Introduction
Renal cell carcinoma (RCC) is the second most common
malignant tumor of the urinary system, with an incidence that is
increasing annually. RCC is associated with >140,000 deaths per
year. The incidence of RCC is more prominent in men, with a
male-to-female ratio of 1.5:1, and peaks at age 60–70 years
(1). Early clinical manifestations
of RCC are not easily detectable, and 1/4-1/3 of patients have
metastasis at the time of clinical diagnosis (2,3).
Therefore, the treatment of RCC is highly challenging. The caspase
recruitment domain (CARD) protein family has three main members,
known as CARD10, CARD11 and CARD14. CARD11 is expressed mainly in
lymphatic tissues, as well as certain hematopoietic organs,
including the spleen and thymus. CARD14 is expressed mainly in the
skin and mucous membranes (4,5).
CARD10, also known as CARMA3 (6),
is widely distributed in all non-hematopoietic tissues, such as the
heart, kidney and liver (6–9).
High expression of CARD10 has also been found in a variety of solid
tumors, such as colorectal (10),
lung (11), pancreatic (12), breast (13), ovarian (14), renal (15) and bladder cancers (16,17).
In colon, lung, pancreatic and breast cancers, studies have shown
that CARD10 promotes growth and invasion of tumor cells, and
inhibits apoptosis (10–13). Furthermore, increased expression of
CARD10 is closely related to malignancy (18–20).
CARD10 exerts its biological functions mainly through the NF-κB
signaling pathway (7,21,22).
This is via a mechanism predominantly involved in the regulation of
the IκB kinase complex by the CARD11-B-cell lymphoma 10
(BCL10)-mucosa-associated lymphoid tissue lymphoma gene 1 (MALT1)
complex, which is composed of downstream BCL10 and MALT1 (7,21,22).
However, the mechanism by which CARD10 modulates signaling pathways
that promote RCC invasion and migration remains unclear. Therefore,
this study investigated the ability of CARD10 to regulate the NF-κB
signaling pathway and promote disease progression in RCC. This
information may provide a novel therapeutic target in RCC.
Materials and methods
Materials
Human RCC cell lines (ACHN and 786-O) and the human
renal tubular epithelial cell line HK-2 were purchased from The
Cell Bank of Type Culture Preservation Committee of the Chinese
Academy of Sciences. Transfection vectors [short hairpin (sh)GFP
and shCARD10] were purchased from Shanghai GeneChem Co., Ltd.
Anti-CARD10 antibody (cat. no. ab36839), anti-IκBα antibody (cat.
no. ab32518), anti-p-65 antibody (cat. no. ab32536),
anti-phosphorylated (p)-p-65 antibody (cat. no. ab86299),
anti-β-actin antibody (cat. no. ab8226), goat anti-rabbit IgG (cat.
no. ab6721) and goat anti-rat IgG (cat. no. ab97057) were purchased
from Abcam. Modified Eagle's Media (MEM), Roswell Park Memorial
Institute (RPMI) 1640 medium, Dulbecco's Modified Eagle's Medium:
Nutrient Mixture F-12 (DMEM/F12) and fetal bovine serum (FBS) were
purchased from Gibco (Thermo Fisher Scientific, Inc.). The MTT cell
proliferation, and cell cycle and apoptosis analysis kits were
purchased from BestBio. Transwell chambers and Matrigel matrix were
purchased from Corning Life Sciences. SDS-PAGE gel preparation kit,
ECL photoluminescence kit, pancreatic cell digestion solution,
bicinchoninic acid (BCA) protein assay kit and PBS were purchased
from Beyotime Institute of Biotechnology.
Cell culture
ACHN, 786-O and HK-2 cells were cultured in MEM,
RPMI 1640 and DMEM/F12 medium, respectively, supplemented with 10%
FBS at 37°C under 5% CO2.
Western blot analysis
Total cellular proteins were extracted using a 100:1
mixed solution of RIPA buffer (Beyotime Institute of Biotechnology)
and PMSF (Beyotime Institute of Biotechnology.). The protein
concentrations were measured using a BCA Protein Assay kit. Target
proteins (30 µg) were separated via SDS-PAGE on a 10% gel and
transferred to PVDF membranes. Non-specific binding was blocked by
incubation in 5% non-fat dried milk for 2 h at room temperature.
Membranes were then incubated overnight at 4°C with primary
antibodies (dilutions: CARD10, 1:200; IκBα, 1:5,000; NF-κB p65,
1:1,000; p-NF-κB p65, 1:1,000). After washing, the membranes were
then incubated with the appropriate HRP-conjugated secondary
antibodies (dilution: 1:10,000) at room temperature for 2 h.
Finally, after washing, immunoreactive protein bands were detected
using an ECL kit and quantified using ImageJ Pro Plus 6.0 software
(National Institutes of Health).
CARD10 shRNA transfection
Cultured RCC cells (ACHN and 786-O; ~90% confluent)
were seeded (2.5×105/ml) in 24-well tissue culture
plates (Beyotime Institute of Biotechnology). Subsequently, when
the cell confluency was ~60%, 20 pmol CARD10 shRNA (or shGFP as the
negative control) was dissolved in 50 µl Opti-MEM (HyClone; GE
Healthcare Life Sciences, and 1 µl Lipofectamine® 2000
reagent (Thermo Fisher Scientific, Inc.) was dissolved in 50 µl
Opti-MEM before both solutions were mixed at room temperature for 5
min. Finally, at ~90% confluence, the culture medium was replaced
with 400 µl serum-free medium and 100 µl transfection mixture was
added. Cells were incubated for 4–6 h before the transfection
medium was replaced with complete medium. After cultivation for
24–48 h, proteins were extracted and successful transfection was
confirmed via western blot analysis.
Transwell cell migration and invasion
assays
For Transwell invasion assays, 100 µl Matrigel (1
mg/ml) was added vertically to the membrane in the Transwell upper
chamber and incubated at 37°C for 4–5 h until dry. The basement
membrane was hydrated by incubation with serum-free medium at 37°C.
