Introduction
Gestational diabetes mellitus (GDM) is a form of
diabetes that first manifests during pregnancy (1). Abnormalities of the placental
barrier, including structural and functional dysfunction, are
evident in the placenta of most women suffering from GDM in the
form of abnormal glucose metabolism, and these changes can lead to
adverse pregnancy outcomes (2,3). The
main structure of the placental barrier is the vasculo-syncytial
membrane (VSM). The transcellular and paracellular pathways are the
two main mechanisms whereby solutes are able to traverse the VSM.
The syncytiotrophoblasts present on the maternal surface of the VSM
lack any obvious fluid-filled paracellular space, but there is an
inverse relationship between the rate of diffusion of inert
hydrophilic solutes on the placenta and their molecular size, which
suggests that a paracellular route exists at the VSM (4). Paracellular permeability is regulated
by tight junctions (TJs) and adherens junctions, and by the
proteins that make up these adhesive cell-cell junction points. TJs
have been reported to be present in both trophoblast cells and in
fetal vessel epithelium (5).
Previous studies have suggested that placental
dysfunction in patients with GDM is caused by hyperglycemia
(6,7). In addition, other studies have
identified that insulin therapy does not improve fetal or newborn
metabolic outcomes (8), and that
it can cause alterations in the placenta (9), indicating factors other than glucose
may be involved in the pathophysiology of GDM. In a number of
studies, advanced glycation end products (AGEs) have been
identified to be present in higher levels in women suffering from
GDM (10,11) and these products are associated
with poor fetal outcomes (12).
AGEs are reportedly involved in increasing the permeability of
retinal vascular and endothelial cells (13,14).
AGEs exert their deleterious effects by either directly damaging
cells or by binding to the specific receptor for AGEs (RAGE). The
binding of AGEs with RAGE stimulates NF-κB pathway activation
(15), enhancing the release of
vascular endothelial growth factor (VEGF) (16).
It was therefore hypothesized that AGEs may be an
important factor worthy of study in the context of placental
barrier dysfunction in patients with GDM. The present study
evaluated placental permeability and the expression of
TJ-associated proteins in rats with GDM, and investigated the
association of GDM with alterations in AGEs, RAGE, NF-κB and VEGF.
In addition, low molecular weight heparin (LMWH), as an
anticoagulant capable of reducing the risk of recurrent
placenta-mediated pregnancy complications (17), has a potent effect on the vascular
endothelium (18,19). Kevane et al (20) identified that the LMWH tinzaparin
serves a protective role in endothelial barrier function. As such,
LMWH was employed to assess whether it had the ability to reduce
placental permeability and to evaluate its relationship with
RAGE.
Materials and methods
Materials
A total of 35 healthy 12-week-old female
Sprague-Dawley (SD) rats (250–300 g) and 15 adult male SD rats
(300–350 g) were acquired from Beijing Vital River Laboratory
Animal Technology Co., Ltd. Animals were maintained in a controlled
environment (25±1°C, 50% humidity and 12-h light/dark cycle) and
were given free access to water and a standard laboratory diet. The
Medical Ethics Committee of Southeast University approved all
animal studies.
The primary antibodies used in this study were:
Rabbit anti-zonular occludens-1 (ZO-1) (cat. no. 61-7300;
Invitrogen; Thermo Fisher Scientific, Inc.), rabbit anti-occludin
(OCLN; cat. no. ab216327; Abcam), rabbit anti-NF-κB p65 (cat. no.
ab16502; Abcam), rabbit anti-GAPDH (cat. no. ab9485; Abcam) and
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibody (cat. no. ab130805; Abcam). The LMWH used was nadroparin
calcium injection, which was purchased from GlaxoSmithKline plc.
Bull serum albumin (BSA) was purchased from Gibco (Thermo Fisher
Scientific, Inc.).
Animal model preparation
Female rats in estrus were allowed to cohabitate for
one night with male rats at a 2:1 ratio in order to facilitate
conception; female rats with detectable sperm in their vaginal
smear were considered pregnant (day 0). The 30 pregnant rats were
randomized into three groups (n=10/group): Normal control (NC)
group, GDM group and GDM + LMWH group. GDM was induced in animals
via intraperitoneally injecting a single dose of streptozotocin (45
mg/kg; Wako Pure Chemical Industries, Ltd.), which had been freshly
prepared. Blood glucose levels of all rats were detected with a
glucometer (Onetouch, Ultra, Johnson & Johnson). Rats
exhibiting a glucose concentration of >16.7 mmol/l for 3 days
after the injection were used in this study. NC animals were
instead injected with 1 ml sodium citrate buffer. On gestational
day 5, rats in the GDM + LMWH group were injected subcutaneously
with LMWH (600 IU/kg/d) to establish an LMWH intervention model,
while other groups were administered an equal volume of 0.9%
saline. On day 16 of pregnancy, animals were sacrificed and the
placentas were removed for western blotting, reverse
transcription-quantitative (RT-q)PCR, transmission electron
microscopy (TEM) and immunohistochemistry (IHC).
ELISA
Blood samples (1 ml) were obtained from the
abdominal aorta on day 16 after sacrifice and were centrifuged at
2,000 × g for 20 min at 5°C. Concentrations of AGEs were measured
from the collected samples using a rat AGEs ELISA kit (cat. no.
RA20685; Bioswamp Wuhan Beinle Biotechnology Co., Ltd.), according
to the manufacturer's protocol. The sensitivity of this assay was
<6 ng/ml, whereas the coefficient of intraplate variation was
≤9%, with interassay variation ≤11%. AGEs were detected using a
microplate reader (ELx800; BioTek Instruments, Inc.) at an emission
wavelength of 450 nm. Standard curves were used to calculate AGEs
concentrations (ng/ml) in serum.
Evans Blue (EB) assay
An EB assay was used to measure VSM leakage. EB (50
mg/kg; Sigma-Aldrich; Merck KGaA) was injected into the tail vein
in 2% PBS. Rats were sacrificed 45 min after injection of EB and
the placentas were harvested, weighed, and incubated in 1 ml
formamide at 55°C for 24 h to extract EB from tissues. The
supernatant was collected after centrifugation at 12,000 × g for
0.5 h at 4°C and a microplate reader was used to measure absorbance
at 630 nm. EB content in placental samples (in ng/ml) was
calculated based upon a standard curve using the following formula:
Placenta EB content (ng/mg)=sample EB content (ng/ml) × formamide
volume (ml)/placental weight (mg) (21).
