Introduction
Neurodegeneration refers to the gradual loss of
neuron structure or function, including neuronal death. Diseases
such as stroke, Parkinson's, Alzheimer's, and Huntington's disease
result from neurodegeneration. Brain tissue is vulnerable to
oxidative stress from either pathological or physiological causes,
which results from the aging processes or neurodegenerative
diseases (1). Oxidative stress in
neurons is a major cause of neuronal apoptosis in neurodegenerative
diseases (2).
The phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (AKT) pathway is known to regulate various intracellular
downstream signals such as apoptosis, cell growth, and
differentiation, through oxidative stimulation (3). In addition, PI3K activates nuclear
transcription of the nuclear factor-E2-related factor 2 (Nrf2),
which promotes neural cell survival (4,5).
Nrf2 has a leading role in the production of phase II enzymes
regulated by antioxidant response elements (AREs) (6,7).
Previous studies have reported the effect of Nrf2 activation and
the subsequent HO-1 expression on nerve damage and oxidative stress
in various models of neurodegenerative disorders (8,9).
HO-1 is instrumental in the regulation of the biological responses
that protect cells against oxidative stress-induced toxicity
(10). In addition, HO-1
expression by inducers is one of the major cytoprotective
mechanisms in HT22 cells with glutamate-induced oxidative toxicity
(11). In addition, the activation
of mitogen-activated protein kinase (MAPK) regulates the expression
of several genes and proteins related to cytoprotective mechanisms,
including the activation of HO-1 (12).
Brassica rapa is composed of numerous
compounds, including flavonoids, phenylpropanoid derivatives,
indole alkaloids, sterol glucosides, and fatty acids (13,14).
However, there has been no study of the molecular targets of B.
rapa and their anti-neurodegenerative biological activity.
Therefore, as part of this screening research project to assess the
neuroprotective potential of natural food components from B.
rapa, we isolated a phenanthrene-derivative named BrPA and
investigated its neuroprotective effects in HT22 cells.
Materials and methods
Materials
BrPA was isolated from B. rapa by a
previously described method (15).
All cell culture reagents were purchased from Gibco; Thermo Fisher
Scientific, Inc.. All inhibitors were purchased from Calbiochem.
Primary and secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc.. All other chemicals were sourced from
Sigma-Aldrich; Merck KGaA, unless otherwise stated.
Cell culture and MTT assay
Mouse hippocampal HT22 cells were donated by
Professor Youn-Chul Kim of Wonkwang University. Mouse hippocampal
HT22 cells were incubated in DMEM containing 1% antibiotic
(Penicillin-Streptomycin) and 10% heat-inactivated FBS at 37°C in a
humidified 5% CO2 and 95% air atmosphere. The media,
antibiotics and FBS used for cell culture were purchased from
Gibco; Thermo Fisher Scientific, Inc.. To test cell viability by
using the MTT assay, mouse hippocampal HT22 cells were maintained
and treated with BrPA (2.5–20 µM) in the absence or presence of 5
mM glutamate for 12 h. The cells were then treated with 500 µg/ml
MTT and incubated at 37°C for 1 h, subsequently the resulting
formazan product in each well was dissolved in DMSO. The dissolved
formazan was detected at a wavelength of 540 nm.
ROS generation assay
To check ROS generation, mouse hippocampal HT22
cells were incubated treat glutamate (5 mM) with or without BrPA
(2.5–20 µM) or SnPP IX (HO inhibitor) (50 µM). Subsequently, the
cells were incubated at 37°C. for 12 h after glutamate treatment.
After washing, Hank's balanced salt solution containing 10 µM
2′,7′-dichlorofluorescein diacetate (DCFDA) was added to each well
of the cell culture plate and stained in the dark for 60 min;
subsequently, the medium was removed, the cells were washed twice,
and extracted with 1% Triton X-100 in PBS at 37°C for 10 min. The
fluorescence of each sample at 490 nm and 525 nm was measured by
using a SpectraMax Gemini XS (Molecular Devices).
Western blotting analysis and the
extraction of cytoplasmic and nuclear cell fractions
The pelleted HT22 cells were washed with PBS, and
then lysed in RIPA buffer. Equal amounts of proteins quantified by
BCA assay were mixed in the sample loading buffer and separated by
SDS-PAGE. The separated proteins were transferred to a
nitrocellulose membrane. Non-specific binding to the membrane was
blocked by incubation in a solution of skimmed milk. The membrane
was incubated with primary antibodies at 4°C overnight, and then
reacted with a horseradish peroxidase-conjugated secondary antibody
from Santa Cruz Biotechnology. To extract the cytoplasmic and
nuclear cell components, HT22 cells were washed with PBS, pelleted,
and lysed with NE-PER reagents (Pierce; Thermo Fisher Scientific,
Inc.). All experimental procedures were performed in accordance
with the manufacturer's data sheet. Cell lysate was used for
western blotting as described above.
Transfections and luciferase
assays
To construct ARE-Luciferase vector, the pGL2
promoter plasmid (Promega Corporation) was introduced into
5′-TGACTCAGCA-3′ at the Nrf2 binding site to bind the
ARE-luciferase vector. The cell lysate for the luciferase assay was
mixed with luciferase substrate solution (Promega Corporation), and
luciferase activity was measured using a luminometer. Luciferase
activity was determined in triplicate for each experiment and
normalized for each sample using β-galactosidase activity. In
addition, siRNA transfections of Nrf2 were tested using a
commercially available reagent Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Briefly, cells were transiently transfected with Nrf2
siRNA for 6 h and recovered in fresh media containing 10% FBS at
37°C for 24 h. Cells transfected with Nrf2 siRNA were cultured with
BrPA (20 µM) for 1.5 h (Nuclear Nrf2) or 12 h (HO-1, GST). In
addition, HT22 cells were pre-treated with or without SnPP (50 µM)
and Nrf2 siRNA, and then cultured with BrPA (20 µM), and later
treated with glutamate (5 mM) for 12 h. The sequences of Nrf2 siRNA
were sense: 5′-GAGGAUGGGAAACCUUACUTT-3′, antisense:
5′-AGUAAGGUUUCCCAUCCUCTT-3′. The sequences of scramble siRNA were
sense: 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense:
5′-ACGUGACACGUUCGGAGAATT-3′.
Glutathione (GSH) determination
To evaluate GSH production, mouse hippocampal HT22
cells were assessed by using a glutathione assay kit from Cayman,
and all experimental procedures were performed in accordance with
the manufacturer's instructions.
