Introduction
Renal ischemia/reperfusion (I/R) injury is a leading
cause of acute kidney injury (AKI) and is associated with severe
morbidity and mortality in both developing and developed countries
(1). A number of mechanisms have
been reported to enhance the susceptibility of elderly patients to
AKI (2). Diabetes mellitus (DM) is
a metabolic disorder associated with a multitude of clinical
syndromes, including atherosclerosis (3). In addition, DM is associated with a
number of severe pathological risks, including the development of
AKI (3,4). Evidence has accumulated supporting
the fact that DM aggravates renal I/R injury (5). Experimental studies have revealed
that diabetic rats develop renal dysfunction faster following IR
injury compared with non-diabetic rats (6,7).
Other research has revealed that progressive hyperglycemia can
cause increased levels of reactive oxygen species (ROS) in the
diabetic kidney following I/R injury (8). However, little is known concerning
the specific mechanisms responsible for how diabetes can result in
an increased vulnerability to I/R injury.
The endoplasmic reticulum (ER) is an intracellular
organelle that plays a key role in protein homeostasis, including
protein folding, processing, conveyancing and degradation (9). However, the ER is also susceptible to
a range of stressors, including hypoxia, Ca2+ overload,
I/R and ROS (10). Stimulation of
the ER by one or more of these stimuli can cause the production of
numerous unfolded or misfolded proteins and the initiation of the
unfolded protein response (UPR), eventually leading to the
activation of ERS (11). Three UPR
pathways have been described, each named after a transmembrane
regulator: Inositol-requiring enzyme 1 α (IRE1α), protein kinase
R-like ER kinase (PERK) and activating transcription factor 6
(ATF6) (12). A large body of data
has also demonstrated that renal I/R injury can induce ERS and
cause AKI (13–15). In addition, in animal models of
diabetes, hyperglycemia can induce the glycation of proteins and
thus impose an enormous burden on the ER with regards to the
abnormal refolding of misfolded or unfolded proteins; this can
result in ERS-induced apoptosis (16). It was therefore hypothesized that
there is a relationship between ERS and I/R injury in the diabetic
kidney. Pyroptosis manifests as a type of inflammatory programmed
cell death induced by inflammatory caspases and exhibits
morphological characteristics that are common to apoptosis and
necrosis (17). However, unlike
necrosis or apoptosis, pyroptosis results in the activation of
pro-inflammatory mediators that are triggered by the release of
cytokines (18). Initially,
pyroptosis activates caspase-1 and then induces the production of
the inflammatory cytokine interleukin-1β (IL-1β) (19). Notably, pyroptosis plays a key role
during renal I/R injury; the overactivation of ERS via the
CHOP/caspase-11 signaling pathway has also been revealed to produce
a significant contribution to this process (20).
Sirtuin 1 (SIRT1) is an NAD+-dependent
histone deacetylase that uses deacetylating multiple factors to
regulate a variety of biological processes, including cell
metabolism, gene transcription, immunological response and glucose
homeostasis (21,22). A recent study demonstrated that
SIRT1 can protect renal function by physically interacting with and
deacetylating eIF2α at lysine (K143) residues (23). This action inhibits the
PERK-eIF2α-ATF4/CHOP axis, thus attenuating ERS-mediated apoptosis
(23). Furthermore, it has been
reported that diabetic rats exhibit reduced levels of SIRT1
signaling (7,24). Notably, research has revealed that
SIRT1 is downregulated by I/R injury and that the overactivation of
SIRT1 attenuates I/R-induced myocardial damage (7,25).
However, whether SIRT1 signaling is downregulated in
diabetes-exacerbated renal I/R injury, and whether ERS is involved
in this process, remains unknown.
The present study aimed to investigate the potential
mechanisms mediating diabetes-aggravated renal I/R injury with
specific emphasis on SIRT1 signaling and its association with ERS,
and the potential role of pyroptosis.
Materials and methods
Animal models
A total of 30 adult male Sprague-Dawley (SD) rats,
aged 6–8 weeks, weighing 220–250 g, were obtained from the Center
of Experimental Animals in the Medical College of Wuhan University.
The animals were placed in a room with a temperature of 23±3°C and
relative humidity of 40–70%, with 12 h day/night cycles. They were
provided with water and a standard diet ad libitum. This
study was approved by the Ethics Committee of Renmin Hospital of
Wuhan University and all procedures complied with the Principles of
Animal Care of Wuhan University (Wuhan, China) and Guide for the
Care and Use of Laboratory Animals.
Materials
Streptozotocin (STZ), dimethyl sulfoxide (DMSO) and
resveratrol were purchased from Sigma-Aldrich; Merck KGaA. Primary
antibodies against SIRT1 (cat. no. DF6033), p-PERK (cat. no.
DF7576), p-eIF2α (cat. no. AF3087), ATF4 (cat. no. AF5416), CHOP
(cat. no. DF6025), IL-1β (cat. no. DF6251), caspase-1 (cat. no.
AF5418) and IL-18 (cat. no. DF6252) were purchased from Affinity
Biosciences. Primary antibody against NLRP3 (cat. no. 19771-1-AP)
was purchased from ProteinTech Group, Inc. Primary antibodies
against PERK (cat. no. ab77654) and eIF2α (cat. no. ab169528) were
purchased from Abcam. A primary antibody against caspase-11 (cat.
no. sc-56038) was purchased from Santa Cruz Biotechnology, Inc. A
primary antibody against β-actin (cat. no. BM0627), goat anti-mouse
(cat. no. BA1051) and goat anti-rabbit (cat. no. BA1054) secondary
antibodies were purchased from Boster Biological Technology.
Type 1 diabetic rat model
STZ [60 mg/kg, dissolved in 0.1 M citrate buffer (pH
4.5)] was administered to each experimental rat by intraperitoneal
(i.p.) injection (26). Prior to
injection, all rats were fasted for 12 h. Normal rats received an
i.p. injection with the same dose of citrate buffer. Commencing 72
h after the injection of STZ, blood glucose levels were measured in
the tail vein of rats for 3 days in succession. Only rats
exhibiting hyperglycemia (a fasting blood glucose level above 16.7
mmol/l) were ultimately regarded as having diabetes (26).
Induction of renal
ischemia/reperfusion injury
Rats were anaesthetized (i.p.) with pentobarbital
(45 mg/kg body weight) and placed on a thermostat to maintain a
body temperature of 37°C during surgery. In each rat, the kidneys
were first exposed through a midline abdominal incision. Then, the
right kidney was resected. Subsequently, the left renal pedicle was
clamped for 45 min using non-invasive vascular forceps and then the
clamp was removed for 24 h to allow reperfusion.
Experimental groups and protocol
The diabetic model was established in 5 weeks.
Diabetic and non-diabetic rats were then randomly divided into 5
groups (6 rats per group): i) The non-diabetic sham group (NS); ii)
the non-diabetic I/R group (NI/R); iii) the diabetic sham group
(DS); iv) the diabetic I/R group (DI/R); v) the diabetic I/R +
resveratrol group (DI/R+Res). In the sham groups, right nephrectomy
was only performed. In the I/R groups, I/R injury was induced, as
aforementioned. Resveratrol is a well-known agonist of SIRT1
(27); rats in the DI/R+Res group
were injected with resveratrol (dissolved in DMSO and delivered
with saline and 30% ethanol) at a dose of 10 mg/kg body-weight
(i.p.) per day for one week; 30 min before surgery, these rats were
administered an injection (i.p.) of the same dose (28). Rats in the other groups were
injected (i.p.) with the same volume of DMSO on the same
timescale.
Renal function and histological
examination
Blood samples were obtained 24 h after reperfusion
to allow the determination of serum blood urea nitrogen (BUN) and
serum creatinine (Scr) using a spectrophotometer (Jiancheng
Biotech). Calculation of these parameters allowed renal function to
be assessed. Renal tissue samples were fixed in 4% paraformaldehyde
at 4°C for 6 h, embedded in paraffin and sectioned at a thickness
of 4 µm. Then, the sections were deparaffinized in dimethylbenzene
at 60°C and hydrated in ethanol (100% twice, 95% twice, 75% twice,
distilled water). Following this the sections were stained with
hematoxylin and eosin (H&E; 4 min with hematoxylin and 4 min
with eosin at room temperature) in order to assess
histopathological kidney injury. Two experienced renal pathologists
then used the sections to independently assess morphological
changes. The severity of injury to the renal tubules was defined
with 5 grades (0–4): 0, no evident visible injury; 1, injury
<25%; 2, injury between 25–50%; 3, injury between 50–75%; and 4,
injury >75% (29).
