Introduction
Age-related macular degeneration (AMD) is an
age-associated disease of the eyes, which is the most common cause
of visual impairment and blindness in the elderly (1). According to a blind register center
survey in Britain, ~50% of blind patients are affected by AMD
(2). The main manifestation of AMD
is decreased retinal pigment epithelial cell digestive ability of
the rod outer segment, which leads to the presence of rod outer
segment residual bodies in the protoplasm. These residual bodies
deposit in Bruch's membrane and form drusen, which lead to impaired
vision or even blindness (3,4).
However, to best of our knowledge, the exact pathogenesis of AMD
remains unclear. The pathogenesis of AMD has been reported to be
related to aging, metabolism, genetic inheritance, smoking, high
blood pressure and obesity (5–8).
Recent studies have demonstrated that antioxidant supplements may
protect retinal pigment epithelial cells from oxidative stress
damage (9–11); however, current AMD therapy remains
ineffective.
Heme oxygenase-1 (HO-1) is an inducible antioxidant
enzyme that exerts cytoprotective effects on various cell types
(12–15). The expression levels of HO-1 are
mainly regulated by the antioxidant response element (ARE), which
activates the nuclear factor 2-related factor 2 (Nrf2). Under
normal conditions, Nrf2 is present in the cytoplasm in combination
with Kelch-like ECH-related protein 1 (Keap1) (16). Activated Nrf2 translocates into the
nucleus and forms heterodimers with transcription factors, which
activate the transcription of HO-1 (17). Studies have demonstrated that the
Nrf2 pathway participates in the development and progression of
oxidative stress injury (18–20).
However, the exact mechanisms of the Nrf2 pathway in the modulation
of H2O2-induced oxidative stress injury in
retinal pigment epithelial cells remain unclear.
Rhizoma Paridis total saponins (RPTS) is a major
effective constituent isolated from the traditional Chinese
medicine, Rhizoma Paridis, which has been demonstrated to possess
numerous biological activities (21,22).
RTPS exhibits antitumor activity in several cancer types, involving
liver (23) and lung cancer
(24). A recent study also
suggested that RPTS attenuates liver fibrosis by regulating
expression of the Ras protein activator-like 1/ERK1/2 signaling
pathway in rats (25). However, it
is unclear whether RPTS may prevent AMD, and little is currently
known about the roles of RPTS in oxidative stress-induced injury on
retinal pigment epithelial cells.
In the present study, the association between RPTS
and H2O2-induced oxidative stress injury was
analyzed. In addition, the roles and mechanisms of RPTS and the
Nrf2 pathway in the protection of
H2O2-induced ARPE-19 cells were examined to
identify a potential therapeutic strategy for preventing AMD.
Materials and methods
Cell culture and reagents
The human retinal pigment epithelial cell line
ARPE-19 was obtained from the Cell Bank of the Chinese Academy of
Sciences. ARPE-19 cells were incubated in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
in an atmosphere containing 5% CO2 at 37°C. RPTS was
obtained from Nanjing DASF Biotechnology Co., Ltd. (cat. no.
dasf0242), and the main components of RPTS were Polyphyllin I,
Polyphyllin II, Polyphyllin VII and Polyphyllin H.
H2O2 was obtained from Xilong Scientific Co.,
Ltd.
Grouping
Cultured ARPE-19 cells (all at 37°C) were separated
into five treatment groups as follows: i) Control, ARPE-19 cells
without treatment; ii) H2O2, ARPE-19 cells
treated with 200 µM H2O2 for 12 h; iii) 10
µg/ml RPTS + H2O2, ARPE-19 cells pretreated
with 10 µg/ml RPTS for 6 h and treated with 200 µM
H2O2 for 12 h; iv) 20 µg/ml RPTS +
H2O2, ARPE-19 cells pretreated with 20 µg/ml
RPTS for 6 h and treated with 200 µM H2O2 for
12 h; and v) 40 µg/ml RPTS + H2O2, ARPE-19
cells pretreated with 40 µg/ml RPTS for 6 h and treated with 200 µM
H2O2 for 12 h.
Cell viability assay
Cell Counting kit-8 (CCK-8; Beyotime Institute of
Biotechnology) was used to detect the effects of various
concentrations of RPTS (10, 20, 40 and 80 µg/ml) at 48 h, and the
effects of H2O2 and RPTS +
H2O2 at 12, 24 and 48 h, on ARPE-19 cell
viability. ARPE-19 cells (~6×103 cells/well) in the
logarithmic phase were seeded into 96-well plates and maintained at
37°C in an atmosphere containing 5% CO2 for 12 h.
Subsequently, CCK reagent (10 µl) was added to each well and the
cells were incubated for 3 h at 37°C. A microplate reader (Bio-Rad
Laboratories, Inc.) was used to record the absorbance at 450 nm.
Cell viability was determined as the percentage of cell survival
compared with the control.
Enzyme-linked immunosorbent assay
(ELISA)
The kits used for the assessment of reactive
malondialdehyde (MDA; cat. no. S0131), superoxide dismutase (SOD;
cat. no. S0101) and glutathione peroxidase (GPx; cat. no. S0056) in
ARPE-19 cells were obtained from Beyotime Institute of
Biotechnology. Cultured ARPE-19 cells (2×105 cells/well)
were seeded into 6-well plates, which were subsequently sealed with
adhesive tape and maintained at 37°C for 90 min. Biotinylated
antibodies (100 µl) were then added to the wells. The wells were
re-sealed and maintained at 37°C for 60 min. Chromogenic substrate
from the kit was added to the wells and the plates were maintained
for 10–15 min in the dark at 37°C. Stop solution was then added to
each well and mixed for 10 min. Optical density was measured at 450
nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Apoptosis assay
Flow cytometry (FCM) was performed to assess the
apoptotic rates of ARPE-19 cells. After washing with PBS, cultured
ARPE-19 cells were trypsinized with 0.25% trypsin (Beyotime
Institute of Biotechnology). The supernatant was removed and the
cells were suspended in incubation buffer (10 mM HEPES, pH 7.4, 140
mM NaCl, 2.5 mM CaCl2) at a density of 1×106
cells/ml. ARPE-19 cells were then incubated with Annexin PE and
7-aminoactinomycin (5 µl; cat. no. 559763; BD Biosciences) at room
temperature in the dark for 15 min. Finally, a FACScalibur flow
cytometer (BD Biosciences) was used to assess apoptosis, and the
data was analyzed using BD CellQuest™ Pro version 1.2 software (BD
Biosciences).
Evaluation of reactive oxygen species
(ROS) and mitochondrial membrane potential (MMP) in ARPE-19
cells
PBS was added to cultured ARPE-19 cells until a cell
density of 1×106 cells/ml was achieved in a 12-well
plate. Subsequently, 2′,7′-dichlorofluorescein diacetate (cat. no.
HY-D0940; MedChem Express LLC) and Fluo-3 acetoxymethyl (cat. no.
70-F1243; Hangzhou MultiSciences Biotech Co., Ltd.) were added to
the ARPE-19 cells to evaluate ROS and MMP, respectively. ARPE-19
cells were incubated at room temperature in the dark for 10 min.
The supernatant was then removed and 100 µl PBS was added to the
cells. FCM was performed to assess ROS levels and MMP in ARPE-19
cells at 488 nm. A total of 10,000 cells were collected from each
sample and analyzed using a flow cytometer, and the data were
analyzed using Summit V4.3 software (Dako; Agilent Technologies,
Inc.).