Statistical analyses
All data were acquired from three independent
experiments and expressed as the mean ± SD. Statistical analyses
were computed by using GraphPad Prism v. 3.03 (GraphPad Software
Inc.). The mean differences were derived using one-way ANOVA and
Newman-Keuls post hoc test, with statistical significance accepted
for values of P<0.05.
Results
Effects of BrPA on glutamate-induced
cell toxicity and ROS generation
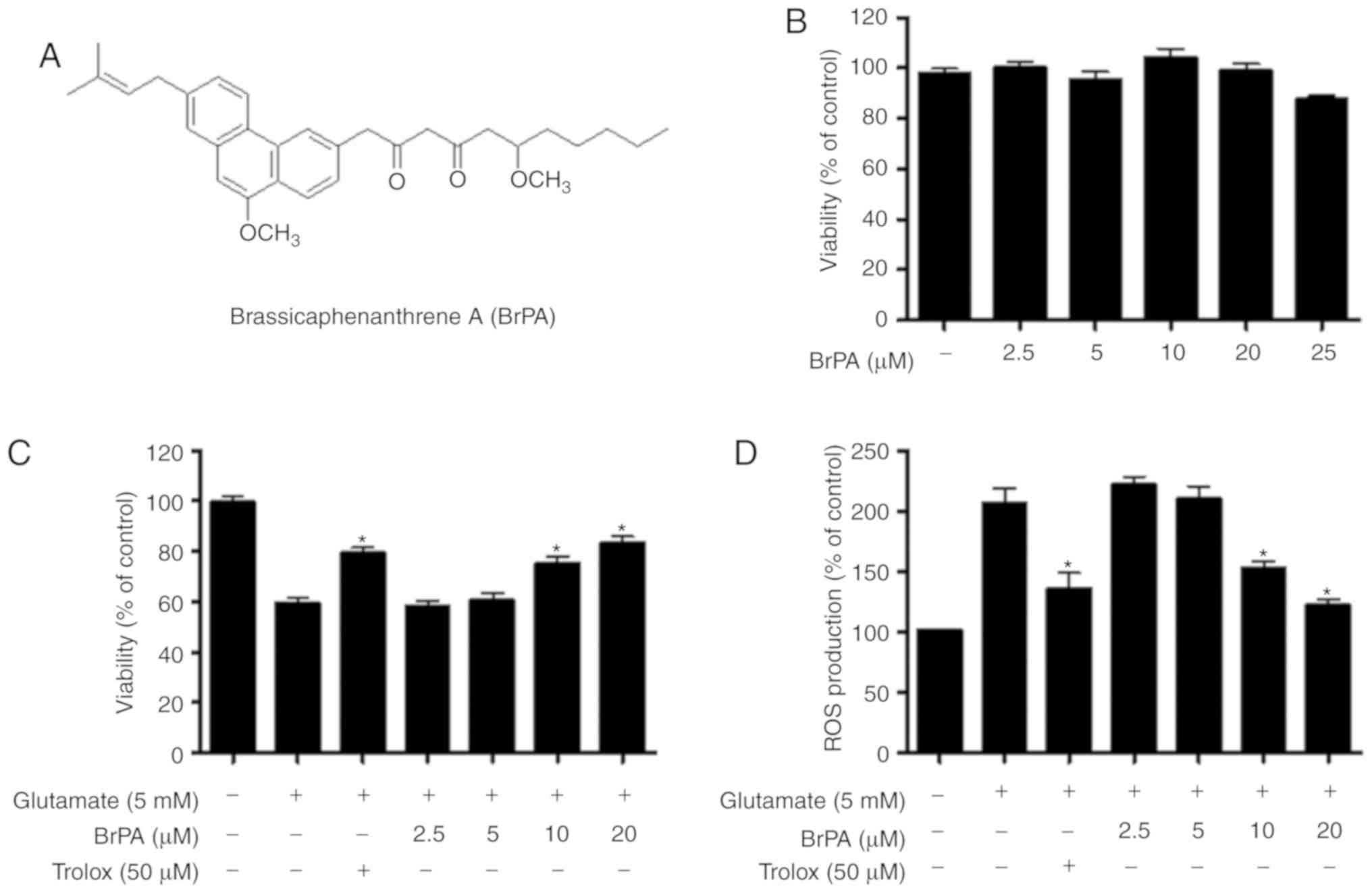
To investigate the cytotoxic potential of BrPA
(Fig. 1A), we first examined the
viability of mouse hippocampal HT22 cells treated with BrPA by
using the MTT assay. No decrease in cell viability was observed at
concentrations below 20 µM (Fig.
1B). To investigate if BrPA exhibited cytoprotective effects
and ROS scavenging effects on glutamate-stimulated cytotoxicity and
ROS production, respectively, HT22 cells pre-incubated with BrPA
were treated with glutamate for 12 h. BrPA increased cytoprotective
activity (Fig. 1C) and ROS
scavenging in glutamate-induced HT22 cells (Fig. 1D).