Western blot analysis
Samples of rat kidneys were collected and
snap-frozen in liquid nitrogen. Total proteins were then extracted
from these tissues using RIPA lysis buffer (Beyotime Institute of
Biotechnology). The bicinchoninic acid (BCA) method was used to
quantify protein levels prior to western blotting. In brief,
protein samples (40 µg/lane) were separated on SDS-PAGE gels (5%
separating, 10% stacking gel) and then transferred to PVDF
membranes. Subsequently, PVDF membranes were blocked with 5%
non-fat milk for 2 h and then incubated at 4°C overnight with
specific antibodies against SIRT1 (1:1,000), p-PERK (1:2,000), PERK
(1:2,000), p-eIF2α (1:1,000), eIF2α (1:1,000), ATF4 (1:100), CHOP
(1:1,000), IL-1β (1:2,000), caspase-1 (1:1,000), caspase-11
(1:200), IL-18 (1:1,000), NLRP3 (1:1,000) and β-actin (1:200). The
next morning, the PVDF membranes were washed three times with TBST
and then incubated with secondary antibodies (horseradish
peroxidase-conjugated goat anti-mouse and goat anti-rabbit;
1:50,000) for 2 h at 37°C. Specific bands were detected by ECL™
(Beijing Pierce Biotechnology) and band densities were quantified
using ImageJ software (v1.8.0; National Institutes of Health).
Statistical analysis
All data are expressed as the mean ± SEM.
Statistical analyses involved one-way ANOVA and Tukey's multiple
comparisons tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Body weight and fasting blood glucose
levels of normal and diabetic rats prior to renal IR injury
Five weeks after establishing the diabetic model,
the diabetic rats exhibited characteristic symptoms of
hyperglycemia, including polydipsia, polyphagia, polyuria and
weight loss, compared with non-diabetic rats (Fig. 1). The blood glucose level of
diabetic rats was significantly higher than that in non-diabetic
rats, and the body weight of diabetic rats was significantly
reduced (P<0.05).
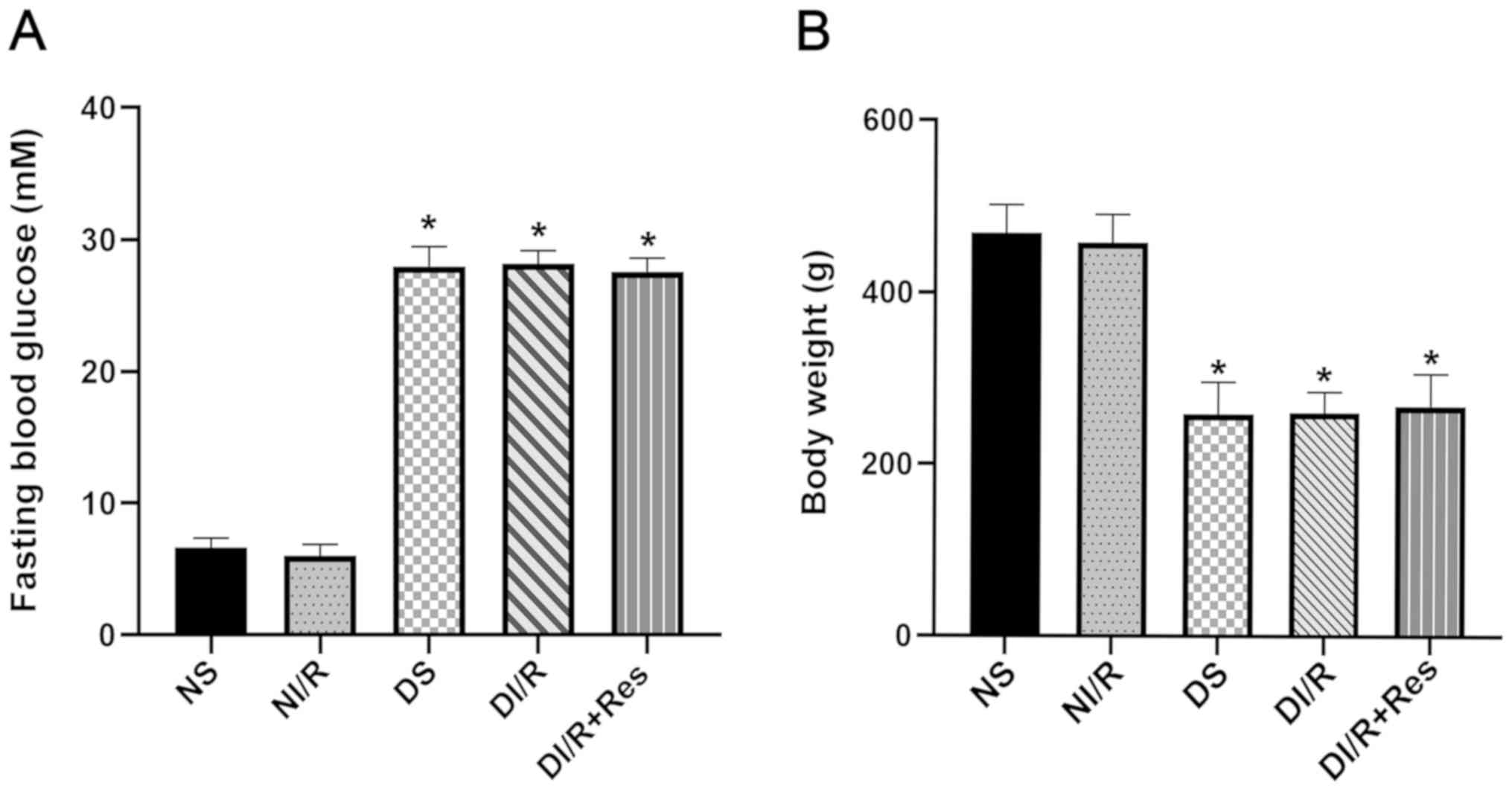 | Figure 1.Comparison of fasting blood glucose
levels and body weights between diabetic and non-diabetic rats.
Five weeks after establishing the diabetic model, (A) fasting blood
glucose levels and (B) body weights of diabetic and non-diabetic
rats were assessed. The results are expressed as the mean ± SEM,
n=6. *P<0.05 vs. NS group and NI/R group. NS and DS groups
received a sham operation. The left renal pedicles of rats in the
NI/R and DI/R groups were clamped for 45 min with non-invasive
vascular forceps followed by 24 h of unclamping for reperfusion.
Rats in the DI/R+Res group received daily i.p. injections of
resveratrol (10 mg/kg of body weight dissolved in DMSO and
delivered with saline and 30% ethanol.) for one week followed by
another injection (i.p.) of the same dose 30 min before surgery.
The groups assessed were as follows: NS, the non-diabetic sham
group; NI/R, the non-diabetic I/R group; DS, the diabetic sham
group; DI/R, the diabetic I/R group; DI/R+Res, the diabetic I/R +
resveratrol group. I/R, ischemia/reperfusion; i.p.,
intraperitoneal; SEM, standard error of the mean. |
Analyses of histopathology and renal
function indicates that DM significantly aggravates renal I/R
injury
The rats in each group underwent either a sham
operation or experienced ischemia for 45 min followed by
reperfusion for 24 h. As revealed in Fig. 2A and B, I/R injury significantly
increased the tubular injury score which was used to evaluate the
degree of kidney injury in both NI/R and DI/R groups (P<0.05,
compared with the NS group). Notably, renal pathological changes in
the NI/R, DS, and DI/R groups exhibited significant damage in the
renal tubules, as evidenced by the loss of brush border, swelling
in the tubular epithelial cells and expansion of the interstitium
(P<0.05). Most importantly, the DI/R group exhibited
significantly more aggravated tissue damage than the NI/R group
(P<0.05). As revealed in Fig. 2C
and D, I/R injury-induced renal dysfunction led to a
significant increase in the levels of Scr and BUN (P<0.05);
these are parameters that are normally used to reflect renal
function. Significantly higher levels of BUN and serum creatinine
were observed in the DI/R group than the NI/R group (P<0.05),
demonstrating that the damage caused by IR was more severe in the
DI/R group. These results illustrated that DM further aggravated
I/R-induced damage in the kidney.