ARE-luciferase activity
ARPE-19 cells (3×104 cells/well) were
seeded into 24-well plates and ARE dual-luciferase reporter plasmid
(50 ng/well; cat. no. 11548ES03; Shanghai Yeasen Biotechnology Co.,
Ltd.) was transfected into cells using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 24 h post-transfection, the
cells were treated with H2O2 + 0, 10, 20 or
40 µg/ml RPTS for 24 h. ARE-luciferase activity was measured at 490
nm following the addition of Luciferase Assay Reagent (Promega
Corporation); Renilla luciferase was used as an internal
control.
Western blot analysis
Proteins were extracted from ARPE-19 cells using
RIPA buffer (Beyotime Institute of Biotechnology), and a
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.) was used
to quantify protein concentration. Protein lysates (25 µg/lane)
were separated by 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and were transferred to a PVDF membrane (EMD
Millipore). The blots were then blocked in TBS + 0.1% Tween-20
containing 5% skimmed milk at 37°C for 1 h and incubated with the
following rabbit anti-human antibodies: Anti-Fas (dilution, 1:100;
cat. no. ab133619), anti-Fas ligand (Fasl; dilution, 1:1,000; cat.
no. ab15285), anti-Bax (dilution, 1:1,000; cat. no. ab32503),
anti-Bcl-2 (dilution, 1:1,000; cat.no. ab32124), anti-caspase-3
(dilution, 1:500; cat. no. ab13847), anti-Nrf2 (dilution, 1:500;
cat. no. ab137550), anti-HO-1 (dilution, 1:2,000; cat. no.
ab13243), anti-γ-glutamyl-cysteine synthetase (γ-GCS; dilution,
1:1,000; cat. no. sc-166382; Santa Cruz Biotechnology, Inc.),
anti-NAD(P)H quinone dehydrogenase 1 (NQO1; dilution, 1:1,000; cat.
no. ab34173) and anti-GAPDH (dilution, 1:2,500; cat. no. ab9485;
all Abcam unless specified) at 4°C overnight. Horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary
antibody (dilution, 1:5,000; cat. no. ab205718; Abcam) was then
added, and the membranes were incubated at room temperature for 1
h. GADPH was used as an internal control. Enhanced
chemiluminescence (ECL) reagents (EMD Millipore) in combination
with an ECL system (GE Healthcare) were used to analyze the
results. Densitometry was performed using Quantity One software
version 2.4 (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cultured ARPE-19 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA was reverse transcribed to cDNA using
PrimeScript™ RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol at 42°C for 1 h and 70°C for 10 min. cDNA
was amplified using the SYBR Fast qPCR Mix (Invitrogen; Thermo
Fisher Scientific, Inc.) on the ABI 7500 Thermocycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: Pretreatment at 94°C for 10 min;
followed by 45 cycles at 95°C for 15 sec and 68°C for 45 sec; and a
final extension step at 75°C for 10 min. The primers were designed
by Invitrogen (Thermo Fisher Scientific, Inc.) as follows: Fas,
forward 5′-GACTCAGAACTTGGAAGGCC-3′, reverse
5′-ACTTGGTATTCTGGGTCCGG-3′ (product, 245 bp); Fasl, forward
5′-TGGTTCTGGTTGCCTTGGTA-3′, reverse 5′-GCATGGACCTTGAGTTGGAC-3′
(product, 210 bp); Bax, forward 5′-CATCATGGGCTGGACATTGG-3′,
reverse, 5′-CCTCAGCCCATCTTCTTCCA-3′ (product, 226 bp); Bcl-2,
forward 5′-TTCTTTGAGTTCGGTGGGGT-3′, reverse
5′-CTTCAGAGACAGCCAGGAGA-3′ (product, 207 bp); caspase-3, forward
5′-TGAGCCATGGTGAAGAAGGA-3′, reverse 5′-TCGGCCTCCACTGGTATTTT-3′
(product, 220 bp); and GAPDH, forward 5′-CCATCTTCCAGGAGCGAGAT-3′
and reverse 5′-TGCTGATGATCTTGAGGCTG-3′ (product, 222 bp). GAPDH was
used as the internal control. The mRNA expression levels were
quantified using the 2−ΔΔCq method (26).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6.0 software (GraphPad Software, Inc.). Data are
presented as the mean ± SD of at least three independent
experiments. The experimental data were analyzed by Kruskal-Wallis
and Tukey's tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
RPTS enhances the viability of ARPE-19
cells treated with H2O2
CCK-8 assay results remonstrated that cell viability
was not affected by 10, 20 and 40 µg/ml RPTS (Fig. 1A). However, 80 µg/ml RPTS reduced
cell viability (P<0.05; Fig.
1A). To further examine whether RPTS affected ARPE-19 cell
viability, the viability of ARPE-19 cells treated with
H2O2 and different concentrations of RPTS was
measured. Compared with control cells, H2O2
treatment significantly inhibited the viability of ARPE-19 cells
(P<0.01); however, in the groups pretreated with RPTS, the
viability of H2O2-treated ARPE-19 cells was
enhanced in a dose-dependent manner (Fig. 1B). These results suggested that
RPTS may increase the viability of ARPE-19 cells treated with
H2O2.
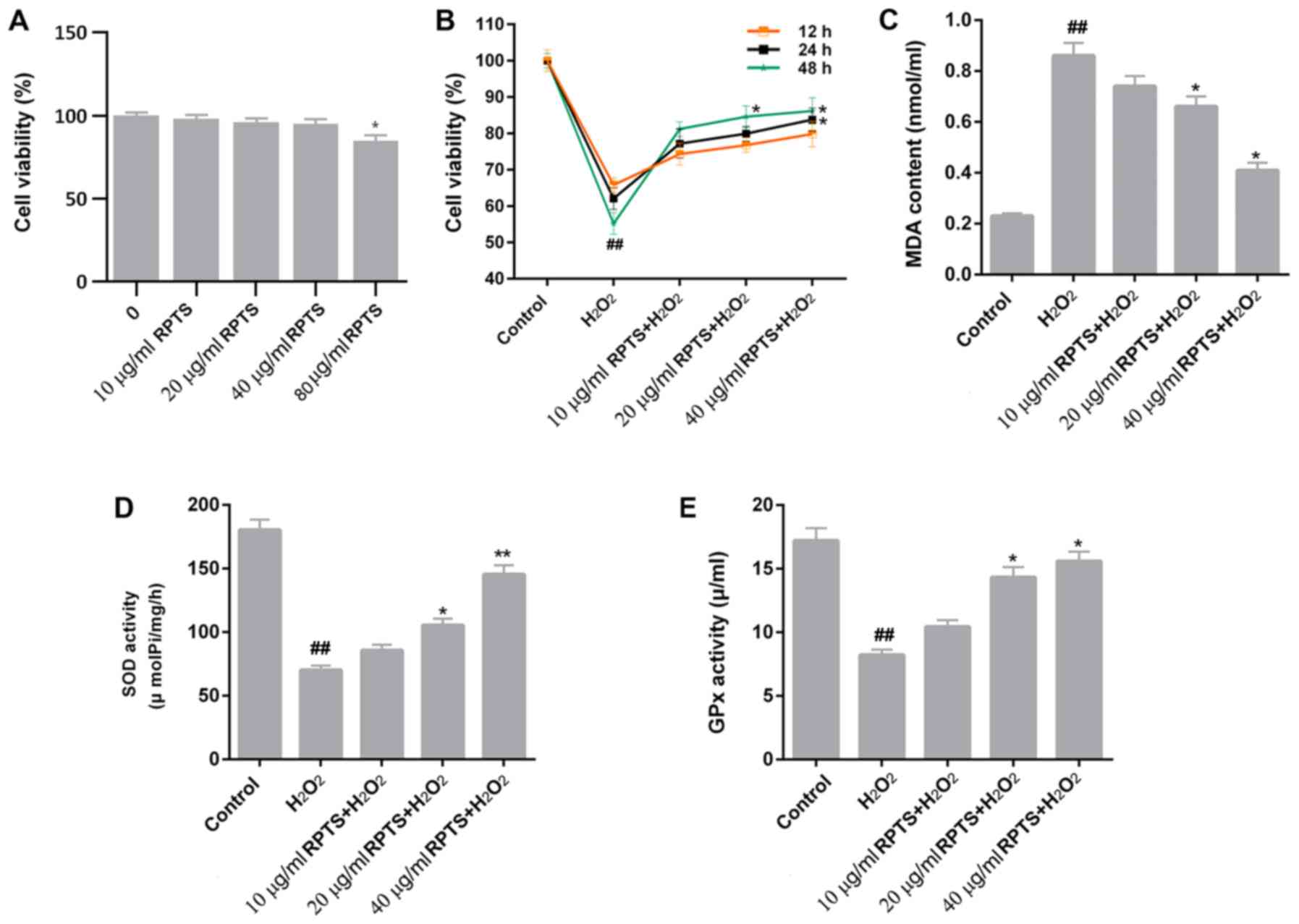 | Figure 1.RPTS affects cell viability and
oxidative stress of ARPE-19 cells treated with
H2O2. (A) ARPE-19 cells were treated with 10,
20, 40 and 80 µg/ml RPTS. Cell viability was detected using the
CCK-8 assay. *P<0.05 vs. 0 µg/ml RPTS. (B) ARPE-19 cells were
treated with H2O2, or pretreated with 10, 20
and 40 µg/ml RPTS and then treated with H2O2.