Effects of BrPA on HO-1 expression in
HT22 cells
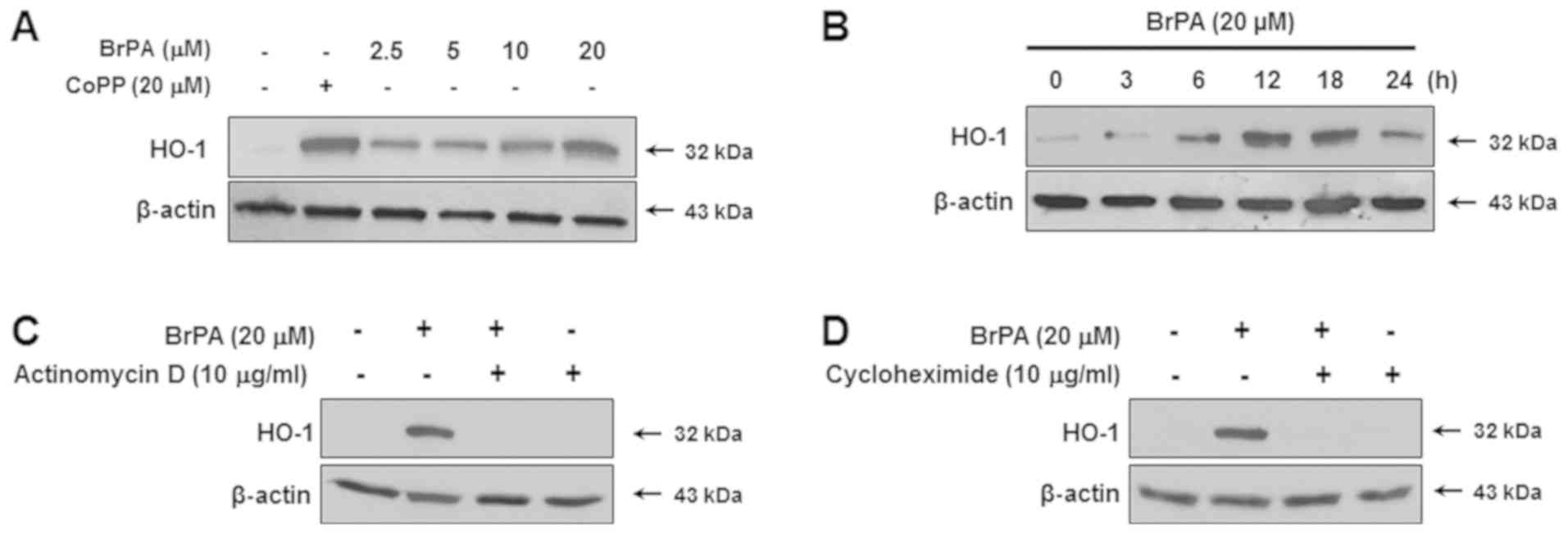
We examined whether BrPA affected in HO-1 protein
expression. As shown in Fig. 2A,
it caused a significant upregulation in HO-1 protein expression,
with a maximum increase caused by 20 µM. At this concentration,
peaks in HO-1 expression were evident at 6 h and approximately 18 h
(Fig. 2B). To determine if the
expression of HO-1 induced by BrPA was transcribed, HT22 cells were
treated with actinomycin D (a transcription inhibitor) or
cycloheximide (a translational inhibitor). Both treatments
completely blocked HO-1 expression (Fig. 2C and D).
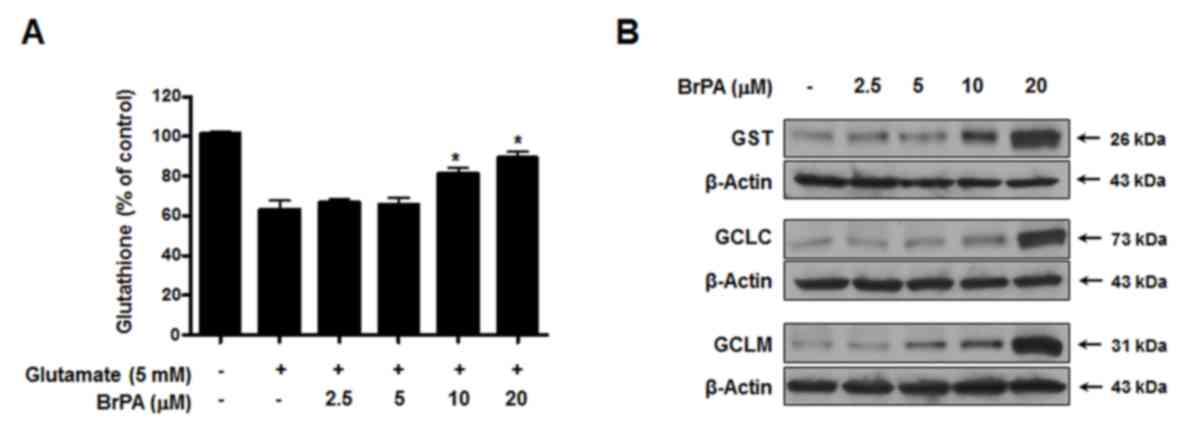
Effects of BrPA on cellular GSH levels
and GSH synthesis enzymes
GSH was depleted in HT22 cells treated with
glutamate, and cells treated with glutamate and BrPA
dose-dependently prevented the glutamate-induced GSH depletion
(Fig. 3A). In addition,
glutathione S-transferase (GST) levels were also upregulated by
treatment with BrPA (Fig. 3B). The
effects of BrPA on glutamate-cysteine ligase (GCL) expression were
also examined. GCL is a rate-limiting enzyme involved in GSH
synthesis, consisting of a catalytic heavy subunit,
glutamate-cysteine ligase catalytic subunit (GCLC), modulatory
light subunit, and a glutamate-cysteine ligase modifier subunit
(GCLM). As shown in Fig. 3B, BrPA
treatment increased GCLC and GCLM in a dose-dependent manner.
Effects of BrPA on the regulation of
Nrf2-related signaling
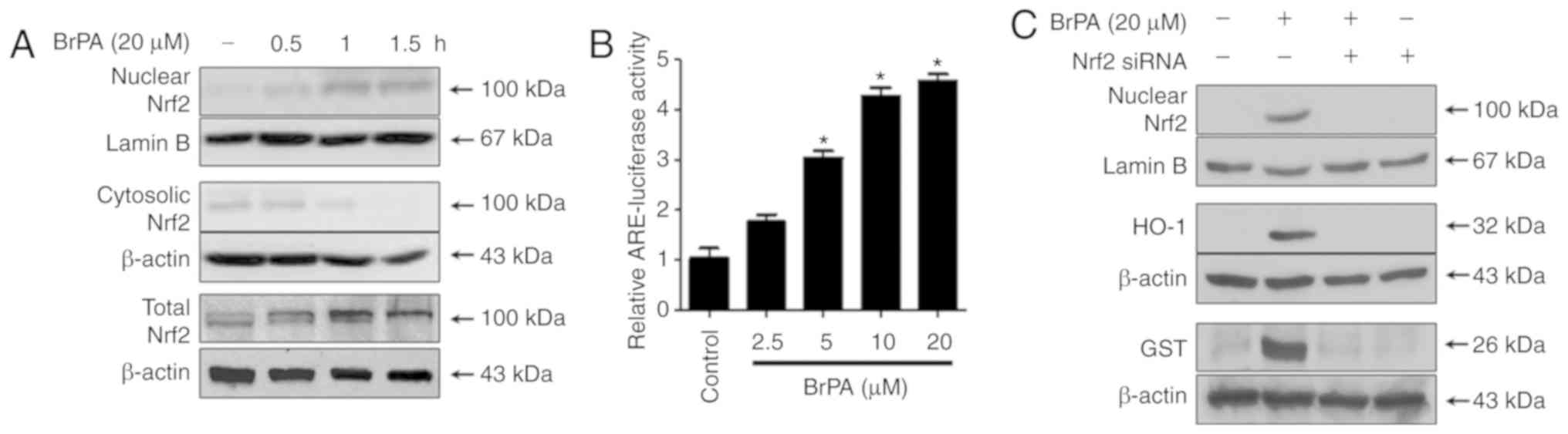
We investigated the translocation of Nrf2 to the
nucleus of HT22 cells treated with 20 µM BrPA for 0.5, 1, and 1.5
h. Nrf2 levels gradually increased in the nuclear fraction, but
were decreased in the cytoplasmic fraction of cells treated with
BrPA, suggesting that BrPA promoted the nuclear translocation of
Nrf2 (Fig. 4A). In addition, BrPA
increased the expression of total Nrf2 (Fig. 4A). BrPA was modulated in
transiently transfected HT22 cells with the ARE-luciferase plasmid
and the modulating effect of luciferase activity was measured by
using the ARE activation assay. BrPA upregulated ARE-driven
luciferase activity (Fig. 4B), and
this activation was closely correlated with the increase in HO-1
and expression of GST (Figs. 2 and
3). Similarly, we tested
BrPA-induced HO-1 and GST expression by using siRNA to silence
Nrf2. Transient transfection of HT22 cells with siRNA Nrf2 was
performed and the cells were treated with 20 µM BrPA for 12 h (HO-1
and GST) or 1.5 h (nuclear Nrf2). Nrf2 siRNA significantly blocked
BrPA-induced Nrf2 nuclear translocation. Transient transfection
with Nrf2 siRNA reduced BrPA-induced GST and HO-1 expression
(Fig. 4C).
