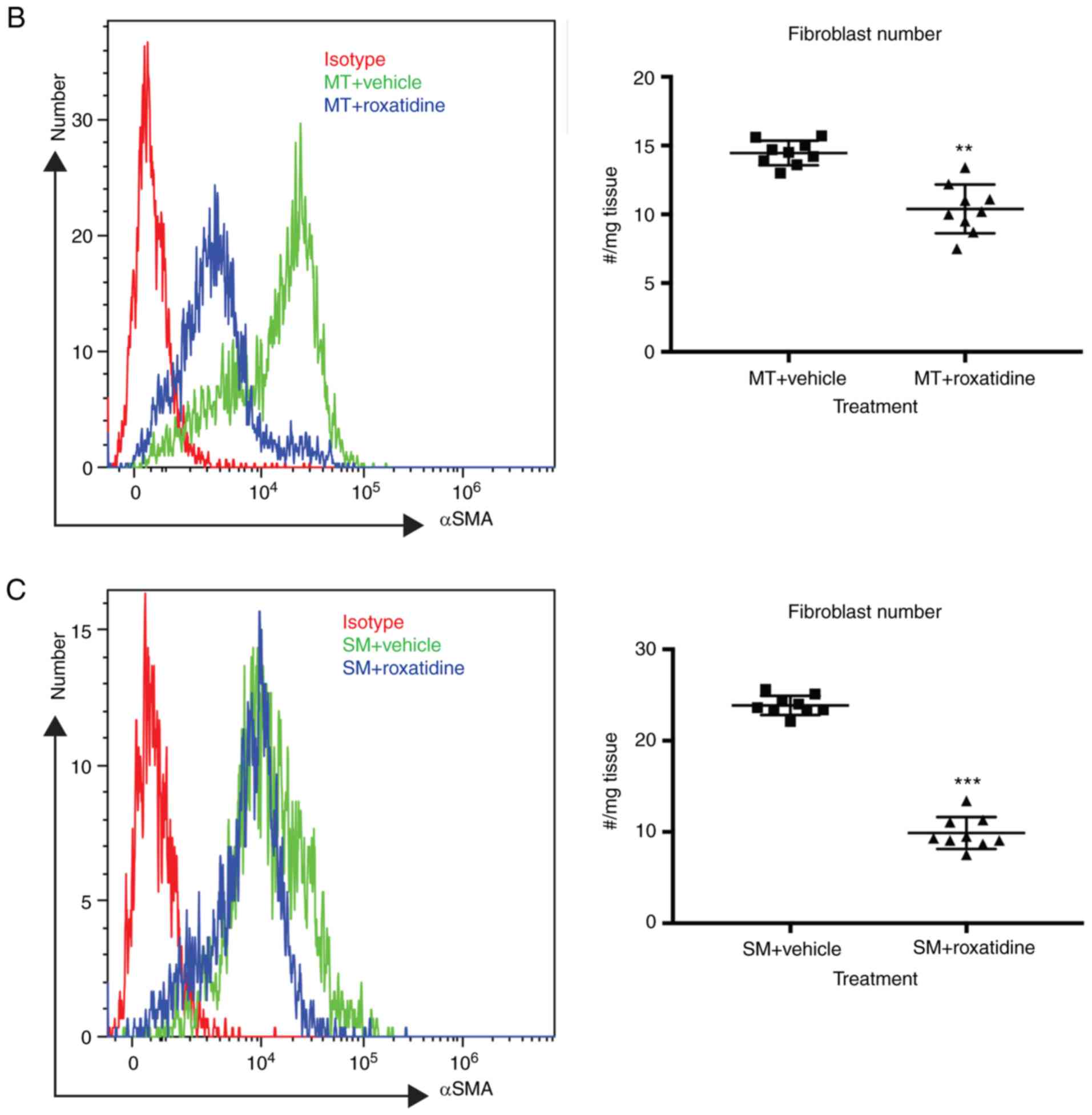 | Figure 6.Treatment with roxatidine in
implant-bearing mice reduced the severity of fibrosis. Implant
materials (5 mm in diameter) were surgically seeded in a
subcutaneous fat pocket in mice as described. Mice were treated
daily by oral gavage with roxatidine (15.62 mg/kg) or PBS (control)
for 14 days. At day 90 post-surgery, serum samples and fibrosis
around implant materials were collected. (A) Administration of
roxatidine reduced levels of TGFβ in the serum. n=9 mice/group,
from one of duplicated experiments. n.s., not significant and
***P<0.001, as indicated. Data were analyzed by one-way ANOVA
followed by the Bonferroni post hoc test. Administration of
roxatidine reduced the number of fibroblasts in surrounding tissue
of (B) MT and (C) SM in mice. Fibroblasts were defined as
αSMA-positive cells by flow cytometry. n=9 mice/group, from one of
duplicated experiments. **P<0.01 and ***P<0.001. Data were
analyzed by the two-tailed Student's t-test. TGF, transforming
growth factor; MT, micro-textured; SM, smooth; SMA, smooth muscle
actin; CTRL, control. |
Discussion
The present study demonstrated that roxatidine
inhibited proinflammatory cytokine production in macrophages
stimulated with breast implant surface materials. Although
roxatidine seemed to have no effect on fibroblast function, the
inhibitory effect of roxatidine on macrophages indirectly resulted
in reduced fibroblast proliferation, potentially via the
TGFβ-dependent forward feedback loop. In vivo studies using
implant-bearing mice also showed that roxatidine provided
protection against fibroblast hyperplasia and TGFβ production.
Inflammation, especially early inflammation during
post-surgery wound healing, plays an important role in fibrosis
(30,31). It has been widely accepted that
aberrant inflammation causes excessive fibrosis and subsequent
capsular contracture in patients with breast implants (5,6).
Based on this speculation, researchers have been seeking to control
inflammation after breast augmentation procedures in order to
prevent capsular contracture (12,32–34).
Applications of anti-inflammatory strategies have been well
reviewed elsewhere (35). Results
from numerous experiments support the idea that limiting
