Introduction
Cockroach sensitization is a risk factor for asthma.
Protease-activated receptors (PAR)-2, Toll-like receptors (TLRs)
and C-type lectin receptors have been suggested to play a role in
the penetration of cockroach allergens through epithelial cells and
allergic inflammation (1). Strong
protease activity in cockroach extracts can degrade protein
components within the extract. Dilution and mixture of cockroach
extract with pollen extracts was reported to cause a loss of
allergen potency (2). The
additions of protease-inhibitor (ε-aminocaproic acid) and sucrose
were reported to increase the shelf life of the protease-rich
American cockroach extract (3);
however, the potential side effects of this protease inhibitor have
not been examined.
More recently, mixtures of cockroach allergens with
other indoor allergens for immunotherapy have been found useful
when more than 50% glycerol is included, even though current
immunotherapy guidelines recommend the separation of high protease
extracts from other products if possible (4,5).
Moreover, cockroach extracts for immunotherapy are currently not
standardized since allergen and protein contents in commercial
extracts vary greatly, and the potency of commercial extracts was
estimated as 10 to 8,570 bioequivalent units (BAU)/ml (6).
In the present study, we determined the optimal
storage conditions for protease-rich German cockroach extracts
prepared in a standardized manner using a Korean isolate (7). The changes in protein and major
allergen (Bla g 1 and Bla g 2, respectively) contents as well as
allergen potency (total IgE reactivity) in the extract stored under
various conditions were examined over a 1-year time period.
Materials and methods
Serum samples
Serum samples were collected from 15 patients (age
range 14–54 years, mean 32 years, 9 males and 6 females) at the
Allergy-Asthma Center at Severance Hospital (Seoul, Korea) between
November 2014 and December 2018. Patient consent was obtained
before the blood collection. Specific IgE to German cockroach was
determined using the ImmunoCAP system (Thermo Fisher Scientific,
Inc.) and serum samples with specific IgE levels higher than 3.5
kUA/l were selected. A pooled serum sample from 13
healthy subjects (age range 25–81 years, mean 48 years, 9 males and
10 females) was used as a negative control. samples were taken
between March 19, 2014 and May 6, 2016 at Severance Hospital,
Seoul, Korea. The Institutional Review Board of the Yonsei
University Health System approved this study (no. 4-2013-0397).
Allergen extraction
Allergen extract was prepared as previously
described (7). In brief,
lyophilized cockroach was homogenized in liquid nitrogen, defatted
with ethyl ether, extracted in phosphate-buffered saline (PBS),
dialyzed against distilled water (DW), filtered (0.22 µm) and
lyophilized again. The protein concentration was determined by
Bradford assay (Bio-Rad Laboratories) after dissolution in
appropriate buffers.
The lyophilized extract was reconstituted in one of
four solutions: normal saline (NS), 50% glycerol in NS, 0.3% phenol
in NS, or 0.3% phenol and 50% glycerol in NS. The dissolved
extracts were aliquoted and maintained at room temperature (RT,
18–26°C) or refrigerated (2–8°C). Samples were taken for testing at
weeks 1, 2, 4, 9, 13, 26 and 52.
Protein analyses and measurement of
allergen content
Protein concentration was measured via Bradford
assay using bovine serum albumin (BSA) as the standard. Allergen
content (Bla g 1 and Bla g 2) was assessed using two-site ELISA
(Indoor Biotechnologies Inc.).
IgE antibody binding inhibition
assay
Allergen extract potency was compared using
inhibition ELISA. Allergen extract (100 µl) at week 0 was coated
overnight at 4°C at a 10 µg/ml concentration in 50 mM carbonate
buffer, pH 9.6. Simultaneously, 4-fold diluted pooled serum from
German cockroach allergy patients (n=15) was pre-incubated
overnight at 4°C with the extract of interest (1/10 volume) or 1%
BSA solution as a control. IgE antibodies were detected with
biotinylated goat anti-human IgE (ε chain specific; Vector
Laboratories Inc.) and streptavidin-peroxidase (Sigma-Aldrich). The
inhibition percentage was calculated as
(1-Ai/A0) ×100, where Ai indicates
the absorbance at 450 nm with an inhibitor and A0
indicates the absorbance without an inhibitor.