TEM
The ultrastructure of placentas was examined by TEM.
The placental samples were placed in cacodylate buffer with 0.05 M
sucrose and stored at 4°C for 24 h, following fixation with 2.5%
glutaraldehyde in cacodylate buffer at 4°C for 1 h. The placental
samples were fixed again in 1% osmium tetroxide for 1 h at 4°C
after dehydration treatment with a graded ethanol series, and then
placed into in a graded series of acetone. Samples were then sliced
into sections (1 µm) after embedding in araldite. The sections were
dyed with 2% uranyl acetate for 10 min and then with 100 µl lead
citrate for 5 min at 25°C. The placental TJ ultrastructure was
systematically investigated via DigitalMicrograph 3.9 (Gatan Inc.)
by electron microscope (EVO MA 10; Zeiss GmbH) at ×25,000
magnification.
IHC
IHC was conducted to investigate differences in the
expression of ZO-1 and OCLN in placental tissue. Placental samples
were fixed in formalin at 22°C for 6 h, dehydrated by different
concentration gradients of ethanol, be transparent in xylene for 30
min, paraffin-embedded, and sectioned at 5 µm. The paraffin slices
were dewaxed and rehydrated by xylene and with a graded ethanol
series, then treated with 3% H2O2 for 10 min,
followed by incubation with appropriate primary antibodies
(dilution: Rabbit anti-ZO-1, 1:200; rabbit anti-OCLN, 1:500) for 24
h at 4°C. Secondary antibodies (1:500) were then used to probe
samples for 50 min at 37°C. The sections were then restained with
1% hematoxylin at 22°C for 1 min, dehydrated, cleared and mounted.
An automated light microscope (DMLA, Leica Microsystems GmbH) was
used to detect the location of dyeing area.
Western blotting
RIPA buffer [5 mM EDTA, 150 mM NaCl, 3 µl PMSF, 1%
NP40 and 50 mM Tris-HCl (pH 7.0)] was used for protein extraction.
BCA protein assay kit (P0009, Beyotime Institute of Biotechnology)
was used to quantify the protein of each samples. Equivalent
protein amounts from each sample (4.25 µg) were then separated via
SDS-PAGE with 10% gels, followed by transfer onto a PVDF membrane
(GE Healthcare Life Sciences). Blots were washed three times using
TBS-0.1% Tween (TBST; 10 min/wash), blocked using 5% fat-free milk
in TBST at 22°C for 4 h and then probed overnight at 4°C with
primary antibodies (dilution: Rabbit anti-ZO-1, 1:1,000; rabbit
anti-OCLN, 1:2,000; rabbit anti-NF-κB p65 1:500; rabbit anti-GAPDH,
1:2,500). Blots were again washed three times with TBST and then
probed with secondary HRP-conjugated goat anti-rabbit IgG (1:3,000
in TBST containing 10% BSA) at 22°C for 1 h. An enhanced
chemiluminescence kit (Tanon 5200; Tanon Science and Technology
Co., Ltd.) was used for protein visualization. Images were recorded
and data were analyzed with Image J 1.8.0 (National Institutes of
Health).
RT-qPCR
RAGE and VEGF-A mRNA expression levels were assessed
using total RNA that was extracted from placental homogenates using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
based on manufacturer's protocols. The first-strand complementary
synthesis reaction was performed using a PrimeScript RT Reagent Kit
(RR047A; Takara Biotechnology Co., Ltd.). RAGE and VEGF expression
were detected using the SLAN real-time PCR detection system
(Shanghai Hongshi Medical Technology Co., Ltd.). The primer
sequences were as follows: VEGF, forward
5′-CAATGATGAAGCCCTGGAGTG-3′, reverse 5′-GCTCATCTCTCCTATGTGCTGG-3′;
RAGE, forward 5′-TGAGACGGGACTCTTCACGCT-3′, reverse
5′-CACCTTCAGGCTCAACCAACA-3′; and β-actin, forward
5′-TGCTATGTTGCCCTAGACTTCG-3′ and reverse
5′-GTTGGCATAGAGGTCTTTACGG-3′. β-actin was used as an internal
control. Amplification conditions were: 95°C for 10 min for enzyme
activation, 40 cycles of denaturation at 95°C for 15 sec, annealing
at 60°C for 10 min, and dissociation curve assessment between 75°C
and 95°C with continuous fluorescence measurement. Only RNA samples
with an OD260/OD280 ratio of 1.8–2.0 were used for RT. Each sample
was measured in triplicate and the 2−ΔΔCq method
(22) was used to assess relative
gene expression.
Statistical analysis
All data were presented as the means ± standard
deviation of triplicate experiments. SPSS 21.0 (SPSS, Inc.) was
used for all analyses, with analysis of variance via one-way
analysis of variance followed by post-hoc Student-Newman-Keuls
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects on placental permeability as
measured by EB assay
EB is a dye with a high affinity for albumin, which
cannot pass the endothelium; therefore, EB bound to albumin is
restricted to blood vessels. In some pathological conditions
associated with high vascular permeability, endothelial cells
partially lose their TJs and allow small molecules such as albumin
to pass through (21), thereby
allowing EB leakage into tissues. In the present study, EB was
injected into the tail vein of rats to evaluate paracellular
permeability of the placenta in each group.