Medium (600–800 µl) containing 10% FBS was added in the lower
chamber and 100–150 µl cell suspension (2×105/ml) was
added to the upper chamber. Plates were incubated at 37°C for 24 h
before cells that had invaded the Matrigel were fixed with 4%
paraformaldehyde for 30 min at room temperature, stained with 0.5%
crystal violet solution for 15–30 min at room temperature and
counted under an inverted light microscope (Olympus Corporation;
CKX31; magnification, ×100).
For Transwell migration assays, the cell suspension
was prepared as described previously. Medium (600–800 µl)
containing 10% FBS was added in the lower chamber and 100–200 µl
cell suspension (3×105/ml) was added to the upper
chamber. Plates were incubated at 37°C for 36 h before cells that
had migrated from the upper chamber to the lower chamber were fixed
with 4% paraformaldehyde for 20 min at room temperature, stained
with 0.5% crystal violet for 30 min at room temperature and counted
under an inverted light microscope (Olympus Corporation; CKX31;
magnification, ×100).
Cell cycle analysis
Cells cultured in 6-well tissue culture plates were
washed and resuspended with 0.5 ml pre-cooled PBS. Cells were then
fixed by the addition of 1.2 ml pre-cooled anhydrous ethanol (final
ethanol concentration was 70%) at −20°C for 1 h. Fixed cells were
harvested by centrifugation at 3,600 × g for 10 min at room
temperature. After removing the supernatant, cells were washed
twice with 1.8 ml PBS and centrifuged at 600 × g at room
temperature. The supernatant was discarded, and the cells were
resuspended in 100 µl RNase-A (50 µg/ml), mixed and digested at
37°C for 30 min. Nuclei were stained with 100 µl propidium iodide
(PI) dye solution (final concentration 50 µg/ml) at 4°C in the dark
for 30 min. Flow cytometric analysis (NAVIOS; Beckman Coulter,
Inc.) of ~3×104 cells was performed immediately using
MultiCycle software version 10.1 (Phoenix Flow Systems, Inc.).
MTT assay
Cells were seeded into 96-well flat-bottomed tissue
culture plates (5-10×103/well in 100 µl medium). The
cells were cultured for 24–96 h at 37°C under 5% CO2
before the addition of 10 µl/well MTT solution (5 mg/ml, 0.5% MTT).
Cells were cultured for a further 4 h before the addition of 150 µl
dimethyl sulfoxide to dissolve crystals. Cell proliferation was
measured at 490 nm by an ELISA plate reader.
Cell apoptosis analysis
Cells (0.5–1×106) were centrifuged at 800
× g for 3 min at room temperature. After washing twice with
pre-cooled PBS, cells were resuspended with 100 µl 1X binding
buffer (BestBio) and 5X allophycocyanin conjugated to Annexin V was
added. After incubation for 15 min at room temperature, 10 µl PI
was added and the cells were incubated at room temperature in the
dark for 5 min. Flow cytometric analysis (NAVIOS) of
~3×106 cells was performed immediately using Kaluza 2.0
software (Beckman Coulter, Inc.).
Activation of NF-κB
The cell suspension (786-O and ACHN cells) was
prepared and inoculated into four Petri dishes at 37°C under 5%
CO2. When the cell density was ~90%, EGF (AF-100-15;
PeproTech, Inc.) was added into the four Petri dishes and the
concentration was adjusted to 10 ng/ml. The cells were treated for
0, 7.5, 15 and 30 min. shGFP and shCARD10 cell lines (786-O and
ACHN cells) were established by transfecting cells with shRNA. EGF
was added to three plates, and the concentration was adjusted to 10
ng/m. The cells were treated for 0, 15 and 30 min. The treatment of
HK-2, 786-O and ACHN cells was the same as before.
Statistical analysis
Data are presented as the mean ± SD. The statistical
analysis was performed using SPSS version 19.0 (IBM, Corp.).
Comparisons were performed with Student's t-test. Statistical
differences among multiple groups were analyzed by one-way ANOVA
and Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was repeated
at least three times.
Results
High expression of CARD10 in RCC
cells
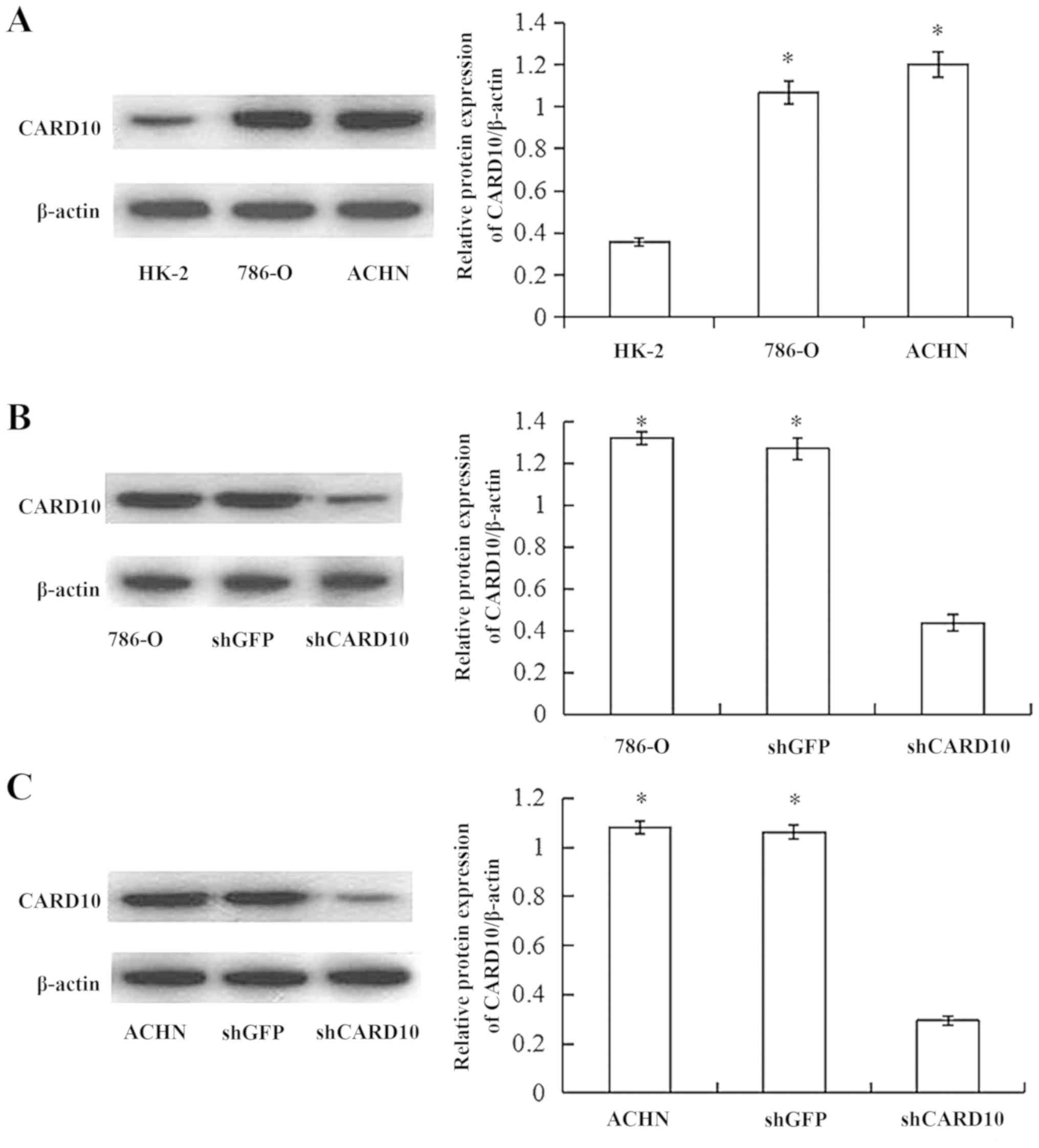
Western blot analysis confirmed that the expression
of CARD10 in RCC cells (786-O and ACHN) was significantly higher
compared with in normal renal tubular epithelial cells (HK-2;
P<0.05; Fig. 1A).
