Introduction
Tuberculosis (TB) remains a major health problem
world worldwide, accounting for 9.6 million new cases and 1.5
million mortalities annually (1).
Although highly effective, isoniazid (INH) and rifampicin (RIF)
combination therapy is associated with hepatotoxicity (2,3).
Hepatotoxicity is the most common serious complication of anti-TB
therapy, and can range from asymptomatic elevation of serum
transferases to hepatitis or hepatic failure (4). Previous studies in rats have shown
that the hepatotoxicity of anti-TB drugs is associated with
inflammatory infiltration, hepatocyte necrosis and fatty
degeneration, as well as changes in serum biochemical markers
(5–7). Although the exact mechanisms of INH
and RIF-induced liver injury remain unclear, several mechanisms
have been suggested, such as oxidative stress, lipid peroxidation,
dose-related toxicity, genotyping-related toxicity and increased
risk of toxic INH metabolites as a result of enzyme induction
(2,3). Endogenous lipid peroxidation has been
reported to be a primary factor driving the cytotoxic actions of
INH and RIF (5). INH and
RIF-mediated oxidative injury is generally attributed to the
accumulation of reactive oxygen species (ROS), which has been
indicated in cell signaling alterations, and can lead to
mitochondrial dysfunction, damage to the cell membrane and
apoptosis in hepatocytes (6).
Antioxidants may therefore serve a protective role against
INH-RIF-induced liver injury (7).
Nuclear factor-κB (NF-κB) is a ubiquitous
transcription factor that mediates immune and inflammatory
responses, cell proliferation and apoptosis (8). Pyrrolidine dithiocarbamate (PDTC), an
antioxidant and potent inhibitor of NF-κB, has previously been
shown to exert extensive anti-inflammatory and antioxidant effects
in rat models of liver cirrhosis (9), immunological liver injury (10), liver failure (11) and cholestatic liver injury
(12). Although it has been
suggested that PDTC can have hepatoprotective effects and
therapeutic value in various types of liver disease, its effects on
anti-TB drug-induced liver injury are yet to be elucidated.
The aim of the present study was to explore the
potential protective effects of PDTC on INH/RIF-induced liver
injury. The potential mechanisms underlying its therapeutic effects
were also investigated.
Materials and methods
Animals and experimental design
Male Sprague-Dawley rats (150–180 g; specific
pathogen-free grade) were obtained from the Experimental Animal
Center of Anhui Medical University (Hefei, China) and housed in
rooms with a maintained temperature of 22–25°C and humidity of
50±5% under a 12-h light-dark cycle, and provided food and water
ad libitum. Rats were acclimatized for 1 week prior to the
initiation of experiments. The animal experiments were approved by
the Animal Care and Use Committee of Anhui Medical University. A
total of 24 rats were randomly assigned to 3 groups (n=8/group) and
treated for 28 consecutive days as follows: Rats in the INH + RIF
and PDTC groups were intragastrically administered INH (50
mg/kg/day) and RIF (50 mg/kg/day; Yan'an Pharmaceutical Co., Ltd.)
following 3 h of abrosia (13,14).
Control rats were intragastrically treated with an equivalent
volume of 0.9% sodium chloride. Additionally, the PDTC group
received 50 mg/kg/day PDTC (Merck KGaA) via an intraperitoneal
injection 2 h following the co-administration of INH and RIF
(10,15,16).
The rats in the control and model groups were intraperitoneally
injected with the equivalent volume of 0.9% sodium chloride
(14). RIF and INH were
solubilized in 1% sodium carboxymethylcellulose and 0.9% sodium
chloride, respectively. PDTC initially was dissolved in DMSO at 10
mg/ml and then diluted by 0.9% sodium chloride to 50 mg/ml. Rats
were anesthetized by an intraperitoneal injection of sodium
pentobarbital (50 mg/kg) the morning following the final drug
treatment. Blood was collected into microtubes from the abdominal
aorta. Rat livers were rapidly excised, leaving the left lobe of
the liver in 10% neutral formalin-fixed buffer for histological
analysis. The remaining livers were snap-frozen and stored in a
liquid nitrogen container until required (17). Then, the rats were sacrificed by
decapitation. Serum samples were obtained from blood following
centrifugation at 4°C, 1,728 × g for 20 min and stored at −20°C for
detection.
Biochemical assays
The serum levels of total bile acid (TBA), total
bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase
(ALT), aspartate aminotransferase (AST) and alkaline phosphatase
(ALP) were measured using common commercial assay kits (TBA, ALT,
AST and ALP kits were obtained from Shanghai Kehua Bio-engineering
Co., Ltd.; TBIL and DBIL kits were obtained from Roche Diagnostics)
using an automatic biochemical analyzer (Roche Diagnostics) in the
clinical laboratory of the First Affiliated Hospital of Anhui
Medical University. Hepatic superoxide dismutase (SOD) activity and
malondialdehyde (MDA) content were detected in the supernatants
from 10% liver homogenates, which were prepared according to the
manufacturer's protocols. SOD and MDA kits were obtained from
Nanjing Jiancheng Bioengineering Institute.
Liver histopathology
Liver tissues were embedded in paraffin following
fixation in 10% formalin at room temperature for 24 h. Tissues were
subsequently cut into 4-µm thick sections 24 h later,
deparaffinized in xylene and hydrated in a graded series of
ethanol, and then routinely stained with hematoxylin for 5 min and
eosin for 10 sec at room temperature. From each sample, 5 adjacent,
non-overlapping fields were selected at random and observed using
routine light microscopy at ×400 magnification.