CCK-8 assay was performed to evaluate the viability of ARPE-19
cells. (C-E) ELISA was performed to assess the levels of (C) MDA,
(D) SOD and (E) GPx in ARPE-19 cells. ##P<0.01 vs.
Control; *P<0.05 and **P<0.01 vs. H2O2.
ARPE-19, Adult Retinal Pigment Epithelial cell line-19; CCK-8, Cell
Counting kit-8; ELISA, enzyme-linked immunosorbent assay; GPx,
glutathione peroxidase; MDA, malondialdehyde; RPTS, Rhizoma Paridis
total saponins; SOD, superoxide dismutase. |
Oxidative stress marker levels in
H2O2-treated ARPE-19 cells are modulated by
RPTS
As oxidative stress contributes to the development
and progression of H2O2-induced injury, the
levels of oxidative stress markers, including MDA, SOD and GPx,
were assessed in ARPE-19 cells treated with
H2O2 and different concentrations of RPTS.
The MDA content in ARPE-19 cells treated with
H2O2 was significantly higher compared with
that in control (P<0.01) cells, whereas RPTS treatment
significantly reduced the levels of MDA in
H2O2-treated ARPE-19 cells (P<0.05;
Fig. 1C). Conversely,
H2O2 treatment significantly reduced the
levels of SOD and GPx in ARPE-19 cells compared with the control
group (P<0.01). The SOD and GPx levels in
H2O2-treated ARPE-19 cells were enhanced in
the groups pretreated with different concentrations of RPTS
compared with in the H2O2 group (P<0.05;
Fig. 1D and E). Therefore, RPTS
pretreatment reduced MDA content, and increased SOD and GPx levels
in H2O2-treated ARPE-19 cells, which
indicated that RPTS may reduce oxidative stress in
H2O2-treated ARPE-19 cells.
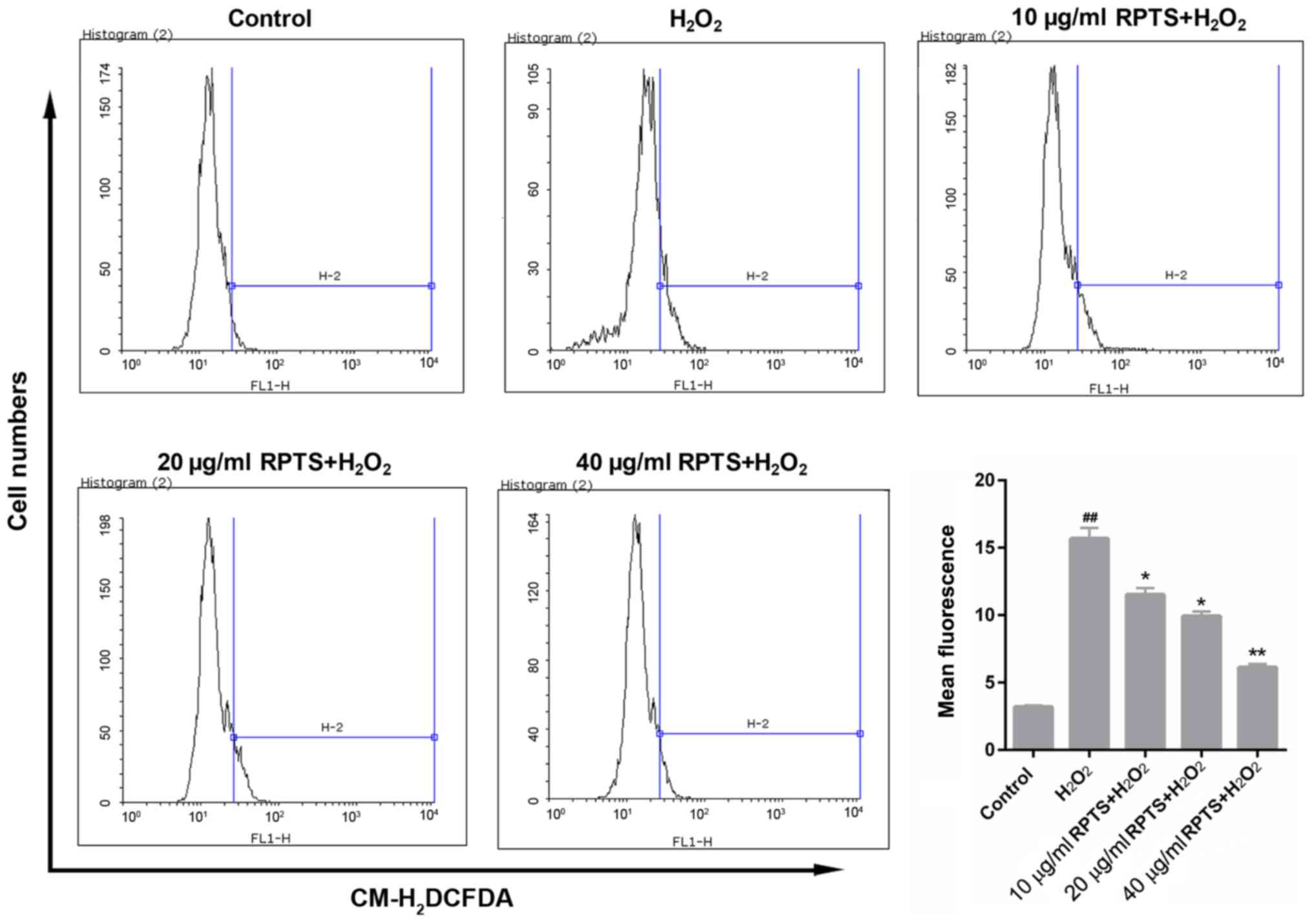
RPTS pretreatment reduces ROS
production in H2O2-treated ARPE-19 cells
ROS production in ARPE-19 cells treated with
H2O2 and with different concentrations of
RPTS was measured. FCM results demonstrated that
H2O2 treatment significantly elevated ROS
levels in ARPE-19 cells compared with in the control group
(P<0.01). In the groups pretreated with RPTS, mean fluorescence
value indicating ROS levels in the
H2O2-treated ARPE-19 cells were decreased
compared with in the H2O2 group (P<0.05;
Fig. 2). Therefore, RPTS may
reduce ROS production in H2O2-treated ARPE-19
cells.