SDS-PAGE and IgE immunoblotting
Protein profile and IgE reactive components were
examined by SDS-PAGE and IgE immunoblotting. Proteins (20 µl) were
separated on 12% SDS-PAGE gels under reducing conditions. Gels were
stained with Coomassie brilliant blue or transferred onto a
polyvinylidene difluoride (PVDF) membrane (GE Waters & Process
Technologies). IgE reactive components were probed with alkaline
phosphate conjugated goat anti-human IgE (1:1,000-dilution) (ε
chain specific; Sigma-Aldrich), and color development was conducted
with nitro blue tetrazolium and
3-bromo-4-chloro-5-indolyl-phosphate (Promega).
Statistical analyses
Statistical differences between test groups were
analyzed by two-way ANOVA followed by Bonferroni correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protein content in German cockroach
extract
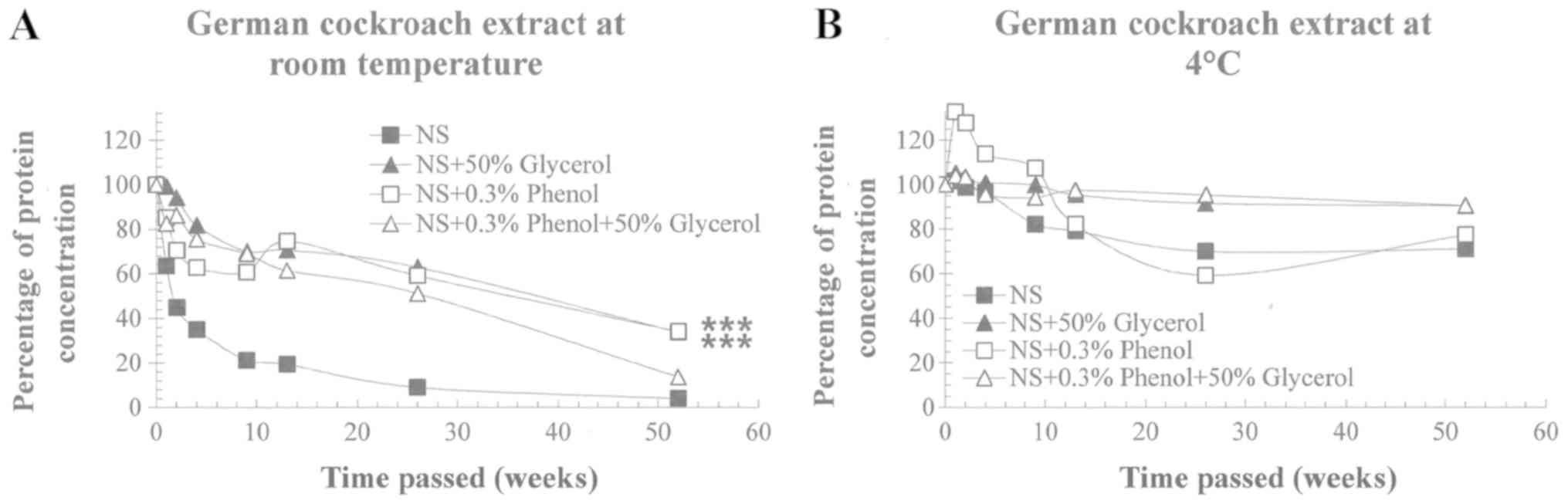
Initial protein concentration was determined to be
0.4 mg/ml. Approximately 33.8% of the original protein
concentration was detected after 52 weeks when 50% glycerol was
added to the extract in NS, whereas 4.1% remained in the extracts
of NS at RT (Fig. 1A). When
refrigerated, >90.0% of the protein was detected when 50%
glycerol NS was added, while <78.0% was detected without
glycerol (Fig. 1B).