Electrophoretic mobility shift assay
(EMSA)
EMSA was performed according to the instructions of
the Light Shift Chemiluminescent EMSA kit (cat. no. 89880; Thermo
Fisher Scientific, Inc.) to detect the DNA binding activity of
NF-κB. Nuclear extracts from liver tissues were prepared using a
nuclear extraction kit (Viagene Biotech, Inc.) and quantified using
a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher
Scientific, Inc.). A biotin-labeled NF-κB probe (Santa Cruz
Biotechnology, Inc.) with a 5′-AGTTGAGGGGACTTTCCCAGGC-3′ sequence
and nuclear proteins (15 µg) were incubated in a total volume of 15
µl in binding buffer at room temperature for 20 min. The extracts
were also incubated with specific NF-κB p65 antibody (2 µg/µl; cat.
no. SC-372X; Santa Cruz Biotechnology, Inc.) at room temperature
for 20 min for a supershift assay. The specificity of binding was
detected by competition with unlabeled 100-fold excess of a cold
and a mutant probe with a 5′-TGGGGAACCTGTGCTGAGTCACTGGAG-3′
sequence (Thermo Fisher Scientific, Inc.). Following incubation,
the DNA-nuclear protein mixtures (15 µl) were subjected to gel
electrophoresis on a 6.5% nondenaturing polyacrylamide gel in 0.25X
Tris-borate-ethylenediaminetetraacetic buffer at 120 V for 60 min,
and then transferred to a presoaked nylon membrane. When the
transfer was complete, DNA was cross-linked to the membrane within
commercial UA-light for 10 min. The biotinylated DNA-protein bands
were detected with streptavidin-horseradish peroxidase (HRP; 1:750)
using a Chemiluminescent Assay (Thermo Fisher Scientific,
Inc.).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from liver tissues using
TRIzol reagent (Sangon Biotech Co., Ltd.) according to the
manufacturer's instructions. RNA concentration was measured by
NanoDrop™ 2000 (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). Then, cDNA was synthesized using a First Strand cDNA
Synthesis kit (Sangon Biotech Co., Ltd.), according to the
manufacturer's protocol. The reverse transcription system was
conducted at a final volume of 20 µl, including total RNA (5 µl),
primer mix (1 µl), dNTP Mix (2 µl), RNase inhibitor (1 µl), AMV
Reverse Transcriptase (2 µl), RNase-free ddH2O (5 µl),
5X RT buffer (4 µl). The PCR system (25 µl) comprised cDNA (0.5
µl), forward primer (0.5 µl), reverse primer (0.5 µl), Taq
polymerase (0.2 µl; Sangon Biotech Co., Ltd.), dNTP (0.5 µl), 10X
Taq Buffer (2.5 µl), MgCl2 (2 µl), RNase-free
ddH2O (18.3 µl). Amplification conditions were as
follows: Pre-denaturation at 95°C for 3 min, followed by 35 cycles
of denaturation at 94°C for 30 sec, annealing at 56°C for 30 sec,
extension at 72°C for 90 sec, final extension at 72°C for 8 min.
Primers were synthesized by Sangon Biotech Co., Ltd. Primer
sequences were as follows: Tumor necrosis factor (TNF)-α, forward
5′-GTCGTAGCAAACCACCAAGC-3′, reverse 5′-TCACAGAGCAATGACTCCAAAG-3′;
bile salt export pump (BSEP), forward 5′-GCACAGTTGCTGGGATTGG-3′,
reverse 5′-TTGGCATAGCTCGGAGTATAAGA-3′; β-actin, forward
5′-GCACAGTTGCTGGGATTGG-3′ and reverse
5′-TTGGCATAGCTCGGAGTATAAGA-3′.
All PCR products were examined by gel
electrophoresis and visualized by ethidium bromide staining. The
analysis of PCR products was performed using a gel documentation
system (Shanghai Furi Technology Co., Ltd.).
Western blot analysis
Liver tissues was homogenized and centrifuged at a
speed of 14,917 × g for 15 min at 4°C. Total proteins of liver
tissues were extracted using RIPA lysate with PMSF (both Beyotime
Institute of Biotechnology). After the protein concentration in the
supernatants was measured using a BCA protein assay kit (Beyotime
Institute of Biotechnology), the protein samples were mixed with 5X
SDS loading buffer at a ratio of 1:4, then denatured at 95°C for 10
min. Equal amounts of protein (30 µg) were separated via 12%
SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD
Millipore). Following incubation with blocking buffer (5% nonfat
milk) for 2 h at room temperature, membranes were incubated
overnight at 4°C with rabbit anti-BSEP (1:500; cat. no. BS71760;
Bioworld Technology, Inc.) and anti-β-actin (1:1,000; cat. no.
TA-09; OriGene Technologies, Inc.) primary antibodies, then washed
with TBST and incubated with an HRP-coupled secondary antibody
(1:10,000; cat. no. ZB-2301; Beijing Zhongshan Golden Bridge
Biotech Co., Ltd; OriGene Technologies, Inc.) for 1 h at room
temperature. The immunoblots were rinsed extensively with TBS-Tween
20 (0.1%) and visualized using an ECL western blot detection kit
(Thermo Fisher Scientific, Inc.). Signals were normalized to
β-actin levels, which was used as the internal standard.
Semi-quantitative evaluation was performed by densitometry using
ImageJ software (version 1.51; National Institutes of Health).
Statistical analysis
SPSS software (version 22.0; IBM Corp.) was used for
the statistical analysis. All data are presented as the mean ± SD.
Statistical analysis of the differences between groups was
performed using one-way analysis of variance (ANOVA) followed by
Student Newman-Keuls test as post hoc. P<0.05 was considered to
indicate a statistically significant difference.
Results
PDTC treatment ameliorates general
performance in INH/RIF-induced liver injury
There was no mortality in any of the groups. Rats in
the INH + RIF group showed loss of appetite, listlessness, rough
hair coat and slow response to external stimuli compared with the
control group. In addition, the skin and mucous membrane of the
nose, ears, paws and tail were dyed orange. Feces and urine were
also clearly orange. The general behavior of the rats in the PDTC
group was almost the same as that of control rats.