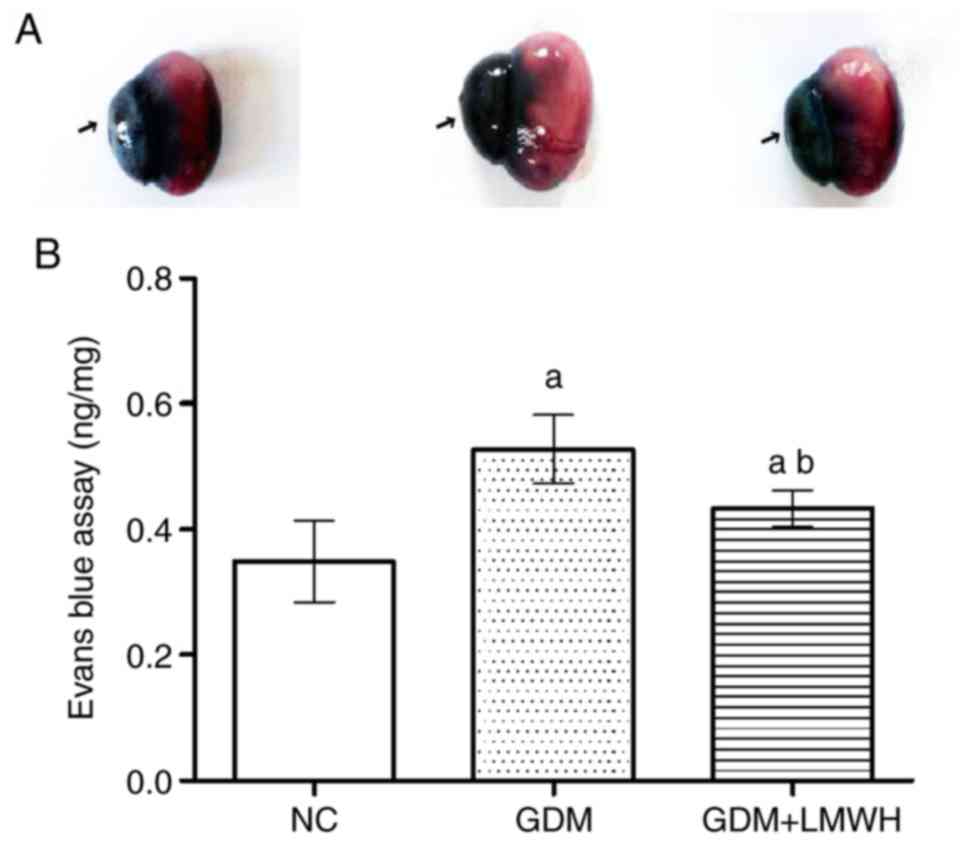
Upon examination, it was evident that placental
coloration differed between groups, with higher EB content
corresponding to a darker color (Fig.
1A). Furthermore, optical density analysis revealed that EB
leakage in GDM group placentas was ~1.5-fold higher than in the NC
group (P<0.05). LMWH-treated GDM rats exhibited lower EV leakage
compared with in the GDM group (P<0.05), although this leakage
was still higher compared with the NC group (P<0.05; Fig. 1B).
Alteration of placental TJ
structure
The ultrastructure of TJs was assessed by TEM. Under
TEM, TJs between syncytiotrophoblasts and vascular endothelial
cells in the placental tissue were loose and damaged in placental
samples from the GDM group. In contrast, animals treated with LMWH
exhibited reduced placental structural damage relative to animals
in the GDM group (Fig. 2A).
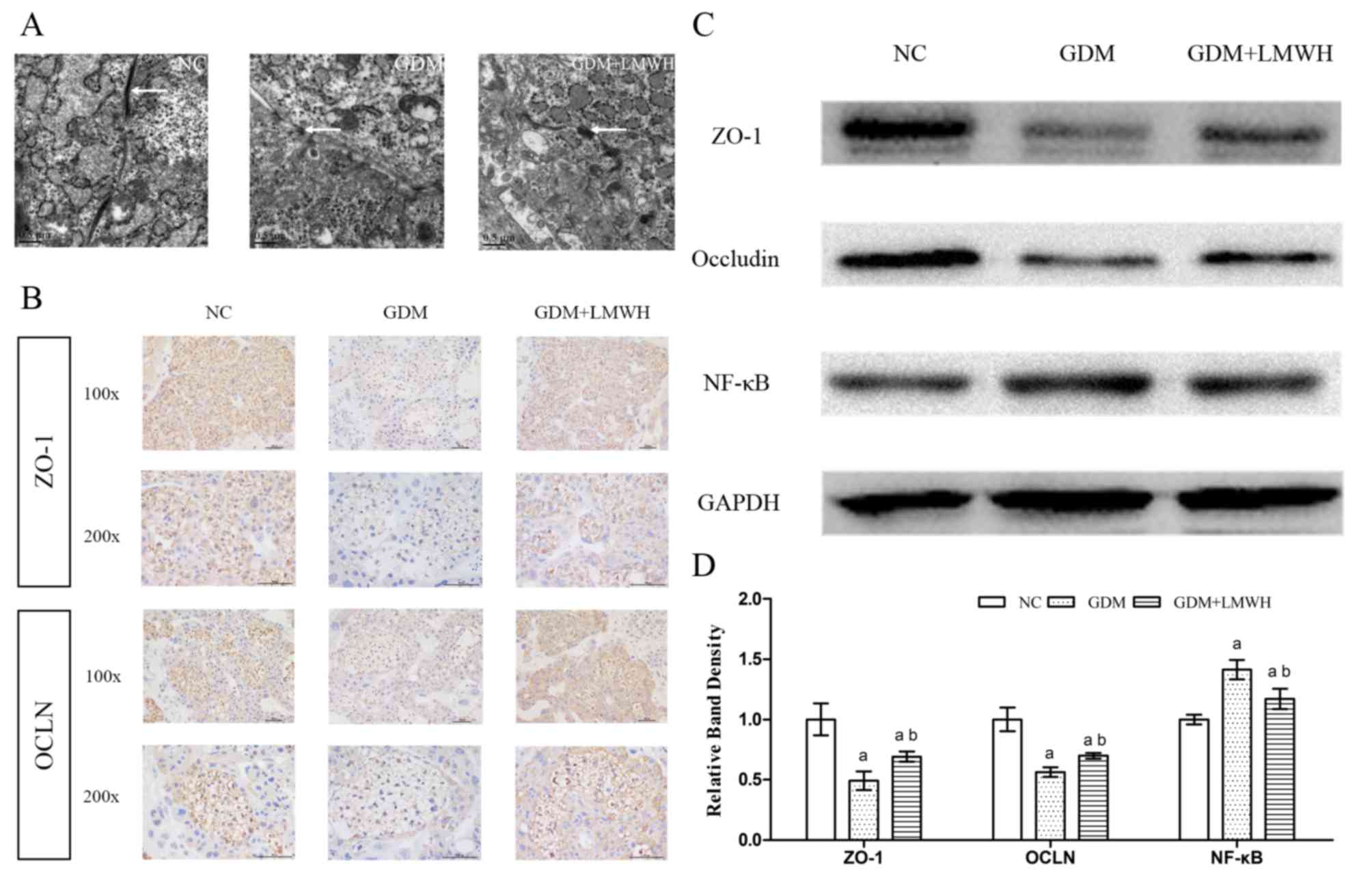 | Figure 2.Alterations in the structure,
location and expression of tight junctions in the placentas of each
group. (A) Electron micrographs of the placental ultrastructure in
each group were captured via transmission electron microscopy at
×25,000 magnification (scale bar, 0.5 µm). White arrows indicate
the TJs on the membrane between cells. (B) Expression and
localization of ZO-1 and OCLN in placental tissues were observed
via IHC (scale bar, 100 µm). Images in the first row correspond to
×100 magnification, while those in the second row correspond to
×200 magnification. (C) Representative western blotting results for
ZO-1, OCLN and NF-κB. GAPDH served as a loading control. (D)
Results of western blot analysis of the expression of ZO-1, OCLN
and NF-κB, with semi-quantification shown. Data represent fold
changes relative to the NC group. Data are presented as the means ±
standard deviation from three independent experiments.
