Introduction
Leber's hereditary optic neuropathy (LHON) is a
maternally inherited disease that affects 1 in 31,000-50,000 people
and culminates in the bilateral loss of central vision (1–3). In
the North East of England, it has been reported that 1:8,500
individuals harbor a primary LHON-causing mutation and 1:31,000
experience visual loss as a result of LHON (4). Patients with LHON may exhibit
abnormal symptoms, including movement disorders, dystonia or
multiplesclerosislike symptoms, which pose a significant challenge
for clinicians (5,6). Few significant improvements in visual
acuity are reported following atrophy of the optic discs. LHON
demonstrates an incomplete penetrance for both vision loss and
gender bias; LHON affects males more frequently than females
(7,8). Three primary mutations including the
NADH dehydrogenase (ND) 4 m.11778G>A, ND6 m.14484T>C
and ND1 m.3460G>A have been identified in 90% of patients
with LHON (9–11). Yet the molecular mechanisms of
these mtDNA mutations in the phenotypic manifestation of LHON have
not been elucidated.
To understand the role of mitochondrial dysfunction
in LHON, an extended genetic screen for mtDNA variants was
performed in a Han Chinese family with a high prevalence of LHON.
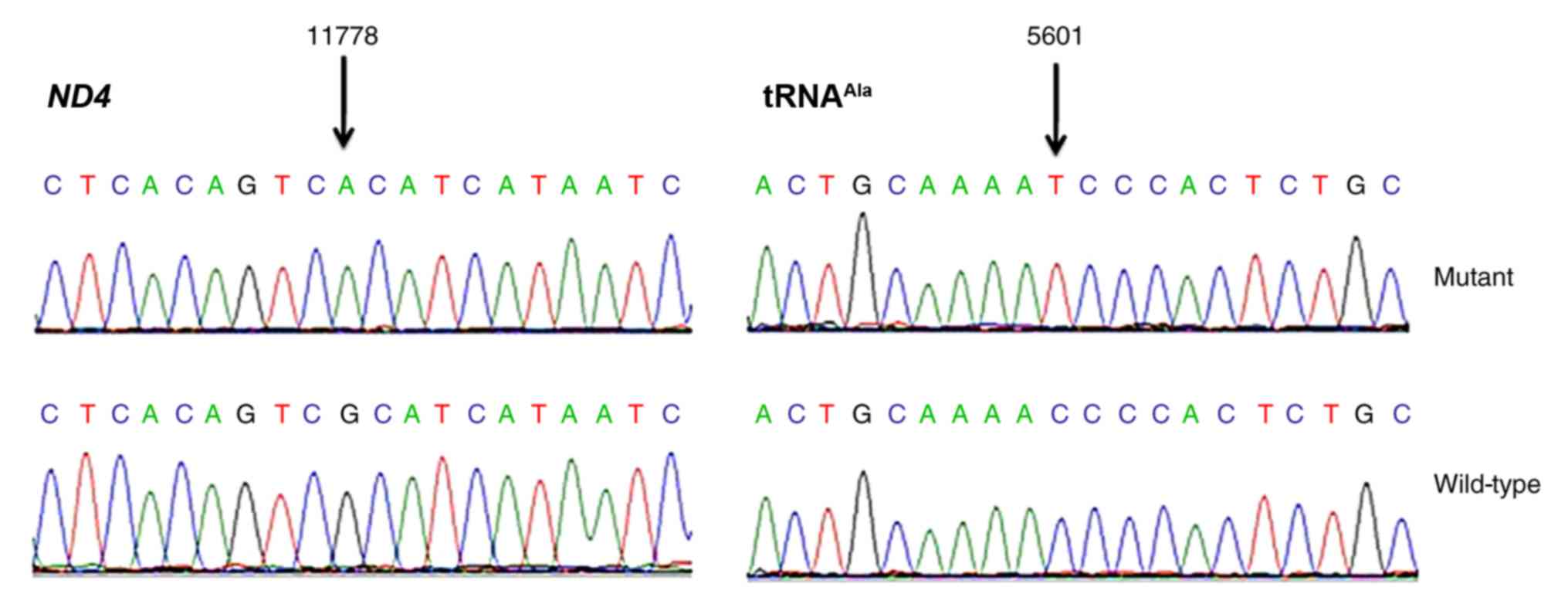
Sequence analysis of the complete mitochondrial genome identified
the occurrence of an ND4 m.11778G>A mutation and an
alanine transfer RNA (tRNAAla) m.5601C>T variant
within matrilineal relatives of the proband. In addition,
bioinformatics analysis was performed in order to explore whether
the m.5601C>T affected the tRNAAla secondary
structure.
Patients and methods
Patients and genetic screening
To identify mtDNA variations in Chinese patients
with LHON, a Han Chinese family was recruited from Hangzhou First
People's Hospital (Hangzhou, China) in January 2018, and blood
samples (5 ml) were collected from each matrilineal relative of the
proband. Blood samples (5 ml) from unrelated control subjects
(n=300) from the same geographical region recruited at the Hangzhou
First People's Hospital were also used in the present study. These
healthy subjects consisted of 200 males and 100 females, aged 11–48
years, and were enrolled from January 2018 to January 2019. The
present study was approved by the Ethics Committee of Hangzhou
First People's Hospital. Written informed consent was obtained from
all participants, or their parent/guardian, prior to enrollment in
the study.