CARD10 promotes the growth of RCC
cells
After 48 h of transfection, the total protein of the
cells was extracted and the effect of transfection was verified via
western blot analysis. CARD10 protein expression was significantly
decreased in cells transfected with CARD10 shRNA (P<0.05;
Fig. 1B and C). The effects of
shRNA-mediated CARD10 silencing on RCC function were investigated.
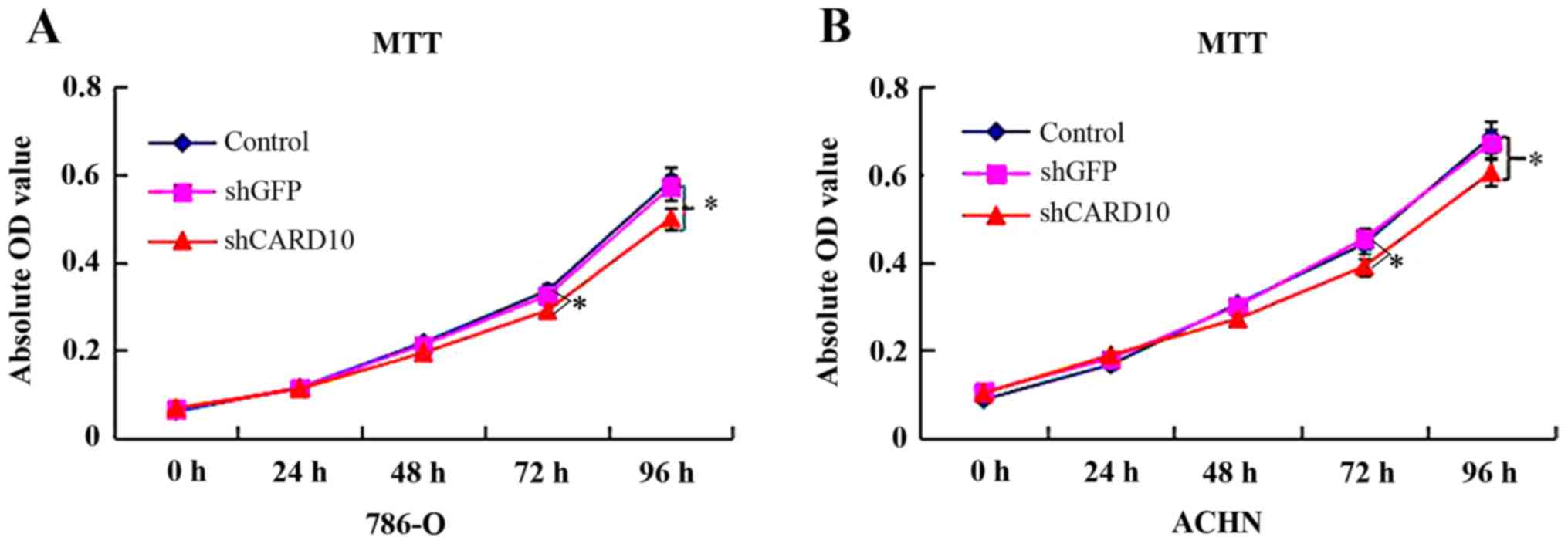
At 48 h after transfection, MTT assays showed that cell
proliferation in the 786-O-shCARD10 group was significantly lower
than that in the 786-O and 786-O-shGFP groups (P<0.05; Fig. 2A). However, there were no
significant differences in the cell proliferation ability between
the 786-O and 786-O-shGFP groups (Fig.