PDTC attenuates INH /RIF-induced liver
injury
Intraperitoneal administration of INH and RIF at a
dose of 50 mg/kg/day over a period of 28 days produced
hepatotoxicity. As presented in Table
I, the co-administration of INH and RIF significantly increased
serum TBA, TBIL, DBIL and ALP activity compared with the control
(P<0.01). There were no significant alterations in the levels of
ALT and AST (P>0.05), whereas PDTC treatment significantly
attenuated the elevation of serum TBA, TBIL, DBIL and ALP compared
with the INH + RIF group (P<0.01), which was suggestive of
reduced injury in liver tissues (Table
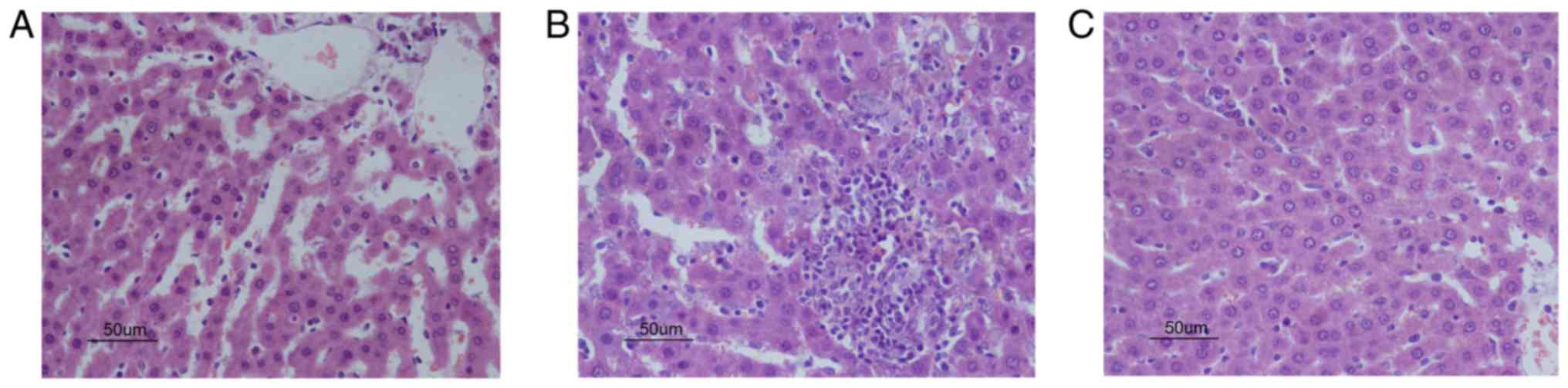
I). Next, as presented in Fig.
1, all animals in the control group showed normal morphology
(Fig. 1A). The INH + RIF group
exhibited moderate to severe lobular inflammation and piecemeal
necrosis (Fig. 1B). The liver
histology of the PDTC group exhibited an almost normal morphology,
with only a low level of liver steatosis and occasional
infiltration of inflammatory cells (Fig. 1C). Liver histopathology was
consistent with serum biochemistry in the present study.
 | Table I.PDTC attenuates liver functional
damage induced by INH and RIF. |
Table I.
PDTC attenuates liver functional
damage induced by INH and RIF.
|
|
| Parameters (mean ±
SD) |
|---|
|
|
|
|
|---|
| Group | N | TBA (µmo1/l) | TBIL (µmol/l) | DBIL (µmol/l) | ALP (U/l) | ALT (U/l) | AST (U/l) |
|---|
| Control | 8 | 22.13±2.70 | 1.02±0.13 | 0.22±0.07 | 82.00±8.38 | 42.34±5.77 | 137.21±12.28 |
| INH + RIF | 8 |
44.26±7.75a |
3.80±0.74a |
2.40±0.58a |
117.88±17.59a | 43.05±11.56 | 146.29±18.53 |
| PDTC | 8 |
22.56±4.08b |
2.20±0.44b |
1.20±0.21b |
93.00±10.07b | 20.32±4.61 | 114.32±9.79 |
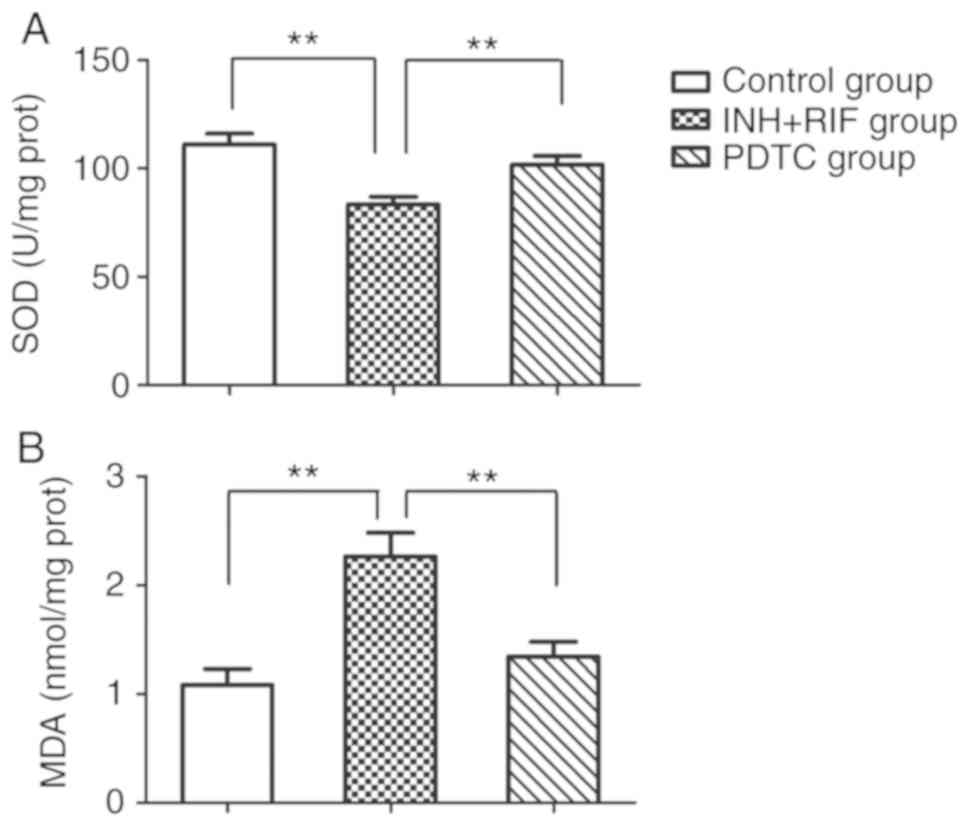
PDTC treatment alleviates
INH/RIF-induced oxidative stress
To evaluate the protective effects of PDTC against
INH/RIF-induced oxidative stress, SOD activity and MDA content in
liver tissue were detected. The SOD activity in liver homogenates
was significantly decreased in the INH + RIF group compared with
the control (P<0.01). However, the administration of PDTC
significantly attenuated the INH/RIF-induced reduction in SOD
activity (P<0.01). Similarly, the MDA content was significantly
elevated following co-administration of INH and RIF as compared
with the control group (P<0.01), whereas PDTC treatment
effectively decreased the INH/RIF-induced increase in MDA content
(P<0.01; Fig. 2).
