Introduction
The histopathological features of acute lung injury
(ALI) include alveolar leukocyte infiltration, lung edema, hyaline
membrane formation, increased alveolar wall thickness and
hemorrhage (1). A wide arrange of
diseases can cause ALI, including pneumonia, sepsis and acute
pancreatitis (2). Studies have
shown that Gram-negative bacterial infection may be crucial in the
development of ALI (3). Several
natural products have been examined in experimental models and have
been shown to inhibit multiple inflammatory pathways associated
with ALI (4,5), however, the morbidity and mortality
rates remain high (6). Therefore,
the development of effective drugs or strategies to treat ALI is
urgently required.
Halofuginone [HF,
7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl] is a
low-molecular-weight plant-derived alkaloid extracted from
Dichroa febrifuga. Previous studies have demonstrated that
HF exhibits antifibrotic activity (7) and offers therapeutic promise in
animal models of fibrotic disease (8). Other studies have indicated that HF
has a beneficial effect in regulating the immune response (9). In addition, HF can reduce the
productions of some pro-inflammatory cytokines, including tumor
necrosis factor-α (TNF-α) (10)
and interleukin (IL)-17 (11), in
inflammatory diseases.
Dexamethasone (DEX) is a potent, long-lasting
synthetic glucocorticoid that possesses potent anti-inflammatory
properties. According to previous reports, DEX can inactivate the
nuclear factor-κB (NF-κB) pathway (12) and inhibit the expression of IL-17
(13), IL-1α (14) and IL-23 (15). For this reason, DEX is widely used
to treat various inflammatory diseases, including rheumatoid
arthritis (16), asthma (17) and ALI (18). According to previous reports, a
combination of DEX with other drugs exerted superior effects on
mitigating ALI (19,20). Therefore, the present study aimed
to examine the synergistic effect of DEX and HF in the treatment of
ALI.
The NF-κB pathway can activate an inflammatory
cascade and lead to the upregulation of several pro-inflammatory
cytokines, including TNF-α and IL-1β (21). According to published reports, the
NF-κB pathway is involved in the pathogenesis of ALI (22–24).
In addition, during the pathological process of ALI, various
pro-inflammatory cytokines are activated, particularly IL-17
(25) and IL-23 (26). This evidence demonstrates that
NF-κB and the inflammatory cytokines are major targets for ALI
therapy.
In the present study, the effect of DEX and HF on
LPS-induced ALI and the underlying molecular mechanisms were
examined in vitro and in vivo. Lipopolysaccharide
(LPS)-induced type II alveolar epithelial cells (HPAEpiC cells) and
ALI rats were treated with DEX, HF or their mixture respectively.
The results demonstrated that there was a synergistic effect of HF
and DEX on LPS-induced ALI in HPAEpiC cells and the rat model.
Materials and methods
Cell culture and treatment
The HPAEpiC human alveolar epithelial cells were
purchased from ScienCell Company (Carlsbad, CA, USA; cat. no. 3200;
http://www.sciencellonline.com/human-pulmonary-alveolar-epithelial-cells.html).
The cells were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. The cells were divided into five groups: Control
group: Normal HPAEPiC cells; LPS group: HPAEPiC cells incubated
with 0.5 µg/ml LPS for 12 h; LPS + DEX group: DEX (100 nM) was
administrated 12 h following LPS treatment; LPS + HF group: HF (100
nM) was administrated 12 h following LPS treatment; LPS + DEX + HF
group: HF (100 nM) and DEX (100 nM) mixture was administrated 12 h
following LPS treatment. All cells were incubated at at 37°C with
5% CO2.
Cell viability measurement
Cell viability was assessed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) assay. The HPAEpiC
cells were seeded into 96-well plates at 5×103
cells/well and incubated in 5% CO2 at 37°C for 12 h. The
culture medium was then replaced with fresh culture medium
containing different doses of HF or DEX at the final concentration
of 0, 1, 2, 5, 10, 20, 50, 100, 200, and 400 nM. The surviving
fractions were determined at 24 and 48 h. Subsequently, the OD
values were detected at 450 nm and normalized to cells treated with
normal medium.
Western blot assay
The HPAEpiC cells subjected to different treatments
for 24 h and the homogenized lung tissue samples were prepared for
the western blot assay. All samples were lysed in RIPA lysis buffer
containing 1% protease inhibitor. In total, 20 µg protein was
loaded in each lane. Proteins were separated by 12% SDS-PAGE and
transferred onto PVDF (EMD Millipore, Billerica, MA, USA)
membranes. The PVDF membranes were then blocked with 5% nonfat milk
and washed with TBS with 5% Tween 20 at room temperature.
Subsequently, the samples were incubated with anti-TNF-α [1:1,000;
cat. no. 11948; Cell Signaling Technology, Inc. (CST)]; anti-IL-1β
(1:1,000; cat. no. 12703; CST); anti-IL-17 (1:1,000; cat. no.
13838; CST); anti-IL-23 (1:100; cat. no. ab45420; Abcam);
anti-IL-10 (1:1,000; cat. no. 12163; CST); anti-P65 (1:1,000; cat.
no. 8242; CST) and anti-GAPDH (1:10,000; cat. no. ab181602; Abcam)
primary antibodies overnight at 4°C. Following extensive washing
with TBST for three times, the membranes were incubated with
horseradish peroxidase-labeled goat anti-rabbit secondary antibody
(cat. no. ab6721; 1:2,000; Abcam) for 1.5 h at room temperature.
The immunoreactive bands were visualized using a ChemiDoc XRS
imaging system and Quantity One analysis software (version 1.42;
Bio-Rad Laboratories, Inc., Franklin Lakes, NJ, USA).
NF-κB nuclear translocation
The HPAEpiC cells on a cover glass were washed with
PBS and fixed in 4% paraformaldehyde. The fixed cells were then
blocked with PBS containing 10% FBS for 30 min at room temperature.
Subsequently, all samples were incubated with primary antibody P65
(1:200; cat. no. ab16502; Abcam) for 1 h at room temperature,
followed by incubation with a secondary antibody conjugated with
FITC (1:20,000; cat. no. ab6662; Abcam) for 30 min at room
temperature. The samples were mounted in medium containing DAPI
(Roche Diagnostics, Basel, Switzerland) for 5 min at room
temperature. The location of NF-κB was measured using a laser
scanning confocal microscope (magnification, ×400; Olympus
Corporation, Tokyo, Japan).
Animals and treatment
HF was purchased from Sigma-Aldrich (Merck KGaA).
Specific-pathogen-free (SPF) Sprague-Dawley male rats (weight,
200–220 g; age, 7 weeks) were obtained from The Laboratory Animal
Center of Yidu Central Hospital Affiliated to Weifang Medical
College (Qingzhou, China). All rats were required to adapt to the
environment for 7 days prior to the experiments with a maintained
temperature of 22°C and a 12-h light/dark cycle at 60% humidity.
The animals were allowed free access to tap water and SPF fodder.
The animal protocols used in the present study were approved by the
Institutional Animal Ethics Committee and according to the
Guidelines of Laboratory Animal Care and Use Committee. A total of
45 rats were randomly divided into five groups (n=9 per group).
