Introduction
Cardiac vascular occlusion leads to the restriction
of blood flow and oxygen to the heart, which causes cardiac
ischemia. Although restoration of blood flow rescues the tissues
from deficiency of oxygen and other supplementary metabolic
products, subsequent lethal injury can induce myocardial damage and
even death, termed ischemia/reperfusion (I/R) injury (1,2).
Previous mechanistic studies demonstrated that calcium overloading,
mitochondrial-mediated apoptosis, redox disorder and inflammation
play central roles in I/R injury (3–6). In
the inflammatory response, the levels of pro-inflammatory
cytokines, including interleukin (IL)-6, IL-18, IL-1β and tumor
necrosis factor (TNF)-α, markedly increased in the progress of
cardiac I/R injury (7). The
inflammatory response was induced quickly by cardiac I/R injury via
the nuclear factor erythroid-2 related factor 2 (Nrf2)
transcription factor-mediated signaling pathway (8). In Nrf2-deficient mice, the
cardiovascular phenotype is more impaired, even in the ischemic
preconditioning model, due to its important function in the
cardiovascular system (9).
Previous studies demonstrated that Nrf2/heme oxygenase-1 (HO-1)
signaling plays an important role in I/R injury in different organs
(10–13). Activation of Nrf2/HO-1 signaling is
an effective method to attenuate myocardial I/R injury in several
previous pharmacological studies (14,15).
In cellular metabolism, the endoplasmic reticulum
(ER) is responsible for protein folding to produce proteins with
biologically functional structures for signal transduction. Extreme
external stress disrupts protein folding and causes ER stress,
which subsequently activates intracellular signaling transduction,
termed the unfolded protein response (UPR) (16). Activating transcription factor 6
(ATF6), double-stranded RNA-activated protein kinase-like ER kinase
(PERK) and inositol requiring enzyme 1 (IRE1), three UPR signal
transducers, combine to function in glucose and lipid metabolism,
leptin and insulin resistance, atherosclerosis and ischemia.
Depending on these mechanisms, the UPR has been implicated in
various physiological conditions, such as obesity, type 2 diabetes
and I/R injury (17). Zhang et
al (18) identified a critical
role of the UPR; ER-induced cell apoptosis was observed under I/R
injury and reversing the UPR could reduce the cardiac infarct
size.
Crocetin (CRO), a natural apocarotenoid dicarboxylic
acid is derived from Crocus sativus L., which originates
from the Qinghai-Tibetan Plateau and can endure low concentrations
of oxygen. Previous studies have demonstrated that CRO reduced
cardiac cytotoxicity and apoptosis by regulating cardiac enzymes
and their function (19,20). Additional previous studies
demonstrated that CRO mechanistically regulated the mitogen
associated protein kinase pathway and nuclear factor-κB signaling
to alleviate myocardial ischemia injury (21,22).
Moreover, CRO significantly reversed spatial learning dysfunction
and attenuated hippocampal injury in an in vivo model of
vascular dementia, which suggested that CRO exhibits more versatile
functions than was previously known (23). A new study has been uncovered that
another similar compound, Crocin, alleviates I/R injury by
regulating ER stress and Nrf2/HO-1 signaling (24). Based on these previous studies, it
was hypothesized that CRO could regulate Nrf2/HO-1 signaling and
the UPR in the cardiovascular system. The aim of the present study
was to evaluate whether CRO can protect the heart against I/R
injury by alleviating inflammation via Nrf2/HO-1 signaling and the
UPR.
Materials and methods
Animals
Sprague Dawley rats (male, 3 months old, 250–300 g,
total number: 120) were obtained from The Experimental Animal
Center of Zhejiang Chinese Medical University (Lot: SCXK; Shanghai
2007-0005). The animals were acclimated for 7 days in a controlled
temperature (20–24°C) with 12-h light/dark cycle and free access to
food and water before the experiments. All experiments were
designed according to The National Institutes of Health Guide for
the Care and Use of Laboratory Animals and approved by The Animal
Care Committee of Zhejiang Chinese Medical University. Every effort
was made to minimize the number and suffering of the animals in the
present study.
Langendorff perfusion and I/R
injury
Rats were anesthetized with sodium pentobarbital (50
mg/kg) containing heparin (300 IU) by intraperitoneal injection.
The rats were sacrificed and hearts were immediately harvested and
mounted on the Langendorff system for retrograde perfusion at a
constant pressure of 75 mmHg with oxygenated (95% O2 and
5% CO2) Krebs-Henseleit (KH) buffer (118 mM NaCl, 4.7 mM
KCl, 1.2 mM MgSO4, 1.25 mM CaCl2, 1.2 mM
KH2PO4, 25 mM NaHCO3 and 11 mM
glucose; pH 7.4) as previously described (25). Hemodynamic measurements for heart
rate (HR), maximal rate of the increase of left ventricular
pressure (dp/dtmax) and left ventricular developed
pressure (LVDP) were assessed during the experiment. After 30 min
for system equilibration, cardiac I/R injury was determined by the
hearts experiencing 30 min ischemia (no flow) and 120 min
reperfusion. The CRO treatment group was subjected to 20 min
equilibration followed by 10 min CRO administration before I/R
injury.
Drugs and chemicals
CRO (P0352; purity ≥95%) was purchased from Shanghai
PureOne Biotechnology and dissolved in DMSO (100 mM) for storage,
and then diluted in KH buffer before use. All the chemical reagents
used in the present study were of analytical grade. Rats were
divided into 5 groups (n=8; other rats were used to verify the
success of the model and to test the surgical procedure): i) Sham,
surgery without occlusion; ii) I/R, surgery with 30 min occlusion,
followed by 120 min reperfusion; iii) I/R + CRO (10 µM); iv) I/R +
CRO (20 µM); and v) I/R + CRO (40 µM).