Allergen content in German cockroach
extract
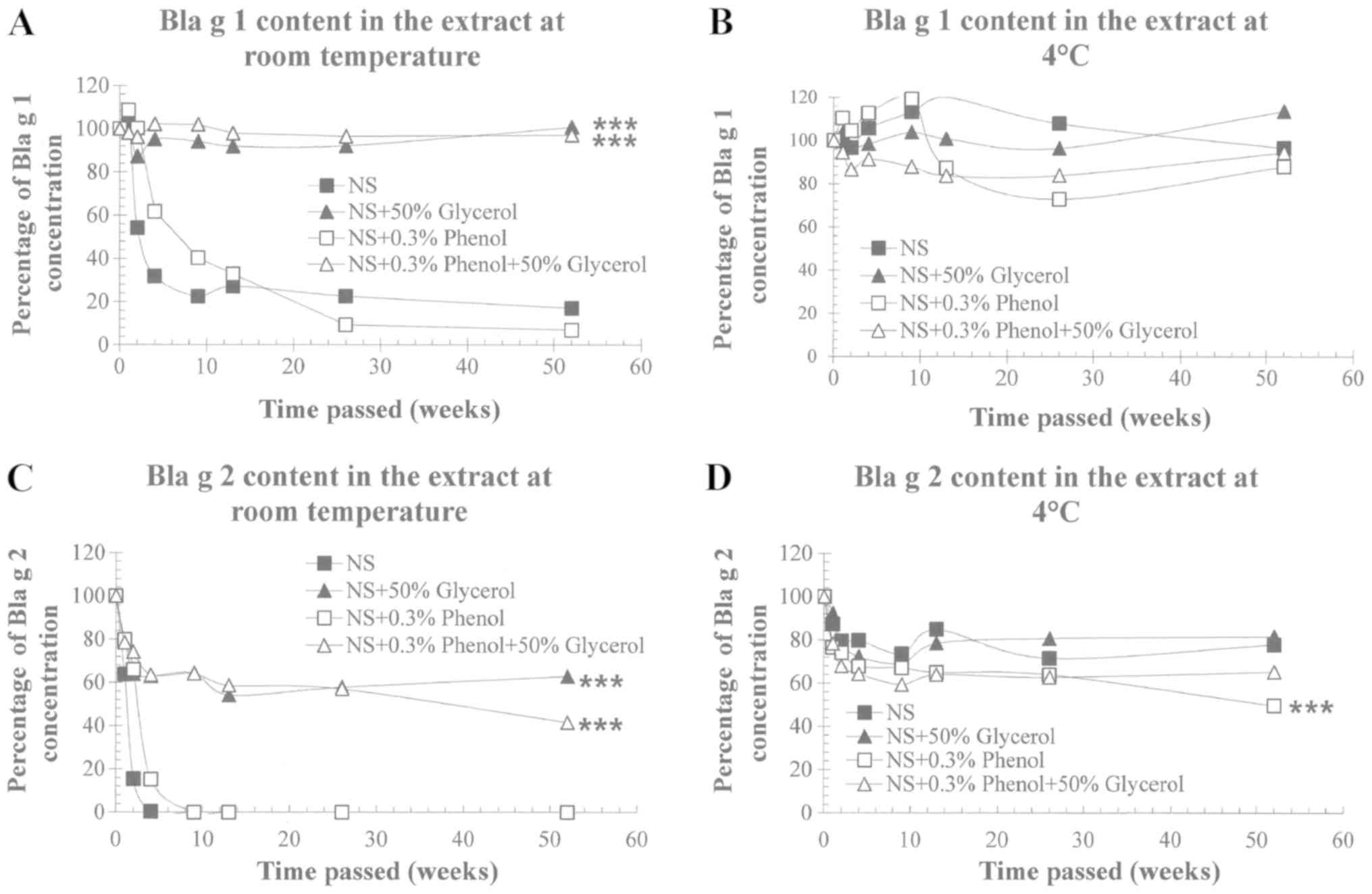
Initial concentrations of Bla g 1 and Bla g 2 were
35.3 and 7.0 µg/ml, respectively. Bla g 1 content in the extract
stored at RT remained at 17.1% in NS, 7.0% in 0.3% phenol NS, 100%
in 50% glycerol NS, and 97.1% in glycerol phenol NS (Fig. 2A). Bla g 1 content detected in the
refrigerated extracts was 96.4% in NS, 100% in 50% glycerol NS,
88.0% in 0.3% phenol NS and 94.4% in glycerol with phenol NS
(Fig. 2B). A similar pattern of
Bla g 2 content was observed, even though Bla g 1 in the extract
was shown to be more stable than Bla g 2 (Fig. 2C and D).