Control group: Normal rats; LPS group: Rats received an LPS (5
mg/kg) intratracheal injection to induce ALI; LPS + DEX group: Rats
were treated with DEX (5 mg/kg/d in PBS) 1 h following LPS
treatment by intraperitoneal injection; LPS + HF group: Rats were
treated with HF (0.1 mg/kg/d in PBS) 1 h following LPS treatment by
intraperitoneal injection; LPS + DEX + HF group: HF (15 mg/kg/d)
and DEX (0.1 mg/kg/d) was simultaneously administrated 1 h
following LPS treatment. The treatment continued for 3 days.
Subsequently, the rats were anesthetized with an intraperitoneal
injection of pentobarbital sodium (30 mg/kg body weight). The lung
tissues were collected from the rats under anesthesia for
subsequent analyses. Following collection, the rats were sacrificed
by intraperitoneal injection of pentobarbital sodium (200 mg/kg
body weight).
Histopathological analysis
The lung tissues were collected and washed with PBS
following sacrifice of the rats. The tissues were then fixed with
10% paraformaldehyde and embedded in paraffin. The samples were cut
into 4-µm sections. Each section was stained with hematoxylin and
eosin (H&E). The histopathological features of ALI were
assessed using light microscopy (magnification, ×400; Olympus
Corporation).
Terminal-deoxynucleotidyl transferase
mediated nick end labeling (TUNEL) staining
According to the manufacturer's protocol, the lung
tissue sections were fixed in acetone and slides were incubated
with terminal deoxynucleotidyl transferase and detection buffer
using the detection kit (Roche Molecular Biochemicals,
Indianapolis, IN, USA). The numbers of TUNEL-positive nuclei were
counted under a light microscope (magnification, ×400; Olympus
Corporation).
Immunohistochemistry assay for
IL-17
The lung tissue sections underwent exposure to 3%
hydrogen peroxide following dewaxing, hydration and antigen
retrieval. The tissue sections were then incubated with anti-IL-17
primary antibodies (1:200; cat. no. ab79056; Abcam) overnight at
4°C. The following day, biotin-labeled secondary antibody (1:250;
cat. no. ab6658; Abcam) was added and incubated for 1 h at room
temperature. The clay bank granules were observed under light
microscopy.
Cytokine ELISA assay
The rats were anesthetized with an intraperitoneal
injection of pentobarbital sodium (30 mg/kg body weight) prior to
cervical dislocation, following which blood was taken from the lung
tissue sections. Following centrifugation at 3,000 × g for 15 min
at 4°C, serum was collected for the measurement of cytokine
concentrations of IL-1β (Invitrogen; Thermo Fisher Scientific,
Inc.), IL-23, TNF-α (PeproTech, Inc, Rocky Hill, NJ, USA), and
IL-10 (eBioscience; Thermo Fisher Scientific, Inc.) using ELISA
kits, following the manufacturer's protocol, at 450 nm.
Determinations were performed in duplicate in three independent
experiments. The results are expressed as pg/ml.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Comparisons between means were performed using one-way
analysis of variance followed by Tukey's post hoc test using SPSS
version17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference for
all types of statistical test.
Results
HF and DEX sustain the survival of
HPAEpiC cells
The structure of HF is illustrated in Fig. 1A. An MTT assay was used to
determine the appropriate concentrations of HF and DEX. The HPAEpiC
cell lines were treated with HF or DEX at different concentrations
(0, 1, 2, 5, 10, 20, 50, 100, 200, and 400 nM), respectively. The
results indicated that no significant decrease was observed in the
HPAEpiC cells treated with HF or DEX concentrations <100 nm.
However, cell viability was markedly reduced at a concentration
≥100 nM (Fig. 1B and C).
Therefore, the concentration of 100 nM was selected for HF and DEX
for the following experiments to exclude cell toxicity. The HPAEpiC
cells were induced by LPS and treated with HF and DEX separately or
together, the results showed that LPS reduced cell survival
compared with the control group. However, the LPS-induced decrease
in cell viability was elevated following treatment with HF or DEZ.
Increased cell survival was observed in the HF + DEX group compared
with that in the DEX group (Fig.
1D).
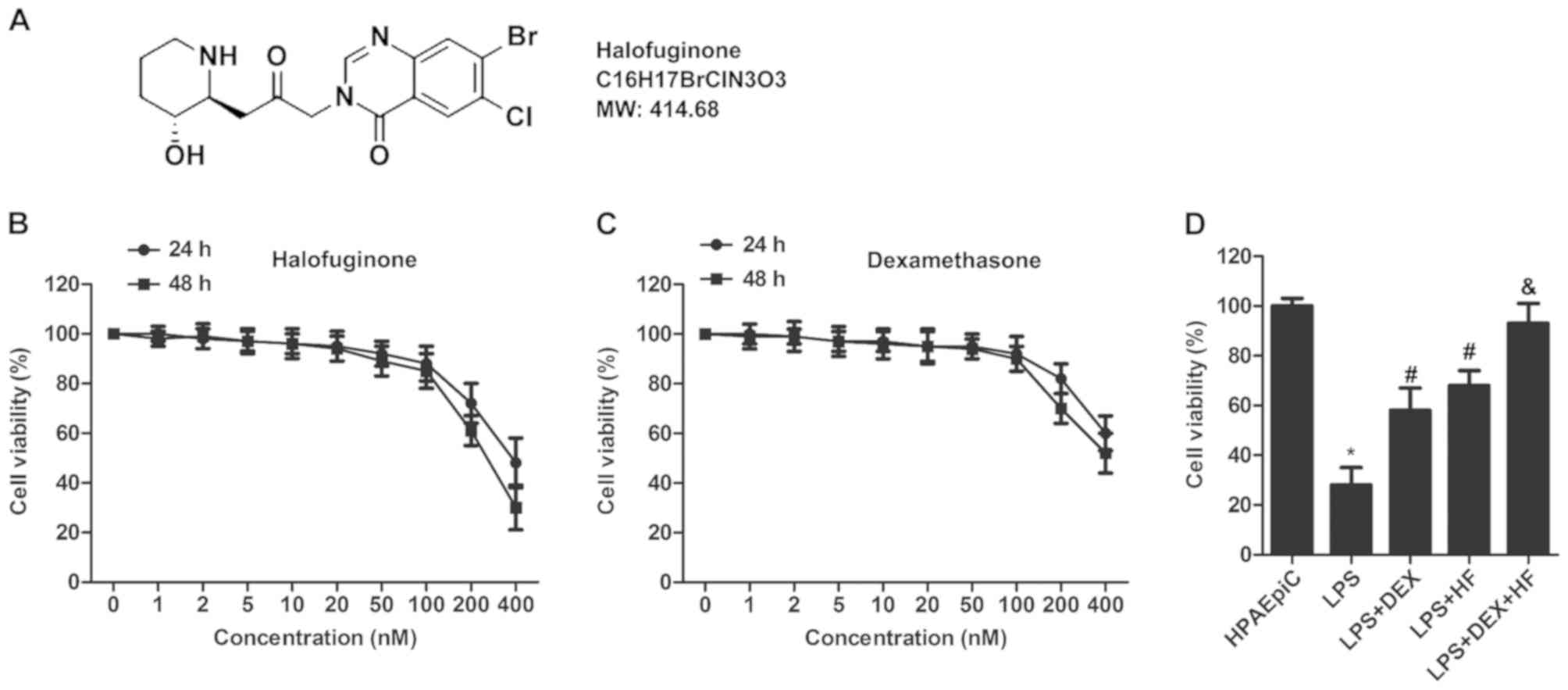 | Figure 1.HF and DEX sustain the survival of
HPAEpiC cells. (A) Structure of HF. (B) HPAEpiC cell viability was
detected using an MTT assay. Cells were treated with HF at
different concentrations (0, 1, 2, 5, 10, 20, 50, 100, 200, and 400
nM) for different durations (24 and 48 h). (C) HPAEpiC cell
viability was measured using an MTT assay. Cells were treated with
DEX at different concentrations (0, 1, 2, 5, 10, 20, 50, 100, 200,
and 400 nM) for different durations (24 and 48 h). (D) HPAEpiC
cells were divided into five groups. Control group: Normal HPAEPiC
cells; LPS group: HPAEPiC cells incubated with 0.5 µg/ml LPS for 12
h; LPS + DEX group: DEX (100 nM) administrated 12 h following LPS
treatment; LPS + HF group: HF (100 nM) administrated 12 h following
LPS treatment; LPS + DEX + HF group: HF (100 nM) + DEX (100 nM)
administrated 12 h following LPS treatment. Cell viability of
HPAEpiC cells was measured using an MTT assay. The experiments were
repeated at least three times (*P<0.05 vs. HPAEpiC group;
#P<0.05 vs. LPS group; &P<0.05 vs.