inflammation during the phase of wound healing inhibits fibrosis
(12,32–34).
In agreement with the aforementioned results (5,6), the
present study also highlighted the important roles of inflammation
in fibrosis as well as the crosstalk between macrophages and
fibroblasts. Moreover, the present study showed that application of
roxatidine inhibited excessive inflammation, as well as the NF-κB
and MAPK signalling pathways.
The present study demonstrated the different roles
of macrophages and fibroblasts during fibrosis. Here, it is
proposed that these two cell types have varied functions in
different phases. Macrophages, as sentinel cells, sensed the
presence of foreign objects, including implant materials, via
activation of the NF-κB and MAPK signaling pathways. Activated
macrophages produced and released proinflammatory cytokines to the
surrounding environment. On the other hand, fibroblasts failed to
sense implant materials, but could be stimulated by the
proinflammatory cytokines produced by activated macrophages,
especially TGFβ. By acting with TGFβ and other proinflammatory
cytokines, fibroblasts displayed increased levels of proliferation
and TGFβ production, which could accelerate fibroblast
proliferation and TGFβ secretion induced by TGFβ. Furthermore,
roxatidine could block activation of macrophages by inhibiting
NF-κB and MAPK signaling pathways to reduce proinflammatory
cytokine production, fibroblast proliferation and fibrosis in
vitro and in vivo.
The present study builds on the understanding of the
cell types that roxatidine has effects on. It has been well
documented that roxatidine influences mast cells during
inflammation (22). However, the
current study revealed that roxatidine could also affect
macrophages by blocking macrophage activation by inhibiting NF-κB
and MAPK signaling pathways. It was hypothesized that roxatidine
blocks NF-κB and MAPK signaling pathways in both mast cells and
macrophages, which are both essential cell types in inflammatory
responses such as allergy and infection (36). Therefore, it is reasonable to
speculate that inhibition of HR2 by roxatidine might have a wide
anti-inflammatory role in a number of conditions including fibrosis
and allergies, which could be explored in further
investigations.