Allergen potency of German cockroach
extract
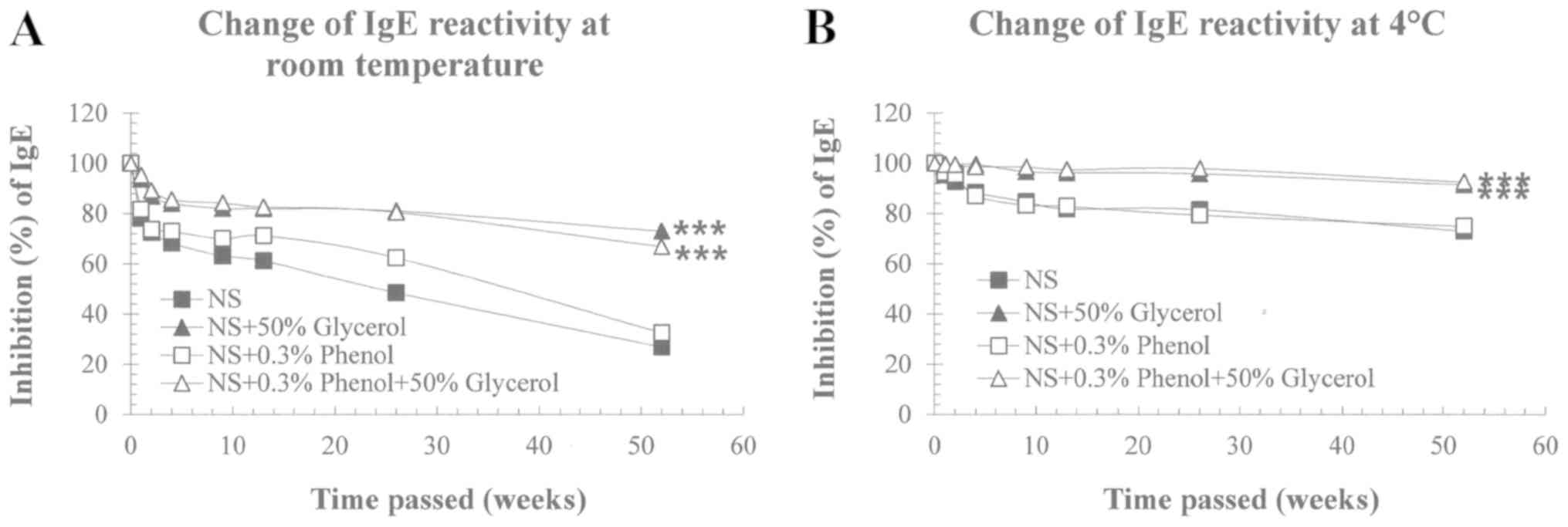
Potency was retained at 73.0% in 50% glycerol NS and
66.9% in 50% glycerol with phenol NS when stored at RT, whereas
26.9% in NS and 32.5% in phenol NS were retained in the absence of
glycerol (Fig. 3A). When
refrigerated, 91.4% potency in 50% glycerol NS and 92.6% potency in
50% glycerol with phenol NS of potency was maintained, while 72.9%
of the allergen potency in NS and 74.9% in phenol NS were retained
in the absence of glycerol (Fig.
3B).