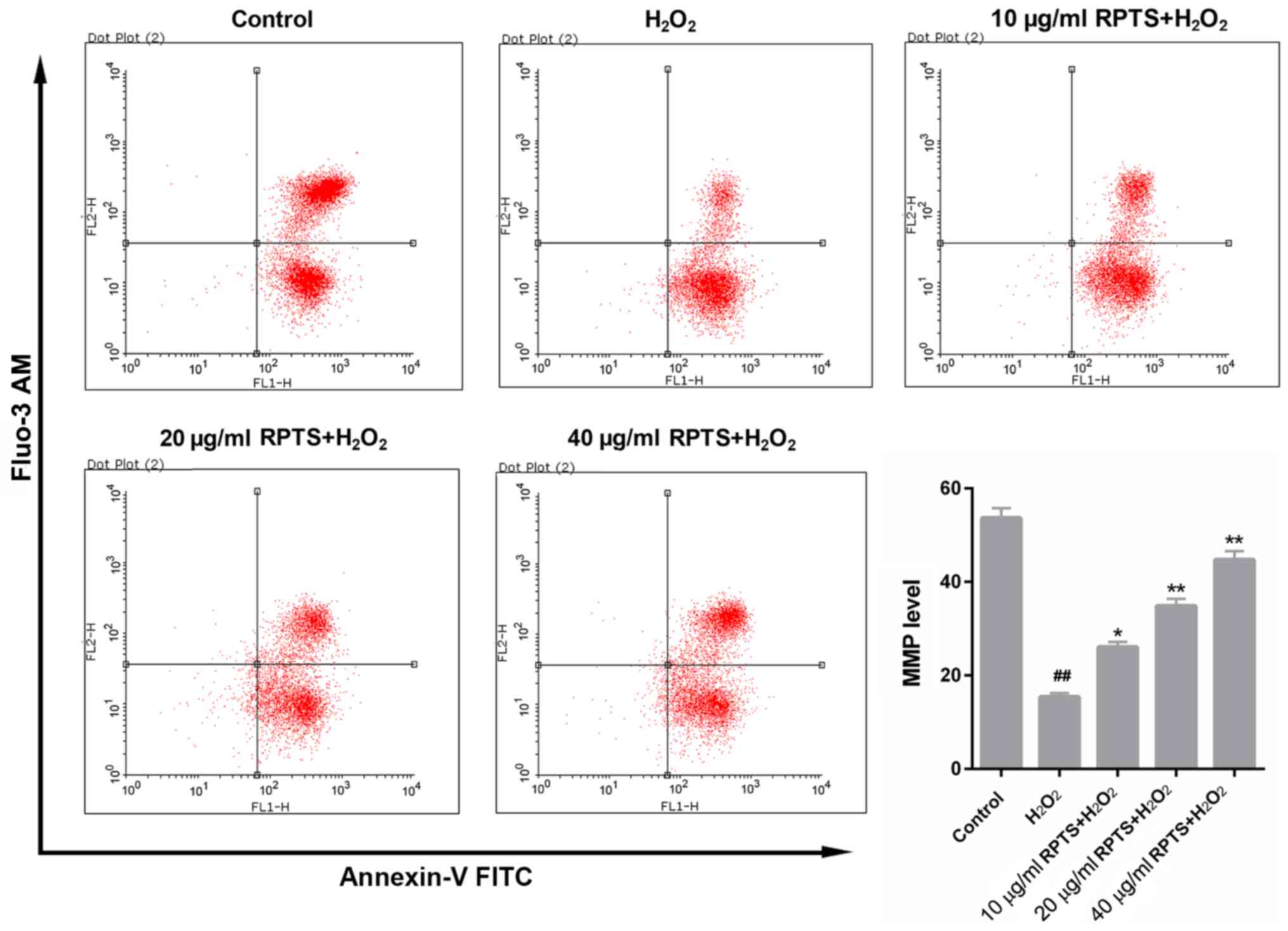
RPTS increases the MMP of
H2O2-treated ARPE-19 cells
FCM analysis of MMP revealed that
H2O2 treatment significantly decreased the
MMP in ARPE-19 cells (P<0.01), whereas the MMP in
H2O2-treated ARPE-19 cells was significantly
enhanced in response to RPTS (P<0.05; Fig. 3). These results suggested that RPTS
may modulate the MMP in ARPE-19 cells treated with
H2O2, which indicated that RPTS may reduce
H2O2-induced oxidative stress in ARPE-19
cells.