PDTC inhibits INH/RIF-induced NF-κB
activation
EMSA was used to further explore the potential role
of NF-κB in INH/RIF-induced liver injury. Following the
co-administration of INH and RIF, the NF-κB DNA-binding activity of
liver homogenates was upregulated significantly (P<0.01); PDTC
treatment markedly inhibited INH/RIF-induced NF-κB activity
(P<0.01; Fig. 3A and B).
Furthermore, supershift analysis using p65 antibody against NF-κB
demonstrated that the band was in accordance with the p65 NF-κB
subunit (Fig. 3A). These results
suggested that NF-κB activation was involved in the pathogenesis of
INH and RIF-induced liver injury.
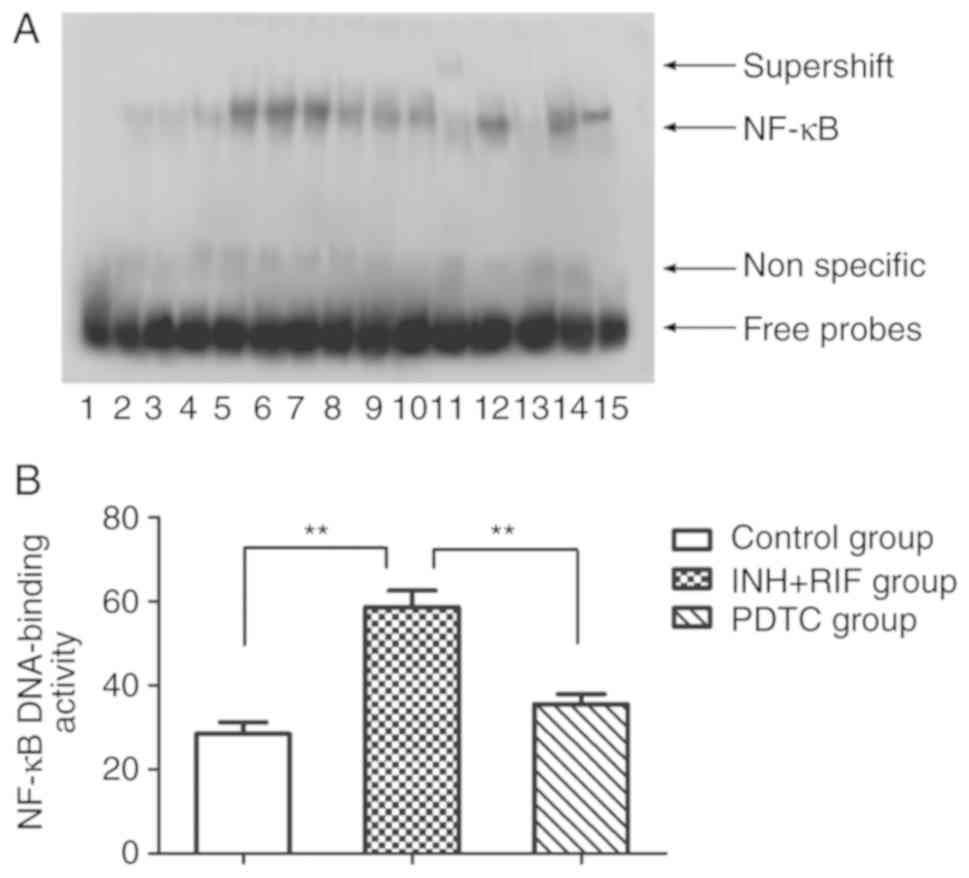 | Figure 3.NF-κB DNA-binding activity of rat
livers by EMSA. (A) Representative autoradiogram for each group.