Clinical examinations
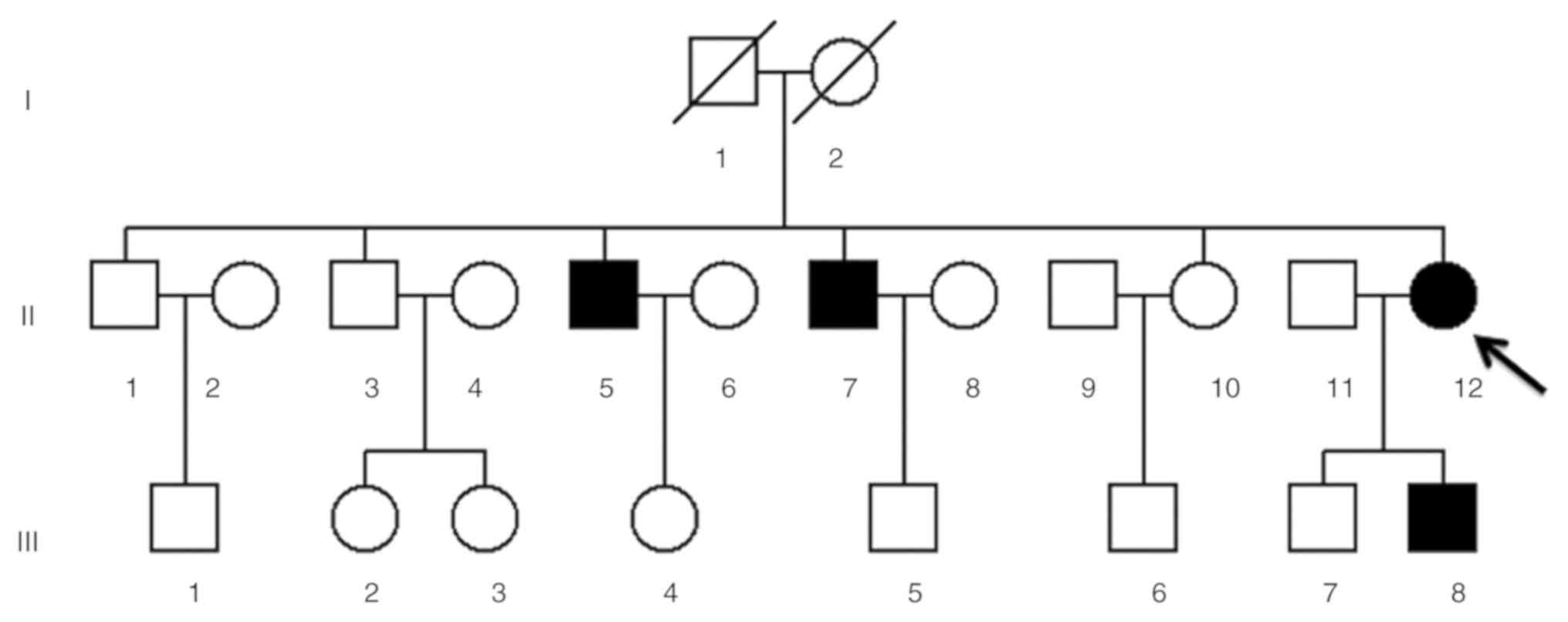
The proband (II-12) and other affected matrilineal
relatives (II-5, II-7 and III-8; Fig.
1) underwent comprehensive ophthalmic examinations, including
visual field tests, examination of visual acuity, fundus
photography, visual evoked potentials and determination of the
degree of visual impairment, performed as previously described
(12,13). The degree of visual impairment was
classified based on the following criteria (12,13):
healthy, ≥0.300; mild, 0.100–0.299; moderate, 0.050–0.099; severe,
0.020–0.049; and profound, <0.020.
PCR and genetic sequencing to identify
mtDNA variants
Genomic DNA from LHON patients and control subjects
was extracted using a DNA extraction kit (QIAamp® DNA
Blood Mini kit; Qiagen GmbH), according to the manufacturer's
protocol. The complete mitochondrial genomes of II-5, II-7, II-12
and III-8 were amplified in 24 overlapping fragments using 200 µM
dNTP, 10X buffer, Taq DNA polymerase and 15 mmol/l Mg2
(cat. no. R004A; Takara Biotechnology, Co., Ltd.). The 24 sequences
of light-strand and heavy-strands oligonucleotide primers for
amplification of mtDNA genes were used according to a previous
report (14). The following
thermocycling conditions were used for PCR: 95°C for 5 min; 30
cycles of 94°C for 10 sec, 60°C for 30 sec and 72°C for 1 min; and
a final extension at 72°C for 5 min. After confirmation of band of
interest, the PCR products were purified using the PureLink Gel
Extraction kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's recommendations. DNA samples with
concentrations >1.0 ng/µl were sequenced using the BigDye™
Terminator Cycle Sequencing reaction kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and an ABI PRISM® 3700
DNA Analyzer. Sequencing data were compared with the updated
Cambridge consensus human mitochondrial genome sequence (accession
no. NC_012920) using DNASTAR version 5.01 (DNASTAR Inc.) (15).