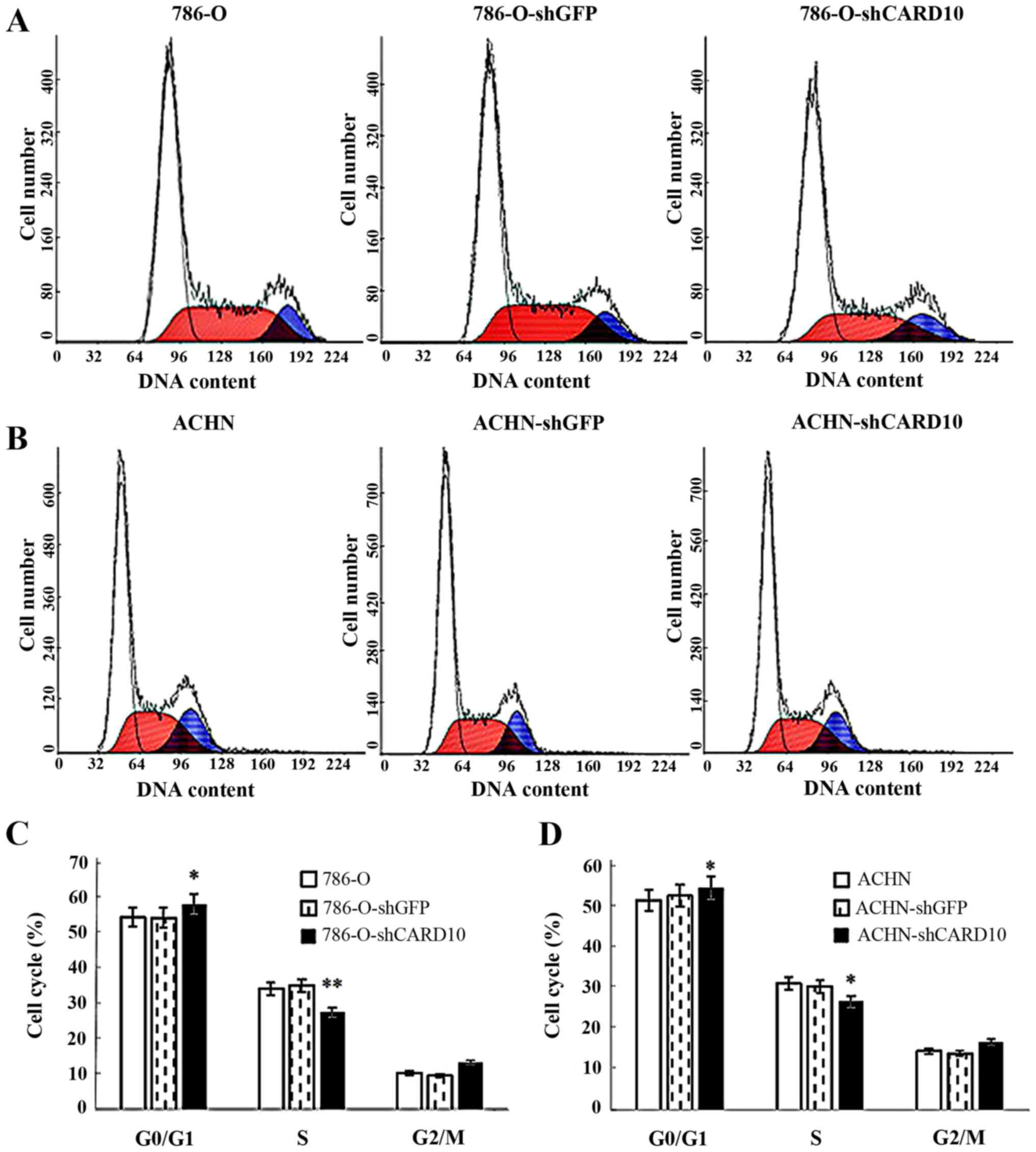
2A). Furthermore, flow cytometric analysis of the cell cycle
distribution in the three groups showed a higher proportion of G1
phase cells in the 786-O-shCARD10 group compared with the other two
groups, while the proportion of S phase cells was significantly
reduced (P<0.05; Fig. 3A and
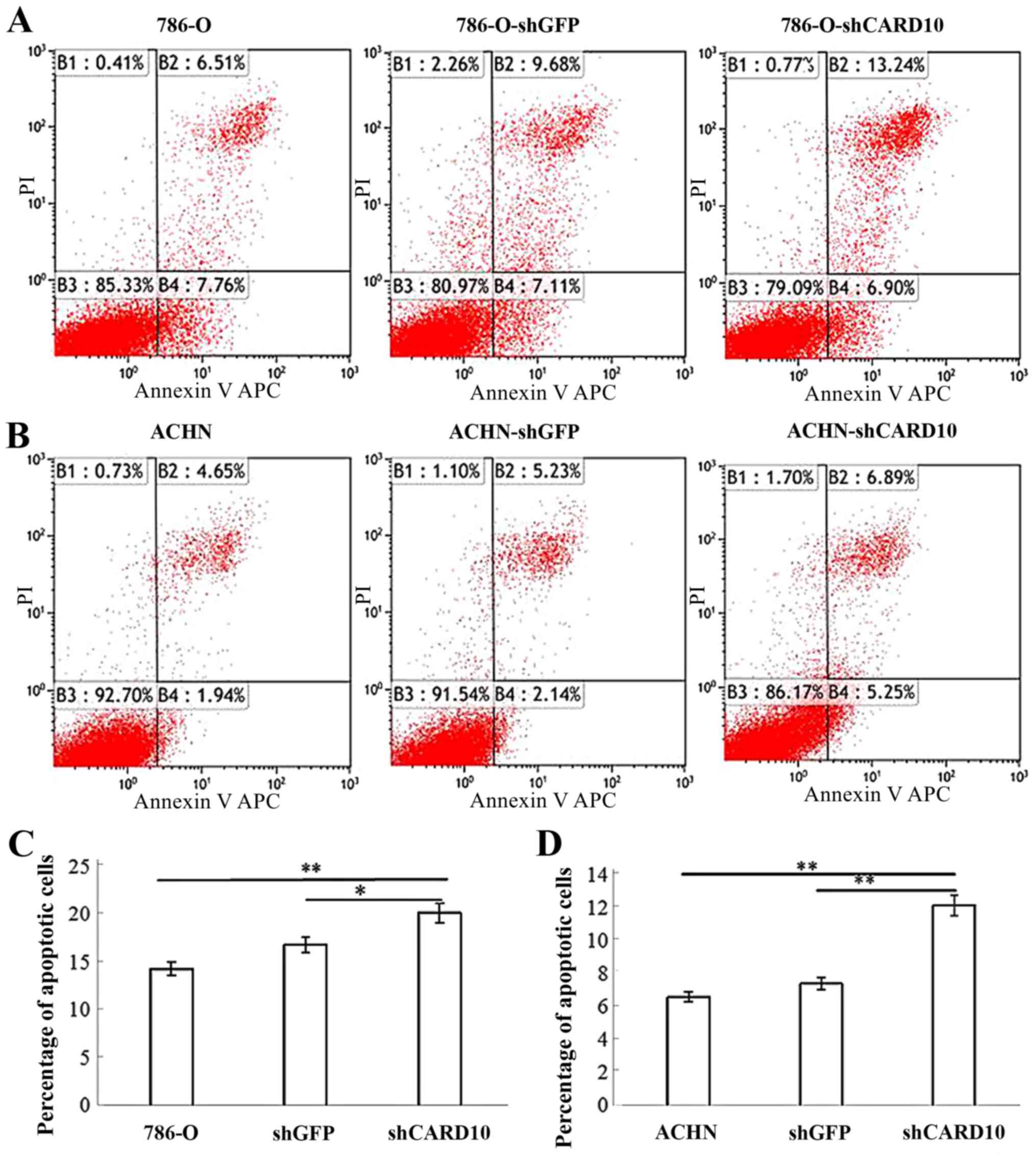
C). To confirm that CARD10 promoted RCC growth, apoptosis in
the three groups of cells was also analyzed. The percentage of
apoptotic cells in the 786-O-shCARD10 group was significantly
higher than that in the other two groups (P<0.05; Fig. 4A and C). Similar results for these
three experiments were obtained in ACHN cells (Figs. 2B, 3B
and D, and 4B and D).