This study also provided more evidence for the
applicability of pharmaceutical therapy for capsular contracture
after breast implant surgery. Previous results from small scale
trials indicate the potential benefit in softening and improving
the appearance of the breast in patients treated with leukotriene
receptor antagonists such as montelukast (37) or zafirlukast (38). Veras-Castillo et al
(39) indicated that pirfenidone
was superior to capsulectomy in patients, displaying a higher
improvement rate and a lower relapse rate. It is worth noting that
the aforementioned trials involved small cohorts, and further
investigations are required.
The major pitfall of the present study is the lack
of exploration into the roles of histamine on macrophages in
fibrosis generation when breast implant materials are present. The
data from the present study suggested that blocking HR2 by
roxatidine had no effect on fibroblasts, but these results
highlight important roles of HR2 on macrophages. However, how the
HR2 is activated and what the cellular sources of histamine are
remain untouched in the current study and in the literature. The
present study presented two theories; however further exploration
is required for clarification. The first theory is that macrophages
sense foreign objects in the tissue and produce histamine. Secreted
histamine acts on macrophages to enhance macrophage activation. The
second is that other cells (for instance, basophils and mast cells)
produce histamine to stimulate macrophages. Moreover, the
protective effects of roxatidine when breast implant materials are
seeded in the submuscular position were not assessed in the present
study. It has been reported that the rate of capsular contracture
is lower when breast implant material is surgically placed in the
submuscular position compared to the subglandular plate (40–42).
Therefore, it is reasonable to presume that the combination of
roxatidine administration and insertion into the submuscular
position should have a synergistic effect in preventing fibrosis
generation. However, this combination requires further
investigation.
In conclusion, the HR2 antagonist roxatidine blocked
activation of macrophages by inhibiting the NF-κB and MAPK
signaling pathways to prevent fibrosis and subsequent capsular
contracture. These results raise the possibility that
administration of roxatidine could aid in preventing capsular
contracture in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Department of
Health of Heilongjiang Province to TW (grant no. 2010-118).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ performed most of the experiments including cell
culture, flow cytometry, and animal surgery. TW performed data
analysis and composed the first draft of the manuscript, as well as
revisions. LT and HS performed statistical analysis and figure
illustration. MG designed the present study and supervised the
whole process. All authors have agreed the final version for
publication.
Ethics approval and consent to
participate
The ethics committee of the Fourth Affiliated
Hospital of Harbin Medical University approved and supervised the
research proposal (approval no.F 170023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vegas MR and Martin del Yerro JL:
Stiffness, compliance, resilience, and creep deformation:
Understanding implant-soft tissue dynamics in the augmented breast:
Fundamentals based on materials science. Aesthetic Plast Surg.
37:922–930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson M: Breast implants: History,
safety, and imaging. Radiol Technol. 84:439M–520M. 2013.PubMed/NCBI
|
|
3
|
Cassileth L, Kohanzadeh S and Amersi F:
One-stage immediate breast reconstruction with implants: A new
option for immediate reconstruction. Ann Plast Surg. 69:134–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cappellano G, Ploner C, Lobenwein S,
Sopper S, Hoertnagl P, Mayerl C, Wick N, Pierer G, Wick G and
Wolfram D: Immunophenotypic characterization of human T cells after
in vitro exposure to different silicone breast implant surfaces.