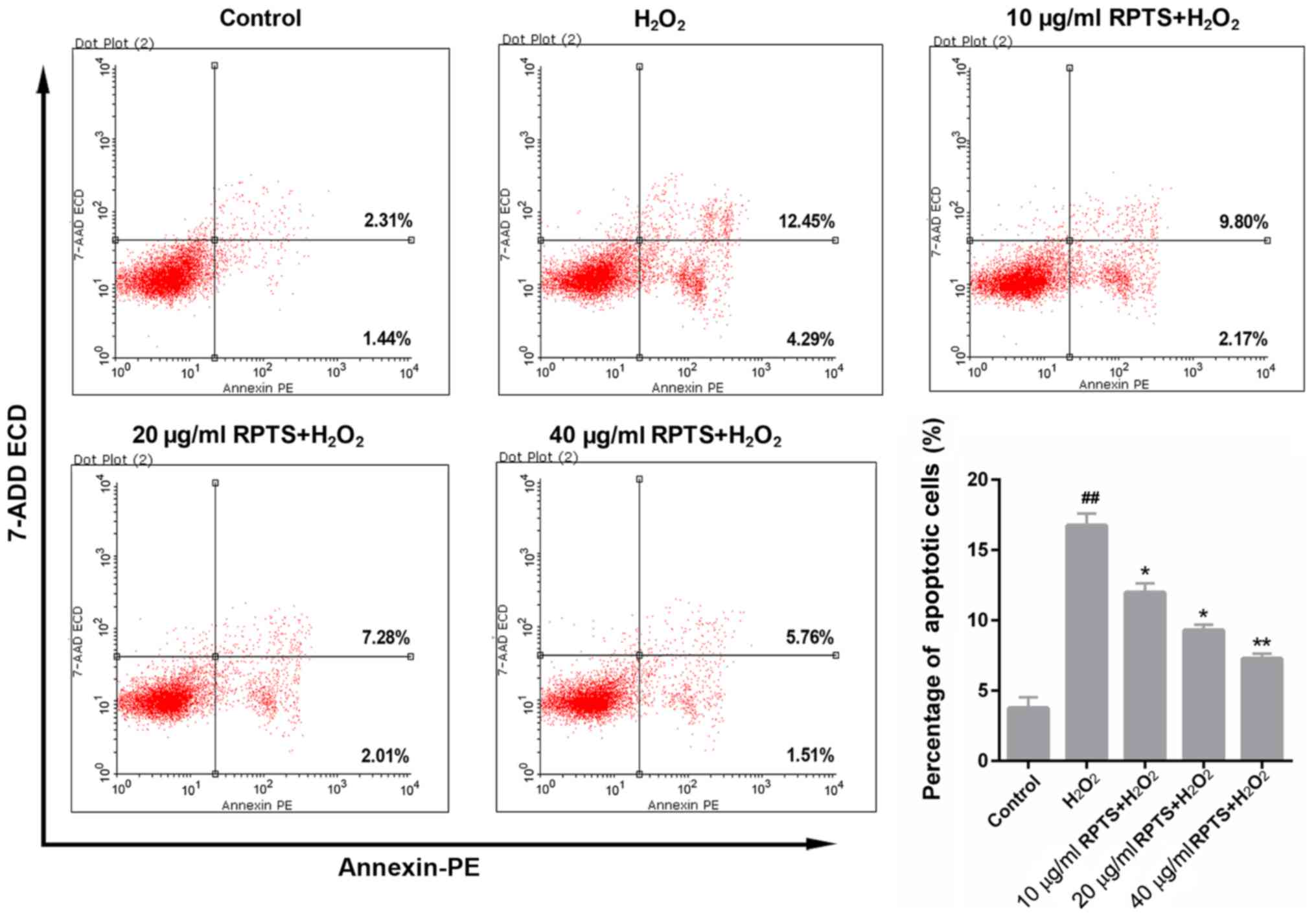
RPTS suppresses
H2O2-mediated apoptosis of ARPE-19 cells
The apoptotic rate of ARPE-19 cells treated with
H2O2 and different concentrations of RPTS was
determined by FCM. The results demonstrated that the proportion of
apoptotic ARPE-19 cells in the H2O2 group was
16.74%, which was significantly higher compared with 3.75% in the
control group (P<0.01; Fig. 4).
By contrast, in the groups pretreated with RPTS, the apoptotic
rates of ARPE-19 cells were significantly decreased compared with
in the H2O2 group (P<0.05; Fig. 4). This suggested that RPTS may
reduce the apoptotic rate of H2O2-treated
ARPE-19 cells in a dose-dependent manner.
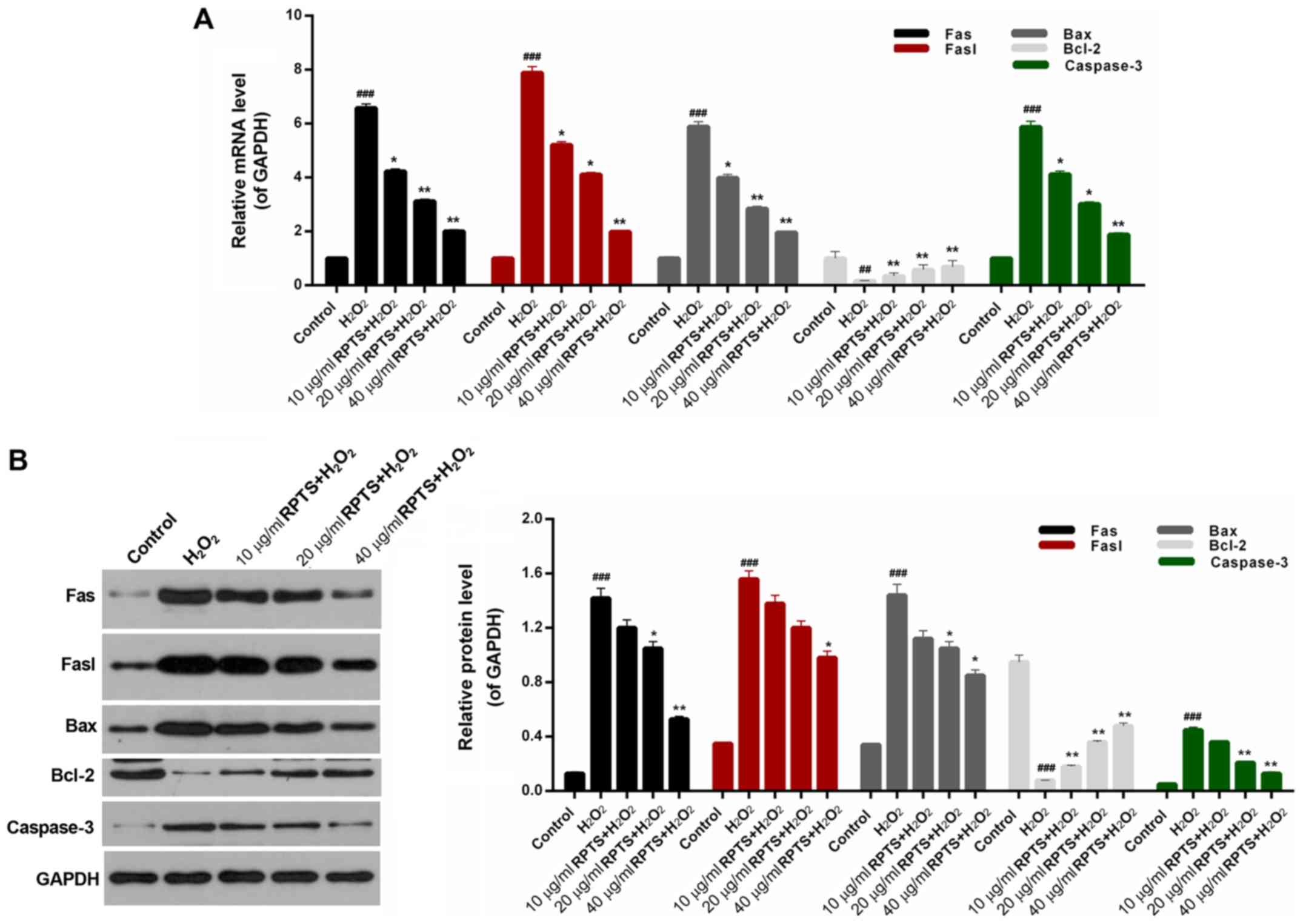
Expression levels of
apoptosis-associated proteins are modulated by RPTS
As RPTS suppressed
H2O2-induced apoptosis of ARPE-19 cells, the
related mechanisms were further investigated. The expression levels
of apoptosis-associated proteins Fas, Fasl, Bax, Bcl-2 and
caspase-3 were measured in ARPE-19 cells treated with
H2O2 and different concentrations of RPTS.
RT-qPCR data demonstrated that the mRNA expression levels of Fas,
Fasl, Bax and caspase-3 in ARPE-19 cells treated with
H2O2 were significantly upregulated compared
with in the control group (P<0.001). However, in the groups
pretreated with RPTS, significant decreases in the expression
levels of Fas, Fasl, Bax and caspase-3 in
H2O2-induced ARPE-19 cells were observed
compared with in the H2O2 group. In addition,
H2O2 treatment significantly reduced Bcl-2
expression in ARPE-19 cells compared with in the control group
(mRNA, P<0.01; protein, P<0.001), whereas RPTS pretreatment
upregulated Bcl-2 expression in H2O2-treated
ARPE-19 cells compared with in the H2O2 group
(P<0.05; Fig. 5A). Western blot
analysis revealed similar trends with regards to Fas, Fasl, Bax,
Bcl-2 and caspase-3 expression in ARPE-19 cells in all groups
(P<0.05; Fig. 5B). These
results indicated that RPTS may suppress the apoptosis of
H2O2-treated ARPE-19 cells by modulating the
expression levels of Fas, Fasl, Bax, Bcl-2 and caspase-3.