Therefore, it can be concluded that CARD10 promotes growth and
inhibits apoptosis in RCC cells.
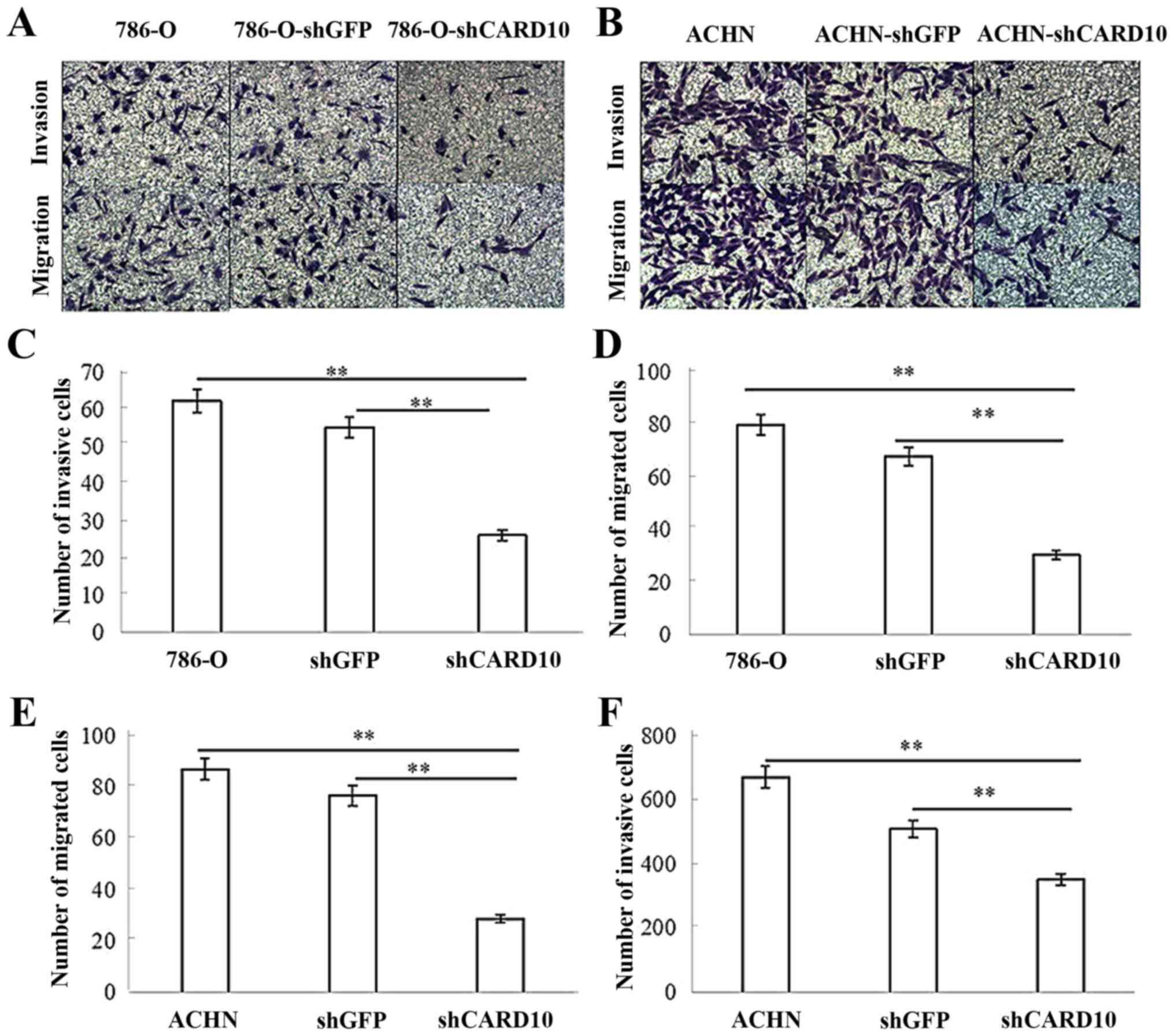
CARD10 promotes the invasion of RCC
cells
To further examine whether CARD10 contributes to the
migration and invasion of RCC cells, migration and Matrigel
invasion assays in Transwell chambers were performed. In invasion
assays, the number of cells passing through the Matrigel in the
786-O-shCARD10 group was significantly reduced compared with in the
786-O and 786-O-shGFP groups, with relative inhibition rates of
57±10.6 and 51.4±12.6%, respectively, after 48 h (P<0.05;
Fig. 5A and C). In migration
assays, the number of cells that migrated from the upper chamber to
the lower chamber in the Transwell plates was significantly lower
in the 786-O-shCARD10 group compared with that in the 786-O and
786-O-shGFP groups, with inhibition rates of 62.3±5.6 and
55.2±7.3%, respectively, at 48 h (P<0.05; Fig. 5A and D). Similarly, the number of
cells passing through the Matrigel in the ACHN-shCARD10 group was
significantly reduced compared to that in the ACHN and ACHN-shGFP
groups, with inhibition rates of 47.5±4.1 and 30.9±4.5%,
respectively, at 48 h (P<0.05; Fig.