PLoS One. 13:e01921082018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Headon H, Kasem A and Mokbel K: Capsular
contracture after breast augmentation: An update for clinical
practice. Arch Plast Surg. 42:532–543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Namnoum JD, Largent J, Kaplan HM, Oefelein
MG and Brown MH: Primary breast augmentation clinical trial
outcomes stratified by surgical incision, anatomical placement and
implant device type. J Plast Reconstr Aesthet Surg. 66:1165–1172.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Little G and Baker JL Jr: Results of
closed compression capsulotomy for treatment of contracted breast
implant capsules. Plast Reconstr Surg. 65:30–33. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams WP Jr: Capsular contracture: What is
it? What causes it? How can it be prevented and managed? Clin Plast
Surg. 36119–126. (vii)2009.PubMed/NCBI
|
|
9
|
Barnsley GP, Sigurdson LJ and Barnsley SE:
Textured surface breast implants in the prevention of capsular
contracture among breast augmentation patients: A meta-analysis of
randomized controlled trials. Plast Reconstr Surg. 117:2182–2190.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuriyama E, Ochiai H, Inoue Y, Sakamoto Y,
Yamamoto N, Utsumi T, Kishi K, Okumoto T and Matsuura A:
Characterization of the capsule surrounding smooth and textured
tissue expanders and correlation with contracture. Plast Reconstr
Surg Glob Open. 5:e14032017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan KT, Wijeratne D, Shih B, Baildam AD
and Bayat A: Tumour necrosis factor-alpha expression is associated
with increased severity of periprosthetic breast capsular
contracture. Eur Surg Res. 45:327–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prantl L, Angele P, Schreml S, Ulrich D,
Poppl N and Eisenmann-Klein M: Determination of serum fibrosis
indexes in patients with capsular contracture after augmentation
with smooth silicone gel implants. Plast Reconstr Surg.
118:224–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolfram D, Rainer C, Niederegger H, Piza H
and Wick G: Cellular and molecular composition of fibrous capsules
formed around silicone breast implants with special focus on local
immune reactions. J Autoimmun. 23:81–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siggelkow W, Faridi A, Spiritus K, Klinge
U, Rath W and Klosterhalfen B: Histological analysis of silicone
breast implant capsules and correlation with capsular contracture.
Biomaterials. 24:1101–1109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brazin J, Malliaris S, Groh B, Mehrara B,
Hidalgo D, Otterburn D, Silver RB and Spector JA: Mast cells in the
periprosthetic breast capsule. Aesthetic Plast Surg. 38:592–601.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brodbeck WG, Voskerician G, Ziats NP,
Nakayama Y, Matsuda T and Anderson JM: In vivo leukocyte cytokine
mRNA responses to biomaterials are dependent on surface chemistry.
J Biomed Mater Res A. 64:320–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brodbeck WG, Shive MS, Colton E, Nakayama
Y, Matsuda T and Anderson JM: Influence of biomaterial surface
chemistry on the apoptosis of adherent cells. J Biomed Mater Res.
55:661–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen IK, Beaven MA, Horakova Z and Keiser
HR: Histamine and collagen synthesis in keloid and hypertrophic
scar. Surg Forum. 23:509–510. 1972.PubMed/NCBI
|
|
20
|
Zdolsek J, Eaton JW and Tang L: Histamine
release and fibrinogen adsorption mediate acute inflammatory
responses to biomaterial implants in humans. J Transl Med.
5:312007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sisti A, Tassinari J, Milonia L, Nisi G
and Grimaldi L: Comparison of allergan, mentor, and sientra
contoured cohesive gel breast implants: A single surgeon's 10-year
experience. Plast Reconstr Surg. 138:548e–549e. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee M, Lee NY, Chung KS, Cheon SY, Lee KT
and An HJ: Roxatidine attenuates mast cell-mediated allergic
inflammation via inhibition of NF-κB and p38 MAPK activation. Sci
Rep. 7:417212017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Wei Y, Tian L, Song H, Ma Y, Yao
Q, Feng M, Wang Y, Gao M and Xue Y: C-C motif chemokine ligand 5
(CCL5) levels in gastric cancer patient sera predict occult
peritoneal metastasis and a poorer prognosis. Int J Surg.
32:136–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frenkiel BA, Temple-Smith P, de Kretser D
and Southwick GJ: Follistatin and the Breast Implant Capsule. Plast
Reconstr Surg Glob Open. 5:e12582017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Sun D, He J, Yang C, Hu T, Zhang L,
Cao H, Tong AP, Song X, Xie Y, et al: Gastroprotective effects of
several H2RAs on ibuprofen-induced gastric ulcer in rats. Life Sci.