Cell culture and small interfering RNA
(siRNA) interference
The H9c2 cardiomyocyte cell line was obtained from
the American Type Culture Collection (cat. no. CRL1446) and
cultured in DMEM containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified incubator (5% CO2) at
37°C. Control siRNA (cat. no. sc-37007; scrambled sequence) and
Nrf2 siRNA (cat. no. sc-37049) was purchased from Santa Cruz
Biotechnology, Inc., and used according to the manufacturer's
protocol. H9c2 cells were transiently transfected with Nrf2 siRNA
(10 µM) using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.) for 6–12 h. Then, the medium was replaced with
DMEM for the following 48 h.
Infarct size
The cardiac infarct size was determined by
2,3,5-triphenyltetrazolium chloride (TTC) staining, as previously
described (26). The heart was
frozen at −20°C for 15 min, then sliced into five 2 mm-thick
transverse sections and immersed in 1% TTC solution in distilled
deionized water for 15 min at 37°C and then fixed in 4%
paraformaldehyde overnight in 4°C. The viable tissue was stained a
deep red color and the infarcted zone was not stained. The
infarcted size of each sliced heart section was measured and the
percentage of the infarcted zone was calculated using ImageJ 1.48V
(National Institutes of Health), image analyzing software.
Creatine kinase (CK), lactate
dehydrogenase (LDH), superoxide dismutase (SOD), malondialdehyde
(MDA) and GSH-PX (glutathione peroxidase)
SOD, CK, LDH, MDA and GSH-PX production from the
coronary flow were measured using a commercial kit (Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
protocol. In brief, after I/R injury, the coronary blood flow was
collected for SOD, CK, LDH, MDA and GSH-PX determination. The
activities of the control group were considered as 1.
Reverse transcription (RT)-PCR
The heart tissue was lysed and homogenized in 400 µl
lysis buffer (RLT Buffer; Qiagen, Inc.). The total RNA of the
tissue was isolated on spin columns with silica-based membranes
(RNeasy Mini kit; Qiagen, Inc.) according to the manufacturer's
protocol. Then, RNA was reverse transcribed (25°C for 10 min,
following at 50°C for 15 min, terminate the reaction by heating at
85°C for 5 min) in a total volume of 20 µl using the RT
High-Capacity RNA-to-cDNA kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Quantitative PCR was performed with the
FastStart Universal SYBR-Green Master Rox (Roche Diagnostics) using
the ViiA™ 7 real-time PCR system. The cycling protocol was as
follows: 95°C for 2 min, followed by 40 cycles at 95°C for 10 sec
and 60°C for 40 sec. All data were normalized to the housekeeping
gene and the relative expression levels were calculated using the
2−ΔΔCq method (27).
Primer sequences for IL-1α, IL-1β, IL-6, IL-8 and TNF-α were used
to quantify the mRNA levels, as well as β-actin used as an
endogenous control. The primer sequences are listed in Table I.
 | Table I.Primer sequences of IL-1α, IL-1β,
IL-6, IL-8, TNF-α and β-actin. |
Table I.
Primer sequences of IL-1α, IL-1β,
IL-6, IL-8, TNF-α and β-actin.
| Name | Forward | Reverse |
|---|
| IL-1α |
5′-CCTCGTCCTAAGTCACTCGC-3′ |
5′-GGCTGGTTCCACTAGGCTTT-3′ |
| IL-1β |
5′-GCACAGTTCCCCAACTGGTA-3′ |
5′-AAGACACGGGTTCCATGGTG-3′ |
| IL-6 |
5′-CCACCCACAACAGACCAGTA-3′ |
5′-GGAACTCCAGAAGACCAGAGC-3′ |
| IL-8 |
5′-CTGCGCCAACACAGAAATTA-3′ |
5′-ATTGCATCTGGCAACCCTAC-3′ |
| TNF-α |
5′-CAGAGGGAAGAGTTCCCCAG-3′ |
5′-CCTTGGTCTGGTAGGAGACG-3′ |
| β-actin |
5′-GCTACAGCTTCACCACCACA-3′ |
5′-ATCGTACTCCTGCTTGCTGA-3′ |
Cell viability
Cell viability was determined by evaluating the
absorbance of MTT. Cells were cultured in 96-well microplates at a
density of 1×104 cells/ml. After siRNA interference and
I/R injury, cells were incubated with medium containing MTT (500
µg/ml) for 3–4 h in the dark. Then, the MTT solution was aspirated
and DMSO (150 µl) was added for 15 min. The absorbance was detected
at 570 nm on a Multi-Mode Detection Platform (SpectraMax Paradigm;
Molecular Devices, LLC). Cell viability of the control group was
considered as 100%.
Immunoblot analysis
Heart tissue was homogenized in ice-cold RIPA buffer
(Cell Signaling Technology, Inc.) containing protease inhibitors
and incubated on ice for 20 min, followed by centrifugation at
13,000 × g for 10 min at 4°C. The supernatant was collected and the
concentration of protein was quantified using the Bio-Rad protein
assay kit (Bio-Rad Laboratories, Inc.). Proteins (30 µg/lane) of
different groups were loaded and separated by SDS-PAGE on 10% gels
and transferred to a PVDF membrane (pore size: 0.45 µm; EMD
Millipore). The membranes were blocked with 5% bovine serum albumin
in TBS with 0.1% Tween 20 (TBST) for 1 h at room temperature and
incubated with Nrf2 (1:1,000, Cell Signaling Technology, Inc.; cat
no. 12721), HO-1 (1:1,000, Cell Signaling Technology, Inc.; cat.
no. 86806), PERK (1:500, Cell Signaling Technology, Inc.; cat. no.