RPTS modulates the Nrf2 pathway
An ARE-luciferase assay was performed to confirm
that RPTS suppressed H2O2-induced oxidative
stress injury. RPTS promoted ARE-luciferase activity in a
dose-dependent manner in H2O2-treated cells
compared with in the H2O2 group (P<0.01;
Fig. 6A). To further investigate
the exact mechanisms underlying the effects of RPTS on the
protection of ARPE-19 cells against H2O2, the
related signaling pathway was identified. Western blotting results
revealed that the expression levels of Nrf2, HO-1, γ-GCS and NQO1
were significantly downregulated following treatment with
H2O2 (P<0.001). By contrast, in the groups
pretreated with RPTS, HO-1, γ-GCS and NQO1 protein expression
levels were significantly increased compared with in the
H2O2 group (P<0.05; Fig. 6B-C). Therefore, RPTS may regulate
the Nrf2 pathway in ARPE-19 cells treated with
H2O2.
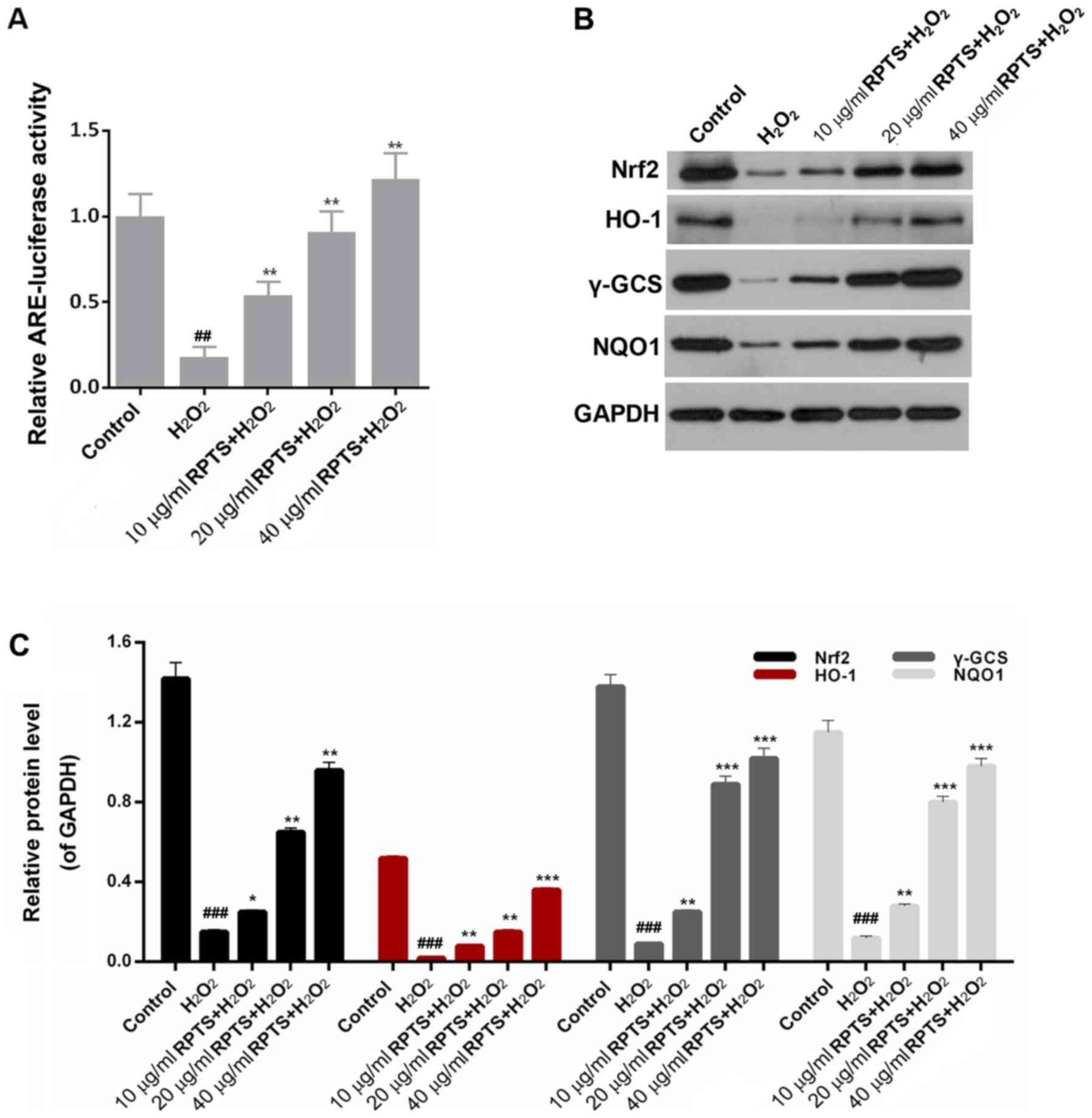 | Figure 6.RPTS modulates the Nrf2 pathway. (A)
ARE-luciferase activity was examined in
H2O2-treated ARPE-19 cells pretreated with
10, 20 and 40 µg/ml RPTS. Renilla luciferase activity was used as
an internal control. (B and C) Western blot analysis was performed
to determine the expression levels of Nrf2, HO-1, γ-GCS and NQO1 in
untreated ARPE-19 cells, ARPE-19 cells treated with
H2O2, and ARPE-19 cells pretreated with 10,
20 and 40 µg/ml RPTS and then treated with
H2O2. ##P<0.01 and
###P<0.001 vs. Control; *P<0.05, **P<0.01 and
***P<0.001 vs. H2O2. ARPE-19, Adult
Retinal Pigment Epithelial cell line-19; ARE, antioxidant response
element; HO-1, heme oxygenase 1; Nrf2, nuclear factor 2-related
factor 2; γ-GCS, γ-glutamyl-cysteine synthetase; NQO1, NAD(P)H
quinone dehydrogenase 1; RPTS, Rhizoma Paridis total saponins. |
Discussion
AMD is associated with multiple factors; increasing
age may enhance oxidative stress, which leads to irreversible
damage to retinal pigment epithelial cells (27). Oxidative stress injury refers to
circumstances, such as ischemia and inflammation, in which the
organism faces various stimuli when highly active molecules such as
ROS and free radicals are overproduced (28). This results in an imbalance between
oxidation and the antioxidant system, which further triggers tissue
damage. To investigate the exact mechanisms underlying oxidative
stress injury in retinal pigment epithelial cells, an experimental
model of ARPE-19 cells treated with H2O2 was
established in the current study. RPTS has been reported to exhibit
numerous biological activities, including antitumor (29), anti-fibrosis and anti-cirrhosis
effects (30). However, the roles
of RPTS in protection against oxidative stress injury in retinal
pigment epithelial cells remain unclear. Therefore, RPTS was
selected as the subject of the present study on
H2O2-induced oxidative stress injury in
ARPE-19 cells. Firstly, the viability of ARPE-19 cells treated with
H2O2 and different concentrations of RPTS was
measured; RPTS enhanced the viability of
H2O2-treated ARPE-19 cells, indicating that
RPTS may serve a function in protecting ARPE-19 cells against
H2O2-induced oxidative stress injury. Further
assessment of the levels of oxidative stress markers in ARPE-19
cells treated with H2O2 and RPTS demonstrated
that RPTS reduced the MDA content, and increased SOD and GPx
levels, in H2O2-treated ARPE-19 cells.
