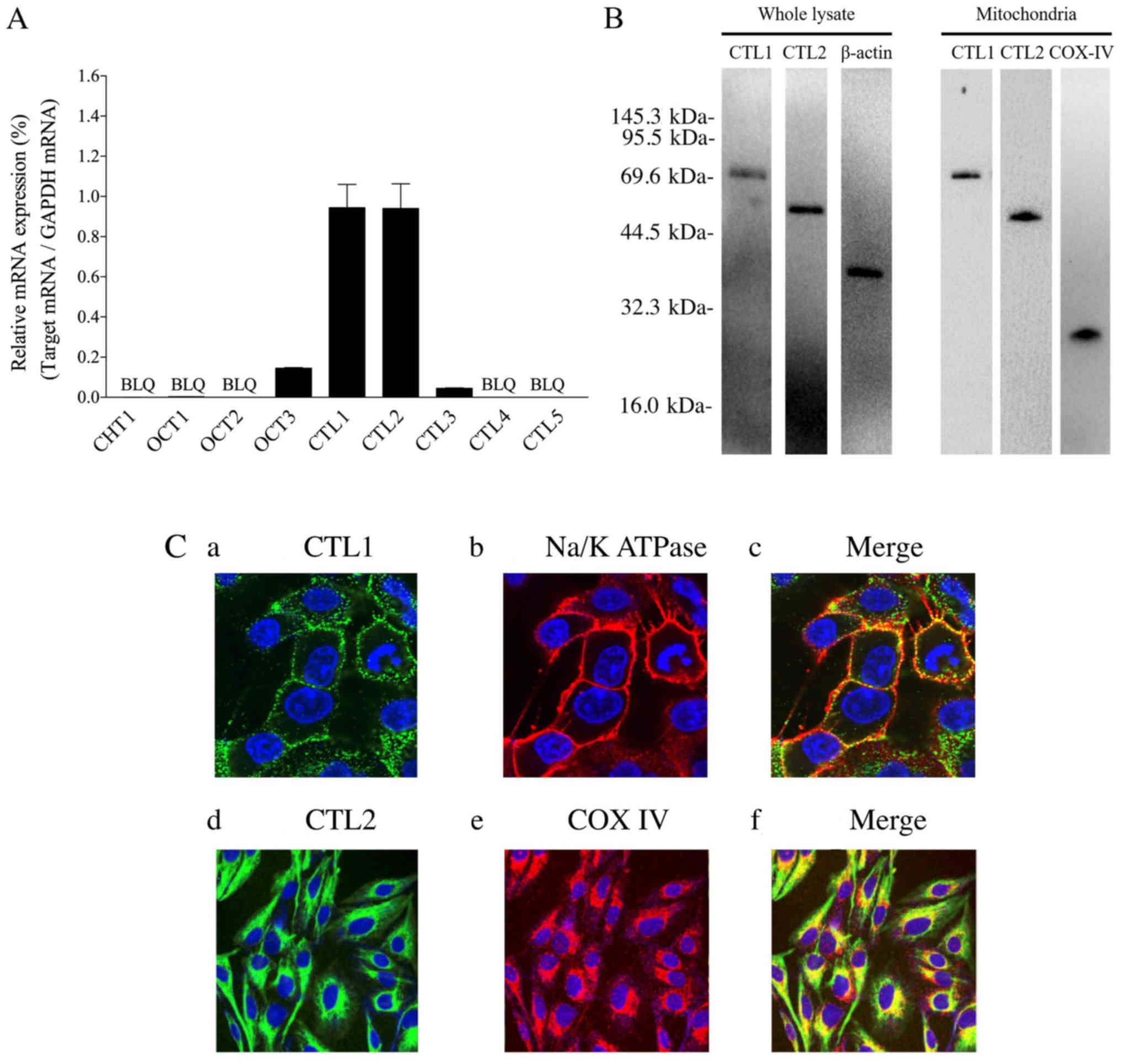
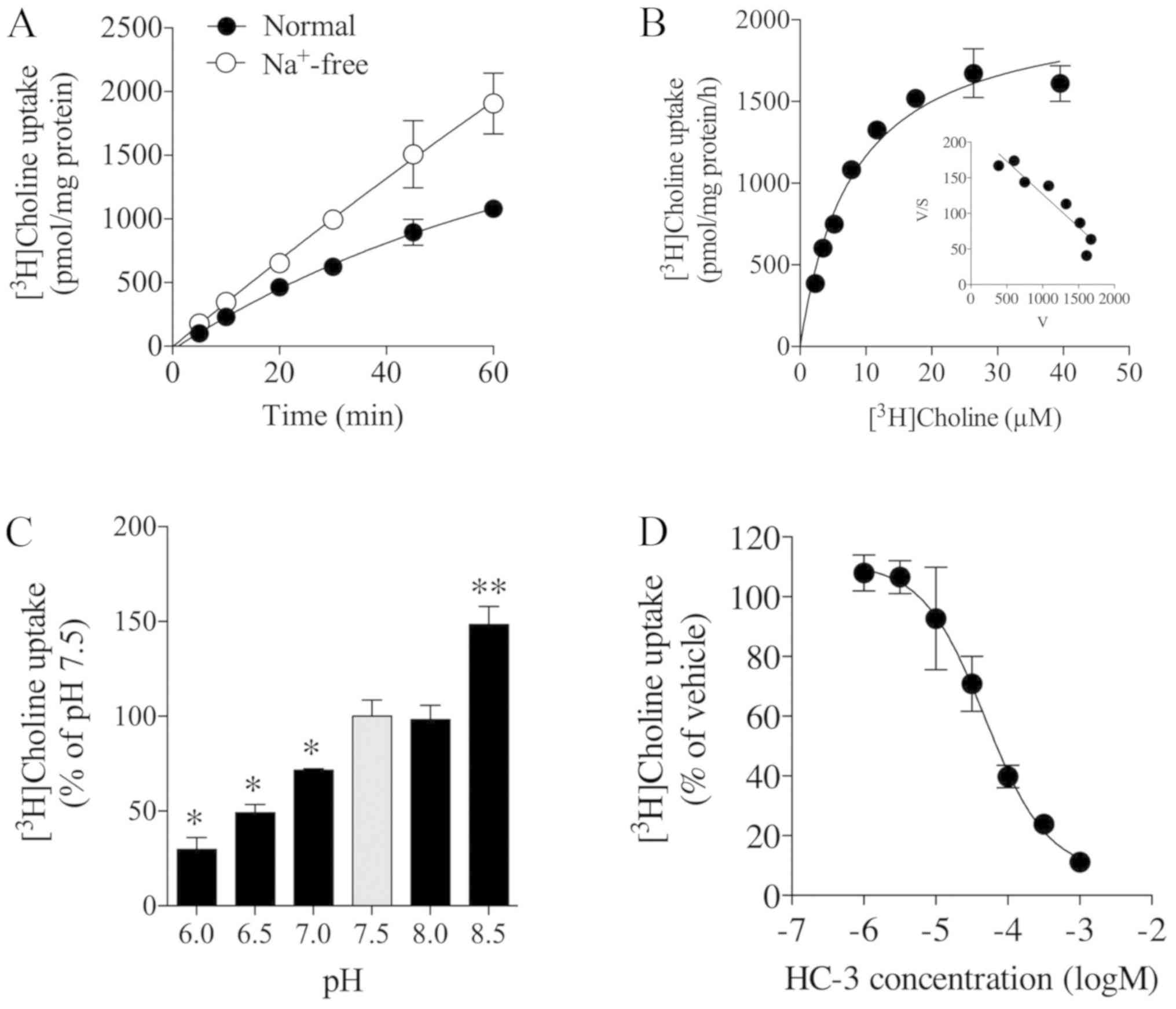
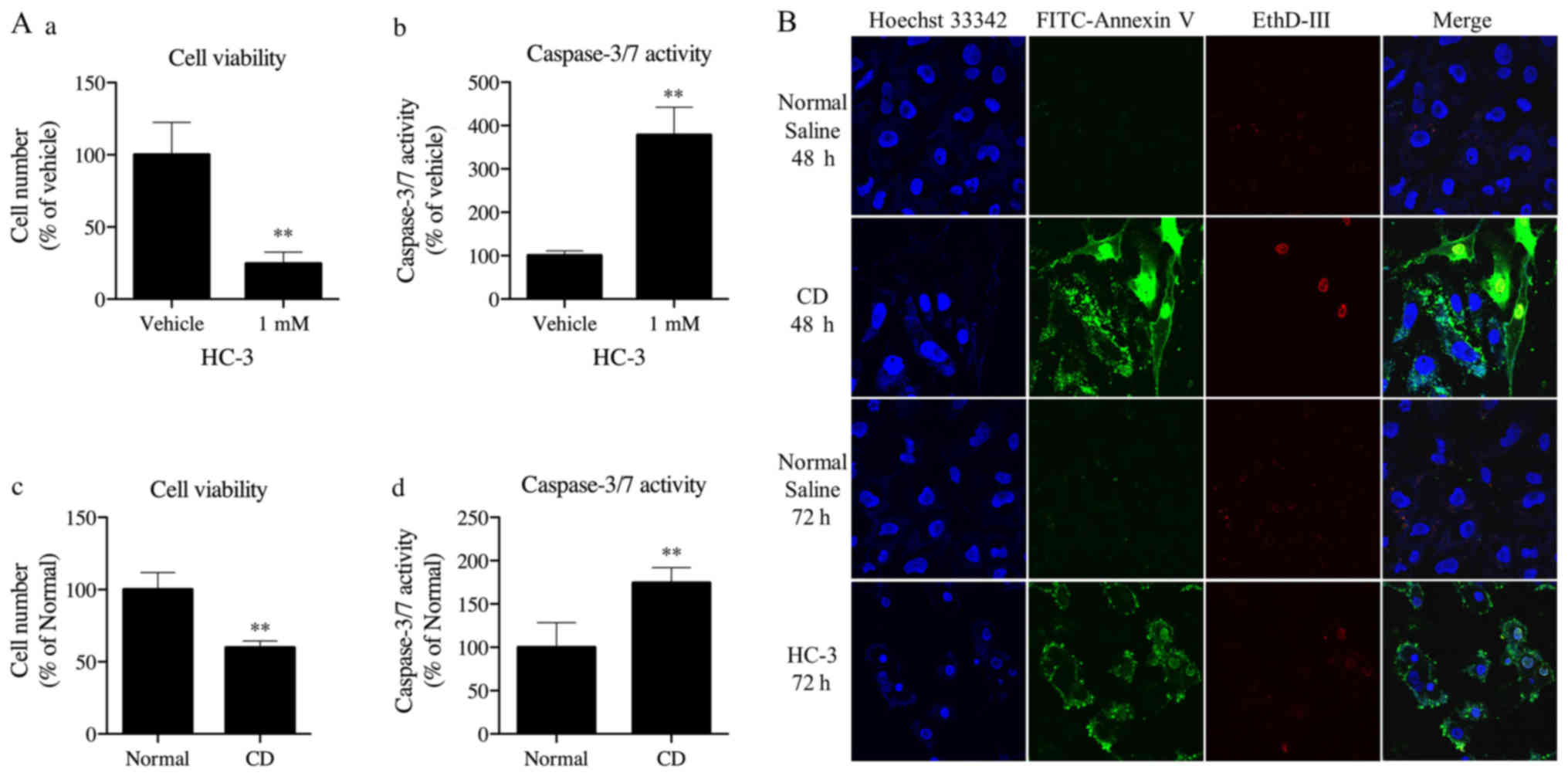
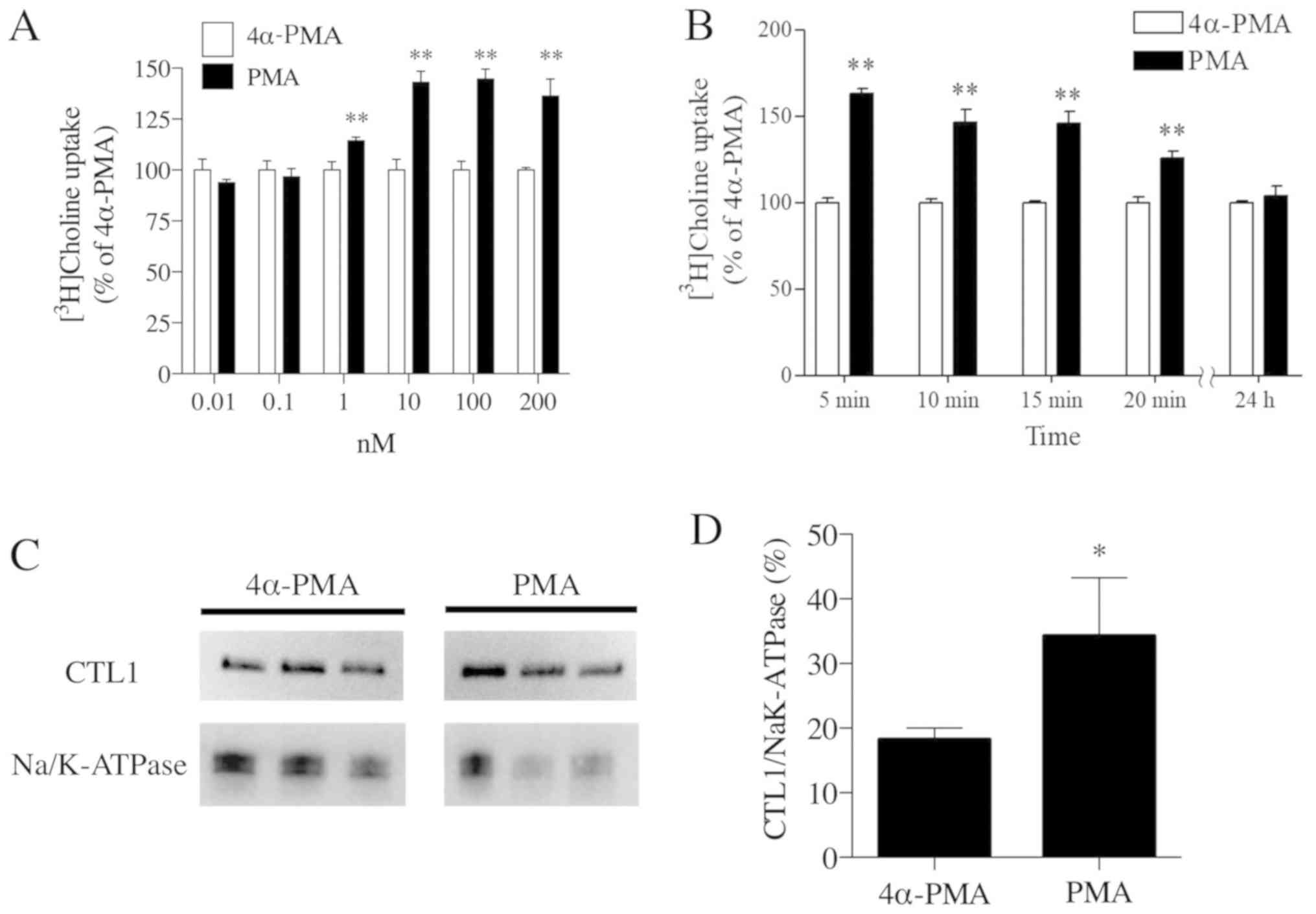
|
1
|
Michel V, Yuan Z, Ramsubir S and Bakovic
M: Choline transport for phospholipid synthesis. Exp Biol Med
(Maywood). 231:490–504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okuda T, Haga T, Kanai Y, Endou H,
Ishihara T and Katsura I: Identification and characterization of
the high-affinity choline transporter. Nat Neurosci. 3:120–125.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koepsell H, Lips K and Volk C:
Polyspecific organic cation transporters: Structure, function,
physiological roles, and biopharmaceutical implications. Pharm Res.
24:1227–1251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Traiffort E, Ruat M, O'Regan S and Meunier
FM: Molecular characterization of the family of choline
transporter-like proteins and their splice variants. J Neurochem.
92:1116–1125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura T, Fujiwara R, Ishiguro N, Oyabu
M, Nakanishi T, Shirasaka Y, Maeda T and Tamai I: Involvement of
choline transporter-like proteins, CTL1 and CTL2, in
glucocorticoid-induced acceleration of phosphatidylcholine
synthesis via increased choline uptake. Biol Pharm Bull.
33:691–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robinson MB: Regulated trafficking of
neurotransmitter transporters: Common notes but different melodies.
J Neurochem. 80:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fullerton MD, Wagner L, Yuan Z and Bakovic
M: Impaired trafficking of choline transporter-like protein-1 at
the plasma membrane and inhibition of choline transport in THP-1
monocyte-derived macrophages. Am J Physiol Cell Physiol.