Phylogenetic conservation
analysis
Phylogenetic analysis was performed to determine the
potential pathogenic role of the identified mtDNA mutations.
Briefly, 17 different species were selected for phylogenetic
analysis (Table I). The
conservation index (CI) was measured by comparing the human
nucleotide alternations with the nucleotide sequences of other
species. CI≥70% was implicated to have functional significance
(16).
 | Table I.Mitochondrial DNA sequence accession
number of 17 vertebrate species used in the phylogenetic
analyses. |
Table I.
Mitochondrial DNA sequence accession
number of 17 vertebrate species used in the phylogenetic
analyses.
| Species | GenBank accession
no. |
|---|
| Homo
sapiens | NC_012920 |
| Cebus
albifron | NC_002763 |
| Gorilla
gorilla | NC_011120 |
| Hylobates
lar | NC_002082 |
| Lemur
catta | NC_004025 |
| Macaca
mulatta | NC_005943 |
| Macaca
sylvanus | NC_002764 |
| Nycticebus
coucang | NC_002765 |
| Pan
paniscus | NC_001644 |
| Pan
troglodytes | NC_001643 |
| Papio
hamadryas | NC_001992 |
| Pongo
pygmaeus | NC_001646 |
| Pongo pygmaeus
abelii | NC_002083 |
| Tarsius
bancanus | NC_002811 |
| Mus
musculus | NC_006914.1 |
| Bos
taurus | HM045018.1 |
| Xenopus
laevis | NC_001573.1 |
Bioinformatics analysis
To determine whether the m.5601C>T variant
affected tRNAAla secondary structure, the RNAfold web
server program was used (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi),
as previously described (17).
Determining the pathogenicity of the
variant
The role of the tRNAAla m.5601C>T
variant was determined using the pathogenicity scoring system, as
described by Yarham et al (18). In brief, mutations were classified
as: ‘neutral polymorphism’, ≤6; ‘possible pathogenic’, 7–10;
‘definitely pathogenic’, ≥11.
Statistical analysis
SPSS 17.0 software (SPSS Inc.) was used for
statistical analysis. Fisher's exact test was used to assess the
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical presentation of a Han Chinese
family with LHON
A pedigree chart from a Han Chinese family with a
history of LHON is presented in Fig.
1. There were four LHON patients presented in the pedigree
(three males and one female), aged 7–39 years old. Medical history
analysis of the proband (II-12) confirmed that no other clinical
disorders, such as deafness, diabetes mellitus, cardiovascular
diseases, cancer or neurological disorders, were present. Following
comprehensive genetic counseling at the Department of Ophthalmology
in Hangzhou First People's Hospital, the proband (age, 39), was
found to have begun suffering from painless and progressive
bilateral loss of vision at the age of 19, manifesting as a dark
cloud in the central vision and difficulty differentiating
different colors. Ophthalmic examination revealed large centrocecal
scotoma in both eyes, a typical clinical feature of LHON (13). A total of three out of seven
matrilineal relatives (II-5, II-7 and III-8), in addition to the
proband (II-12), suffered from moderate to profound visual
impairment (Table II).
 | Table II.Summary of the clinical data for the
proband (II-12) and matrilineal relatives (II-5, II-7 and III-8) in
the Han Chinese family with maternally inherited Leber's hereditary
optic neuropathy. |
Table II.
Summary of the clinical data for the
proband (II-12) and matrilineal relatives (II-5, II-7 and III-8) in
the Han Chinese family with maternally inherited Leber's hereditary
optic neuropathy.
|
|
|
|
| Visual impairment
score |
|
|---|
|
|
|
|
|
|
|
|---|
| Subject | Sex | Age at onset
(years) | Age at test
(years) | Right eye | Left eye | Degree of visual
impairmenta |
|---|
| II-5 | Male | 11 | 35 | 0.03 | 0.05 | Severe |
| II-7 | Male | 16 | 33 | 0.1 | 0.2 | Moderate |
| II-12 | Female | 19 | 39 | 0.01 | 0.01 | Profound |
| III-8 | Male | 3 | 7 | 0.02 | 0.01 | Profound |
Screening for mtDNA mutations
To investigate the molecular basis of LHON, II-5,
II-7, II-12 and III-8 were screened for mutations following PCR
amplification of the mtDNA genomes. Sequence analysis of the mtDNA
PCR products revealed 32 genetic polymorphisms (Table III), all of which belonged to the
human mtDNA B5b1 haplogroup (19).
Of these, there were nine variants in the D-loop gene, two variants
in the 12S rRNA gene, one variant in the 16S rRNA gene and one
variant in a tRNA gene (m.5601C>T). The other variants were
mainly localized within oxidative phosphorylation encoding genes.
Notably, five missense mutations were identified: Mitochondrial
encoded NADH dehydrogenase 1 (ND1) m.3593T>C (p.V96A),
ND2 m.5442T>C (p.F325L), ATP 6 m.9103T>C
(p.F193L), ND3 m.10398A>G (p.T114A) and ND4
m.11778G>A (p.R340H). The CIs of these variants were
investigated between different species, including mouse, bovine and
Xenopus laevis (20–22).