5683), phosphorylated (p)-PERK (1:500, Cell Signaling Technology,
Inc.; cat. no. 3179), IRE1 (1:1,000, Cell Signaling Technology,
Inc.; cat. no. 3294), X-box binding protein 1 (XBP1; 1:1,000, Cell
Signaling Technology, Inc.; cat no. 40435) and ATF6 (1:1,000; Cell
Signaling Technology, Inc.; cat no. 65880) primary antibodies
overnight at 4°C. β-Actin (1:10,000; Cell Signaling Technology,
Inc.; cat no. 3700) was used as the loading control. After the
primary antibody incubation, the membrane was washed with TBST 3
times (5 min) and incubated with the anti-mouse and anti-rabbit IgG
HRP secondary antibodies (1:10,000; Cell Signaling Technology,
Inc.; cat. nos. 7076, 7074) for 1 h at room temperature. The
intensity of the bands was detected using the ChemiDoc Touch
Imaging System (Bio-Rad Laboratories, Inc.; series no.
732BR2237).
Statistical analysis
All data are presented as the mean ± standard error
of the mean with GraphPad Prism 7.0 (GraphPad Software, Inc.). The
Student's t-test was used to analyze the differences between 2
groups and one-way analysis of variance with multiple comparisons
(using Tukey's test) was used to analyze the differences between 3
or more groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
CRO alleviates myocardial I/R
injury
The present study established a Langendorff
perfusion system, an ex vivo model of I/R, to evaluate the
cardiac protective effect of CRO in rat hearts. CRO was dissolved
in perfusion KH buffer and administered for 10 min before 30 min
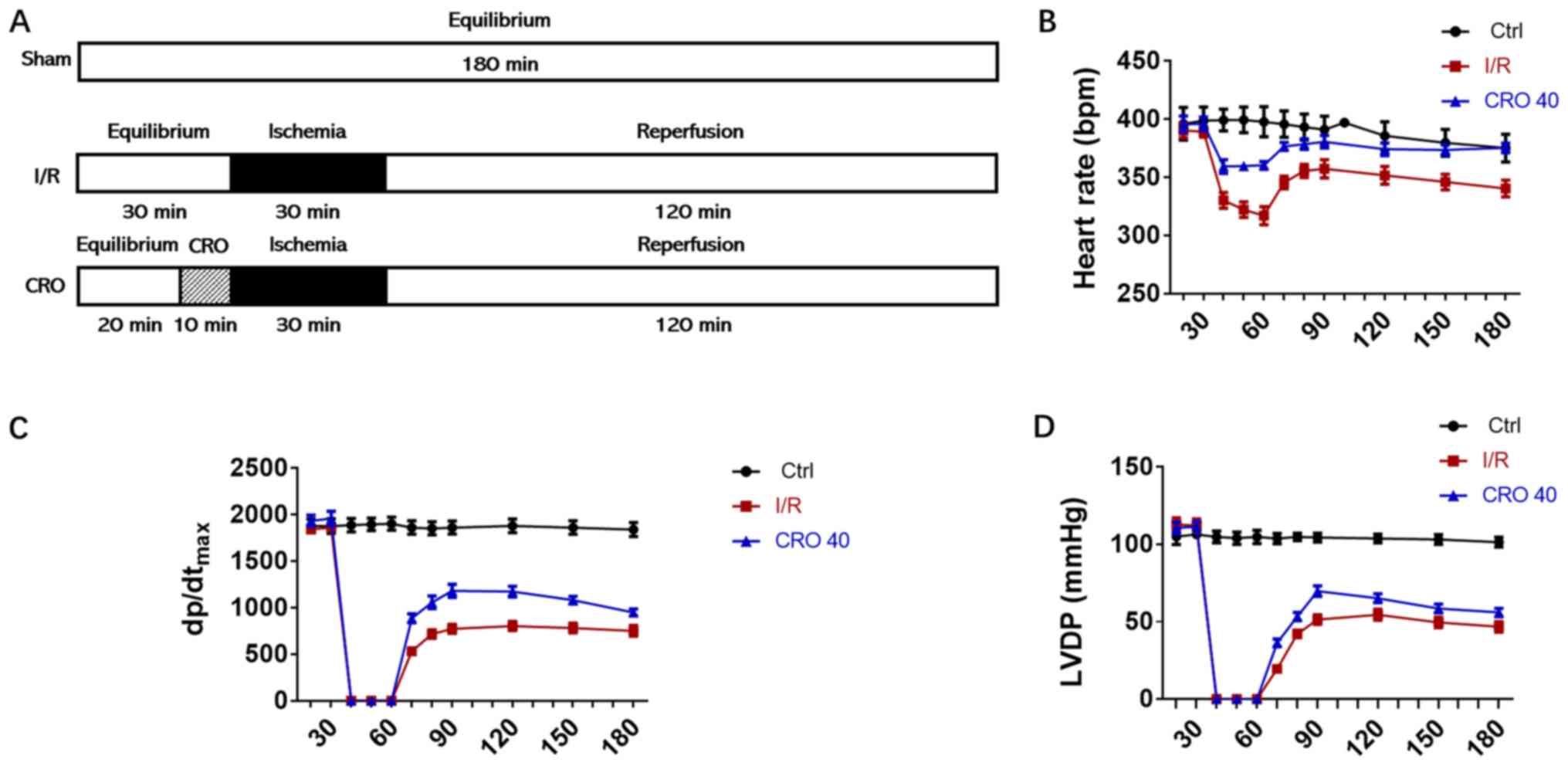
ischemia followed by 120 min reperfusion as shown in Fig. 1A. During the whole process of the
experiment, cardiac functional parameters were recorded and
analyzed. Long-term I/R injury decreased the HR,
dp/dtmax and LVDP. CRO (40 µM) markedly enhanced these
cardiac functional parameters in the period of reperfusion,
suggesting that CRO exhibited cardioprotection in rats (Fig. 1B-D).