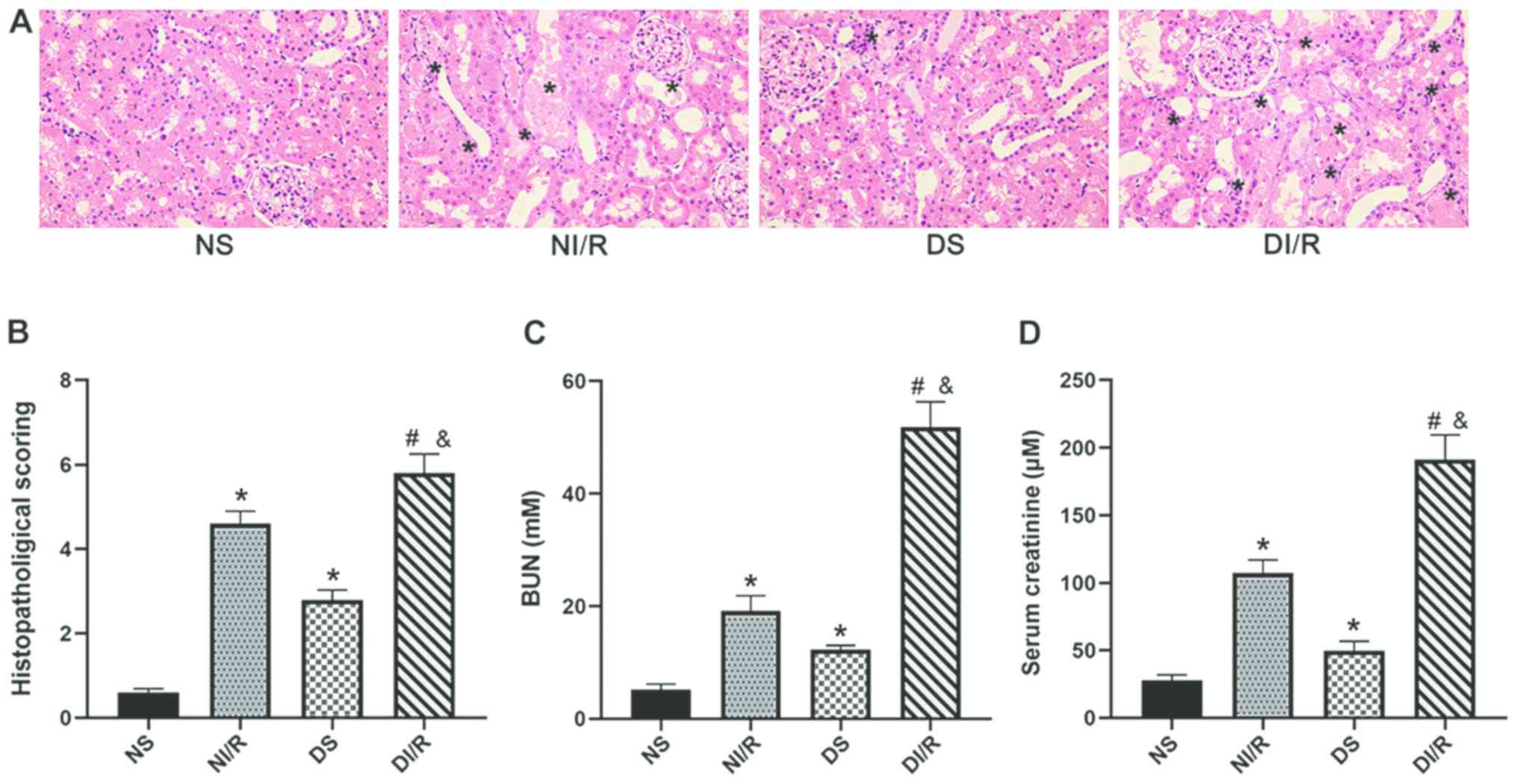 | Figure 2.Rats were subjected to sham surgery
or renal I/R injury. Twenty-four hours after reperfusion, serum and
kidneys were collected for urea and creatinine testing and H&E
staining. (A) H&E staining of kidney sections (magnification,
×400); ‘*’ symbol in the images of this part represents the
pathological changes in the kidney, including tubular epithelial
cell swelling, interstitial expansion, intertubular hemorrhaging
and necrotic tubules. (B) Histopathological scoring. (C) Serum BUN
concentration. (D) Serum creatinine concentration. Data are
presented as the mean ± SEM, n=6. *P<0.05 vs. NS group;
#P<0.05 vs. DS group; &P<0.05 vs.
NI/R group. The groups assessed were as follows: NS, the
non-diabetic sham group; NI/R, the non-diabetic I/R group; DS, the
diabetic sham group; DI/R, the diabetic I/R group. I/R,
ischemia/reperfusion; H&E, hematoxylin and eosin; BUN, blood
urea nitrogen; SEM, standard error of the mean. |
DM exacerbates renal I/R injury by
enhancing ERS
The ERS response in diabetic and non-diabetic rats
when subjected to renal I/R injury was next investigated by
assessing PERK/eIF2α/ATF4-mediated renal ERS. As revealed in
Fig. 3A-E, the expression levels
of p-PERK, p-eIF2α, ATF4, and CHOP were significantly higher in the
diabetic kidney compared with the NS group (P<0.05). The DI/R
group exhibited further exacerbation in terms of the ERS response
compared with the NI/R group (P<0.05). These data indicated that
an enhancement in ERS may be involved in the aggravation of renal
I/R injury in DM rats.
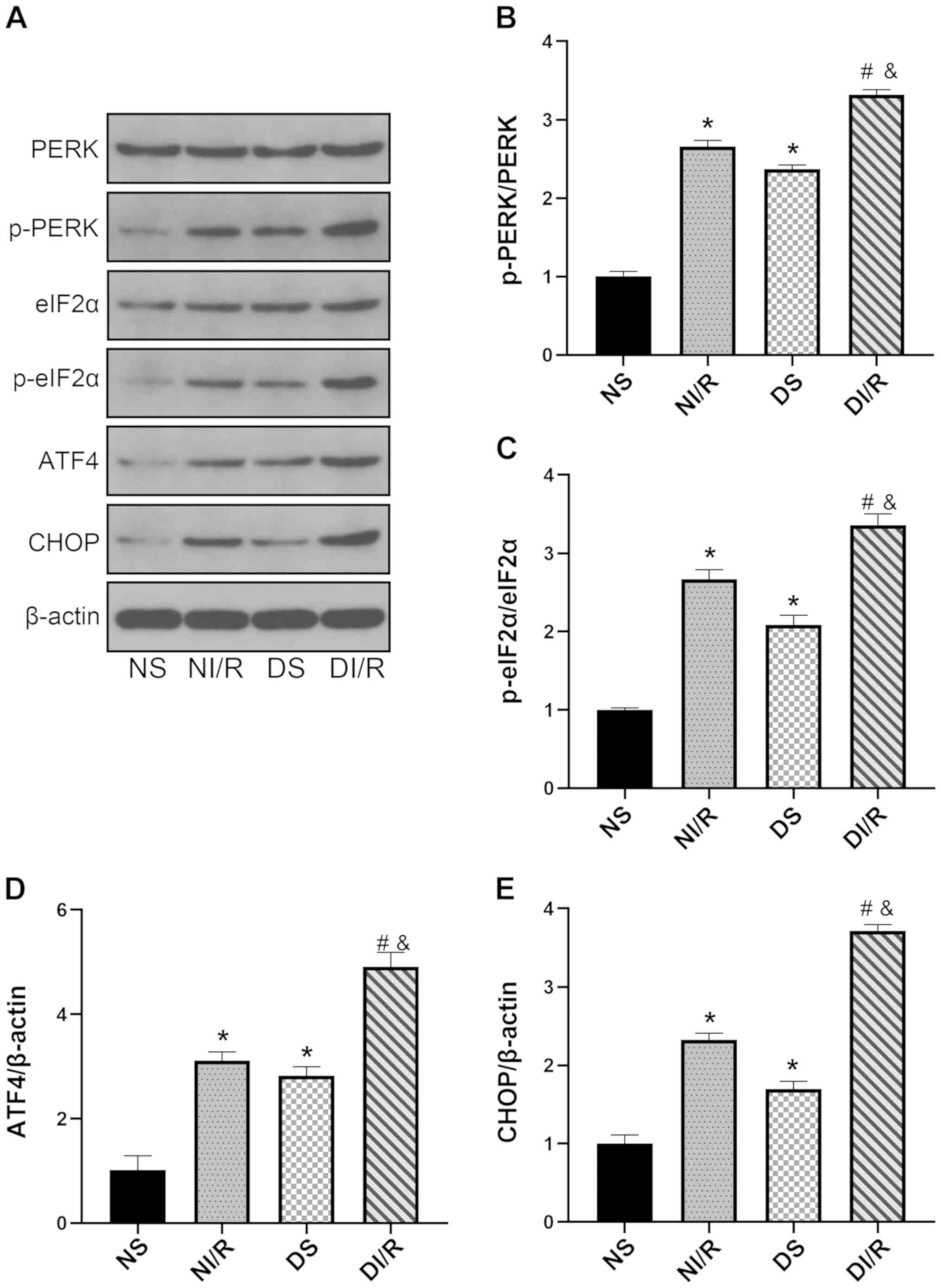 | Figure 3.DM enhances endoplasmic reticulum
stress. Western blot analysis was performed after 24 h of
reperfusion. (A) Representative blots and histograms showing (B)
p-PERK/PERK ratio; (C) p-eIF2α/eIF2α ratio; (D) ATF4 expression;
and (E) CHOP expression. The results are expressed as the mean ±
SEM, n=6. *P<0.05 vs. NS group; #P<0.05 vs. DS
group; &P<0.05 vs. NI/R group. The groups
assessed were as follows: NS, the non-diabetic sham group; NI/R,
the non-diabetic I/R group; DS, the diabetic sham group; DI/R, the
diabetic I/R group. DM, diabetes mellitus; SEM, standard error of
the mean; I/R, ischemia/reperfusion; p-, phosphorylated; eIF2α,
eukaryotic translation initiation factor 2 subunit α; ATF4,
activating transcription factor 4; CHOP, C/EBP homologous
protein. |
Renal SIRT1 signaling is impaired in
DM and pyroptosis is a crucial event during renal IR injury
The expression levels of SIRT1 and
pyroptosis-related proteins were assessed in all experimental and
control rats. As revealed in Fig. 4A
and B, SIRT1 expression was significantly downregulated in the
kidney following ischemia reperfusion injury in the DI/R group
compared with the NS group (P<0.05). Furthermore, the expression
levels of SIRT1 were significantly lower in the DI/R group compared
with the NI/R group (P<0.05). As shown in Fig. 4A and C-H), pyroptosis-associated
proteins, including caspase-1, caspase-11, NLRP3, IL-18, and IL-1β,
were markedly increased by I/R injury in the NI/R and DI/R groups
compared with the NS group (P<0.05). In addition, following
renal I/R, the levels of proteins related to pyroptosis were
significantly higher in the diabetic group than those in the NI/R
group (P<0.05). These data demonstrated that DM impaired renal
SIRT1 signaling. Following renal I/R, the levels of SIRT1 were
further reduced, thus triggering pyroptosis and resulting in
AKI.