Change of protein profile and IgE
reactive components
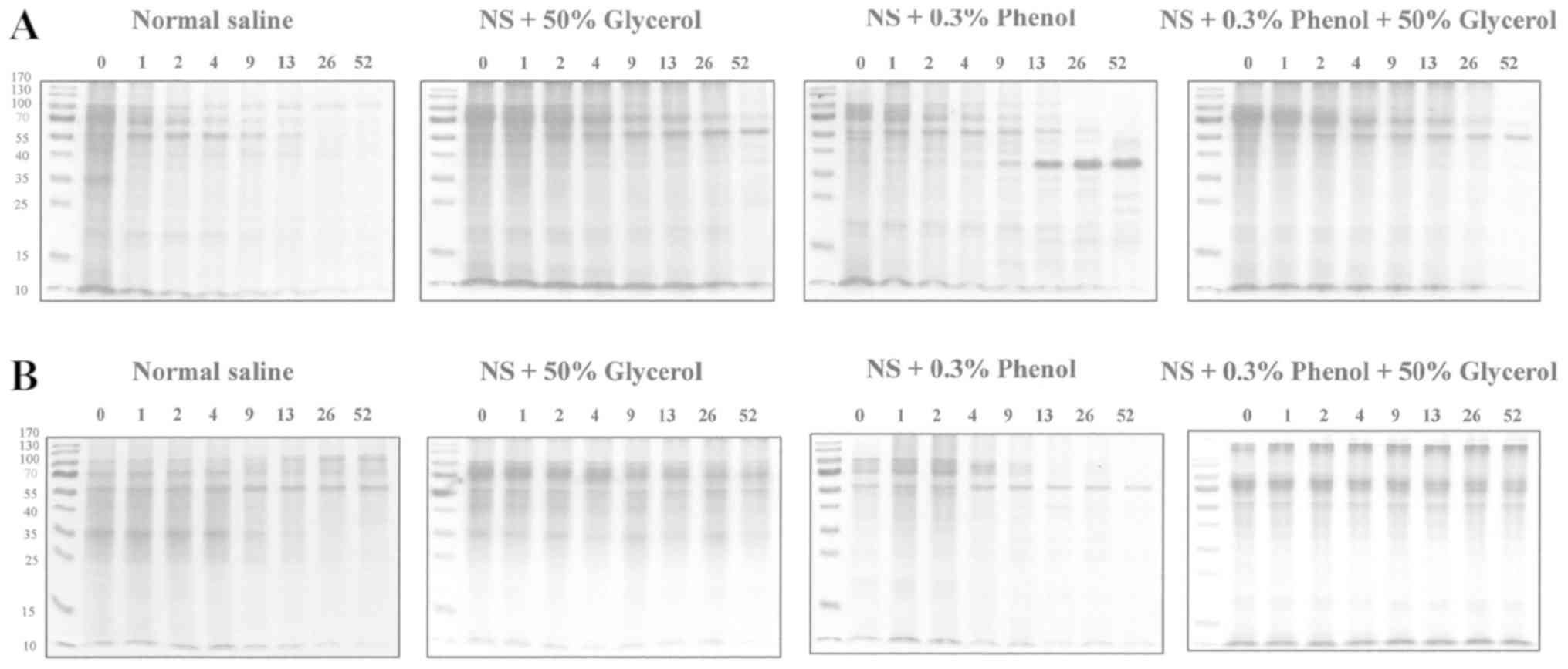
Notably, a 35-kDa component and components >55
kDa were shown to be degraded when the extracts were maintained at
RT (Fig. 4A). Slow disruption of
the 35 kDa band was observed in the refrigerated extract in 50%
glycerol NS and also bands around 70 kDa in the refrigerated
extract in 0.3% phenol NS (Fig.
4B). Notably, some partially degraded proteins produced bands
of ~55 kDa in 50% glycerol NS at RT, 50% glycerol with 0.3% phenol
NS at RT, and 50% glycerol with 0.3% phenol NS and refrigerated,
~38 kDa in 0.3% phenol NS at RT.
Strong IgE reactions were detected between 55 and
100 kDa components when extracts were dissolved in a solution with
50% glycerol NS (regardless of 0.3% phenol) and kept refrigerated
(Fig. 4B, C and D). IgE reaction
to these components seemed to correlate with potency. IgE
reactivity to the 34 kDa band was detected only in the refrigerated
samples and within the first few weeks of the sampling at RT
(Fig. 4C and D).