Of all identified variants, only tRNAAla m.5601C>T
and ND4 m.11778G>A were conserved. Notably, the
m.5601C>T and m.11778G>A mutations were absent in the 300
control subjects compared with the mtDNA genomes of the matrilineal
relatives (P<0.05). Taken together, these results indicated that
tRNAAla m.5601C>T and ND4 m.11778G>A may
have active roles in the pathogenesis of LHON.
 | Table III.Sequence analysis of mitochondrial
DNA mutations in a Han Chinese family with maternally inherited
Leber's hereditary optic neuropathy. |
Table III.
Sequence analysis of mitochondrial
DNA mutations in a Han Chinese family with maternally inherited
Leber's hereditary optic neuropathy.
| Gene | Position | Base change | Conservation
(H/B/M/X)a | CI (%) | Previously
reportedb |
|---|
| D-loop | 73 | A>G |
|
| Yes |
|
| 152 | T>C |
|
| Yes |
|
| 189 | A>C |
|
| Yes |
|
| 263 | A>G |
|
| Yes |
|
| 489 | T>C |
|
| Yes |
|
| 16117 | T>C |
|
| Yes |
|
| 16172 | T>C |
|
| Yes |
|
| 16223 | T>C |
|
| Yes |
|
| 16519 | T>C |
|
| Yes |
| 12S rRNA | 709 | G>A | G/A/A/- |
| Yes |
|
| 1438 | A>G | A/A/A/G |
| Yes |
| 16S rRNA | 2706 | A>G | A/G/A/A |
| Yes |
| ND1 | 3593 | T>C
(p.V96A) | V/I/I/A | 25 | Yes |
|
| 4102 |
|
|
| Yes |
| ND2 | 4769 | A>G |
|
| Yes |
|
| 4833 | A>G |
|
| Yes |
|
| 5108 | T>C |
|
| Yes |
|
| 5442 | T>C (p.
F325L) | F/F/M/L | 23 | Yes |
|
tRNAAla | 5601 | C>T | C/C/C/C | 100 | Yes |
| CO1 | 7028 | C>T |
|
| Yes |
|
| 7600 | G>A |
|
| Yes |
| CO2 | 8167 | C>T |
|
| Yes |
| ATP6 | 8547 | C>T |
|
| Yes |
|
| 8748 | C>T |
|
| Yes |
|
| 9103 | T>C (p.
F193L) | F/F/F/S | 52 | Yes |
| ND3 | 10398 | A>G (p.
T114A) | T/T/T/A | 36 | Yes |
| ND4 | 11719 | G>A |
|
| Yes |
|
| 11778 | G>A (p.
R340H) | R/R/R/R | 100 | Yes |
| ND5 | 12705 | C>T |
|
| Yes |
| ND6 | 14668 | C>T |
|
| Yes |
| Cyt b | 15043 | G>A |
|
| Yes |
|
| 15301 | G>A |
|
| Yes |
In addition, the results revealed that the ratio
between affected males and females carrying ND4
m.11778G>A mutations in this case study was 3:1, which was
similar to previous studies on families with LHON carrying
ND4 m.11778G>A mutations (Table IV) (23–28).
These findings suggested that the m.11778G>A mutation may be the
molecular basis for the LHON phenotype.
 | Table IV.Clinical and molecular data for eight
Han Chinese pedigrees carrying the ND4 11778G>A primary
mutation in LHON. |
Table IV.
Clinical and molecular data for eight
Han Chinese pedigrees carrying the ND4 11778G>A primary
mutation in LHON.