aP<0.05 vs. NC group, bP<0.05 vs. GDM
group. GDM, gestational diabetes mellitus; LMWH, low molecular
weight heparin; NC, normal control; OCLN, occludin; TJ, tight
junction; ZO-1, zonular occludens-1. |
TJ protein expression analysis
IHC was used to observe the localization and
expression of TJ proteins, including ZO-1 and OCLN. As expected,
ZO-1 and OCLN were mostly located in the syncytiotrophoblast cell
membrane, localizing to the TJs between cells. The two proteins did
not exhibit apparent redistribution in the GDM group or the GDM +
LMWH group compared with in the NC group (Fig. 2B). The expression levels of ZO-1
and OCLN were lower in the GDM group compared with in the NC
group.
Western blotting was used to confirm differences in
OCLN and ZO-1 protein levels, demonstrating clear reductions in
these proteins in GDM placental tissues compared with in the NC
group (P<0.05) (Fig. 2C and D),
whereas LMWH upregulated the expression levels of these proteins
(P<0.05), albeit to levels lower compared with the NC group
(P<0.05).
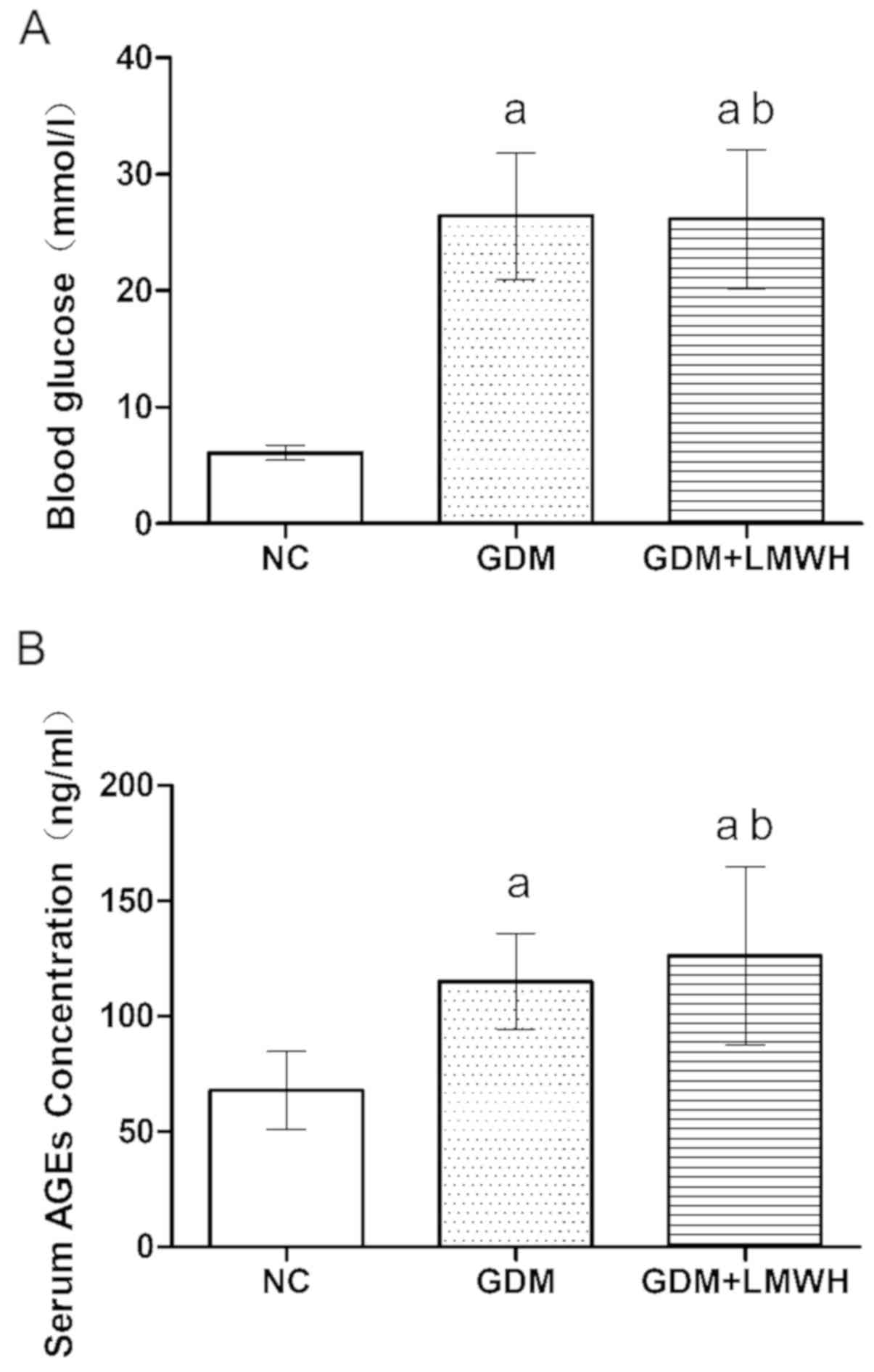
Increased blood glucose and serum AGEs
levels in the GDM model
On day 16 of pregnancy, blood glucose and serum AGEs
levels were assessed in all groups. Animals in the GDM and GDM +
LMWH groups exhibited significantly higher blood glucose levels
compared with in the NC group (P<0.05), but no significant
differences were observed between the GDM and GDM + LMWH groups
(P>0.05; Fig. 3A). AGEs serum
levels exhibited variations similar to those of blood glucose in
the GDM and GDM + LMWH groups (Fig.