5B and E). Furthermore, the number of cells that migrated from
the upper chamber to the lower chamber in the Transwell plates in
the ACHN-shCARD10 group was significantly reduced compared with
that in the ACHN and ACHN-shGFP groups, with inhibition rates of
62.3±5.6 and 55.2±7.3%, respectively, at 48 h (P<0.05; Fig. 5B and F).
CARD10 knockdown inhibits activation
of the NF-κB signaling pathway in RCC cells
To investigate the mechanism by which CARD10
promoted the progression of RCC, the effect of CARD10-knockdown on
IκBα, NF-κB p65 and p-p65 levels was tested. First, 786-0 cells
were stimulated with epidermal growth factor (EGF; 10 ng/ml) for 0,
7.5, 15 and 30 min. The expression of proteins related to
activation of the NF-κB signaling pathway was then evaluated via
western blot analysis. IκBα protein expression decreased
significantly in a time-dependent manner, while the p-p65/p65
content increased (P<0.05; Fig.
6A). These results demonstrated that EGF stimulated activation
of the NF-κB signaling pathways in RCC cells. Then, 786-O-shGFP and
786-O-shCARD10 cells were treated with EGF (10 ng/ml) for 0, 15 and
30 min; 786-O-shGFP cells were used as a negative control group.
Western blot analysis showed that there was a significantly higher
IκBα protein content and lower p-p65/p65 content in the
786-O-shCARD10 group compared with that in the negative control
group when measured at the same time point (P<0.05; Fig. 6B). These results demonstrated that
CARD10 knockdown inhibited the activation of the NF-κB signaling
pathway-related proteins in 786-0 cells. Similar results were
obtained when the experiments were repeated with ACHN cells
(Fig. 6C and D). NF-κB activation
in HK-2 cells and RCC cells was further validated following
prolonged EGF (10 ng/ml) treatment. The results showed
significantly higher IκBα protein content and decreased p65
phosphorylation in HK-2 cells than that in the RCC cells when
measured at the same time point (P<0.05; Fig. 6E).
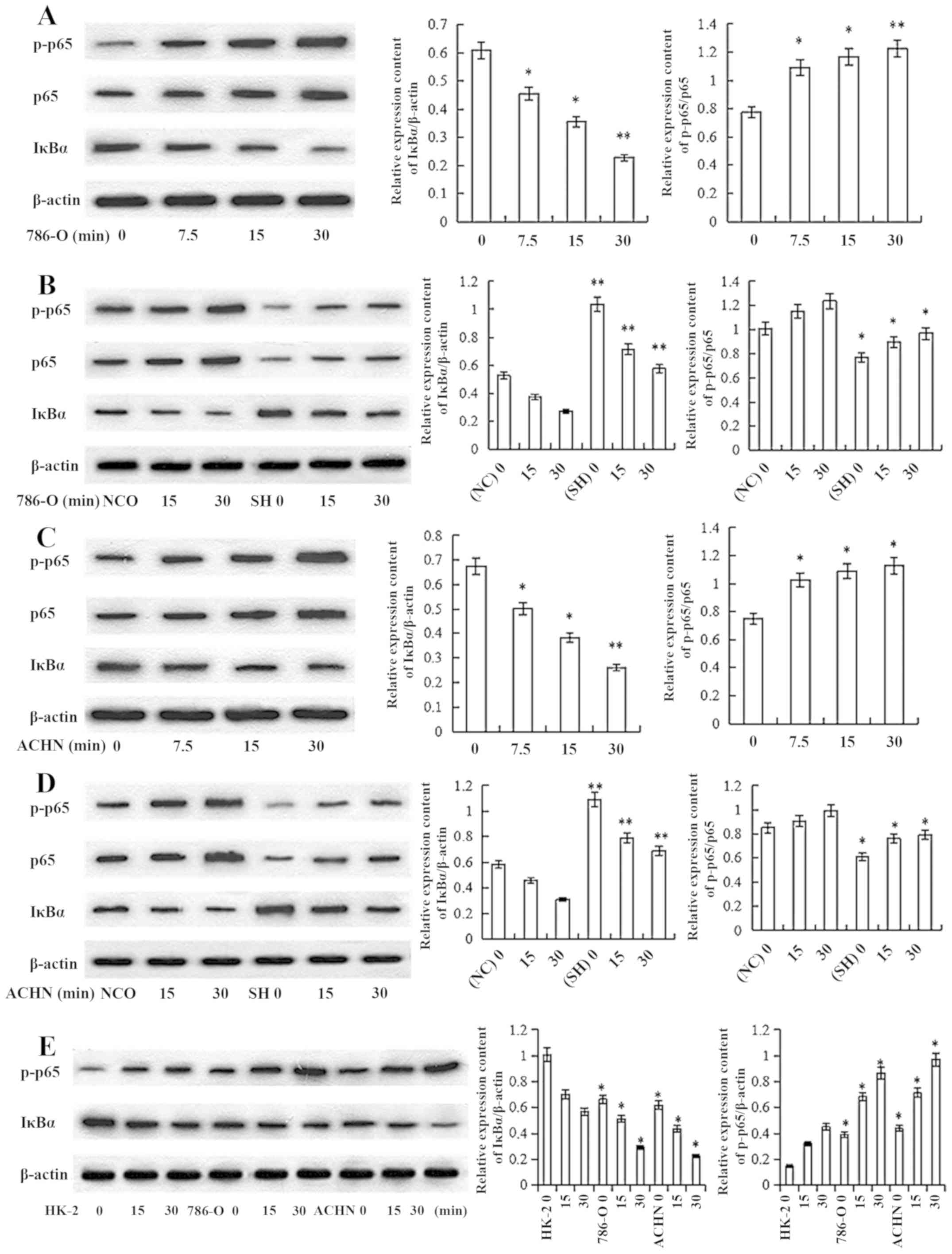 | Figure 6.Inhibiting the expression of CARD10
can inhibit the activation of NF-κB signaling pathways in RCC. (A)
786-0 cells were stimulated with EGF (10 ng/ml) for 0, 7.5, 15 and
30 min. With the prolongation of treatment time, IкBα levels were
increasingly downregulated, while p-p65/p65 content showed an
upward trend. *P<0.05, **P<0.01 vs. 0 ng/ml. (B) 786-O NC
(shGFP transfection) group and the experimental group (shCARD
transfection) were treated with EGF (10 ng/ml) for 0, 15 and 30
min. At the same treatment time, the experimental group had higher
IкBα protein content and lower p-p65/p65 content than the negative
control group. The bar graph indicates relative expression of
various proteins. *P<0.05, **P<0.01 vs. NC. (C) ACHN cell
were stimulated with EGF (10 ng/ml) for 0, 7.5, 15 and 30 min. With
the prolongation of treatment time, IкBα levels were increasingly
downregulated, while p-p65/p65 content showed an upward trend.