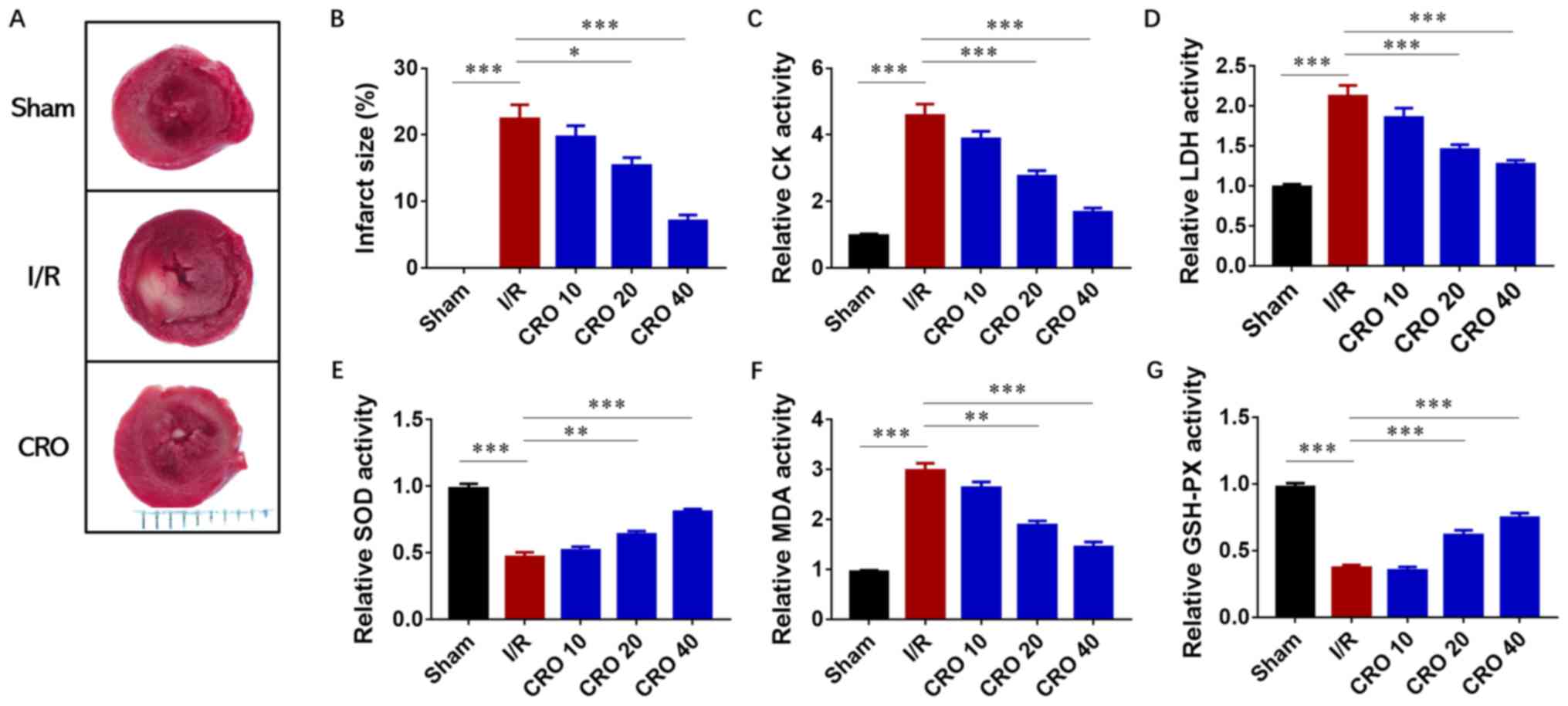
After I/R injury, the heart was harvested for
evaluation of the infarct size and the perfused buffer from the
coronary flow was collected for determination of cardiac enzyme
activities. A significant increase in the infarcted area was
observed in the I/R group, ~20% compared with the sham group
(P<0.001). Dose-dependent decreases in infarct size were
identified in the CRO pretreatment group, particularly in the CRO
40 group in which the infarct size was <10% (Fig. 2A and B). Then, the activities of
two enzymes, CK and LDH, were analyzed, which represented cardiac
toxicity in the perfused buffer from the coronary flow. Both CK and
LDH displayed downregulation in I/R hearts pretreated with CRO,
suggesting that CRO could reduce cardiac toxicity from I/R injury
(Fig. 2C and D). The antioxidant
effect of CRO was further evaluated by determining the activities
of SOD, MDA and GSH-PX. The changes of these three enzymes were
significant in the I/R group and CRO regulated their activities in
a dose-dependent manner (P<0.001; Fig. 2E-G). Collectively, these
observations demonstrated that CRO exhibited a protective effect to
alleviate cardiac I/R injury.