3B).
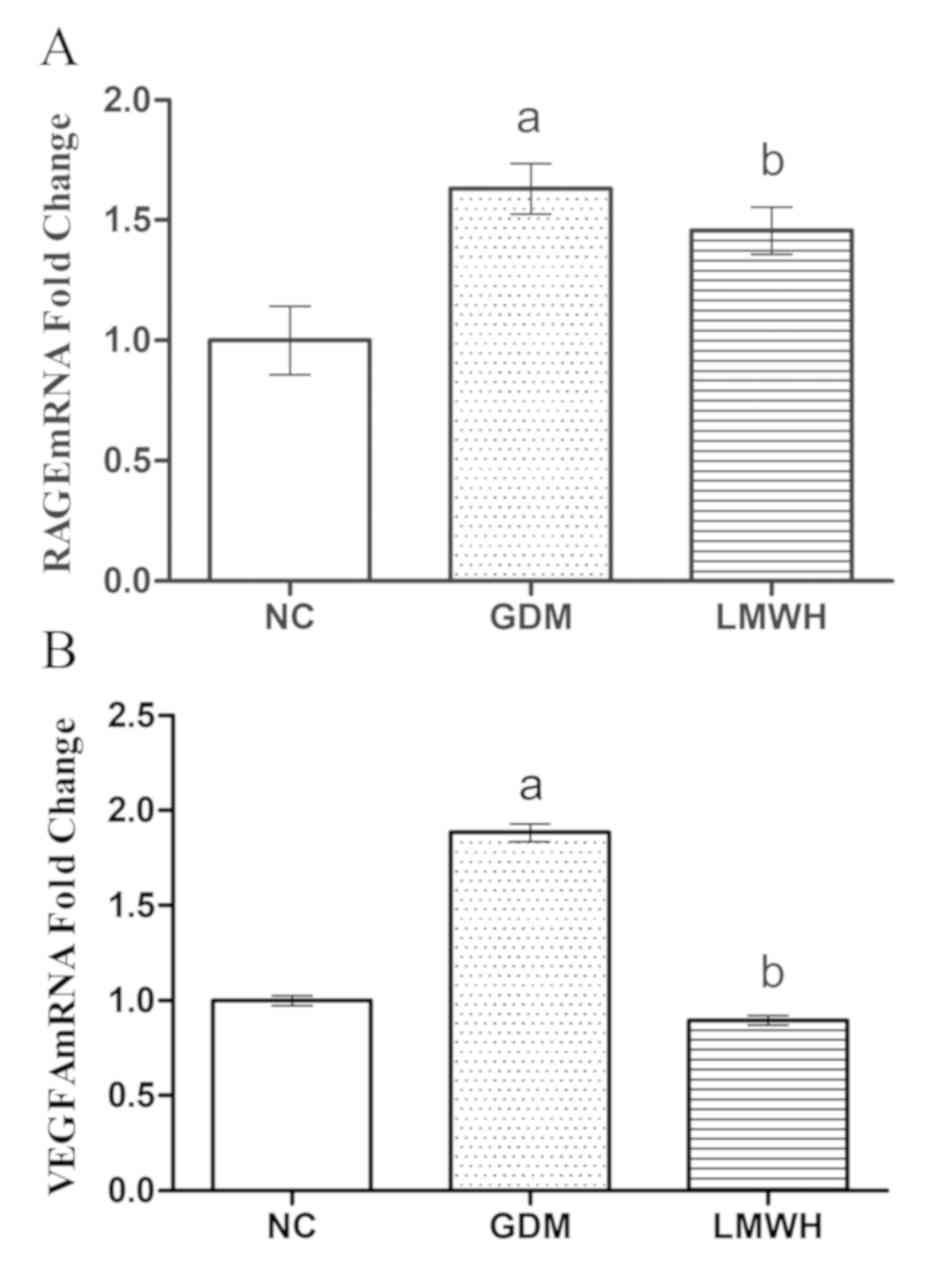
Altered placental RAGE/NF-κB pathway
activation
In order to clarify the signaling pathways
associated with altered placental permeability in this model, the
RAGE/NF-κB signaling pathway was analyzed in the placental samples.
The mRNA expression levels of RAGE and VEGF were assessed via
RT-qPCR. RAGE expression was significantly increased in samples
from the GDM and GDM + LMWH groups compared with in the NC group
(P<0.05). LMWH treatment partially decreased this increase in
RAGE expression in animals with GDM (P<0.05; Fig. 4A). Compared to normal rats, GDM
rats exhibited a significant upregulation of VEGF mRNA (P<0.05),
whereas LMWH treatment reduced VEGF expression to levels similar to
in the NC group (P<0.05; Fig.
4B). These results suggested that LMWH treatment may reduce the
expression of RAGE and VEGF at the mRNA level.
NF-κB protein levels were assessed via western
blotting (Fig. 2C). Compared with
in NC animals, rats in the GDM group exhibited significantly
increased placental NF-κB levels (P<0.05). NF-κB levels were
reduced following treatment with LMWH in GDM rats (P<0.05) to
levels higher compared with NC animals (P<0.05; Fig. 2D).
Discussion
The present study established a rat model of GDM to
observe the changes in placental permeability and TJs, the effects
of LMWH on these parameters, and the potential underlying
mechanisms. It was identified that the GDM rats exhibited a
significant increase in EB leakage and a lower expression of the TJ
proteins OCLN and ZO-1 compared with in NC animals. Significant
changes in the placentas of patients with GDM have previously been
identified, including decreased syncytiotrophoblasts apical
microvilli density, a thickened placental barrier and increased ST
vacuoles (23). Hayward et
al (24) observed changes in
placental glucose and neutral amino acids in the context of GDM;
however, the changes in placental transfer of macromolecules, such
as albumin, in GDM remain to be elucidated The leakage of EB bound
to albumin in the present study provided evidence of the transfer
of albumin through the placenta. Reductions in TJ proteins in the
placenta of GDM indicate dysfunction of the TJ barriers, thus
increasing macromolecule flux via the paracellular route (6,25).