*P<0.05, **P<0.01 vs. 0 ng/ml. (D) ACHN NC (shGFP
transfection) group and the experimental group (shCARD
transfection) were treated with EGF (10 ng/ml) for 0, 15 and 30
min. At the same treatment time, the experimental group had higher
IкBα protein content and lower p-p65/p65 content than the negative
control group. The bar graph indicates relative expression of
various proteins. *P<0.05, **P<0.01 vs. NC. (E) Normal HK-2
cells and RCC cell lines were treated with EGF (10 ng/ml) for 0, 15
and 30 min. At the same treatment time, there was significantly
higher IκBα protein content and lower p-p65 content in HK-2 cells
than in the RCC cells. *P<0.05 vs. HK-2. CARD10, caspase
recruitment domain 10; EGF, epidermal growth factor; NC, negative
control; p, phosphorylated; RCC, renal cell carcinoma; sh, short
hairpin (RNA). |
Discussion
A previous study in the literature demonstrated that
CARD10 expression in RCC is significantly higher than that in
adjacent non-cancerous tissues (15). In the present study, it was first
verified that CARD10 was expressed at higher levels in RCC cells
than in normal renal tubular epithelial cells. Secondly, because
CARD10 has been found to promote the growth of a variety of types
of tumors, such as rectal (10),
lung (11), and breast cancer
(13), the aim was to verify the
involvement of CARD10 in the proliferation and invasion of RCC. The
ACHN and 786-O renal cancer cell lines were transfected with CARD10
shRNA to silence CARD10 expression. Finally, the proliferative,
invasive and migratory abilities of RCC cell lines were examined
after transfection with CARD10 shRNA compared with a negative
control. These results showed that CARD10 promoted proliferation
and invasion in two different RCC cell lines.
NF-κB, which is a protein with multiple
transcriptional regulatory functions, participates in the
transcriptional regulation of genes related to inflammation, stress
response, immune cell activation, proliferation, differentiation,
apoptosis and tumorigenesis (23,24).
NF-κB is activated via classical and non-classical pathways,
although the classical pathway is more common (24). In the stationary state, NF-κB
exists in the cell cytoplasm in the inactive state combined with
the inhibitor IκB to form a trimer p50-p65-IκB. Following
activation, IκB is released from the p50-p65-IκB timer to form a
p50-p65 dimer (25–27). Studies have shown that
guanosine-binding protein-coupled receptor (28), EGF receptor (28–30)
and protein kinase C (31) induce
NF-κB activation (32). EGF
consists of 53 amino acids and is characterized by its ability to
promote cell proliferation. EGF is the prototypical
growth-promoting cytokine, but also has well-established roles in
stimulating tumor-initiating stem cells and tumorigenesis (33). EGF induces NF-κB activation in a
variety of tumors, such as breast, colon, non-small cell lung, and
pancreatic cancers (34–37). To verify EGF-induced NF-κB
activation in RCC cells, a previous study treated tumor cells with
10 ng/ml EGF (38). The results
showed a time-dependent decrease in IκB content and increasing
p-p65/p65 content. Therefore, it can be concluded that EGF induces
NF-κB activation in RCC cells. To further demonstrate the ability
of CARD10 to mediate EGF-induced NF-κB activation, CARD10
shRNA-transfected RCC cells were treated with EGF. The results
showed a significant decrease in NF-κB activation in the
shRNA-transfected group compared with that in the negative control
group at the same time point. As the expression of CARD10 in RCC is
higher than that in normal renal tubular epithelial cells, the
activation of the NF-κB signaling pathway was further analyzed with
the prolongation of EGF treatment time. The results showed that
NF-κB activation in RCC cell lines was significantly higher than
that in normal renal tubular epithelial cells. Therefore, it was
concluded that CARD10 mediates NF-κB activation in RCC cells.
In conclusion, the expression of CARD10 was
significantly increased in RCC cells. Furthermore, CARD10 promoted
proliferation, invasion and migration of RCC cells, while also
inhibiting the apoptosis of RCC cells via the regulation of the
NF-κB signaling pathway. This study preliminarily explored the
mechanism by which CARD10 regulates the development and progression
of RCC. This information is important for the early diagnosis of
RCC and the design of targeted therapy.
Acknowledgements
Not applicable.
Funding
The National Natural Science Foundation of China
(grant no. 81572507) funded the present study.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LP participated in the design of the study and
analysis of data, and drafted the manuscript. LB and DY designed
the study and drafted the manuscript. KH, ZC, JW and QW were
involved in analysis and interpretation of data. Authors whose
names appear on the submission have contributed sufficiently to the
scientific work and therefore share collective responsibility and
accountability for the results.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuusk T, Grivas N, de Bruijn R and Bex A:
The current management of renal cell carcinoma. Minerva Med.
108:357–369. 2017.PubMed/NCBI
|
|
3
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zotti T, Polvere I, Voccola S, Vito P and
Stilo R: CARD14/CARMA2 signaling and its role in inflammatory skin
disorders. Front Immunol. 9:21672018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scudiero I, Vito P and Stilo R: The three
CARMA sisters: So different, so similar: A portrait of the three
CARMA proteins and their involvement in human disorders. J Cell
Physiol. 229:990–997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stilo R, Liguoro D, Di Jeso B, Formisano
S, Consiglio E, Leonardi A and Vito P: Physical and functional
interaction of CARMA1 and CARMA3 with Ikappa kinase gamma-NFkappaB
essential modulator. J Biol Chem. 279:34323–34331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blonska M and Lin X: NF-κB signaling
pathways regulated by CARMA family of scaffold proteins. Cell Res.