CRO alleviates myocardial I/R injury
via regulation of inflammation
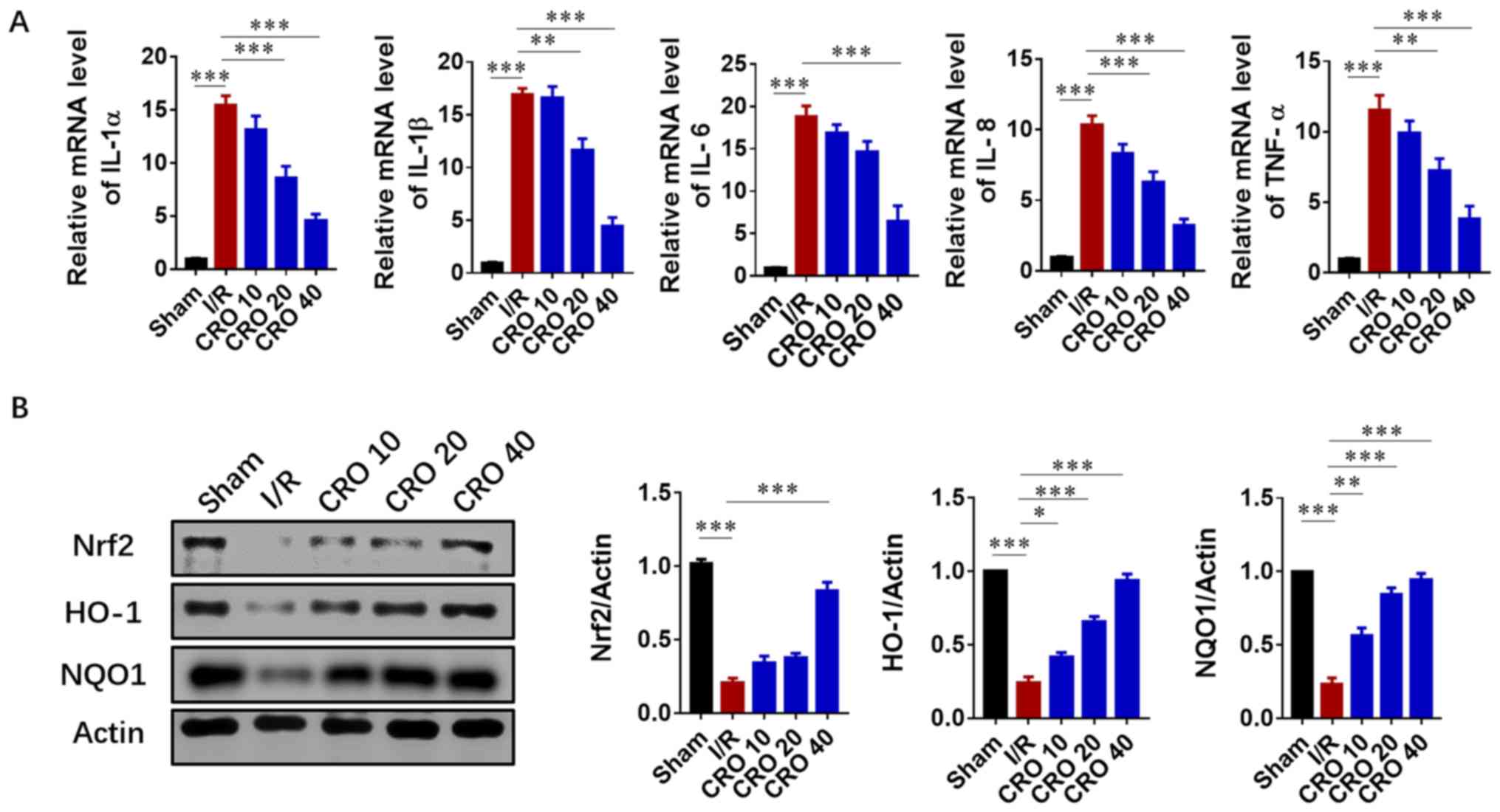
As the inflammatory response accelerated tissue
injury in the reperfusion period after the lethal ischemic damage,
the levels of pro-inflammatory cytokines were detected. Cardiac
mRNA levels of IL-1α, IL-1β, IL-6, IL-18 and TNF-α significantly
increased in the I/R group (P<0.001). CRO significantly reduced
the mRNA expression of these pro-inflammatory cytokines in a
dose-dependent manner (P<0.001; Fig. 3A). Nrf2 is sensitive to oxidative
stress and activates the transcription of HO-1 and NAD(P)H: Quinine
oxidoreductase 1 (NQO1) in I/R injury (15). Nrf2-mediated signaling plays a key
role in the innate immune/inflammatory pathway and regulates
pro-inflammatory biomarkers, including IL-1 and TNF-α (28). Due to the importance of Nrf2/HO-1
signaling in the inflammatory response induced by I/R, their
protein expression was evaluated. I/R injury significantly reduced
the protein expression of Nrf2, HO-1 and NQO1, and CRO upregulated
their expression in a dose dependent fashion (P<0.001; Fig. 3B). These results suggested that CRO
could attenuate cardiac I/R injury via Nrf2/HO-1-mediated
anti-inflammation signaling.
CRO alleviates myocardial I/R injury
via regulation of UPR signaling
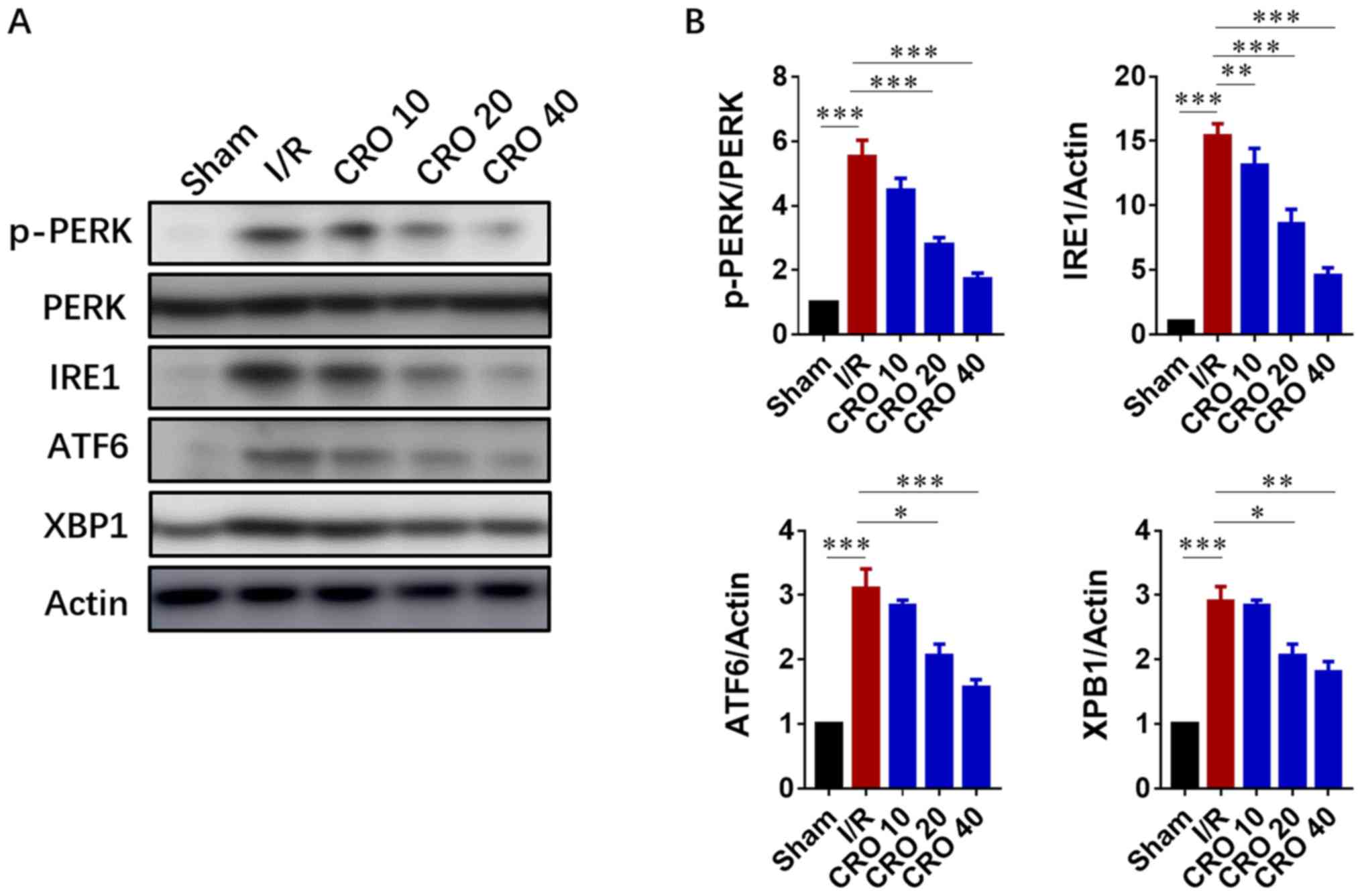
The UPR is composed of three principle branches;
IRE1, PERK and ATF6. The IRE1 branch is the central branch of the