The results of TEM and IHC in the present study identified that TJs
were located in endothelial and trophoblast cells, which is
consistent with previous studies (25,26).
GDM placentas have been shown to exhibit a significant reduction in
the TJ proteins, ZO-1 and OCLN, and the adherens junction proteins,
VE-cadherin and β-catenin, particularly upon exposure to
hyperglycemia during the first trimester when the vascular
remodeling phase of placental growth occurs (25). Alterations in placental
permeability and the expression of TJ proteins in GDM placentas may
account for adverse fetal and neonatal outcomes (6,8).
In the current study, the blood glucose and serum
AGEs levels in GDM rats were higher compared with in normal rats.
Previous work by Li and Yang (27)
indicated that the level of serum AGEs was positively associated
with glucose levels. Chronic hyperglycemia has been highlighted as
a possible contributing factor in diabetic vascular complications
(28) and may be the cause of
increased vascular permeability in the context of diabetic
retinopathy (29). Under
conditions of chronic hyperglycemia, AGEs are actively formed and
accumulate in circulation and in various tissues, as they are
produced due to the non-enzymatic glycation of proteins, lipids and
nucleic acids (30). The diabetic
vascular complications triggered by AGEs are dominant and
independent of hyperglycemia in those with diabetes (31). Previously, AGEs were reported to
increase the permeability of retinal vascular and endothelial cells
by upregulating certain chemokines or promoting actin rearrangement
(32). In Alzheimer's disease,
AGEs have been identified as disrupting TJs in the blood-brain
barrier, thus increasing its permeability (33). Therefore, both hyperglycemia and
AGEs have an effect on vascular permeability.
AGEs exert their deleterious effects by either
directly damaging cells or through a receptor-mediated pathway
(15). RAGE is the most studied
receptor of AGEs and is a member of the immunoglobulin superfamily,
present primarily on vascular, endothelial and smooth muscle cells,
and on monocyte/macrophage membranes (34). Once AGEs are recognized by RAGE on
the cell membrane, downstream signaling leads to oxidative stress
and inflammation in cells via the activation of NF-κB (35). The expression of RAGE can be
upregulated by AGEs, as evidenced by the fact that RAGE protein
levels are increased in settings where AGEs are abundant (15). The present study identified that
the expression of placental RAGE mRNA was increased in GDM group
animals that exhibited elevated levels of serum AGEs. NF-κB is a
downstream target of RAGE and was significantly elevated in the GDM
group in the present study. Mice with enhanced NF-κB expression
exhibit greater sensitivity to lipopolysaccharide-induced toxemia,
which is associated with an increase in vascular permeability and a
clear reduction in the formation of TJs (35). Upon activation of RAGE, the
transcription factor NF-κB undergoes nuclear translocation and
binds to the promoter region of RAGE, thereby inducing RAGE gene
expression (15). It was therefore
inferred that the RAGE/NF-κB pathway was activated in GDM placentas
in response to high AGEs levels.
As a master regulator of inflammation, NF-κB is
capable of controlling the transcription of a range of genes
related to the inflammatory response, including VEGF, which is
known to enhance vascular permeability (36). VEGF binding to its cell surface
receptors triggers the disassembly of TJs and promotes an increase
in vascular permeability (36).
Increased vascular permeability, deficiency of VE-cadherin and
elevated levels of VEGF have been reported in patients with GDM
(26,37). Previous studies of barrier systems,
such as the blood-brain barrier (33), the blood-retinal barrier (13) and the blood-placenta barrier
(26), have confirmed the
relationship between VEGF and TJs. Research into the blood-brain
barrier has revealed that the expression of VEGF is upregulated by
hypoxia, thereby increasing blood-brain barrier permeability
(33). In addition, blood-retinal
barrier dysfunction has been recovered by targeting ZO-1 through a
reduction in VEGF (38).
Hypoxia-induced TJ dysfunction in trophoblasts can be improved by
downregulation of VEGF (26). A
recent study identified that VEGF165b, intercellular
adhesion molecule 1 (ICAM-1) and AGEs in GDM were higher, and
VEGF165b/total VEGF ratio was higher in GDM, which was
correlated with ICAM-1 and AGEs (39). The present study also identified
that the expression of VEGF-A mRNA was increased in the placentas
of GDM rats, but did not detect the expression of each isoform of
VEGF-A. VEGF165 and VEGF121 are the most
highly expressed VEGF-A isoforms, whereas VEGF121 has no
affinity for heparin (40).
Therefore it was hypothesized that VEGF165 serves a
major role in regulating placental permeability in GDM. It was
further hypothesized that the RAGE/NF-κB pathway in the placenta of
animals with GDM can upregulate VEGF expression, making this an
important mechanism underlying dysfunction at the GDM placental
barrier.
The present study reported that LMWH attenuated the
increase in VSM permeability in rats with GDM; this was accompanied
by a partial recovery in the expression of ZO-1 and OCLN. There was
no significant difference in blood glucose or serum AGEs
concentrations between GDM and GDM + LMWH animals, indicating that
LMWH has no role in regulating blood glucose or serum AGEs. This
effect may instead be due to the non-anticoagulant effects of LMWH
(40). According to previous
studies, nadroparin, which was used in the present study, has
anti-inflammatory, anti-metastatic and anti-fibrotic activities
(41–44). Yalniz et al (44) reported that nadroparin exerts
anti-oxidative and anti-inflammatory effects by regulating the
NF-κB and nuclear factor erythroid 2-related factor 2/heme
oxygenase 1 pathways. In contrast to tinzaparin, fondaparinux or to
direct oral anticoagulants, enoxaparin increases the permeability
of podocytes to albumin, as reported by Delézay et al
(45). The heterogeneity of LMWHs
may explain these inconsistent and even opposite conclusions.
In the present study, LMWH intervention resulted in
a decrease in RAGE and VEGF mRNA expression levels, which were
upregulated in animals in the GDM group, whereas it markedly
reduced NF-κB protein levels. The LMWH tinzaparin has been
identified to serve a protective role in endothelial barrier
function (20). Bentzer et
al (46) revealed that
heparins are potential inhibitors of hypertension-induced increases
in vascular permeability.
