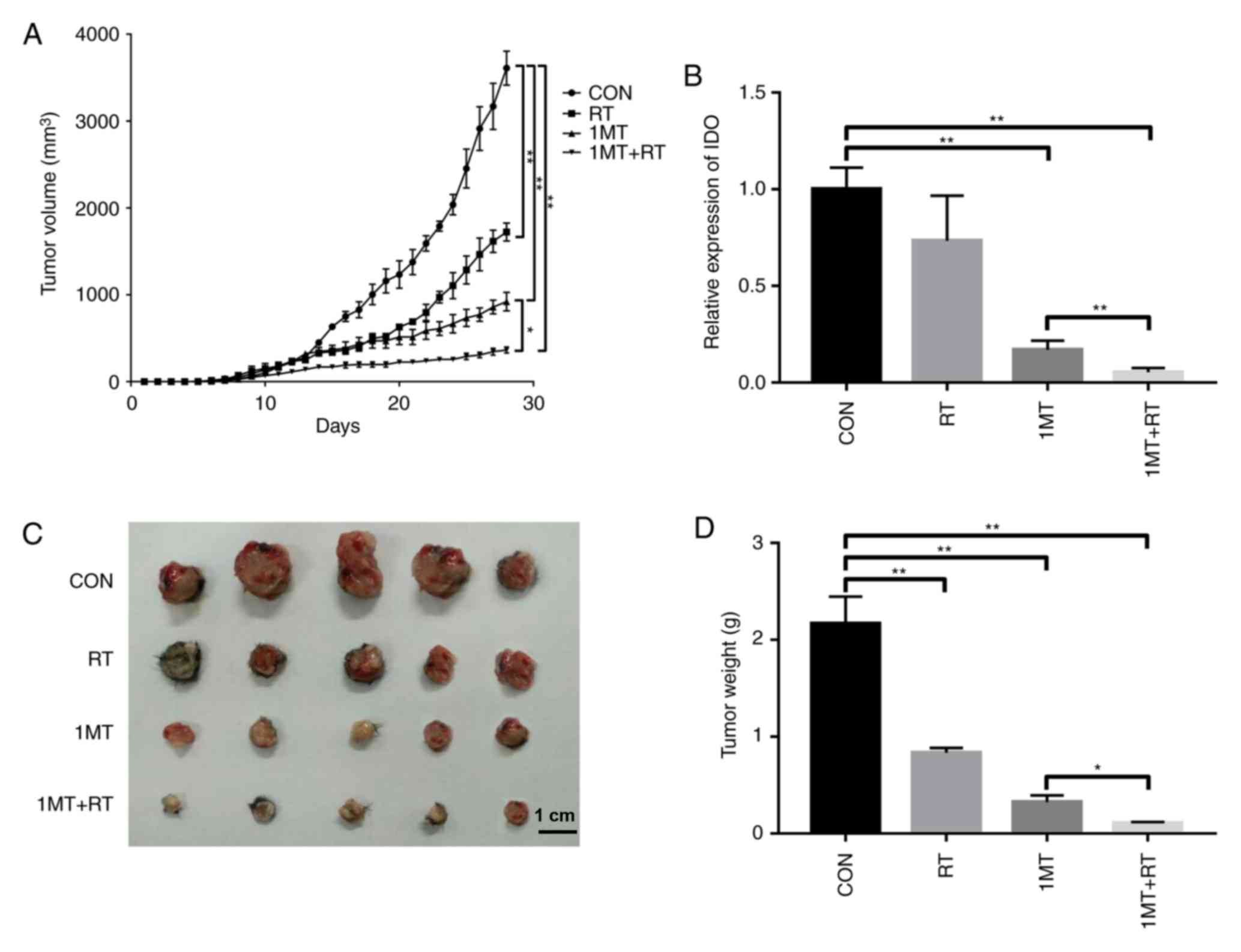
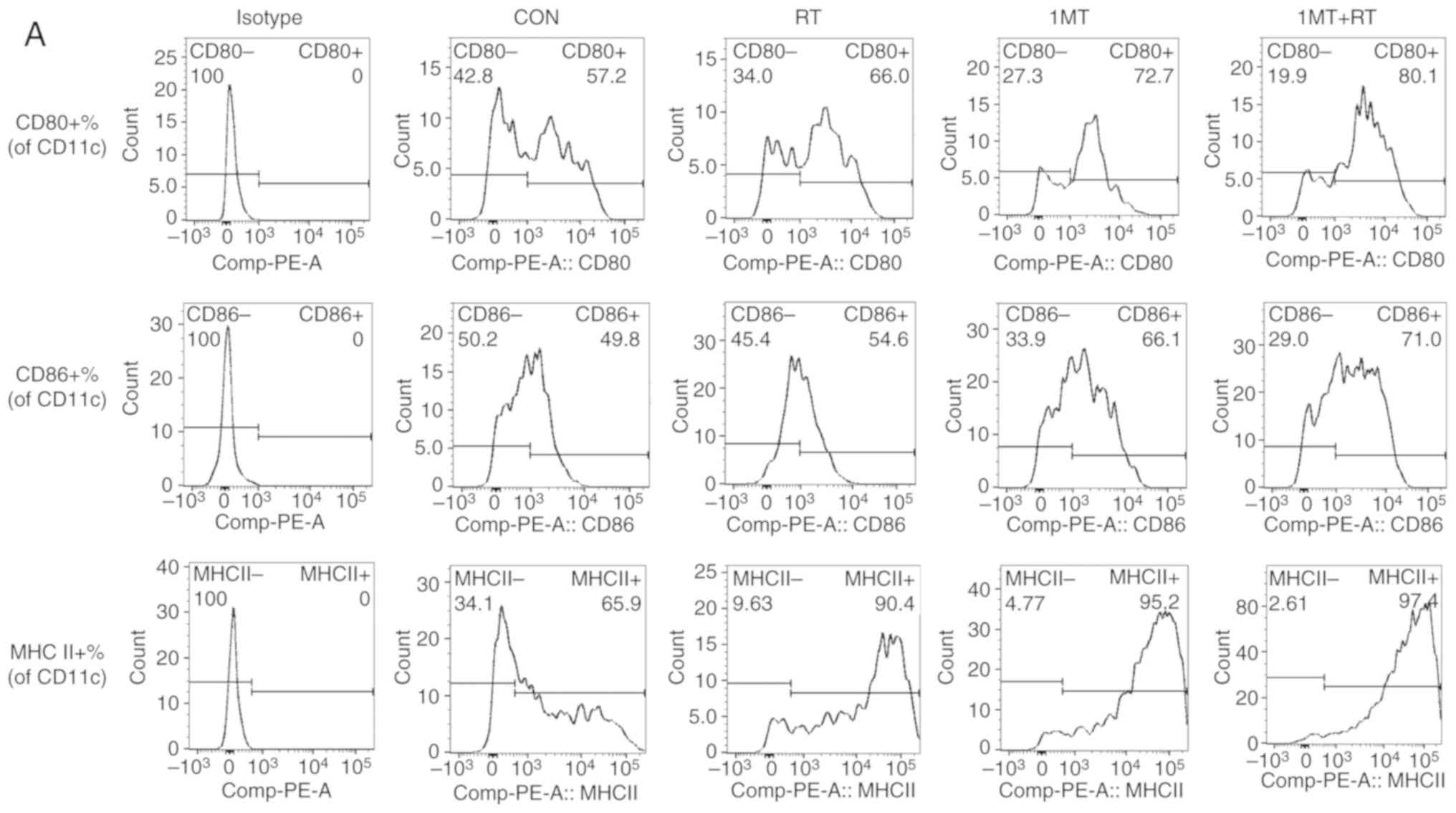
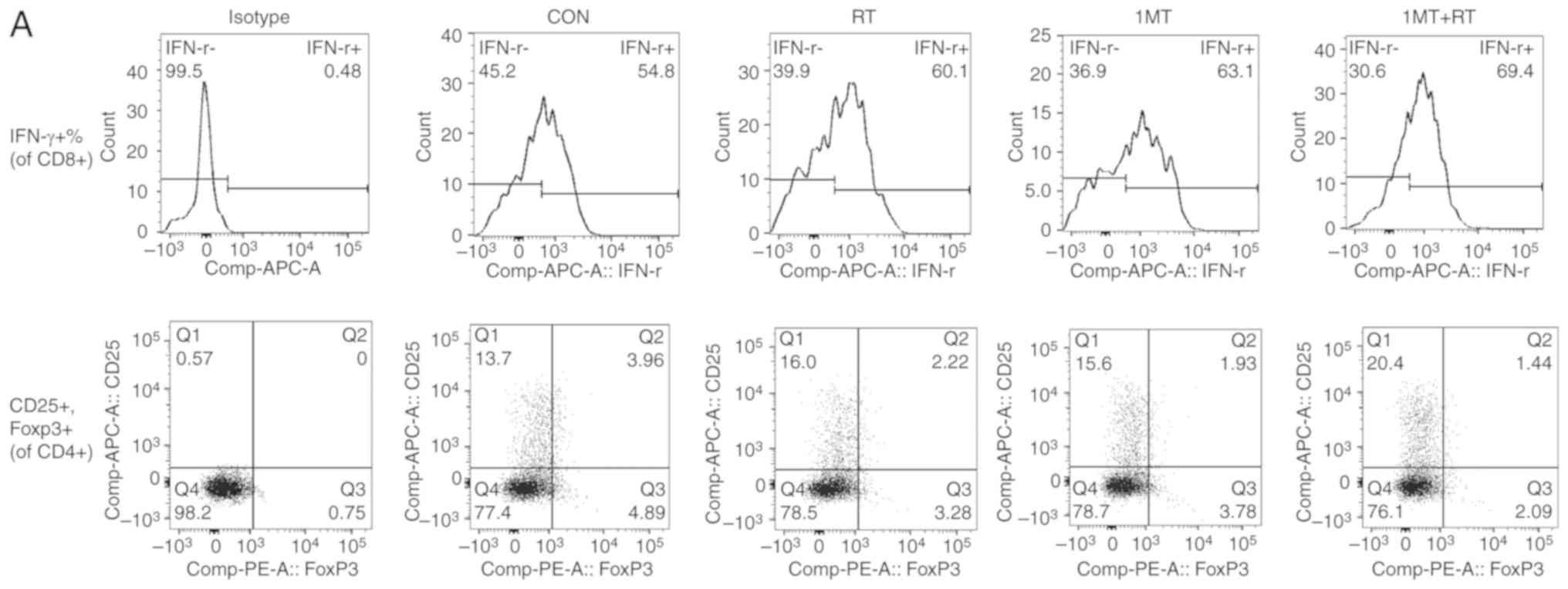
|
1
|
O'Donnell JS, Smyth MJ and Teng MW:
Acquired resistance to anti-PD1 therapy: Checkmate to checkpoint
blockade? Genome Med. 8:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weinlich R, Oberst A, Beere HM and Green
DR: Necroptosis in development, inflammation and disease. Nat Rev
Mol Cell Biol. 18:127–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Postow MA, Callahan MK, Barker CA, Yamada
Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al:
Immunologic correlates of the abscopal effect in a patient with
melanoma. N Engl J Med. 366:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levy A, Chargari C, Marabelle A,
Perfettini JL, Magné N and Deutsch E: Can immunostimulatory agents