UPR; its activation cleaves the mRNA of XBP-1 and activates XBP-1
to translocate to the nucleus for transcription of genes involved
in ER stress (16,17). The UPR has been demonstrated to
become activated when myocardial reperfusion occurs. Drugs with
cardioprotective effects are demonstrated to attenuate the toxic
UPR (29). Due to the important
role of the UPR in I/R injury, immunoblot analysis was performed to
evaluate the expression of proteins in the UPR system. The results
showed that expression of p-PERK, IRE1, ATF6 and XBP-1
significantly increased in cells induced by I/R, suggesting I/R
injury activated ER-stress signaling (P<0.001; Fig. 4). Pretreatment with CRO
significantly decreased the expression levels of these proteins,
displaying a beneficial effect by downregulating ER-stress
signaling (P<0.05; Fig. 4).
CRO alleviates myocardial I/R injury
possibly in a Nrf2-dependent manner
Nrf2 transcription activated antioxidant and
anti-inflammatory gene expression against cardiac I/R injury.
Therefore, it was further evaluated whether CRO protected the heart
against I/R injury in a Nrf2-dependent manner. An in vitro
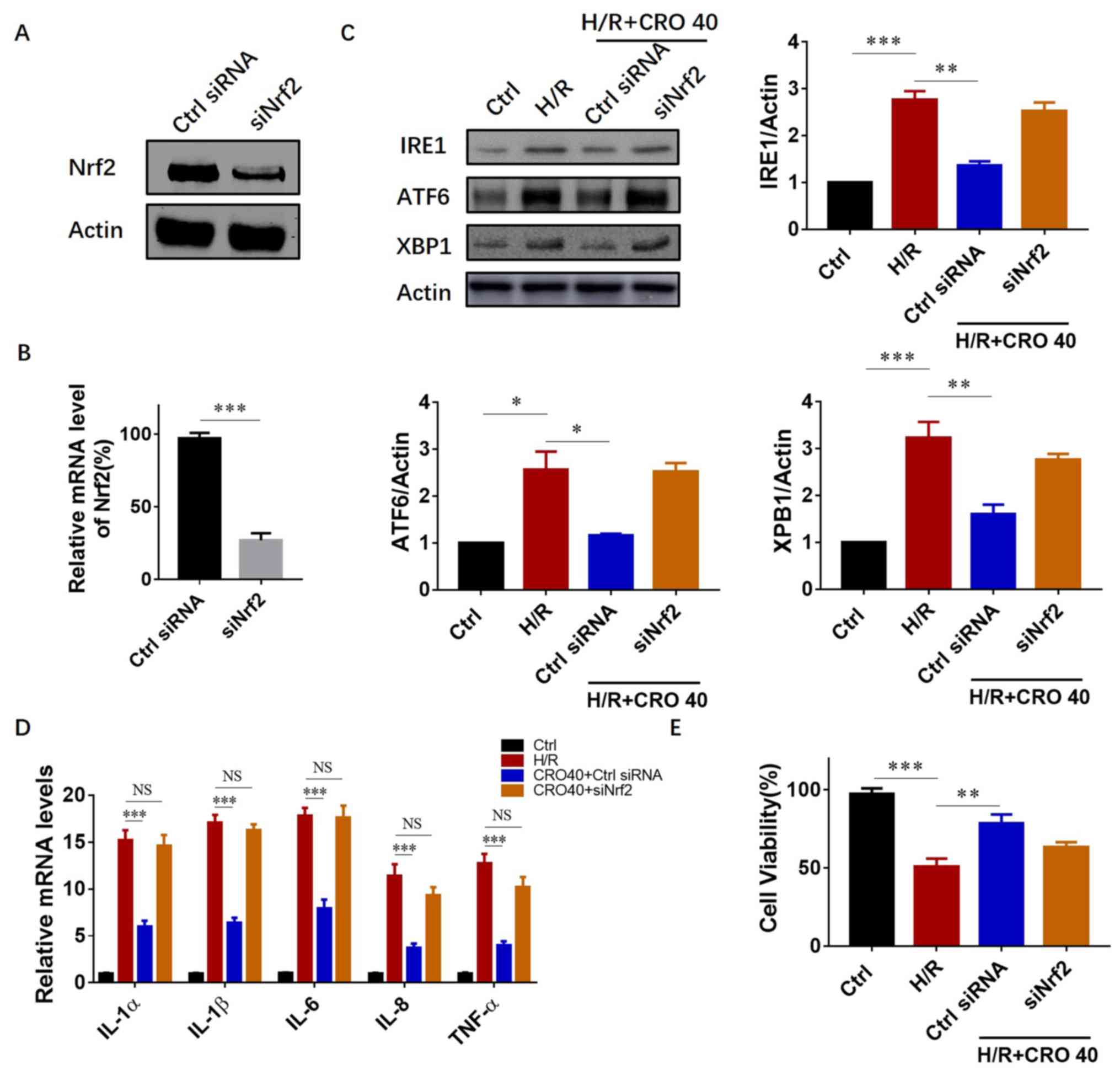
system was first applied using Nrf2 siRNA to downregulate Nrf2
expression in H9c2 cardiomyocytes. The results showed that both the
protein and mRNA expression reduced >1/2 after 54–60 h siRNA
treatment (Fig. 5A and B).