LMWH has been reported to be a competitive
antagonist of RAGE that competes with AGEs for RAGE binding,
inhibiting its activation. This was identified by Takeuchi et
al (47), who demonstrated
that LMWH attenuates changes in diabetic kidneys by inhibiting
RAGE. This finding suggests that LMWH may serve as an inhibitor of
RAGE, which would alter the placental permeability in GDM. It was
hypothesized that LMWH acts as a competitive antagonist of RAGE,
whose expression was downregulated, thereby inactivating the
RAGE/NF-κB pathway. Furthermore the expression of VEGF was
decreased, thereby reducing placental permeability. A limitation of
the present study is the lack of an in vitro study to
further confirm the relationship between GDM and LMWH. In addition,
the effects of GDM on the offspring were not analyzed.
In conclusion, the AGEs-RAGE system may represent a
novel target for treating placental barrier dysfunction in GDM.
LMWH may be a potential drug for the treatment of diseases related
to the AGEs-RAGE system.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 659000095) and
Science and Technology Development Foundation of Nanjing Medical
University (grant no. NMUB2018249).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YS, JQ, FZ and BJ performed experiments and data
analysis; YS, BJ and JQ drafted the manuscript; YS, QZ, XL, YH, YY,
DS and LJ participated in the study design, data collection and
revision process. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Medical Ethics Committee of Southeast University
approved all animal studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AGEs
|
advanced glycation end products
|
|
EB
|
Evans blue
|
|
GDM
|
gestational diabetes mellitus
|
|
IHC
|
immunohistochemistry
|
|
LMWH
|
low molecular weight heparin
|
|
OCLN
|
occludin
|
|
RAGE
|
receptor for advanced glycation end
products
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
TEM
|
transmission electron microscopy
|
|
TJ
|
tight junction
|
|
VEGF
|
vascular endothelial growth factor
|
|
VSM
|
vasculo-syncytial membrane
|
|
ZO-1
|
zonular occludens-1
|
References
|
1
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 37 (Suppl
1):S81–S90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jarmuzek P, Wielgos M and Bomba-Opon D:
Placental pathologic changes in gestational diabetes mellitus.
Neuro Endocrinol Lett. 36:101–105. 2015.PubMed/NCBI
|
|
3
|
Aires MB and Dos Santos AC: Effects of
maternal diabetes on trophoblast cells. World J Diabetes.
6:338–344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sibley CP, Brownbill P, Glazier JD and
Greenwood SL: Knowledge needed about the exchange physiology of the
placenta. Placenta. 64 (Suppl 1):S9–S15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leach L, Lammiman MJ, Babawale MO, Hobson
SA, Bromilou B, Lovat S and Simmonds MJ: Molecular organization of
tight and adherens junctions in the human placental vascular tree.
Placenta. 21:547–557. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leach L, Taylor A and Sciota F: Vascular
dysfunction in the diabetic placenta: Causes and consequences. J
Anat. 215:69–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sobrevia L, Salsoso R, Fuenzalida B,
Barros E, Toledo L, Silva L, Pizarro C, Subiabre M, Villalobos R,
Araos J, et al: Insulin is a key modulator of fetoplacental
endothelium metabolic disturbances in gestational diabetes
mellitus. Front Physiol. 7:1192016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subiabre M, Silva L, Toledo F, Paublo M,
López MA, Boric MP and Sobrevia L: Insulin therapy and its
consequences for the mother, foetus, and newborn in gestational
diabetes mellitus. Biochim Biophys Acta Mol Basis Dis.
1864:2949–2956. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baumuller S, Lehnen H, Schmitz J, Fimmers
R and Müller AM: The impact of insulin treatment on the expression
of vascular endothelial cadherin and beta-catenin in human
fetoplacental vessels. Pediatr Dev Pathol. 18:17–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartakova V, Kollarova R, Kuricova K,
Sebekova K, Belobradkova J and Kankova K: Serum
carboxymethyl-lysine, a dominant advanced glycation end product, is
increased in women with gestational diabetes mellitus. Biomed Pap
Med Fac Univ Palacky Olomouc Czech Repub. 160:70–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harsem NK, Braekke K, Torjussen T, Hanssen
K and Staff AC: Advanced glycation end products in pregnancies
complicated with diabetes mellitus or preeclampsia. Hypertens
Pregnancy. 27:374–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guosheng L, Hongmei S, Chuan N, Haiying L,
Xiaopeng Z and Xianqiong L: The relationship of serum AGE levels in
diabetic mothers with adverse fetal outcome. J Perinatol.
29:483–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stitt AW, Bhaduri T, McMullen CB, Gardiner
TA and Archer DB: Advanced glycation end products induce
blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell
Biol Res Commun. 3:380–388. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo XH, Huang QB, Chen B, Wang SY, Li Q,
Zhu YJ, Hou FF, Fu N, Brunk UT and Zhao M: Advanced glycation end
products induce actin rearrangement and subsequent
hyperpermeability of endothelial cells. APMIS. 114:874–883. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie J, Mendez JD, Méndez-Valenzuela V and
Aguilar-Hernández MM: Cellular signalling of the receptor for
advanced glycation end products (RAGE). Cell Signal. 25:2185–2197.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boulanger E, Grossin N, Wautier MP, Taamma
R and Wautier JL: Mesothelial RAGE activation by AGEs enhances VEGF
release and potentiates capillary tube formation. Kidney Int.
71:126–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodger MA, Gris JC, de Vries JIP,
Martinelli I, Rey É, Schleussne E, Middeldorp S, Kaaja R, Langlois
NJ, Ramsay T, et al: Low-molecular-weight heparin and recurrent
placenta-mediated pregnancy complications: A meta-analysis of
individual patient data from randomised controlled trials. Lancet.
388:2629–2641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wat JM, Hawrylyshyn K, Baczyk D, Greig IR
and Kingdom JC: Effects of glycol-split low molecular weight
heparin on placental, endothelial, and anti-inflammatory pathways
relevant to preeclampsia. Biol Reprod. 99:1082–1090. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gasowska K, Naumnik B, Klejna K and
Myśliwiec M: The influence of unfractionated and low-molecular
weight heparins on the properties of human umbilical vein
endothelial cells (HUVEC). Folia Histochem Cytobiol. 47:17–23.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kevane B, Egan K, Allen S, Maguire P,
Neary E, Lennon Á and Ní Áinle FN: Endothelial barrier protective
properties of low molecular weight heparin: A novel potential tool
in the prevention of cancer metastasis. Res Pract Thromb Haemost.
1:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radu M and Chernoff J: An in vivo assay to
test blood vessel permeability. J Vis Exp. 73:e500622013.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao C,
Gao L, Liu J and Cui Y: Ultrastructure of placenta of gravidas with
gestational diabetes mellitus. Obstet Gynecol Int. 2015:2831242015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayward CE, Jones RL and Sibley CP:
Mechanisms of transfer across the human placenta. Placenta
Intrauterine Environment. 12:121–133. 2008.
|
|
25
|
Babawale MO, Lovat S, Mayhew TM, Lammiman
MJ, James DK and Leach L: Effects of gestational diabetes on
junctional adhesion molecules in human term placental vasculature.
Diabetologia. 43:1185–1196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhao HJ, Xia XR, Diao FY, Ma X,
Wang J, Gao L, Liu J, Gao C, Cui YG and Liu JY: Hypoxia-induced and
HIF1 a-VEGF-mediated tight junction dysfunction in choriocarcinoma
cells: Implications for preeclampsia. Clin Chim Acta. 489:203–211.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S and Yang H: Relationship between
advanced glycation end products and gestational diabetes mellitus.
J Matern Fetal Neonatal Med. 32:2783–2789. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holman RR, Paul SK, Bethel MA, Matthews DR
and Neil HA: 10-year follow-up of intensive glucose control in type
2 diabetes. New Engl J Med. 359:1577–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antonetti DA, Lieth E, Barber AJ and
Gardner TW: Molecular mechanisms of vascular permeability in
diabetic retinopathy. Semin Ophthalmol. 14:240–248. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh R, Barden A, Mori T and Beilin L:
Advanced glycation end-products: A review. Diabetologia.
44:129–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chilelli NC, Burlina S and Lapolla A:
AGEs, rather than hyperglycemia, are responsible for microvascular
complications in diabetes: A ‘glycoxidation-centric’ point of view.
Nutr Metab Cardiovasc Dis. 23:913–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Svensjö E, Cyrino F, Michoud E, Ruggiero
D, Bouskela E and Wiernsperger N: Vascular permeability increase as
induced by histamine or bradykinin is enhanced by advanced
glycation endproducts (AGEs). J Diabetes Complications. 13:187–190.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wan W, Chen H and Li Y: The potential
mechanisms of Aβ-receptor for advanced glycation end-products
interaction disrupting tight junctions of the blood-brain barrier
in Alzheimer's disease. Int J Neurosci. 124:75–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmidt AM, Yan SD, Yan SF and Stern DM:
The biology of the receptor for advanced glycation end products and
its ligands. Biochim Biophys Acta. 1498:99–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kisseleva T, Song L, Vorontchikhina M,
Feirt N, Kitajewski J and Schindler C: NF-kappaB regulation of
endothelial cell function during LPS-induced toxemia and cancer. J
Clin Invest. 116:2955–2963. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vandenbroucke E, Mehta D, Minshall R and
Malik AB: Regulation of endothelial junctional permeability. Ann N
Y Acad Sci. 1123:134–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leach L, Gray C, Staton S, Babawale MO,
Gruchy A, Foster C, Mayhew TM and James DK: Vascular endothelial
cadherin and beta-catenin in human fetoplacental vessels of
pregnancies complicated by Type 1 diabetes: Associations with
angiogenesis and perturbed barrier function. Diabetologia.
47:695–709. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Obert E, Strauss R, Brandon C, Grek C,
Ghatnekar G, Gourdie R and Rohrer B: Targeting the tight junction
protein, zonula occludens-1, with the connexin43 mimetic peptide,
aCT1, reduces VEGF-dependent RPE pathophysiology. J Mol Med (Berl).
95:535–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krishnasamy S, Ravi V, Rajaraman B, Kumar
Thulasingam S, Dhevasena CS, Pathak A, Swaminathan K, Sundaresan M,
Ayyappa KA, Arunkumar G, et al: Role of
VEGF165b/VEGFTOTAL ratio in gestational
diabetes mellitus. Gynecol Endocrinol. 35:811–814. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
41
|
Yan Y, Ji Y, Su N, Mei X, Wang Y, Du S,
Zhu W, Zhang C, Lu Y and Xing XH: Non-anticoagulant effects of low
molecular weight heparins in inflammatory disorders: A review.
Carbohydr Polym. 160:71–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdel-Salam OM, Baiuomy AR, Ameen A and
Hassan NS: A study of unfractionated and low molecular weight
heparins in a model of cholestatic liver injury in the rat.
Pharmacol Res. 51:59–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nagy Z, Turcsik V and Blaskó G: The effect
of LMWH (Nadroparin) on tumor progression. Pathol Oncol Res.
15:689–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yalniz M, Demirel U, Orhan C, Bahcecioglu
IH, Ozercan IH, Aygun C, Tuzcu M and Sahin K: Nadroparin sodium
activates Nrf2/HO-1 pathway in acetic acid-induced colitis in rats.
Inflammation. 35:1213–1221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Delézay O, Hé Z, Sabido O, Hodin S, Bin V,
Saleem MA, Mismetti P and Delavenne X: Effects of heparin and
derivatives on podocytes: An in vitro functional and morphological
evaluation. J Cell Physiol. Jan 26–2019.doi: 10.1002/jcp.28191
(Epub ahead of print). View Article : Google Scholar
|
|
46
|
Bentzer P, Fisher J, Kong HJ, Mörgelin M,
Boyd JH, Walley KR, Russell JA and Linder A: Heparin-binding
protein is important for vascular leak in sepsis. Intensive Care
Med Exp. 4:332016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takeuchi A, Yamamoto Y, Munesue S,
Harashima A, Watanabe T, Yonekura H, Yamamoto H and Tsuchiya H: Low
molecular weight heparin suppresses receptor for advanced glycation
end products-mediated expression of malignant phenotype in human
fibrosarcoma cells. Cancer Sci. 104:740–749. 2013. View Article : Google Scholar : PubMed/NCBI
|