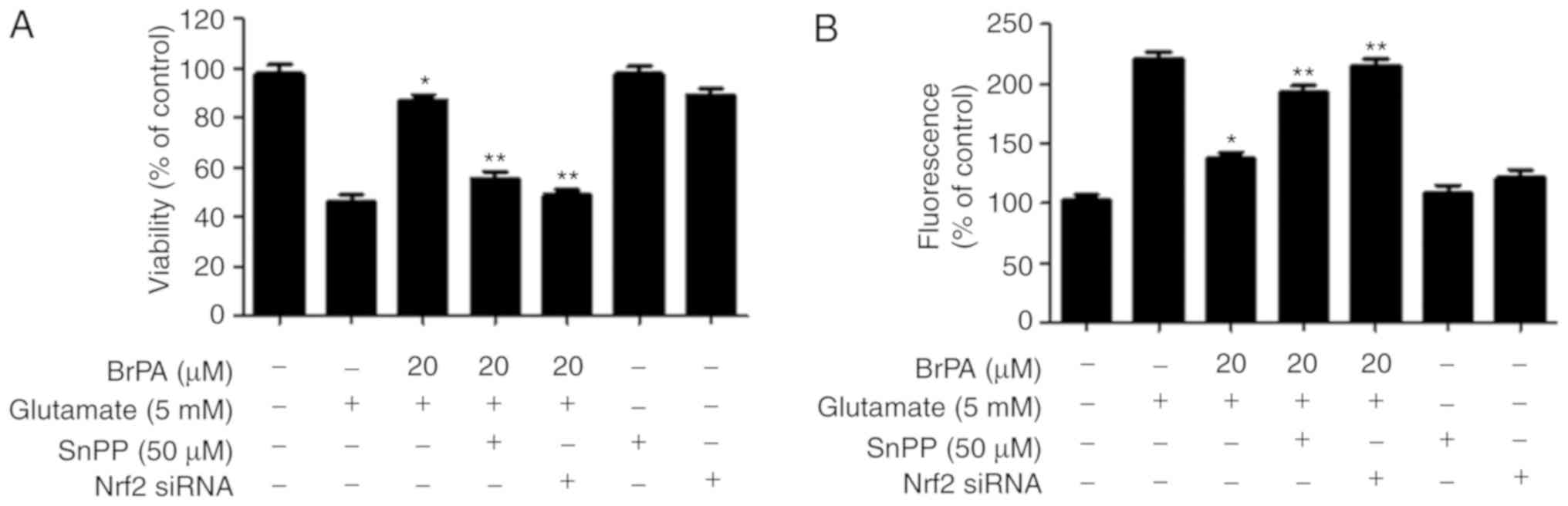
Effects of BrPA on cell protection
through the Nrf2-regulated HO-1 expression
In this experiment, we examined whether the
BrPA-induced protein expression of HO-1 was involved in
cytoprotection or ROS inhibition in HT22 cells. The cells were
treated with 20 µM BrPA for 12 h in the presence or absence of SnPP
IX, an inhibitor of HO-1 activity. SnPP IX markedly inhibited
BrPA-mediated protection (Fig.
5A). The expression of HO-1 by BrPA was also important for the
inhibition of glutamate-induced ROS production (Fig. 5B). Similarly, we evaluated the role
of BrPA-induced Nrf2 translocation in cell protection and ROS
inhibition. The transient transfection of HT22 cells with Nrf2
siRNA was performed and the cells were treated with 20 µM BrPA and
stimulated with glutamate. As shown in Fig. 5, when the cells were transfected
with Nrf2 siRNA, the cytoprotective and ROS inhibitory effects of
BrPA were decreased.
Involvement of the MAPK pathway in
BrPA-induced HO-1 expression
When oxidative stress increases within a cell, MAPK
signaling is activated. The activation of the MAPK pathway affects
the expression of HO-1 (16). The
largest increase in BrPA-induced expression of HO-1 occurred at a
concentration of 20 µM BrPA (Fig.
2). BrPA (20 µM) activated the JNK pathway and upregulated JNK
phosphorylation. As shown in Fig.
6A, the phosphorylation of JNK was observed 30 min after BrPA
treatment. In contrast, p38 and ERK kinases were not phosphorylated
in any of the time periods tested. In addition, specific inhibitors
of MEK 1/2 (the kinases upstream of ERK 1/2; PD98059), JNK
(SP600125), and p38 (SB203580) were used to confirm the association
of BrPA-induced MAPK regulation and HO-1 expression with
cytoprotective effects. The JNK inhibitor (SP600125) markedly
attenuated BrPA-increased HO-1 expression, whereas the p38 and ERK
inhibitors were ineffective (Fig.
6B). As expected, the JNK inhibitor (SP600125) reduced the
cytoprotective effect of BrPA, but not the p38 and ERK inhibitors
(Fig. 6C).