| Author, year | Pedigree
number | Affected ratio
(male:female) | Penetrance of LHON
(%) | Secondary
variants | MthNA
haplogroup | (Refs.) |
|---|
| Ding et al,
2019 | 1 | 3:1 | 40 | tRNAAla
m.5601C>T | B5b1 | – |
| Qu et al,
2006 | 2 | 3:1 | 61.5 | tRNAMet
m.4435A>G | D5 | (23) |
| Li et al,
2006 | 3 | 2:1 | 60 | tRNAThr
m.15951A>G | D4 | (24) |
| Zhang et al,
2010 | 4 | 3:0 | 37.5 | ND1
m.3394T>C | M9a | (25) |
| Qu et al,
2007 | 5 | 3.5:1 | 33 | ND4
m.11696G>A | D4 | (26) |
| Zhang et al,
2010 | 6 | 1:1 | 57.1 | ND6
m.14502T>C | M10a | (27) |
| Qu et al,
2009 | 7 | 1:0 | 14.2 | None | M8a2 | (28) |
| Qu et al,
2009 | 8 | 2:0 | 8 | None | D4g2 | (28) |
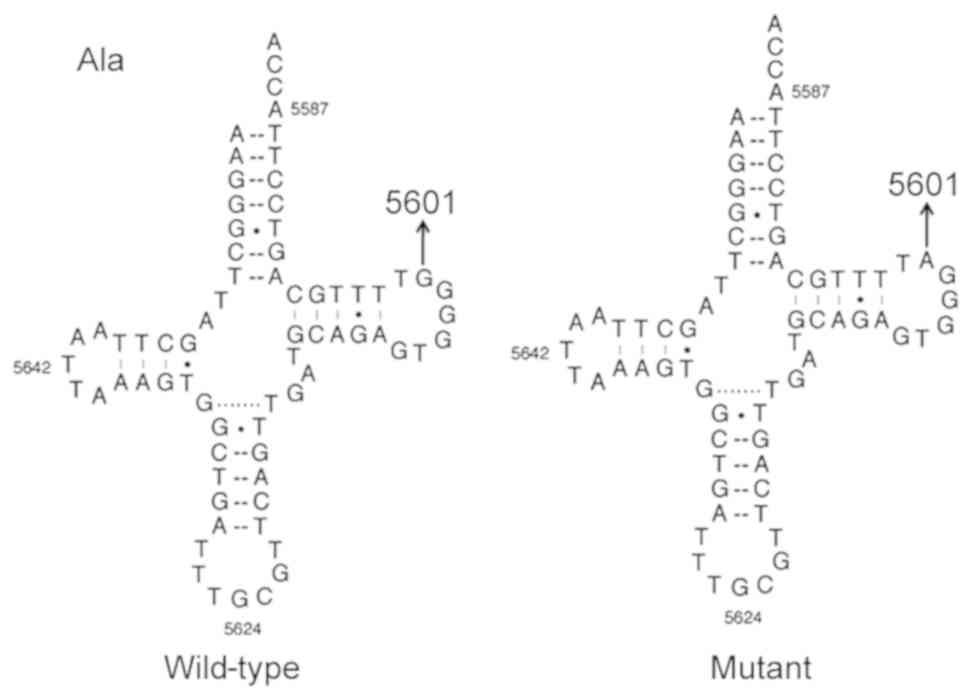
m.5601C>T variant alters the
tRNAAla structure
The m.5601C>T variant is located at a highly
conserved position in the TψC loop within the tRNAAla
(29); thus, the point mutation
results in a missense mutation that creates a novel base pairing
(55T-59C) (Figs. 2 and 3). Subsequent bioinformatics analysis
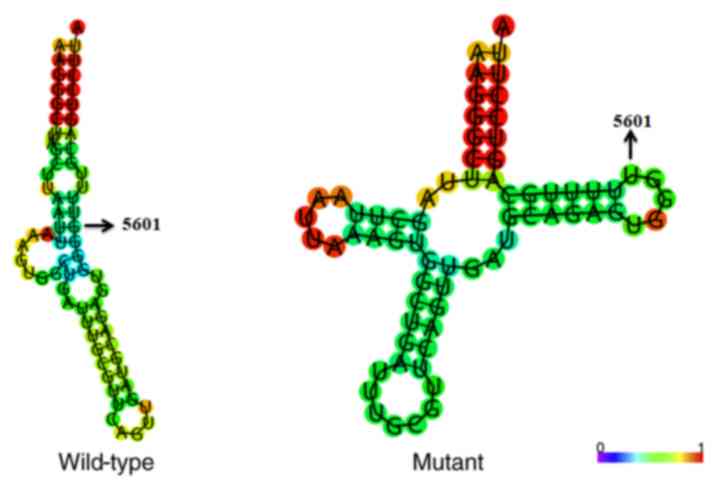
revealed that the m.5601C>T variant caused a structural
alteration of tRNAAla (Fig.
4), which indicated that m.5601C>T may have an impact on
tRNAAla function.
m.5601C>T variant is ‘possibly
pathogenic’ for LHON
The pathogenicity scoring system described by Yarham
et al (18) was used to
determine the role of the tRNAAla m.5601C>T variant.
As presented in Table V, the total
pathogenicity score for the m.5601C>T variant was 8, placing it
within the ‘possibly pathogenic’ category for LHON.
 | Table V.Pathogenicity scoring system for the
m.5601C>T mutation. |
Table V.
Pathogenicity scoring system for the
m.5601C>T mutation.
| Scoring
criteria | m.5601C>T
mutation | Score | Classification |
|---|
| More than one
independent report | Yes | 2 |
|
| Evolutionary
conservation of the base pair | No changes | 2 |
|
| Variant
heteroplasmy | No | 0 |
|
| Segregation of the
mutation with disease | Yes | 2 |
|
| Histochemical
evidence of mitochondrial disease | Strong
evidence | 2 |
|
| Biochemical defect
in complex I, III or IV | No | 0 |
|
| Evidence of
mutation segregation with biochemical defect from single-fiber
studies | No | 0 |
|
| Mutant mt-tRNA
steady-state level or evidence of pathogenicity in
trans-mitochondrial cybrid studies | No | 0 |
|
| Total score |
| 8 | Possibly
pathogenic |
Discussion
In the present study, a Han Chinese family with
maternally inherited LHON was clinically and molecularly
characterized. One of the most common features of LHON is bilateral
loss of vision in the matrilineal relatives of the proband
(9); this preferential effect on
vision has facilitated the positive association between mtDNA
mutations and LHON (30). Clinical
evaluation of this family revealed that the age of onset for visual
impairment between 3 and 19 years. The association between m.