149:65–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joseph J, Variathu KT and Mohanty M:
Mediatory role of interleukin-6 in alpha smooth muscle actin
induction and myofibroblast formation around silicone tissue
expander. J Biomed Mater Res A. 101:2967–2973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho EJ, An HJ, Shin JS, Choi HE, Ko J, Cho
YW, Kim HM, Choi JH and Lee KT: Roxatidine suppresses inflammatory
responses via inhibition of NF-κB and p38 MAPK activation in
LPS-induced RAW 264.7 macrophages. J Cell Biochem. 112:3648–3659.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shaw TJ and Martin P: Wound repair at a
glance. J Cell Sci. 122:3209–3213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez AC, Costa TF, Andrade ZA and
Medrado AR: Wound healing-A literature review. An Bras Dermatol.
91:614–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katzel EB, Koltz PF, Tierney R, Williams
JP, Awad HA, O'Keefe RJ and Langstein HN: A novel animal model for
studying silicone gel-related capsular contracture. Plast Reconstr
Surg. 126:1483–1491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prantl L, Poppl N, Horvat N, Heine N and
Eisenmann-Klein M: Serologic and histologic findings in patients
with capsular contracture after breast augmentation with smooth
silicone gel implants: Is serum hyaluronan a potential predictor?
Aesthetic Plast Surg. 29:510–518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ulrich D, Lichtenegger F, Eblenkamp M,
Repper D and Pallua N: Matrix metalloproteinases, tissue inhibitors
of metalloproteinases, aminoterminal propeptide of procollagen type
III, and hyaluronan in sera and tissue of patients with capsular
contracture after augmentation with Trilucent breast implants.
Plast Reconstr Surg. 114:229–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Araco A, Caruso R, Araco F, Overton J and
Gravante G: Capsular contractures: A systematic review. Plast
Reconstr Surg. 124:1808–1819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dobranowski P and Sly LM: SHIP negatively
regulates type II immune responses in mast cells and macrophages. J
Leukoc Biol. 2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang CK and Handel N: Effects of
Singulair (montelukast) treatment for capsular contracture. Aesthet
Surg J. 30:404–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schlesinger SL, Ellenbogen R, Desvigne MN,
Svehlak S and Heck R: Zafirlukast (Accolate): A new treatment for
capsular contracture. Aesthet Surg J. 22:329–336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Veras-Castillo ER, Cardenas-Camarena L,
Lyra-Gonzalez I, Muñoz-Valle JF, Lucano-Landeros S, Guerrerosantos
J, Gonzalez-Ulloa B, Mercado-Barajas JL, Sanchez-Parada MG,
Azabache-Wennceslao R and Armendariz-Borunda J: Controlled clinical
trial with pirfenidone in the treatment of breast capsular
contracture: Association of TGF-β polymorphisms. Ann Plast Surg.
70:16–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Motosko CC, Khouri KS, Poudrier G, Sinno S
and Hazen A: Evaluating platelet-rich therapy for facial aesthetics
and alopecia: A critical review of the literature. Plast Reconstr
Surg. 141:1115–1123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stevens WG, Harrington J, Alizadeh K,
Berger L, Broadway D, Hester TR, Kress D, d'Incelli R, Kuhne J and
Beckstrand M: Five-year follow-up data from the U.S. clinical trial
for Sientra's U.S. Food and Drug Administration-approved Silimed(R)
brand round and shaped implants with high-strength silicone gel.
Plast Reconstr Surg. 130:973–981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hammond DC, Migliori MM, Caplin DA, Garcia
ME and Phillips CA: Mentor Contour Profile Gel implants: Clinical
outcomes at 6 years. Plast Reconstr Surg. 129:1381–1391. 2012.
View Article : Google Scholar : PubMed/NCBI
|