enhance the abscopal effect of radiotherapy? Eur J Cancer.
62:36–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Golden EB, Chhabra A, Chachoua A, Adams S,
Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg
J, et al: Local radiotherapy and granulocyte-macrophage
colony-stimulating factor to generate abscopal responses in
patients with metastatic solid tumours: A proof-of-principle trial.
Lancet Oncol. 16:795–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seyedin SN, Tang C and Welsh JW: Author's
view: Radiation and immunotherapy as systemic therapy for solid
tumors. Oncoimmunology. 4:e9864022015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ungaro A, Orsi F, Casadio C, Galdy S,
Spada F, Cella CA, Tonno CD, Bonomo G, Vigna PD, Murgioni S, et al:
Successful palliative approach with high-intensity focused
ultrasound in a patient with metastatic anaplastic pancreatic
carcinoma: A case report. Ecancermedicalscience. 10:6352016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Espenel S, Vallard A, Rancoule C, Garcia
MA, Guy JB, Chargari C, Deutsch E and Magné N: Melanoma: Last call
for radiotherapy. Crit Rev Oncol Hematol. 110:13–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu CS and Liu JH: Pneumonitis in cancer
patients receiving anti-PD-1 and radiotherapies: Three case
reports. Medicine (Baltimore). 96:e57472017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye Q, Wang C, Xian J, Zhang M and Cao Y
and Cao Y: Expression of programmed cell death protein 1 (PD-1) and
indoleamine 2,3-dioxygenase (IDO) in the tumor microenvironment and
in tumor-draining lymph nodes of breast cancer. Hum Pathol.