LPS+DEX group). HF, halofuginone; DEX, dexamethasone; LPS,
lipopolysaccharide; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
HF and DEX weaken LPS-induced
inflammatory response
To investigate the impact of HF and DEX on the
inflammatory response, typical inflammatory cytokines were detected
in the non-LPS or LPS-induced HPAEpiC cell lines. The western blot
assays demonstrated that the expression levels of TNF-α, IL-1β,
IL-17 and IL-23 were markedly increased, whereas that of IL-10 was
markedly decreased, in the LPS-induced HPAEpiC cells compared with
the normal HPAEpiC cells. In addition, the expression levels of
TNF-α, IL-1β, IL-17, IL-23 were reduced, whereas that of IL-10 was
elevated, in the LPS-induced cells treated with DEX or HF compared
with the LPS-induced cells. Furthermore, the expression levels of
TNF-α, IL-1β, IL-17 and IL-23 were suppressed, whereas that of
IL-10 was upregulated, in the LPS-induced cells treated with DEX +
HF, compared with those treated with DEX alone (Fig. 2A-F). These results indicated that
the combination of HF and DEX can weaken the LPS-induced
inflammatory response more than either HF or DEX used alone.
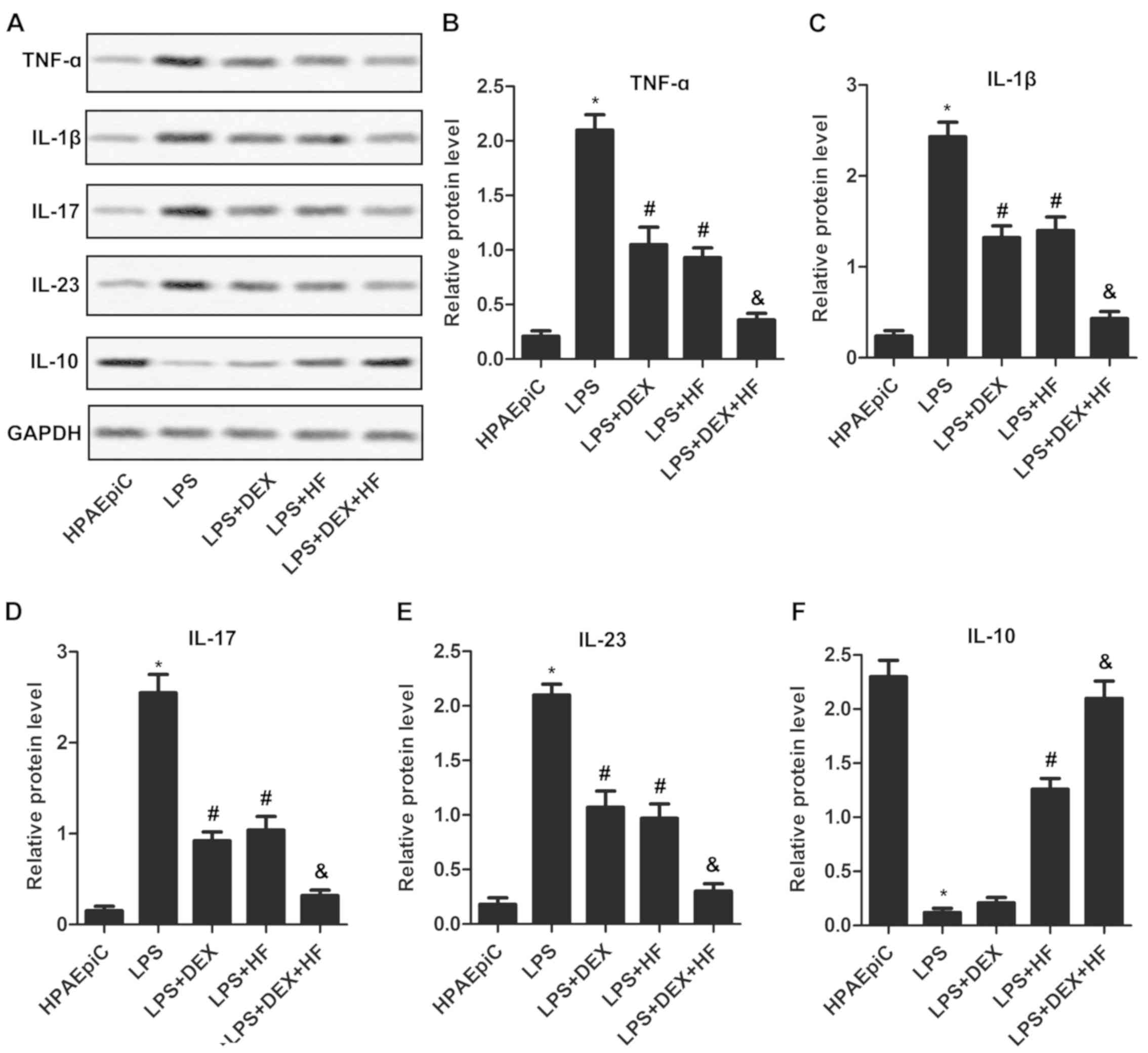 | Figure 2.HF and DEX weaken the LPS-induced
inflammatory response. (A) Expression levels of inflammatory
cytokines (TNF-α, IL-1β, IL-17, IL-23 and IL-10) were detected
using western blot analysis. GAPDH was the endogenous reference.
Histograms showing the expression levels of (B) TNF-α, (C) IL-1β,
(D) IL-17, (E) IL-23 and (F) IL-10 in HPAEpiC cells according to
the results of western blot analysis. The experiments were repeated
at least three times, and data are presented as the mean + standard
deviation (*P<0.05 vs. HPAEpiC group; #P<0.05 vs.