Channel 1, negative control; channels 2–4, control group; channels
5–7, INH + RIF group; channels 8–10, PDTC group; channel 11,
nuclear extracts incubated with anti-p65 antibody; channel 12,
negative control of P65 antibody; channel 13, cold competition
control with a 100-fold excess of unlabeled probe; channel 14, cold
competition control with a 100-fold excess of unlabeled mutant
probe; channel 15, positive control of NF-κB activity. (B) Relative
amount of NF-κB DNA-binding activity. **P<0.01. NF-κB, nuclear
factor-κB. EMSA, Electrophoretic mobility shift assay; INH,
isoniazid; RIF, rifampicin; PDTC, pyrrolidine dithiocarbamate. |
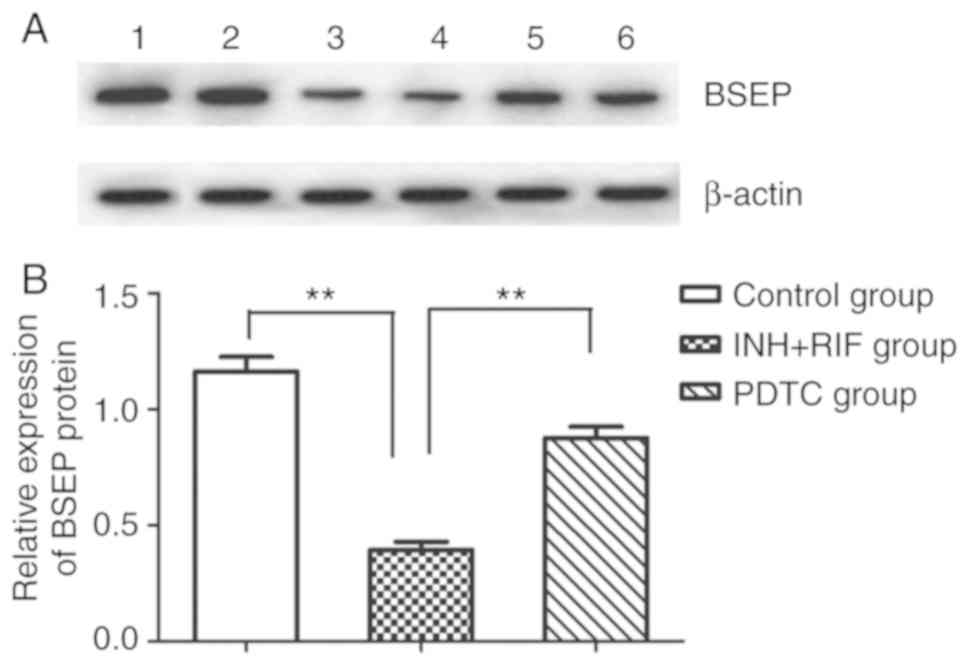
PDTC prevents INH/RIF-induced
upregulation of TNF-α and downregulation BSEP
To further study the possible mechanisms via which
PDTC alleviates INH and RIF-induced liver injury, the levels of
TNF-α and BSEP mRNA gene expression were detected in liver tissues
via RT-PCR, and the protein expression of BSEP was detected via
western blot analysis. Compared with the control group, the mRNA
expression of TNF-α and BSEP was significantly up- and
downregulated, respectively, in the INH + RIF group (both
P<0.01; Fig. 4A and B), and the
protein expression of BSEP was also significantly decreased in the
INH + RIF group (P<0.01; Fig. 5A
and B). Conversely, compared with the INH + RIF group, the mRNA
expression of TNF-α and BSEP was significantly decreased and
increased, respectively, in the PDTC group (P<0.01 and
P<0.05, respectively; Fig. 4A and
B), and the protein expression of BSEP was also significantly
elevated in the PDTC group (P<0.01; Fig. 5A and B). These results revealed
that PDTC could prevent INH/RIF-induced increases in TNF-α and
decreases in BSEP.
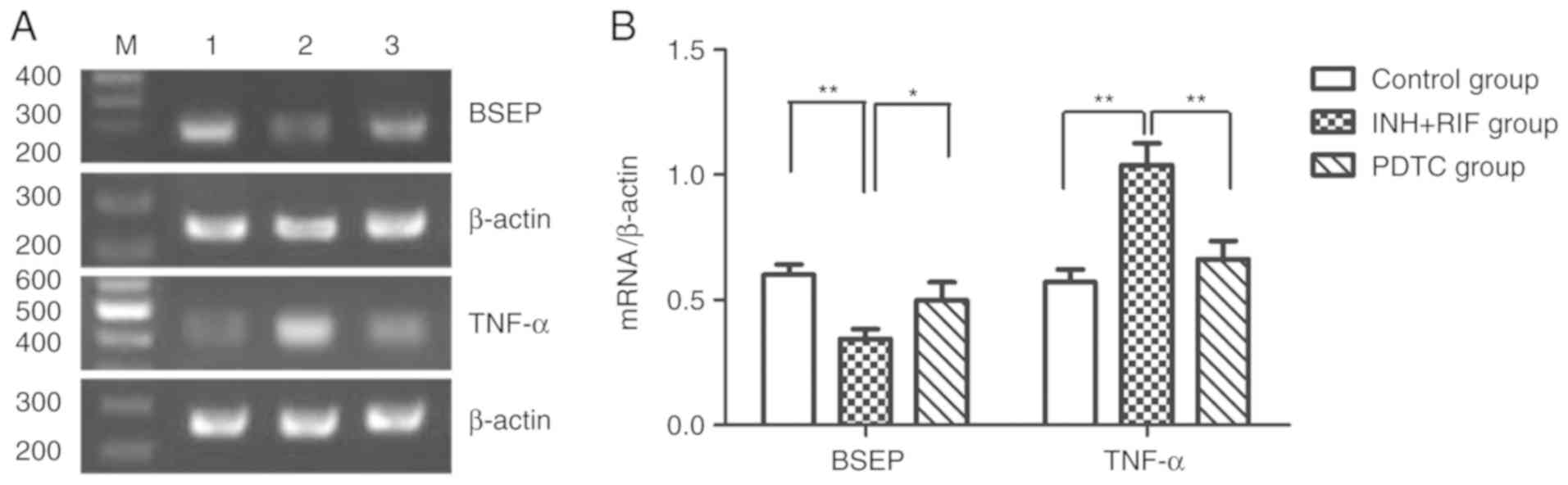 | Figure 4.Effects of PDTC on the mRNA
expression of BSEP and TNF-α in liver tissues, as determined via
reverse transcription-PCR. (A) Representative autoradiogram
results. 1, control group; 2, INH + RIF group; 3, PDTC group. (B)
Ratio of BSEP and TNF-α normalized to the β-actin mRNA level.
*P<0.05, **P<0.01. INH, isoniazid; RIF, rifampicin; PDTC,
pyrrolidine dithiocarbamate; BSEP, bile salt export pump; TNF,
tumor necrosis factor. |
Discussion
Liver injury is a highly prevalent clinical disease
worldwide, with its main types being viral hepatitis, drug-induced
liver injury (DILI), alcoholic liver disease, hepatic
ischemia-reperfusion injury, non-alcoholic fatty liver disease,
hepatocellular carcinoma and hepatic parasitic diseases (18–20).
Drug-related hepatotoxicity is one of the main challenges faced by
clinicians, as it is a major cause of liver failure characterized
by morphological and metabolic aberrations in the liver, owing to
the fact that the liver is the main drug detoxification organ
(5). In the present study, the
co-administration of INH and RIF (50 mg/kg/day for 28 days)
successfully induced a hepatotoxic model of drug-induced liver
injury in rats characterized by the elevation of serum markers,
hepatocellular steatosis, inflammatory cell infiltration, and
spotty and focal necrosis, consistent with previous studies
(13,14,21).