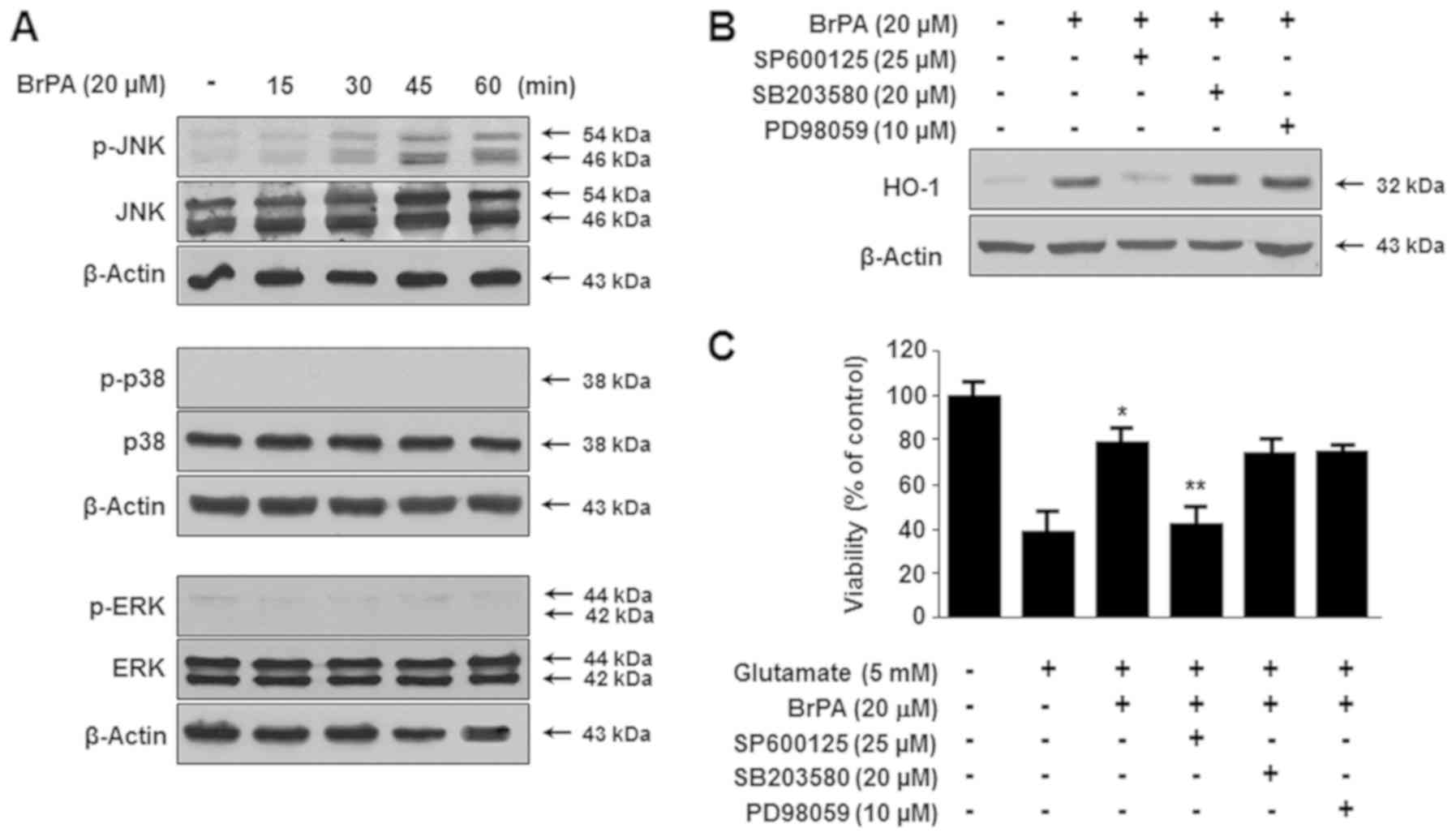 | Figure 6.BrPA upregulated HO-1 expression via
the phosphorylation of JNK MAPK. HT22 cells were cultured with BrPA
(20 µM) for the indicated time period (0–60 min), and the
expression of phosphorylated JNK, p38, or ERK MAPK was determined
using (A) western blotting. The cells were pre-incubated with
specific inhibitors, SP600125 (25 µM, JNK inhibitor), SB203580 (20
µM, p38 inhibitor), or PD98059 (10 µM, ERK inhibitor) for 1 h, and
then (B) cultured with BrPA (20 µM) for 12 h. Each specific
inhibitor was treated for 1 h in cells exposed or not exposed to
BrPA (20 µM) for 12 h, and the cells were then (C) exposed to 5 mM
glutamate for 12 h. The results are presented as the mean ±
standard deviation (n=3). *P<0.05 vs. glutamate.
**P<0.05 vs. glutamate with BrPA (20 µM). BrPA,
brassicaphenanthrene A; HO, heme oxygenase. |
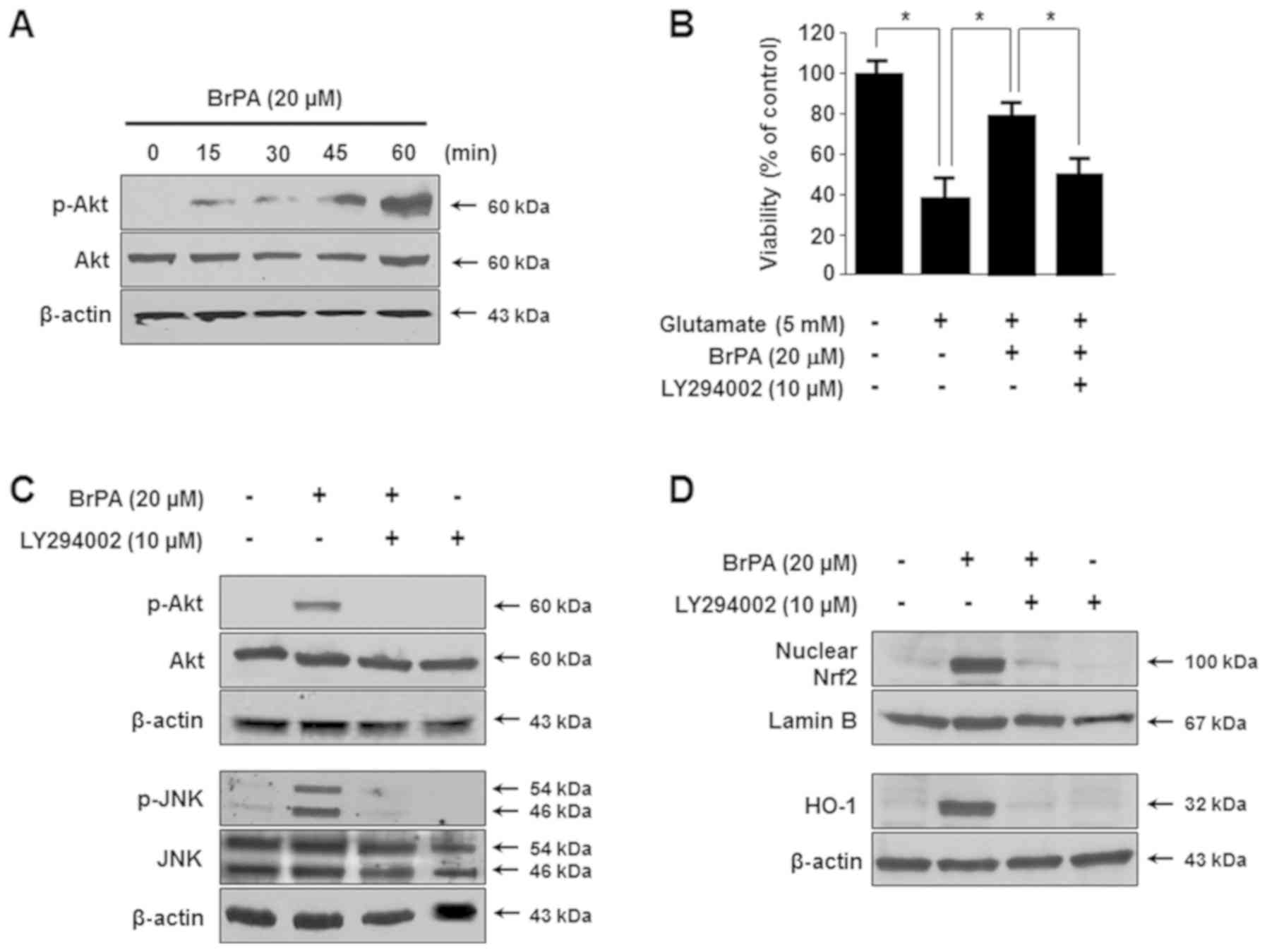
Involvement of the PI3K/Akt pathway in
the BrPA-induced HO-1 expression
Phosphatidylinositol 3-kinase (PI3K), which is
regulated by various phytochemicals, is known to be related to the
expression of HO-1 (17). To
verify an association between BrPA-induced Akt activation and HO-1
expression, Akt phosphorylation mediated by BrPA was examined in
HT22 cells by using an anti-phospho-Akt antibody. After treatment
with BrPA, Akt was phosphorylated for 15–60 min, after which the
phosphorylation decreased gradually (Fig. 7A). The inhibitor of the PI3K
pathway, LY294002, inhibited BrPA-enhanced cytoprotection (Fig. 7B). Furthermore, pre-treatment with
LY294002 attenuated BrPA-induced phosphorylation of Akt and JNK
(Fig. 7C). Pre-treatment with
LY294002 caused a similar attenuation of the nuclear translocation
of BrPA-induced Nrf2, and of HO-1 expression in the cells (Fig. 7D).
Discussion
B. rapa ssp. campestris is a staple
constituent of kimchi, a traditional Korean food. It is a
type of turnip commercially cultivated in Ganghwa Island, Korea
(18). B. rapa is known to
relieve chronic constipation and liver illnesses and ameliorate
kidney function (19). However, no
study has yet been conducted on the molecular mechanisms of the
neuroprotective effects on the pharmacological activities of its
constituents. This study has provided evidence that
brassicaphenanthrene A (BrPA) isolated from the roots of B.
rapa exerted neuroprotection through upregulation the Nrf2/MAPK
pathway, following the activation of HO-1 expression. This study
has presented the first evidence that natural extracts from the
roots of B. rapa can exert antioxidant effects against
glutamate-induced neurotoxicity by the activation of Nrf2/HO-1
signaling in HT22 cells.
BrPA resulted in a significant dose-dependent
reduction in glutamate-induced reactive oxygen generation in
immortalized mouse hippocampal HT22 cells. It is known that
neuronal cells, such as primary neuronal cells and tissue slices,
can be damaged by oxidative glutamate toxicity (20). Therefore, glutamate-induced