LPS group; &P<0.05 vs. LPS+DEX group). HF,
halofuginone; DEX, dexamethasone; LPS, lipopolysaccharide; TNF-α,
tumor necrosis factor-α; IL, interleukin. |
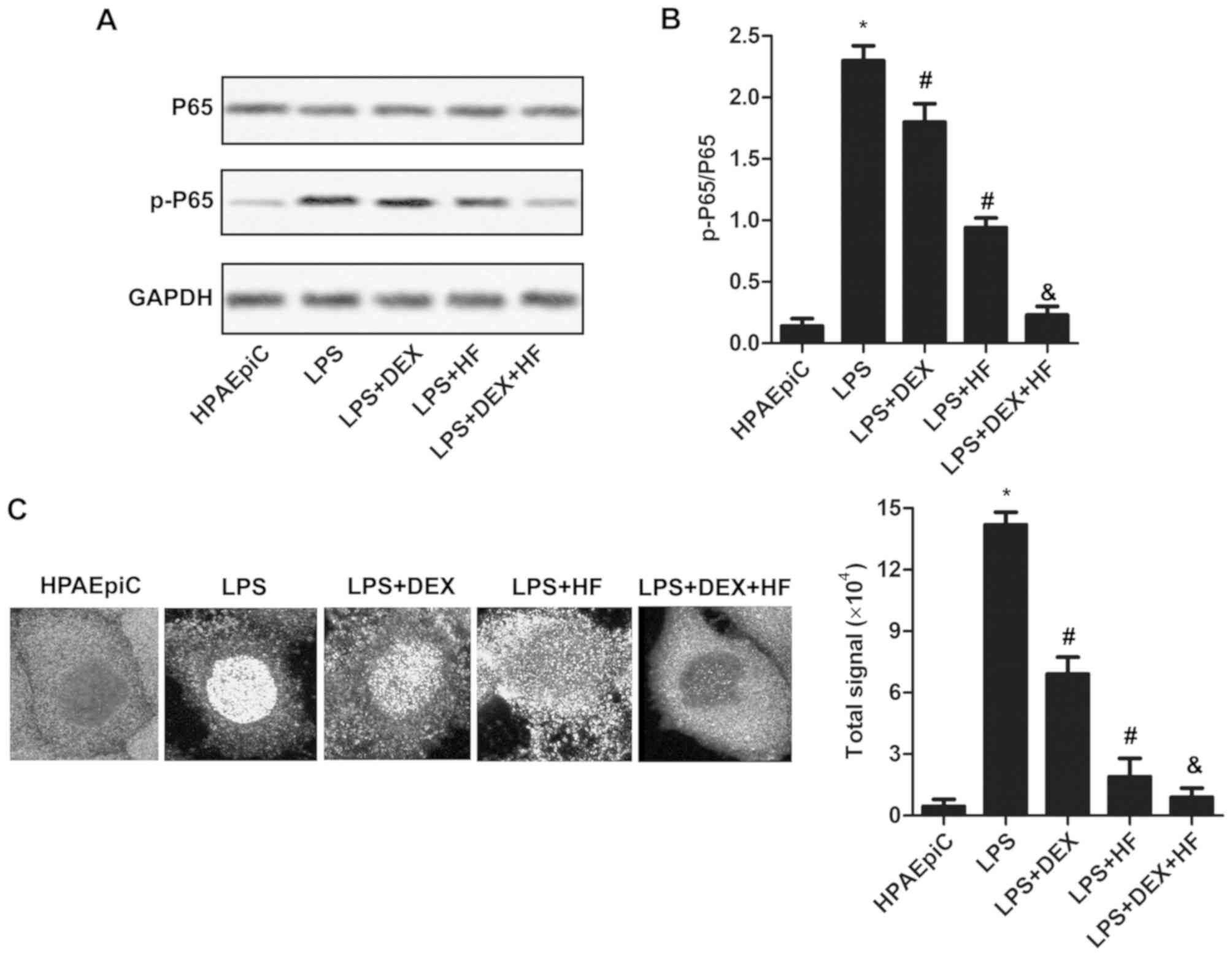
HF and DEX reduce NF-κB activity via
suppressing the phosphorylation of P65
To determine the mechanism underlying the effect of
HF and DEX on the pro-inflammatory responses of HPAEpiC cells, the
expression level of P65 and its phosphorylated form was measured by
western blot analysis. As shown in Fig. 3A and B, the level of p-P65/P65 was
upregulated in the LPS-induced HPAEpiC cells compared with that in
the normal HPAEpiC cells. In addition, the expression of p-P65/P65
was reduced in the LPS-induced cells treated with DEX or HF,
compared with that in the LPS group. Furthermore, the expression of
p-P65/P65 was decreased in the LPS-induced cells treated with DEX +
HF compared with those treated with DEX alone. To further validate
this result, the subcellular localization of P65 was investigated
using the immunofluorescence technique (Fig. 3C). The results indicated that the
combination of DEX + HF decreased the nuclear translocation of
p65.
HF and DEX alleviate acute lung injury
in an LPS-induced rat model
To observe the impact of HF and DEX on morphological
changes of lung tissues, H&E staining was used. The results
showed that, in the rats suffering from LPS-induced ALI with no
treatment, minimal typical histomorphology of the lung was
observed. By contrast, in the LPS-induced ALI rats treated with HF
+ DEX, the degree of perivascular inflammation and neutrophil
infiltration was significantly decreased, compared with that in the
LPS-induced ALI rats treated with DEX or HF alone. Furthermore, the
combination of the two drugs reduced the congestion and edema of
pulmonary alveoli, thickness of the pulmonary septum and fibrosis
compared with the LPS-induced ALI rats treated with DEX or HF alone
(Fig. 4A). Apoptotic bodies in the
lung tissue were examined using a TUNEL assay. As shown in Fig. 4B and C, the numbers of apoptotic
bodies were increased in the LPS-induced lung tissues compared with
those in the normal lung tissues. In addition, apoptotic numbers
were reduced in the LPS-induced lung tissues treated with DEX or HF
compared with those in LPS-induced lung tissues. Furthermore, the
numbers of apoptotic bodies were decreased in LPS-induced lung
tissues treated with DEX + HF compared with those in the
LPS-induced lung tissues treated with DEX alone (Fig. 4B and C). To confirm the effect of
DEX and HF on inflammatory factors in LPS-induced lung tissues,
immunohistochemical staining was used to investigate the expression
of IL-17. The results suggested that the expression of IL-17 was
markedly upregulated in the LPS-induced lung tissues compared with
that in normal lung tissues. In addition, the expression of IL-17
was reduced in the LPS-induced lung tissues treated with HF or DEX
compared with that in LPS-induced lung tissues. The expression
level of IL-17 was decreased in the LPS-induced lung tissues
treated with HF + DEX compared with that in LPS-induced lung
tissues treated with DEX alone (Fig.
4D and E). To identify whether DEX and HF activated the NF-κB
pathway, western blot analysis was performed to measure the
expression of P65 and its phosphorylated form in the lung tissues.