NF-κB activation has been implicated in the
pathogenesis of hepatocyte injury in a series of experimental
models of liver damage (9–12,16),
suggesting that the inhibition of NF-κB could serve as a potential
protective candidate against various types of liver disease. The
present study demonstrated that NF-κB was markedly activated by INH
+ RIF. PDTC treatment protected against INH-RIF-induced liver
injury by attenuating increases in serum TBA, TBIL, DBIL and ALP
levels, and improving histopathological changes. These results
suggested a protective effect of PDTC against anti-TB drug-induced
liver injury. The results also showed that the PDTC group exhibited
a lower level of ALT and AST than the control group; however, this
was not significant. A previous study reported that the level of
ALT was lower in a PDTC group compared with a control group in a
rat model of liver injury induced by intestinal
ischemia-reperfusion, which is a similar result to the present
study (22). However, to the best
of the authors' knowledge, no study has yet elucidated the specific
mechanism underlying this phenomenon. Therefore, the results should
be interpreted with caution, due to the limited number of reports,
and further large-scale, well-designed randomized controlled trials
on this topic are required.
Previous studies have shown that oxidative stress
serves an important role in the pathogenesis of INH-RIF-induced
liver injury (21,23), which is generally attributed to the
accumulation of ROS, also known as a secondary messenger to
activate NF-κB (24,25). The present study showed that
hepatic SOD activity was markedly decreased following INH + RIF
administration. Furthermore, the hepatic MDA content was
significantly elevated, supporting previous observations that
oxidative stress may play a partial role in INH and RIF-induced
liver injury.
PDTC exerts direct antioxidant effects by inhibiting
free radical generation and scavenging free radicals via its
chelating activity for metal ions, which may catalyze the formation
of free radicals (26).
Additionally, PDTC has been shown to alleviate oxidative injury via
the inhibition of oxidative stress in drug-induced hepatic
fibrosis, drug-induced acute kidney injury and gastroesophageal
reflux disease in experimental models (9,27,28).
In the present study, it was observed that PDTC administration
significantly reversed the reduction in SOD activity and attenuated
the elevation of the MDA level. These results indicated that PDTC
might protect against INH/RIF-induced liver injury via its
antioxidant effects.
A growing body of evidence has indicated that TNF-α
plays an important role in the pathogenesis of DILI by mediating
direct hepatocyte injury and accelerating inflammatory cytokine
production (29). TNF-α binding to
the TNF receptor initiates NF-κB activation, inducing the
expression of inflammatory genes and the release of a large number
of inflammatory factors (30). In
addition, TNF-α has been shown to result in ROS generation, mainly
through mitochondrial pathways or NADPH oxidase (31,32),
which upregulate inflammatory cascades and promote liver injury.
Results from the present study showed that a marked inflammatory
response in INH and RIF-induced liver injury, demonstrated by
inflammatory cell infiltration, markedly activated hepatic NF-κB
and upregulated hepatic TNF-α mRNA. These results indicated that
the NF-κB-mediated upregulation of TNF-α may be involved in the
pathogenesis of INH/RIF-induced liver injury. Several studies have
shown that PDTC can prevent inflammatory injury via the inhibition
of NF-κB and the subsequent downregulation of TNF-α (27,33).
In the present study, it was found that the administration of PDTC
reduced inflammation, suppressed NF-κB activation and resulted in
the downregulation of TNF-α mRNA, indicating that the
anti-inflammatory action of PDTC partially contributed to liver
protection.
In the present study, it was observed that the
INH/RIF-induced clinical liver dysfunction of rats predominantly
manifested as increased levels of TBA, TBIL, DBIL and ALP, which
indicated a cholestatic DILI (34). It has been reported that
cholestatic liver injury may be associated with impaired
hepatobiliary transporters expressed at the apical membrane of
hepatocytes (35). BSEP, an
ATP-binding cassette transporter encoded by the ABCB11 gene,
is primarily localized on the canalicular membrane of hepatocytes
and regulates conjugated bile acid excretion, as well as hepatic
excretion of various forms of drugs and their metabolites into bile
(36). The inhibition of BSEP
activity could lead to decreased biliary excretion, accumulation of
bile salts inside hepatocytes and cholestatic liver disease
(37). It has been reported that
RIF is a competitive inhibitor of BSEP (38). An in vitro study also
demonstrated that anti-TB drug administration markedly
downregulated hepatogenic BSEP (39). Results from the present study were
consistent with previous reports, showing a reduction in BSEP
coupled with decreased levels of TBA, TBIL, DBIL and ALP in INH +
RIF-treated rats, which implied that the inhibition of BSEP
resulting in the disruption of bile acid homeostasis may be a
potential mechanism underlying INH/RIF-induced liver injury. PDTC
treatment markedly upregulated BSEP and reversed the elevation of
serum markers. These results suggested that PDTC may protect
against INH/RIF-induced hepatic injury by increasing the expression
of BSEP. However, the underlying mechanisms of PDTC-induced BSEP
upregulation remain poorly understood.
It has previously been indicated that inflammation
plays a crucial role in cholestatic liver injury (40). Certain studies have suggested that
BSEP expression can be reduced by proinflammatory cytokines
activating specific signaling pathways during inflammation
(41–43). In the present study, it was found
that TNF-α was markedly elevated when NF-κB was activated in INH
and RIF-treated rats with a concurrent decrease in BSEP expression,
indicating a possible crosstalk between BSEP and the NF-κB/TNF-α
signaling pathways. These data were consistent with the hypothesis
that inflammation leads to the downregulation of BSEP, and that the
restoration of its expression by inhibiting inflammation in the
liver may re-establish bile acid homeostasis (44). PDTC therapy may therefore
upregulate the expression of BSEP and improve cholestasis by
controlling inflammatory pathways as a result of NF-κB
inhibition.