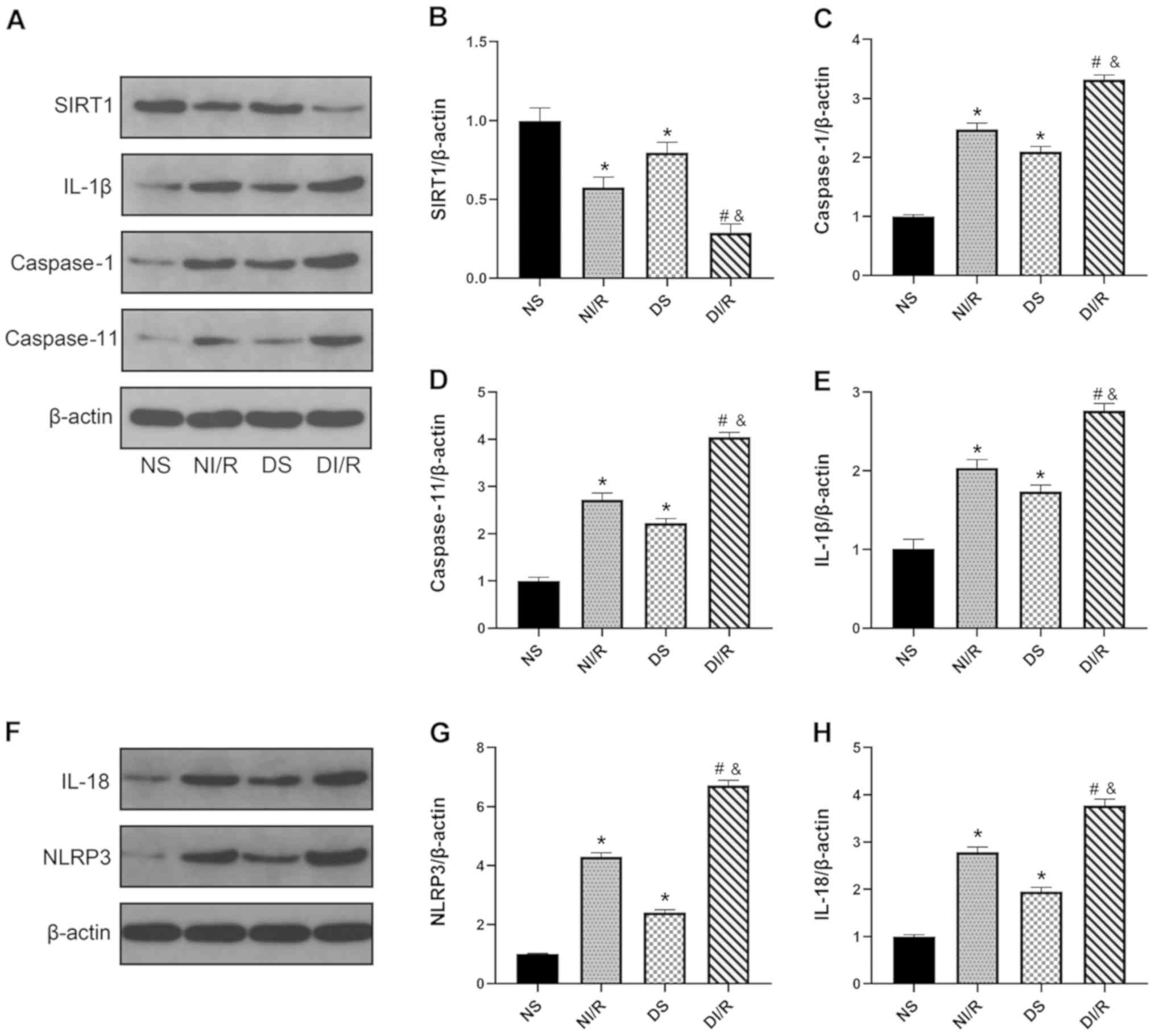 | Figure 4.DM impairs myocardial SIRT1 signaling
and aggravates pyroptosis in both renal I/R-injured and control
rats. Western blot analysis was performed after 24 h of
reperfusion. (A) Representative blots and histograms showing
expression of (B) SIRT1; (C) active caspase-1; (D) active
caspase-11; and (E) IL-1β. (F) Representative blots. Histograms
showing expression of (G) NLRP3 and (H) IL-18. The results are
expressed as the mean ± SEM, n=6. *P<0.05 vs. NS group;
#P<0.05 vs. DS group; &P<0.05 vs.
NI/R group. The groups assessed were as follows: NS, the
non-diabetic sham group; NI/R, the non-diabetic I/R group; DS, the
diabetic sham group; DI/R, the diabetic I/R group. DM, diabetes
mellitus; SIRT1, sirtuin 1; I/R, ischemia/reperfusion; SEM,
standard error of the mean; IL, interleukin; NLRP3, NLR family
pyrin domain containing 3. |
Analysis of histopathology and renal
function reveals that resveratrol, an established agonist of SIRT1,
protects against renal I/R injury in diabetic rats
In order to further evaluate the effect of SIRT1
signaling on renal I/R injury in the animal model of type 1 DM,
rats were pretreated with resveratrol, an established agonist of
SIRT1, for 7 consecutive days prior to surgery. As revealed in
Fig. 5A and B, I/R significantly
increased the renal tubular injury scores related to pathological
changes in the DI/R group compared with the DS group (P<0.05),
while resveratrol pre-treatment effectively ameliorated renal
injury in the DI/R+Res group compared with the DI/R group
(P<0.05). As revealed in Fig. 5C
and D, significantly aggravated kidney dysfunction was evident
in the DI/R group compared with the DS group (P<0.05). However,
resveratrol significantly reduced the levels of BUN and serum
creatinine in the DI/R+Res group compared with the DI/R group
(P<0.05). Collectively, these data indicated that the
re-activation of SIRT1 partially protected against renal I/R injury
in diabetic rats.