75:81–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang C, Peng L, Zeng S, Zhao Q, Tang L,
Jiang X, Zhang J, Yan N and Chen Y: Investigation of indoleamine
2,3-dioxygenase 1 expression in uveal melanoma. Exp Eye Res.
181:112–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brahmer J, Reckamp KL, Baas P, Crinó L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Younes A, Brody J, Carpio C,
Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, Nagler A, Ozcan M,
Avivi I, Bosch F, et al: Safety and activity of ibrutinib in
combination with nivolumab in patients with relapsed non-Hodgkin
lymphoma or chronic lymphocytic leukaemia: A phase 1/2a study.
Lancet Haematol. 6:e67–e78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prendergast GC, Malachowski WJ, Mondal A,
Scherle P and Muller AJ: Indoleamine 2,3-dioxygenase and its
therapeutic inhibition in cancer. Int Rev Cell Mol Biol.
336:175–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozuma Y, Takada K, Toyokawa G, Kohashi K,
Shimokawa M, Hirai F, Tagawa T, Okamoto T, Oda Y and Maehara Y:
Indoleamine 2,3-dioxygenase 1 and programmed cell death-ligand 1
co-expression correlates with aggressive features in lung
adenocarcinoma. Eur J Cancer. 101:20–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li A, Barsoumian HB, Schoenhals JE,
Cushman TR, Caetano MS, Wang X, Valdecanas DR, Niknam S, Younes AI,
Li G, et al: Indoleamine 2,3-dioxygenase 1 inhibition targets
anti-PD1-resistant lung tumors by blocking myeloid-derived
suppressor cells. Cancer Lett. 431:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanizaki Y, Kobayashi A, Toujima S, Shiro
M, Mizoguchi M, Mabuchi Y, Yagi S, Minami S, Takikawa O and Ino K:
Indoleamine 2,3-dioxygenase promotes peritoneal metastasis of
ovarian cancer by inducing an immunosuppressive environment. Cancer
Sci. 105:966–973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moreno ACR, Porchia B, Pagni RL, Souza
PDC, Pegoraro R, Rodrigues KB, Barros TB, Aps LRMM, de Araújo EF,
Calich VLG and Ferreira LCS: The combined use of melatonin and an
indoleamine 2,3-dioxygenase-1 inhibitor enhances vaccine-induced
protective cellular immunity to HPV16-associated tumors. Front
Immunol. 9:19142018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alahdal M, Xing Y, Tang T and Liang J:
1-Methyl-D-tryptophan reduces tumor CD133+ cells,
Wnt/β-catenin and NF-kappaβp65 while enhances lymphocytes NF-κβ2,
STAT3, and STAT4 pathways in murine pancreatic adenocarcinoma. Sci
Rep. 8:98692018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5:200ra1162013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams DK, Markwalder JA, Balog AJ, Chen
B, Chen L, Donnell J, Haque L, Hart AC, Mandal SK, Nation A, et al:
Development of a series of novel o-phenylenediamine-based
indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors. Bioorg Med Chem
Lett. 28:732–736. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vatner RE, Cooper BT, Vanpouille-Box C,
Demaria S and Formenti SC: Combinations of immunotherapy and
radiation in cancer therapy. Front Oncol. 4:3252014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia H, Ren W, Feng Y, Wei T, Guo M, Guo J,
Zhao J, Song X, Wang M, Zhao T, et al: The enhanced antitumour
response of pimozide combined with the IDO inhibitor L-MT in
melanoma. Int J Oncol. 53:949–960. 2018.PubMed/NCBI
|
|
24
|
Leary S, Underwood W, Anthony R, Cartner
S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA,
Meyer R, et al: AVMA Guidelines for the Euthanasia of Animals: 2013
edition. University of Alaska Anchorage; 2013. 2013, PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holling TM, Schooten E and van Den Elsen
PJ: Function and regulation of MHC class II molecules in
T-lymphocytes: Of mice and men. Hum Immunol. 65:282–290. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kursunel MA and Esendagli G: The untold
story of IFN-γ in cancer biology. Cytokine Growth Factor Rev.