Further studies are required to identify which component of RPTS is
responsible for these results.
Since RPTS reduced the levels of oxidative stress
markers, it was hypothesized that RPTS may affect ROS levels and
MMP in ARPE-19 cells treated with H2O2. An
appropriate level of ROS is necessary in the aging process, whereas
overproduction of ROS can lead to an imbalance in the internal
environment, which results in cell death, structural and functional
tissue damage, and organ damage (31,32).
Therefore, increased endogenous ROS production is a marker of a
high level of oxidative stress. Retinal pigment epithelial cells
are necessary in the function of vision, and they transports
nutrients and ions between photoreceptors and the choriocapillaris
(33). Therefore, ROS production
in ARPE-19 cells treated with H2O2 and RPTS
was further analyzed. RPTS distinctly reduced ROS production in
ARPE-19 cells treated with H2O2, which
suggested that RPTS may serve as a therapeutic agent of oxidative
stress injury.
MMP participates in the development and progression
of cell apoptosis (34). The MMP
of ARPE-19 cells was assessed in the present study; the results
demonstrated that RPTS enhanced the MMP of
H2O2-treated ARPE-19 cells. These results
suggested that RPTS served a function of alleviating the oxidative
stress injury induced by H2O2 on ARPE-19
cells.
Previous studies have demonstrated that oxidative
stress aggravates the degeneration, dysfunction and apoptosis of
age-related retinal pigment epithelial cells (35,36).
In the present study, the apoptosis of ARPE-19 cells treated with
H2O2 and RPTS was tested, which indicated
that RPTS significantly reduced the apoptotic rate of
H2O2-treated ARPE-19 cells. In addition, RPTS
downregulated the expression levels of Fas, Fasl, Bax and
caspase-3, and upregulated Bcl-2 expression in
H2O2-treated ARPE-19 cells. These results
suggested that RPTS suppressed the apoptosis of
H2O2-treated ARPE-19 cells by modulating the
expression levels of Fas, Fasl, Bax, Bcl-2 and caspase-3.
The Nrf2 pathway serves crucial roles in oxidative
stress injury-induced cell apoptosis (37,38).
However, no studies have focused on the association between RPTS
and the Nrf2 pathway in oxidative stress injury, to the best of our
knowledge. Nrf2-driven free radical detoxification pathways are
important endogenous homeostatic mechanisms, and previous studies
have reported that, under oxidative stress, cellular antioxidant
defenses depend primarily on Nrf2 dissociation from Keap1 and its
subsequent translocation to the nucleus, where the activation of
antioxidant genes occurs (39,40).
The results of the present study demonstrated that Nrf2, HO-1,
γ-GCS and NQO1 protein levels were significantly decreased by
H2O2 stimulation and partially rescued by
RPTS pretreatment, which indicated that Nrf2 signaling may be
involved in RPTS-induced protection of ARPE-19 cells against
oxidative injury. In addition, several studies have reported the
effects of H2O2 on Nrf2 expression that were
consistent with the present study (41,42).
Under oxidation conditions in vitro, cells
may be unable to spontaneously produce an antioxidant defense.
Therefore, in vitro experiments and the use of only one
retinal cell line were potential limitations of the present study;
further in vivo experiments and other retinal cell lines are
required to validate the effects of H2O2 and
RPTS on the Nrf2 pathway. Other limitations included the lack of
different detection methods and markers for oxidative stress and
apoptosis.
The results of the present study demonstrated that
RPTS reduced oxidative stress and suppressed the apoptosis of
ARPE-19 cells treated with H2O2 by increasing
Nrf2 and antioxidative enzyme expression. Previous studies have
suggested that total saponins of several natural products, such as
Panax notoginseng saponins (43), Platycodon grandiflorum
saponins (44) and Aralia
taibaiensis saponins (45),
exert a protective effect on oxidative injury. Therefore, the
present study suggested that RPTS may alleviate oxidative stress
injury induced by H2O2 by upregulating the
Nrf2 pathway. These results may provide novel insight into the
pathogenesis of oxidative stress injury and may lead to novel
approaches to the treatment of oxidative stress injury.
In conclusion, the present study demonstrated that
RPTS alleviated oxidative stress injury induced by
H2O2 by upregulating the Nrf2 pathway. These
results are crucial for the understanding the mechanisms underlying
RPTS activity in retinal pigment epithelial cells. The potential
protective effects of RPTS on H2O2-treated
ARPE-19 cells suggested that RPTS may be an effective therapeutic
agent for the treatment of oxidative stress injury, opening up a
novel direction in AMD research and treatment.
Acknowledgements
Not applicable.
Funding
This work was supported by the 1351 Personnel
Training Program of Beijing Chao-Yang Hospital Affiliated to
Capital Medical University (grant no. CYXZ-2017-09).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
BZ, ZW and GW conceived and designed the study. JH,
ZL and BY acquired, analyzed and interpreted the data. BZ and ZW
drafted and critically revised the manuscript for important
intellectual content. All authors agree to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy and integrity of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheng CY, Yamashiro K, Jia Chen L, Ahn J,
Huang L, Huang L, Cheung CM, Miyake M, Cackett PD, Yeo IY, et al:
Corrigendum: New loci and coding variants confer risk for
age-related macular degeneration in East Asians. Nat Commun.
6:68172015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans J and Wormald R: Is the incidence of
registrable age-related macular degeneration increasing? Br J
Ophthalmol. 80:9–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kagan DB, Liu H and Hutnik CM: Efficacy of
various antioxidants in the protection of the retinal pigment
epithelium from oxidative stress. Clin Ophthalmol. 6:1471–1476.
2012.PubMed/NCBI
|
|
4
|
Age-Related Eye Disease Study Research
Group, ; SanGiovanni JP, Chew EY, Clemons TE, Ferris FL III,
Gensler G, Lindblad AS, Milton RC, Seddon JM and Sperduto RD: The
relationship of dietary carotenoid and vitamin A, E, and C intake
with age-related macular degeneration in a case-control study:
AREDS Report No. 22. Arch Ophthalmol. 125:1225–1232. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim EK, Kim H, Vijayakumar A, Kwon O and
Chang N: Associations between fruit and vegetable, and antioxidant
nutrient intake and age-related macular degeneration by smoking
status in elderly Korean men. Nutr J. 16:772017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seddon JM, Gensler G, Klein ML and Milton
RC: C-reactive protein and homocysteine are associated with dietary
and behavioral risk factors for age-related macular degeneration.
Nutrition. 22:441–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yildirim Z, Ucgun NI and Yildirim F: The
role of oxidative stress and antioxidants in the pathogenesis of
age-related macular degeneration. Clinics (Sao Paulo). 66:743–746.
2011.PubMed/NCBI
|
|
8
|
Zhou H, Zhao X, Johnson EJ, Lim A, Sun E,
Yu J, Zhang Y, Liu X, Snellingen T, Shang F and Liu N: Serum
carotenoids and risk of age-related macular degeneration in a
chinese population sample. Invest Ophthalmol Vis Sci. 52:4338–4344.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Macdonald L: Review: In age-related
macular degeneration, antioxidant multivitamins and zinc
supplements each decrease progression. Ann Intern Med.