oxidative damage is one of the key contributors to pathological
neuronal cell damage (21). To
combat this, natural compounds have an intrinsic antioxidant effect
on glutamate-induced oxidative toxicity and can provide a strategy
for the development of therapeutic agents, involving the induction
of an intracellular cascade of protective pathways. In our previous
studies, certain plant-derived chemicals were confirmed to exert a
protective effect against glutamate-stimulated oxidative cell death
in HT22 cells (22–24).
HO-1 is highly expressed in oxidative conditions in
neurons (25). Cerebellar granule
cells are obtained from neurons of transgenic mice designed to
overexpress HO-1 and appear to be relatively resistant to
glutamate- and H2O2-mediated oxidative stress
in vitro (26,27). The dose-dependent induction of HO-1
expression is evidence of the induction of HO-1 by BrPA in HT22
cells. In addition, we observed that both cycloheximide and
actinomycin D completely blocked HO-1 expression. These results
suggested that BrPA-mediated HO-1 expression required de
novo RNA and protein synthesis. Furthermore, the pre-incubation
of HT22 cells with BrPA resulted in improved resistance to
glutamate-induced oxidative stress; this effect was partially
attributable to the induction of HO-1 as SnPP IX, which prevents HO
enzyme activity and markedly decreases BrPA-induced cytoprotection.
The induction of HO-1 expression was also needed to attenuate
glutamate-induced ROS production. Our results demonstrated that in
our experimental conditions, BrPA-induced cytoprotective effects
were mediated through HO-1 expression. Furthermore, several
researchers have reported that phytochemicals such as resveratrol
(28), curcumin (29), and epigallocatechin-3-gallate
(30) showed a noteworthy
therapeutic advantage in oxidative stress-induced neuronal damage,
via the induction of HO-1.
Nrf2 plays a crucial role in the oxidative
stress-related induction of ARE-mediated expression of antioxidant
enzymes and other inducible genes via various stimuli (7). Among phase 2 detoxifying and
antioxidant enzymes, GSH and GST are well known as pivotal
antioxidants that induce the protection of various cells against
oxidative injury through the reduction of ROS and nitrogen radicals
(31). GCL is composed of two
subunits that contain ARE sequences in their promoters: A modifier
subunit (GCLM) and a catalytic subunit (GCLC). GCL is the
rate-limiting enzyme in the synthesis of GSH (32). In addition, the expression of GCLM
and GCLC is modulated by antioxidants and anti-inflammatory agents
(33). In this study, BrPA-induced
dose-dependent protection against GSH depletion induced by
glutamate was observed, as well as the upregulation of GST and GCL
levels. It has been reported that GSH, GST, and GCL, known to
transcribe many oxidative stress-inducible genes, are also
modulated via Nrf2 induction in response to exposure to
electrophiles (34). Nrf2, which
is bound to an inhibitor protein Keap-1, resides in the cytosol.