No IgE reactive component was detected by IgE
immunoblotting with the extracts at week 0, which should include
all the allergenic components (Fig. 4E
and G), using a pooled serum sample with healthy subjects
(Fig. 4F and H).
Discussion
Commercial cockroach extracts are highly variable in
terms of allergen content and potency. However, high protease
activity in cockroach extracts makes it more difficult to
standardize the allergen extracts. Glycerol is a well-known
stabilizer of allergen extracts (8). However, stability of German cockroach
extract has not been described. Therefore, we aimed to observe what
occurs with German cockroach extract which has strong protease
activity. In this study, the protein concentration was well
conserved when refrigerated, and the addition of 50% glycerol NS
seemed to inhibit the protease activity and enhanced cockroach
extract stability.
The change of IgE reactive components were
investigated by SDS-PAGE and IgE immunoblotting. However, it was
not certain concerning the identity of each protein band. It seems
that a high salt concentration affects the solubility of various
components in the extracts and produces high molecular weight bands
in SDS-PAGE. However, these components were not allergenic, as
examined by IgE immunoblotting. Furthermore, phenol, which is a
reagent commonly used to eliminate protein in a laboratory, also
influenced the banding pattern by SDS-PAGE. Some protein
degradation by phenol may have caused the different protein profile
and IgE reaction. The phenol effect is thought to be minimized by
the addition of glycerol. In this study, Bla g 1 and Bla g 2
content in the cockroach extract were examined because two-site
ELSIA kits for these allergens are commercially available. However,
a growing body of evidence suggests that Bla g 1 may not be a major
allergen. None of the cockroach allergens identified to date appear
to be immunodominant (6,9,10).
In recent studies, Bla g 5, Bla g 11 and Bla g 2 were determined as
relatively important (6,11). A more convenient and cheap assay
for the quantification of Bla g 5 and Bla g 11 is required.
Moreover, partially degraded allergens could retain IgE epitopes,
although allergens could not be quantified with the two-site ELISA
that recognizes two different epitopes. Liquid chromatography and
multiple reaction monitoring mass spectrometry (LC-MRM MS) may
offer rapid and accurate quantification of allergens. However,
current LC-MRM MS technology is unlikely to detect and reflect the
change in conformational epitopes in the extracts (12).
Extract potency is thought to better reflect extract
reactivity stability for allergic reactions that are elicited by
allergen-specific IgE antibodies. In general, potency was well
preserved when 50% glycerol NS was added and refrigerated, as
protein and allergen content were preserved. Notably, slower
deterioration of potency was observed compared to the protein and
allergen contents, reflecting the fact that some of the partially
degraded allergens retained IgE reactivity.
Abrupt deterioration of cockroach extract in the
absence of 50% glycerol NS is in line with the analyses of
commercial aqueous and glycerinated cockroach extracts (13). On the other hand, the commonly used
bacteriostatic agent phenol seems to have little effect on the
stability of cockroach extract, although it has been shown to be
disadvantageous over time with some pollen extracts (14).
The present study has several limitations. First,
the effect of human serum albumin (HSA), a common reagent used as a
stabilizer for allergen dilution, was not included. HSA is commonly
added to protect allergen adsorption to glass vials and protein
digestion. However, HSA is known to be effective in diluted
extracts. Second, temperature excursions during shipping and
clinical use were not tested; therefore, the results could be
applied to the concentrated extract but not to diluted extracts.
Finally, the change of protease activity over time was not
examined. Proteases can elicit inflammatory reactions; however,
allergenicity is not determined by protease activity but instead by
IgE reactivity.
Taken together, cockroach extract with high protease
activity can be stored for at least one year by the addition of 50%
glycerol NS and refrigeration. The present study could facilitate
the further refinement of allergen extract standardization with
high protease activity and the development of improved diagnostics
and immunotherapeutics. There is still great need for a surrogate
system to assess the quality of cockroach extract.
Acknowledgements
Not applicable.
Funding
The present study was supported by a fund (grant no.