11778G>A and LHON was reported as early as 1988 (31). In the present study, patients
harboring the m.11778G>A mutation had different mtDNA
haplogroups, suggesting that the m.11778G>A mutation occurred
sporadically and multiplied through evolution of the mtDNA in
China. The varying degree of visual impairment in this Chinese
family suggested that modifying factors, such as nuclear genes,
environmental factors and mitochondrial genetic polymorphisms, may
also contribute to LHON penetrance (32). In particular, secondary
LHON-associated variants, such as ND1 m.4216T>C and
ND5 m.13708G>A mutations in the mtDNA haplogroup J, may
increase the penetrance and severity of LHON, in combination with
the primary mutations, in European populations (33). mtDNA haplogroups M7b1′2 and M8a
have been implicated in the clinical expression of the
LHON-associated ND4 m.11778G>A mutation (33).
In the present study, sequencing of the complete
mitochondrial genomes of the matrilineal relatives (II-5, II-7,
II-12 and III-8) revealed a set of genetic polymorphisms from the
Asian mtDNA haplogroup B5b1 (19).
Of these variants, tRNAAla m.5601C>T was of most
interest because this variant is located at a highly conserved
nucleotide in the TψC loop of tRNAAla (position 59),
which is thought to be involved in tertiary interactions between
the TψC loop and the truncated D-arm (34). Bioinformatics analysis revealed
that the m.5601C>T variant created a novel Watson-Crick
base-pairing (55T-59C). The tRNAAla m.5601C>T variant
has previously been associated with maternally inherited
hypertension and mitochondrial myopathy, encephalopathy, lactic
acidosis, and stroke-like episodes (35,36).
Therefore, the m.5601C>T may alter the secondary structure of
tRNAAla and impair the mt-tRNA metabolism and protein
translation, and contribute to the LHON phenotype. A previous study
has demonstrated that the m.12192G>A mutation, occurring at a
similar position on tRNAHis, modulates the clinical
expression of deafness in a Chinese pedigree (37), whereas the m.5601C>T variant may
increase the penetrance of the hypertension-associated
tRNAMet m.4435A>G mutation (35). Zhou et al have previously
described the association between the tRNAAla
m.5601C>T variant and LHON in seven Han Chinese families
(38). However, these families
only carried the m.5601C>T variant, and did not harbor the three
LHON-associated primary mutations (ND4 m.11778G>A,
ND6 m.14484T>C and ND1 m.3460G>A), thus
exhibiting very low penetrance and severity of visual impairment
(4.5–25.0%) (38). In the present
study, the penetrance of LHON-induced visual impairment was 40%,
which suggested that the combination of the ND4
m.11778G>A mutation and the tRNAAla m.5601C>T
variant may be responsible for the higher prevalence of LHON in
this family.
Results from the present study suggested that the
tRNAAla m.5601C>T variant could increase both the
prevalence and the expression of the LHON-associated ND4
m.11778G>A mutation. Evidence to support this includes the fact
that the mutation occurs at a highly conserved nucleotide of
tRNAAla, which is critical for basal tRNA activity and
normal function (29). The present
data demonstrated that the m.5601C>T variant alters the
secondary structure of the tRNAAla gene. Finally, the
pathogenicity scoring system generated indicated that the
m.5601C>T variant was ‘possibly pathogenic’ (18). Therefore, the mitochondrial
dysfunction, caused by the ND4 m. 11778G>A mutation, may
be worsened by the m. 5601C>T variant. In conclusion, the m.
5601C>T variant may have a modified role in clinical expression
of LHON-associated m. 11778G>A mutation in this family.
Nevertheless, the incomplete penetrance of visual
impairment in this family (as evidenced by family members harboring
these mutations but exhibiting normal vision) indicated that the
ND4 m.11778G>A and tRNAAla m.5601C>T
variants are insufficient alone to produce the observed clinical
phenotypes. Therefore, it is likely that other risk factors,
including environmental factors, nuclear genes and epigenetic
modifications, may contribute to the clinical manifestation of LHON
in this pedigree. The main limitation of this study is the lack of
functional analysis of the tRNAAla m.5601C>T variant.
Further studies, such as the use of cytoplasmic hybrid cells
carrying the tRNAAla m.5601C>T variant are required
to confirm our conclusions and to identify additional contributing
risk factors.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Hangzhou
Health and Family Planning Commission (grant no. 2015A04), The
Hangzhou Bureau of Science and Technology (grant no. 20150633B16),
The Zhejiang Provincial Administration of Traditional Chinese
Medicine (grant no. 2018ZB082) and The Ministry of Public Health
from Zhejiang Province (grant nos. 2013KYA158 and 2018ZH019).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD and YFY designed the study. MYL and BHX performed
the molecular analysis. YFY collected the samples and performed the
clinical examinations. JHL analyzed the datasets and carried out
the phylogenetic analysis. JHL and YD wrote the paper. All authors
discussed the results and implications and commented on the
manuscript at all stages. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Hangzhou First People's Hospital (Hangzhou, China).