The results demonstrated that the expression of p-P65/P65 was
markedly increased in LPS-induced lung tissues compared with that
in normal lung tissues. In addition, the expression of p-P65/P65
was reduced in the LPS-induced lung tissues treated with HF or DEX
compared with that in LPS-induced lung tissues. Furthermore, the
expression level of p-P65/P65 was decreased in the LPS-induced lung
tissues treated with HF + DEX compared with that in LPS-induced
lung tissues treated with DEX alone (Fig. 4F and G). Finally, the LPS-induced
elevated levels of TNF-α, IL-1β and IL-23 were decreased in the
DEX- or HF-treated cells. The decreased levels of TNF-α, IL-1β and
IL-23 and increased level of IL-10 were measured in the DEX + HF
group and compared with those in the cells treated with DEX alone
(Fig. 4H). These results suggested
that the combination of HF and DEX led to further alleviation of
ALI in the LPS-induced rat model.
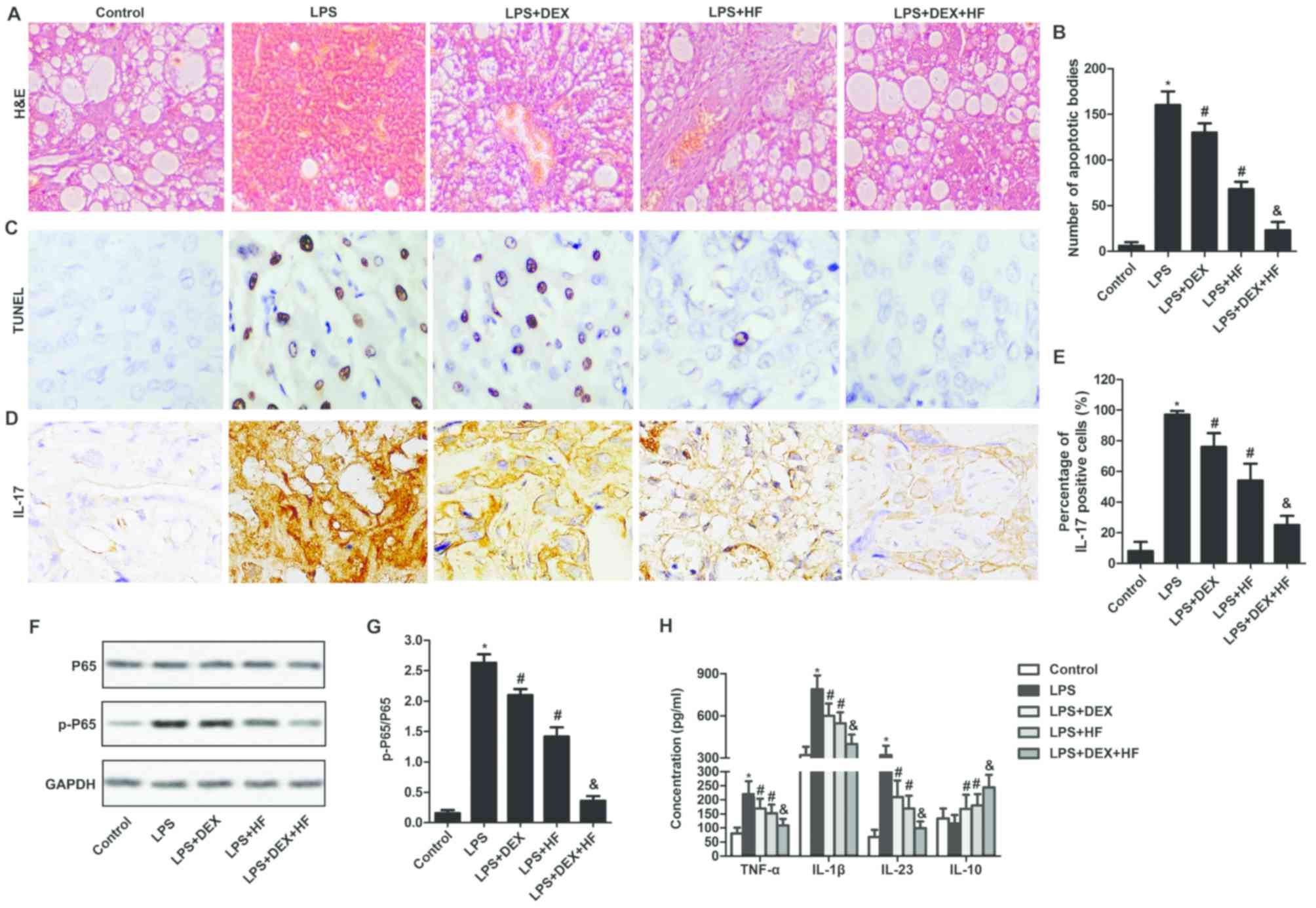 | Figure 4.HF and DEX alleviate acute lung
injury in an LPS-induced rat model. (A) H&E staining to examine
the morphological changes of lung tissues (magnification, ×400).
(B) Data summary and analysis of the number of apoptotic bodies
according to the results of the TUNEL assay. (C) TUNEL assay used
to detect the number of apoptotic bodies (magnification, ×400). (D)
Expression of IL-17 was observed through an immunohistochemical
assay. Magnification, ×400. (E) Histogram represents the percentage
of IL-17-positive cells in lung tissue according to the results of
immunohistochemical assay. (F) Expression levels of P65 and p-P65
were evaluated through western blot analysis. GAPDH was used as an
endogenous reference. (G) Histogram showing the expression of
p-P65/P65according to the results of western blot analysis. (H)
Concentrations of TNF-α, IL-1β, IL-23 and IL-10 were measured using
an ELISA assay. The experiments were repeated at least three times
and data are presented as the mean + standard deviation (*P<0.05
vs. healthy control group; #P<0.05 vs. LPS group;
&P<0.05 vs. LPS+DEX group). HF, halofuginone;
DEX, dexamethasone; LPS, lipopolysaccharide; IL-17, interleukin-17;
TNF-α, tumor necrosis factor-α; p-, phosphorylated; H&E,
hematoxylin and eosin; TUNEL, terminal-deoxynucleotidyl transferase
mediated nick end labeling. |
Discussion
ALI is a clinical syndrome of severe lung failure.
The complications of ALI include persistent respiratory failure,
prolonged dependence on mechanical ventilation, multi-organ
dysfunction and mortality (27).
Despite progress having been made in drug exploration and
understanding mechanisms, effective pharmacotherapy for ALI remains
limited. Until now, a number of studies have demonstrated that
several extracts from Chinese herbs can be of importance in
treating ALI, including Sarcandra glabra (28), wogonin (29), flos lonicerae japonicae (30) and triptolide (31). These reports indicate the potential
of Chinese herbs in treating ALI.
HF, a nontoxic antiparasitic alkaloid derivative of
Dichroa febrifuga roots, has been widely used in the
treatment of multiple inflammatory diseases, including colitis
(32), autoimmune arthritis
(9) and acute viral myocarditis
(33). However, the use of HF to
treat ALI has not been reported. DEX, a well-known
anti-inflammatory agent, is also widely applied to treat various
inflammatory diseases. According to published reports, DEX has been
used to treat inflammatory bowel disease (34), inflammatory airway disease
(35), corneal inflammation
(36) and ALI (37). These studies suggest that the
combination of HF and DEX is a promising therapeutic regime for the
treatment of ALI.