290:C1230–C1238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schenkel LC, Sivanesan S, Zhang J, Wuyts
B, Taylor A, Verbrugghe A and Bakovic M: Choline supplementation
restores substrate balance and alleviates complications of Pcyt2
deficiency. J Nutr Biochem. 26:1221–1234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michel V and Bakovic M: The ubiquitous
choline transporter SLC44A1. Cent Nerv Syst Agents Med Chem.
12:70–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Butler LM, Arning E, Wang R, Bottiglieri
T, Govindarajan S, Gao YT and Yuan JM: Prediagnostic levels of
serum one-carbon metabolites and risk of hepatocellular carcinoma.
Cancer Epidemiol Biomarkers Prev. 22:1884–1893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghoshal AK and Farber E: The induction of
liver cancer by dietary deficiency of choline and methionine
without added carcinogens. Carcinogenesis. 5:1367–1370. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rinella ME, Elias MS, Smolak RR, Fu T,
Borensztajn J and Green RM: Mechanisms of hepatic steatosis in mice
fed a lipogenic methionine choline-deficient diet. J Lipid Res.
49:1068–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishiyama R, Nagashima F, Iwao B, Kawai Y,
Inoue K, Midori A, Yamanaka T, Uchino H and Inazu M: Identification
and functional analysis of choline transporter in tongue cancer: A
novel molecular target for tongue cancer therapy. J Pharmacol Sci.
131:101–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haubrich DR and Gerber NH: Choline
dehydrogenase. Assay, properties and inhibitors. Biochem Pharmacol.
30:2993–3000. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wiedeman AM, Dyer RA, Green TJ, Xu Z, Barr
SI, Innis SM and Kitts DD: Variations in plasma choline and
metabolite concentrations in healthy adults. Clin Biochem.
60:77–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Apparsundaram S, Ferguson SM, George AL Jr
and Blakely RD: Molecular cloning of a human,
hemicholinium-3-sensitive choline transporter. Biochem Biophys Res
Commun. 276:862–867. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burckhardt G and Wolff NA: Structure of
renal organic anion and cation transporters. Am J Physiol Renal
Physiol. 278:F853–F866. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gründemann D, Liebich G, Kiefer N, Köster
S and Schömig E: Selective substrates for non-neuronal monoamine
transporters. Mol Pharmacol. 56:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kouji H, Inazu M, Yamada T, Tajima H, Aoki
T and Matsumiya T: Molecular and functional characterization of
choline transporter in human colon carcinoma HT-29 cells. Arch
Biochem Biophys. 483:90–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uchida Y, Inazu M, Takeda H, Yamada T,
Tajima H and Matsumiya T: Expression and functional
characterization of choline transporter in human keratinocytes. J
Pharmacol Sci. 109:102–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagashima F, Nishiyama R, Iwao B, Kawai Y,
Ishii C, Yamanaka T, Uchino H and Inazu M: Molecular and functional
characterization of choline transporter-like proteins in esophageal
cancer cells and potential therapeutic targets. Biomol Ther.
26:399–408. 2018. View Article : Google Scholar
|
|
22
|
Inazu M, Yamada T, Kubota N and Yamanaka
T: Functional expression of choline transporter-like protein 1
(CTL1) in small cell lung carcinoma cells: A target molecule for
lung cancer therapy. Pharmacol Res. 76:119–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yara M, Iwao B, Hara N, Yamanaka T, Uchino
H and Inazu M: Molecular and functional characterization of choline
transporter in the human trophoblastic cell line JEG-3 cells.
Placenta. 36:631–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaplan CP, Porter RK and Brand MD: The
choline transporter is the major site of control of choline
oxidation in isolated rat liver mitochondria. FEBS Lett. 321:24–26.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang XH, Zhao C and Ma ZA: The increase
of cell-membranous phosphatidylcholines containing polyunsaturated
fatty acid residues induces phosphorylation of p53 through
activation of ATR. J Cell Sci. 120:4134–4143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai H, Erhardt P, Troppmair J, Diaz-Meco
MT, Sithanandam G, Rapp UR, Moscat J and Cooper GM: Hydrolysis of
phosphatidylcholine couples Ras to activation of Raf protein kinase
during mitogenic signal transduction. Mol Cell Biol. 13:7645–7651.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beckman ML, Bernstein EM and Quick MW:
Multiple G protein-coupled receptors initiate protein kinase C
redistribution of GABA transporters in hippocampal neurons. J
Neurosci. 19:RC91999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jayanthi LD, Samuvel DJ, Blakely RD and
Ramamoorthy S: Evidence for biphasic effects of protein kinase C on
serotonin transporter function, endocytosis, and phosphorylation.