Nrf2 released from Keap-1 by cell stimulation enters the nucleus
and binds to the AREs in the promoter of the target gene (6,7). In
this study, we found that BrPA markedly induced Nrf2 levels and
efficiently promoted its translocation into the nucleus. Thus,
BrPA-induced Nrf2 nuclear translocation is associated with an
increase in its ARE transcriptional activity. Transient
transfection with Nrf2 siRNA completely diminished BrPA-induced
HO-1 and GST expression, and reversed the BrPA-induced decrease in
cell protection and ROS inhibition. Overall, these observations
indicated that Nrf2-dependent increases in the protein expression
of GCL, GST, and HO-1 induced by BrPA, conferred cytoprotection
against glutamate-induced oxidative damage in HT22 cells.
Moreover, the JNK pathways appear to be related to
BrPA-mediated HO-1 expression. When cells were treated with a
specific protein kinase inhibitor, the JNK pathway proved to have a
decisive role in HO-1 induction. Previous studies reported that
MAPK expression mediated the regulation of basic cellular processes
such as transcription factor phosphorylation, proliferation, stress
response, apoptosis, and immune defense. In addition, MAPK
expression modulated the expression of several genes and proteins,
such as HO-1 (16). Because the
inhibitor SP600125 completely blocked HO-1 induction, BrPA-induced
HO-1 expression was directly correlated with the JNK pathway.
The PI3K/Akt pathway is reportedly connected with
the regulation of Nrf2 nuclear translocation and the expression of
ARE-related phase II enzymes (35,36).
The neuroprotective mechanisms of PI3K/Akt signaling may be related
to the activation of Nrf2 transcription, which is widely viewed as
a mediator of neuroprotection, as it upregulates various
antioxidant enzymes (4,5). Therefore, we also observed if the
activation of the PI3K/Akt and JNK pathways was related to
BrPA-induced Nrf2-mediated HO-1 expression. The specific PI3K
inhibitor LY294002 attenuated BrPA-induced Akt and JNK activation,
Nrf2 nuclear translocation, HO-1 expression, and cytoprotective
effects in HT22 cells. These results indicated that the PI3K/Akt
cascade and JNK pathways, activated by BrPA, participate in the
early-stage expression of HO-1 in HT22 cells. This study suggests
that BrPA isolated from the roots of B. rapa effectively
prevented oxidative neuronal cell death and BrPA-induced HO-1
regulation via PI3K/Akt, JNK, and Nrf2 signals, eventually playing
a significant role in the protection of HT22 cells. The further
studies are required to evaluate the nuclear translocation of some
molecules using such as the immunofluorescence staining to confirm
the detailed mechanism by BrPA.
In conclusion, these results indicated that BrPA
isolated from the roots of B. rapa, effectively prevented
glutamate-induced mouse hippocampal cell damage. In addition, HO-1
expression through the PI3K/Akt, JNK, and Nrf2 pathways regulated
by BrPA, appeared to have an important role in neuronal cell
protection. Further, this study has shown that BrPA exerts
neuroprotective effects through the regulation of Nrf2-HO-1
signaling.
Acknowledgements
Not applicable.
Funding
The current study was supported by a research fund
from Chosun University in 2016 (K207334001).
Authors' contributions
WK, NIB, YCK and DSL conceived of the study. HL, WK,
AC, BL and DSL performed formal analysis. HL, WK, AC, BL and DSL
performed investigation. NIB, HO, and YCK provided the resources.
HL, WK, AC, BL and DSL curated the data. HL, WK, and DSL wrote and
prepared the draft of the manuscript. HL, WK, SCK, ERW and DSL
wrote, reviewed and edited the manuscript. SCK, HO, ERW, NIB, YCK
and DSL performed the visualization. NIB, YCK and DSL supervised
the study.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hald A and Lotharius J: Oxidative stress
and inflammation in Parkinson's disease: Is there a causal link?
Exp Neurol. 193:279–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simonian NA and Coyle JT: Oxidativec
stress in neurodegenerative diseases. Annu Rev Pharmacol Toxiol.
36:83–106. 1996. View Article : Google Scholar
|
|
3
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enomoto A, Itoh K, Nagayoshi E, Haruta J,
Kimura T, O'Connor T, Harada T and Yamamoto M: High sensitivity of
Nrf2 knockout mice to acetaminophen hepatotoxicity associated with
decreased expression of ARE-regulated drug metabolizing enzymes and
antioxidant genes. Toxicol Sci. 59:169–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Narasimhan M, Mahimainathan L, Rathinam
ML, Riar AK and Henderson GI: Overexpression of Nrf2 protects
cerebral cortical neurons from ethanolinduced apoptotic death. Mol
Pharmacol. 80:988–999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balogun E, Hoque M, Gong P, Killeen E,
Green CJ, Foresti R, Alam J and Motterlini R: Curcumin activates
the heme oxygenase-1 gene via regulation of Nrf2 and the
antioxidant-responsive element. Biochem J. 371:887–895. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Vries HE, Witte M, Hondius D,
Rozemuller AJ, Drukarch B, Hoozemans J and van Horssen J:
Nrf2-induced: A promising target to counteract ROS-mediated damage
in neurodegenerative disease antioxidant protection? Free Radic
Biol Med. 45:1375–1383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee DS and Jeong GS: Arylbenzofuran
isolated from Dalbergia odorifera suppresses
lipopolysaccharide-induced mouse BV2 microglial cell activation,
which protects mouse hippocampal HT22 cells death from
neuroinflammation-mediated toxicity. Eur J Pharmacol. 728:1–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maines MD: Heme oxygenase: Function,
multiplicity, regulatory mechanisms, and clinical applications.