167:JC572017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen C, Ma W, Zheng W, Huang H, Xia R, Li
C and Zhu X: The antioxidant effects of riluzole on the APRE-19
celll model injury-induced by t-BHP. BMC Ophthalmol. 17:2102017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Gong HM, Zou HH, Liang L and Wu
XY: Isorhamnetin prevents H2O2-induced
oxidative stress in human retinal pigment epithelial cells. Mol Med
Rep. 17:648–652. 2018.PubMed/NCBI
|
|
12
|
Calabrese V, Cornelius C, Trovato A,
Cavallaro M, Mancuso C, Di Rienzo L, Condorelli D, De Lorenzo A and
Calabrese EJ: The hormetic role of dietary antioxidants in free
radical-related diseases. Curr Pharm Des. 16:877–883. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durante W: Targeting heme oxygenase-1 in
vascular disease. Curr Drug Targets. 11:1504–1516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrándiz ML and Devesa I: Inducers of
heme oxygenase-1. Curr Pharm Des. 14:473–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, Hamid T, Keith RJ, Zhou G,
Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, et
al: Cardioprotective and antiapoptotic effects of heme oxygenase-1
in the failing heart. Circulation. 121:1912–1925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tkachev VO, Menshchikova EB and Zenkov NK:
Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry
(Mosc). 76:407–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayes JD and McMahon M: Molecular basis
for the contribution of the antioxidant responsive element to
cancer chemoprevention. Cancer Lett. 174:103–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen F, Zhang N, Ma X, Huang T, Shao Y, Wu
C and Wang Q: Naringin alleviates diabetic kidney disease through
inhibiting oxidative stress and inflammatory reaction. PLoS One.
10:e01438682015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui G, Luk SC, Li RA, Chan KK, Lei SW,
Wang L, Shen H, Leung GP and Lee SM: Cytoprotection of baicalein
against oxidative stress-induced cardiomyocytes injury through the
Nrf2/Keap1 pathway. J Cardiovasc Pharmacol. 65:39–46. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Y, Zhang YJ, Liu WW, Shi AW and Gu N:
Salidroside suppresses HUVECs cell injury induced by oxidative
stress through activating the Nrf2 signaling pathway. Molecules.
21:E10332016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Man S, Chai H, Cui J, Yao J, Ma L and Gao
W: Antitumor and anti-metastatic mechanisms of Rhizoma
paridis saponins in Lewis mice. Environ Toxicol. 33:149–155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Man S, Li Y, Fan W, Gao W, Liu Z, Li N,
Zhang Y and Liu C: Curcuma increasing antitumor effect of
Rhizoma paridis saponins through absorptive enhancement of
paridis saponins. Int J Pharm. 454:296–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng ZX, Liu BR, Qian XP, Ding YT, Hu WJ,
Sun J and Yu LX: Proteomic analysis of anti-tumor effects by
Rhizoma Paridis total saponin treatment in HepG2 cells. J
Ethnopharmacol. 120:129–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Man S, Li J, Qiu P, Liu J, Liu Z, Ma L and
Gao W: Inhibition of lung cancer in diethylnitrosamine-induced mice
by Rhizoma paridis saponins. Mol Carcinog. 56:1405–1413.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong Y, Han YQ, Wang YZ, Gao JR, Li YX,
Liu Q and Xia LZ: Paridis Rhizoma Sapoinins attenuates liver
fibrosis in rats by regulating the expression of RASAL1/ERK1/2
signal pathway. J Ethnopharmacol. 192:114–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lim LS, Mitchell P, Seddon JM, Holz FG and
Wong TY: Age-related macular degeneration. Lancet. 379:1728–1738.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higgins GC, Beart PM, Shin YS, Chen MJ,
Cheung NS and Nagley P: Oxidative stress: Emerging mitochondrial
and cellular themes and variations in neuronal injury. J Alzheimers
Dis. 20 (Suppl 2):S453–S473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao X, Zou J, Bui-Nguyen TM, Bai P, Gao
L, Liu J, Liu S, Xiao J, Chen X, Zhang X and Wang H: Paris saponin
II of Rhizoma Paridis-a novel inducer of apoptosis in human
ovarian cancer cells. Biosci Trends. 6:201–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Man S, Fan W, Gao W, Li Y, Wang Y, Liu Z
and Li H: Anti-fibrosis and anti-cirrhosis effects of Rhizoma
paridis saponins on diethylnitrosamine induced rats. J
Ethnopharmacol. 151:407–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kitada M and Koya D: SIRT1 in Type 2
diabetes: Mechanisms and therapeutic potential. Diabetes Metab J.
37:315–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salminen A, Kaarniranta K and Kauppinen A:
Crosstalk between oxidative stress and SIRT1: Impact on the aging
process. Int J Mol Sci. 14:3834–3859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai J, Nelson KC, Wu M, Sternberg P Jr and
Jones DP: Oxidative damage and protection of the RPE. Prog Retin
Eye Res. 19:205–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y,
Hou D and Zhang X: Astaxanthin protects ARPE-19 cells from
oxidative stress via upregulation of Nrf2-regulated phase II
enzymes through activation of PI3K/Akt. Mol Vis. 19:1656–1666.
2013.PubMed/NCBI
|
|
37
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen B, Lu Y, Chen Y and Cheng J: The role
of Nrf2 in oxidative stress-induced endothelial injuries. J
Endocrinol. 225:R83–R99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hiramatsu K, Tsuneyoshi T, Ogawa T and
Morihara N: Aged garlic extract enhances heme oxygenase-1 and
glutamate-cysteine ligase modifier subunit expression via the
nuclear factor erythroid 2-related factor 2-antioxidant response
element signaling pathway in human endothelial cells. Nutr Res.
36:143–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu Z, Shi Z, Xie C, Gong W, Hu Z and Peng
Y: A novel mechanism of Gamma-aminobutyric acid (GABA) protecting
human umbilical vein endothelial cells (HUVECs) against
H2O2-induced oxidative injury. Comp Biochem
Physiol C Toxicol Pharmacol. 217:68–75. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui W, Leng B and Wang G: Klotho protein
inhibits H2O2-induced oxidative injury in
endothelial Cells via regulation of PI3K/Akt/Nrf2/HO-1 Pathways.
Can J Physiol Pharmacol. 97:370–376. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang M, Guan Y, Xu J, Qin J, Li C, Ma X,
Zhang Z, Zhang B and Tang J: Evaluating the protective mechanism of
panax notoginseng saponins against oxidative stress damage
by quantifying the biomechanical properties of single cell. Anal
Chim Acta. 1048:186–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leng J, Wang Z, Fu CL, Zhang J, Ren S, Hu
JN, Jiang S, Wang YP, Chen C and Li W: NF-κB and AMPK/PI3K/Akt
signaling pathways are involved in the protective effects of
Platycodon grandiflorum saponins against
acetaminophen-induced acute hepatotoxicity in mice. Phytother Res.
32:2235–2246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li YN, Guo Y, Xi MM, Yang P, Zhou XY, Yin
S, Hai CX, Li JG and Qin XJ: Saponins from Aralia
taibaiensis attenuate D-galactose-induced aging in rats by
activating FOXO3a and Nrf2 pathways. Oxid Med Cell Longev.
2014:3205132014. View Article : Google Scholar : PubMed/NCBI
|