Mol Pharmacol. 67:2077–2087. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daniels GM and Amara SG: Regulated
trafficking of the human dopamine transporter. Clathrin-mediated
internalization and lysosomal degradation in response to phorbol
esters. J Biol Chem. 274:35794–35801. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gates J Jr, Ferguson SM, Blakely RD and
Apparsundaram S: Regulation of choline transporter surface
expression and phosphorylation by protein kinase C and protein
phosphatase 1/2A. J Pharmacol Exp Therap. 310:536–545. 2004.
View Article : Google Scholar
|
|
31
|
Hariparsad N, Carr BA, Evers R and Chu X:
Comparison of immortalized Fa2N-4 cells and human hepatocytes as in
vitro models for cytochrome P450 induction. Drug Metab Dispos.
36:1046–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramboer E, Vanhaecke T, Rogiers V and
Vinken M: Immortalized human hepatic cell lines for in vitro
testing and research purposes. Methods Mol Biol. 1250:53–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu N, Hu M, Duan R, Liu C, Guo H, Zhang M,
Yu Y, Wang X, Liu L and Liu X: Increased levels of fatty acids
contributed to induction of hepatic CYP3A4 activity induced by
diabetes-in vitro evidence from HepG2 cell and Fa2N-4 cell lines. J
Pharmacol Sci. 124:433–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rumsby M, Schmitt J, Sharrard M, Rodrigues
G, Stower M and Maitland N: Human prostate cell lines from normal
and tumourigenic epithelia differ in the pattern and control of
choline lipid headgroups released into the medium on stimulation of
protein kinase C. Br J Cancer. 104:673–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bouma ME, Rogier E, Verthier N, Labarre C
and Feldmann G: Further cellular investigation of the human
hepatoblastoma-derived cell line HepG2: Morphology and
immunocytochemical studies of hepatic-secreted proteins. In Vitro
Cell Dev Bio. 25:267–275. 1989. View Article : Google Scholar
|
|
36
|
Greene MW, Burrington CM, Lynch DT,
Davenport SK, Johnson AK, Horsman MJ, Chowdhry S, Zhang J, Sparks
JD and Tirrell PC: Lipid metabolism, oxidative stress and cell
death are regulated by PKC delta in a dietary model of nonalcoholic
steatohepatitis. PLoS One. 9:e858482014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greene MW, Burrington CM, Ruhoff MS,
Johnson AK, Chongkrairatanakul T and Kangwanpornsiri A: PKC{delta}
is activated in a dietary model of steatohepatitis and regulates
endoplasmic reticulum stress and cell death. J Biol Chem.
285:42115–42129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakatsuka A, Matsuyama M, Yamaguchi S,
Katayama A, Eguchi J, Murakami K, Tesgigawara S, Ogawa D, Wada N,
Yasunaka T, et al: Insufficiency of phosphatidylethanolamine
N-methyltransferase is a risk for lean non-alcoholic
steatohepatitis. Sci Rep. 6:217212016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu D, Shu XO, Xiang YB, Li H, Yang G, Gao
YT, Zheng W and Zhang X: Higher dietary choline intake is
associated with lower risk of nonalcoholic fatty liver in
normal-weight Chinese women. J Nutr. 144:2034–2040. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou RF, Chen XL, Zhou ZG, Zhang YJ, Lan
QY, Liao GC, Chen YM and Zhu HL: Higher dietary intakes of choline
and betaine are associated with a lower risk of primary liver
cancer: A case-control study. Sci Rep. 7:6792017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roppongi M, Izumisawa M, Terasaki K,
Muraki Y and Shozushima M: 18F-FDG and
11C-choline uptake in proliferating tumor cells is
dependent on the cell cycle in vitro. Ann Nucl Med. 33:237–243.
2018. View Article : Google Scholar : PubMed/NCBI
|