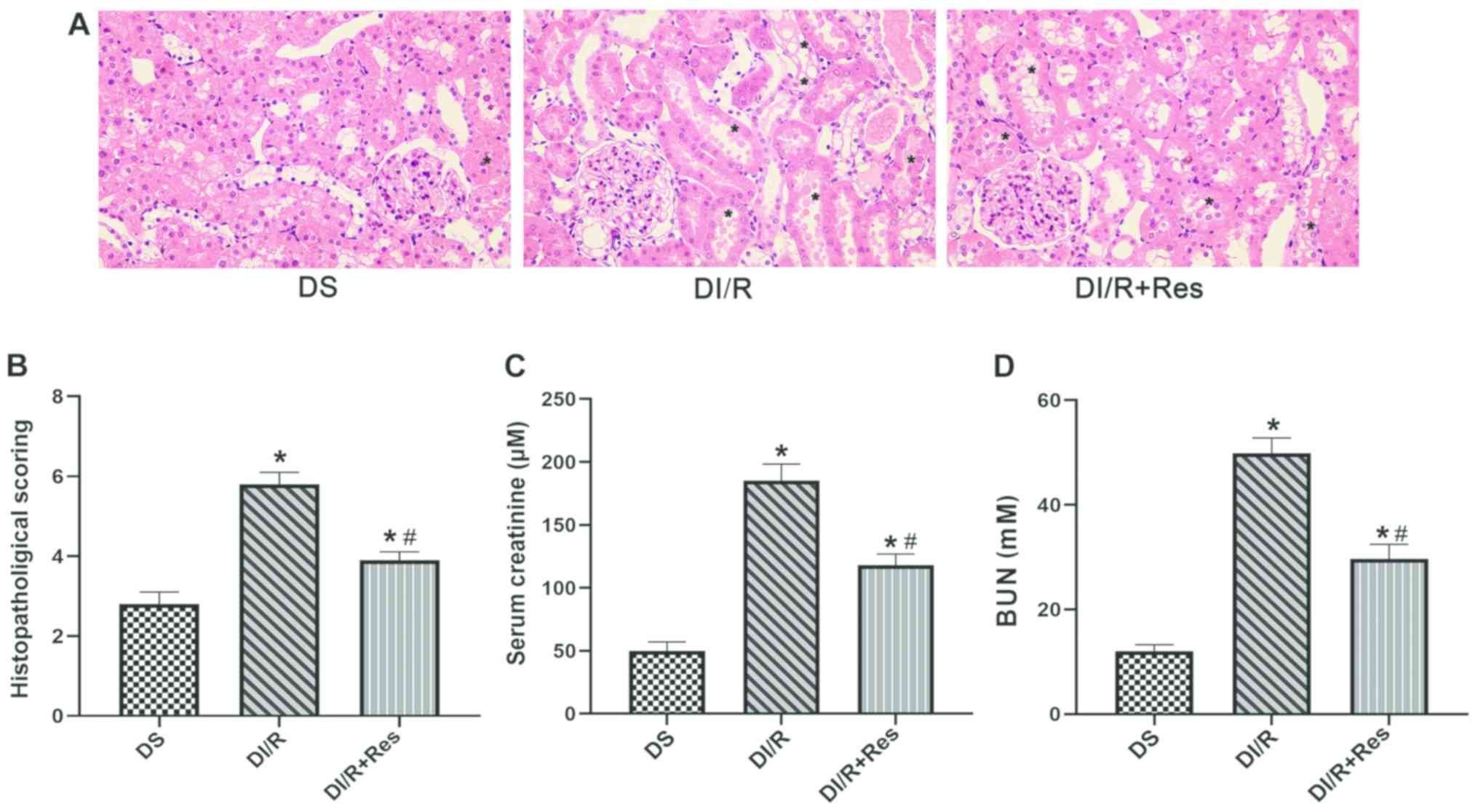 | Figure 5.Resveratrol pre-treatment
significantly attenuates kidney dysfunction in terms of the serum
levels of creatine, BUN and histopathological scoring. (A) H&E
staining of kidney sections (magnification, ×400); ‘*’ symbol
represents the pathological changes in the kidney, including
tubular epithelial cell swelling, interstitial expansion,
intertubular hemorrhaging and necrotic tubules. (B)
Histopathological scoring. (C) Serum creatinine concentration. (D)
Serum BUN concentration. Data are presented as the mean ± SEM.
*P<0.05 vs. the DS group; #P<0.05 vs. the DI/R
group. The groups assessed were as follows: DS, the diabetic sham
group; DI/R, the diabetic I/R group; DI/R+Res, the diabetic I/R +
resveratrol group. BUN, blood urea nitrogen; I/R,
ischemia/reperfusion; SEM, standard error of the mean. |
Resveratrol supplementation markedly
increases SIRT1 expression thus reducing the levels of ERS and
alleviating renal pyroptosis
Next, the relationship between SIRT1 expression and
ERS-mediated pyroptosis was explored in diabetic animals subjected
to I/R injury. As revealed in Fig. 6F
and G, I/R injury resulted in an evident reduction in the
expression of SIRT1 compared with the DS group (P<0.05).
Moreover, resveratrol treatment led to a significantly higher level
of SIRT1 in the DI/R+Res group than those in the DI/R group
(P<0.05). As revealed in Fig.
6A-E, the activation of SIRT1 significantly reduced the
expression of p-PERK, p-eIF2α, ATF4, and CHOP in the group treated
with resveratrol (P<0.05). Furthermore, as revealed in Fig. 6F and H-M, the expression of
caspase-1, caspase-11, IL-1β, NLRP3, and IL-18 (which reflected the
level of pyroptosis) was significantly reduced in the DI/R+Res
group compared with the DI/R group (P<0.05). Collectively, these
data further indicated that resveratrol suppressed renal ERS levels
by upregulating SIRT1 signaling in diabetic rats. Furthermore, the
enhancement of SIRT1 resulted in the alleviation of renal
pyroptosis.
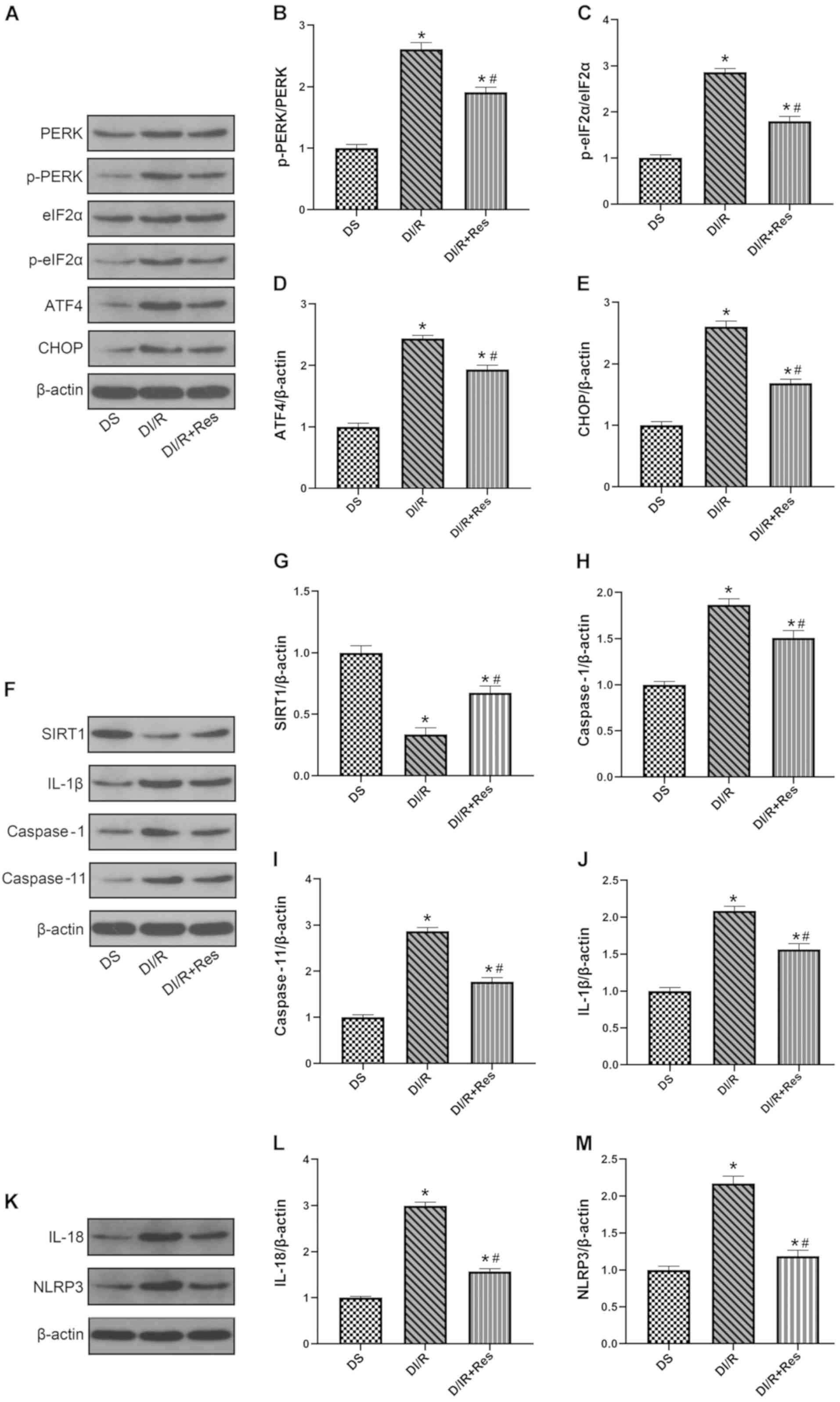 | Figure 6.Resveratrol pre-treatment upregulates
SIRT1 expression, reduces endoplasmic reticulum stress and
attenuates pyroptosis induced by I/R injury in diabetic rats.