Subsequently, it was investigated whether downregulation of UPR
protein expression by CRO pretreatment was dependent on Nrf2
activation. In the group with control siRNA (non-target) treatment,
CRO pretreatment significantly reduced the expression of IRE1, ATF6
and XBP1. However, in the group with Nrf2 siRNA, the expression of
IRE1, ATF6 and XBP1 did not reduce even with CRO pretreatment,
suggesting that CRO attenuated the I/R-induced UPR in a
Nrf2-dependent manner (Fig. 5C).
The mRNA levels of pro-inflammatory cytokines were also measured
and the results clearly showed that deficiency of Nrf2 suppressed
the anti-inflammatory ability of CRO (Fig. 5D). From these results, it was
determined whether reduction of Nrf2 expression inhibited the
cardioprotective effect of CRO. An MTT assay was performed to
evaluate cell survival and the results showed that CRO
significantly increased cell viability in H9c2 cells induced by
I/R. In the group with Nrf2 siRNA, CRO could not enhance cell
survival, suggesting that the cardioprotective effect of CRO
required Nrf2 activation (Fig.
5E).
Discussion
The present study provided insight into the
cardioprotective effect of CRO, a natural compound and its
functional mechanisms. A classical ex vivo model of cardiac
I/R injury was used, termed the Langendorff perfusion system, to
establish a working experimental evaluation system. The limitation
of the animal experiment is lack of an in vivo
pharmacological study. In further pharmacological evaluation, the
authors are going to perform I/R injury in a whole animal and study
the protective effect and concentration of CRO. Moreover,
histological analysis of an in vivo sample can also be
collected to evaluate the pathological parameters.
Classical mechanical hypotheses suggest that CRO
protected against I/R injury in the brain and heart via an
antioxidant response. However, previous studies demonstrated that
inflammation served an important role in reperfusion injury,
causing a secondary cascade of lethal damage (2,12).
Therefore, it was suggested that CRO had the ability to reduce the
inflammatory response induced by reperfusion injury. In recent
years, the UPR system, also mitochondrial UPR, has become a very
important cellular adaptive system to regulate the stress-induced
signaling pathway, especially in I/R injury (18,30).
Based on the beneficial effect of CRO to the heart and previous
references (21–23), it was hypothesized that CRO can
regulate the inflammation response and the UPR system.
The present results demonstrated that CRO
upregulated Nrf2/HO-1 signaling to reduce the inflammation response
and downregulated UPR protein activities. As Nrf2 is a
transcription factor, it activates a series of anti-inflammatory
molecules and a previous study showed loss of Nrf2 reduced the
activities of ER stress-related protein expression (24). Therefore, siRNA was used to
downregulate Nrf2 expression, to determine whether Nrf2 was
essential for CRO to display its cardiac protective effects. The
present results found that the cardioprotective effect of CRO was
reduced in Nrf2-deficient cells, indicating that CRO protected
cardiac functions in a Nrf2-dependent manner, including its
regulation of UPR signaling. However, the relationship between the
Nrf2-mediated inflammatory response and UPR signaling remains
unknown. Additionally, further investigation is required to
elucidate the underlying mechanism of CRO and its Nrf2-specific
binding domains and whether overexpressed Nrf2 may protect the
heart from I/R injury via downregulation of UPR activation or
conversely, whether downregulation of UPR activation may affect
Nrf2/HO-1 signaling activation by CRO. Future studies may consider
whether CRO could be applied to treat other cardiomyopathies and
reperfusion-induced cellular toxicity.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
Traditional Chinese Medicine Key Research Project (grant no.
2014ZZ004) and The National Natural Science Foundation of China
(grant no. 81771520).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MY designed and finished most of the experiments of
this project. GM provided general support in experiments and
analyzed part of data. LO and CS helped to feed and prepare
animals, and also performed some of the biochemical kit
measurements. PH and SH provided funding, controlled the progress
of this study and made substantial contributions to the conception
of this work. In addition, SH drafted the manuscript.
Ethics approval and consent to
participate
All experiments were designed according to The
National Institutes of Health Guide for the Care and Use of
Laboratory Animals and approved by The Animal Care Committee of
Zhejiang Chinese Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frank A, Bonney M, Bonney S, Weitzel L,
Koeppen M and Eckle T: Myocardial ischemia reperfusion injury: From
basic science to clinical bedside. Semin Cardiothorac Vasc Anesth.