21:55–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang C and Lin X: Regulation of NF-κB by
the CARD proteins. Immunol Rev. 246:141–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Juilland M and Thome M: Holding all the
CARDs: How MALT1 controls CARMA/CARD-dependent signaling. Front
Immunol. 9:19272018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Qian L, Li X and Yan J:
MicroRNA-195 inhibits colorectal cancer cell proliferation,
colony-formation and invasion through targeting CARMA3. Mol Med
Rep. 10:473–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang YW, Chiu CF, Lee KY, Hong CC, Wang
YY, Cheng CC, Jan YH, Huang MS, Hsiao M, Ma JT and Su JL: CARMA3
represses metastasis suppressor NME2 to promote lung cancer
stemness and metastasis. Am J Respir Crit Care Med. 192:64–75.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du S, Jia L, Zhang Y, Fang L, Zhang X and
Fan Y: CARMA3 is upregulated in human pancreatic carcinoma, and its
depletion inhibits tumor proliferation, migration, and invasion.
Tumour Biol. 35:5965–5970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ekambaram P, Lee JL, Hubel NE, Hu D,
Yerneni S, Campbell PG, Pollock N, Klei LR, Concel VJ, Delekta PC,
et al: The CARMA3-Bcl10-MALT1 signalosome drives NFκB activation
and promotes aggressiveness in angiotensin II receptor-positive
breast cancer. Cancer Res. 78:1225–1240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie C, Han Y, Fu L, Li Q, Qiu X and Wang
E: Overexpression of CARMA3 is associated with advanced tumor
stage, cell cycle progression, and cisplatin resistance in human
epithelial ovarian cancer. Tumour Biol. 35:7957–7964. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu GL, Yuan JL, Huang XD, Rong JF, Zhang
LX, Liu YP and Wang FL: Evaluating the expression of CARMA3 as a
prognostic tumor marker in renal cell carcinoma. Tumour Biol.
34:3431–3435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Liu X, Jing Z, Bi J, Li Z, Liu X,
Li J, Li Z, Zhang Z and Kong C: The circINTS4/miR-146b/CARMA3 axis
promotes tumorigenesis in bladder cancer. Cancer Gene Ther.
2019.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Man X, He J, Kong C, Zhu Y and Zhang Z:
Clinical significance and biological roles of CARMA3 in human
bladder carcinoma. Tumour Biol. 35:4131–4136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mahanivong C, Chen HM, Yee SW, Pan ZK,
Dong Z and Huang S: Protein kinase C alpha-CARMA3 signaling axis
links Ras to NF-kappa B for lysophosphatidic acid-induced urokinase
plasminogen activator expression in ovarian cancer cells. Oncogene.
27:1273–1280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang D, You Y, Lin PC, Xue L, Morris SW,
Zeng H, Wen R and Lin X: Bcl10 plays a critical role in NF-kappaB
activation induced by G protein-coupled receptors. Proc Natl Acad
Sci USA. 104:145–150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Zhang C, Liu W, Zheng W, Zhang Y,
Wang S, Huang D, Liu X and Bai Z: MicroRNA-24 upregulation inhibits
proliferation, metastasis and induces apoptosis in bladder cancer
cells by targeting CARMA3. Int J Oncol. 47:1351–1360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Senftleben U, Cao Y, Xiao G, Greten FR,
Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC and Karin M:
Activation by IKKalpha of a second, evolutionary conserved,
NF-kappa B signaling pathway. Science. 293:1495–1499. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S and Lin X: CARMA3: Scaffold
protein involved in NF-κB signaling. Front Immunol. 10:1762019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med.
8:227–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cildir G, Low KC and Tergaonkar V:
Noncanonical NF-κB signaling in health and disease. Trends Mol Med.
22:414–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McAuley JR, Freeman TJ, Ekambaram P, Lucas
PC and McAllister-Lucas LM: CARMA3 is a critical mediator of G
protein-coupled receptor and receptor tyrosine kinase-driven solid
tumor pathogenesis. Front Immunol. 9:18872018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang C and Lin X: Analysis of epidermal
growth factor-induced NF-κB signaling. Methods Mol Biol.
1280:75–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan D and Lin X: Epithelial growth factor
receptor-activated nuclear factor κB signaling and its role in
epithelial growth factor receptor-associated tumors. Cancer J.
19:461–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan D, Zhu Y, Zhou Z, Wang T, You H, Jiang
C and Lin X: The CBM complex underwrites NF-κB activation to
promote HER2-associated tumor malignancy. Mol Cancer Res.
14:93–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang C, Zhou Z, Quan Y, Zhang S, Wang T,
Zhao X, Morrison C, Heise MT, He W, Miller MS and Lin X: CARMA3 is
a host factor regulating the balance of inflammatory and antiviral
responses against viral infection. Cell Rep. 14:2389–2401. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen R, Jin G and McIntyre TM: The soluble
protease ADAMDEC1 released from activated platelets hydrolyzes
platelet membrane pro-epidermal growth factor (EGF) to active
high-molecular-weight EGF. J Biol Chem. 292:10112–10122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elbaz M, Nasser MW, Ravi J, Wani NA,
Ahirwar DK, Zhao H, Oghumu S, Satoskar AR, Shilo K, Carson WE III
and Ganju RK: Modulation of the tumor microenvironment and
inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of
Cannabidiol in breast cancer. Mol Oncol. 9:906–919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Lin Z, Chen B, Chen S, Jiang Z, Zhou
T, Hou Z and Wang Y: Ezrin/NF-kB activation regulates
epithelial-mesenchymal transition induced by EGF and promotes
metastasis of colorectal cancer. Biomed Pharmacother. 92:140–148.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu W, Jaspers I, Zhang W, Graves LM and
Samet JM: Role of Ras in metal-induced EGF receptor signaling and
NF-kappaB activation in human airway epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 282:L1040–L1048. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J
Cancer. 105:735–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang T, Grabiner B, Zhu Y, Jiang C, Li H,
You Y, Lang J, Hung MC and Lin X: CARMA3 is crucial for
EGFR-Induced activation of NF-κB and tumor progression. Cancer Res.
71:2183–2192. 2011. View Article : Google Scholar : PubMed/NCBI
|