Western blotting was performed, (A) representative blots and
histograms showing (B) p-PERK/PERK ratio; (C) p-eIF2α/eIF2α ratio;
(D) ATF4 expression; and (E) CHOP expression. (F) Representative
blots and histograms showing expression of (G) SIRT1; (H) active
caspase-1; (I) active caspase-11; and (J) IL-1β. (K) Representative
blots and histograms showing expression of (L) IL-18 and (M) NLRP3.
Date are expressed as the mean ± SEM, n=6. *P<0.05 vs. DS group;
#P<0.05 vs. DI/R group. The groups assessed were as
follows: DS, the diabetic sham group; DI/R, the diabetic I/R group;
DI/R+Res, the diabetic I/R + resveratrol group. SIRT1, sirtuin 1;
I/R, ischemia/reperfusion; SEM, standard error of the mean; p,
phosphorylated; eIF2α, eukaryotic translation initiation factor 2
subunit α; ATF4, activating transcription factor 4; CHOP, C/EBP
homologous protein; IL, interleukin; NLRP3, NLR family pyrin domain
containing 3. |
Discussion
The present analysis indicated that SIRT1 signaling
was impaired in type 1 diabetic rats. Following renal I/R injury,
the levels of SIRT1 expression were further attenuated. Notably,
resveratrol, a known agonist of SIRT1 signaling, alleviated
ERS-mediated pyroptosis, resulting in the amelioration of
I/R-induced kidney injury. Fig. 7
outlines the proposed induction of pyroptosis. In the diagram renal
injury in patients with DM induces hyperglycemia, while also
initiating ROS and an inflammatory response, as well as
downregulating the expression of SIRT1 (a protein that normally
protects the ER from stress). This results in ER stress via the
PERK/eIF2α/ATF4/CHOP pathways, thus triggering pyroptosis. The
present data therefore revealed a potential mechanism for the
exacerbation of renal injury following I/R in diabetics.
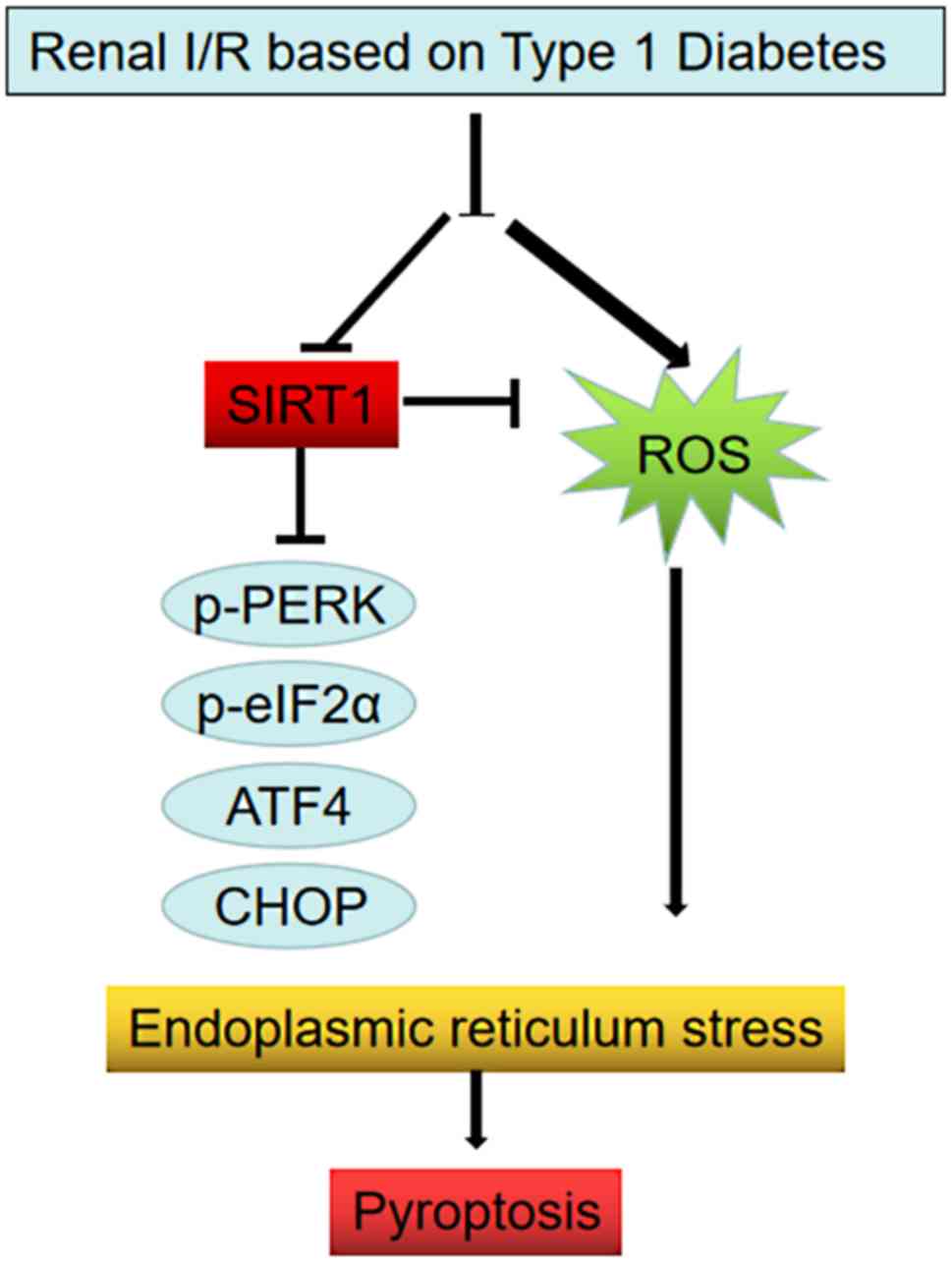 | Figure 7.Schematic diagram of the induction of
pyroptosis via ER stress and the effects of SIRT1 on this process.
DM induces hyperglycemia and initiates cellular oxidative stress
and an inflammatory response. Collectively, these processes result
in ER stress via the PERK/eIF2α/ATF4/CHOP pathways. Furthermore, DM
downregulated the expression of SIRT1, a protein that normally
protects the ER from stress. ER, endoplasmic reticulum; SIRT1,
sirtuin 1; DM, diabetes mellitus; I/R, ischemia/reperfusion; p,
phosphorylated; eIF2α, eukaryotic translation initiation factor 2
subunit α; ATF4, activating transcription factor 4; CHOP, C/EBP
homologous protein; ROS, reactive oxygen species. |
Renal I/R injury can induce dysfunction in a range
of organs, including AKI (30).
Furthermore, clinical trials have demonstrated that AKI is
associated with high morbidity rates (31). It is well known that DM is a
serious risk factor for renal disease (32) and that renal I/R injury,
accompanied by diabetes, can aggravate AKI; this condition has a
poor prognosis (33). Previous
studies have reported that the accumulation of ROS plays a
significant role in I/R-induced kidney injury in diabetic patients
(34). A previous study reported
increased levels of BUN, serum creatinine and proinflammatory
cytokines in diabetic rats that had experienced I/R injury
(6). In the present study, it was
observed that diabetes aggravated renal I/R injury through acute
tubular damage and exacerbated kidney dysfunction; these effects
were reflected by the higher levels of BUN and serum creatinine.
These results were in line with those reported by previous
publications.