16:123–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhalla NS, Elmoselhi AB, Hata T and Makino
N: Status of myocardial antioxidants in ischemia-reperfusion
injury. Cardiovasc Res. 47:446–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bolli R: Cardioprotective function of
inducible nitric oxide synthase and role of nitric oxide in
myocardial ischemia and preconditioning: An overview of a decade of
research. J Mol Cell Cardiol. 33:1897–1918. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garlid KD, Dos Santos P, Xie ZJ, Costa AD
and Paucek P: Mitochondrial potassium transport: The role of the
mitochondrial ATP-sensitive K(+) channel in cardiac function and
cardioprotection. Biochim Biophys Acta. 1606:1–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kingery JR, Hamid T, Lewis RK, Ismahil MA,
Bansal SS, Rokosh G, Townes TM, Ildstad ST, Jones SP and Prabhu SD:
Leukocyte iNOS is required for inflammation and pathological
remodeling in ischemic heart failure. Basic Res Cardiol.
112:192017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen QM and Maltagliati AJ: Nrf2 at the
heart of oxidative stress and cardiac protection. Physiol Genomics.
50:77–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jakobs P, Serbulea V, Leitinger N, Eckers
A and Haendeler J: Nuclear factor (Erythroid-Derived 2)-like 2 and
thioredoxin-1 in atherosclerosis and ischemia/reperfusion injury in
the heart. Antioxid Redox Signal. 26:630–644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji Q, Gao J, Zheng Y, Liu X, Zhou Q, Shi
C, Yao M and Chen X: Inhibition of microRNA-153 protects neurons
against ischemia/reperfusion injury in an oxygen-glucose
deprivation and reoxygenation cellular model by regulating
Nrf2/HO-1 signaling. J Biochem Mol Toxicol. 31:2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zu G, Zhou T, Che N and Zhang X:
Salvianolic acid A protects against oxidative stress and apoptosis
induced by intestinal ischemia-reperfusion injury Through
activation of Nrf2/HO-1 pathways. Cell Physiol Biochem.
49:2320–2332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong F and Zhou X: The Nrf2/HO-1 pathway
mediates the antagonist effect of L-arginine on renal
ischemia/reperfusion injury in rats. Kidney Blood Press Res.
42:519–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Hu X and Jiang H: ERS-PERK
signaling pathway-mediated Nrf2/ARE-HO-1 axis: A novel therapeutic
target for attenuating myocardial ischemia and reperfusion injury.
Int J Cardiol. 203:779–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng L, Jin Z, Zhao R, Ren K, Deng C and
Yu S: Resveratrol attenuates inflammation and oxidative stress
induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE
pathway. Int J Clin Exp Med. 8:10420–10428. 2015.PubMed/NCBI
|
|
15
|
Zeng X, Li J and Li Z: Ginsenoside Rd
mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1
signaling pathway. Int J Clin Exp Med. 8:14497–1504.
2015.PubMed/NCBI
|
|
16
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee J and Ozcan U: Unfolded protein
response signaling and metabolic diseases. J Biol Chem.
289:1203–1211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Tang Y, Li Y, Xie L, Zhuang W,
Liu J and Gong J: Unfolded protein response plays a critical role
in heart damage after myocardial ischemia/reperfusion in rats. PLoS
One. 12:e01790422017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Li Y and Ge Z: Cardiaprotective
effect of crocetin by attenuating apoptosis in isoproterenol
induced myocardial infarction rat model. Biomed Pharmacother.
93:376–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalalinia F, Ghasim H, Amel Farzad S,
Pishavar E, Ramezani M and Hashemi M: Comparison of the effect of
crocin and crocetin, two major compounds extracted from saffron, on
osteogenic differentiation of mesenchymal stem cells. Life Sci.
208:262–267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Z, Nan C, Wang H, Su Q, Xue W, Chen
Y, Shan X, Duan J, Chen G and Tao W: Crocetin ester improves
myocardial ischemia via Rho/ROCK/NF-kappaB pathway. Int
Immunopharmacol. 38:186–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishizuka F, Shimazawa M, Umigai N,
Ogishima H, Nakamura S, Tsuruma K and Hara H: Crocetin, a
carotenoid derivative, inhibits retinal ischemic damage in mice.
Eur J Pharmacol. 703:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tashakori-Sabzevar F, Hosseinzadeh H,
Motamedshariaty VS, Movassaghi AR and Mohajeri SA: Crocetin
attenuates spatial learning dysfunction and hippocampal injury in a
model of vascular dementia. Curr Neurovasc Res. 10:325–334. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Yuan B, Cheng B, Liu Y, Zhang B,
Wang X, Lin X, Yang B and Gong G: Crocin alleviates myocardial
ischemia/reperfusion-induced endoplasmic reticulum stress via
regulation of miR-34a/Sirt1/Nrf2 pathway. Shock. 51:123–130. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Facundo HT, Carreira RS, de Paula JG,
Santos CC, Ferranti R, Laurindo FR and Kowaltowski AJ: Ischemic
preconditioning requires increases in reactive oxygen release
independent of mitochondrial K+ channel activity. Free Radic Biol
Med. 40:469–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Liu Y, Ren X, Zhou H, Wang K,
Zhang H and Luo P: Caffeoylquinic acid derivatives extract of
erigeron multiradiatus alleviated acute Myocardial ischemia
reperfusion injury in rats through inhibiting NF-KappaB and JNK
activations. Mediators Inflamm. 2016:79619402016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:4022001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Y, Li W, Su ZY and Kong AN: The
complexity of the Nrf2 pathway: Beyond the antioxidant response. J
Nutr Biochem. 26:1401–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takatori O, Usui S, Okajima M, Kaneko S,
Ootsuji H, Takashima SI, Kobayashi D, Murai H, Furusho H and
Takamura M: Sodium 4-phenylbutyrate attenuates myocardial
reperfusion injury by reducing the unfolded protein response. J
Cardiovasc Pharmacol Ther. 22:283–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bi X, Zhang G, Wang X, Nguyen C, May HI,
Li X, Al-Hashimi AA, Austin RC, Gillette TG, Fu G, et al:
Endoplasmic reticulum chaperone GRP78 protects heart from
ischemia/reperfusion injury through Akt activation. Circ Res.
122:1545–1554. 2018. View Article : Google Scholar : PubMed/NCBI
|