Accumulated evidence has demonstrated that HF can
affect various inflammatory cytokines. For example, the
administration of HF has been shown to result in marked decreases
in the levels of pro-inflammatory factors, including IL-6, TNF-α
and IL-1β (38). In addition, it
has been reported that HF treatment decreased IL-17A and improved
features of chronic lung allograft dysfunction in a mouse
orthotopic lung transplant model (11). According to Carlson et al
(39), pro-inflammatory IL-23
responses can be selectively repressed by activation of the
HF-induced amino acid starvation response in mature Th17 memory
cells. DEX has been reported to affect multiple inflammatory
cytokines to reduce inflammatory responses. It has been shown that
stimulation of DEX with β-glucans markedly increased the secretion
of IL-10 and phosphorylation of Syk, and decreased the production
of IL-12, IL-23 and TNF-α (40).
Furthermore, DEX was reported to suppress the serum level of IL-17
in a bleomycin A5-induced rat model of pulmonary fibrosis (13). Similarly, in the present study, HF
or DEX used alone reduced the expression of pro-inflammatory
cytokines (TNF-α, IL-1β, IL-17 and IL-23) and elevated the
expression of the anti-inflammatory IL-10 cytokine. The combination
of these two drugs led to a more marked decrease in
pro-inflammatory cytokine and increase in anti-inflammatory
cytokine expression.
The NF-κB signaling pathway is a crucial pathway
which has been reported to be involved in the regulation of
pro-inflammatory mediator generation (41). A number of studies have indicated
that the NF-κB signaling pathway is activated in ALI (22,23,42).
In previous studies, DEX effectively decreased the activity of
NF-κB P65 (40,43). In addition, increasing evidence
suggests that various monomers of Chinese herbs can inactivate the
NF-κB signaling pathway to prevent the development of inflammatory
diseases. According to Xu et al (44), β-glucan-induced macrophage
activation can be suppressed by tetrandrine through inhibiting
signal transducer and activator of transcription 3, extracellular
signal-regulated kinase and NF-κB signaling pathways. Others have
reported that the activation of NF-κB can be reduced by shikonin,
leading to inhibited oxidized low-density lipoprotein-induced
monocyte adhesion in EA.hy926 endothelial cells (45). A similar result was obtained in the
present study; treatment with DEX or HF alone reduced the activity
of NF-κB via suppressing the phosphorylation of P65. However, the
simultaneous application of these two drugs decreased the
phosphorylation of P65 more markedly than that with HF or DEX
alone. In addition, other signaling pathways have been associated
with LPS-induced lung injury. A study by Li et al (46) demonstrated that apigenin
C-glycosides inhibit acute inflammation and apoptosis by
suppressing activation of the toll-like receptor 4/transient
receptor potential cation channel, subfamily C, member 6 signaling
pathway in a murine model of ALI. Cordycepin exerted a protective
effect on injuries in lung tissues via the nuclear factor,
erythroid 2 like 2/heme oxygenase-1 pathway (47). The p38 mitogen-activated protein
kinase signaling pathway was also shown to be involved in the
LPS-induced excessive inflammatory responses in ALI (48). These pathways are to be examined in
subsequent investigations to examine the compounds effects.
Progressive fibroproliferative diseases include
liver cirrhosis, kidney fibrosis and pulmonary fibrosis. Pulmonary
fibrosis is a chronic, incurable clinical disease, the therapeutic
options for which are usually of limited success. Until now, an
increasing number of studies have demonstrated that HF is vital in
treating fibroproliferative diseases. According to Liang et
al (38), the oral
administration of HF had a potent effect against liver fibrosis by
decreasing inflammation-mediated liver damage and the deposition of
collagen I. In addition, it has been shown that esophageal and
hypopharyngeal fibrosis can be safely prevented by HF (49). Nagler et al (50) observed that HF was a potent
inhibitor of bleomycin-induced pulmonary fibrosis in vivo,
and was suitable for use as a novel therapeutic regimen for the
treatment of this dysfunction. In addition to this, DEX has also
been reported to exhibit its protective effect in certain
fibroproliferative diseases. Wang et al stated briefly that
DEX defended against bleomycin A5-induced pulmonary fibrosis
through decreasing the level of IL-17 in the serum of rats
(13). A similar result was
obtained in the present study; HF used alone suppressed lung
fibrosis and the combination of DEX with HF enhance the curative
effect.
The ALI model can be induced by different reagents;
a rodent model of ALI can be induced by sulfur dioxide with DEX to
evaluate whether the inflammatory response and lung fibrosis can be
counteracted (51). According to a
report by Xu et al (52),
an ALI model was induced by oleic acid to measure the cell
apoptotic pathway. The ALI model in the present study was
established by intratracheal injection of LPS (5 mg/kg) in rats.
According to a previous report, the intratracheal instillation of
LPS induced a robust pulmonary pro-inflammatory response with
endothelial barrier dysfunction (53). Dong et al (54) also used a rat model of ALI induced
by LPS and an LPS-induced cell model to investigate the effect of
carbon monoxide on ALI. As the present study focused on the role of
immune adjustment by HF and DEX, LPS was selected to induce the ALI
model. LPS-induced ALI has also been associated with sepsis and
acute respiratory distress syndrome (55) in addition to the cecal ligation and
puncture model of sepsis (56).
The synergistic effect of HF and DEX on sepsis is to be
investigated in our subsequent investigations.
Based on the experimental results, the present study
suggested that HF enhanced the effect of DEX in sustaining survival
and LPS-induced inflammatory response of HPAEpiC cells. The
mechanism may be associated with inhibition of the phosphorylation
of NF-κB. In addition, HF may assist DEX in alleviating cell
apoptosis and inflammatory responses in the LPS-induced rat model.
These findings progress current understanding of the NFκB pathway
associated with ALI elicited by the combination of DEX and HF.
Acknowledgements
The authors would like to thank Dr Feng Luan, Dr
Yong Xu, Dr Jingwei Wang and Dr Ling Lu of Yidu Central Hospital
Affiliated to Weifang Medical College (Qingzhou, China), for
providing helpful discussions and technical support for the present
study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HLD analyzed and interpreted the main data regarding
cell viability and ALI model establishment. ADZ was responsible for
pathological and statistical analyses. HY was responsible for the
design and drafting of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments in the present study were
approved by the Animal Care and Research Committee of Yidu Central
Hospital Affiliated to Weifang Medical College (Qingzhou, China).
All experiments were performed in compliance with relevant laws and
guidelines. All experiments were conducted according to the
institutional guidelines of Yidu Central Hospital Affiliated to
Weifang Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
HF
|
halofuginone
|
|
DEX
|
dexamethasone
|
|
LPS
|
lipopolysaccharide
|
|
HPAEpiC
|
type II alveolar epithelial cells
|
|
NF-κB
|
nuclear factor κB
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-17
|
interleukin-17
|
|
IL-23
|
interleukin-23
|
|
IL-1β
|
interleukin-1β
|
|
SD rats
|
Sprague-Dawley rats
|
References
|
1
|
Miyashita T, Ahmed AK, Nakanuma S, Okamoto
K, Sakai S, Kinoshita J, Makino I, Nakamura K, Hayashi H, Oyama K,
et al: A three-phase approach for the early identification of acute
lung injury induced by severe sepsis. In Vivo. 30:341–349.