2015-ER6602-00) from the Research of Korea Centers for Disease
Control & Prevention. This research was also supported by a
grant from the Korea Healthcare Technology R&D Project through
the Korean Health Industry Development Institute, which is funded
by the Republic of Korea's Ministry of Health and Welfare (grant
no. HI14C1324).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KYJ drafted the manuscript. JL and JEY performed
SDS-PAGE and IgE immunoblotting experiments. KHP, JHL and JWP
collected serum samples. JDK prepared and provided cockroach
extracts. KYJ, KHP, JHL and JWP designed the study and experiments.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Patient consent was obtained before the blood
collection. The Institutional Review Board of the Yonsei University
Health System approved this study (no. 4-2013-0397).
Patient consent for publication
Not applicable.
Competing interests
KYJ, KHP, JHL, JDK and JWP have stocks in Prolagen.
KYJ receives a consultancy fee from Prolagen.
Authors' information
KYJ is a technical advisor, JDK is a CEO, and JWP is
a CTO of Prolagen.
References
|
1
|
Do DC, Zheo Y and Gao P: Cockroach
allergen exposure and risk of asthma. Allergy. 71:463–474. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nelson HS, Iklé D and Buchmeier A: Studies
of allergen extract stability: The effects of dilution and mixing.
J Allergy Clin Immunol. 98:382–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sudha VT, Srivastava D, Arora N, Gaur SN
and Singh BP: Stability of protease-rich Periplaneta americana
allergen extract during storage: Formulating preservatives to
enhance shelf life. J Clin Immunol. 27:294–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grier TJ, Hall DM, Duncan EA and Goyne TC:
Mixing compatibilities of Aspergillus and American cockroach
allergens with other high-protease fungal and insect extracts. Ann
Allergy Asthma Immunol. 114:233–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grier TJ, Hall DM, Duncan EA and Gada SM:
Allergen stabilities and compatibilities in immunotherapy mixtures
that contain cat, dog, dust mite, and cockroach extracts. Ann
Allergy Asthma Immunol. 115:496–502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slater JE, James R, Pongracic JA, Liu AH,
Sarpong S, Sampson HA, Satinover SM, Woodfolk JA, Mitchell HE,
Gergen PJ and Eggleston PA: Biological potency of German cockroach
allergen extracts determined in an inner city population. Clin Exp
Allergy. 37:1033–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong KY, Choi SY, Lee JH, Lee JS, Yong
TS, Hong CS and Park JW: Preparation and characterization of an
extract of German cockroach from a Korean source. Allergy Asthma
Immunol Res. 5:102–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Plunkett G: Update: Stability of allergen
extracts to establish expiration dating. Curr Opin Otolaryngol Head
Neck Surg. 24:261–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satinover SM, Reefer AJ, Pomes A, Chapman
MD, Platts-Mills TA and Woodfolk JA: Specific IgE and IgG
antibody-binding patterns to recombinant cockroach allergens. J
Allergy Clin Immunol. 115:803–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khurana T, Dobrovolskaia E, Shartouny JR
and Slater JE: Multiplex assay for protein profiling and potency
measurement of German cockroach allergen extracts. PLoS One.
10:e01402252015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong KY, Kim CR, Park J, Han IS, Park JW
and Yong TS: Identification of novel allergenic components from
German cockroach fecal extract by a proteomic approach. Int Arch
Allergy Immunol. 161:315–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mindaye ST, Spiric J, David NA, Rabin RL
and Slater JE: Accurate quantification of 5 German cockroach (GCr)
allergens in complex extracts using multiple reaction monitoring
mass spectrometry (MRM MS). Clin Exp Allergy. 47:1661–1670. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patterson ML and Slater JE:
Characterization and comparison of commercially available German
and American cockroach allergen extracts. Clin Exp Allergy.
32:721–727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niemeijer NR, Kauffman HF, van Hove W,
Dubois AE and de Moncy JG: Effect of dilution, temperature, and
preservatives on the long-term stability of standardized inhalant
allergen extracts. Ann Allergy Asthma Immunol. 76:535–540. 1996.
View Article : Google Scholar : PubMed/NCBI
|