Written informed consent was obtained from all participants, or
their parent/guardian, prior to enrollment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu-Wai-Man P, Turnbull DM and Chinnery PF:
Leber hereditary optic neuropathy. J Med Genet. 39:162–169. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carelli V, Rugolo M, Sgarbi G, Ghelli A,
Zanna C, Baracca A, Lenaz G, Napoli E, Martinuzzi A and Solaini G:
Bioenergetics shapes cellular death pathways in Leber's hereditary
optic neuropathy: A model of mitochondrial neurodegeneration.
Biochim Biophys Acta. 1658:172–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Puomila A, Hämäläinen P, Kivioja S,
Savontaus ML, Koivumäki S, Huoponen K and Nikoskelainen E:
Epidemiology and penetrance of Leber hereditary optic neuropathy in
Finland. Eur J Hum Genet. 15:1079–1089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu-Wai-Man P, Griffiths PG, Brown DT,
Howell N, Turnbull DM and Chinnery PF: The epidemiology of Leber
hereditary optic neuropathy in the North East of England. Am J Hum
Genet. 72:333–339. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu-Wai-Man P, Griffiths PG and Chinnery
PF: Mitochondrial optic neuropathies-disease mechanisms and
therapeutic strategies. Prog Retin Eye Res. 30:81–114. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia X, Li S, Xiao X, Guo X and Zhang Q:
Molecular epidemiology of mtDNA mutations in 903 Chinese families
suspected with Leber hereditary optic neuropathy. J Hum Genet.
51:851–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fraser JA, Biousse V and Newman NJ: The
neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol.
55:299–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mackey DA, Oostra RJ, Rosenberg T,
Nikoskelainen E, Bronte-Stewart J, Poulton J, Harding AE, Govan G,
Bolhuis PA and Norby S: Primary pathogenic mtDNA mutations in
multigeneration pedigrees with Leber hereditary optic neuropathy.
Am J Hum Genet. 59:481–485. 1996.PubMed/NCBI
|
|
9
|
Catarino CB, Ahting U, Gusic M, Iuso A,
Repp B, Peters K, Biskup S, von Livonius B, Prokisch H and
Klopstock T: Characterization of a Leber's hereditary optic
neuropathy (LHON) family harboring two primary LHON mutations
m.11778G>A and m.14484T>C of the mitochondrial DNA.
Mitochondrion. 36:15–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu D, Jia X, Zhang AM, Guo X, Zhang YP,
Zhang Q and Yao YG: Molecular characterization of six Chinese
families with m.3460G>A and Leber hereditary optic neuropathy.
Neurogenetics. 11:349–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asanad S, Meer E, Tian JJ, Fantini M,
Nassisi M and Sadun AA: Leber's hereditary optic neuropathy: Severe
vascular pathology in a severe primary mutation. Intractable Rare
Dis Res. 8:52–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang M, Jiang P, Li F, Zhang J, Ji Y, He
Y, Xu M, Zhu J, Meng X, Zhao F, et al: Frequency and spectrum of
mitochondrial ND6 mutations in 1218 Han Chinese subjects with
leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci.
55:1321–1331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang P, Liang M, Zhang J, Gao Y, He Z, Yu
H, Zhao F, Ji Y, Liu X, Zhang M, et al: Prevalence of mitochondrial
ND4 mutations in 1281 Han Chinese subjects with leber's hereditary
optic neuropathy. Invest Ophthalmol Vis Sci. 56:4778–4788. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rieder MJ, Taylor SL, Tobe VO and
Nickerson DA: Automating the identification of DNA variations using
quality-based fluorescence re-sequencing: Analysis of the human
mitochondrial genome. Nucleic Acids Res. 26:967–973. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andrews RM, Kubacka I, Chinnery PF,
Lightowlers RN, Turnbull DM and Howell N: Reanalysis and revision
of the Cambridge reference sequence for human mitochondrial DNA.
Nat Genet. 23:1471999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruiz-Pesini E and Wallace DC: Evidence for
adaptive selection acting on the tRNA and rRNA genes of human
mitochondrial DNA. Hum Mutat. 27:1072–1081. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding Y, Xia BH, Zhang CJ and Zhuo GC:
Mitochondrial tRNALeu(UUR) C3275T, tRNAGln
T4363C and tRNALys A8343G mutations may be associated
with PCOS and metabolic syndrome. Gene. 642:299–306. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yarham JW, Al-Dosary M, Blakely EL, Alston
CL, Taylor RW, Elson JL and McFarland R: A comparative analysis
approach to determining the pathogenicity of mitochondrial tRNA
mutations. Hum Mutat. 32:1319–1325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong QP, Bandelt HJ, Sun C, Yao YG, Salas
A, Achilli A, Wang CY, Zhong L, Zhu CL, Wu SF, et al: Updating the
East Asian mtDNA phylogeny: A prerequisite for the identification
of pathogenic mutations. Hum Mol Genet. 15:2076–2086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bibb MJ, Van Etten RA, Wright CT, Walberg
MW and Clayton DA: Sequence and gene organization of mouse
mitochondrial DNA. Cell. 26:167–180. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gadaleta G, Pepe G, De Candia G,
Quagliariello C, Sbisa E and Saccone C: The complete nucleotide
sequence of the rattus norvegicus mitochondrial genome: Cryptic
signals revealed by comparative analysis between vertebrates. J Mol
Evol. 28:497–516. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roe BA, Ma DP, Wilson RK and Wong JF: The
complete nucleotide sequence of the xenopus laevis mitochondrial
genome. J Biol Chem. 260:9759–9774. 1985.PubMed/NCBI
|
|
23
|
Qu J, Li R, Zhou X, Tong Y, Lu F, Qian Y,
Hu Y, Mo JQ, West CE and Guan MX: The novel A4435G mutation in the
mitochondrial tRNAMet may modulate the phenotypic expression of the
LHON-associated ND4 G11778A mutation. Invest Ophthalmol Vis Sci.