2016.PubMed/NCBI
|
|
2
|
Zimmerman JJ, Akhtar SR, Caldwell E and
Rubenfeld GD: Incidence and outcomes of pediatric acute lung
injury. Pediatrics. 124:87–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sawa T: The molecular mechanism of acute
lung injury caused by Pseudomonas aeruginosa: From bacterial
pathogenesis to host response. J Intensive Care. 2:102014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Y, Ren J, Wang L, Zhao X, Zhang M,
Shimizu K and Zhang C: Protective effects of total alkaloids from
Dendrobium crepidatum against LPS-induced acute lung injury in mice
and its chemical components. Phytochemistry. 149:12–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel VJ, Biswas Roy S, Mehta HJ, Joo M
and Sadikot RT: Alternative and natural therapies for acute lung
injury and acute respiratory distress syndrome. Biomed Res Int.
2018:24768242018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson ER and Matthay MA: Acute lung
injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med
Pulm Drug Deliv. 23:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dent P, Yacoub A, Contessa J, Caron R,
Amorino G, Valerie K, Hagan MP, Grant S and Schmidt-Ullrich R:
Stress and radiation-induced activation of multiple intracellular
signaling pathways. Radiat Res. 159:283–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pines M, Snyder D, Yarkoni S and Nagler A:
Halofuginone to treat fibrosis in chronic graft-versus-host disease
and scleroderma. Biol Blood Marrow Transplant. 9:417–425. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park MK, Park JS, Park EM, Lim MA, Kim SM,
Lee DG, Baek SY, Yang EJ, Woo JW, Lee J, et al: Halofuginone
ameliorates autoimmune arthritis in mice by regulating the balance
between Th17 and Treg cells and inhibiting osteoclastogenesis.
Arthritis Rheumatol. 66:1195–1207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng S, Wang K, Huang M, Qiu Q, Xiao Y,
Shi M, Zou Y, Yang X, Xu H and Liang L: Halofuginone inhibits
TNF-α-induced the migration and proliferation of fibroblast-like
synoviocytes from rheumatoid arthritis patients. Int
Immunopharmacol. 43:187–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oishi H, Martinu T, Sato M, Matsuda Y,
Hirayama S, Juvet SC, Guan Z, Saito T, Cypel M, Hwang DM, et al:
Halofuginone treatment reduces interleukin-17A and ameliorates
features of chronic lung allograft dysfunction in a mouse
orthotopic lung transplant model. J Heart Lung Transplant.
35:518–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao W, Tong D, Li Q, Huang P and Zhang F:
Dexamethasone promotes regeneration of crushed inferior alveolar
nerve by inhibiting NF-κB activation in adult rats. Arch Oral Biol.
80:101–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang A, Wang F, Yin Y, Zhang M and Chen P:
Dexamethasone reduces serum level of IL-17 in Bleomycin-A5-induced
rats model of pulmonary fibrosis. Artif Cells Nanomed Biotechnol.
46:783–787. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roach BL, Kelmendi-Doko A, Balutis EC,
Marra KG, Ateshian GA and Hung CT: Dexamethasone release from
within engineered cartilage as a chondroprotective strategy against
interleukin-1α. Tissue Eng Part A. 22:621–632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palma L, Sfara C, Antonelli A and Magnani
M: Dexamethasone restrains ongoing expression of interleukin-23p19
in peripheral blood-derived human macrophages. BMC Pharmacol.
11:82011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Jiang H, Li Y, Chen W, Li H, Peng
K, Zhang Z and Sun X: Targeting NF-κB signaling with polymeric
hybrid micelles that co-deliver siRNA and dexamethasone for
arthritis therapy. Biomaterials. 122:10–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaughnessy AF: Single-dose dexamethasone
an option for acute adult asthma. Am Fam Physician.
95:Online2017.
|
|
18
|
Kosutova P, Mikolka P, Balentova S,
Adamkov M, Kolomaznik M, Calkovska A and Mokra D: Intravenous
dexamethasone attenuated inflammation and influenced apoptosis of
lung cells in an experimental model of acute lung injury. Physiol
Res. 65 (Suppl 5):S663–S672. 2016.PubMed/NCBI
|
|
19
|
Liu F, Pauluhn J, Trubel H and Wang C:
Single high-dose dexamethasone and sodium salicylate failed to
attenuate phosgene-induced acute lung injury in rats. Toxicology.
315:17–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Chen X, Devshilt I, Yun Q, Huang
C, An L, Dorjbat S and He X: Fennel main constituent,
trans-anethole treatment against LPS-induced acute lung injury by
regulation of Th17/Treg function. Mol Med Rep. 18:1369–1376.
2018.PubMed/NCBI
|
|
21
|
Park GY and Christman JW: Nuclear factor
kappa B is a promising therapeutic target in inflammatory lung
disease. Curr Drug Targets. 7:661–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho YC, Lee SS, Yang ML, Huang-Liu R, Lee
CY, Li YC and Kuan YH: Zerumbone reduced the inflammatory response
of acute lung injury in endotoxin-treated mice via Akt-NFκB
pathway. Chem Biol Interact. 271:9–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang T, Hou W and Fu Z: Preventative
effect of OMZ-SPT on lipopolysaccharide-induced acute lung injury
and inflammation via nuclear factor-kappa B signaling in mice.
Biochem Biophys Res Commun. 485:284–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li N, Song Y, Zhao W, Han T, Lin S,
Ramirez O and Liang L: Small interfering RNA targeting NF-κB
attenuates lipopolysaccharide-induced acute lung injury in rats.
BMC Physiol. 16:72016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Gu Y, Tu Q, Wang K, Gu X and Ren T:
Blockade of Interleukin-17 restrains the development of acute lung
injury. Scand J Immunol. 83:203–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan B, Chen F, Xu L, Xing J and Wang X:
HMGB1-TLR4-IL23-IL17A axis promotes paraquat-induced acute lung
injury by mediating neutrophil infiltration in mice. Sci Rep.