31:73–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gondek DC, Lu LF, Quezada SA, Sakaguchi S
and Noelle RJ: Cutting edge: Contact-mediated suppression by
CD4+CD25+ regulatory cells involves a granzyme B-dependent,
perforin-independent mechanism. J Immunol. 174:1783–1786. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fife BT and Pauken KE: The role of the
PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad
Sci. 1217:45–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blackburn SD, Shin H, Haining WN, Zou T,
Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA and Wherry
EJ: Coregulation of CD8+ T cell exhaustion by multiple inhibitory
receptors during chronic viral infection. Nat Immunol. 10:29–37.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haymaker CL, Wu RC, Ritthipichai K,
Bernatchez C, Forget MA, Chen JQ, Liu H, Wang E, Marincola F, Hwu P
and Radvanyi LG: BTLA marks a less-differentiated
tumor-infiltrating lymphocyte subset in melanoma with enhanced
survival properties. Oncoimmunology. 4:e10142462015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vanpouille-Box C, Alard A, Aryankalayil
MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN,
Formenti SC and Demaria S: DNA exonuclease Trex1 regulates
radiotherapy-induced tumour immunogenicity. Nat Commun.
8:156182017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou
F and Zhou C: Combined radiotherapy and Anti-PD-L1 antibody
synergistically enhances antitumor effect in non-small cell lung
cancer. J Thorac Oncol. 12:1085–1097. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herter-Sprie GS, Koyama S, Korideck H, Hai
J, Deng J, Li YY, Buczkowski KA, Grant AK, Ullas S, Rhee K, et al:
Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung
cancer. JCI Insight. 1:e874152016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brown ZJ, Yu SJ, Heinrich B, Ma C, Fu Q,
Sandhu M, Agdashian D, Zhang Q, Korangy F and Greten TF:
Indoleamine 2,3-dioxygenase provides adaptive resistance to immune
checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol
Immunother. 67:1305–1315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lim JY, Lee SE, Park G, Choi EY and Min
CK: Inhibition of indoleamine 2,3-dioxygenase by stereoisomers of
1-methyl tryptophan in an experimental graft-versus-tumor model.
Exp Hematol. 42:862–866.e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiao B, Liu B, Song Y, Yu Z and Guo S:
Local cytotoxic T-lymphocyte-associated antigen-4 immunoglobulin
inhibition of rejection response is dependent on indoleamine
2,3-dioxygenase activities in the allograft. Transplant Proc.
46:3637–3640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ostrand-Rosenberg S, Horn LA and
Ciavattone NG: Radiotherapy both promotes and inhibits
myeloid-derived suppressor cell function: Novel strategies for
preventing the tumor-protective effects of radiotherapy. Front
Oncol. 9:2152019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oweida A, Hararah MK, Phan A, Binder D,
Bhatia S, Lennon S, Bukkapatnam S, Van Court B, Uyanga N, Darragh
L, et al: Resistance to radiotherapy and PD-L1 blockade is mediated
by TIM-3 upregulation and regulatory T-cell infiltration. Clin
Cancer Res. 24:5368–5380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu JF, Zhao K, Huang BJ, Xiong P, Fang M,
Shen GX and Gong FL: Clone and expression of murine BTLA
extracellular domain gene and its effect on the expression of B7 on
dendritic cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 22:413–416.
2006.(In Chinese). PubMed/NCBI
|
|
42
|
Shukuya T and Carbone DP: Predictive
markers for the efficacy of Anti-PD-1/PD-L1 antibodies in lung
cancer. J Thorac Oncol. 11:976–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Du W, Yang M, Turner A, Xu C, Ferris RL,
Huang J, Kane LP and Lu B: TIM-3 as a target for cancer
immunotherapy and mechanisms of action. Int J Mol Sci. 18(pii):
E6452017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ritthipichai K, Haymaker CL, Martinez M,
Aschenbrenner A, Yi X, Zhang M, Kale C, Vence LM, Roszik J,
Hailemichael Y, et al: Multifaceted role of BTLA in the control of
CD8+ T-cell Fate after antigen encounter. Clin Cancer
Res. 23:6151–6164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang W, Chan CK, Weissman IL, Kim BYS and
Hahn SM: Immune priming of the tumor microenvironment by radiation.
Trends Cancer. 2:638–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hirata E and Sahai E: Tumor
microenvironment and differential responses to therapy. Cold Spring
Harb Perspect Med. 7(pii): a0267812017. View Article : Google Scholar : PubMed/NCBI
|