47:475–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li R, Qu J, Zhou X, Tong Y, Hu Y, Qian Y,
Lu F, Mo JQ, West CE and Guan MX: The mitochondrial tRNA(Thr)
A15951G mutation may influence the phenotypic expression of the
LHON-associated ND4 G11778A mutation in a Chinese family. Gene.
376:79–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang M, Zhou X, Li C, Zhao F, Zhang J,
Yuan M, Sun YH, Wang J, Tong Y, Liang M, et al: Mitochondrial
haplogroup M9a specific variant ND1 T3394C may have a modifying
role in the phenotypic expression of the LHON-associated ND4
G11778A mutation. Mol Genet Metab. 101:192–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu J, Li R, Zhou X, Tong Y, Yang L, Chen
J, Zhao F, Lu C, Qian Y, Lu F and Guan MX: Cosegregation of the ND4
G11696A mutation with the LHON-associated ND4 G11778A mutation in a
four generation Chinese family. Mitochondrion. 7:140–146. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Zhou X, Zhou J, Li C, Zhao F,
Wang Y, Meng Y, Wang J, Yuan M, Cai W, et al: Mitochondrial ND6
T14502C variant may modulate the phenotypic expression of
LHON-associated G11778A mutation in four Chinese families. Biochem
Biophys Res Commun. 399:647–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu J, Zhou X, Zhang J, Zhao F, Sun YH,
Tong Y, Wei QP, Cai W, Yang L, West CE and Guan MX: Extremely low
penetrance of Leber's hereditary optic neuropathy in 8 Han Chinese
families carrying the ND4 G11778A mutation. Ophthalmology.
116:558–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Florentz C, Sohm B, Tryoen-Toth P, Putz J
and Sissler M: Human mitochondrial tRNAs in health and disease.
Cell Mol Life Sci. 60:1356–1375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wallace DC and Lott MT: Leber hereditary
optic neuropathy: Exemplar of an mtDNA disease. Handb Exp
Pharmacol. 240:339–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wallace DC, Singh G, Lott MT, Hodge JA,
Schurr TG, Lezza AM, Elsas LJ II and Nikoskelainen EK:
Mitochondrial DNA mutation associated with Leber's hereditary optic
neuropathy. Science. 242:1427–1430. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Ji Y, Lu Y, Fu R, Xu M, Liu X and
Guan MX: Leber's hereditary optic neuropathy (LHON)-associated ND5
12338T > C mutation altered the assembly and function of complex
I, apoptosis and mitophagy. Hum Mol Genet. 27:1999–2011. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji Y, Zhang AM, Jia X, Zhang YP, Xiao X,
Li S, Guo X, Bandelt HJ, Zhang Q and Yao YG: Mitochondrial DNA
haplogroups M7b1′2 and M8a affect clinical expression of leber
hereditary optic neuropathy in Chinese families with the
m.11778G->a mutation. Am J Hum Genet. 83:760–768. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ueda T, Yotsumoto Y, Ikeda K and Watanabe
K: The T-loop region of animal mitochondrial tRNA(Ser)(AGY) is a
main recognition site for homologous seryl-tRNA synthetase. Nucleic
Acids Res. 20:2217–2222. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng P, Li S, Liu C, Zha Z, Wei X and
Yuan Y: Mitochondrial tRNAAla C5601T mutation may
modulate the clinical expression of tRNAMet A4435G
mutation in a Han Chinese family with hypertension. Clin Exp
Hypertens. 40:595–600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanaka M, Ino H, Ohno K, Ohbayashi T,
Ikebe S, Sano T, Ichiki T, Kobayashi M, Wada Y and Ozawa T:
Mitochondrial DNA mutations in mitochondrial myopathy,
encephalopathy, lactic acidosis, and stroke-like episodes (MELAS).
Biochem Biophys Res Commun. 174:861–868. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding Y, Teng YS, Zhuo GC, Xia BH and Leng
JH: The mitochondrial tRNAHis G12192A mutation may modulate the
clinical expression of deafness-associated tRNAThr G15927A mutation
in a Chinese pedigree. Curr Mol Med. 19:136–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou HH, Dai XN, Lin B, Mi H, Liu XL, Zhao
FX, Zhang JJ, Zhou XT, Sun YH, Wei QP, et al: The analysis of
Leber's hereditary optic neuropathy associated with mitochondrial
tRNAAla C5601T mutation in seven Han Chinese families. Yi Chuan.
34:1031–1042. 2012.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|