SIRT1, an NAD+-dependent deacetylase, can
exert a marked effect in a number of cellular functions, including
transcriptional reprogramming, DNA repair, stress resistance and
apoptosis (35). In a recent
study, Li et al (21)
demonstrated that SIRT1 is a significant age-related protective
factor against renal I/R-induced injury. Moreover, several studies
have demonstrated that SIRT1 may represent an effective therapeutic
option for diabetes by controlling insulin secretion, regulating
fatty acid oxidation (36,37) and defending against cellular
oxidative damage and inflammation (38). Notably, Yu et al (39) demonstrated that DM downregulated
SIRT1 signaling, and it has been shown that this effect was further
impaired by I/R injury in cardiomyocytes (39). In accordance with previous
research, in the present study it was observed that the expression
of SIRT1 was decreased in both I/R groups, especially in the DI/R
group. These results indicated that diabetes leads to a reduction
in SIRT1 signaling, thus exacerbating the damage caused by renal
I/R injury-induced oxidative stress. It was revealed that IR
injury-induced kidney dysfunction and tissue damage in diabetic
rats was markedly alleviated by treatment with resveratrol, a known
agonist of SIRT1. Collectively, this data supported the hypothesis
that SIRT1 was notably attenuated in diabetic rats and was further
impaired by I/R injury. However, the reason that I/R treatment
downregulates the expression of SIRT1 may be due to the
overactivation of oxidative stress or other factors, and the
underlying mechanism requires further exploration in a future
study.
Numerous research studies have verified the
relevance of ERS in the pathophysiological process of diabetic I/R
injury in cardiomyocytes (40,41).
For example, Yu et al (39)
revealed that the inhibition of oxidative stress, and the
attenuation of SIRT1-mediated ERS, could improve myocardial
I/R-induced damage in diabetic state. More recently, Guo et
al (42) revealed that ERS
levels were downregulated by SIRT1 in diabetic rats. However, very
little is known about kidney injury. In the present study, it was
observed that the upregulation of ERS was mediated by the
PERK/eIF2α/ATF4 pathway in both of the I/R groups but especially in
the DI/R group. In addition, resveratrol was used in the DI/R
groups to further confirm the effects of SIRT1. As anticipated, the
SIRT1 agonist attenuated the ERS levels. To the best of our
knowledge, there is no previous research describing the fact that
DM exacerbates renal I/R injury by enhancing SIRT1-mediated levels
of ERS. In addition, the specific mechanisms responsible for the
effect of SIRT1 on ERS in cases of I/R injury in the diabetic
kidney have yet to be fully elucidated. Previous studies
highlighted the potential role of SIRT1, predominantly because this
protein is associated with the circulatory system and has the
ability to regulate antioxidative stress (43,44).
It was speculated in the present study that DM impaired SIRT1
signaling and that the increased levels of oxidative stress in
diabetic rats may contribute to enhanced ERS via the
PERK/eIF2α/ATF4 pathway, thus leading to the aggravation of I/R
injury-induced kidney damage.
A previous study has demonstrated that via the
activation ERS, I/R injury can induce several types of cell death,
including autophagy, apoptosis, and necroptosis (20). Pyroptosis, which is dependent upon
the levels of caspase-1, can cause the plasma membrane to burst and
the activation of a range of inflammatory mediators; this results
in a form of inflammatory cell death that differs from apoptosis
(20,45). Furthermore, the activation of
caspase-1 can result in the separate conversion of pro-inflammatory
forms of IL-1β and IL-18 into mature IL-1β and IL-18 (46). Subsequently, these active
inflammatory cytokines are delivered to the internal environment to
promote inflammation (47). Qiu
et al (48) further
demonstrated that the activation of inflammatory mediators,
including caspase-1, IL-1β and IL-18 was elevated in a diabetic
animal model. Furthermore, this study showed that when these
diabetic rats were subjected to myocardial I/R insult, there were
further increases in the levels of NLRP3 inflammasomes, activated
caspase-1 and IL-1β. In another study, Wang et al (49) revealed that pyroptosis was
associated with the development of I/R in renal tubular cells. The
present study revealed that diabetes and ischemia both
significantly induce cellular pyroptosis and that this effect was
exacerbated in diabetic states. It was also revealed that
resveratrol ameliorated pyroptosis-mediated renal damage. Previous
studies reported that ERS is an essential pathway in pyroptosis
(50,20). The present data concurred with
these previous findings in that the increased expression of
caspase-1, caspase-11 and IL-1β was observed in the NI/R group,
especially in the DI/R group. In addition, resveratrol ameliorated
this effect. Collectively, the data generated during this study
indicated that DM aggravates renal I/R injury by downregulating the
SIRT1 pathway. However, diabetes is a complex metabolic disease
involving aberrant levels of glucose, lipids and inflammation
(3,7). As for the specific factors of
diabetes that may be associated with the SIRT1 pathway and ERS,
further research is required.
The experimental data of the present study revealed
that SIRT1 signaling was impaired in diabetic rats, thus
aggravating ERS. This induced cellular pyroptosis following renal
I/R injury, which ultimately led to AKI. The present research
enhanced our understanding of why the diabetic kidney is
susceptible to I/R injury. In addition, SIRT1 appears to represent
a promising therapeutic target for diabetic patients with renal I/R
injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Wuhan Morning
Light Plan of Youth Science and Technology (grant no.
2017050304010281), The Natural Science Foundation of Hubei Province
(grant nos. 2016CFB114 and 2017CFB181) and The Research Project of
Wuhan University (grant no. 2042017kf0097).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, DG, YY and LW conceived and designed the
research, performed experiments and approved the final version of
the manuscript. JZ, ZC and XL interpreted the results and prepared
the figures. JZ, XL and LW analyzed the data, drafted, edited and
revised the manuscript. All authors reviewed and approved the final
manuscript, certify that they have participated sufficiently in the
present study, and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
Renmin Hospital of Wuhan University, and all procedures complied
with the Principles of Animal Care of Wuhan University (Wuhan,
China) and Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li PK, Burdmann EA and Mehta RL: World
kidney day 2013: Acute kidney injury-global health alert. Am J
Kidney Dis. 61:359–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Bonventre JV and Parrish AR: The
aging kidney: Increased susceptibility to nephrotoxicity. Int J Mol
Sci. 15:15358–15376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lejay A, Fang F, John R, Van JA, Barr M,
Thaveau F, Chakfe N, Geny B and Scholey JW: Ischemia reperfusion
injury, ischemic conditioning and diabetes mellitus. J Mol Cell
Cardiol. 91:11–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muroya Y, He X, Fan L, Wang S, Xu R, Fan F
and Roman RJ: Enhanced renal ischemia-reperfusion injury in aging
and diabetes. Am J Physiol Renal Physiol. 315:F1843–F1854. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melin J, Hellberg O, Akyurek LM, Kallskog
O, Larsson E and Fellstrom BC: Ischemia causes rapidly progressive
nephropathy in the diabetic rat. Kidney Int. 52:985–991. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Hu F, Wen J, Wei X, Zeng Y, Sun
Y, Luo S and Sun L: Effects of sevoflurane on NF-κB and TNF-α
expression in renal ischemia-reperfusion diabetic rats. Inflamm
Res. 66:901–910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi S, Lei S, Tang C, Wang K and Xia Z:
Melatonin attenuates acute kidney ischemia/reperfusion injury in
diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling
pathway. Biosci Rep. 39:BSR201816142019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu Y, Wu Y, Meng M, Luo M, Zhao H, Sun H
and Gao S: GYY4137 protects against myocardial ischemia/reperfusion
injury via activation of the PHLPP-1/Akt/Nrf2 signaling pathway in
diabetic mice. J Surg Res. 225:29–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bravo R, Parra V, Gatica D, Rodriguez AE,
Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E,
et al: Endoplasmic reticulum and the unfolded protein response:
Dynamics and metabolic integration. Int Rev Cell Mol Biol.
301:215–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao X, Fu L, Xiao M, Xu C, Sun L, Zhang T,
Zheng F and Mei C: The nephroprotective effect of
tauroursodeoxycholic acid on ischaemia/reperfusion-induced acute
kidney injury by inhibiting endoplasmic reticulum stress. Basic
Clin Pharmacol Toxicol. 111:14–23. 2012.PubMed/NCBI
|
|
11
|
Gu Y, Huang F, Wang Y, Chen C, Wu S, Zhou
S, Hei Z and Yuan D: Connexin32 plays a crucial role in
ROS-mediated endoplasmic reticulum stress apoptosis signaling
pathway in ischemia reperfusion-induced acute kidney injury. J
Transl Med. 16:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan M, Shu S, Guo C, Tang C and Dong Z:
Endoplasmic reticulum stress in ischemic and nephrotoxic acute
kidney injury. Ann Med. 50:381–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Wang L, Weng X, Chen H, Du Y, Diao
C, Chen Z and Liu X: Inhibition of Brd4 alleviates renal
ischemia/reperfusion injury-induced apoptosis and endoplasmic
reticulum stress by blocking FoxO4-mediated oxidative stress. Redox
Biol. 24:1011952019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su M, Ren S, Zhong W and Han X: Impact of
propofol on renal ischemia/reperfusion endoplasmic reticulum
stress. Acta Cir Bras. 32:533–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu H, Fan Y, Sun H, Chen L and Man X:
Curcumin inhibits endoplasmic reticulum stress induced by cerebral
ischemia- reperfusion injury in rats. Exp Ther Med. 14:4047–4052.