FASEB J. 2:2557–2568. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rössler OG, Bauer I, Chung HY and Thiel G:
Glutamate-induced cell death of immortalized murine hippocampal
neurons: Neuroprotective activity of heme oxygenase-1, heat shock
protein 70, and sodium selenite. Neurosci Lett. 362:253–257. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi BM, Kim HJ, Oh GS, Pae HO, Oh H,
Jeong S, Kwon TO, Kim YM and Chung HT:
1,2,3,4,6-Penta-O-galloyl-beta-D-glucose protects rat neuronal
cells (Neuro 2A) from hydrogen peroxide-mediated cell death via the
induction of heme oxygenase-1. Neurosci Lett. 328:185–189. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schonhof I, Krumbein A and Brückner B:
Genotypic effects on glucosinolates and sensory properties of
broccoli and cauliflower. Nahrung. 48:25–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bang MH, Lee DY, Han MW, Oh YJ, Chung HG,
Jeong TS, Choi MS, Lee KT and Baek NI: Development of biologically
active compounds from edible plant sources-XX: Isolation of lipids
from the roots of Brassica campestris ssp. rapa. J
Korean Soc App Biol Chem. 50:233–237. 2007.
|
|
15
|
Wu Q, Cho JG, Yoo KH, Jeong TS, Park JH,
Kim SY, Kang JH, Chung IS, Choi MS, Lee KT, et al: A new
phenanthrene derivative and two diarylheptanoids from the roots of
Brassica rapa ssp. campestris inhibit the growth of
cancer cell lines and LDL-oxidation. Arch Pharm Res. 36:423–429.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kietzmann T, Samoylenko A and Immenschuh
S: Transcriptional regulation of heme oxygenase-1 gene expression
by MAP kinases of the JNK and p38 pathways in primary cultures of
rat hepatocytes. J Biol Chem. 278:17927–17936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin D, Rojo AI, Salinas M, Diaz R,
Gallardo G, Alam J, De Galarreta CM and Cuadrado A: Regulation of
heme oxygenase-1 expression through the phosphatidylinositol
3′-kinase/Akt pathway and the Nrf2 transcription factor in response
to the antioxidant phytochemical carnosol. J Biol Chem.
279:8919–8929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung UJ, Baek NI, Chung HG, Bang MH, Jeong
TS, Lee KT, Kang YJ, Lee MK, Kim HJ, Yeo J and Choi MS: Effects of
the ethanol extract of the roots of Brassica rapa on glucose
and lipid metabolism in C57BL/KsJ-db/db mice. Clin Nutr.
27:158–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Poppel G, Verhoeven DT, Verhagen H and
Goldbohm RA: Brassica vegetables and cancer prevention.
Epidemiology and mechanisms. Adv Exp Med Biol. 472:159–168. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vornov JJ and Coyle JT: Glutamate
neurotoxicity and the inhibition of protein synthesis in the
hippocampal slice. J Neurochem. 56:996–1006. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coyle JT and Puttfarcken P: Oxidative
stress, glutamate, and neurodegenerative disorders. Science.
262:689–695. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong GS, Li B, Lee DS, Byun E, An RB, Pae
HO, Chung HT, Youn KH and Kim YC: Lavandulyl flavanones from
Sophora flavescens protect mouse hippocampal cells against
glutamate-induced neurotoxicity via the induction of heme
oxygenase-1. Biol Pharm Bull. 31:1964–1967. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong GS, Li B, Lee DS, Kim KH, Lee IK,
Lee KR and Kim YC: Cytoprotective and anti-inflammatory effects of
spinasterol via the induction of heme oxygenase-1 in murine
hippocampal and microglial cell lines. Int Immunopharmacol.
10:1587–1594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Lee DS, Jeong GS and Kim YC:
Involvement of heme oxygenase-1 induction in the cytoprotective and
immunomodulatory activities of 6,4′-dihydroxy-7-methoxyflavanone in
murine hippocampal and microglia cells. Eur J Pharmacol.
674:153–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dwyer BE, Nishimura RN and Lu SY:
Differential expression of heme oxygenase-1 in cultured cortical
neurons and astrocytes determined by the aid of a new heme
oxygenase antibody. Response to oxidative stress. Brain Res Mol
Brain Res. 30:37–47. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le WD, Xie WJ and Appel SH: Protective
role of heme oxygenase-1 in oxidative stress-induced neuronal
injury. J Neurosci Res. 56:652–658. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen K, Gunter K and Maines MD: Neurons
overexpressing heme oxygenase-1 resist oxidative stress-mediated
cell death. J Neurochem. 75:304–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CY, Jang JH, Li MH and Surh YJ:
Resveratrol upregulates heme oxygenase-1 expression via activation
of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res
Commun. 331:993–1000. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang C, Zhang X, Fan H and Liu Y: Curcumin
upregulates transcription factor Nrf2, HO-1 expression and protects
rat brains against focal ischemia. Brain Res. 1282:133–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romeo L, Intrieri M, D'Agata V, Mangano
NG, Oriani G, Ontario ML and Scapagnini G: The major green tea
polyphenol, (−)-epigallocatechin-3-gallate, induces heme oxygenase
in rat neurons and acts as an effective neuroprotective agent
against oxidative stress. J Am Coll Nutr. 28 (Suppl):492S–499S.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dickinson DA and Forman HJ: Cellular
glutathione and thiols metabolism. Biochem Pharmacol. 64:1019–1026.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rushworth SA, Ogborne RM, Charalambos CA
and O'Connell MA: Role of protein kinase C delta in
curcumin-induced antioxidant response element-mediated gene
expression in human monocytes. Biochem Biophys Res Commun.
341:1007–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thimmulappa RK, Fuchs RJ, Malhotra D,
Scollick C, Traore K, Bream JH, Trush MA, Liby KT, Sporn MB,
Kensler TW and Biswal S: Preclinical evaluation of targeting the
Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection
from LPS-induced inflammatory response and reactive oxygen species
in human peripheral blood mononuclear cells and neutrophils.
Antioxid Redox Signal. 9:1963–1970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rushworth SA and MacEwan DJ: The role of
Nrf2 and cytoprotection in regulating chemotherapy resistance of
human leukemia Cells. Cancers (Basel). 3:1605–1621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen C, Pung D, Leong V, Hebbar V, Shen G,
Nair S, Li W and Kong AN: Induction of detoxifying enzymes by
garlic organosulfur compounds through transcription factor Nrf2:
Effect of chemical structure and stress signals. Free Radic Biol
Med. 37:1578–1590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen G, Hebbar V, Nair S, Xu C, Li W, Lin
W, Keum YS, Han J, Gallo MA and Kong AN: Regulation of Nrf2
transactivation domain activity. The differential effects of
mitogen-activated protein kinase cascades and synergistic
stimulatory effect of Raf and CREB-binding protein. J Biol Chem.
279:23052–23060. 2004. View Article : Google Scholar : PubMed/NCBI
|