In conclusion, the findings of the present study
indicated that PDTC exerted protective effects against
INH/RIF-induced hepatic injury. The anti-inflammatory and
antioxidant effects, as well as upregulation of BSEP, may underlie
the therapeutic actions of PDTC in the treatment of INH/RIF-induced
liver injury. Moreover, it is important to investigate whether
these experimental observations can be validated in liver cell
models. Therefore, future studies will focus on the effect of PDTC
on anti-TB-drug-induced liver injury in vitro.
Acknowledgements
Not applicable.
Funding
This work was funded by Natural Science Foundation
of Anhui Province (grant no. 1208085MH155).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH was involved in the acquisition and analysis of
data, and drafting the article. YS contributed to the experimental
design, the interpretation of data, revising the manuscript, and
supervision and guidance. LW was involved in the acquisition and
analysis of data. JX contributed to the experimental design,
revised the manuscript and provided supervision and guidance. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures for treating animals in
this study were performed according to National Regulations on the
Administration of Laboratory Animals and approved by the Animal
Care and Use Committee of Anhui Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
INH
|
isoniazid
|
|
RIF
|
rifampicin
|
|
TB
|
tuberculosis
|
|
NF-κB
|
nuclear factor-κB
|
|
PDTC
|
pyrrolidine dithiocarbamate
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
ALP
|
alkaline phosphatase
|
|
TBA
|
total biliary acid
|
|
TBIL
|
total bilirubin
|
|
DBIL
|
direct bilirubin
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
BSEP
|
bile salt export pump
|
|
TNF-α
|
tumor necrosis factor-α
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
World Health Organization, . Global
tuberculosis report 2015. World Health Organization; Geneva:
2015
|
|
2
|
Yew WW and Leung CC: Antituberculosis
drugs and hepatotoxicity. Respirology. 11:699–707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yew WW, Chang KC and Chan DP: Oxidative
stress and first-line antituberculosis drug-induced hepatotoxicity.
Antimicrob Agents Chemother. 62:e02637–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baskaran UL and Sabina EP: Clinical and
experimental research in antituberculosis drug-induced
hepatotoxicity: A review. J Integr Med. 15:27–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santhosh S, Sini TK, Anandan R and Mathee
PT: Hepatoprotective activity of chitosan against isoniazid and
rifampicin-induced toxicity in experimental rats. Eur J Pharmacol.
572:69–73. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chowdhury A, Santra A, Bhattacharjee K,
Ghatak S, Saha DR and Dhali GK: Mitochondrial oxidative stress and
penneability transition in isoniaizd and Rifampicin induced liver
injury in mice. J Hepatol. 45:117–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicoletti NF, Rodrigues-Junior V, Santos
AA Jr, Leite CE, Dias AC, Batista EL Jr, Basso LA, Campos MM,
Santos DS and Souto AA: Protective effects of resveratrol on
hepatotoxicity induced by isoniazid and rifampicin via SIRT1
modulation. J Nat Prod. 77:2190–2195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leotoing L, Chereau F, Baron S, Hube F,
Valencia HJ, Bordereaux D, Demmers JA, Strouboulis J and Baud V:
A20-binding inhibitor of nuclear factor-kappaB (NF-kappaB)-2
(ABIN-2) is an activator of inhibitor of NF-kappaB (IkappaB) kinase
alpha (IKKalpha)-mediated NF-kappaB transcriptional activity. J
Biol Chem. 286:32277–32288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruck R, Schey R, Aeed H, Hochman A,
Genina O and Pines M: A protective effect of pyrrolidine
dithiocarbamate in a rat model of liver cirrhosis. Liver Int.
24:169–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin JD, Cao ZH, Li XF, Kang XL, Xue Y, Li
YL, Zhang D, Liu XY and Xue YZ: Effect of ammonium pyrrolidine
dithiocarbamate (PDTC) on NF-κB activation and CYP2E1 content of
rats with immunological liver injury. Pharm Biol. 52:1460–1466.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang F, Li X, Wang LK, Wang LW, Han XQ,
Zhang H and Gong ZJ: Inhibitions of NF-κB and TNF-α result in
differential effects in rats with acute on chronic liver failure
induced by d-Gal and LPS. Inflammation. 37:848–857. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demirbilek S, Akin M, Gürünlüoğlu K, Aydin
NE, Emre MH, Taş E, Aksoy RT and Ay S: The NF-kappaB inhibitors
attenuate hepatic injury in bile duct ligated rats. Pediatr Surg
Int. 22:655–663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rana SV, Pal R, Vaiphie K and Singh K:
Effect of different oral doses of isoniazid-rifampicin in rats. Mol
Cell Biochem. 289:39–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pal R, Vaiphei K, Sikander A, Singh K and
Rana SV: Effect of garlic on isoniazid and rifampicin-induced
hepatic injury in rats. World J Gastroenterol. 12:636–639. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsui N, Kasajima K, Hada M, Nagata T,
Senga N, Yasui Y, Fukuishi N and Akagi M: Inhibiton of NF-kappaB
activation during ischemia reduces hepatic ischemia/reperfusion
injury in rats. J Toxicol Sci. 30:103–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heiman J, Wallin M, Gustafsson BI, Friman
S and Delbro D: Pharmacological of rat liver by up-regulation of
heme oxygenase 1. Transplant Proc. 38:2705–2707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan M, Song YL, Xu JM and Gan HZ:
Melatonin ameliorates nonalcoholic fatty liver induced by high-fat
diet in rats. J Pineal Res. 41:79–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Işık A, Fırat D, Korkmaz S, Demiryılmaz İ
and Yılmaz İ: Atipik prezente kist hidatik: Pankreas Başında Kitle.
Sakarya Tıp Dergisi. 8:149–152. 2018.
|
|
20
|
Işık A, Sayar İ, Gülhan B and Firat D:
Fascioliasis: A rare case mimicking cholelithiasis. J Kartal TR.