2017.PubMed/NCBI
|
|
16
|
Pandey VK, Mathur A and Kakkar P: Emerging
role of Unfolded Protein Response (UPR) mediated proteotoxic
apoptosis in diabetes. Life Sci. 216:246–258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong T, Liao D, Liu X and Lei X: Using
small molecules to dissect non-apoptotic programmed cell death:
Necroptosis, ferroptosis, and pyroptosis. Chembiochem.
16:2557–2561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jorgensen I, Lopez JP, Laufer SA and Miao
EA: IL-1β, IL-18, and eicosanoids promote neutrophil recruitment to
pore-induced intracellular traps following pyroptosis. Eur J
Immunol. 46:2761–2766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL,
Zhan J, Tong YN, Lin LR and He YN: Ischemia-reperfusion induces
renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J
Physiol Renal Physiol. 306:F75–F84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li WF, Yang K, Zhu P, Zhao HQ, Song YH,
Liu KC and Huang WF: Genistein ameliorates
ischemia/reperfusion-induced renal injury in a SIRT1-dependent
manner. Nutrients. 9:E4032017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelly GS: A review of the sirtuin system,
its clinical implications, and the potential role of dietary
activators like resveratrol: Part 2. Altern Med Rev. 15:313–328.
2010.PubMed/NCBI
|
|
23
|
Prola A, Pires Da Silva J, Guilbert A,
Lecru L, Piquereau J, Ribeiro M, Mateo P, Gressette M, Fortin D,
Boursier C, et al: SIRT1 protects the heart from ER stress-induced
cell death through eIF2α deacetylation. Cell Death Differ.
24:343–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koka S, Aluri HS, Xi L, Lesnefsky EJ and
Kukreja RC: Chronic inhibition of phosphodiesterase 5 with
tadalafil attenuates mitochondrial dysfunction in type 2 diabetic
hearts: Potential role of NO/SIRT1/PGC-1α signaling. Am J Physiol
Heart Circ Physiol. 306:H1558–H1568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai
M, Pei H, Wang X, Zhang H, Meng Q, et al: Melatonin
receptor-mediated protection against myocardial
ischemia/reperfusion injury: Role of SIRT1. J Pineal Res.
57:228–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue R, Lei S, Xia ZY, Wu Y, Meng Q, Zhan
L, Su W, Liu H, Xu J, Liu Z, et al: Selective inhibition of PTEN
preserves ischaemic post-conditioning cardioprotection in
STZ-induced Type 1 diabetic rats: Role of the PI3K/Akt and
JAK2/STAT3 pathways. Clin Sci (Lond). 130:377–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W, Luo J, Yang F, Wang Y, Yin Y,
Strom A, Gustafsson JÅ and Guan X: BRCA1 inhibits AR-mediated
proliferation of breast cancer cells through the activation of
SIRT1. Sci Rep. 6:220342016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun J, Guo E, Yang J, Yang Y, Liu S, Hu J,
Jiang X, Dirsch O, Dahmen U, Dong W and Liu A: Carbon monoxide
ameliorates hepatic ischemia/reperfusion injury via sirtuin
1-mediated deacetylation of high-mobility group box 1 in rats.
Liver Transpl. 23:510–526. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye
Z and Zhang X: Ischemic preconditioning attenuates
ischemia/reperfusion-induced kidney injury by activating autophagy
via the SGK1 signaling pathway. Cell Death Dis. 9:3382018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Liu X, Chen H, Chen Z, Weng X, Qiu
T and Liu L: Effect of picroside II on apoptosis induced by renal
ischemia/reperfusion injury in rats. Exp Ther Med. 9:817–822. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Melin J, Hellberg O and Fellström B:
Hyperglycaemia and renal ischaemia-reperfusion injury. Nephrol Dial
Transplant. 18:460–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumaş M, Eşrefoğlu M, Karataş E, Duymaç N,
Kanbay S, Ergün IS, Üyüklü M and Koçyiğit A: Investigation of
dose-dependent effects of berberine against renal
ischemia/reperfusion injury in experimental diabetic rats.
Nefrologia. 39:411–423. 2019.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abu-Saleh N, Awad H, Khamaisi M, Armaly Z,
Karram T, Heyman SN, Kaballa A, Ichimura T, Holman J and Abassi Z:
Nephroprotective effects of TVP1022, a non-MAO inhibitor S-isomer
of rasagiline, in an experimental model of diabetic renal ischemic
injury. Am J Physiol Renal Physiol. 306:F24–F33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grubisha O, Smith BC and Denu JM: Small
molecule regulation of Sir2 protein deacetylases. FEBS J.
272:4607–4616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gerhart-Hines Z, Rodgers JT, Bare O, Lerin
C, Kim SH, Mostoslavsky R, Alt FW, Wu Z and Puigserver P: Metabolic
control of muscle mitochondrial function and fatty acid oxidation
through SIRT1/PGC-1alpha. EMBO J. 26:1913–1923. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bordone L, Motta MC, Picard F, Robinson A,
Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A,
et al: Sirt1 regulates insulin secretion by repressing UCP2 in
pancreatic beta cells. PLoS Biol. 4:e312006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kitada M and Koya D: SIRT1 in Type 2
diabetes: Mechanisms and therapeutic potential. Diabetes Metab J.
37:315–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai
M, Yang Y, Chen W, Liu J, Yi W, et al: Reduced silent information
regulator 1 signaling exacerbates myocardial ischemia-reperfusion
injury in type 2 diabetic rats and the protective effect of
melatonin. J Pineal Res. 59:376–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu J, Zhou Q, Xu W and Cai L: Endoplasmic
reticulum stress and diabetic cardiomyopathy. Exp Diabetes Res.
2012:8279712012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo W, Jiang T, Lian C, Wang H, Zheng Q
and Ma H: QKI deficiency promotes FoxO1 mediated nitrosative stress
and endoplasmic reticulum stress contributing to increased
vulnerability to ischemic injury in diabetic heart. J Mol Cell
Cardiol. 75:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chong ZZ, Wang S, Shang YC and Maiese K:
Targeting cardiovascular disease with novel SIRT1 pathways. Future
Cardiol. 8:89–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luo XY, Qu SL, Tang ZH, Zhang Y, Liu MH,
Peng J, Tang H, Yu KL, Zhang C, Ren Z and Jiang ZS: SIRT1 in
cardiovascular aging. Clin Chim Acta. 437:106–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bergsbaken T and Cookson BT: Macrophage
activation redirects yersinia-infected host cell death from
apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog.
3:e1612007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fantuzzi G and Dinarello CA:
Interleukin-18 and interleukin-1 beta: Two cytokine substrates for
ICE (caspase-1). J Clin Immunol. 19:1–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chou X, Ding F, Zhang X, Ding X, Gao H and
Wu Q: Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum
stress and pyroptosis through XBP-1s deacetylation in human renal
tubular epithelial cells. Arch Toxicol. 93:965–986. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M,
Meng Q, Zhou B, Leng Y and Xia ZY: NLRP3 Inflammasome
activation-mediated pyroptosis aggravates myocardial
ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev.
2017:97432802017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang L, Chen Z, Weng X, Wang M, Du Y and
Liu X: Combined ischemic postconditioning and ozone
postconditioning provides synergistic protection against renal
ischemia and reperfusion injury through inhibiting pyroptosis.
Urology. 123:296.e1–296.e8. 2019. View Article : Google Scholar
|
|
50
|
Yang CC, Yao CA, Yang JC and Chien CT:
Sialic acid rescues repurified lipopolysaccharide-induced acute
renal failure via inhibiting TLR4/PKC/gp91-mediated endoplasmic
reticulum stress, apoptosis, autophagy, and pyroptosis signaling.
Toxicol Sci. 141:155–165. 2014. View Article : Google Scholar : PubMed/NCBI
|





