7:5972017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
D'Alessio FR, Tsushima K, Aggarwal NR,
West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM,
McDyer JF and King LS: CD4+CD25+Foxp3+ Tregs resolve experimental
lung injury in mice and are present in humans with acute lung
injury. J Clin Invest. 119:2898–2913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu TY and Chen SB: Sarcandra
glabra combined with lycopene protect rats from
lipopolysaccharide induced acute lung injury via reducing
inflammatory response. Biomed Pharmacother. 84:34–41. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei CY, Sun HL, Yang ML, Yang CP, Chen LY,
Li YC, Lee CY and Kuan YH: Protective effect of wogonin on
endotoxin-induced acute lung injury via reduction of p38 MAPK and
JNK phosphorylation. Environ Toxicol. 32:397–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kao ST, Liu CJ and Yeh CC: Protective and
immunomodulatory effect of flos Lonicerae japonicae by augmenting
IL-10 expression in a murine model of acute lung inflammation. J
Ethnopharmacol. 168:108–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Zhang L, Duan W, Liu B, Gong P,
Ding Y and Wu X: Anti-inflammatory effects of triptolide by
inhibiting the NF-κB signalling pathway in LPS-induced acute lung
injury in a murine model. Mol Med Rep. 10:447–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Xiao HT, Wang HS, Mu HX, Zhao L, Du
J, Yang D, Wang D, Bian ZX and Lin SH: Halofuginone reduces the
inflammatory responses of DSS-induced colitis through metabolic
reprogramming. Mol Biosyst. 12:2296–2303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun XH, Fu J and Sun DQ: Halofuginone
alleviates acute viral myocarditis in suckling BALB/c mice by
inhibiting TGF-β1. Biochem Biophys Res Commun. 473:558–564. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dianzani C, Foglietta F, Ferrara B, Rosa
AC, Muntoni E, Gasco P, Della Pepa C, Canaparo R and Serpe L: Solid
lipid nanoparticles delivering anti-inflammatory drugs to treat
inflammatory bowel disease: Effects in an in vivo model. World J
Gastroenterol. 23:4200–4210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leguillette R, Tohver T, Bond SL, Nicol JA
and McDonald KJ: Effect of dexamethasone and fluticasone on airway
hyperresponsiveness in horses with inflammatory airway disease. J
Vet Intern Med. 31:1193–1201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soiberman U, Kambhampati SP, Wu T, Mishra
MK, Oh Y, Sharma R, Wang J, Al Towerki AE, Yiu S, Stark WJ and
Kannan RM: Subconjunctival injectable dendrimer-dexamethasone gel
for the treatment of corneal inflammation. Biomaterials. 125:38–53.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kozan A, Kilic N, Alacam H, Guzel A,
Guvenc T and Acikgoz M: The effects of dexamethasone and L-NAME on
acute lung injury in rats with lung contusion. Inflammation.
39:1747–1756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang J, Zhang B, Shen RW, Liu JB, Gao MH,
Li Y, Li YY and Zhang W: Preventive effect of halofuginone on
concanavalin A-induced liver fibrosis. PLoS One. 8:e822322013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carlson TJ, Pellerin A, Djuretic IM,
Trivigno C, Koralov SB, Rao A and Sundrud MS: Halofuginone-induced
amino acid starvation regulates Stat3-dependent Th17 effector
function and reduces established autoimmune inflammation. J
Immunol. 192:2167–2176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kotthoff P, Heine A, Held SAE and Brossart
P: Dexamethasone induced inhibition of Dectin-1 activation of
antigen presenting cells is mediated via STAT-3 and NF-κB signaling
pathways. Sci Rep. 7:45222017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yeh YC, Yang CP, Lee SS, Horng CT, Chen
HY, Cho TH, Yang ML, Lee CY, Li MC and Kuan YH: Acute lung injury
induced by lipopolysaccharide is inhibited by wogonin in mice via
reduction of Akt phosphorylation and RhoA activation. J Pharm
Pharmacol. 68:257–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu X, Pu Y, Kong W, Tang X, Zhou J, Gou H,
Song X, Zhou H, Gao N and Shen J: Antidesmone, a unique
tetrahydroquinoline alkaloid, prevents acute lung injury via
regulating MAPK and NF-κB activities. Int Immunopharmacol.
45:34–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He J, Zhou J, Yang W, Zhou Q, Liang X,
Pang X, Li J, Pan F and Liang H: Dexamethasone affects cell
growth/apoptosis/chemosensitivity of colon cancer via
glucocorticoid receptor α/NF-κB. Oncotarget. 8:67670–67683.
2017.PubMed/NCBI
|
|
44
|
Xu J, Liu D, Yin Q and Guo L: Tetrandrine
suppresses β-glucan-induced macrophage activation via inhibiting
NF-κB, ERK and STAT3 signaling pathways. Mol Med Rep. 13:5177–5184.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang CS, Lin AH, Yang TC, Liu KL, Chen HW
and Lii CK: Shikonin inhibits oxidized LDL-induced monocyte
adhesion by suppressing NFκB activation via up-regulation of
PI3K/Akt/Nrf2-dependent antioxidation in EA.hy926 endothelial
cells. Biochem Pharmacol. 93:352–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li K, He Z, Wang X, Pineda M, Chen R, Liu
H, Ma K, Shen H, Wu C, Huang N, et al: Apigenin C-glycosides of
Microcos paniculata protects lipopolysaccharide induced apoptosis
and inflammation in acute lung injury through TLR4 signaling
pathway. Free Radic Biol Med. 124:163–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qing R, Huang Z, Tang Y, Xiang Q and Yang
F: Cordycepin alleviates lipopolysaccharide-induced acute lung
injury via Nrf2/HO-1 pathway. Int Immunopharmacol. 60:18–25. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen LJ, Ding YB, Ma PL, Jiang SH, Li KZ,
Li AZ, Li MC, Shi CX, Du J and Zhou HD: The protective effect of
lidocaine on lipopolysaccharide-induced acute lung injury in rats
through NF-κB and p38 MAPK signaling pathway and excessive
inflammatory responses. Eur Rev Med Pharmacol Sci. 22:2099–2108.
2018.PubMed/NCBI
|
|
49
|
Yavas G, Calik M, Calik G, Yavas C, Ata O
and Esme H: The effect of Halofuginone in the amelioration of
radiation induced-lung fibrosis. Med Hypotheses. 80:357–359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nagler A, Firman N, Feferman R, Cotev S,
Pines M and Shoshan S: Reduction in pulmonary fibrosis in vivo by
halofuginone. Am J Respir Crit Care Med. 154:1082–1086. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wigenstam E, Elfsmark L, Ågren L, Akfur C,
Bucht A and Jonasson S: Anti-inflammatory and anti-fibrotic
treatment in a rodent model of acute lung injury induced by sulfur
dioxide. Clin Toxicol (Phila). 56:1185–1194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu X, Zhu Q, Niu F, Zhang R, Wang Y, Wang
W, Sun D, Wang X and Wang A: A2BAR activation attenuates acute lung
injury by inhibiting alveolar epithelial cell apoptosis both in
vivo and in vitro. Am J Physiol Cell Physiol. 315:C558–C570. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ehrentraut H, Weisheit C, Scheck M, Frede
S and Hilbert T: Experimental murine acute lung injury induces
increase of pulmonary TIE2-expressing macrophages. J Inflamm
(Lond). 15:122018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dong SA, Zhang Y, Yu JB, Li XY, Gong LR,
Shi J and Kang YY: Carbon monoxide attenuates
lipopolysaccharide-induced lung injury by mitofusin proteins via
p38 MAPK pathway. J Surg Res. 228:201–210. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takaoka Y, Goto S, Nakano T, Tseng HP,
Yang SM, Kawamoto S, Ono K and Chen CL: Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced,
sepsis-related severe acute lung injury in mice. Sci Rep.
4:52042014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Q, Liu X, Yao Z, Mao S, Wei Q and
Chang Y: Penehyclidine hydrochloride inhibits the release of
high-mobility group box 1 in lipopolysaccharide-activated RAW264.7
cells and cecal ligation and puncture-induced septic mice. J Surg
Res. 186:310–317. 2014. View Article : Google Scholar : PubMed/NCBI
|