27:145–146. 2016.
|
|
21
|
Sodhi CP, Rana SV, Mehta SK, Vaiphei K,
Attari S and Mehta S: Study of oxidative-stress in
isoniazid-rifampicin induced hepatic injury in young rats. Drug
Chem Toxicol. 20:255–269. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian XF, Yao JH, Li YH, Gao HF, Wang ZZ,
Yang CM and Zheng SS: Protective effect of pyrrolidine
dithiocarbamate on liver injury induced by intestinal
ischemia-reperfusion in rats. Hepatobiliary Pancreat Dis Int.
5:90–95. 2006.PubMed/NCBI
|
|
23
|
Rana SV, Pal R, Vaiphei K, Ola RP and
Singh K: Hepatoprotection by carotenoids in isoniazid-rifampicin
induced hepatic injury in rats. Biochem Cell Biol. 88:819–834.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narayanan A, Amaya M, Voss K, Chung M,
Benedict A, Sampey G, Kehn-Hall K, Luchini A, Liotta L, Bailey C,
et al: Reactive oxygen species activate NFκB (p65) and p53 and
induce apoptosis in RVFV infected liver cells. Virology.
449:270–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kastl L, Sauer SW, Ruppert T, Beissbarth
T, Becker MS, Süss D, Krammer PH and Gülow K: TNF-α mediates
mitochondrial uncoupling and enhances ROS-dependent cell migration
via NF-κB activation in liver cells. FEBS Lett. 588:175–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu JW, Wang H, Yan-Li J, Zhang C, Ning H,
Li XY, Zhang H, Duan ZH, Zhao L, Wei W and Xu DX: Differential
effects of pyrrolidine dithiocarbamate on TNF-alpha-mediated liver
injury in two different models of fulminant hepatitis. J Hepatol.
48:442–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu HX, Wang XL, Zhang LN, Zhang J and Zhao
W: Involvement of the TLR4/NF-κB signaling pathway in the repair of
esophageal mucosa injury in rats with gastroesophageal reflux
disease. Cell Physiol Biochem. 51:1645–1657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Borghi SM, Fattori V, Ruiz-Miyazawa KW,
Bertozzi MM, Lourenco-Gonzalez Y, Tatakihara RI, Bussmann AJC,
Mazzuco TL, Casagrande R and Verri WA Jr: Pyrrolidine
dithiocarbamate inhibits mouse acute kidney injury induced by
diclofenac by targeting oxidative damage, cytokines and NF-κB
activity. Life Sci. 208:221–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masson MJ, Collins LA and Pohl LR: The
role of cytokines in the mechanism of adverse drug reactions. Handb
Exp Pharmacol. 195–231. 2010.doi: 10.1007/978-3-642-00663-0_8.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blaser H, Dostert C, Mak TW and Brenner D:
TNF and ROS crosstalk in inflammation. Trends Cell Biol.
26:249–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morgan MJ and Liu ZG: Reactive oxygen
species in TNFalpha-induced signaling and cell death. Mol Cells.
30:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moe KT, Khairunnisa K, Yin NO,
Chin-Dusting J, Wong P and Wong MC: Tumor necrosis factor-α-induced
nuclear factor-kappaB activation in human cardiomyocytes is
mediated by NADPH oxidase. J Physiol Biochem. 70:769–779. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He CY, Jiang LP, Wang CY and Zhang Y:
Inhibition of NF-κB by pyrrolidine dithiocarbamate prevents the
inflammatory response in a ligature-induced peri-implantitis model:
A canine study. Cell Physiol Biochem. 49:610–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Padda MS, Sanchez M, Akhtar AJ and Boyer
JL: Drug-induced cholestasis. Hepatology. 53:1377–1387. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Corsini A and Bortolini M: Drug-induced
liver injury: The role of drug metabolism and transport. J Clin
Pharmacol. 53:463–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kubitz R, Dröge C, Kluge S, Stindt J and
Häussinger D: Genetic variations of bile salt transporters. Drug
Discov Today Technol. 12:e55–e67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kis E, Ioja E, Rajnai Z, Jani M, Méhn D,
Herédi-Szabó K and Krajcsi P: BSEP inhibition: In vitro screens to
assess cholestatic potential of drugs. Toxicol In Vitro.
26:1294–1299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stieger B: The role of the
sodium-taurocholate cotransporting polypeptide (NTCP) and of the
bile salt export pump (BSEP) in physiology and pathophysiology of
bile formation. Handb Exp Pharmacol. 205–259. 2011.doi:
10.1007/978-3-642-14541-4_5. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo YX, Xu XF, Zhang QZ, Li C, Deng Y,
Jiang P, He LY and Peng WX: The inhibition of hepatic bile acids
transporters Ntcp and Bsep is involved in the pathogenesis of
isoniazid/rifampicin-induced hepatotoxicity. Toxicol Mech Methods.
25:382–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sato K, Hall C, Glaser S, Francis H, Meng
F and Alpini G: Pathogenesis of kupffer cells in cholestatic liver
injury. Am J Pathol. 186:2238–2247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Le Vee M, Lecureur V, Stieger B and Fardel
O: Regulation of drug transporter expression in human hepatocytes
exposed to the proinflammatory cytokines tumor necrosis
factor-alpha or interleukin-6. Drug Metab Dispos. 37:685–693. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Donner MG, Schumacher S, Warskulat U,
Heinemann J and Häussinger D: Obstructive cholestasis induces
TNF-alpha- and IL-1-mediated periportal downregulation of Bsep and
zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol
Gastrointest Liver Physiol. 293:G1134–G1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Diao L, Li N, Brayman TG, Hotz KJ and Lai
Y: Regulation of MRP2/ABCC2 and BSEP/ABCB11 expression in sandwich
cultured human and rat hepatocytes exposed to inflammatory
cytokines TNF-{alpha}, IL-6, and IL-1{beta}. J Biol Chem.
285:31185–31192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Y, Song X, Valanejad L, Vasilenko A,
More V, Qiu X, Chen W, Lai Y, Slitt A, Stoner M, et al: Bile salt
export pump is dysregulated with altered farnesoid X receptor
isoform expression in patients with hepatocellular carcinoma.
Hepatology. 57:1530–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|